Development of Statistically Optimized Chemically Cross-Linked Hydrogel for the Sustained-Release Delivery of Favipiravir
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Hydrogel Formulations
2.2. Drug Loading
2.3. Numerical Optimization
2.4. Characterization of Hydrogels
2.4.1. Organoleptic Evaluation
2.4.2. Swelling Studies
2.4.3. Sol–Gel Fraction
2.4.4. Porosity Measurement
2.4.5. In Vitro Drug Release Studies
2.4.6. Drug Entrapment Efficiency
2.4.7. Drug Release Kinetics
- Zero-Order Equation
- First Order Equation:
- Higuchi
- ’s Equation:
- Hixson-Crowell Model:
- Korsmeyer–Peppas Model:
2.4.8. FTIR Analysis
2.4.9. X-Ray Diffraction
2.4.10. Scanning Electron Microscopy (SEM)
3. Results and Discussions
3.1. Swelling Studies
3.1.1. Effect of Crosslinker (MBA) on Hydrogel Swelling
3.1.2. Effect of Monomer on Hydrogel Swelling
3.2. Sol–Gel Fraction
3.3. Porosity
3.4. Drug Entrapment Efficiency
3.5. In Vitro Drug Release Studies
3.5.1. Effect of Concentration of β-CD on In Vitro Drug Release
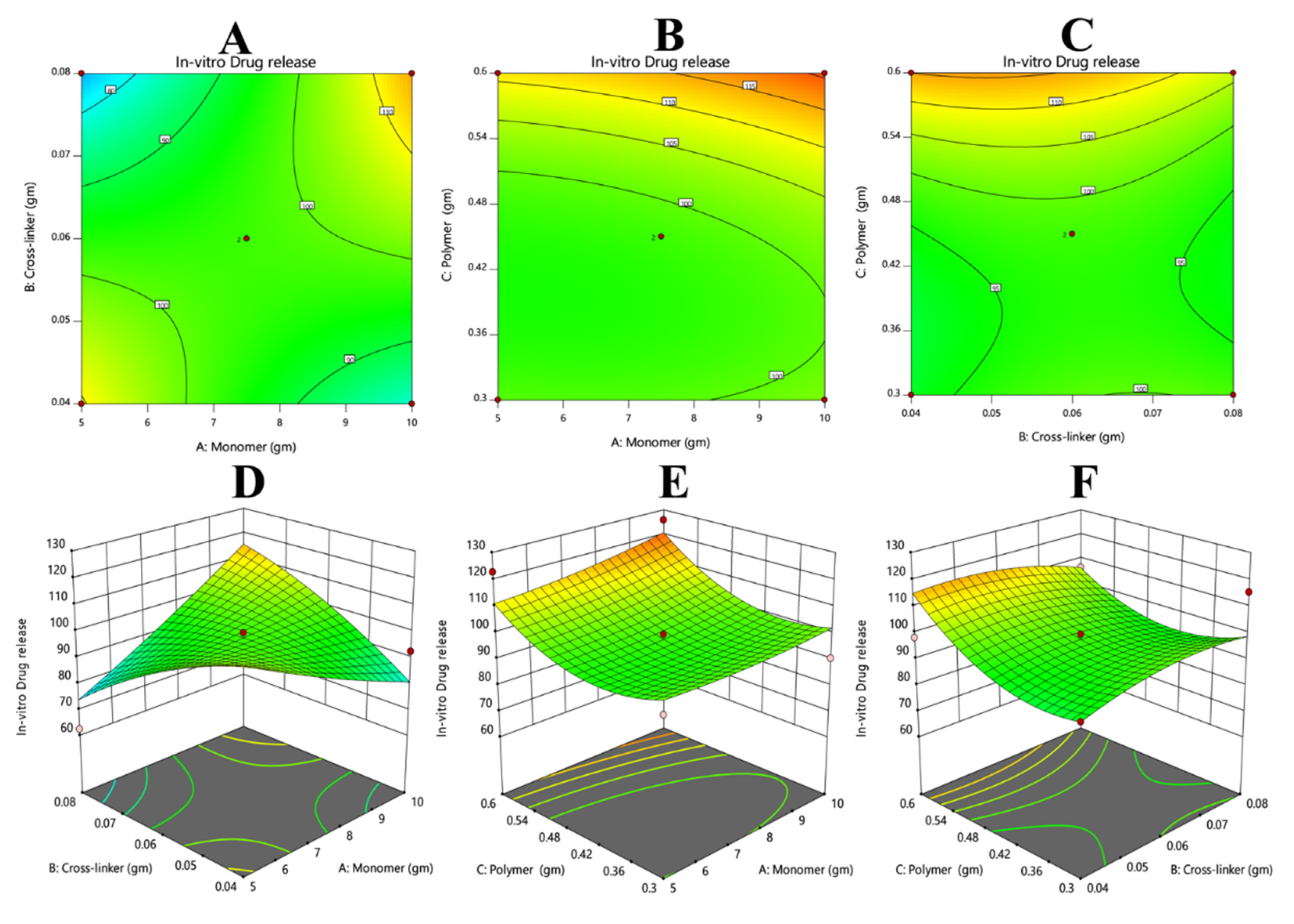
3.5.2. Effects of Monomer and Crosslinker Concentrations on In Vitro Drug Release
3.6. Drug Release Kinetics
3.7. Mathematical Modeling
3.7.1. Response 1: Degree of Swelling
3.7.2. Response 2: Gel Fraction
