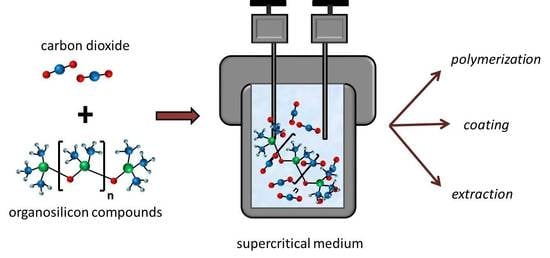
Organosilicone Compounds in Supercritical Carbon Dioxide
Abstract
1. Introduction
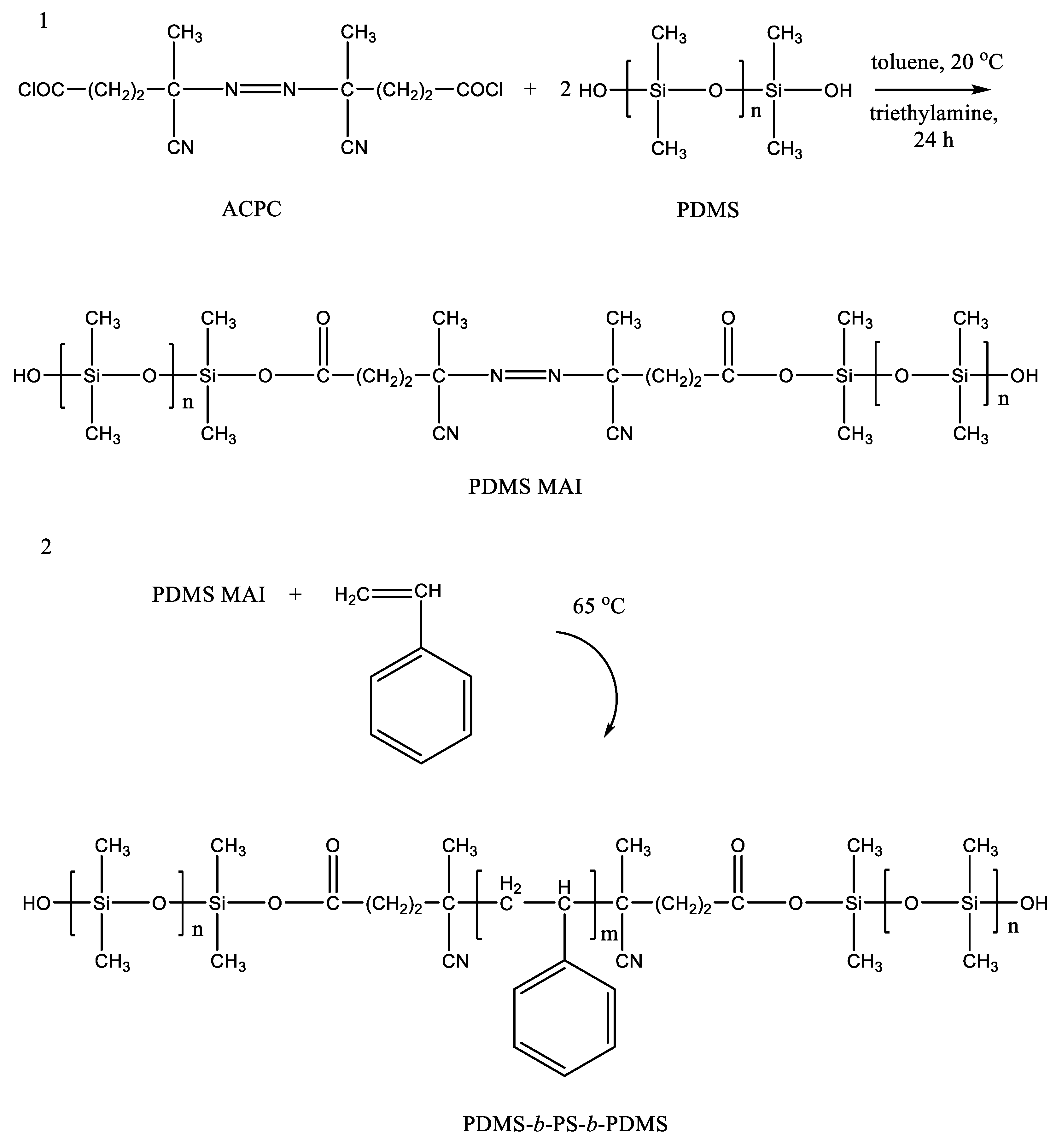
2. Synthesis in scCO2
2.1. Benefits of scCO2
2.2. Obtaining Aerogels in scCO2
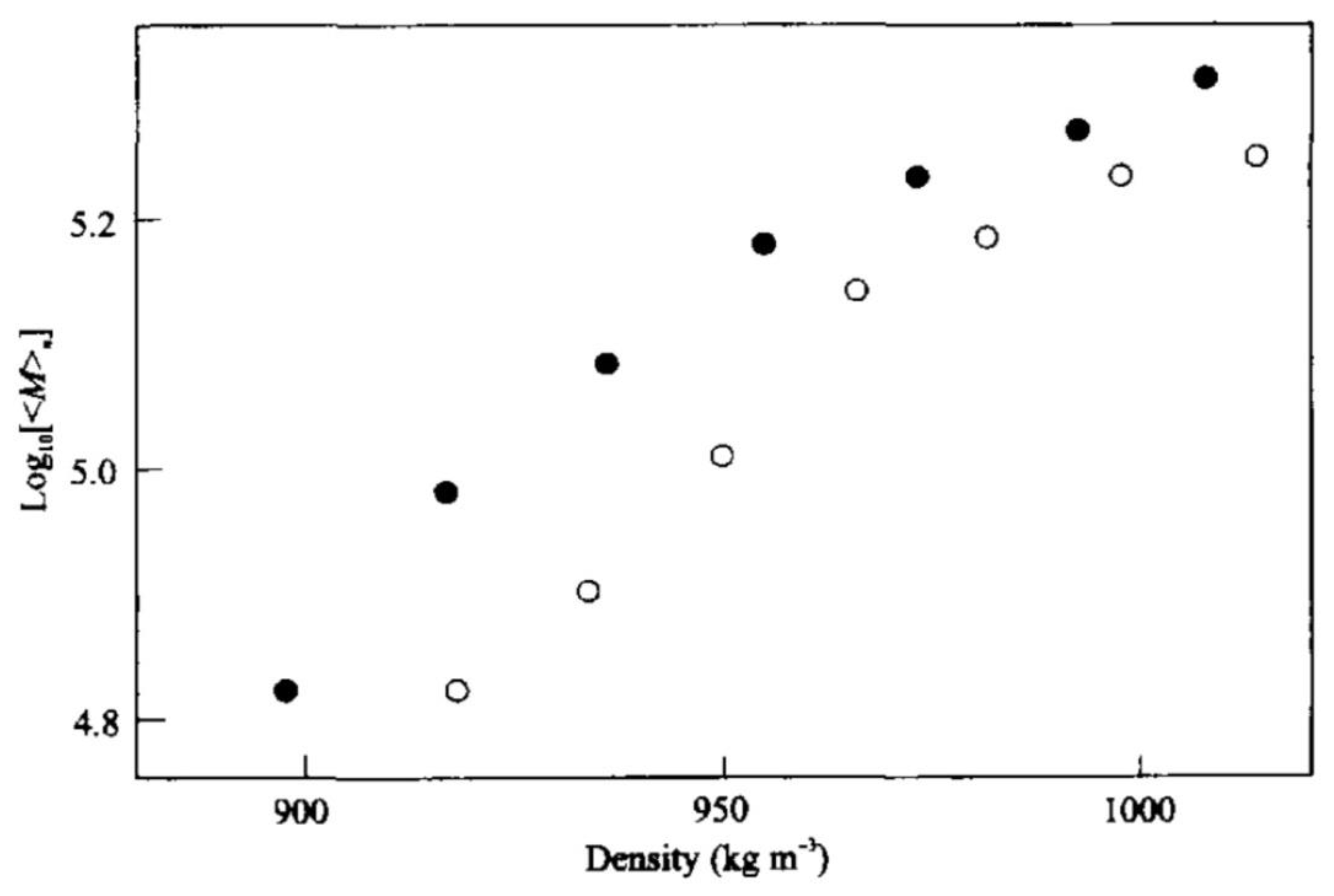
2.3. Copolymers Based on Organosilicon Compounds in scCO2
2.4. Spatially Regular Well-Defined Mesoporous Organosilicon Materials
2.5. Composites Formed in scCO2
3. Silicone Coatings, Silylation
4. PDMS Role in Pore Nucleation Process during Decompression
5. Polysiloxanes as Polymerization Stabilizers
6. Extraction and Fractionation in scCO2
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tutek, K.; Masek, A.; Kosmalska, A.; Cichosz, S. Application of fluids in supercritical conditions in the polymer industry. Polymers 2021, 13, 729. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.D.; Kiran, E. Formation of polymer particles with supercritical fluids: A review. J. Supercrit. Fluids 2005, 34, 287–308. [Google Scholar] [CrossRef]
- Haldorai, Y.; Shim, J.J.; Lim, K.T. Synthesis of polymer-inorganic filler nanocomposites in supercritical CO2. J. Supercrit. Fluids 2012, 71, 45–63. [Google Scholar] [CrossRef]
- Kiran, E. Supercritical fluids and polymers—The year in review—2014. J. Supercrit. Fluids 2016, 110, 126–153. [Google Scholar] [CrossRef]
- Pigaleva, M.A.; Elmanovich, I.V.; Temnikov, M.N.; Gallyamov, M.O.; Muzafarov, A.M. Organosilicon compounds in supercritical carbon dioxide: Synthesis, polymerization, modification, and production of new materials. Polym. Sci. Ser. B 2016, 58, 235–270. [Google Scholar] [CrossRef]
- McClain, J.B.; Londono, D.; Combes, J.R.; Romack, T.J.; Canelas, D.A.; Betts, D.E.; Wignall, G.D.; Samulski, E.T.; DeSimone, J.M. Solution properties of a CO2-soluble fluoropolymer via small angle neutron scattering. J. Am. Chem. Soc. 1996, 118, 917–918. [Google Scholar] [CrossRef]
- Chillura-Martino, D.; Triolo, R.; McClain, J.B.; Combes, J.R.; Betts, D.E.; Canelas, D.A.; DeSimone, J.M.; Samulski, E.T.; Cochran, H.D.; Londono, J.D.; et al. Neutron scattering characterization of homopolymers and graft-copolymer micelles in supercritical carbon dioxide. J. Mol. Struct. 1996, 383, 3–10. [Google Scholar] [CrossRef][Green Version]
- Loy, D.A.; Russick, E.M.; Yamanaka, S.A.; Baugher, B.M.; Shea, K.J. Direct Formation of Aerogels by Sol-Gel Polymerizations of Alkoxysilanes in Supercritical Carbon Dioxide. Chem. Mater. 1997, 9, 2264–2268. [Google Scholar] [CrossRef]
- Park, S.J.; Kwak, T.Y.; Mansoori, G.A. Statistical mechanical description of supercritical fluid extraction and retrograde condensation. Int. J. Thermophys. 1987, 8, 449–471. [Google Scholar] [CrossRef]
- Zou, F.; Peng, L.; Fu, W.; Zhang, J.; Li, Z. Flexible superhydrophobic polysiloxane aerogels for oil-water separation via one-pot synthesis in supercritical CO2. RSC Adv. 2015, 5, 76346–76351. [Google Scholar] [CrossRef]
