Bioinspired Hydrogels as Platforms for Life-Science Applications: Challenges and Opportunities
Abstract
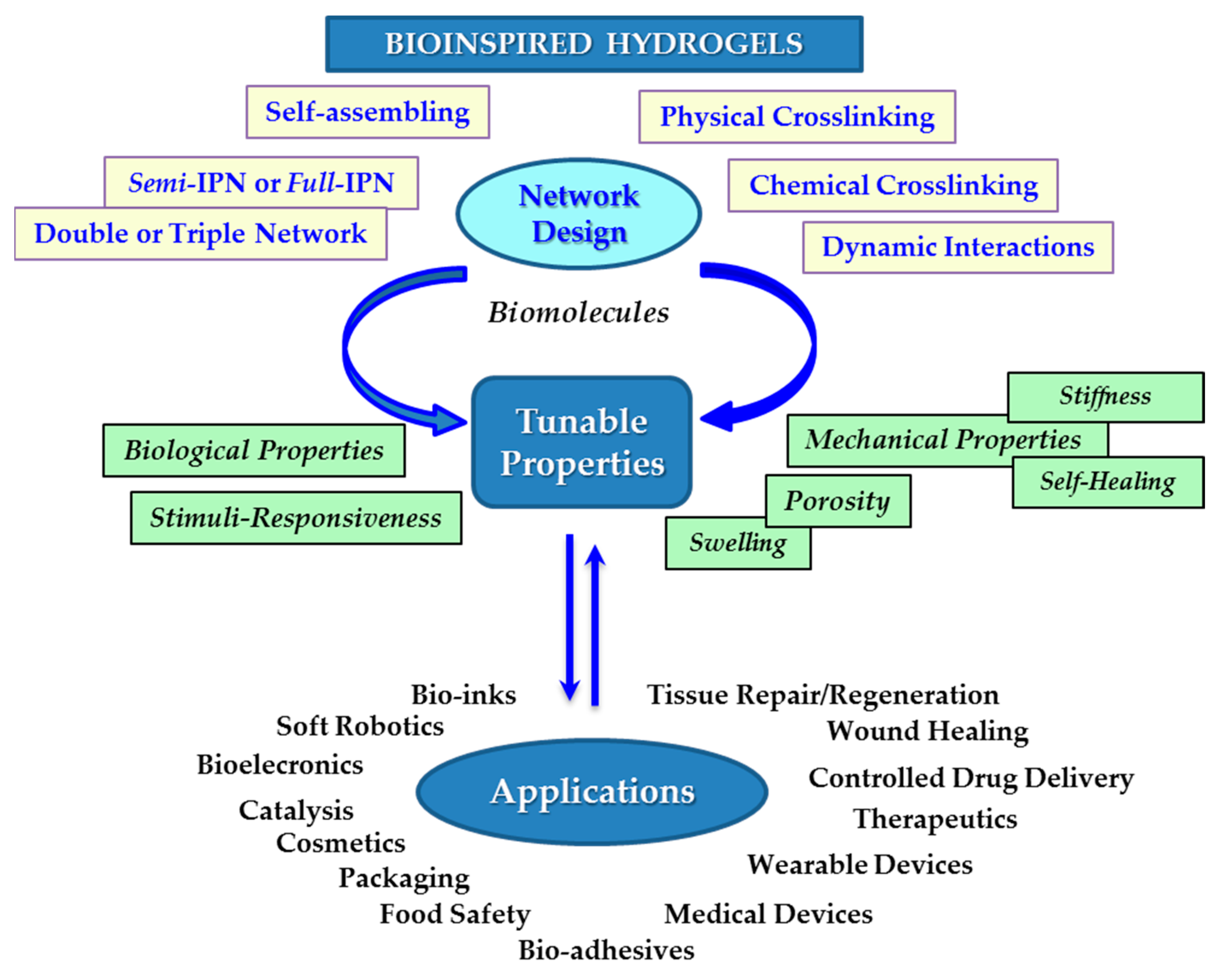
:1. Introduction
2. Hydrogel Design as a Function of the Targeted Application
2.1. Bioinspired Approaches for the Design of Hydrogels with Targeted Properties
- -
- Synthesis of new molecules (monomers, functionalized peptides) that are then assembled in 3D networks;
- -
- Use of network structures (natural or synthetic molecules) in various combinations to give them new features and functionalities.
2.2. Tunable Characteristics of Hydrogels
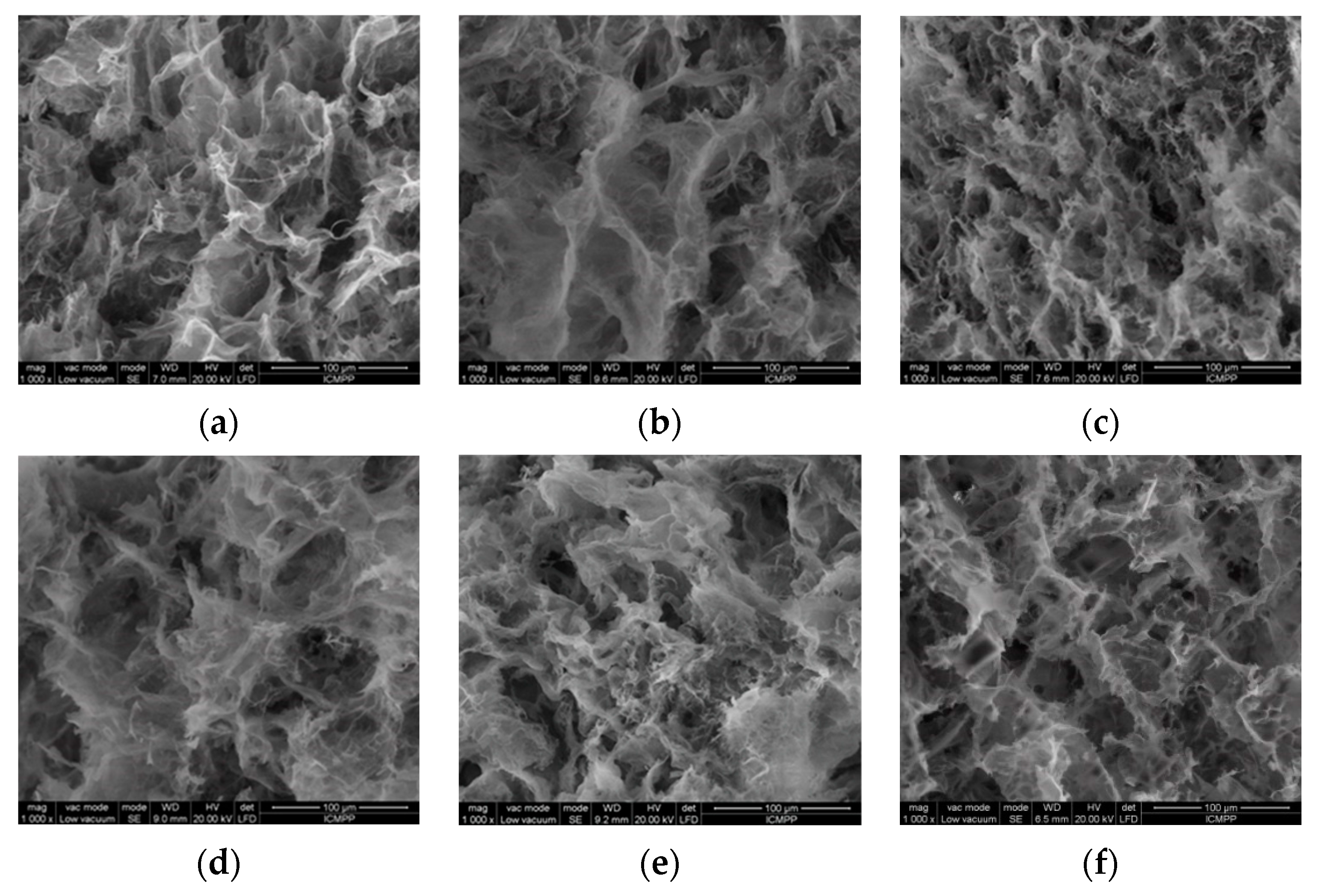
2.2.1. Porosity
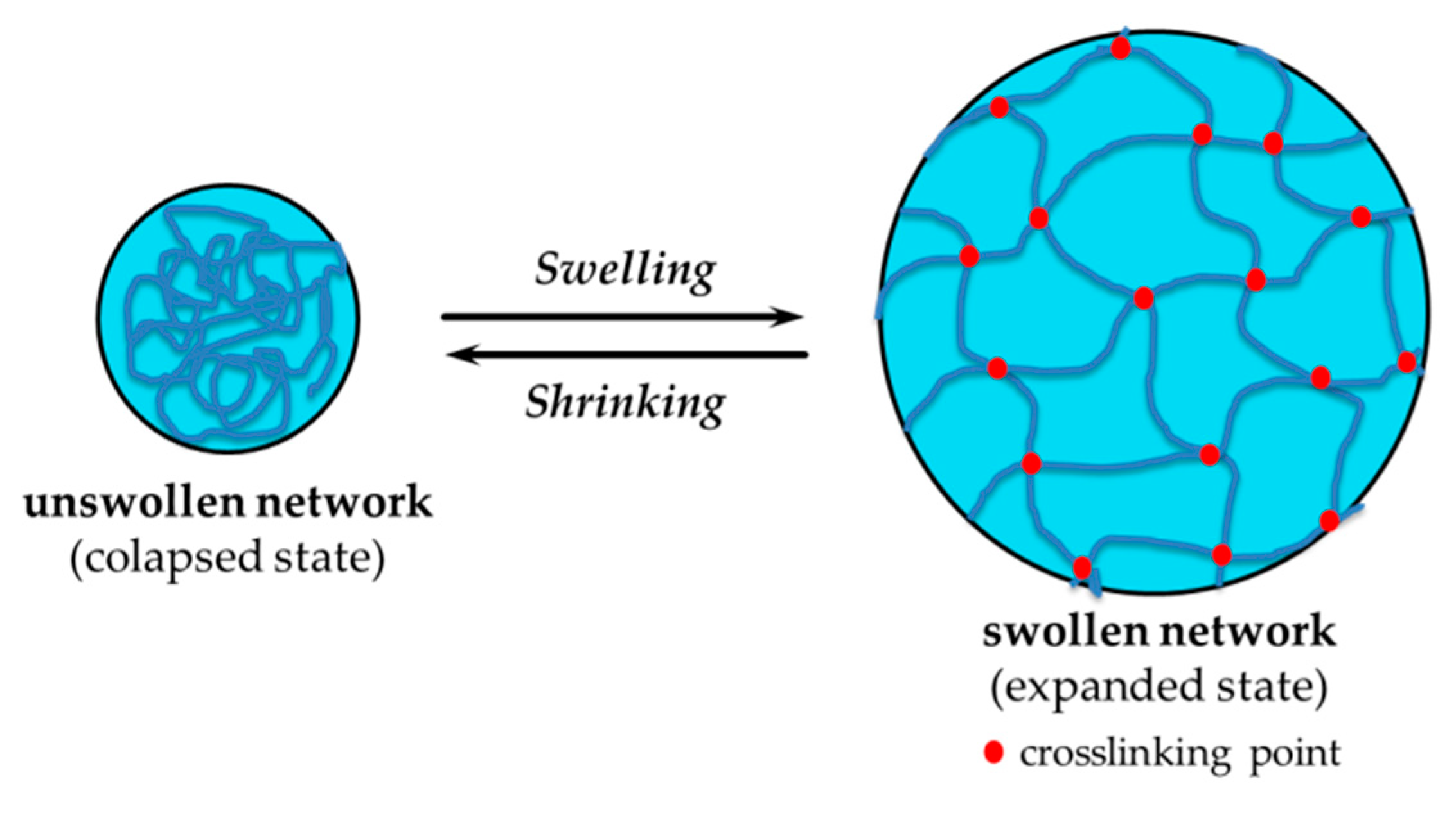
2.2.2. Swelling
2.2.3. Biological Properties
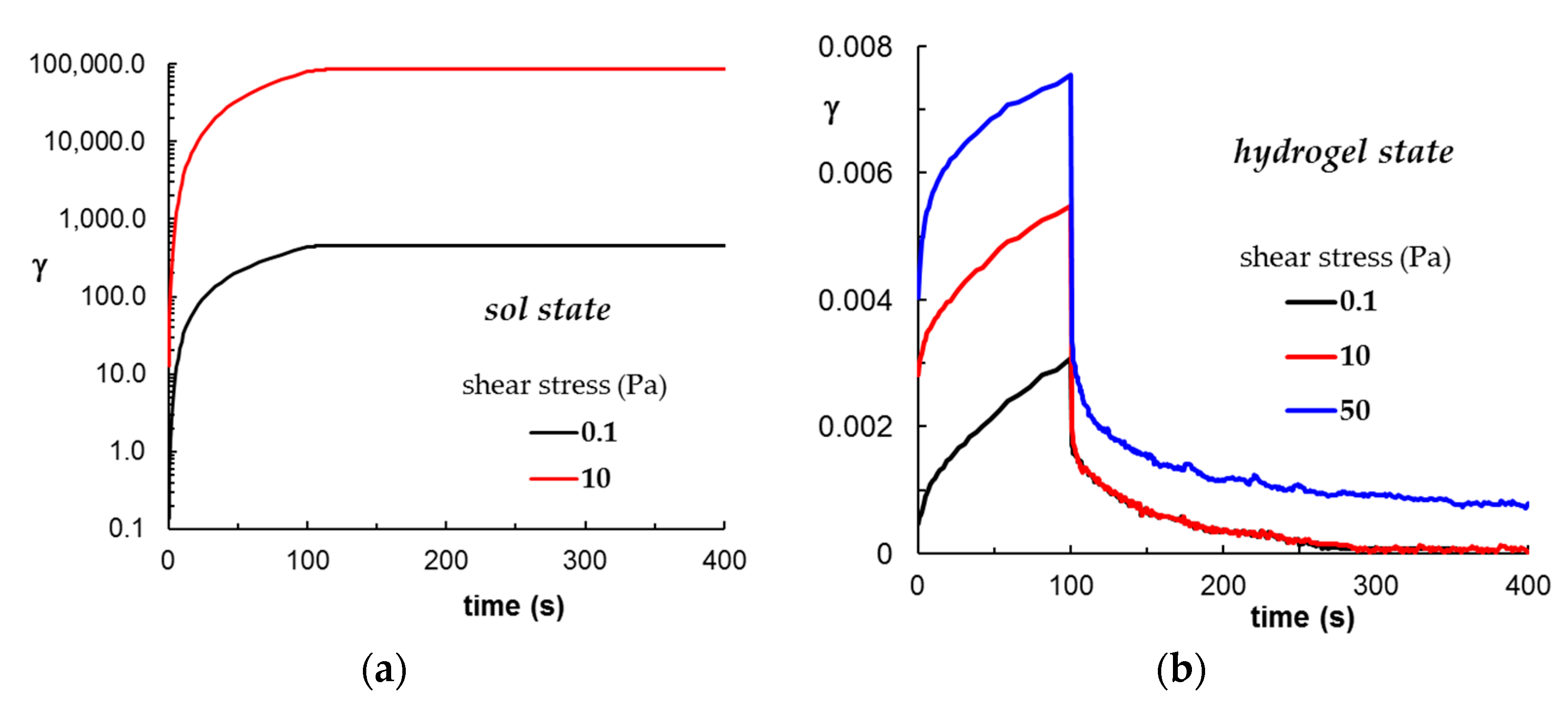
2.2.4. Mechanical Properties
2.2.5. Self-Healing Ability
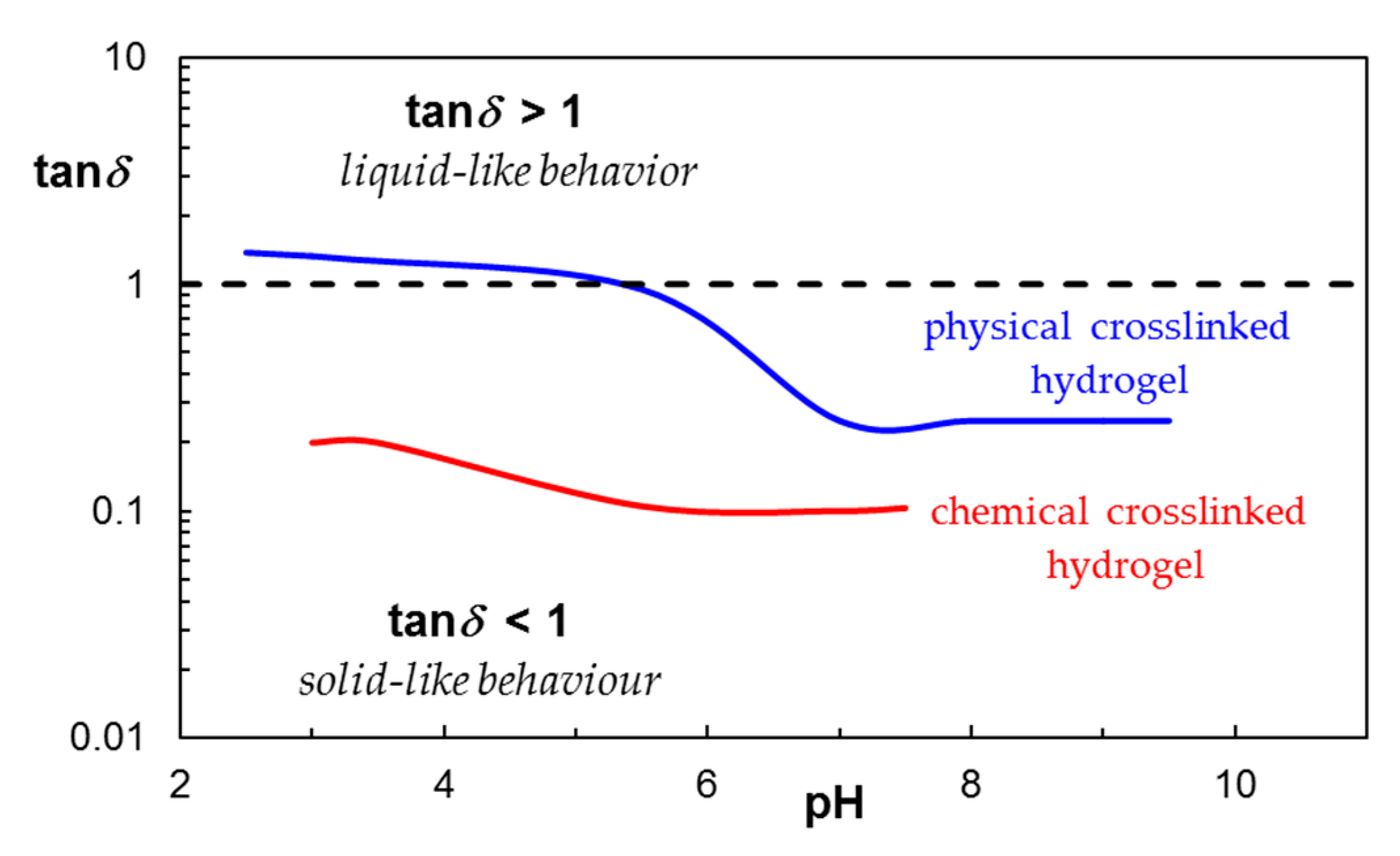
2.2.6. Stimuli-Responsiveness
3. Biomacromolecules Provide an Important Source for Biocompatible/Biodegradable Hydrogels
3.1. Chitosan and Chitin
3.2. Alginate
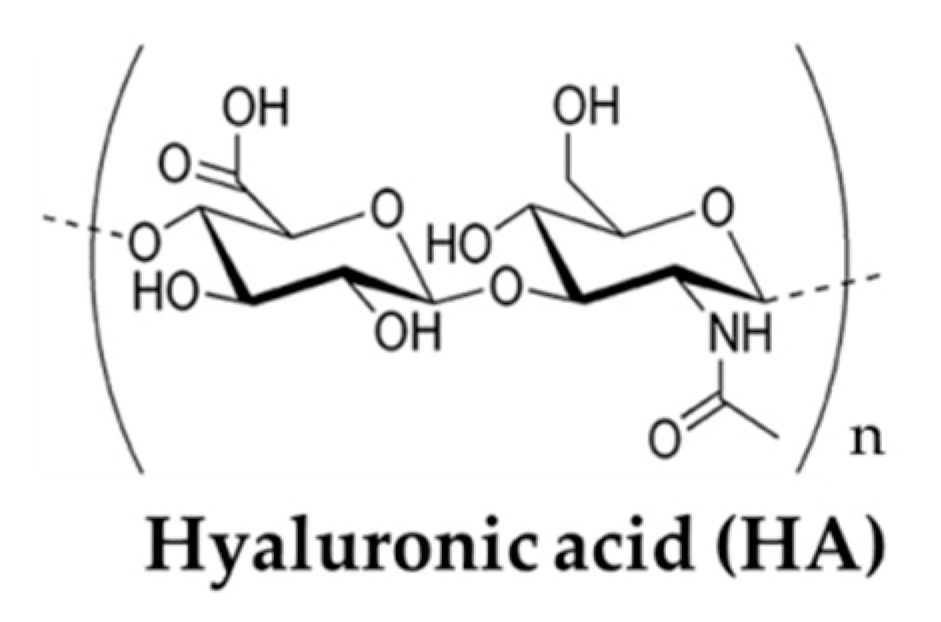
3.3. Hyaluronic Acid
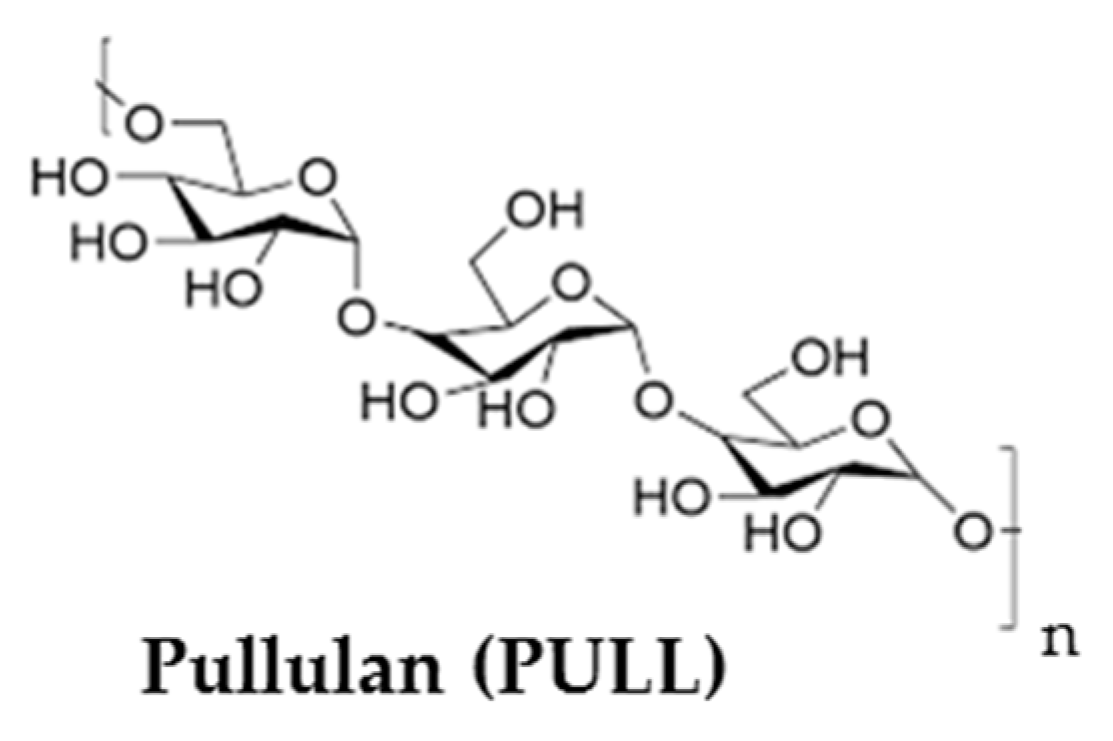
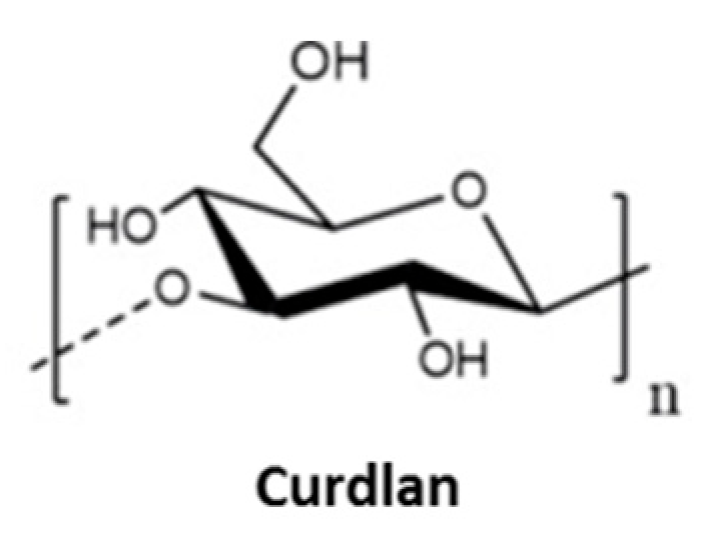
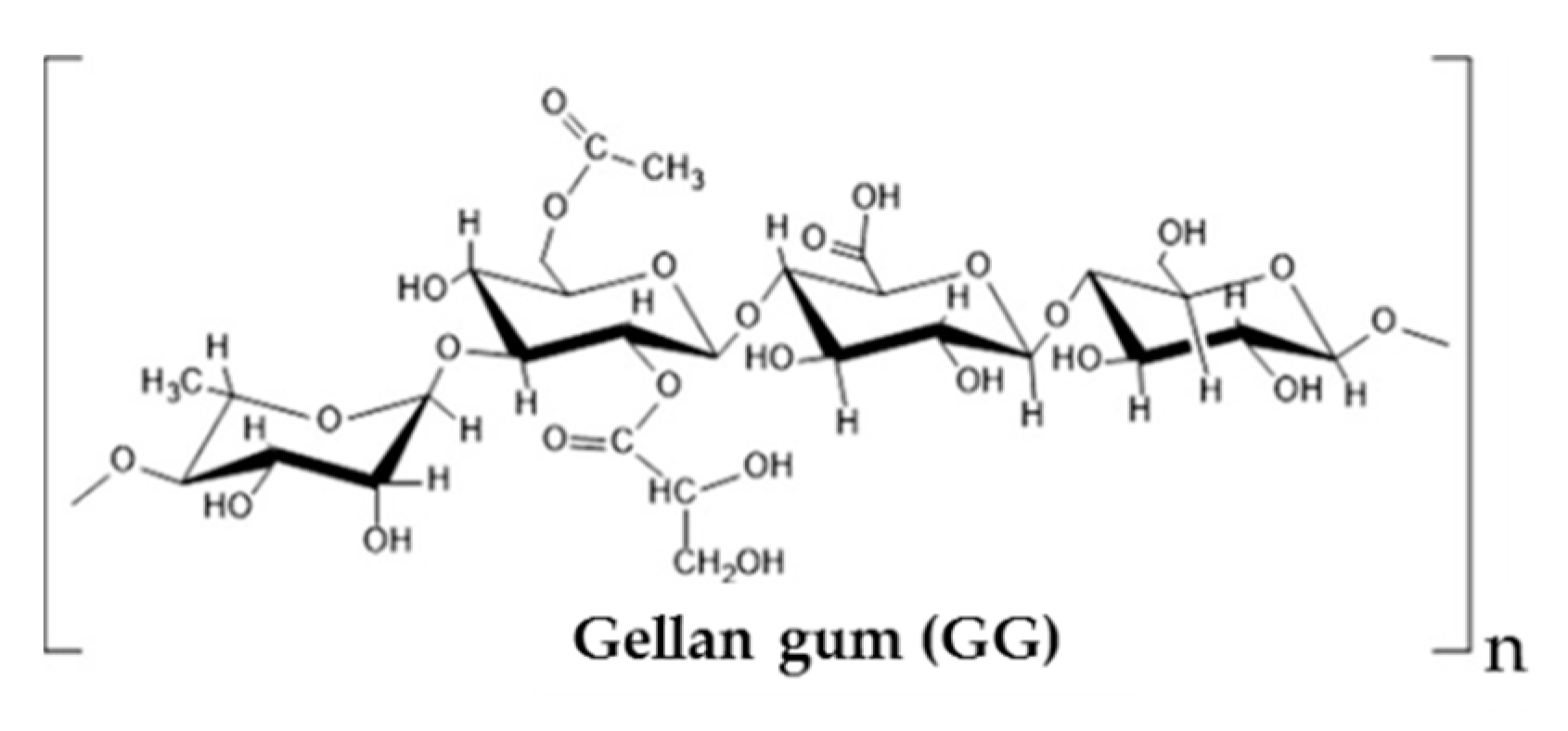
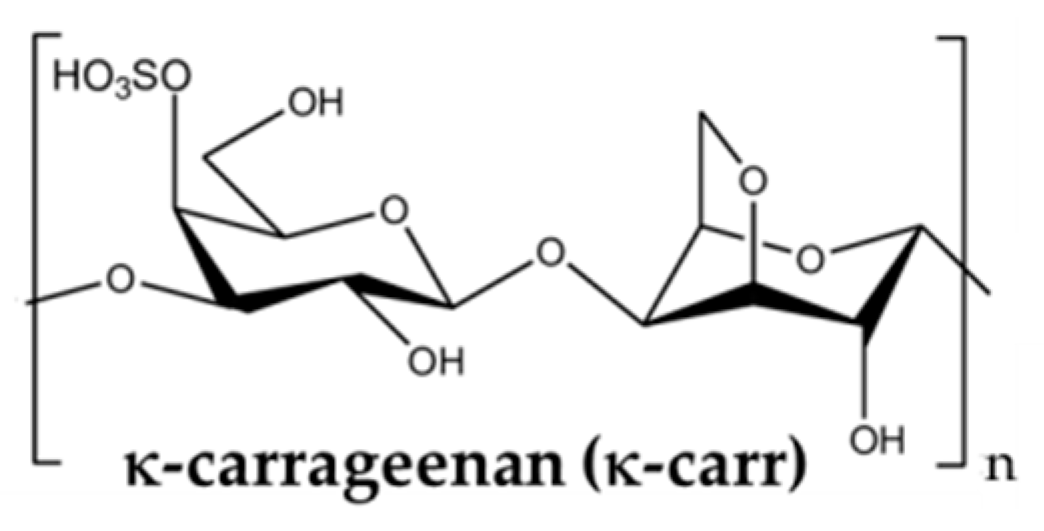
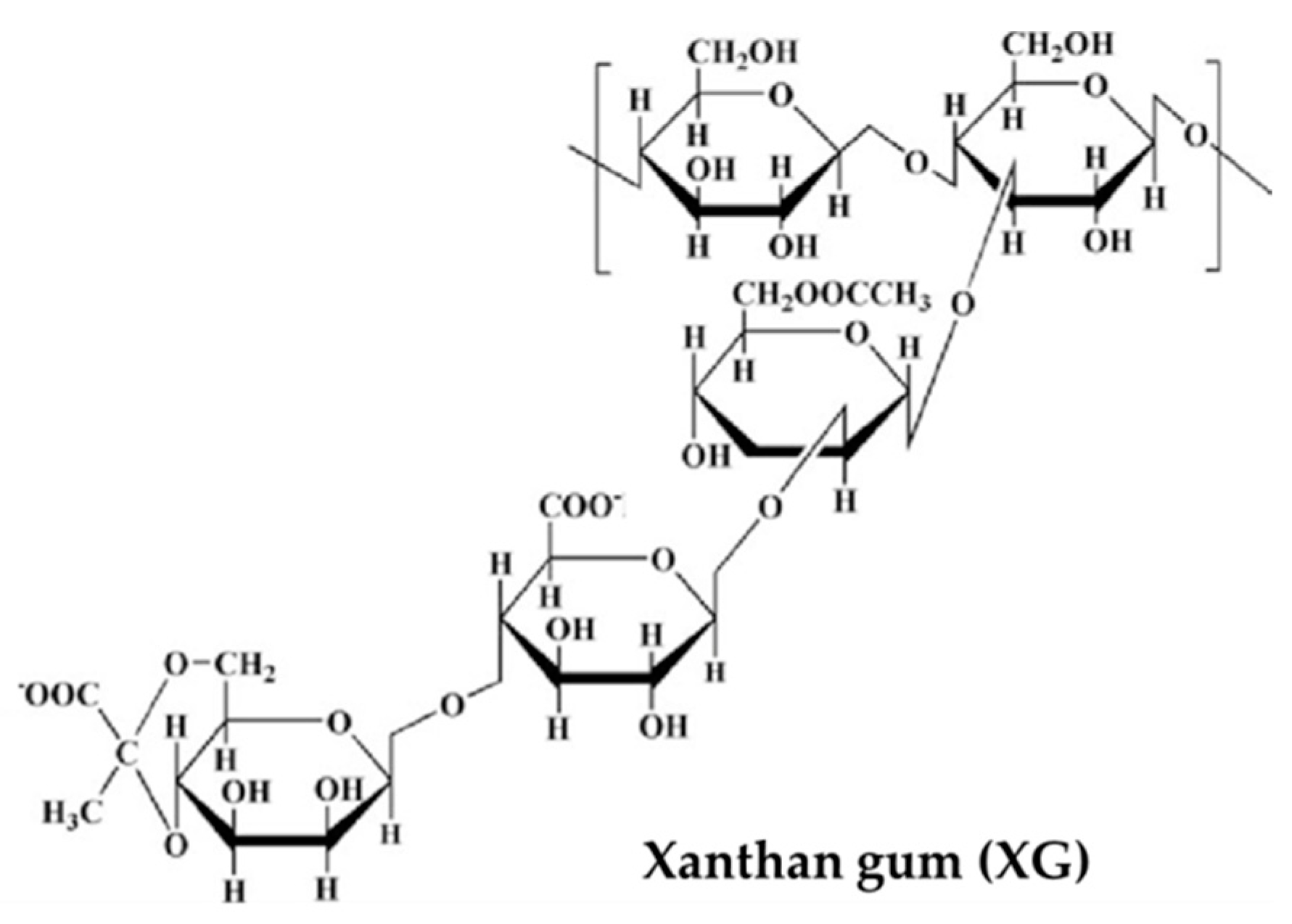
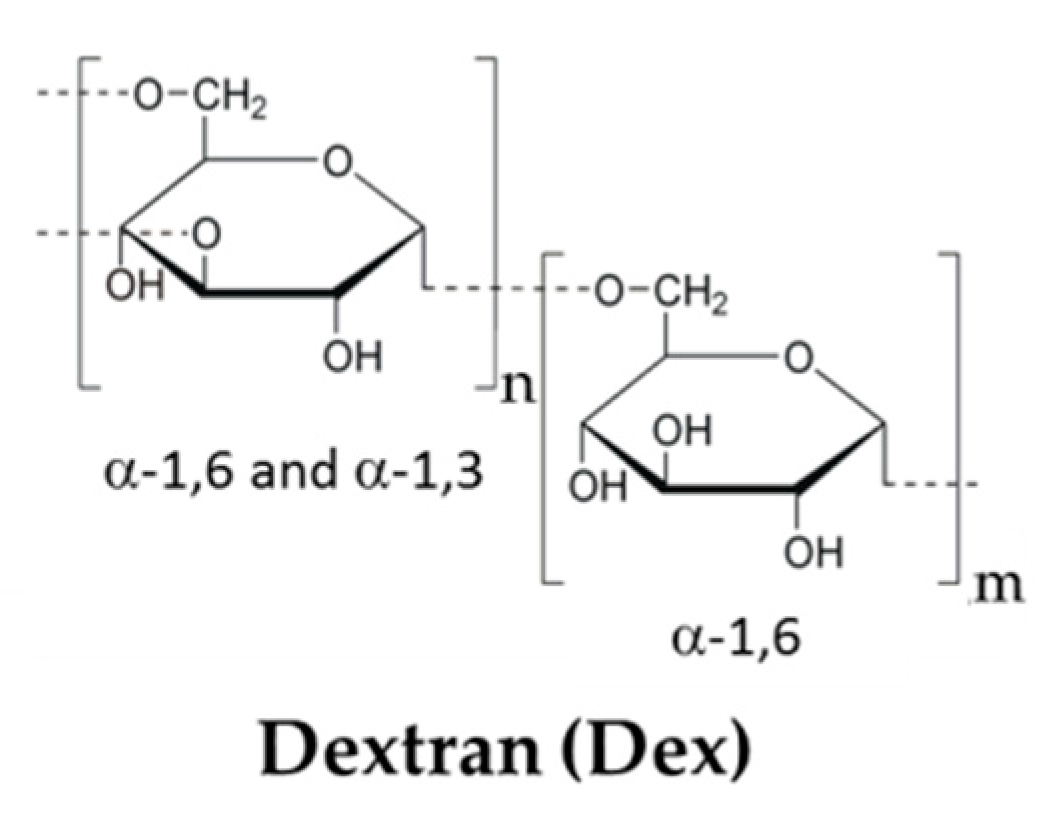
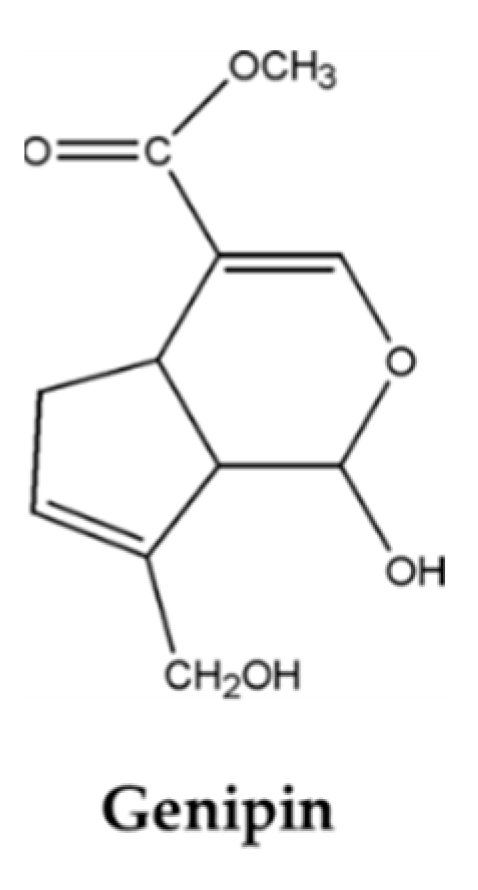
3.4. Other Polyssacharides
3.5. Collagen
3.6. Gelatin
4. Concluding Remarks
5. Future Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BSA | bovine serum albumin |
| CNF | cellulose nanofiber |
| ECM | extracellular matrix |
| IPN | interpenetrated polymer network |
| NP | nanoparticle |
| Alg-Na | sodium alginate |
| CB[n] | cucurbit[n]uril |
| CH | chitin |
| CMC | carboxymethyl cellulose |
| CS | chitosan |
| GMA | methacryloyl gelatin |
| HA | hyaluronic acid |
| HPC | hydroxypropyl cellulose |
| κ-carr | κ-carrageenan |
| PAA | poly(acrylic acid) |
| PAAm | polyacrylamide |
| P(AAm-co-LMA) | poly(acrylamide-co-lauryl methacrylate) |
| P(AAm-co-AA) | poly (acrylamide-co-acrylic acid) |
| PCL | polycaprolactone |
| PEG | poly(ethylene glycol) |
| PEI | poly(ethylene imine) |
| PNiPAm | poly(N-isopropylacrylamide) |
| P(NiPAm-co-NiPMAAm) | poly(N-isopropylacrylamide-co-N-isopropylmethacrylamide) |
| PEO | poly(ethylene oxide) |
| PVA | poly(vinyl alcohol) |
| PULL | pullulan |
| SiO2-g-PBA | SiO2-g-poly(butyl acrylate) |
References
- Yang, D. Recent advances in hydrogels. Chem. Mater. 2022, 34, 1987–1989. [Google Scholar] [CrossRef]
- Wang, B.X.; Xu, W.; Yang, Z.; Wu, Y.; Pi, F. An overview on recent progress of the hydrogels: From material resources, properties, to functional applications. Macromol. Rapid Commun. 2022, 43, 2100785. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef]
- Amrita; Arora, A.; Sharma, P.; Katti, D.S. Pullulan-based composite scaffolds for bone tissue engineering: Improved osteoconductivity by pore wall mineralization. Carbohydr. Polym. 2015, 123, 180–189. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Carpa, R.; Remizovschi, A.; Culda, C.A.; Butiuc-Keul, A.L. Inherent and composite hydrogels as promising materials to limit antimicrobial resistance. Gels 2022, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Ying, B.; Liu, X. Skin-like hydrogel devices for wearable sensing, soft robotics and beyond. iScience 2021, 24, 103174. [Google Scholar] [CrossRef]
- Ziai, Y.; Petronella, F.; Rinoldi, C.; Nakielski, P.; Zakrzewska, A.; Kowalewski, T.A.; Augustyniak, W.; Li, X.; Calogero, A.; Sabała, I.; et al. Chameleon-inspired multifunctional plasmonic nanoplatforms for biosensing applications. NPG Asia Mater. 2022, 14, 18. [Google Scholar] [CrossRef]
- Wei, J.; Xie, J.; Zhang, P.; Zou, Z.; Ping, H.; Wang, W.; Xie, H.; Shen, J.Z.; Lei, L.; Fu, Z. Bioinspired 3D printable, self-healable, and stretchable hydrogels with multiple conductivities for skin-like wearable strain sensors. ACS Appl. Mater. Interfaces 2021, 13, 2952–2960. [Google Scholar] [CrossRef]
- Mishra, A.K.; Pan, W.; Giannelis, E.P.; Shepherd, R.F.; Wallin, T.J. Making bioinspired 3D-printed autonomic perspiring hydrogel actuators. Nat. Protoc. 2021, 16, 2068–2087. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Darabi, M.A.; Liu, Y.; He, Y.; Zhong, W.; Mequanin, K.; Li, B.; Lu, F.; Xing, M.M.Q. Hydrogels from natural egg white with extraordinary stretchability, direct-writing 3D printability and self-healing for fabrication of electronic sensors and actuators. J. Mater. Chem. A 2019, 7, 24626–24640. [Google Scholar] [CrossRef]
- Paikar, A.; Novichkov, A.I.; Hanopolskyi, A.I.; Smaliak, V.A.; Sui, X.; Kampf, N.; Skorb, E.V.; Semenov, S.N. Spatiotemporal regulation of hydrogel actuators by autocatalytic reaction networks. Adv. Mater. 2022, 34, 2106816. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.Y.; Mao, S.; Yuan, J.; Wang, S.; He, X.; Zhang, X.; Du, C.; Zhang, D.; Wu, Z.L.; Yang, J. Molecularly engineered zwitterionic hydrogels with high toughness and self-healing capacity for soft electronics applications. Chem. Mater. 2021, 33, 8418–8429. [Google Scholar] [CrossRef]
- Bashari, A.; Rouhani Shirvan, A.; Shakeri, M. Cellulose-based hydrogels for personal care products. Polym. Adv. Technol. 2018, 29, 2853–2867. [Google Scholar] [CrossRef]
- Enawgaw, H.; Tesfaye, T.; Yilma, K.T.; Limeneh, D.Y. Synthesis of a cellulose-Co-AMPS hydrogel for personal hygiene applications using cellulose extracted from corncobs. Gels 2021, 7, 236. [Google Scholar] [CrossRef]
- Huang, J.-F.; Zhong, J.; Chen, G.-P.; Lin, Z.-T.; Deng, Y.; Liu, Y.-L.; Cao, P.-Y.; Wang, B.; Wei, Y.; Wu, T.; et al. A hydrogel-based hybrid theranostic contact lens for fungal keratitis. ACS Nano 2016, 10, 6464–6473. [Google Scholar] [CrossRef]
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef]
- Li, J.; Jia, X.; Yin, L. Hydrogel: Diversity of structures and applications in food science. Food Rev. Int. 2021, 37, 313–372. [Google Scholar] [CrossRef]
- Bucatariu, F.; Teodosiu, C.; Morosanu, I.; Fighir, D.; Ciobanu, R.; Petrila, L.M.; Mihai, M. An overview on composite sorbents based on polyelectrolytes used in advanced wastewater treatment. Polymers 2021, 13, 3963. [Google Scholar] [CrossRef]
- Sahiner, N.; Butun, S.; Ozay, O.; Dibek, B. Utilization of smart hydrogel–metal composites as catalysis media. J. Colloid Interface Sci. 2012, 373, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Hapiot, F.; Menuel, S.; Monflier, E. Thermoresponsive hydrogels in catalysis. ACS Catal. 2013, 3, 1006–1010. [Google Scholar] [CrossRef]
- Pauly, J.; Gröger, H.; Patel, A.V. Catalysts encapsulated in biopolymer hydrogels for chemoenzymatic one-pot processes in aqueous media. Chem. Cat Chem. 2019, 11, 1503–1509. [Google Scholar] [CrossRef]
- Bercea, M. Self-healing behavior of polymer/protein hybrid hydrogels. Polymers 2022, 14, 130. [Google Scholar] [CrossRef]
- Wang, W.; Xiang, L.; Diaz-Dussan, D.; Zhang, J.; Yang, W.; Gong, L.; Chen, J.; Narain, R.; Zeng, H. Dynamic flexible hydrogel network with biological tissue-like self-protective functions. Chem. Mater. 2020, 32, 10545–10555. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, S.; Yan, T.; Fan, X.; Li, F.; Yang, X.; Ren, B.; Xu, J.; Liu, J. Injectable and fast self-healing protein hydrogels. Soft Matter 2019, 15, 7583–7589. [Google Scholar] [CrossRef]
- Bercea, M.; Biliuta, G.; Avadanei, M.; Baron, R.I.; Butnaru, M.; Coseri, S. Self-healing hydrogels of oxidized pullulan and poly(vinyl alcohol). Carbohydr. Polym. 2019, 206, 210–219. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Rusu, A.G.; Bercea, M.; Ghilan, A.; Dumitriu, R.; Mititelu-Tartau, L. New self-healing hydrogels based on reversible physical interactions and their potential applications. Eur. Polym. J. 2019, 118, 176–185. [Google Scholar] [CrossRef]
- Rusu, A.; Nita, L.E.; Bercea, M.; Tudorachi, N.; Diaconu, A.; Pamfil, D.; Rusu, D.; Ivan, F.E.; Chiriac, A. Interpenetrated polymer network with modified chitosan in composition and self-healing properties. Int. J. Biol. Macromol. 2019, 132, 374–384. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft materials by design: Unconventional polymer networks give extreme properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar] [CrossRef]
