Effect of Solvent on Superhydrophobicity Behavior of Tiles Coated with Epoxy/PDMS/SS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of SS from POFA
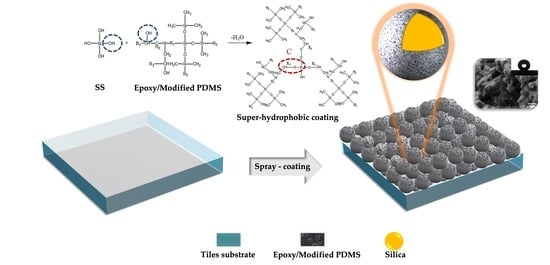
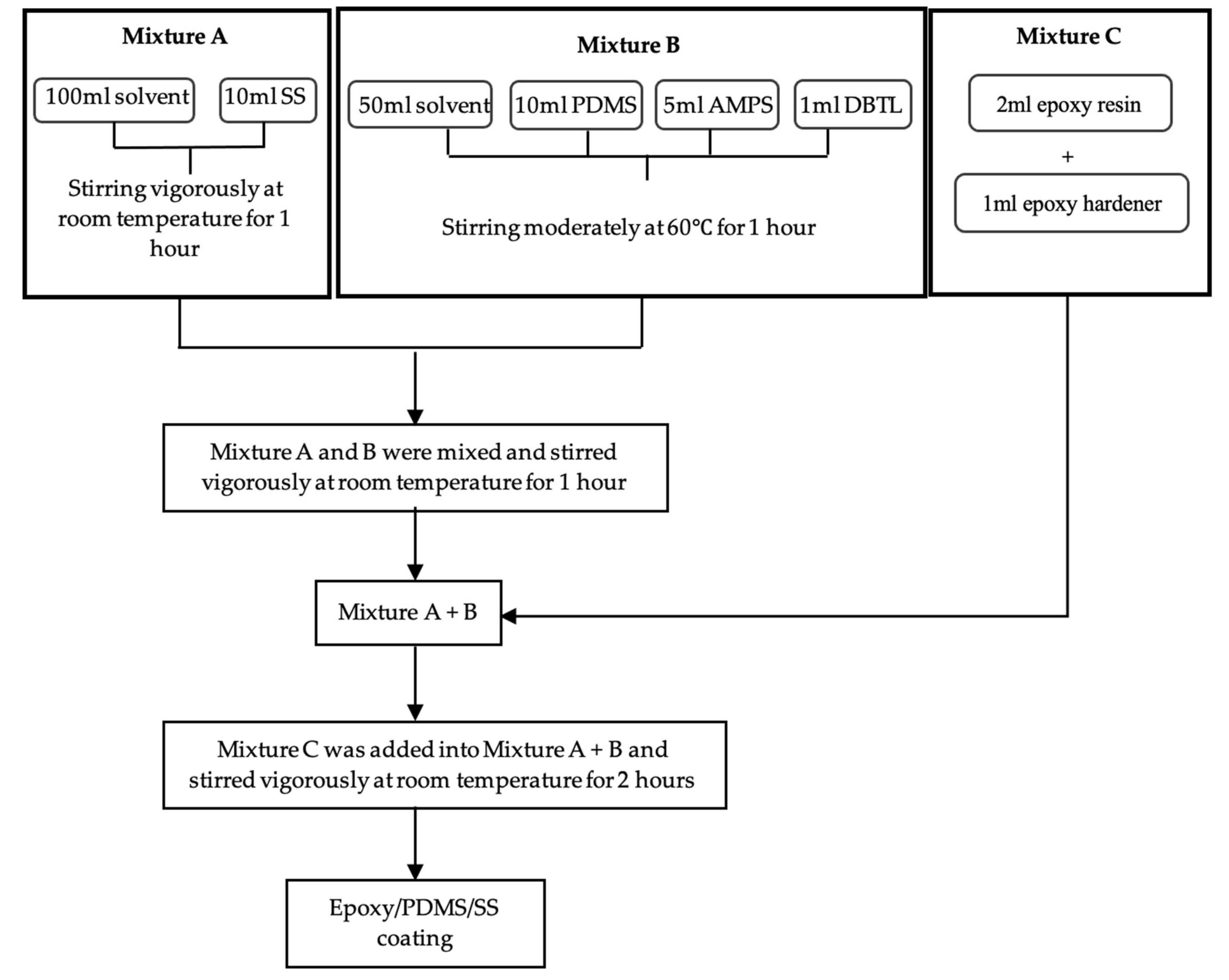
2.3. Preparation of the Hydrophobic Solution
2.4. Fabrication of Hydrophobic Coating on Tiles Substrate
2.5. Characterizations and Analysis
2.5.1. Water Contact Angle (WCA) and Tilting Angle (TA) Measurement
2.5.2. Atomic Force Microscopy (AFM)
2.5.3. Field-Emission Scanning Electron Microscopy (FESEM)
2.5.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.5. Peel-Off Test
3. Results and Discussion
3.1. Effect of Solvent on Wettability
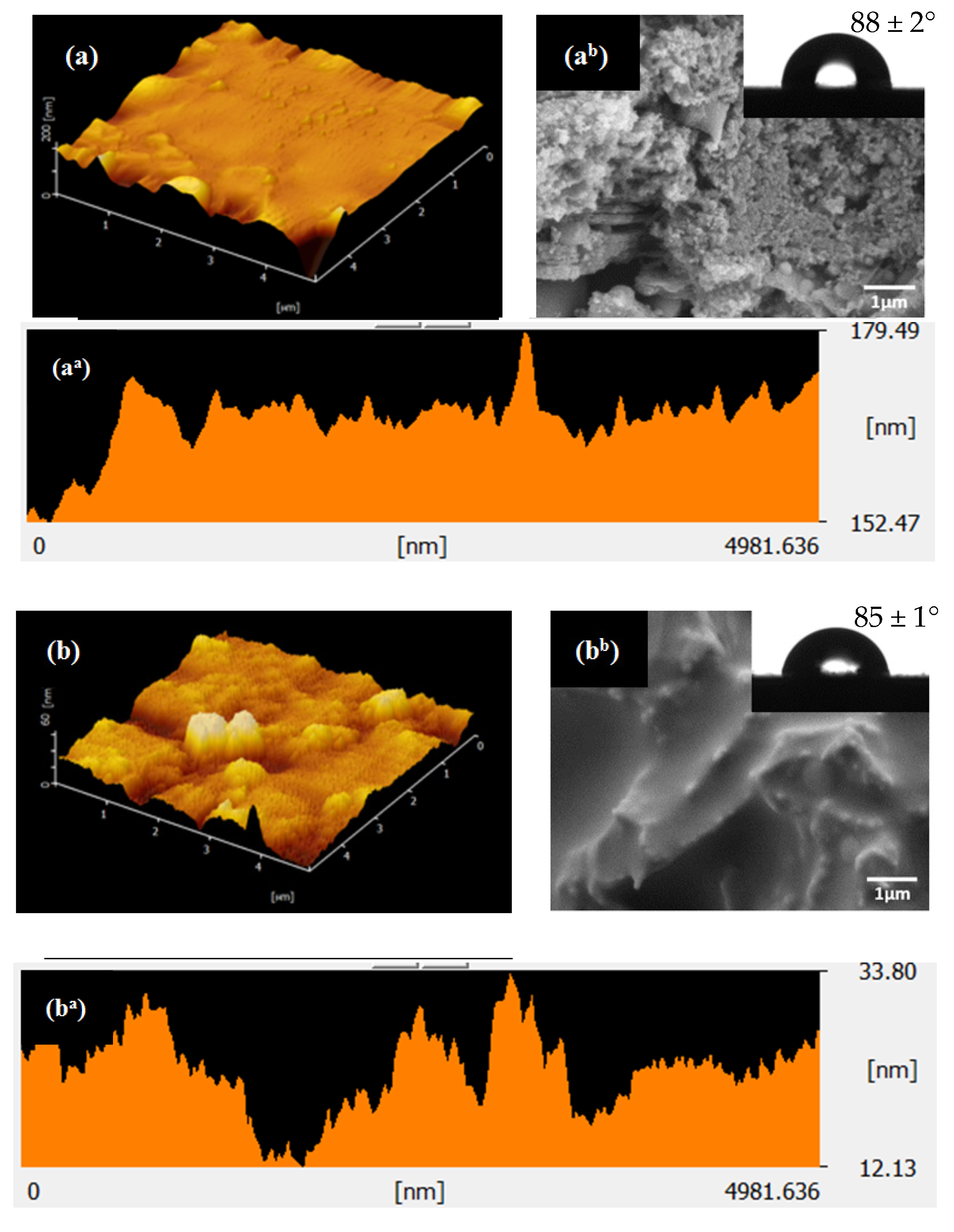
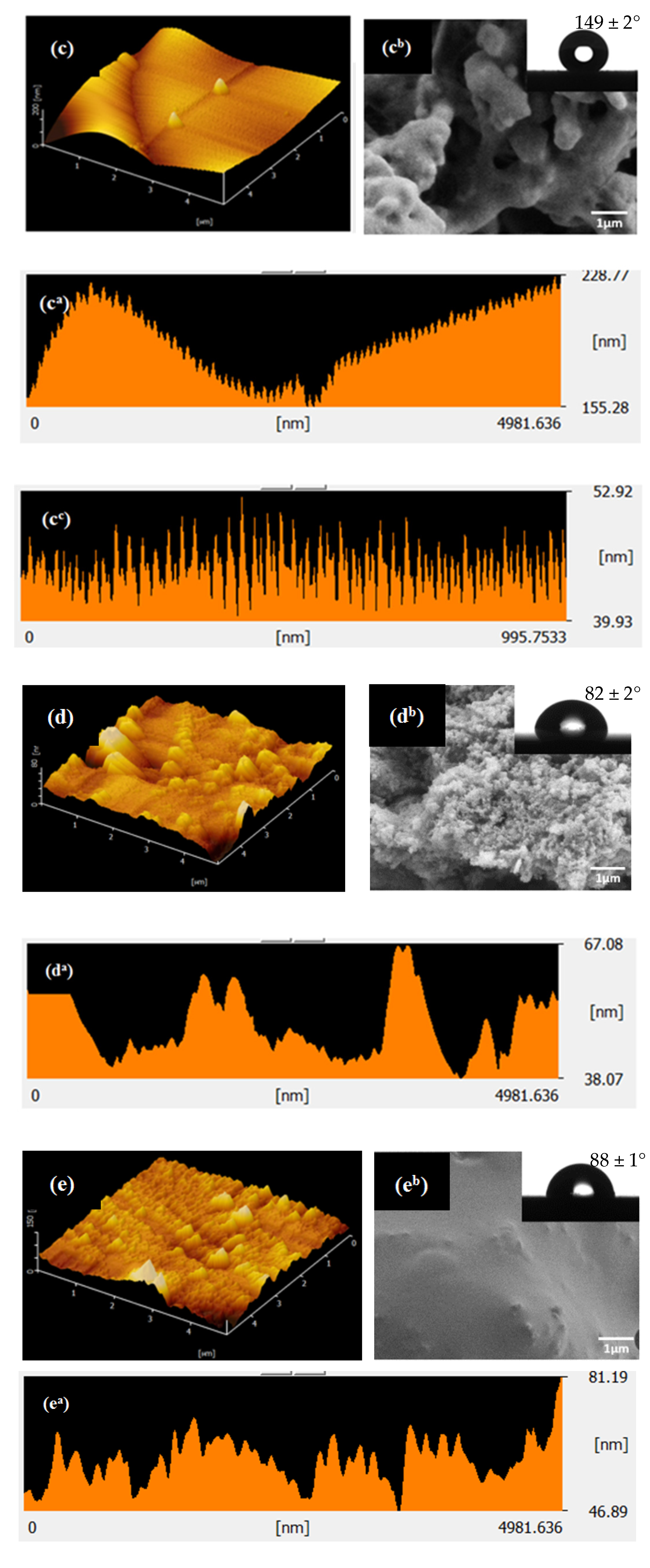
3.2. Effect of Solvent on Surface Morphology and Surface Roughness
3.3. Effect of Solvent on Dispersion of Coating Solution
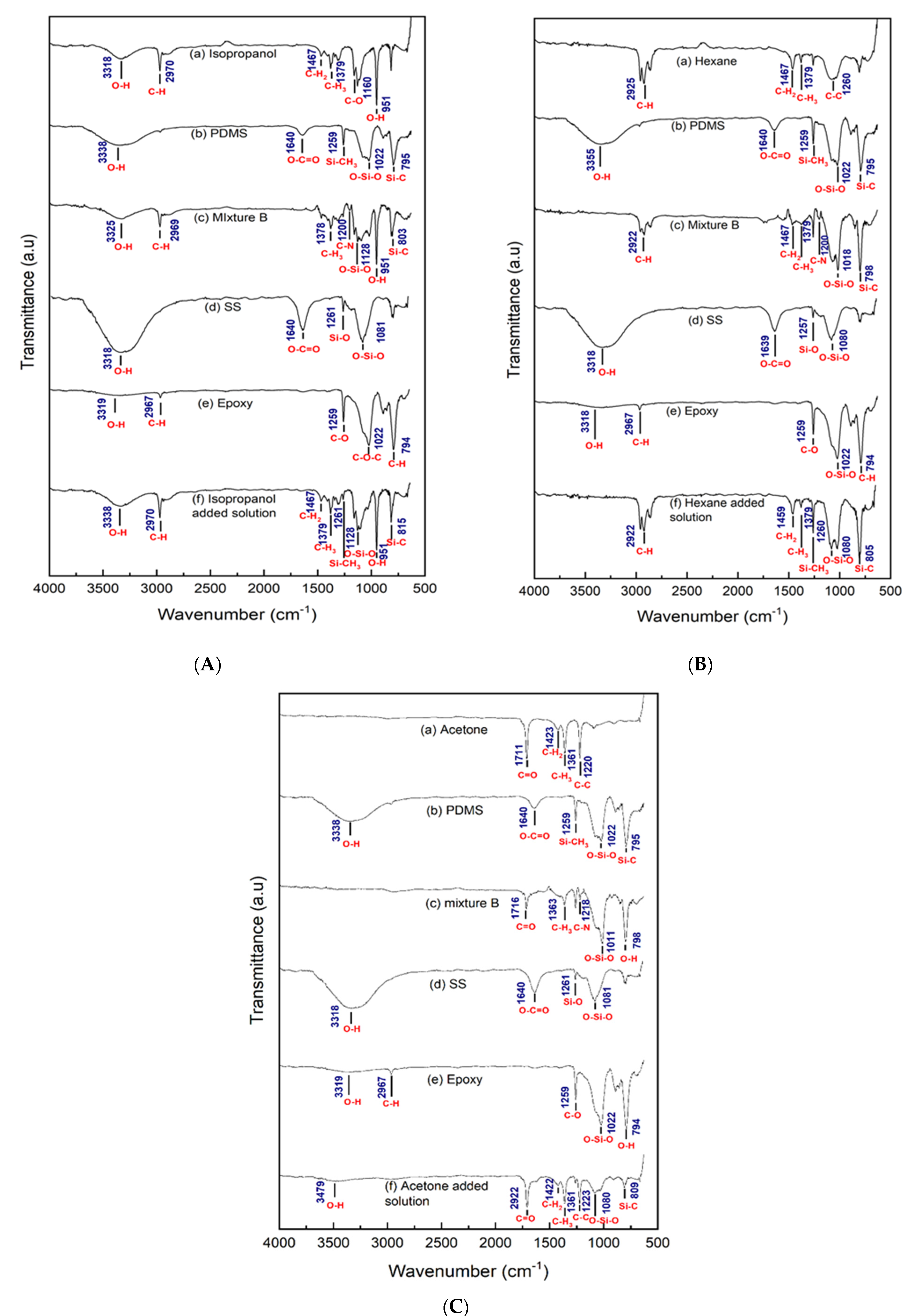
3.4. FTIR of Different Types of Solvent and Coating Solution
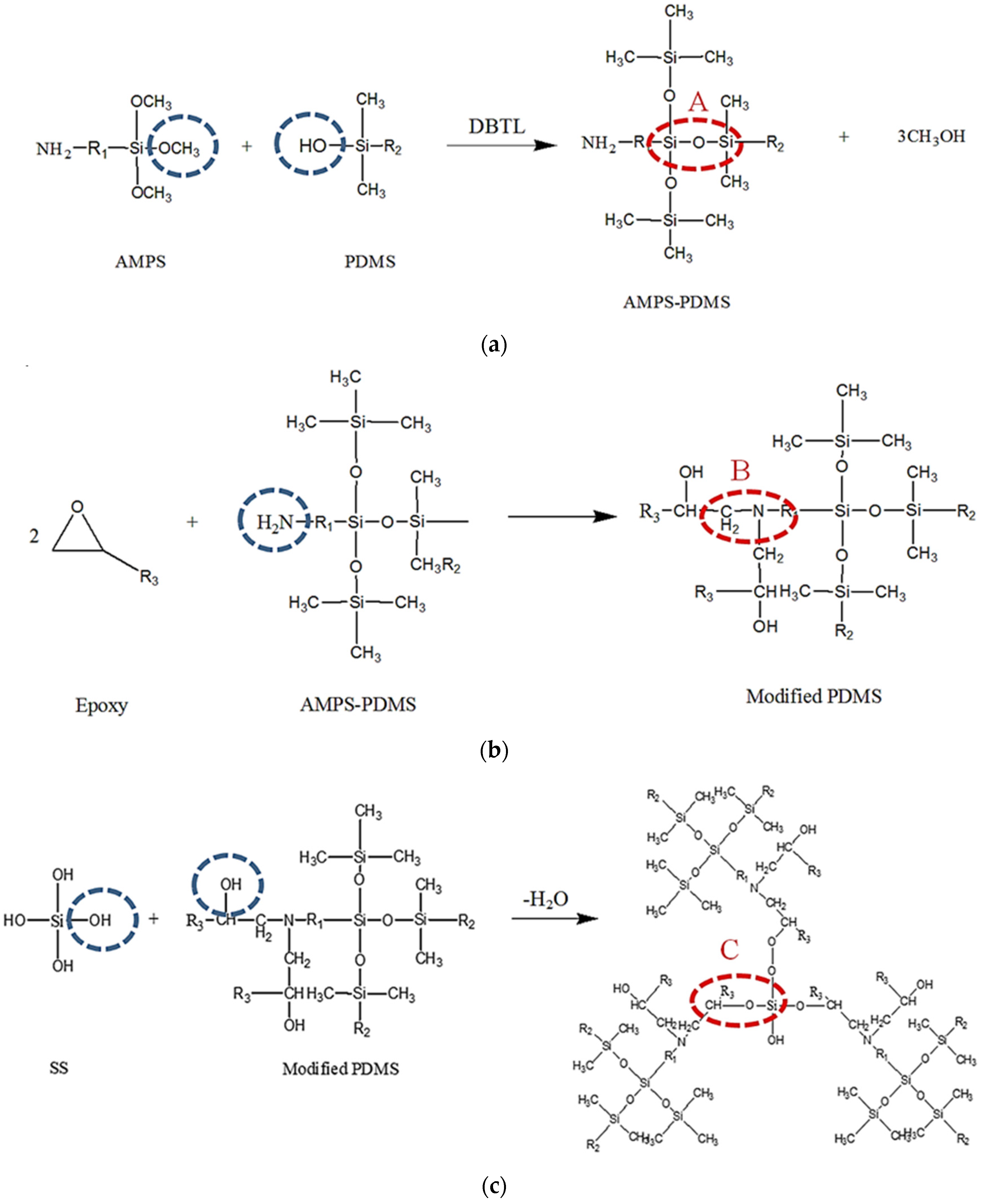
3.5. The Mechanism of Reaction of S3 Solution
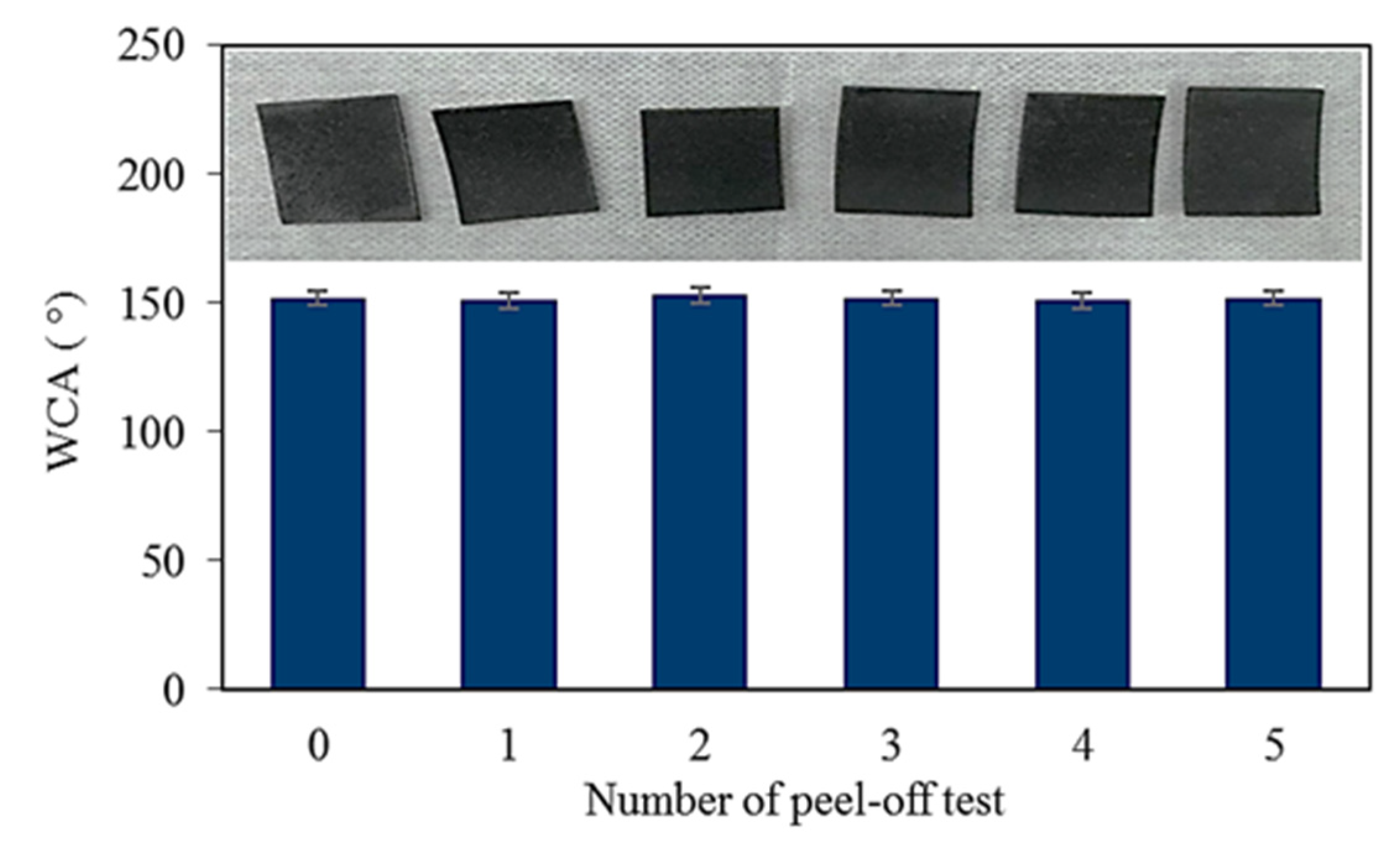
3.6. Peel of Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varshney, P.; Mohapatra, S.S. Fabrication of durable and regenerable superhydrophobic coatings on metallic surfaces for potential industrial applications. In Proceedings of the 2018 8th International Conference on Mechanical and Intelligent Manufacturing Technologies (ICMIMT), Cape Town, South Africa, 3–6 February 2017; IEEE: Piscataway, NJ, USA, 2018; pp. 16–20. [Google Scholar]
- Barati Darband, G.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and engineering of superhydrophobic surfaces: Review of corrosion resistance, Chemical and Mechanical Stability. Arab. J. Chem. 2018, 13, 1763–1802. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Zhao, X.; Tian, N.; Zhang, J. Totally waterborne, nonfluorinated, mechanically robust, and self-healing superhydrophobic coatings for actual anti-icing. ACS Appl. Mater. Interfaces 2018, 10, 39391–39399. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Hakonsen, V.; He, Z.; Xiao, S.; He, J.; Zhang, Z. Enhancing the mechanical durability of icephobic surfaces by introducing autonomous self-healing function. ACS Appl. Mater. Interfaces 2018, 10, 11972–11978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2015, 13, 11–29. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ren, F.; Liu, Y. A superhydrophobic EP/PDMS nanocomposite coating with high gamma radiation stability. Appl. Surf. Sci. 2018, 436, 405–410. [Google Scholar] [CrossRef]