- Elmanovich, I.V.; Pryakhina, T.A.; Vasil’ev, V.G.; Gallyamov, M.O.; Muzafarov, A.M. A study of the hydrosilylation approach to a one-pot synthesis of silicone aerogels in supercritical CO2. J. Supercrit. Fluids 2018, 133, 512–518. [Google Scholar] [CrossRef]
- Elmanovich, I.V.; Pryakhina, T.A.; Gallyamov, M.O.; Migulin, D.A.; Meshkov, I.B.; Vasil’ev, V.G.; Muzafarov, A.M. Silicone aerogels with tunable mechanical properties obtained via hydrosilylation reaction in supercritical CO2. J. Supercrit. Fluids 2019, 149, 120–126. [Google Scholar] [CrossRef]
- Mahadik, D.B.; Lee, Y.K.; Chavan, N.K.; Mahadik, S.A.; Park, H.H. Monolithic and shrinkage-free hydrophobic silica aerogels via new rapid supercritical extraction process. J. Supercrit. Fluids 2016, 107, 84–91. [Google Scholar] [CrossRef]
- Kanamori, B.K.; Aizawa, M.; Nakanishi, K.; Hanada, T. New Transparent Methylsilsesquioxane Aerogels and Xerogels with Improved Mechanical Properties. Adv. Mater. 2007, 19, 1589–1593. [Google Scholar] [CrossRef]
- Kanamori, K.; Nakanishi, K.; Hanada, T. Elastic aerogels and xerogels synthesized from methyltrimethoxysilane (MTMS). MRS Proc. 2009, 1134, 1134-BB07-06. [Google Scholar] [CrossRef]
- Zu, G.; Kanamori, K.; Maeno, A.; Kaji, H.; Nakanishi, K. Superflexible Multifunctional Polyvinylpolydimethylsiloxane-Based Aerogels as Efficient Absorbents, Thermal Superinsulators, and Strain Sensors. Angew. Chemie 2018, 57, 9722–9727. [Google Scholar] [CrossRef] [PubMed]
- Zu, G.; Kanamori, K.; Shimizu, T.; Zhu, Y.; Maeno, A.; Kaji, H.; Nakanishi, K.; Shen, J. Versatile Double-Cross-Linking Approach to Transparent, Machinable, Supercompressible, Highly Bendable Aerogel Thermal Superinsulators. Chem. Mater. 2018, 30, 2759–2770. [Google Scholar] [CrossRef]
- Zu, G.; Kanamori, K.; Wang, X.; Nakanishi, K.; Shen, J. Superelastic Triple-Network Polyorganosiloxane-Based Aerogels as Transparent Thermal Superinsulators and Efficient Separators. Chem. Mater. 2020, 32, 1595–1604. [Google Scholar] [CrossRef]
- Kholodkov, D.N.; Arzumanyan, A.V.; Novikov, R.A.; Kashin, A.S.; Polezhaev, A.V.; Vasil’Ev, V.G.; Muzafarov, A.M. Silica-Based Aerogels with Tunable Properties: The Highly Efficient BF3-Catalyzed Preparation and Look inside Their Structure. Macromolecules 2021, 54, 1961–1975. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.K.; Park, H.H.; Kang, E.S.; Nadargi, D.Y. Silica aerogel: Synthesis and applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef]
- Yadav, P.; Agrawal, M.; Alexander, A.; Patel, R.; Siddique, S.; Saraf, S.; Ajazuddin. Polymer production and processing using supercritical carbon dioxide. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Supercritical Carbon Dioxide as Green Solvent; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128173886. [Google Scholar]
- Stakhanov, A.I.; Said-Galiev, E.E.; Izmailov, B.A.; Vasnev, V.A.; Khokhlov, A.R. Synthesis of poly(arylate-siloxane)s in supercritical carbon dioxide. Polym. Sci. Ser. B 2008, 50, 11–15. [Google Scholar] [CrossRef]