- Contessi Negrini, N.; Angelova Volponi, A.; Sharpe, P.T.; Celiz, A.D. Tunable cross-linking and adhesion of gelatin hydrogels via bioorthogonal click chemistry. ACS Biomater. Sci. Eng. 2021, 7, 4330–4346. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Nguyen, H.; Chang, C.-Y.; Lin, C.-C. Dual functionalization of gelatin for orthogonal and dynamic hydrogel cross-linking. ACS Biomater. Sci. Eng. 2021, 7, 4196–4208. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for therapeutic delivery: Current developments and future directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Tavafoghi, M.; Sheikhi, A.; Tutar, R.; Jahangiry, J.; Baidya, A.; Haghniaz, R.; Khademhosseini, A. Engineering tough, injectable, naturally derived, bioadhesive composite hydrogels. Adv. Health Mater. 2020, 9, 1901722. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.; Cosman, B.; Bercea, M.; Ailiesei, G.L.; Fundueanu, G. Thermosensitive poloxamer-graft-carboxymethyl pullulan: A potential injectable hydrogel for drug delivery. Polymers 2021, 13, 3025. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, D.-M.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. New insights of scaffolds based on hydrogels in tissue engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef]
- Varma, D.M.; Gold, G.T.; Taub, P.J.; Nicoll, S.B. Injectable carboxymethylcellulose hydrogels for soft tissue filler applications. Acta Biomater. 2014, 10, 4996–5004. [Google Scholar] [CrossRef]
- Dankers, P.Y.W.; Harmsen, M.C.; Brouwer, L.A.; van Luyn, M.J.A.; Meijer, E.W. A modular and supramolecular approach to bioactive scaffolds for tissue engineering. Nat. Mater. 2005, 4, 568–574. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, D.; Ji, N.; Lee, S.; Wang, G.; Zheng, Y.; Zhang, X.; Yang, L.; Qin, Z.; Yang, Y. Bioinspired design of sericin/chitosan/Ag@MOF/GO hydrogels for efficiently combating resistant bacteria, rapid hemostasis, and wound healing. Polymers 2021, 13, 2812. [Google Scholar] [CrossRef]
- Bercea, M.; Gradinaru, L.M.; Morariu, S.; Plugariu, I.A.; Gradinaru, R.V. Tailoring the properties of PVA/HPC/BSA hydrogels for wound dressing applications. React. Funct. Polym. 2022, 170, 105094. [Google Scholar] [CrossRef]
- Mandru, M.; Bercea, M.; Gradinaru, L.M.; Ciobanu, C.; Drobota, M.; Vlad, S. Polyurethane/poly (vinyl alcohol) hydrogels: Preparation, characterization and drug delivery. Eur. Polym. J. 2019, 118, 137–145. [Google Scholar] [CrossRef]
- Tamo, A.K.; Doench, I.; Walter, L.; Montembault, A.; Sudre, G.; David, L.; Morales-Helguera, A.; Selig, M.; Rolauffs, B.; Bernstein, A.; et al. Development of bioinspired functional chitosan/cellulose nanofiber 3D hydrogel constructs by 3D printing for application in the engineering of mechanically demanding tissues. Polymers 2021, 13, 1663. [Google Scholar] [CrossRef] [PubMed]
- Wiraja, C.; Ning, X.; Cui, M.; Xu, C. Hydrogel-based technologies for the diagnosis of skin pathology. Technologies 2020, 8, 47. [Google Scholar] [CrossRef]
- Zhu, Y.; Haghniaz, R.; Hartel, M.C.; Mou, L.; Tian, X.; Rosario Garrido, P.; Wu, Z.; Hao, T.; Guan, S.; Ahadian, S.; et al. Recent advances in bioinspired hydrogels materials, devices, and biosignal computing. ACS Biomater. Sci Eng. 2022. [Google Scholar] [CrossRef]
- Verhulsel, M.; Vignes, M.; Descroix, S.; Malaquin, L.; Vignjevic, D.M.; Viovy, J.L. A review of microfabrication and hydrogel engineering for micro-organs on chips. Biomaterials 2014, 35, 1816–1832. [Google Scholar] [CrossRef]
- Varaprasad, K.; Vimala, K.; Raghavendra, G.M.; Jayaramudu, T.; Sadiku, E.R.; Ramam, K. Cell Encapsulation in Polymeric Self-Assembled Hydrogels. In Nanotechnology Applications for Tissue Engineering; Thomas, S., Grohens, Y., Ninan, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 149–171. [Google Scholar]
- Ma, Y.; Hua, M.; Wu, S.; Du, Y.; Pei, X.; Zhu, X.; Zhou, F.; He, X. Bioinspired high-power-density strong contractile hydrogel by programmable elastic recoil. Sci. Adv. 2020, 6, eabd2520. [Google Scholar] [CrossRef]
- Sekine, Y.; Nankawa, T.; Yunoki, S.; Sugita, T.; Nakagawa, H.; Yamada, T. Eco-friendly carboxymethyl cellulose nanofiber hydrogels prepared via freeze cross-linking and their applications. ACS Appl. Polym. Mater. 2020, 2, 5482–5491. [Google Scholar] [CrossRef]
- Baron, R.I.; Bercea, M.; Avadanei, M.; Lisa, G.; Biliuta, G.; Coseri, S. Green route for the fabrication of self-healable hydrogels based on tricarboxy cellulose and poly(vinyl alcohol). Int. J. Biol. Macromol. 2019, 123, 744–751. [Google Scholar] [CrossRef]
- Yang, X.; Guo, L.; Fan, Y.; Zhang, X. Preparation and characterization of macromolecule cross-linked collagen hydrogels for chondrocyte delivery. Int. J. Biol. Macromol. 2013, 61, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Bruce, J.E. Chemical cross-linking for protein-protein interaction studies. Methods Mol. Biol. 2009, 492, 283–293. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, T.; Song, Y.; Sun, W. Assessment of various crosslinking agents on collagen/chitosan scaffolds for myocardial tissue engineering. Biomed. Mater. 2020, 15, 045003. [Google Scholar] [CrossRef]
- Breul, K.; Stengelin, E.; Urschbach, M.; Mondeshki, M.; Wüst, L.; Sirleaf, J.; Seitel, S.; Emt, T.; Pschierer, S.; Besenius, P.; et al. Cell adhesion on UV-crosslinked polyurethane gels with adjustable mechanical strength and thermoresponsiveness. Macromol. Rapid Commun. 2021, 42, 2100505. [Google Scholar] [CrossRef]
- Fernandes-Cunha, G.M.; Chen, K.M.; Chen, G.; Le, P.; Han, J.H.; Mahajan, L.A.; Lee, H.J.; Na, K.S.; Myung, D. In situ-forming collagen hydrogel crosslinked via multi-functional PEG as a matrix therapy for corneal defects. Sci. Rep. 2020, 10, 16671. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Fan, H.; Gong, J.P. Fabrication of bioinspired hydrogels: Challenges and opportunities. Macromolecules 2020, 53, 2769–2782. [Google Scholar] [CrossRef]
- Mastrullo, V.; Cathery, W.; Velliou, E.; Madeddu, P.; Campagnolo, P. Angiogenesis in tissue engineering: As nature intended? Front. Bioeng. Biotechnol. 2020, 8, 188. [Google Scholar] [CrossRef]
- Ullah, A.; Lima, S.I. Bioinspired tunable hydrogels: An update on methods of preparation, classification, and biomedical and therapeutic applications. Int. J. Pharm. 2022, 612, 121368. [Google Scholar] [CrossRef]
- Peters, J.T.; Wechsler, M.E.; Peppas, N.A. Advanced biomedical hydrogels: Molecular architecture and its impact on medical applications. Regen. Biomater. 2021, 8, 1–21. [Google Scholar] [CrossRef]
- Clegg, J.R.; Wagner, A.M.; Shin, S.R.; Hassan, S.; Peppas, N.A. Modular fabrication of intelligent material-tissue interfaces for bioinspired and biomimetic devices. Prog. Mater. Sci. 2019, 106, 100589. [Google Scholar] [CrossRef]
- Cella, C.; La Spina, R.; Mehn, D.; Fumagalli, F.; Ceccone, G.; Valsesia, A.; Gilliland, D. Detecting micro- and nanoplastics released from food packaging: Challenges and analytical strategies. Polymers 2022, 14, 1238. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Wancura, M.; Gilchrist, A.E.; Toubbeh, S.; Harley, B.A.C.; Cosgriff-Hernandez, E.; Peppas, N.A. Precise control of synthetic hydrogel network structure via linear, independent synthesis-swelling relationships. Sci. Adv. 2021, 7, eabe3245. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A. The swollen polymer network hypothesis: Quantitative models of hydrogel swelling, stiffness, and solute transport. Prog. Polym. Sci. 2020, 105, 101243. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Rusu, A.G.; Bercea, M.; Diaconu, A.; Tudorachi, N. Interpenetrating polymer network systems based on poly(dimethylaminoethyl methacrylate) and a copolymer containing pendant spiroacetal moieties. Mater. Sci. Eng. C 2018, 87, 22–31. [Google Scholar] [CrossRef]
- Nistor, M.T.; Chiriac, A.P.; Nita, L.E.; Vasile, C.; Bercea, M. Semi-interpenetrated polymer networks of hyaluronic acid modified with poly(aspartic acid). J. Polym. Res. 2013, 20, 86. [Google Scholar] [CrossRef]
- Dragan, E.S.; Perju, M.M.; Dinu, M.V. Preparation and characterization of IPN composite hydrogels based on polyacrylamide and chitosan and their interaction with ionic dyes. Carbohydr. Polym. 2012, 88, 270–281. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, S.J.; Kim, S.I. Swelling behavior of interpenetrating polymer network hydrogels composed of poly (vinyl alcohol) and chitosan. React. Funct. Polym. 2003, 55, 53–59. [Google Scholar] [CrossRef]
- Ugrinovic, V.; Panic, V.; Spasojevic, P.; Seslija, S.; Bozic, B.; Petrovic, R.; Janackovic, D.; Veljovic, D. Strong and tough, pH sensible, interpenetrating network hydrogels based on gelatin and poly(methacrylic acid). Polym. Eng. Sci. 2022, 62, 622–636. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; Luo, J.; Gao, Q.; Mao, A.; Li, J. Research progress on double-network hydrogels. Mater. Today Commun. 2021, 29, 102757. [Google Scholar] [CrossRef]