- Su, X.; Li, H.; Lai, X.; Zhang, L.; Liang, T.; Feng, Y.; Zeng, X. Polydimethylsiloxane-based superhydrophobic surfaces on steel substrate: Fabrication, reversibly extreme wettability and oil–water separation. ACS Appl. Mater. Interfaces 2017, 9, 3131–3141. [Google Scholar] [CrossRef]
- Gao, S.; Dong, X.; Huang, J.; Li, S.; Li, Y.; Chen, Z.; Lai, Y. Rational construction of highly transparent superhydrophobic coatings based on a non-particle, fluorine-free and water-rich system for versatile oil-water separation. Chem. Eng. J. 2018, 333, 621–629. [Google Scholar] [CrossRef]
- Qing, Y.; Yang, C.; Sun, Y.; Zheng, Y.; Wang, X.; Shang, Y.; Wang, L.; Liu, C. Facile fabrication of superhydrophobic surfaces with corrosion resistance by nanocomposite coating of TiO2 and polydimethylsiloxane. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 471–477. [Google Scholar] [CrossRef]
- Zhang, Z.; Ge, B.; Men, X.; Li, Y. Mechanically durable, superhydrophobic coatings prepared by dual-layer method for anti-corrosion and self-cleaning. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 182–188. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, J.; Xu, W.; Yu, T.; Chen, Z.; Duan, J. Eco-friendly anticorrosion superhydrophobic Al2O3@PDMS coating with salt deliquescence self-coalescence behaviors under high atmospheric humidity. Front. Mater. 2022, 9, 839948. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Chang, W.; Fan, X.; Li, C.; Shi, Y. A facile cost-effective method for preparing robust self-cleaning transparent superhydrophobic coating. Appl. Phys. A 2016, 122, 916. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, S.; Lv, Z.; Fan, L.; Huang, Y.; Liu, X. A facile method to prepare superhydrophobic coatings for various substrates. Appl. Sci. 2022, 12, 1–9. [Google Scholar] [CrossRef]
- Acikbas, G.; Acikbas, N.C. Nanoarchitectonics for polymer-ceramic hybrid coated ceramic tiles for antibacterial activity and wettability. Appl. Phys. A 2021, 127, 794. [Google Scholar] [CrossRef]
- Acikbas, G.; Acikbas, N.C. The effect of sintering regime on superhydrophobicity of silicon nitride modified ceramic surfaces. J. Asian Ceram. Soc. 2021, 9, 733–743. [Google Scholar] [CrossRef]
- Abbas, A.; Zhang, C.; Asad, M.; Waqas, A.; Khatoon, A.; Hussain, S.; Husain Mir, S. Recent developments in artificial super-wettable surfaces based on bioinspired polymeric materials for biomedical applications. Polymers 2022, 14, 238. [Google Scholar] [CrossRef]