- Akgün, M.; Deniz, S.; Baran, N.; Uzun, N.I.; Akgün, N.A.; Dinçer, S. Synthesis of polydimethylsiloxane-block-polystryrene-block- polydimethylsiloxane via polysiloxane-based macroinitiator in supercritical CO2. Polym. Int. 2005, 54, 374–380. [Google Scholar] [CrossRef]
- Hu, H.; Chen, M.; Cheng, R. Siloxane-modified poly(acrylic acid) synthesized in supercritical CO2. Polymer 2002, 44, 341–345. [Google Scholar] [CrossRef]
- Shiho, H.; DeSimone, J.M. Preparation of Silicone-Graft Copolymers by Homogeneous Radical Copolymerization in Supercritical Carbon Dioxide. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1139–1145. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Wang, W.; Mokaya, R.; Poliakoff, M. Synthesis of siliceous hollow spheres with large mesopore wall structure by supercritical CO2-in-water interface templating. Chem. Commun. 2005, 210–212. [Google Scholar] [CrossRef]
- Huang, X.; Li, W.; Wang, M.; Tan, X.; Wang, Q.; Zhang, M.; Wang, C.; Zhang, H. Synthesis of multiple-shelled organosilica hollow nanospheres via a dual-template method by using compressed CO2. Microporous Mesoporous Mater. 2017, 247, 66–74. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Huang, X.; Wang, Q.; Liu, L.; Wang, M.; Tan, X.; Luo, T.; Patil, A.J. Compressed CO2 mediated synthesis of bifunctional periodic mesoporous organosilicas with tunable porosity. Chem. Commun. 2016, 52, 9668–9671. [Google Scholar] [CrossRef]
- Pigaleva, M.A.; Elmanovich, I.V.; Kononevich, Y.N.; Gallyamov, M.O.; Muzafarov, A.M. A biphase H2O/CO2 system as a versatile reaction medium for organic synthesis. RSC Adv. 2015, 5, 103573–103608. [Google Scholar] [CrossRef]
- Pai, R.A.; Humayun, R.; Schulberg, M.T.; Sengupta, A.; Sun, J.N.; Watkins, J.J. Mesoporous Silicates Prepared Using Preorganized Templates in Supercritical Fluids. Science 2004, 303, 507–510. [Google Scholar] [CrossRef]
- Pai, R.A.; Watkins, J.J. Synthesis of mesoporous organosilicate films in supercritical carbon dioxide. Adv. Mater. 2006, 18, 241–245. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Small is different: Shape-, size-, and composition-dependent properties of some colloidal semiconductor nanocrystals. Acc. Chem. Res. 2004, 37, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A. Functional materials: From hard to soft porous frameworks. Angew. Chemie 2010, 49, 8328–8344. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.Z.; Yu, C.Z.; Xing, W.; Hu, Q.H.; Djojoputro, H.; Lu, G.Q. Synthesis and bio-adsorptive properties of large-pore periodic mesoporous organosilica rods. Chem. Mater. 2005, 17, 6172–6176. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, M.; Wang, M.; Li, W.; Wang, C.; Hou, X.; Luan, S.; Wang, Q. Gold/Periodic Mesoporous Organosilicas with Controllable Mesostructure by Using Compressed CO2. Langmuir 2018, 34, 3642–3653. [Google Scholar] [CrossRef]
- Kazarian, S.G. Polymer Processing with Supercritical Fluids. Polym. Sci. Ser. C 2000, 42, 78–101. [Google Scholar]