3.7.3. Response 3: Porosity
3.7.4. Response 4: Entrapment Efficiency
3.7.5. Response 5: In Vitro Drug Release
3.8. Numerical Optimization
3.9. Fourier Transform Infrared Spectroscopy (FTIR)
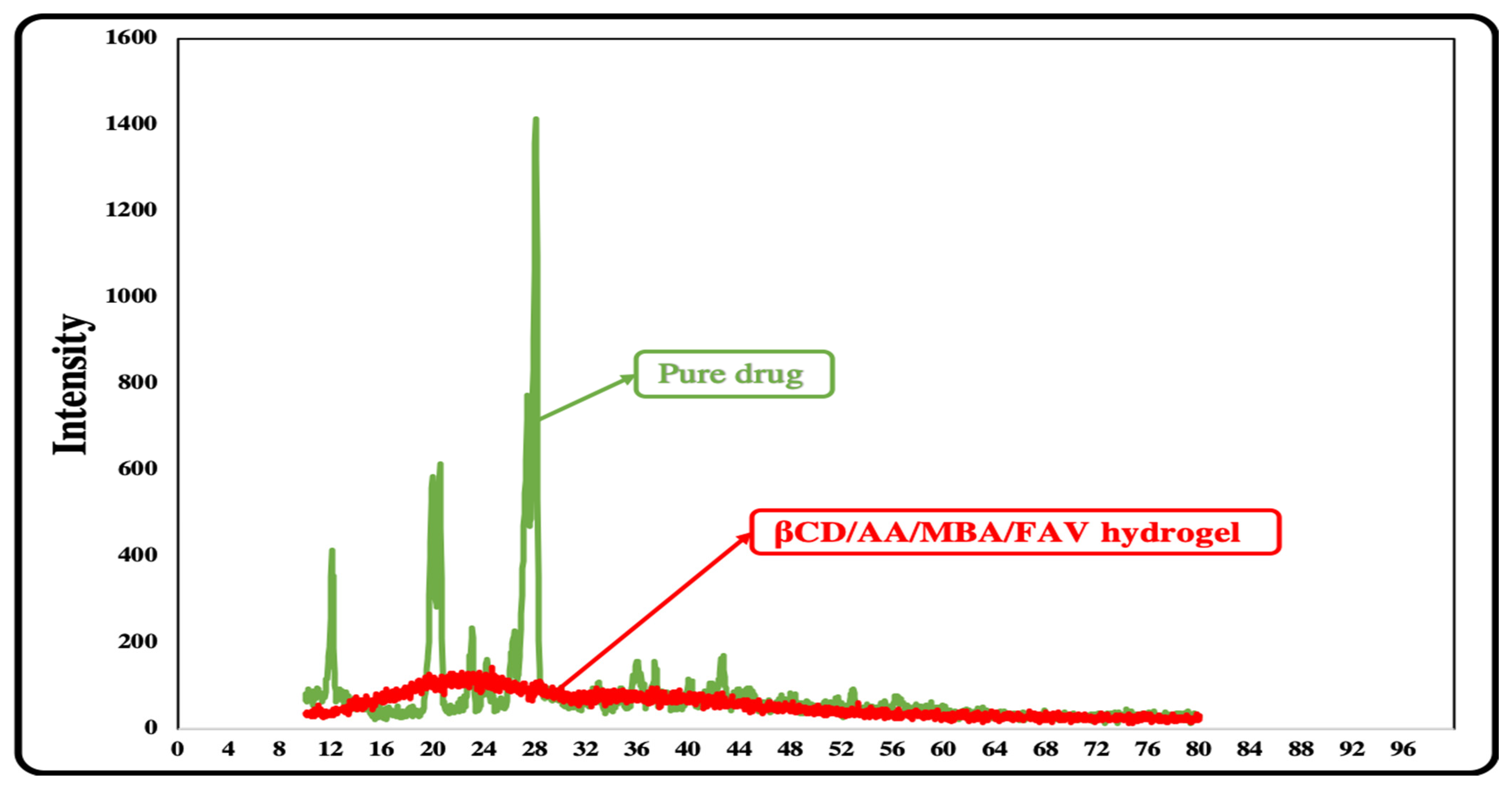
3.10. X-Ray Diffraction (XRD)
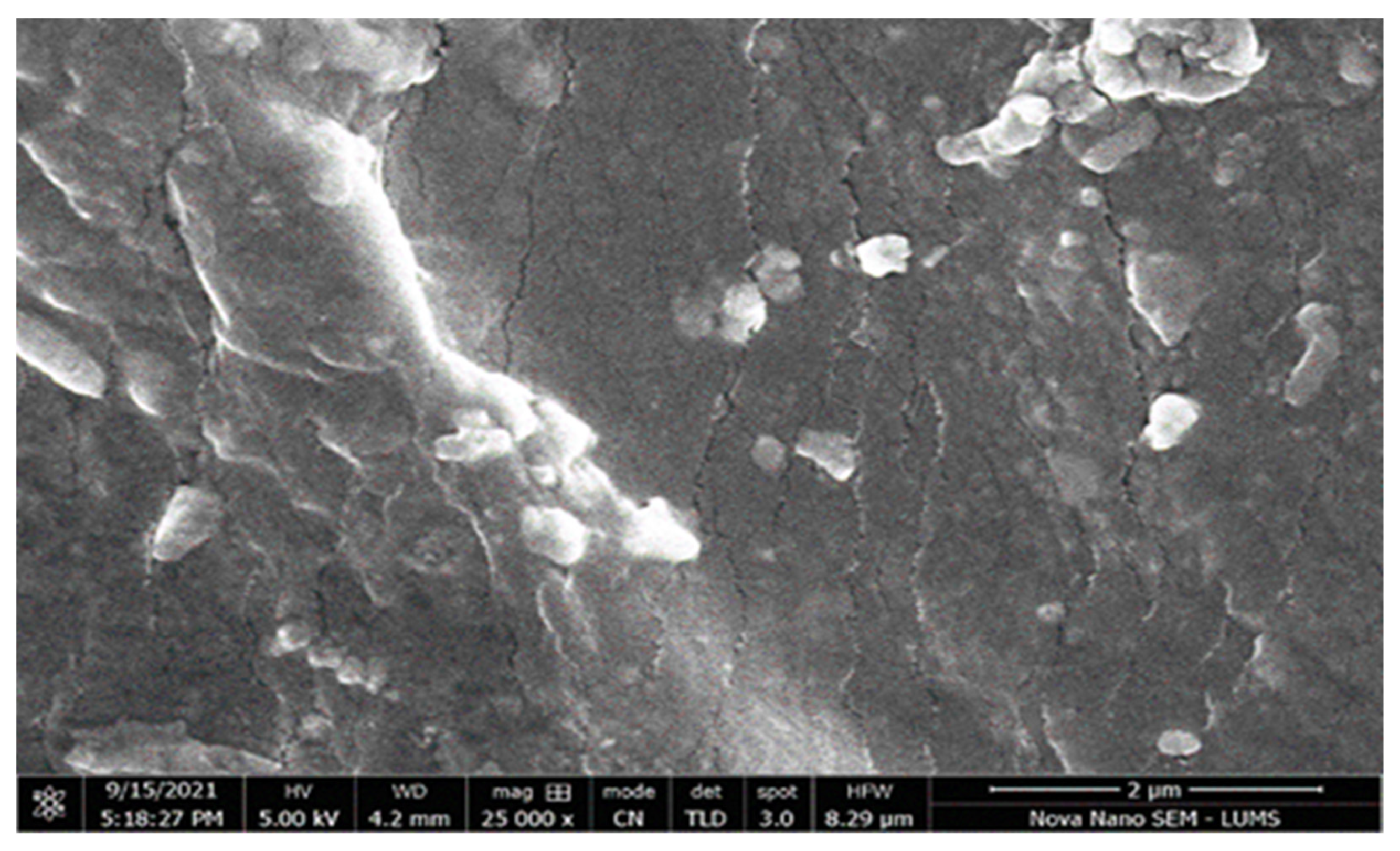
3.11. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef]
- Sharpe, L.A.; Daily, A.M.; Horava, S.D.; Peppas, N.A. Therapeutic applications of hydrogels in oral drug delivery. Expert Opin. Drug Deliv. 2014, 11, 901–915. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, B.; Paul, S.R.; Nayak, S.K.; Qureshi, D.; Pal, K. Synthesis and biomedical applications of filled hydrogels. In Polymeric Gels; Elsevier: Amsterdam, The Netherlands, 2018; pp. 283–302. [Google Scholar]
- Lin, H.-C.; Anggelia, M.R.; Cheng, C.-C.; Ku, K.-L.; Cheng, H.-Y.; Wen, C.-J.; Wang, A.Y.L.; Lin, C.-H.; Chu, I. A mixed thermosensitive hydrogel system for sustained delivery of tacrolimus for immunosuppressive therapy. Pharmaceutics 2019, 11, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Silberberg, M. Cyclodextrin as a Drug Carrier Increasing Drug Solubility. Sci. J. Lander Coll. Arts Sci. 2017, 11, 28–35. [Google Scholar]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [Green Version]