- Wu, M.; An, N.; Li, Y.; Sun, J. Layer-by-layer assembly of fluorine-free polyelectrolyte–surfactant complexes for the fabrication of self-healing superhydrophobic films. Langmuir 2016, 32, 12361–12369. [Google Scholar] [CrossRef]
- Guo, X.-J.; Xue, C.-H.; Li, M.; Li, X.; Ma, J.-Z. Fabrication of robust, superhydrophobic, electrically conductive and UV-blocking fabrics via layer-by-layer assembly of carbon nanotubes. RSC Adv. 2017, 7, 25560–25565. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Huang, J.; Chen, Z.; Chen, G.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y. Robust translucent superhydrophobic PDMS/PMMA film by facile one-step spray for self-cleaning and efficient emulsion separation. Chem. Eng. J. 2017, 330, 26–35. [Google Scholar] [CrossRef]
- El Dessouky, W.I.; Abbas, R.; Sadik, W.A.; El Demerdash, A.G.M.; Hefnawy, A. Improved adhesion of superhydrophobic layer on metal surfaces via one step spraying method. Arab. J. Chem. 2015, 10, 368–377. [Google Scholar] [CrossRef]
- Hejazi, I.; Seyfi, J.; Sadeghi GM, M.; Jafari, S.H.; Khonakdar, H.A.; Drechsler, A.; Davachi, S.M. Investigating the interrelationship of superhydrophobicity with surface morphology, topography and chemical composition in spray-coated polyurethane/silica nanocomposites. Polymer 2017, 128, 108–118. [Google Scholar] [CrossRef]
- Sung, Y.H.; Kim, Y.D.; Choi, H.-J.; Shin, R.; Kang, S.; Lee, H. Fabrication of superhydrophobic surfaces with nano-in-micro structures using UV-nanoimprint lithography and thermal shrinkage films. Appl. Surf. Sci. 2015, 349, 169–173. [Google Scholar] [CrossRef]
- Kothary, P.; Dou, X.; Fang, Y.; Gu, Z.; Leo, S.-Y.; Jiang, P. Superhydrophobic hierarchical arrays fabricated by a scalable colloidal lithography approach. J. Colloid Interface Sci. 2017, 487, 484–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Sakai, M.; Fujishima, A. Development of sol–gel processed semi-transparent and self-cleaning superhydrophobic coatings. J. Mater. Chem. A 2014, 2, 5548–5553. [Google Scholar] [CrossRef]
- Kumar, D.; Wu, X.; Fu, Q.; Ho JW, C.; Kanhere, P.D.; Li, L.; Chen, Z. Hydrophobic sol–gel coatings based on polydimethylsiloxane for self-cleaning applications. Mater. Des. 2015, 86, 855–862. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Zhang, J.; Liu, J.; Han, Z.; Ren, L. Corrosion inhibition of biomimetic super-hydrophobic electrodeposition coatings on copper substrate. Corros. Sci. 2015, 94, 190–196. [Google Scholar] [CrossRef]