- Means, A.K.; Ehrhardt, D.A.; Whitney, L.V.; Grunlan, M.A. Thermoresponsive double network hydrogels with exceptional compressive mechanical properties. Macromol. Rapid Commun. 2017, 38, 1700351. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Chen, H.; Yan, H.; Huang, L.; Yang, J.; Zheng, J. A novel design strategy for fully physically linked double network hydrogels with tough, fatigue resistant, and self-healing properties. Adv. Funct. Mater. 2015, 25, 1598–1607. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of double network hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Engineering of tough double network hydrogels. Macromol. Chem. Phys. 2016, 217, 1022–1036. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Ren, B.; Zhang, Y.; Ma, Y.; Xu, L.; Chen, Q.; Zheng, J. Super bulk and interfacial toughness of physically crosslinked double-network hydrogels. Adv. Funct. Mater. 2017, 27, 1703086. [Google Scholar] [CrossRef]
- Kurokawa, T.; Furukawa, H.; Wang, W.; Tanaka, Y.; Gong, J.P. Formation of a strong hydrogel-porous solid interface via the double-network principle. Acta Biomater. 2010, 6, 1353–1359. [Google Scholar] [CrossRef]
- Liu, J.; Lin, S.; Liu, X.; Qin, Z.; Yang, Y.; Zang, J.; Zhao, X. Fatigue-resistant adhesion of hydrogels. Nat Commun. 2020, 11, 1071. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Zhao, C.; Wang, Q.; Zheng, J. A robust, one-pot synthesis of highly mechanical and recoverable double network hydrogels using thermoreversible sol-gel polysaccharide. Adv. Mater. 2013, 25, 4171–4176. [Google Scholar] [CrossRef]
- Mahajan, A.; Singh, A.; Datta, D.; Katti, D.S. Bioinspired injectable hydrogels dynamically stiffen and contract to promote mechanosensing-mediated chondrogenic commitment of stem cells. ACS Appl. Mater. Interfaces 2022, 14, 7531–7550. [Google Scholar] [CrossRef]
- Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Designing smart triple-network cationic cryogels with outstanding efficiency and selectivity for deep cleaning of phosphate. Chem. Eng. J. 2021, 426, 131411. [Google Scholar] [CrossRef]
- Liu, J.; Lan, Y.; Yu, Z.; Tan, C.S.Y.; Parker, R.M.; Abell, C.; Scherman, O.A. Cucurbit[n]uril-based microcapsules self-assembled within microfluidic droplets: A versatile approach for supramolecular architectures and materials. Acc. Chem. Res. 2017, 50, 208–217. [Google Scholar] [CrossRef]
- Tan, C.S.Y.; Agmon, G.; Liu, J.; Hoogland, D.; Janecek, E.-R.; Appel, E.; Scherman, O.A. Distinguishing relaxation dynamics in transiently crosslinked polymeric networks. Polym. Chem. 2017, 8, 5336–5343. [Google Scholar] [CrossRef]
- Resmerita, A.M.; Assaf, K.I.; Lazar, A.I.; Nau, W.M.; Farcas, A. Polyrotaxanes based on PEG-amine with cucurbit [7]uril, α-cyclodextrin and its tris-O-methylated derivative. Eur. Polym. J. 2017, 93, 323–333. [Google Scholar] [CrossRef]
- Tan, C.S.Y.; Liu, J.; Groombridge, A.S.; Barrow, S.J.; Dreiss, C.A.; Scherman, O.A. Controlling spatiotemporal mechanics of supramolecular hydrogel networks with highly branched Cucurbit [8]uril polyrotaxanes. Adv. Funct. Mater. 2018, 28, 1702994. [Google Scholar] [CrossRef]
- Khare, E.; Holten-Andersen, N.; Buehler, M.J. Transition-metal coordinate bonds for bioinspired macromolecules with tunable mechanical properties. Nat. Rev. Mater. 2021, 6, 421–436. [Google Scholar] [CrossRef]
- Wei, P.; Chen, T.; Chen, G.; Hou, K.; Zhu, M. Ligament-inspired tough and anisotropic fibrous gel belt with programed shape deformations via dynamic stretching. ACS Appl. Mater. Interfaces 2021, 13, 19291–19300. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, N.L.; Pothineni, S.; Wu, B.; Harper, J.M.; Burton, J.C. Pore-size dependence and slow relaxation of hydrogel friction on smooth surfaces. Proc. Natl. Acad. Sci. USA 2020, 117, 11247–11256. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, Y.; Zhao, X.; Ji, Z.; Ma, Z.; Gao, X.; Ma, S.; Wang, X.; Zhou, F. Bioinspired design of a cartilage-like lubricated composite with mechanical robustness. ACS Appl. Mater. Interfaces 2022, 14, 9899–9908. [Google Scholar] [CrossRef]
- Chen, L.; Hu, W.X.; Du, M.; Song, Y.; Wu, Z.; Zheng, Q. Bioinspired, recyclable, stretchable hydrogel with boundary ultralubrication. ACS Appl. Mater. Interfaces 2021, 13, 42240–42249. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Yu, W.J.; Yu, Y.; Li, R.Y.; Gao, Q.; Ren, K.F.; Ji, J. A bioinspired hydrogel-elastomer hybrid surface for enhanced mechanical properties and lubrication. ACS Appl. Mater. Interfaces 2021, 13, 50461–50469. [Google Scholar] [CrossRef]
- Lin, S.; Liu, J.; Liu, X.; Zhao, X. Muscle-like fatigue-resistant hydrogels by mechanical training. Proc. Natl. Acad. Sci. USA 2019, 116, 10244–10249. [Google Scholar] [CrossRef] [PubMed]
- Morariu, S.; Bercea, M.; Teodorescu, M.; Avadanei, M. Tailoring the properties of PVA/PVP hydrogels for biomedical applications. Eur. Polym. J. 2016, 84, 313–325. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Properties of water bound in hydrogels. Gels 2017, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gunasekaran, S. State of water in chitosan-PVA hydrogel. J. Appl. Polym. Sci. 2006, 101, 3227–3232. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Razaq, S.I.A.; Mehboob, H.; Rehman, S.; Al-Arjan, W.S.; Amin, R. Antibacterial and hemocompatible pH-responsive hydrogel for skin wound healing application: In vitro drug release. Polymers 2021, 13, 3703. [Google Scholar] [CrossRef] [PubMed]
- Bercea, M.; Bibire, L.E.; Morariu, S.; Carja, G. Chitosan/poly(vinyl alcohol)/LDH biocomposites with pH-sensitive properties. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 628–636. [Google Scholar] [CrossRef]
- Fu, P.S.; Wang, J.C.; Lai, P.L.; Liu, S.M.; Chen, Y.S.; Chen, W.C.; Hung, C.C. Effects of gamma radiation on the sterility assurance, antibacterial ability, and biocompatibility of impregnated hydrogel macrosphere protein and drug release. Polymers 2021, 13, 938. [Google Scholar] [CrossRef]
- Ramazani, A.; Aghahosseini, H. The biological properties of hydrogels based on natural polymers. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 247–269. [Google Scholar] [CrossRef]
- Malmsten, M. Antimicrobial and antiviral hydrogels. Soft Matter 2011, 7, 8725–8736. [Google Scholar] [CrossRef]
- Ykada, Y. Biocompatibility of hydrogels. In Gels Handbook; Volume 1: The Fundamentals; Osada, Y., Kajiwara, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 388–407. [Google Scholar]
- Okada, T.; Ikada, Y. Surface modification of silicone for percutaneous implantation. J. Biomater. Sci. Polym. Ed. 1995, 7, 171–180. [Google Scholar] [CrossRef]
- Kato, K.; Kikumura, Y.; Yamamoto, M.; Tomita, N.; Yamada, S.; Ikada, Y. Collagen immobilization onto the surface of artificial hair for improving the tissue adhesion. J. Adhes. Sci. Technol. 2000, 14, 635–650. [Google Scholar] [CrossRef]
- Li, L.; Xie, L.; Zheng, R.; Sun, R. Self-assembly dipeptide hydrogel: The structures and properties. Front. Chem. 2021, 9, 739791. [Google Scholar] [CrossRef]
- Bai, S.; Pappas, C.; Debnath, S.; Frederix, P.W.J.M.; Leckie, J.; Fleming, S.; Ulijn, R.V. Stable emulsions formed by self-assembly of interfacial networks of dipeptide derivatives. ACS Nano 2014, 8, 7005–7013. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeon, S.I.; Sim, S.B.; Byun, Y.; Ahn, C.H. A supramolecular host-guest interaction-mediated injectable hydrogel system with enhanced stability and sustained protein release. Acta Biomater. 2021, 131, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.J.; Liu, J.; Yu, Z.; Zhou, H.; Deng, X.; Abell, C.; Scherman, O.A. Viscoelastic hydrogel microfibers exploiting cucurbit [8]uril host-guest chemistry and microfluidics. ACS Appl. Mater. Interfaces 2020, 12, 17929–17935. [Google Scholar] [CrossRef] [PubMed]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (poly)phenols, volatile compounds from vegetables, medicinal and aromatic plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef]
- Casadey, R.; Broglia, M.; Barbero, C.; Criado, S.; Rivarola, C. Controlled release systems of natural phenolic antioxidants encapsulated inside biocompatible hydrogels. React. Funct. Polym. 2020, 156, 104729. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef]
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial hydrogels: Promising materials for medical application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef]