- Sarfraz, R.M.; Ahmad, M.; Mahmood, A.; Akram, M.R.; Abrar, A. Development of β-cyclodextrin-based hydrogel microparticles for solubility enhancement of rosuvastatin: An in vitro and in vivo evaluation. Drug Des. Dev. Ther. 2017, 11, 3083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Tian, B.; Liu, Y.; Wan, J.-B. Cyclodextrin-Containing Hydrogels: A Review of Preparation Method, Drug Delivery, and Degradation Behavior. Int. J. Mol. Sci. 2021, 22, 13516. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.; Seyfoddin, A.; Fatihhi, S. Acrylic acid/acrylamide based hydrogels and its properties—A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Tholkappiyan, R.; Vishista, K. NN-methylene bis acrylamide: A novel fuel for combustion synthesis of zinc ferrite nanoparticles and studied by X-ray photoelectron spectroscopy. Int J. ChemTech. Res. 2014, 6, 2834–2842. [Google Scholar]
- Rajan, K.P.; Thomas, S.P.; Gopanna, A.; Chavali, M. Polyurethane Nanostructures for Drug Delivery Applications. Nano-Microscale Drug Deliv. Syst. 2017, 299–319. [Google Scholar] [CrossRef]
- Encinas, A.H.; Vaquero, J.M.; Dios, A.Q. Interdisciplinary Tasks: Mathematics to Solve Specific Engineering Problems.