- Ashoka, S.; Saleema, N.; Sarkar, D. Tuning of superhydrophobic to hydrophilic surface: A facile one step electrochemical approach. J. Alloy. Compd. 2017, 695, 1528–1531. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, D.; Lu, X.; Lu, Q. Transparent, thermally and mechanically stable superhydrophobic coating prepared by an electrochemical template strategy. J. Mater. Chem. A 2015, 3, 3801–3807. [Google Scholar] [CrossRef]
- Mohamed AM, A.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef] [Green Version]
- Fihri, A.; Bovero, E.; Al-Shahrani, A.; Al-Ghamdi, A.; Alabedi, G. Recent progress in superhydrophobic coatings used for steel protection: A review. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 378–390. [Google Scholar] [CrossRef]
- Khaskhoussi, A.; Calabrese, L.; Patané, S.; Proverbio, E. Effect of chemical surface texturing on the superhydrophobic behavior of micro–nano-roughened AA6082 surfaces. Materials 2021, 14, 7161. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, W.K.; Qu, Z.G.; Ren, G.F.; Wang, H.C. Surface roughness dominated wettability of carbon fiber in gas diffusion layer materials revealed by molecular dynamics simulations. Int. J. Hydrogen Energy 2021, 46, 26489–26498. [Google Scholar] [CrossRef]
- Saleem, M.S. Development of Super Hydrophobic Surfaces Using Silica Nanoparticles. Master’s Thesis, CUNY City College, New York, NY, USA, 2015. [Google Scholar]
- Wang, X.; Li, X.; Lei, Q.; Wu, Y.; Li, W. Fabrication of superhydrophobic composite coating based on fluorosilicone resin and silica nanoparticles. R. Soc. Open Sci. 2018, 5, 180598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Shao, H.; Lv, P.; Tang, C.; He, Z.; Zhou, Y.; Shuai, M.; Mei, J.; Lau, W.-M. Fast preparation of mechanically stable superhydrophobic surface by UV cross-linking of coating onto oxygen-inhibited layer of substrate. Chem. Eng. J. 2018, 338, 440–449. [Google Scholar] [CrossRef]
- Saharudin, K.A.; Sreekantan, S.; Basiron, N.; Chun, L.K.; Kumaravel, V.; Abdullah, T.K.; Ahmad, Z.A. Improved super-hydrophobicity of eco-friendly coating from palm oil fuel ash (POFA) waste. Surf. Coat. Technol. 2018, 337, 126–135. [Google Scholar] [CrossRef]
- Qing, Y.; Yang, C.; Hu, C.; Zheng, Y.; Liu, C. A facile method to prepare superhydrophobic fluorinated polysiloxane/ZnO nanocomposite coatings with corrosion resistance. Appl. Surf. Sci. 2015, 326, 48–54. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, D.; Yang, S. Spray-coating of superhydrophobic aluminum alloys with enhanced mechanical robustness. J. Colloid Interface Sci. 2014, 423, 101–107. [Google Scholar] [CrossRef]