- Cao, Z.; Luo, Y.; Li, Z.; Tan, L.; Liu, X.; Li, C.; Zheng, Y.; Cui, Z.; Yeung, K.W.K.; Liang, Y.; et al. Antibacterial hybrid hydrogels. Macromol. Biosci. 2021, 21, 2000252. [Google Scholar] [CrossRef]
- Zhou, F.; Yang, Y.; Zhang, W.; Liu, S.; Shaikh, A.B.; Yang, L.; Lai, Y.; Ouyang, H.; Zhu, W. Bioinspired, injectable, tissue-adhesive and antibacterial hydrogel for multiple tissue regeneration by minimally invasive therapy. Appl. Mater. Today 2022, 26, 101290. [Google Scholar] [CrossRef]
- Pan, Z.; Ye, H.; Wu, D. Recent advances on polymeric hydrogels as wound dressings. APL Bioeng. 2021, 5, 011504. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Bellotto, O.; Semeraro, S.; Bandiera, A.; Tramer, F.; Pavan, N.; Marchesan, S. Polymer conjugates of antimicrobial peptides (AMPs) with D-amino acids (D-aa): State of the art and future opportunities. Pharmaceutics 2022, 14, 446. [Google Scholar] [CrossRef]
- Silva, A.R.P.; Guimarães, M.S.; Rabelo, J.; Belén, L.H.; Perecin, C.J.; Farías, J.G.; Santos, J.H.P.M.; Rangel-Yagui, C.O. Recent advances in the design of antimicrobial peptide conjugates. J. Mater. Chem. B 2022, 10, 2384–2429. [Google Scholar] [CrossRef] [PubMed]
- Doğan, E.E.; Tokcan, P.; Diken, M.E.; Yilmaz, B.; Kizilduman, B.K.; Sabaz, P. Synthesis, characterization and some biological properties of PVA/PVP/PN hydrogel nanocomposites: Antibacterial and biocompatibility. Adv. Mater. Sci. 2019, 19, 32–45. [Google Scholar] [CrossRef]
- Liu, L.; Shi, J.; Sun, X.; Zhang, Y.; Qin, J.; Peng, S.; Xu, J.; Song, L.; Zhang, Y. Thermo-responsive hydrogel-supported antibacterial material with persistent photocatalytic activity for continuous sterilization and wound healing. Compos. B Eng. 2022, 229, 109459. [Google Scholar] [CrossRef]
- Nainu, F.; Permana, A.D.; Djide, N.J.N.; Anjani, Q.K.; Utami, R.N.; Rumata, N.R.; Zhang, J.; Emran, T.B.; Simal-Gandara, J. Pharmaceutical approaches on antimicrobial resistance: Prospects and challenges. Antibiotics 2021, 10, 981. [Google Scholar] [CrossRef]
- Chen, Z.; Xing, L.; Fan, Q.; Cheetham, A.G.; Lin, R.; Holt, B.; Chen, L.; Xiao, Y.; Cui, H. Drug-bearing supramolecular filament hydrogels as anti-inflammatory agents. Theranostics 2017, 7, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.S.; Ahmad, M.; Minhas, M.U.; Tulain, R.; Barkat, K.; Khalid, I.; Khalid, Q. Chitosan/xanthan gum based hydrogels as potential carrier for an antiviral drug: Fabrication, characterization, and safety evaluation. Front. Chem. 2020, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Rokhade, A.P.; Patil, S.A.; Aminabhavi, T.M. Synthesis and characterization of semi-interpenetrating polymer network microspheres of acrylamide grafted dextran and chitosan for controlled release of acyclovir. Carbohydr. Polym. 2007, 67, 605–613. [Google Scholar] [CrossRef]
- Teodorescu, M.; Morariu. S. Drug delivery system based on PVA and clay for potential treatment of COVID-19. J. Polym. Res. 2022, 29, 67. [Google Scholar] [CrossRef]
- Sultan, A.S.; Vila, T.; Hefni, E.; Karlsson, A.J.; Jabra-Rizk, M.A. Evaluation of the antifungal and wound-healing properties of a novel peptide-based bioadhesive hydrogel formulation. Antimicrob. Agents Chemother. 2019, 63, e00888-19. [Google Scholar] [CrossRef]
- Zumbuehl, A.; Ferreira, L.; Kuhn, D.; Astashkina, A.; Long, L.; Yeo, Y.; Iaconis, T.; Ghannoum, M.; Fink, G.R.; Langer, R.; et al. Antifungal hydrogels. Proc. Natl. Acad. Sci. USA 2007, 104, 12994–12998. [Google Scholar] [CrossRef]
- Sen, M.; Uzun, C.; Güven, O. Controlled release of terbinafine hydrochloride from pH sensitive poly(acrylamide/maleic acid) hydrogels. Int. J. Pharm. 2000, 203, 149–157. [Google Scholar] [CrossRef]
- Sen, M.; Yakar, A. Controlled release of antifungal drug terbinafine hydrochloride from poly(N-vinyl 2-pyrrolidone/itaconic acid) hydrogels. Int. J. Pharm. 2001, 228, 33–41. [Google Scholar] [CrossRef]
- Paskiabi, F.A.; Bonakdar, S.; Shokrgozar, M.A.; Imani, M.; Jahanshiri, Z.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Terbinafine-loaded wound dressing for chronic superficial fungal infections. Mater. Sci. Eng. C 2017, 73, 130–136. [Google Scholar] [CrossRef]
- Bercea, M.; Morariu, S.; Teodorescu, M. Rheological investigation of poly(vinyl alcohol)/poly(N-vinyl pyrrolidone) mixtures in aqueous solution and hydrogel state. J. Polym. Res. 2016, 23, 142. [Google Scholar] [CrossRef]
- Bercea, M.; Morariu, S.; Rusu, D. In situ gelation of aqueous solutions of entangled poly(vinyl alcohol). Soft Matter 2013, 9, 1244–1253. [Google Scholar] [CrossRef]
- Bercea, M.; Morariu, S.; Brunchi, C.-E. Influence of non-solvent on the gelation phenomenon of polymer solutions. Rev. Roum. Chim. 2008, 53, 769–776. [Google Scholar]
- Bercea, M.; Bibire, L.E.; Morariu, S.; Teodorescu, M.; Carja, G. pH influence on rheological and structural properties of chitosan/poly(vinyl alcohol)/layered double hydroxide composites. Eur. Polym. J. 2015, 70, 147–156. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Wellard, R.M.; Woodruff, M.; Klein, T. Tailoring hydrogel viscoelasticity with physical and chemical crosslinking. Polymers 2015, 7, 2650–2669. [Google Scholar] [CrossRef]
- Bercea, M.; Darie, R.N.; Nita, L.E.; Morariu, S. Temperature responsive gels based on Pluronic F127 and poly(vinyl alcohol). Ind. Eng. Chem. Res. 2011, 50, 4199–4206. [Google Scholar] [CrossRef]
- Croitoriu, A.; Nita, L.E.; Chiriac, A.P.; Rusu, A.G.; Bercea, M. New physical hydrogels based on co-assembling of Fmoc-amino-acids. Gels 2021, 7, 208. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Bercea, M.; Ghilan, A.; Rusu, A.G.; Dumitriu, R.; Mititelu Tartau, L. Multifunctional hybrid 3D network based on hyaluronic acid and a copolymer containing pendant spiroacetal moieties. Int. J. Biol. Macromol. 2019, 125, 191–202. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between structure and rheology of hydrogels for various applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Kulichikhin, V.G.; Malkin, A.Y. The role of structure in polymer rheology: Review. Polymers 2022, 14, 1262. [Google Scholar] [CrossRef]
- Bercea, M.; Gradinaru, L.M.; Barbalata-Mandru, M.; Vlad, S.; Nita, L.E.; Plugariu, I.A.; Albulescu, R. Shear flow of associative polymers in aqueous solutions. J. Mol. Struct. 2021, 1238, 130441. [Google Scholar] [CrossRef]
- Mattei, G.; Cacopardo, L.; Ahluwalia, A. Engineering gels with time-evolving viscoelasticity. Materials 2020, 13, 438. [Google Scholar] [CrossRef]
- Arafune, H.; Watarai, Y.; Kamijo, T.; Honma, S.; Sato, T. Mechanical and lubrication properties of double network ion gels obtained by a one-step process. Materials 2022, 15, 2113. [Google Scholar] [CrossRef]
- Bercea, M.; Gradinaru, L.M.; Plugariu, I.A.; Mandru, M.; Tigau, D.L. Viscoelastic behaviour of self-assembling polyurethane and poly(vinyl alcohol). Polym. Int. 2020, 69, 149–155. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Ji, Y.; Xu, J.; Ye, Z.; Wang, S.; Du, X. Highly transparent, mechanical, and self-adhesive zwitterionic conductive hydrogels with polyurethane as a cross-linker for wireless strain sensors. J. Mater. Chem. B 2022, 10, 2933–2943. [Google Scholar] [CrossRef]
- Wu, S.; Hua, M.; Alsaid, Y.; Du, Y.; Ma, Y.; Zhao, Y.; Lo, C.Y.; Wang, C.; Wu, D.; Yao, B.; et al. Poly(vinyl alcohol) hydrogels with broad-range tunable mechanical properties via the Hofmeister effect. Adv. Mater. 2021, 33, 2007829. [Google Scholar] [CrossRef]
- Guo, Y.; An, X.; Fan, Z. Aramid nanofibers reinforced polyvinyl alcohol/tannic acid hydrogel with improved mechanical and antibacterial properties for potential application as wound dressing. J. Mech. Behav. Biomed. Mater. 2021, 118, 104452. [Google Scholar] [CrossRef]
- Lu, X.; Si, Y.; Zhang, S.; Yu, J.; Ding, B. In situ synthesis of mechanically robust, transparent nanofiber-reinforced hydrogels for highly sensitive multiple sensing. Adv. Funct. Mater. 2021, 31, 2103117. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Zhang, Y.; Chi, C.; Dong, G.; Lin, J.; Chen, Q. Superelastic, antifreezing, antidrying, and conductive organohydrogels for wearable strain sensors. ACS Appl. Mater. Interfaces 2021, 13, 51546–51555. [Google Scholar] [CrossRef]
- Xia, S.; Song, S.; Ren, X.; Gao, G. Highly tough, anti-fatigue and rapidly self-recoverable hydrogels reinforced with core-shell inorganic-organic hybrid latex particles. Soft Matter 2017, 13, 6059–6067. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Q.; Song, S.; Duan, L.; Gao, G. Bioinspired dynamic cross-linking hydrogel sensors with skin-like strain and pressure sensing behaviors. Chem. Mater. 2019, 31, 9522–9531. [Google Scholar] [CrossRef]