- Du, Y.X.; Chen, X.P. Favipiravir: Pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin. Pharmacol. Ther. 2020, 108, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuta, Y.; Gowen, B.B.; Takahashi, K.; Shiraki, K.; Smee, D.F.; Barnard, D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir. Res. 2013, 100, 446–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, N.S.; Ahmad, M.; Minhas, M.U. Cross-linked β-cyclodextrin and carboxymethyl cellulose hydrogels for controlled drug delivery of acyclovir. PLoS ONE 2017, 12, e0172727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Teng, L.; Yu, K.; Sun, X.; Fan, C.; Long, C.; Liu, N.; Li, S.; Wu, B.; Xu, Q. Design of hydrogels of 5-hydroxymethyl tolterodine and their studies on pharmacokinetics, pharmacodynamics and transdermal mechanism. Eur. J. Pharm. Sci. 2017, 96, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Rosli, N.A.; Ahmad, I.; Mat Lazim, A.; Mohd Amin, M.C.I. Synthesis and swelling behavior of pH-sensitive semi-IPN superabsorbent hydrogels based on poly (acrylic acid) reinforced with cellulose nanocrystals. Nanomaterials 2017, 7, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Li, P.; Tang, W.; Du, S.; Yu, M.; Lu, H.; Tan, H.; Xing, X. A facile injectable carbon dot/oxidative polysaccharide hydrogel with potent self-healing and high antibacterial activity. Carbohydr. Polym. 2021, 251, 117040. [Google Scholar] [CrossRef]
- Nanda, S.; Sood, N.; Reddy, B.; Markandeywar, T.S. Preparation and characterization of poly (vinyl alcohol)-chondroitin sulphate hydrogel as scaffolds for articular cartilage regeneration. Indian J. Mater. Sci. 2013, 2013, 516021. [Google Scholar] [CrossRef] [Green Version]
- Sarfraz, R.M.; Akram, M.R.; Ali, M.R.; Mahmood, A.; Khan, M.U.; Ahmad, H.; Qaisar, M.N. Development and in-vitro evaluation of pH responsive polymeric nano hydrogel carrier system for gastro-protective delivery of naproxen sodium. Adv. Polym. Technol. 2019, 2019, 6090965. [Google Scholar] [CrossRef] [Green Version]