- Alekseev, E.S.; Alentiev, A.Y.; Belova, A.S.; Bogdan, V.I.; Bogdan, T.V.; Bystrova, A.V.; Gafarova, E.R.; Golubeva, E.N.; Grebenik, E.A.; Gromov, O.I.; et al. Supercritical fluids in analytical chemistry. Russ. Chem. Rev. 2020, 89, 1337–1427. [Google Scholar] [CrossRef]
- Su, L.; Pei, S.; Li, L.; Li, H.; Zhang, Y.; Yu, W.; Zhou, C. Preparation of polysiloxane/perfluorosulfonic acid nanocomposite membranes in supercritical carbon dioxide system for direct methanol fuel cell. Int. J. Hydrogen Energy 2009, 34, 6892–6901. [Google Scholar] [CrossRef]
- Su, L.; Li, L.; Li, H.; Tang, J.; Zhang, Y.; Yu, W.; Zhou, C. Preparation of polysiloxane modified perfluorosulfonic acid composite membranes assisted by supercritical carbon dioxide for direct methanol fuel cell. J. Power Sources 2009, 194, 220–225. [Google Scholar] [CrossRef]
- Simonov, A.S.; Kondratenko, M.S.; Elmanovich, I.V.; Sizov, V.E.; Kharitonova, E.P.; Abramchuk, S.S.; Nikolaev, A.Y.; Fedosov, D.A.; Gallyamov, M.O.; Khokhlov, A.R. Modification of Nafion with silica nanoparticles in supercritical carbon dioxide for electrochemical applications. J. Memb. Sci. 2018, 564, 106–114. [Google Scholar] [CrossRef]
- Sanli, D.; Erkey, C. Silylation from supercritical carbon dioxide: A powerful technique for modification of surfaces. J. Mater. Sci. 2015, 50, 7159–7181. [Google Scholar] [CrossRef]
- Domingo, C.; Loste, E.; Fraile, J. Grafting of trialkoxysilane on the surface of nanoparticles by conventional wet alcoholic and supercritical carbon dioxide deposition methods. J. Supercrit. Fluids 2006, 37, 72–86. [Google Scholar] [CrossRef]
- López-aranguren, P.; Saurina, J.; Vega, L.F.; Domingo, C. Microporous and Mesoporous Materials Sorption of tryalkoxysilane in low-cost porous silicates using a supercritical CO2 method. Microporous Mesoporous Mater. 2012, 148, 15–24. [Google Scholar] [CrossRef]
- Rébiscoul, D.; Perrut, V.; Renault, O.; Rieutord, F.; Olivier, S.; Haumesser, P. Alkoxysilane layers deposited by SC CO2 process on silicon oxide for microelectronics applications. J. Supercrit. Fluids 2009, 51, 287–294. [Google Scholar] [CrossRef]
- Israel, S.S.; Rebiscoul, D.; Odorico, M.; Flaud, V.; Ayral, A. Surface properties of alkoxysilane layers grafted in supercritical carbon dioxide. Langmuir 2019, 35, 2792–2800. [Google Scholar] [CrossRef]
- Israel, S.S.; Tardif, S.; Larrey, V.; Ayral, A. Impact of Silica Surface Nanoconfinement on the Microstructure of Alkoxysilane Layers Grafted by Supercritical Carbon Dioxide. J. Phys. Chem. C 2019, 123, 12305–12312. [Google Scholar] [CrossRef]
- Gorman, B.P.; Zhang, Z.; Matz, P.D.; Mueller, D.W.; Reidy, R.F.; Gorman, B.P.; Zhang, Z. Rapid repair of plasma ash damage in low-k dielectrics using supercritical CO2. J. Vac. Sci. Technol. B 2004, 22, 1210. [Google Scholar] [CrossRef]