- Xia, S.; Song, S.; Gao, G. Robust and flexible strain sensors based on dual physically cross-linked double network hydrogels for monitoring human-motion. Chem. Eng. J. 2018, 354, 817–824. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, Y.; Zhang, H.; Wang, Y.; Fan, X.; Liu, T. Fast-recoverable, self-healable, and adhesive nanocomposite hydrogel consisting of hybrid nanoparticles for ultrasensitive strain and pressure sensing. Chem. Mater. 2021, 33, 6146–6157. [Google Scholar] [CrossRef]
- Luo, C.; Xie, S.; Deng, X.; Sun, Y.; Shen, Y.; Li, M.; Fu, W. From micelle-like aggregates to extremely-stretchable, fatigue-resistant, highly-resilient and self-healable hydrogels. Eur. Polym. J. 2022, 167, 111047. [Google Scholar] [CrossRef]
- Wang, J.; Dai, T.; Wu, H.; Ye, M.Y.; Yuan, G.; Jia, H. Tannic acid-Fe3+ activated rapid polymerization of ionic conductive hydrogels with high mechanical properties, self-healing, and self-adhesion for flexible wearable sensors. Compos. Sci. Technol. 2022, 221, 109345. [Google Scholar] [CrossRef]
- Fu, C.; Lin, J.; Tang, Z.; Chen, L.; Huang, F.; Kong, F.; Ni, Y.; Huang, L. Design of asymmetric-adhesion lignin reinforced hydrogels with anti-interference for strain sensing and moist air induced electricity generator. Int. J. Biol. Macromol. 2022, 201, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Geng, L.; Wu, J.; Huang, A.; Peng, X. Highly strong and sensitive bilayer hydrogel actuators enhanced by cross-oriented nanocellulose networks. Compos. Sci. Technol. 2022, 225, 109494. [Google Scholar] [CrossRef]
- Wang, J.; Dai, T.; Lin, Y.; Jia, H.; Ji, Q.; Yuan, G. Polysaccharide-based high-strength, self-healing and ultra-sensitive wearable sensors. Ind. Crop. Prod. 2022, 178, 114618. [Google Scholar] [CrossRef]
- Yi, B.; Xu, Q.; Liu, W. An overview of substrate stiffness guided cellular response and its applications in tissue regeneration. Bioact. Mater. 2022, 15, 82–102. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cho, B.; Kim, S.; Kim, J. Direct conversion of fibroblasts to osteoblasts as a novel strategy for bone regeneration in elderly individuals. Exp. Mol. Med. 2019, 51, 54. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, M.; Chen, G.; Xu, Z.; Lv, Y. Demineralized bone scaffolds with tunable matrix stiffness for efficient bone integration. ACS Appl. Mater. Interfaces 2018, 10, 27669–27680. [Google Scholar] [CrossRef]
- Chen, G.; Dong, C.; Yang, L.; Lv, Y. 3D scaffolds with different stiffness but the same microstructure for bone tissue engineering. ACS Appl. Mater. Interfaces 2015, 7, 15790–15802. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Tan, B.; Zhu, L.; Wang, Y.; Liao, J. A review on the design of hydrogels with different stiffness and their effects on tissue repair. Front. Bioeng. Biotechnol. 2022, 10, 817391. [Google Scholar] [CrossRef]
- Zhao, D.; Pang, B.; Zhu, Y.; Cheng, W.; Cao, K.; Ye, D.; Si, C.; Xu, G.; Chen, C.; Yu, H. A stiffness-switchable, biomimetic smart material enabled by supramolecular reconfiguration. Adv. Mater. 2022, 34, 2107857. [Google Scholar] [CrossRef]
- Speck, O.; Speck, T. An overview of bioinspired and biomimetic self-repairing materials. Biomimetics 2019, 4, 26. [Google Scholar] [CrossRef]
- Cremaldi, J.C.; Bhushan, B. Bioinspired self-healing materials: Lessons from nature. Beilstein J. Nanotechnol. 2018, 9, 907–935. [Google Scholar] [CrossRef] [PubMed]
- Ailincai, D.; Rosca, I.; Morariu, S.; Mititelu-Tartau, L.; Marin, L. Iminoboronate-chitooligosaccharides hydrogels with strong antimicrobial activity for biomedical applications. Carbohydr. Polym. 2022, 276, 118727. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, W.; Wu, W.H.; Xue, B.; Xiang, D.; Li, Y.; Qin, M.; Sun, F.; Wang, W.; Zhang, W.B.; et al. Reversible hydrogels with tunable mechanical properties for optically controlling cell migration. Nano Res. 2018, 11, 5556–5565. [Google Scholar] [CrossRef]
- Guo, M.; Pitet, L.M.; Wyss, H.M.; Vos, M.; Dankers, P.Y.W.; Meijer, E.W. Tough stimuli-responsive supramolecular hydrogels with hydrogen-bonding network junctions. J. Am. Chem. Soc. 2014, 136, 6969–6977. [Google Scholar] [CrossRef]
- Degtyar, E.; Harrington, M.J.; Politi, Y.; Fratzl, P. The mechanical role of metal ions in biogenic protein-based materials. Angew. Chem. Int. Ed. 2014, 53, 12026. [Google Scholar] [CrossRef]
- Yoshida, S.; Ejima, H.; Yoshie, N. Tough elastomers with superior self-recoverability induced by bioinspired multiphase design. Adv. Funct. Mater. 2017, 27, 1701670. [Google Scholar] [CrossRef]
- Cohades, A.; Branfoot, C.; Rae, S.; Bond, I.; Michaud, V. Progress in self-healing fiber-reinforced polymer composites. Adv. Mater. Interfaces 2018, 5, 1800177. [Google Scholar] [CrossRef]
- Li, P.; Zhong, Y.; Wang, X.; Hao, J. Enzyme-regulated healable polymeric hydrogels. ACS Cent. Sci. 2020, 6, 1507–1522. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional hydrogels with tunable structures and properties for tissue engineering applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, H.C.; Bhokisham, N.; Li, J.; Hong, K.L.; Quan, D.N.; Tsao, C.Y.; Bentley, W.E.; Payne, G.F. Biofabricating functional soft matter using protein engineering to enable enzymatic assembly. Bioconjug. Chem. 2018, 29, 1809–1822. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Dastjerdi, M.B.; Ai, A.; Ahmadi, A.; Godarzi, A.; Rahimi, A.; Ai, J. Horseradish peroxidase-catalyzed hydrogelation for biomedical applications. Biomater. Sci. 2018, 6, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; He, C.; Xiao, C.; Li, G.; Chen, X. Injectable glycopolypeptide hydrogels as biomimetic scaffolds for cartilage tissue engineering. Biomaterials 2015, 51, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jawaid, M.; Raveendran, S.N.; Asiri, A.M.A. (Eds.) Self-Healing Composite Materials. From Design to Applications; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Sikdar, P.; Uddin, M.M.; Dip, T.M.; Islam, S.; Hoque, M.S.; Dhar, A.K.; Wu, S. Recent advances in the synthesis of smart hydrogels. Mater. Adv. 2021, 2, 4532–4573. [Google Scholar] [CrossRef]
- Mazidi, Z.; Javanmardi, S.; Naghib, S.M.; Mohammadpour, Z. Smart stimuli-responsive implantable drug delivery systems for programmed and on-demand cancer treatment: An overview on the emerging materials. Chem. Eng. J. 2022, 433, 134569. [Google Scholar] [CrossRef]
- Van Gheluwe, L.; Chourpa, I.; Gaigne, C.; Munnier, E. Polymer-based smart drug delivery systems for skin application and demonstration of stimuli-responsiveness. Polymers 2021, 13, 1285. [Google Scholar] [CrossRef]
- Hoque, J.; Sangaj, N.; Varghese, S. Stimuli-responsive supramolecular hydrogels and their applications in regenerative medicine. Macromol. Biosci. 2019, 19, 1800259. [Google Scholar] [CrossRef]
- Deng, K.; Bellmann, C.; Fu, Y.; Rohn, M.; Guenther, M.; Gerlach, G. Miniaturized force-compensated hydrogel-based pH sensors. Sens. Actuators B 2018, 255, 3495–3504. [Google Scholar] [CrossRef]
- Cheng, Y.; Ren, K.; Yang, D.; Wei, J. Bilayer-type fluorescence hydrogels with intelligent response serve as temperature/pH driven soft actuators. Sens. Actuators B 2018, 255, 3117–3126. [Google Scholar] [CrossRef]
- Hapuarachchige, S.; Artemov, D. Theranostic pretargeting drug delivery and imaging platforms in cancer precision medicine. Front. Oncol. 2020, 10, 1131. [Google Scholar] [CrossRef]
- Werner, R.A.; Higuchi, T.; Pomper, M.G.; Rowe, S.P. Theranostics in oncology-thriving, now more than ever. Diagnostics 2021, 11, 805. [Google Scholar] [CrossRef]
- Dong, Y.C.; Bouché, M.; Uman, S.; Burdick, J.A.; Cormode, D.A. Detecting and monitoring hydrogels with medical imaging. ACS Biomater. Sci. Eng. 2021, 7, 4027–4047. [Google Scholar] [CrossRef] [PubMed]
- Kolosnjaj-Tabi, J.; Gibot, L.; Fourquaux, I.; Golzio, M.; Rols, M.P. Electric field-responsive nanoparticles and electric fields: Physical, chemical, biological mechanisms and therapeutic prospects. Adv. Drug. Deliv. Rev. 2019, 138, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Fundueanu, G.; Constantin, M.; Bucatariu, S.; Ascenzi, P. Poly(N-isopropylacrylamide-co-N-isopropylmethacrylamide) thermo-responsive microgels as self-regulated drug delivery system. Macromol. Chem. Phys. 2016, 217, 22. [Google Scholar] [CrossRef]