- Nautiyal, U.; Devi, A.; Charanjeet, A.S. Development and Evaluation of Interpenetrating Polymer Network Hydrogel for Controlled Release of Cefadroxil. Int. J. Health Biol. Sci. 2019, 2, 1–15. [Google Scholar]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical modeling of release kinetics from supramolecular drug delivery systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, R.; Zaman, M.; Salawi, A.; Khan, M.A.; Iqbal, M.O.; Riaz, R.; Ahmed, M.M.; Butt, M.H.; Alvi, M.N.; Almoshari, Y. Synthesis of Chemically Cross-Linked pH-Sensitive Hydrogels for the Sustained Delivery of Ezetimibe. Gels 2022, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.; Hussain, Z.; Niazi, M.B.K.; Liaqat, U.; Afzal, M. Biogenic synthesis of silver nanoparticles and evaluation of physical and antimicrobial properties of Ag/PVA/starch nanocomposites hydrogel membranes for wound dressing application. J. Drug Deliv. Sci. Technol. 2019, 52, 403–414. [Google Scholar] [CrossRef]
- Ninciuleanu, C.M.; Ianchiş, R.; Alexandrescu, E.; Mihăescu, C.I.; Scomoroşcenco, C.; Nistor, C.L.; Preda, S.; Petcu, C.; Teodorescu, M. The Effects of Monomer, Crosslinking Agent, and Filler Concentrations on the Viscoelastic and Swelling Properties of Poly (methacrylic acid) Hydrogels: A Comparison. Materials 2021, 14, 2305. [Google Scholar] [CrossRef]
- Sindhu, S.; Siddaramaiah, D.; Gowda, V.; Datta, V.; Srivastava, A. Formulation and Evaluation of pH Sensitive Poly (Acrylic Acid-Co-Hydroxy Ethyl Methacrylate) Hydrogels for Specific Site Drug Delivery. Der. Pharma. Chem. 2015, 7, 35–45. [Google Scholar]
- Barkat, K.; Ahmad, M.; Usman Minhas, M.; Khalid, I.; Nasir, B. Development and characterization of pH-responsive polyethylene glycol-co-poly (methacrylic acid) polymeric network system for colon target delivery of oxaliplatin: Its acute oral toxicity study. Adv. Polym. Technol. 2018, 37, 1806–1822. [Google Scholar] [CrossRef]