- Sahoo, B.N.; Nanda, S.; Kozinski, J.A.; Mitra, S.K. PDMS/camphor soot composite coating: Towards a self-healing and a self-cleaning superhydrophobic surface. RSC Adv. 2017, 7, 15027–15040. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, B.T.; Alla, J.P.; Essomba, J.S.; Nishter, N.F. Non-fluorinated superhydrophobic spray coatings for oil-water separation applications: An eco-friendly approach. J. Clean. Prod. 2020, 256, 120693. [Google Scholar] [CrossRef]
- Caldona, E.B.; Sibaen, J.W.; Tactay, C.B.D.; Mendiola, S.L.; Abance, C.B.; Añes, M.P.; DSerrano, F.D.; SDe Guzman, M.M. Preparation of spray coated surfaces from green formulated superhydrophobic coatings. SN Appl. Sci. 2019, 1, 1657. [Google Scholar] [CrossRef] [Green Version]
- Latthe, S.S.; Sutar, R.S.; Kodag, V.S.; Bhosale, A.K.; Madhan Kumarc, A.; Sadasivuni, K.K.; Xing, R.; Liu, S. Self–cleaning superhydrophobic coatings: Potential industrial applications. Prog. Org. Coat. 2019, 128, 52–58. [Google Scholar] [CrossRef]
- Gong, X.; He, S. Highly Durable Superhydrophobic Polydimethylsiloxane/Silica Nanocomposite Surfaces with Good Self-Cleaning Ability. ACS Omega 2020, 5, 4100–4108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, A.; Zhao, W. Analysis of the Acid and Alkali Resistance of Superhydrophobic Paper Mulch. Cellulose 2021, 28, 8705–8718. [Google Scholar] [CrossRef]
- Luo, X.; Hu, W.; Cao, M.; Ren, H.; Feng, J.; Wei, M. An environmentally friendly approach for the fabrication of conductive superhydrophobic coatings with sandwich-like structures. Polymers 2018, 10, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghavan, S.R.; Walls, H.; Khan, S.A. Rheology of silica dispersions in organic liquids: New evidence for solvation forces dictated by hydrogen bonding. Langmuir 2000, 16, 7920–7930. [Google Scholar] [CrossRef]
- Lee, D.H.; Jeong, J.; Han, S.W.; Kang, D.P. Superhydrophobic surfaces with near-zero sliding angles realized from solvent relative permittivity mediated silica nanoparticle aggregation. J. Mater. Chem. A 2014, 2, 17165–17173. [Google Scholar] [CrossRef]
- Chang, H.; Tu, K.; Wang, X.; Liu, J. Fabrication of mechanically durable superhydrophobic wood surfaces using polydimethylsiloxane and silica nanoparticles. RSC Adv. 2015, 5, 30647–30653. [Google Scholar] [CrossRef]
