1. Introduction
Wound healing is a dynamic and complex process involving hemostasis, inflammation, proliferation, and remodeling stages, as well as engaging a range of cells, genes, and cytokines for repairing the injured tissue [
1,
2]. Natural and synthetic traditional materials such as bandages, gauzes, and cotton wool have been used for protecting wounds and absorbing exudates [
2]. However, they have no therapeutic effects on the different wound healing stages. Therefore, many efforts have been dedicated to developing new wound dressings with beneficial properties for wound healing [
3,
4]. Materials such as gelatin (GEL) [
5,
6], chitosan [
2,
4,
7], alginate [
8], collagen [
9], polycaprolactone (PCL) [
5,
10,
11], poly(α-L-glutamic acid) [
12], antibiotics [
13,
14], and phytotherapeutics [
5,
10,
11,
15,
16] are being used for such purpose.
GEL is considered a promising biomaterial due to its biocompatibility and biodegradability, amphoteric character, good affinity with proteins, and capacity for potential modifications [
17]. GEL promotes cell adhesion and proliferation when it is used in wound dressing applications [
5,
18]. On the other hand, PCL is a synthetic aliphatic polyester widely used in biomedicine, food, and other industrial applications [
10,
11,
19] due to its biocompatibility, biodegradability, and desired mechanical properties [
20]. However, certain disadvantages limit the use of both polymers as wound dressings. For instance, GEL exhibits poor mechanical strength [
20,
21], whereas PCL has a low degradation rate in aqueous solution and its hydrophobic nature limits cell adhesion [
10,
19]. Thus, PCL/GEL blends with improved mechanical and biological features have been developed to overcome those drawbacks [
5,
20,
21,
22].
Plant-derived metabolites have been widely used by humans for hundreds of years due to their healing properties [
10,
23]. Among them, the
Pinus radiata bark extracts (PEs), composed of a high concentration of condensed tannins [
24], have shown suitable antioxidant and anti-inflammatory properties, among other bioactivities [
25,
26]. However, the low solubility, high sensitivity against environmental changes, instability in physiological medium, and volatility of phenolic compounds have limited their use in biomedicine [
10,
15]. Electrospinning of PE–biopolymer systems could provide protection to PE against external factors to which the bark extract is vulnerable (light, heat, moisture, and oxidation). Electrospinning has been established as an outstanding and versatile technique to produce electrospun fibers with high specific surface area, excellent fluid drainage, and controlled drug delivery [
5,
10,
11,
19]. In the literature, different bioactive molecules have been entrapped into PCL/GEL electrospun fibers for wound dressing applications. For instance, Ramalingam et al. [
20] demonstrated that natural herbal extracts (
Gymnema sylvestre) loaded into PCL/GEL electrospun nanofibrous structures improved the antimicrobial activity of their materials. According to Mohamadi et al. [
21], PCL/GEL nanofibers containing 20%
w/w coconut oil improved biocompatibility and antibacterial activity against
S. aureus and
E. coli bacteria, which are desirable for wound healing. Similarly, Unalan et al. [
5] fabricated PCL/GEL electrospun nanofibers loaded with clove essential oil (CLV). Their results showed that PCL/GEL/CLV nanofibers exhibited excellent antibacterial activity against
S. aureus and
E. coli bacteria and were considered promising candidates for wound healing applications.
According to the authors’ knowledge, incorporating PE into nanofibrous structures of PCL/GEL for biomedical applications has not been investigated before. Thus, this study aimed to develop PCL/GEL electrospun nanofibers loaded with Pinus radiata bark extracts for wound healing applications for the first time. In this regard, the effects of loading different PE concentrations (0.18% and 0.36% w/w) into PCL/GEL electrospun nanofibers were analyzed in terms of surface morphology, average fiber diameter, functional groups, total phenol content, wettability, degradation and release behavior, antibacterial activity, cell biocompatibility, and in vitro wound healing.