- Shabir, F.; Erum, A.; Tulain, U.R.; Hussain, M.A.; Ahmad, M.; Akhter, F. Preparation and characterization of pH sensitive crosslinked Linseed polysaccharides-co-acrylic acid/methacrylic acid hydrogels for controlled delivery of ketoprofen. Des. Monomers Polym. 2017, 20, 485–495. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Minhas, M.U.; Tulain, R.; Barkat, K.; Khalid, I.; Khalid, Q. Chitosan/xanthan gum based hydrogels as potential carrier for an antiviral drug: Fabrication, characterization, and safety evaluation. Front. Chem. 2020, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, A.A.; Elbakry, A.M.; Esmaeil, A.H.; Khaleel, S.A. Pharmaceutical and pharmacokinetic evaluation of novel rectal mucoadhesive hydrogels containing tolmetin sodium. J. Pharm. Investig. 2018, 48, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Dong, K.; Dong, Y.; You, C.; Xu, W.; Huang, X.; Yan, Y.; Zhang, L.; Wang, K.; Xing, J. Assessment of the drug loading, in vitro and in vivo release behavior of novel pH-sensitive hydrogel. Drug Deliv. 2016, 23, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Bueno, V.B.; Bentini, R.; Catalani, L.H.; Petri, D.F.S. Synthesis and swelling behavior of xanthan-based hydrogels. Carbohydr. Polym. 2013, 92, 1091–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.A.; Bhaskar, B. Preliminary investigation of drug impurities associated with the anti-influenza drug Favipiravir—An insilico approach. Comput. Theor. Chem. 2021, 1204, 113375. [Google Scholar] [CrossRef] [PubMed]
- Sawant, V.; Bamane, S. PEG-beta-cyclodextrin functionalized zinc oxide nanoparticles show cell imaging with high drug payload and sustained pH responsive delivery of curcumin in to MCF-7 cells. J. Drug Deliv. Sci. Technol. 2018, 43, 397–408. [Google Scholar] [CrossRef]
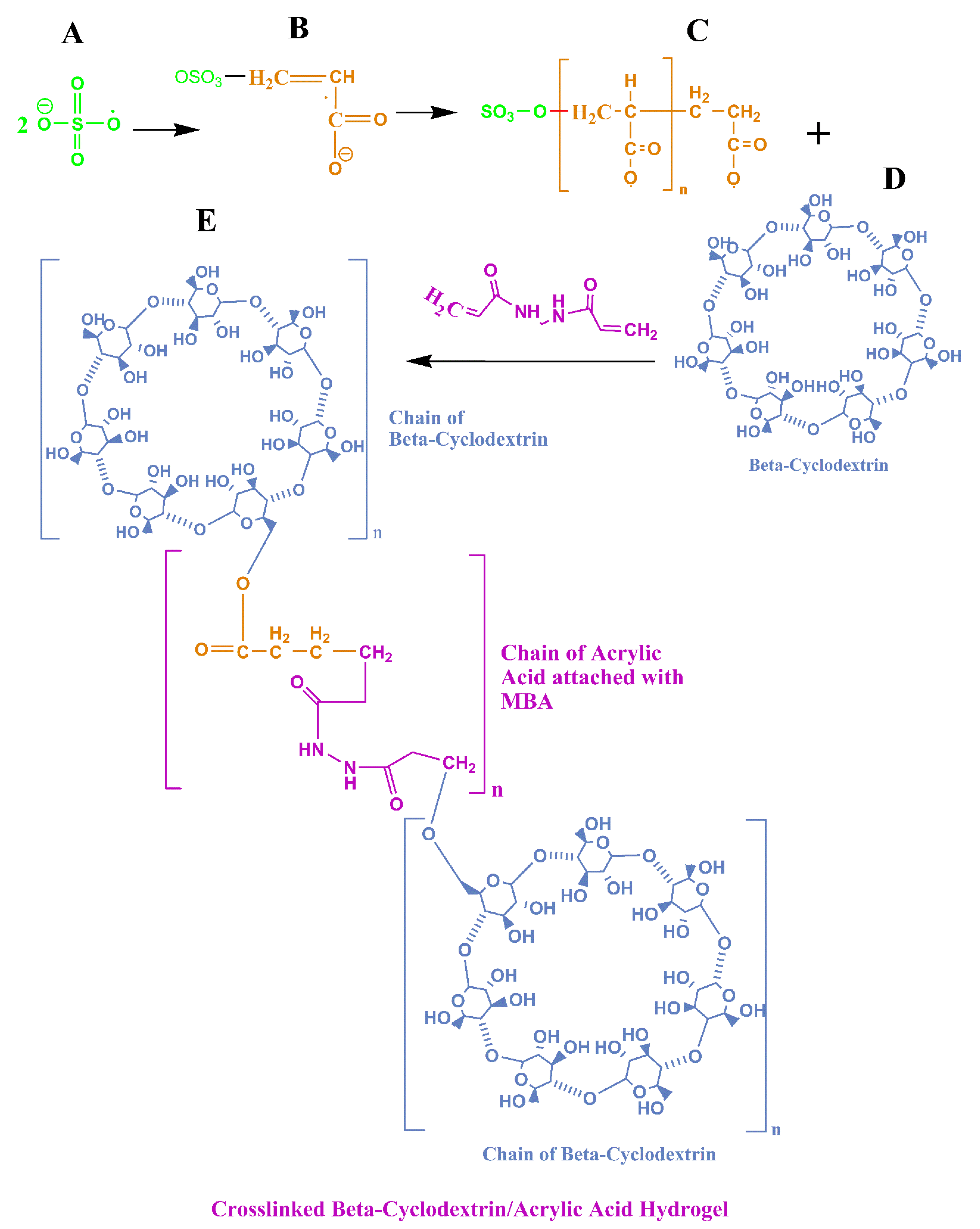

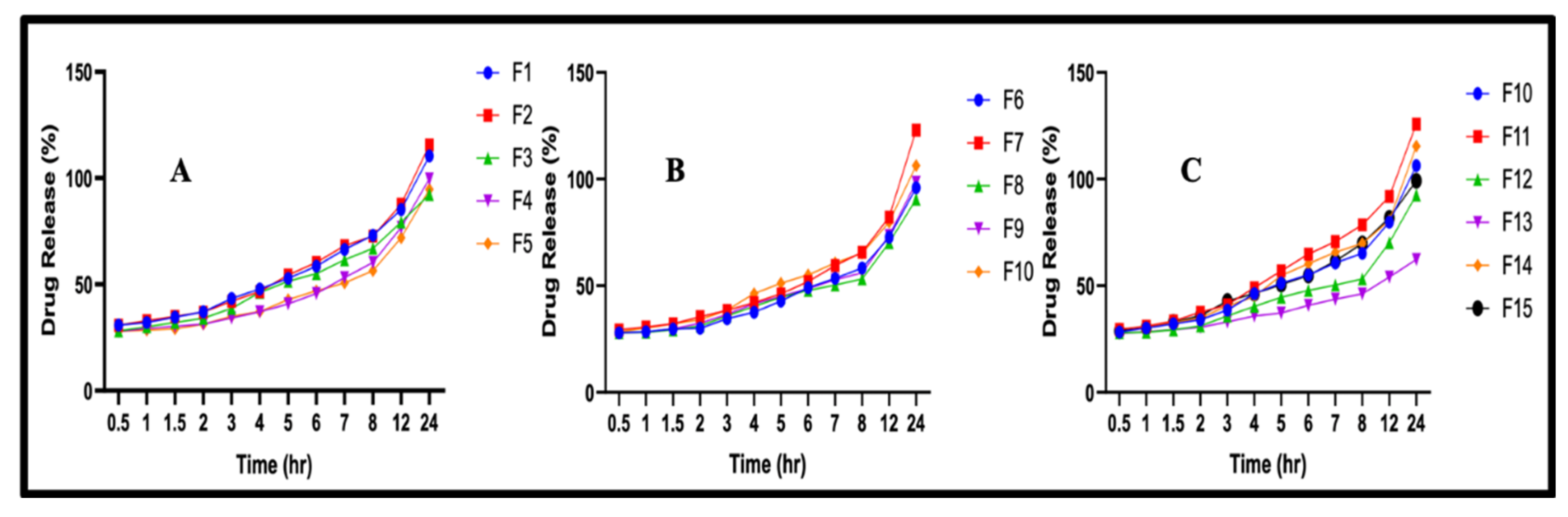
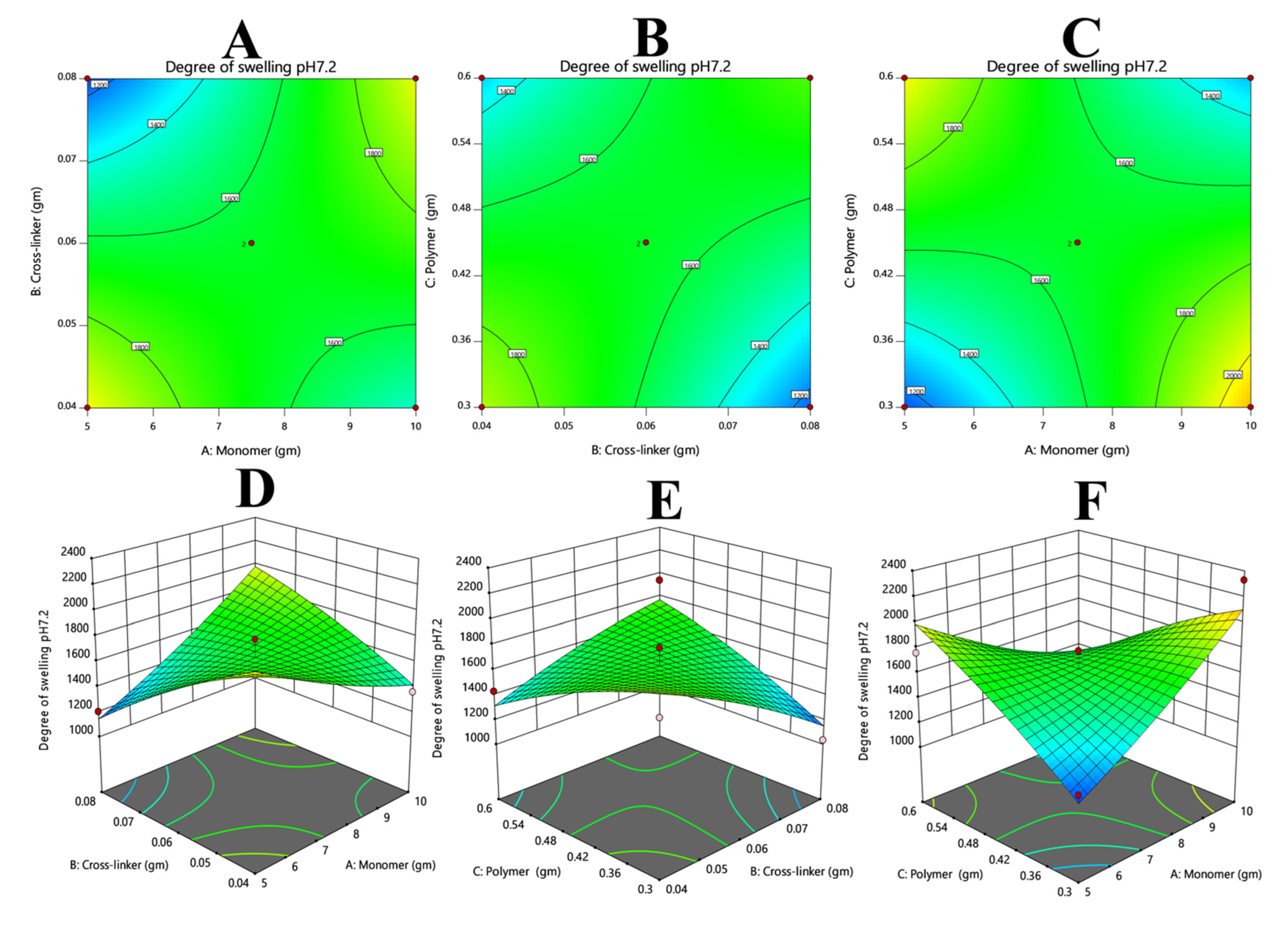


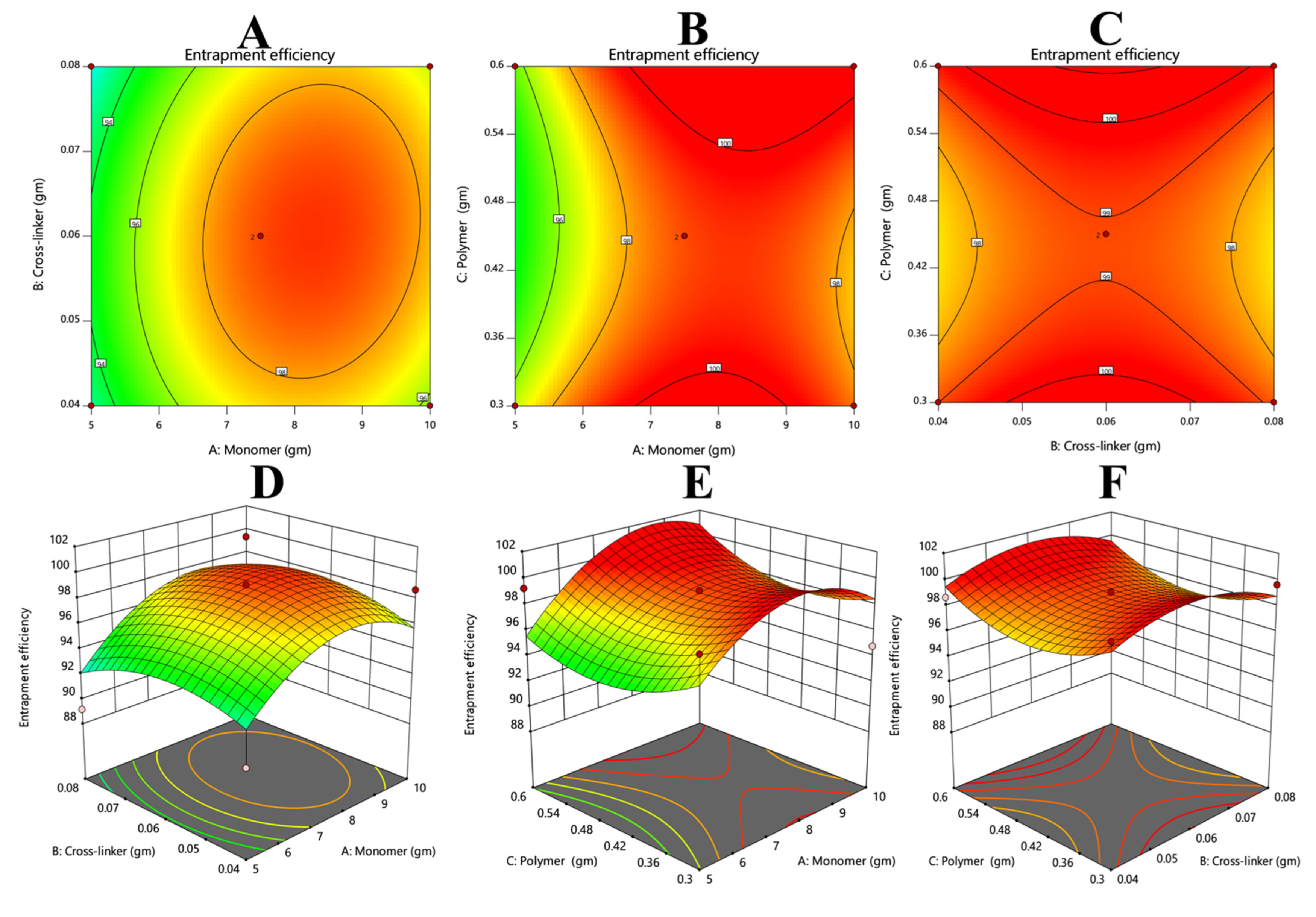

| Serial Number | Monomer (Acrylic Acid) (G) | Crosslinker (MBA) (G) | Oligomer (β-CD) (G) |
|---|---|---|---|
| X1 | X2 | X3 | |
| 1 | 5 | 0.04 | 0.225 |
| 2 | 2.5 | 0.02 | 0.225 |
| 3 | 3.75 | 0.02 | 0.15 |
| 4 | 3.75 | 0.03 | 0.225 |
| 5 | 2.5 | 0.03 | 0.15 |
| 6 | 3.75 | 0.03 | 0.225 |
| 7 | 2.5 | 0.03 | 0.3 |
| 8 | 5 | 0.03 | 0.15 |
| 9 | 3.75 | 0.02 | 0.3 |
| 10 | 3.75 | 0.04 | 0.3 |
| 11 | 5 | 0.03 | 0.3 |