- Lahlouh, B.; Lubguban, J.A.; Sivaraman, G.; Gale, R.; Gangopadhyay, S. Silylation Using a Supercritical Carbon Dioxide Medium to Repair Plasma-Damaged Porous Organosilicate Films. Electrochem. Solid-State Lett. 2004, 7, 338–341. [Google Scholar] [CrossRef]
- Xie, B.; Muscat, A.J. Silylation of porous methylsilsesquioxane films in supercritical carbon dioxide. Microelectron. Eng. 2004, 76, 52–59. [Google Scholar] [CrossRef]
- Mok, J.; Seok, H.; Lee, W.; Choi, B.; Gyu, H.; Taek, K. Microelectronic Engineering Repair of plasma-damaged p-SiOCH dielectric films in supercritical CO2. Microelectron. Eng. 2010, 87, 1680–1684. [Google Scholar] [CrossRef]
- Rajagopalan, T.; Lahlouh, B.; Lubguban, J.A.; Biswas, N. Investigation on hexamethyldisilazane vapor treatment of plasma-damaged nanoporous organosilicate films. Appl. Surf. Sci. 2006, 252, 6323–6331. [Google Scholar] [CrossRef]
- Kalinina, A.A.; Kholodkov, D.N.; Meshkov, I.B.; Pigaleva, M.A.; Elmanovich, I.V.; Molodtsova, Y.A.; Gallyamov, M.O.; Muzafarov, A.M. Hydrolytic polycondensation of methylalkoxysilanes under pressure. Russ. Chem. Bull. 2016, 65, 1104–1109. [Google Scholar] [CrossRef]
- Wang, M.; Ma, J.; Chu, R.; Park, C.B.; Nanqiao, Z. Effect of the Introduction of Polydimethylsiloxane on the Foaming Behavior of Block-Copolymerized Polypropylene. J. Appl. Polym. Sci. 2012, 123, 2726–2732. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, S.; Xin, Z.; Shi, Y.; Zhao, S. Polydimethylsiloxane assisted supercritical CO2 foaming behavior of high melt strength polypropylene grafted with styrene. Front. Chem. Sci. Eng. 2016, 10, 396–404. [Google Scholar] [CrossRef]
- Qiang, W.; Hu, D.; Liu, T.; Zhao, L. The Journal of Supercritical Fluids Strategy to control CO2 diffusion in polystyrene microcellular foaming via CO2 -philic additives. J. Supercrit. Fluids 2019, 147, 329–337. [Google Scholar] [CrossRef]
- Qiang, W.; Zhao, L.; Gao, X.; Liu, T.; Liu, Z.; Yuan, W. The Journal of Supercritical Fluids Dual role of PDMS on improving supercritical CO2 foaming of polypropylene: CO2-philic additive and crystallization nucleating agent. J. Supercrit. Fluids 2020, 163, 104888. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, W.; Jung, K.; Park, B.; Park, J.; Ko, J.; Cho, H. A highly sensitive and flexible capacitive pressure sensor based on a porous three-dimensional PDMS/microsphere composite. Polymers 2020, 12, 1412. [Google Scholar] [CrossRef]
- Li, S.; Dong, K.; Li, R.; Huang, X.; Chen, T.; Xiao, X. Capacitive pressure sensor inlaid a porous dielectric layer of superelastic polydimethylsiloxane in conductive fabrics for detection of human motions. Sens. Actuators A Phys. 2020, 312, 112106. [Google Scholar] [CrossRef]
- Kim, D.H.; Jung, Y.; Jung, K.; Kwak, D.H.; Park, D.M.; Shin, M.G.; Tak, H.J.; Ko, J.S. Hollow polydimethylsiloxane (PDMS) foam with a 3D interconnected network for highly sensitive capacitive pressure sensors. Micro Nano Syst. Lett. 2020, 8, 24. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Choi, S.J.; Kwon, T.H.; Im, H.; Moon, D.I.; Baek, D.J.; Seol, M.L.; Duarte, J.P.; Choi, Y.K. A polydimethylsiloxane (PDMS) sponge for the selective absorption of oil from water. ACS Appl. Mater. Interfaces 2011, 3, 4552–4556. [Google Scholar] [CrossRef] [PubMed]