- Qiang, S.; Chen, K.; Yin, Y.; Wang, C. Robust UV-cured superhydrophobic cotton fabric surfaces with self-healing ability. Mater. Des. 2017, 116, 395–402. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Ben, K.; Chen, Z.; Wang, Y.; Guan, Z. Transparent, durable and thermally stable PDMS-derived superhydrophobic surfaces. Appl. Surf. Sci. 2015, 339, 94–101. [Google Scholar] [CrossRef]
- Schaeffer, D.A.; Polizos, G.; Smith, D.B.; Lee, D.F.; Hunter, S.R.; Datskos, P.G. Optically transparent and environmentally durable superhydrophobic coating based on functionalized SiO2 nanoparticles. Nanotechnology 2015, 26, 055602. [Google Scholar] [CrossRef]
- Long, M.; Peng, S.; Deng, W.; Yang, X.; Miao, K.; Wen, N.; Miao, X.; Deng, W. Robust and thermal-healing superhydrophobic surfaces by spin-coating of polydimethylsiloxane. J. Colloid Interface Sci. 2017, 508, 18–27. [Google Scholar] [CrossRef]
- ECHA. Substance Information: N-Hexane; ECHA: Helsinki, Finland, 2018; Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.003.435 (accessed on 13 November 2018).
- Smallwood, I. Handbook of Organic Solvent Properties; Butterworth-Heinemann: Oxford, UK, 2012. [Google Scholar]
- Chen, I.-J.; Lindner, E. The stability of radio-frequency plasma-treated polydimethylsiloxane surfaces. Langmuir 2007, 23, 3118–3122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhou, A.G.; Sun, B.R.; Chen, K.S.; Yu, H.-Z. Functional and versatile superhydrophobic coatings via stoichiometric silanization. Nat. Commun. 2021, 12, 982. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, K.; Zhang, D.; Guo, Z. Stable and Durable Conductive Superhydrophobic Coatings Prepared by Double-Layer Spray Coating Method. Nanomaterials 2021, 11, 1506. [Google Scholar] [CrossRef] [PubMed]
- Chuah, W.H. Investigation on Hydrophobic Coating for Improved Functionality and Durability; Universiti Sains Malaysia: Pulau Pinang, Malaysia, 2015. [Google Scholar]
- Tu, K.; Kong, L.; Wang, X.; Liu, J. Semitransparent, durable superhydrophobic polydimethylsiloxane/SiO2 nanocomposite coatings on varnished wood. Holzforschung 2016, 70, 1039–1045. [Google Scholar] [CrossRef]
- Celia, E.; Darmanin, T.; de Givenchy, E.T.; Amigoni, S.; Guittard, F. Recent advances in designing superhydrophobic surfaces. J. Colloid Interface Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef]
- Polizos, G.; Tuncer, E.; Sauers, I.; More, K.L. Physical properties of epoxy resin/titanium dioxide nanocomposites. Polym. Eng. Sci. 2011, 51, 87–93. [Google Scholar] [CrossRef]