| 12 | 5 | 0.02 | 0.225 |
| 13 | 2.5 | 0.04 | 0.225 |
| 14 | 3.75 | 0.04 | 0.15 |
| Formulation | Absorbance | Total Drug Loaded (mg/mL) | Amount of Drug Recovered (mg/mL) | % Entrapment Efficiency |
|---|---|---|---|---|
| F1 | 1.645 | 5 | 3.97375 | 79.475 |
| F2 | 2.004 | 5 | 4.87125 | 97.425 |
| F3 | 1.766 | 5 | 4.27625 | 85.525 |
| F4 | 1.786 | 5 | 4.32625 | 86.525 |
| F5 | 1.656 | 5 | 4.00125 | 80.025 |
| F6 | 1.65 | 5 | 3.98625 | 79.725 |
| F7 | 1.712 | 5 | 4.14125 | 82.825 |
| F8 | 1.756 | 5 | 4.25125 | 85.025 |
| F9 | 1.765 | 5 | 4.27375 | 85.475 |
| F10 | 1.732 | 5 | 4.19125 | 83.825 |
| F11 | 1.656 | 5 | 4.00125 | 80.025 |
| F12 | 1.702 | 5 | 4.11625 | 82.325 |
| F13 | 1.976 | 5 | 4.80125 | 96.025 |
| F14 | 1.876 | 5 | 4.55125 | 91.025 |
| F15 * | 2.041 | 5 | 4.96375 | 99.275 |
| Formulation | Zero-Order | First Order | Higuchi Model | Korsmeyer-Peppas Model | Hixson-Crowell Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | K0 | R2 | K1 | R2 | KH | R2 | KKP | N | R2 | KHC | |
| F1 | 0.1431 | 6.296 | 0.8153 | 0.178 | 0.9415 | 24.318 | 0.9742 | 29.209 | 0.417 | 0.7717 | 0.049 |
| F2 | 0.0057 | 6.481 | 0.8171 | 0.182 | 0.9546 | 24.874 | 0.9727 | 28.678 | 0.436 | 0.7854 | 0.051 |
| F3 | 0.5570 | 5.593 | 0.7887 | 0.154 | 0.8823 | 21.967 | 0.9689 | 28.769 | 0.377 | 0.7162 | 0.043 |
| F4 | 0.1417 | 5.450 | 0.7777 | 0.125 | 0.9276 | 20.713 | 0.9346 | 22.773 | 0.457 | 0.7477 | 0.036 |
| F5 | 0.0333 | 5.224 | 0.7406 | 0.120 | 0.9272 | 20.007 | 0.9481 | 23.328 | 0.431 | 0.6852 | 0.034 |
| F6 | 0.0008 | 5.315 | 0.7709 | 0.124 | 0.9346 | 20.350 | 0.9525 | 23.481 | 0.435 | 0.7213 | 0.035 |
| F7 | 0.4206 | 6.333 | 0.7757 | 0.152 | 0.9509 | 23.673 | 0.9513 | 23.076 | 0.511 | 0.7618 | 0.042 |
| F8 | 0.2832 | 5.095 | 0.6804 | 0.119 | 0.9079 | 19.700 | 0.9569 | 24.494 | 0.401 | 0.5960 | 0.034 |
| F9 | 0.0223 | 5.405 | 0.7566 | 0.128 | 0.9397 | 20.701 | 0.9603 | 24.099 | 0.431 | 0.7033 | 0.036 |
| F10 | 0.0278 | 5.939 | 0.8191 | 0.155 | 0.9565 | 22.819 | 0.9779 | 26.592 | 0.431 | 0.7736 | 0.043 |
| F11 | 0.2447 | 6.916 | 0.8389 | 0.197 | 0.9776 | 26.279 | 0.9804 | 27.927 | 0.473 | 0.8440 | 0.061 |