- Pawłowska, S.; Rinoldi, C.; Nakielski, P.; Ziai, Y.; Urbanek, O.; Li, X.; Kowalewski, A.T.; Ding, B.; Pierini, F. Ultraviolet light-assisted electrospinning of core–shell fully cross-linked P(NIPAAm-co-NIPMAAm) hydrogel-based nanofibers for thermally induced drug delivery self-regulation. Adv. Mater. Interfaces 2020, 7, 2000247. [Google Scholar] [CrossRef]
- Huang, S.; Ma, Z.; Sun, C.; Zhou, Q.; Li, Z.; Wang, S.; Yan, Q.; Liu, C.; Hou, B.; Zhang, C. An injectable thermosensitive hydrogel loaded with a theranostic nanoprobe for synergistic chemo–photothermal therapy for multidrug-resistant hepatocellular carcinoma. J. Mater. Chem. B 2022, 10, 2828–2843. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V.; Ghiorghita, C.A. Chitosan-based polyelectrolyte complex cryogels with elasticity, toughness and delivery of curcumin engineered by polyions pair and cryostructuration steps. Gels 2022, 8, 240. [Google Scholar] [CrossRef]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Benko, A.; Palka, K.; Wojcik, M.; Przekora, A. Elastic and biodegradable chitosan/agarose film revealing slightly acidic pH for potential applications in regenerative medicine as artificial skin graft. Int. J. Biol. Macromol. 2020, 164, 172–183. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; de Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for developing physical gels of chitosan and of chitosan derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef]
- Duceac, I.A.; Verestiuc, L.; Dimitriu, C.D.; Maier, V.; Coseri, S. Design and preparation of new multifunctional hydrogels based on chitosan/acrylic polymers for drug delivery and wound dressing applications. Polymers 2020, 12, 1473. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef] [PubMed]
- Sviben, S.; Spaeker, O.; Bennet, M.; Albéric, M.; Dirks, J.-H.; Moussian, B.; Fratzl, P.; Bertinetti, L.; Politi, Y. Epidermal cell surface structure and chitin-protein coassembly determine fiber architecture in the locust cuticle. ACS Appl. Mater. Interfaces 2020, 12, 25581–25590. [Google Scholar] [CrossRef] [PubMed]
- Serbezeanu, D.; Bercea, M.; Butnaru, M.; Enache, A.A.; Rimbu, C.M.; Vlad-Bubulac, T. Development of histamine reinforced poly(vinyl alcohol)/chitosan blended films for potential biomedical applications. J. Appl. Polym. Sci. 2022, 139, 51912. [Google Scholar] [CrossRef]
- Hou, J.; Aydemir, B.E.; Dumanli, A.G. Understanding the structural diversity of chitins as a versatile biomaterial. Phil. Trans. R. Soc. A 2021, 379, 20200331. [Google Scholar] [CrossRef]
- Behr, M.; Ganesan, K. Improving polysaccharide-based chitin/chitosan-aerogel materials by learning from genetics and molecular biology. Materials 2022, 15, 1041. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A.; Parveen, A. Thermosensitive chitosan-based injectable hydrogel as an efficient anticancer drug carrier. ACS Omega 2020, 5, 20450–20460. [Google Scholar] [CrossRef]
- Balan, V.; Verestiuc, L. Strategies to improve chitosan hemocompatibility: A review. Eur. Polym. J. 2014, 53, 171–188. [Google Scholar] [CrossRef]
- Yao, H.-Y.; Lin, H.-R.; Sue, G.-P.; Lin, Y.-J. Chitosan-based hydrogels prepared by UV polymerization for wound dressing. Polym. Polym. Compos. 2019, 27, 155–167. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Yang, K.; Fu, Y.V.; Xu, T.; Li, S.; Zhang, D.; Wang, L.-N.; Lee, C.-S. A novel double-crosslinking-double-network design for injectable hydrogels with enhanced tissue adhesion and antibacterial capability for wound treatment. Adv. Funct. Mater. 2020, 30, 1904156. [Google Scholar] [CrossRef]
- Morariu, S.; Bercea, M.; Brunchi, C.E. Effect of cryogenic treatment on the rheological properties of chitosan/poly(vinyl alcohol) hydrogels. Ind. Eng. Chem. Res. 2015, 54, 11475–11482. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Yu, W. Bioinspired anisotropic chitosan hybrid hydrogel. ACS Appl. Bio Mater. 2020, 3, 6959–6966. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Liang, X.; Guo, J.; Zhu, K.; Zhang, L. Ultra-stretchable and force-sensitive hydrogels reinforced with chitosan microspheres embedded in polymer networks. Adv. Mater. 2016, 28, 8037–8044. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, J.; Chen, Y.; Zhang, L.; Zhou, J. Dual physical crosslinking strategy to construct moldable hydrogels with ultrahigh strength and toughness. Adv. Funct. Mater. 2018, 28, 1800739. [Google Scholar] [CrossRef]
- Liu, S.; Li, L. Unique gelation of chitosan in an alkali/urea aqueous solution. Polymer 2018, 141, 124–131. [Google Scholar] [CrossRef]
- Li, C.; Han, Q.; Guan, Y.; Zhang, Y. Thermal gelation of chitosan in an aqueous alkali-urea solution. Soft Matter 2014, 10, 8245–8253. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Lee, C.; Shin, J.; Lee, J.S.; Byun, E.; Ryu, J.H.; Um, S.H.; Kim, D.; Lee, H.; Cho, S.W. Bioinspired, calcium-free alginate hydrogels with tunable physical and mechanical properties and improved biocompatibility. Biomacromolecules 2013, 14, 2004–2013. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Munir, A. Bioinspired sodium alginate based thermosensitive hydrogel membranes for accelerated wound healing. Int. J. Biol. Macromol. 2020, 155, 751–765. [Google Scholar] [CrossRef]
- Cui, L.; Hu, J.; Wang, W.; Yan, C.; Guo, Y.; Tu, C. Smart pH response flexible sensor based on calcium alginate fibers incorporated with natural dye for wound healing monitoring. Cellulose 2020, 27, 6367–6381. [Google Scholar] [CrossRef]
- Miguel, S.P.; Cabral, C.S.D.; Moreira, A.F.; Correia, I.J. Production and characterization of a novel asymmetric 3D printed construct aimed for skin tissue regeneration. Colloids Surf. B 2019, 181, 994–1003. [Google Scholar] [CrossRef]
- Chen, H.; Xing, X.; Tan, H.; Jia, Y.; Zhou, T.; Chen, Y.; Ling, Z.; Hu, X. Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing. Mater. Sci. Eng. C 2017, 70, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From food industry to biomedical applications and management of metabolic disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.-A.; Muzzin, N.; Toufanian, S.; Slick, R.A.; Lawlor, M.W.; Seifried, B.; Moquin, P.; Latulippe, D.; Hoare, T. Drug-impregnated, pressurized gas expanded liquid-processed alginate hydrogel scaffolds for accelerated burn wound healing. Acta Biomater. 2020, 112, 101–111. [Google Scholar] [CrossRef]
- Leppiniemi, J.; Lahtinen, P.; Paajanen, A.; Mahlberg, R.; Metsä-Kortelainen, S.; Pinomaa, T.; Pajari, H.; Vikholm-Lundin, I.; Pursula, P.; Hytönen, V.P. 3D-printable bioactivated nanocellulose-alginate hydrogels. ACS Appl. Mater. Interfaces 2017, 9, 21959–21970. [Google Scholar] [CrossRef]
- Han, C.; Wang, X.; Ni, Z.; Ni, Y.; Huan, W.; Lv, Y.; Bai, S. Effects of nanocellulose on alginate/gelatin bio-inks for extrusion-based 3D printing. BioResources 2020, 15, 7357–7373. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G. Potential and limitations of nanocelluloses as components in biocomposite inks for three-dimensional bioprinting and for biomedical devices. Biomacromolecules 2018, 19, 701–711. [Google Scholar] [CrossRef]
- Im, S.; Choe, G.; Seok, J.M.; Yeo, S.J.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. An osteogenic bioink composed of alginate, cellulose nanofibrils, and polydopamine nanoparticles for 3D bioprinting and bone tissue engineering. Int. J. Biol. Macromol. 2022, 205, 520–529. [Google Scholar] [CrossRef]
- Wang, S.; Xiong, Y.; Chen, J.; Ghanem, A.; Wang, Y.; Yang, J.; Sun, B. Three dimensional printing bilayer membrane scaffold promotes wound healing. Front. Bioeng. Biotechnol. 2019, 7, 348. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhao, L.; Wang, J.; Wang, S.; Liu, Y.; Liu, X. High-strength and high-toughness sodium alginate/polyacrylamide double physically crosslinked network hydrogel with superior self-healing and self-recovery properties prepared by a one-pot method. Colloids Surf. A 2020, 589, 124402. [Google Scholar] [CrossRef]
- Amaral, A.J.R.; Gaspar, V.M.; Lavrador, P.; Mano, J.F. Double network laminarin-boronic/alginate dynamic bioink for 3D bioprinting cell-laden constructs. Biofabrication 2021, 13, 035045. [Google Scholar] [CrossRef]
- Geng, Z.; Ji, Y.; Yu, S.; Liu, Q.; Zhou, Z.; Guo, C.; Lu, D.; Pei, D. Preparation and characterization of a dual cross-linking injectable hydrogel based on sodium alginate and chitosan quaternary ammonium salt. Carbohydr. Res. 2021, 507, 108389. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Front. Veter Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic acid: The influence of molecular weight on structural, physical, physico-chemical, and degradable properties of biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Rebenda, D.; Vrbka, M.; Čípek, P.; Toropitsyn, E.; Nečas, D.; Pravda, M.; Hartl, M. On the dependence of rheology of hyaluronic acid solutions and frictional behavior of articular cartilage. Materials 2020, 13, 2659. [Google Scholar] [CrossRef]