- Gallyamov, M.O.; Nikolaev, A.Y.; Nikitin, L.N. Polystyrene Foamed with Supercritical CO2 as Possible Model System of the Membrane Materials for Flow Batteries. Polym. Sci. Ser. A 2018, 60, 507–514. [Google Scholar] [CrossRef]
- Kondratenko, M.S.; Elmanovich, I.V.; Gallyamov, M.O. Polymer materials for electrochemical applications: Processing in supercritical fluids. J. Supercrit. Fluids 2017, 127, 229–246. [Google Scholar] [CrossRef]
- Sizov, V.E.; Kondratenko, M.S.; Gallyamov, M.O.; Stevenson, K.J. Advanced porous polybenzimidazole membranes for vanadium redox batteries synthesized via a supercritical phase-inversion method. J. Supercrit. Fluids 2018, 137, 111–117. [Google Scholar] [CrossRef]
- Gritskova, I.A.; Lakhtin, V.G.; Shragin, D.I.; Ezhova, A.A.; Sokolskaya, I.B.; Krizhanovsky, I.N.; Storozhenko, P.A.; Muzafarov, A.M. Synthesis of oligosiloxanes with 3-aminopropyl groups and their testing as surfactants in the preparation of polymer microspheres. Russ. Chem. Bull. 2018, 67, 1908–1914. [Google Scholar] [CrossRef]
- Klein, S.M.; Manoharan, V.N.; Pine, D.J.; Lange, F.F. Preparation of monodisperse PMMA microspheres in nonpolar solvents by dispersion polymerization with a macromonomeric stabilizer. Colloid Polym. Sci. 2003, 282, 7–13. [Google Scholar] [CrossRef]
- Richez, A.P.; Farrand, L.; Goulding, M.; Wilson, J.H.; Lawson, S.; Biggs, S.; Cayre, O.J. Erratum: Poly(dimethylsiloxane)-stabilized polymer particles from radical dispersion polymerization in nonpolar solvent: Influence of stabilizer properties and monomer type (Langmuir (2014) 30:5 (1220-1228) 10.1021/la4039304). Langmuir 2014, 30, 5046. [Google Scholar] [CrossRef]
- Canelas, D.A.; DeSimone, J.M. Polymerizations in Liquid and Supercritical Carbon Dioxide. Adv. Polym. Sci. 1997, 133, 102–140. [Google Scholar] [CrossRef]
- Boyère, C.; Jérôme, C.; Debuigne, A. Input of supercritical carbon dioxide to polymer synthesis: An overview. Eur. Polym. J. 2014, 61, 45–63. [Google Scholar] [CrossRef]
- McAllister, T.D.; Farrand, L.D.; Howdle, S.M. Improved Particle Size Control for the Dispersion Polymerization of Methyl methacrylate in Supercritical Carbon Dioxide. Macromol. Chem. Phys. 2016, 217, 2294–2301. [Google Scholar] [CrossRef]
- Shaffer, K.A.; Jones, T.A.; Canelas, D.A.; DeSimone, J.M.; Wilkinson, S.P. Dispersion polymerizations in carbon dioxide using siloxane-based stabilizers. Macromolecules 1996, 29, 2704–2706. [Google Scholar] [CrossRef]
- Oliveira, P.F.; Machado, R.A.F.; Barth, D.; Acosta, E.D. Dispersion polymerization of methyl methacrylate in supercritical carbon dioxide using vinyl terminated poly(dimethylsiloxane). Chem. Eng. Process. Process Intensif. 2016, 103, 46–52. [Google Scholar] [CrossRef]