| F12 | 0.1924 | 5.148 | 0.6978 | 0.119 | 0.9175 | 19.837 | 0.9556 | 24.155 | 0.411 | 0.6225 | 0.034 |
| F13 | 3.0774 | 3.929 | 0.8107 | 0.085 | 0.3238 | 15.913 | 0.9458 | 26.483 | 0.263 | 1.2802 | 0.024 |
| F14 | 0.1024 | 6.321 | 0.8255 | 0.170 | 0.9646 | 24.148 | 0.9754 | 27.051 | 0.449 | 0.7885 | 0.047 |
| OF | 0.3685 | 5.844 | 0.8035 | 0.162 | 0.9127 | 22.781 | 0.9728 | 28.801 | 0.393 | 0.7437 | 0.045 |
| Parameter | Predicted Outcomes | Obtained Results |
|---|---|---|
| Porosity | 200.000% | 187% |
| Gel Fraction | 93.745% | 92.8% |
| Entrapment Efficiency | 99.033% | 98.73% |
| Degree of Swelling pH 6.8 | 147.629% | 1362% |
| Degree of swelling pH 7.2 | 1556.189% | 1770% |
| In vitro drug release | 99.998% | 98.419% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salawi, A.; Khan, A.; Zaman, M.; Riaz, T.; Ihsan, H.; Butt, M.H.; Aman, W.; Khan, R.; Majeed, I.; Almoshari, Y.; et al. Development of Statistically Optimized Chemically Cross-Linked Hydrogel for the Sustained-Release Delivery of Favipiravir. Polymers 2022, 14, 2369. https://doi.org/10.3390/polym14122369
Salawi A, Khan A, Zaman M, Riaz T, Ihsan H, Butt MH, Aman W, Khan R, Majeed I, Almoshari Y, et al. Development of Statistically Optimized Chemically Cross-Linked Hydrogel for the Sustained-Release Delivery of Favipiravir. Polymers. 2022; 14(12):2369. https://doi.org/10.3390/polym14122369
Chicago/Turabian StyleSalawi, Ahmad, Arooj Khan, Muhammad Zaman, Tehseen Riaz, Hafsa Ihsan, Muhammad Hammad Butt, Waqar Aman, Rahima Khan, Imtiaz Majeed, Yosif Almoshari, and et al. 2022. "Development of Statistically Optimized Chemically Cross-Linked Hydrogel for the Sustained-Release Delivery of Favipiravir" Polymers 14, no. 12: 2369. https://doi.org/10.3390/polym14122369
APA StyleSalawi, A., Khan, A., Zaman, M., Riaz, T., Ihsan, H., Butt, M. H., Aman, W., Khan, R., Majeed, I., Almoshari, Y., & Alshamrani, M. (2022). Development of Statistically Optimized Chemically Cross-Linked Hydrogel for the Sustained-Release Delivery of Favipiravir. Polymers, 14(12), 2369. https://doi.org/10.3390/polym14122369