- Cassuto, D.; Bellia, G.; Schiraldi, C. An overview of soft tissue fillers for cosmetic dermatology: From filling to regenerative medicine. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1857–1866. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acidBased wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Luo, X.; Liu, Y.; Zheng, C.; Huo, Q.; Liu, X. Development of novel hyaluronic acid/human-like collagen bio-composite membranes: A facile “surface modification-assembly” approach. Int. J. Biol. Macromol. 2021, 193, 378–386. [Google Scholar] [CrossRef]
- Hilšer, P.; Suchánková, A.; Mendová, K.; Filipič, K.E.; Daniel, M.; Vrbka, M. A new insight into more effective viscosupplementation based on the synergy of hyaluronic acid and phospholipids for cartilage friction reduction. Biotribology 2021, 25, 100166. [Google Scholar] [CrossRef]
- Bayer, I.S. Hyaluronic acid and controlled release: A review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Shou, X.; Ni, D.; Kong, T.; Zhao, Y. Bio-inspired lubricant drug delivery particles for the treatment of osteoarthritis. Nanoscale 2020, 12, 17093–17102. [Google Scholar] [CrossRef] [PubMed]
- Ehsanipour, A.; Nguyen, T.; Aboufadel, T.; Sathialingam, M.; Cox, P.; Xiao, W.; Walthers, C.M.; Seidlits, S.K. Injectable, Hyaluronic acid-based scaffolds with macroporous architecture for gene delivery. Cell. Mol. Bioeng. 2019, 12, 399–413. [Google Scholar] [CrossRef]
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef]
- Park, H.; Lee, H.J.; An, H.; Lee, K.Y. Alginate hydrogels modified with low molecular weight hyaluronate for cartilage regeneration. Carbohydr. Polym. 2017, 162, 100–107. [Google Scholar] [CrossRef]
- Kodavaty, J. Poly (vinyl alcohol) and hyaluronic acid hydrogels as potential biomaterial systemsA comprehensive review. J. Drug Deliv. Sci. Technol. 2022, 71, 103298. [Google Scholar] [CrossRef]
- Lee, H.Y.; Hwang, C.H.; Kim, H.-E.; Jeong, S.-H. Enhancement of bio-stability and mechanical properties of hyaluronic acid hydrogels by tannic acid treatment. Carbohydr. Polym. 2018, 186, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Gao, H.; Lic, Q.; Cao, X. Injectable dual cross-linked adhesive hyaluronic acid multifunctional hydrogel scaffolds for potential applications in cartilage repair. Polym. Chem. 2020, 11, 3169–3178. [Google Scholar] [CrossRef]
- Dromel, P.C.; Singh, D.; Andres, E.; Likes, M.; Kurisawa, M.; Alexander-Katz, A.; Spector, M.; Young, M. A bioinspired gelatin-hyaluronic acid-based hybrid interpenetrating network for the enhancement of retinal ganglion cells replacement therapy. Regen. Med. 2021, 6, 85. [Google Scholar] [CrossRef]
- Bermejo-Velasco, D.; Doub, W.; Heerschap, A.; Ossipov, D.; Hilborn, J. Injectable hyaluronic acid hydrogels with the capacity for magnetic resonance imaging. Carbohydr. Polym. 2018, 197, 641–648. [Google Scholar] [CrossRef]
- Sahiner, N.; Umut, E.; Suner, S.S.; Sahiner, M.; Culha, M.; Ayyala, R.S. Hyaluronic acid (HA)-Gd(III) and HA-Fe(III) microgels as MRI contrast enhancing agents. Carbohydr. Polym. 2022, 277, 118873. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Rikkers, M.; Mihajlovic, M.; Viola, M.; Schuiringa, G.; Ilochonwu, B.C.; Masereeuw, R.; Vonk, L.; Malda, J.; Ito, K.; et al. Viscoelastic chondroitin sulfate and hyaluronic acid double-network hydrogels with reversible cross-links. Biomacromolecules 2022, 23, 1350–1365. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.B.; Danti, S.; De Clerck, K.; Lazzeri, A.; Morganti, P. Pullulan for advanced sustainable body- and skin-contact applications. J. Funct. Biomater. 2020, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Badwhar, P.; Dubey, K.K. Insights of microbial pullulan production: A bioprocess engineer assessment. Curr. Biotechnol. 2018, 7, 262–272. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, R.; Yang, S.; Guan, J.; Zhang, D.; Ma, Y.; Liu, H. Research progress of self-assembled nanogel and hybrid hydrogel systems based on pullulan derivatives. Drug Deliv. 2018, 25, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Tabasum, S.; Noreen, A.; Maqsood, M.F.; Umar, H.; Akram, N.; Nazli, Z.; Chatha, S.A.S.; Zia, K.M. A review on versatile applications of blends and composites of pullulan with natural and synthetic polymers. Int. J. Biol. Macromol. 2018, 120, 603–632. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, A.G. Drug delivery systems using pullulan, a biocompatible polysaccharide produced by fungal fermentation of starch. Environ. Chem. Lett. 2019, 17, 1209–1223. [Google Scholar] [CrossRef]
- Navpreet, S.S.; Kaur, N.; Hassan, M.; Kennedy, J.F. Pullulan in biomedical research and developmentA review. Int. J. Biol. Macromol. 2021, 166, 694–706. [Google Scholar] [CrossRef]
- Bae, H.; Ahari, A.F.; Shin, H.; Nichol, J.W.; Hutson, C.B.; Masaeli, M.; Kim, S.-H.; Aubin, H.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered pullulan methacrylate hydrogels promote cell proliferation and 3D cluster formation. Soft Matter 2011, 467, 1903–1911. [Google Scholar] [CrossRef]
- Baron, R.I.; Culica, M.E.; Biliuta, G.; Bercea, M.; Gherman, S.; Zavastin, D.; Ochiuz, L.; Avadanei, M.; Coseri, S. Physical hydrogels of oxidized polysaccharides and poly(vinyl alcohol) for wound dressing applications. Materials 2019, 12, 1569. [Google Scholar] [CrossRef]
- Spatareanu, A.; Bercea, M.; Budtova, T.; Harabagiu, V.; Sacarescu, L.; Coseri, S. Synthesis, characterization and solution behaviour of oxidized pullulan. Carbohydr. Polym. 2014, 111, 63–71. [Google Scholar] [CrossRef]
- Coseri, S.; Bercea, M.; Harabagiu, V.; Budtova, T. Oxydation vs. degradation in polysaccharides: PullulanA case study. Eur. Polym. J. 2016, 85, 82–91. [Google Scholar] [CrossRef]
- Chen, H.; Wei, X.; Chen, H.; Wei, H.; Wang, Y.; Nan, W.; Zhang, Q.; Wen, X. The study of establishment of an in vivo tumor model by three-dimensional cells culture systems methods and evaluation of antitumor effect of biotin-conjugated pullulan acetate nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, T.; Sohail, M.; Shah, S.A.; Mahmood, A.; Kousar, M.; Jabeen, N. Bioactive and multifunctional keratin-pullulan based hydrogel membranes facilitate re-epithelization in diabetic model. Int. J. Biol. Macromol. 2022; in press. [Google Scholar] [CrossRef]
- Suflet, D.M.; Popescu, I.; Prisacariu, A.I.; Pelin, I.M. Synthesis and characterization of curdlanPhosphorylated curdlan based hydrogels for drug release. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 870–879. [Google Scholar] [CrossRef]
- Suflet, D.M.; Pelin, I.M.; Dinu, M.V.; Lupu, M.; Popescu, I. Hydrogels based on monobasic curdlan phosphate for biomedical applications. Cell. Chem. Technol. 2019, 53, 897–906. [Google Scholar] [CrossRef]
- Tong, X.; Qi, X.; Mao, R.; Pan, W.; Zhang, M.; Wu, X.; Chen, G.; Shen, J.; Deng, H.; Hu, R. Construction of functional curdlan hydrogels with bio-inspired polydopamine for synergistic periodontal antibacterial therapeutics. Carbohydr. Polym. 2020, 245, 116585. [Google Scholar] [CrossRef]
- Zhou, Z.; Xiao, J.; Guan, S.; Geng, Z.; Zhao, R.; Gao, B. A hydrogen-bonded antibacterial curdlan-tannic acid hydrogel with an antioxidant and hemostatic function for wound healing. Carbohydr. Polym. 2022, 285, 119235. [Google Scholar] [CrossRef] [PubMed]
- Khani, A.; Eskandani, M.; Derakhshankhah, H.; Soleimani, K.; Nakhjavani, S.A.; Massoumi, B.; Jahanban-Esfahlan, R.; Moloudi, K.; Jaymand, M. A novel stimuli-responsive magnetic hydrogel based on nature-inspired tragacanth gum for chemo/hyperthermia treatment of cancerous cells. J. Polym. Res. 2022, 29, 149. [Google Scholar] [CrossRef]
- Muthukumar, T.; Song, J.E.; Khang, G. Biological role of gellan gum in improving scaffold drug delivery, cell adhesion properties for tissue engineering applications. Molecules 2019, 24, 4514. [Google Scholar] [CrossRef]
- Learmonth, D.A.; Costa, P.M.; Veloso, T.R.; Cunha, C.B.; Cautela, M.P.; Correia, C.; Vallejo, M.C.; Sousa, R.A. Synthesis and biological evaluation of a bioinspired, tissue-adhesive gellan gum-based hydrogel designed for minimally invasive delivery and retention of chondrogenic cells. Biomater. Sci. 2020, 8, 3697–3711. [Google Scholar] [CrossRef]