- Hollande, L.; Do Marcolino, I.; Balaguer, P.; Domenek, S.; Gross, R.A.; Allais, F. Preparation of renewable epoxy-amine resins with tunable thermo-mechanical properties, wettability and degradation abilities from lignocellulose-and plant oils-derived components. Front. Chem. 2019, 7, 159. [Google Scholar] [CrossRef]
- Gindl, M.; Sinn, G.; Stanzl-Tschegg, S.E. The effects of ultraviolet light exposure on the wetting properties of wood. J. Adhes. Sci. Technol. 2006, 20, 817–828. [Google Scholar] [CrossRef]
- Tsuda, Y. Surface wettability controllable polyimides by UV light irradiation for printed electronics. J. Photopolym. Sci. Technol. 2016, 29, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Chaban, V.V.; Maciel, C.; Fileti, E.E. Does the like dissolves like rule hold for fullerene and ionic liquids? J. Solut. Chem. 2014, 43, 1019–1031. [Google Scholar] [CrossRef] [Green Version]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons Ltd.: New York, NJ, USA, 2006. [Google Scholar]
- Li, K.-M.; Jiang, J.-G.; Tian, S.-C.; Chen, X.-J.; Yan, F. Influence of Silica Types on Synthesis and Performance of Amine−Silica Hybrid Materials Used for CO2 Capture. J. Phys. Chem. C 2014, 118, 2454–2462. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, S.; Wu, L. Facile fabrication of self-repairing superhydrophobic coatings. Chem. Commun. 2014, 50, 11891–11894. [Google Scholar] [CrossRef] [PubMed]
- Ernault, E.; Richaud, E.; Fayolle, B. Thermal oxidation of epoxies: Influence of diamine hardener. Polym. Degrad. Stab. 2016, 134, 76–86. [Google Scholar] [CrossRef] [Green Version]
- Krauklis, A.E.; Echtermeyer, A.T. Mechanism of yellowing: Carbonyl formation during hygrothermal aging in a common amine epoxy. Polymers 2018, 10, 1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Low Surface Energy Material | Solvent | Nanoparticle | Substrate | WCA (°) | TA (°) | Surface Roughness (µm) | Reference |
|---|---|---|---|---|---|---|---|
| PU | Hexadecyl polysiloxane modified SiO2 | Glass | 163.9 | 3.7 | [3] | ||
| PDMS | Ethyl acetate | SiO2 | Glass | 157 | 5.357 | [10] | |
| PDMS | Hexane | SiO2 | 164 | 2 | 0.023 | [12] | |
| OCTES | Ethanol, water | SiO2 | Metal | 155 | <5 | [18] | |
| PU | THF | SiO2 | Glass | 160 | <2 | 2.550 | [20] |
| Fluorosilicone | Ethyl acetate, butyl acetate | SiO2 | Glass | 153 | 2.5 | [34] | |
| PDMS, PMMS | Hexane | SiO2 | Polyurethane acrylate | 160 | 5 | [35] | |
| PDMS | Hexane | SiO2 | Glass | 156 | 1 | [36] | |