- Fehrenbacher, U.; Ballauff, M.; Muth, O.; Hirth, T. Investigation of heterogeneous radical polymerization of methyl methacrylate with polydimethylsiloxane as stabilizing agent in supercritical CO2 by turbidimetry. Appl. Organomet. Chem. 2001, 15, 613–616. [Google Scholar] [CrossRef]
- Lee, K.N.; Lee, H.J.; Kim, J.H. Synthesis of phenolic/furfural gel microspheres in supercritical CO2. J. Supercrit. Fluids 2000, 17, 73–80. [Google Scholar] [CrossRef]
- Santos, T.M.M.; Chaves, B.B.; Cerqueira, J.S.; Canario, M.M.; Bresolin, D.; Pinto, J.C.; MacHado, R.A.F.; Cabral-Albuquerque, E.C.M.; Vieira De Melo, S.A.B. Dispersion Polymerization of Methyl Methacrylate in Supercritical CO2: A Preliminary Evaluation of in Situ Incorporation of Copaiba Oil. Ind. Eng. Chem. Res. 2020, 59, 9398–9407. [Google Scholar] [CrossRef]
- Zhan, S.P.; Huang, X.; Zhao, Q.C.; Chen, L.; Hou, W.M.; Zhang, S.; Deng, J.J. A high efficiency PDMS-based stabilizer for dispersion polymerization of L-lactide in supercritical carbon dioxide. J. Macromol. Sci. Part A Pure Appl. Chem. 2013, 50, 1070–1074. [Google Scholar] [CrossRef]
- Wang, W.; Griffiths, R.M.T.; Naylor, A.; Giles, M.R.; Irvine, D.J.; Howdle, S.M. Preparation of cross-linked microparticles of poly(glycidyl methacrylate) by dispersion polymerization of glycidyl methacrylate using a PDMS macromonomer as stabilizer in supercritical carbon dioxide. Polymer 2002, 43, 6653–6659. [Google Scholar] [CrossRef]
- Zhan, S.; Deng, J.; Wang, J.; Hou, W.; Li, Z. Synthesis of triblock stabilizers with and without end-capped for dispersion polymerization in supercritical carbon dioxide. J. Polym. Res. 2019, 26, 229. [Google Scholar] [CrossRef]
- Mendes, R.L.; Fernandes, H.L.; Coelbo, J.P.; Reis, E.C.; Cabral, J.M.S.; Novaid, J.M.; Palavrab, A.F. Analytical, Nutritional and Clinical Methoak Section Supercritical CO, extraction of carotenuids and other lipids from Chlorella vd’garis. Food Chem. 1995, 53, 99–103. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D. Supercritical CO2 extraction of Eucalyptus leaves oil and comparison with Soxhlet extraction and hydro-distillation methods. Sep. Purif. Technol. 2014, 133, 443–451. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G.; Senatore, F. Supercritical CO2 Extraction and Fractionation of Lavender Essential Oil and Waxes. J. Agric. Food Chem. 1995, 43, 1654–1658. [Google Scholar] [CrossRef]
- Anklam, E.; Berg, H.; Mathiasson, L.; Sharman, M.; Ulberth, F. Supercritical fluid extraction (SFE) in food analysis: A review. Food Addit. Contam. 1998, 15, 729–750. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, J.A.; Herrero, M.; Castro-Puyana, M.; Ibáñez, E. Supercritical fluid extraction. RSC Green Chem. 2013, 1, 196–230. [Google Scholar] [CrossRef]
- Vandenburg, H.J.; Clifford, A.A.; Bartle, K.D.; Carroll, J.; Newton, I.; Garden, L.M.; Dean, J.R.; Costley, C.T. Analytical extraction of additives from polymers. Analyst 1997, 122, 101–115. [Google Scholar] [CrossRef]