- Laradji, A.; Shui, Y.-B.; Karakocak, B.B.; Evans, L.; Hamilton, P.; Ravi, N. Bioinspired thermosensitive hydrogel as a vitreous substitute: Synthesis, properties, and progress of animal studies. Materials 2020, 13, 1337. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Piculell, L.; Borgström, J. Rheology of kappa-carrageenan in mixtures of sodium and cesium iodide: Two types of gels. Carbohydr. Polym. 1996, 31, 215–225. [Google Scholar] [CrossRef]
- Bercea, M.; Wolf, B.A. Associative behavior of κ-carrageenan in aqueous solutions and its modification by different monovalent salts as reflected by viscometric parameters. Int. J. Biol. Macromol. 2019, 140, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.C.; Li, C.Y.; Du, M.; Song, Y.H.; Wu, Z.L.; Zheng, Q. Improved toughness and stability of kappa-carrageenan/polyacryl- amide double-network hydrogels by dual cross-linking of the first network. Macromolecules 2019, 52, 629–638. [Google Scholar] [CrossRef]
- Patel, A.; Sant, V.; Velankar, S.; Dutta, M.; Balasubramanian, V.; Sane, P.; Agrawal, V.; Wilson, J. Self-assembly of multiscale anisotropic hydrogels through interfacial polyionic complexation. J Biomed. Mater. Res. 2020, 108, 2504–2518. [Google Scholar] [CrossRef]
- Patel, A.; Zaky, S.H.; Schoedel, K.; Li, H.; Sant, V.; Beniash, E.; Sfeir, C.; Stolz, D.B.; Sant, S. Design and evaluation of collagen-inspired mineral-hydrogel nanocomposites for bone regeneration. Acta Biomater. 2020, 112, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Zaky, S.H.; Li, H.; Schoedel, K.; Almarza, A.J.; Sfeir, C.; Sant, V.; Sant, S. Bottom-up self-assembled hydrogel-mineral composites regenerate rabbit ulna defect without added growth factors. ACS Appl. Bio Mater. 2020, 3, 5652–5663. [Google Scholar] [CrossRef]
- Bercea, M.; Morariu, S. Real-time monitoring the order-disorder conformational transition of xanthan gum. J. Mol. Liq. 2020, 309, 113168. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Hu, Q.; Chen, G.; Ren, Y.; Wu, X.; Ren, J. Bioinspired anti-digestive hydrogels selected by a simulated gut microfluidic chip for closing gastrointestinal fistula. iScience 2018, 8, 40–48. [Google Scholar] [CrossRef]
- Li, S.; Chen, N.; Li, X.; Li, Y.; Xie, Z.; Ma, Z.; Zhao, J.; Hou, X.; Yuan, X. Bioinspired double-dynamic-bond crosslinked bioadhesive enables post-wound closure care. Adv. Funct. Mater. 2020, 30, 2000130. [Google Scholar] [CrossRef]
- Du, Z.; Li, N.; Hua, Y.; Shi, Y.; Bao, C.; Zhang, H.; Yang, Y.; Lin, Q.; Zhu, L. Physiological pH-dependent gelation for 3D printing based on the phase separation of gelatin and oxidized dextran. Chem. Commun. 2017, 53, 13023–13026. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing: Collagen-based biomaterials. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Saroia, J.; Yanen, W.; Wei, Q.; Zhang, K. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Des Manuf. 2018, 1, 265–279. [Google Scholar] [CrossRef]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. Part B 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Zheng, P.J.; Cui, Z.D.; Zhao, N.Q.; Wang, Y.F.; De Yao, K. Swelling behaviour and elastic properties of gelatin gels. Polym. Int. 1997, 44, 448–452. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef]
- Alcala-Orozco, C.R.; Mutreja, I.; Cui, X.; Kumar, D.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. Design and characterisation of multi-functional strontium-gelatin nanocomposite bioinks with improved print fidelity and osteogenic capacity. Bioprinting 2020, 18, e00073. [Google Scholar] [CrossRef]
- Moshayedi, S.; Sarpoolaky, H.; Khavandi, A. Fabrication, swelling behavior, and water absorption kinetics of genipin-crosslinked gelatin–chitosan hydrogels. Polym. Eng. Sci. 2021, 61, 3094–3103. [Google Scholar] [CrossRef]
- Pettinelli, N.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Feijoo-Bandín, S.; Lagode, F. Carrageenan-based physically crosslinked injectable hydrogel for wound healing and tissue repairing applications. Int. J. Pharm. 2020, 589, 119828. [Google Scholar] [CrossRef]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials 2012, 33, 3143–3152. [Google Scholar] [CrossRef]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. Gellan gum microgel-reinforced cell-laden gelatin hydrogels. J. Mater. Chem. B 2014, 2, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.F.; Lee, S.C. Effect of gelatin on the swelling behavior of organic hybrid gels based on N-isopropylacrylamide and gelatin. J. Appl. Polym. Sci. 2005, 98, 1092–1099. [Google Scholar] [CrossRef]
- Touyama, R.; Takeda, Y.; Inoue, K.; Kawamura, I.; Yatsuzuka, M.; Ikumoto, T.; Shingu, T.; Yokoi, T.; Inouye, H. Studies on the blue pigments produced from genipin and methylamine. I. Structures of the brownish-red pigments, intermediates leading to the blue pigments. Chem. Pharm. Bull. 1994, 42, 668–673. [Google Scholar] [CrossRef]
- Kerscher, P.; Kaczmarek, J.A.; Head, S.E.; Ellis, M.E.; Seeto, W.J.; Kim, J.; Bhattacharya, S.; Suppiramaniam, V.; Lipke, E.A. Direct production of human cardiac tissues by pluripotent stem cell encapsulation in gelatin methacryloyl. ACS Biomater. Sci. Eng. 2017, 3, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Van Hoorick, J.; Tytgat, L.; Dobos, A.; Ottevaere, H.; Van Erps, J.; Thienpont, H.; Ovsianikov, A.; Dubruel, P.; Van Vlierberghe, S. (Photo-)crosslinkable gelatin derivatives for biofabrication applications. Acta Biomater. 2019, 97, 46–73. [Google Scholar] [CrossRef]
- Wen, C.; Lu, L.; Li, X. Enzymatic and ionic crosslinked gelatin/κ-carrageenan IPN hydrogels as potential biomaterials. J. Appl. Polym. Sci. 2014, 131, 1–8. [Google Scholar] [CrossRef]
- Fu, F.; Chen, Z.; Zhao, Z.; Wang, H.; Shang, L.; Gu, Z.; Zhao, Y. Bio-inspired self-healing structural color hydrogel. Proc. Natl. Acad. Sci. USA 2017, 114, 5900–5905. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Ponzini, S.; De Stefano, P.; Ortoleva, G.; Vignati, L.; Draghi, L. Cross-linking optimization for electrospun gelatin: Challenge of preserving fiber topography. Polymers 2020, 12, 2472. [Google Scholar] [CrossRef]
- Koshy, S.; Desai, R.M.; Joly, P.; Li, J.; Bagrodia, R.K.; Lewin, S.A.; Joshi, N.S.; Mooney, D.J. Click-crosslinked injectable gelatin hydrogels. Adv. Healthc. Mater. 2016, 5, 541–547. [Google Scholar] [CrossRef]
- Gao, T.; Gillispie, G.J.; Copus, J.S.; Kumar, A.; Asari, P.R.; Seol, Y.J.; Atala, A.; Yoo, J.J.; Lee, S.J. Optimization of gelatin-alginate composite bioink printability using rheological parameters: A systematic approach. Biofabrication 2019, 10, 034106. [Google Scholar] [CrossRef]
- Green, J.J.; Elisseeff, J.H. Mimicking biological functionality with polymers for biomedical applications. Nature 2016, 540, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Lee, H. Engineering hydrogels for the development of three-dimensional in vitro models. Int. J. Mol. Sci. 2022, 23, 2662. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wei, Z.; Han, C.; Weng, X. Nanomaterials as promising theranostic tools in nanomedicine and their applications in clinical disease diagnosis and treatment. Nanomaterials 2021, 11, 3346. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Yang, J.; Suo, Z. Fatigue of hydrogels. Eur. J. Mech. A Solids 2019, 74, 337–370. [Google Scholar] [CrossRef]
- Mierke, C.T. Viscoelasticity acts as a marker for tumor extracellular matrix characteristics. Front. Cell Dev. Biol. 2021, 9, 785138. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Almawash, S.; Osman, S.K.; Mustafa, G.; El Hamd, M.A. Current and future prospective of injectable hydrogelsDesign challenges and limitations. Pharmaceuticals 2022, 15, 371. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bercea, M. Bioinspired Hydrogels as Platforms for Life-Science Applications: Challenges and Opportunities. Polymers 2022, 14, 2365. https://doi.org/10.3390/polym14122365
Bercea M. Bioinspired Hydrogels as Platforms for Life-Science Applications: Challenges and Opportunities. Polymers. 2022; 14(12):2365. https://doi.org/10.3390/polym14122365
Chicago/Turabian StyleBercea, Maria. 2022. "Bioinspired Hydrogels as Platforms for Life-Science Applications: Challenges and Opportunities" Polymers 14, no. 12: 2365. https://doi.org/10.3390/polym14122365
APA StyleBercea, M. (2022). Bioinspired Hydrogels as Platforms for Life-Science Applications: Challenges and Opportunities. Polymers, 14(12), 2365. https://doi.org/10.3390/polym14122365