| Fluorinated polysiloxane | Butyl acetate | ZnO | Steel | 166 | 4 | [37] | |
| Glass resin | Isopropanol | SiO2 | Aluminium | 155 | 4 | [38] | |
| PDMS | THF | Camphor soot particles | Glass | 171 | 5 | 1.491 | [39] |
| HMDS | γ-Aminopropyltriethoxysilane | SiO2 | Glass, wood, filter paper, cotton, plastic, stone, fabric and aluminium foil | 161 | 6.5 | [40] | |
| Hexadecyltrimethoxysilane (HDTMS) | Ethanol | SiO2 | White rice husks and cabbage | 155 | - | [41] | |
| Hydrophobic silica | Hexane | SiO2 | Body of motorcycle, building wall | 160 | 6 | [42] | |
| PDMS | Hexane | SiO2 | Glass, paper, and plastic | 156.4 | 5 | [43] | |
| Paper mulch | Anhydrous ethanol | SiO2 | 160.6 | 4.2 | [44] |
| Solvent | Relative Permittivity, ε | WCA (°) | Reference |
|---|---|---|---|
| Xylene | 2.30 | 154 | [6] |
| 156.8 | [13] | ||
| Tetrahydrofuran | 7.60 | 152 | [48] |
| 153 | [49] | ||
| Toluene | 2.38 | 154 | [33] |
| Hexane | 1.90 | 160 | [7] |
| 164 | [12] | ||
| 160 | [35] | ||
| 160 | [42] | ||
| 156.4 | [43] | ||
| 163 160 | [50] [51] | ||
| 158 | [52] | ||
| 172 | [56] | ||
| Ethyl | 25 | 149 | [57] |
| Sample | Type of Solvent | WCA (°) | TA (°) | RMS (nm) | Surface Energy (J/m2) |
|---|---|---|---|---|---|
| S1 | Acetone | 88 ± 2 | 20 ± 2 | 7.09 | 31 ± 1 |
| S2 | Hexane | 85 ± 1 | 46 ± 1 | 13.01 | 33 ± 1 |
| S3 | Isopropanol | 149 ± 2 | 10 ± 2 | 18.57 | 2 ± 0 |
| S1-UV | Acetone | 82 ± 2 | 25 ± 2 | 9.934 | 34 ± 1 |
| S2-UV | Hexane | 88 ± 1 | 38 ± 1 | 11.57 | 31 ± 1 |
| S3-UV | Isopropanol | 152 ± 2 | 7 ± 2 | 21.80 | 1 ± 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sreekantan, S.; Yong, A.X.; Basiron, N.; Ahmad, F.; De’nan, F. Effect of Solvent on Superhydrophobicity Behavior of Tiles Coated with Epoxy/PDMS/SS. Polymers 2022, 14, 2406. https://doi.org/10.3390/polym14122406
Sreekantan S, Yong AX, Basiron N, Ahmad F, De’nan F. Effect of Solvent on Superhydrophobicity Behavior of Tiles Coated with Epoxy/PDMS/SS. Polymers. 2022; 14(12):2406. https://doi.org/10.3390/polym14122406
Chicago/Turabian StyleSreekantan, Srimala, Ang Xue Yong, Norfatehah Basiron, Fauziah Ahmad, and Fatimah De’nan. 2022. "Effect of Solvent on Superhydrophobicity Behavior of Tiles Coated with Epoxy/PDMS/SS" Polymers 14, no. 12: 2406. https://doi.org/10.3390/polym14122406
APA StyleSreekantan, S., Yong, A. X., Basiron, N., Ahmad, F., & De’nan, F. (2022). Effect of Solvent on Superhydrophobicity Behavior of Tiles Coated with Epoxy/PDMS/SS. Polymers, 14(12), 2406. https://doi.org/10.3390/polym14122406