- Daimon, H.; Hirata, Y. Directly coupled supercritical-fluid extraction/capillary supercritical-fluid chromatography of polymer additives. Chromatographia 1991, 32, 549–554. [Google Scholar] [CrossRef]
- Hirata, Y.; Okamoto, Y. Supercritical fluid extraction combined with microcolumn liquid chromatography for the analysis of polymer additives. J. Microcolumn Sep. 1989, 1, 46–50. [Google Scholar] [CrossRef]
- Ryan, T.W.; Yocklovich, S.G.; Watkins, J.C.; Levy, E.J. Quantitative analysis of additives in polymers using coupled supercritical fluid extraction-supercritical fluid chromatography. J. Chromatogr. A 1990, 505, 273–282. [Google Scholar] [CrossRef]
- Yilgör, I.; McGrath, J.E. Novel supercritical fluid techniques for polymer fractionation and purification. Polym. Bull. 1984, 12, 491–497. [Google Scholar] [CrossRef]
- McGrath, J.E.; Riffle, J.S.; Banthia, A.K.; Yilgor, I.; Wilkes, G.L. Overview of the Polymerization of Cyclosiloxanes. ACS Symp. Ser. 1983, 212, 145–172. [Google Scholar] [CrossRef]
- Clifford, A.A.; Bartle, K.D.; Gelebart, I.; Zhu, S. Fractionation of polymers using supercritical fluid extraction. J. Chromatogr. A 1997, 785, 395–401. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.S.; Lee, S.B. Phase behaviors and fractionation of polymer solutions in supercritical carbon dioxide. J. Supercrit. Fluids 1998, 13, 99–106. [Google Scholar] [CrossRef]
- Zhao, X.; Watkins, R.; Barton, S.W. Strategies for supercritical CO2 fractionation of polydimethylsiloxane. J. Appl. Polym. Sci. 1995, 55, 773–778. [Google Scholar] [CrossRef]
- Sanli, D.; Erkey, C. Demixing pressures of hydroxy-terminated poly(dimethylsiloxane)-carbon dioxide binary mixtures at 313.2 K, 323.2 K and 333.2 K. J. Supercrit. Fluids 2014, 92, 264–271. [Google Scholar] [CrossRef]
- Xiong, Y.; Kiran, E. Miscibility, density and viscosity of poly(dimethylsiloxane) in supercritical carbon dioxide. Polymer 1995, 36, 4817–4826. [Google Scholar] [CrossRef]
- Bayraktar, Z.; Kiran, E. Miscibility, phase separation, and volumetric properties in solutions of poly(dimethylsiloxane) in supercritical carbon dioxide. J. Appl. Polym. Sci. 2000, 75, 1397–1403. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sizov, V.E.; Zefirov, V.V.; Gallyamov, M.O.; Muzafarov, A.M. Organosilicone Compounds in Supercritical Carbon Dioxide. Polymers 2022, 14, 2367. https://doi.org/10.3390/polym14122367
Sizov VE, Zefirov VV, Gallyamov MO, Muzafarov AM. Organosilicone Compounds in Supercritical Carbon Dioxide. Polymers. 2022; 14(12):2367. https://doi.org/10.3390/polym14122367
Chicago/Turabian StyleSizov, Victor E., Vadim V. Zefirov, Marat O. Gallyamov, and Aziz M. Muzafarov. 2022. "Organosilicone Compounds in Supercritical Carbon Dioxide" Polymers 14, no. 12: 2367. https://doi.org/10.3390/polym14122367
APA StyleSizov, V. E., Zefirov, V. V., Gallyamov, M. O., & Muzafarov, A. M. (2022). Organosilicone Compounds in Supercritical Carbon Dioxide. Polymers, 14(12), 2367. https://doi.org/10.3390/polym14122367