2. Materials and Methods
2.1. Materials
Gelatin (type A, 300 g Bloom), glacial acetic acid (GAA, used as a solvent), PCL (Mw = 80,000 Da), fetal bovine serum (FBS, F2442), Folin–Ciocalteu reagent (F9252), and sodium carbonate were purchased from Sigma-Aldrich (Darmstadt, Germany). Phosphate-buffered saline (PBS, biotech grade, pH = 7.4) was obtained from Merck. The microorganism strains of S. aureus (ATCC25823) and E. coli (ACTC25922) were used. Luria/Miller agar (X969.1) and lysogeny broth medium (Luria/Miller, 6673.1) were supplied by Carl Roth GmbH (Karlsruhe, Germany). A human keratinocyte (HaCaT) cell line was obtained from Cell Lines Services GmbH (CLS, 300493, Baden-Württemberg, Germany). Dulbecco’s modified Eagle’s medium (DMEM, 31885-023), trypsin/EDTA (25200-056), and penicillin/streptomycin (PS, 15140-122) were purchased from Thermo Scientific (Schwerte, Germany). Pinus radiata bark was supplied by Technological Development Unit (UDT, Concepción, Chile). All reagents and solvents were of analytical grade.
2.2. Pinus radiata Bark Extract Production
Pinus radiata bark extracts were produced through a pilot-scale extraction process, as described by Bocalandro et al. [
24] For this purpose, a reactor volume of 4 m
3 and a vapor heating system composed of a shell and a tube heat exchanger with 6 m
2 heat transference area were used. In addition, a recirculation circuit for the extracted solution was implemented. Briefly, the
Pinus radiata bark was ground with a double-knife mill to an average size lower than 20 mm. Then, the bark was dried at room temperature until a humidity of 24.5% (
dry weight), and 100 kg (
dry weight) of bark was soaked in an ethanol/water solution at a 1:20 ratio (
w/v) for 120 min at 120 °C. Subsequently, the ethanol was evaporated under a vacuum (absolute pressure 0.05 bar) at room temperature. Thus, the water-insoluble particulate material after decanting and the water-soluble polyphenol fraction were obtained. Finally, the water-soluble polyphenols were lyophilized at room temperature and the obtained extracts were stored in sealed amber glass containers for further analysis.
2.3. Preparation of Electrospinning Solutions
The electrospinning solutions were fabricated according to the protocol established by Unalan et al. [
5], with slight modifications. Briefly, GEL powder (4.8%
w/w) was dissolved in 10 mL of GAA (90%
v/v) at 45 °C for 4 h. PCL pellets (1.12 g) were added to the previous solution and stirred overnight at room temperature. After 30 min of adding PCL pellets to prepare the PE-containing solution, different concentrations of PE (0.18% and 0.36%
w/w) were separately added to each PCL/GEL solution. All prepared solutions were stirred overnight at room temperature to achieve homogeneity. Finally, each solution was sonicated for 15 min at room temperature before electrospinning.
2.4. Electrospinning Process
The electrospinning process was carried out as previously described by Unalan et al. [
5]. Briefly, PCL/GEL and PE-loaded PCL/GEL solutions were separately loaded into a plastic syringe (3 mL) fitted with a 21G needle. A commercially available device (Electrospinning Starter Kit, Linari Engineering Srl; Pisa; Italy) was used to produce the electrospun nanofibers by applying a voltage of 18 kV. The distance between the aluminum foil-wrapped collector and the needle tip was 12 cm. The flow rate used to produce electrospun nanofibers was 0.6 mL h
−1. Finally, electrospinning was carried out under defined environmental conditions (temperature: 23 ± 2 °C and relative humidity: 26 ± 2%). The electrospun nanofibers were stored at 4 °C in the dark until further analysis. The nomenclature used to identify the electrospun nanofibers was PCL/GEL, PCL/GEL/0.18%PE, and PCL/GEL/0.36%PE. The above percentages (
w/
w) indicate the PE content loaded into each PCL/GEL solution.
2.5. Characterization of Electrospun Nanofibers
The surface morphology of the electrospun nanofibers was analyzed by scanning electron microscopy (SEM) (ETH: 2 kV, Everhart-Thornley detector (SE2), AURIGA base 55, Carl Zeiss). The samples were coated with a layer of gold before SEM analysis. The average fiber diameter was calculated using 50 random pores obtained from the SEM images in ImageJ software (NIH, Bethesda, MD, USA).
The functional groups of PE, PCL/GEL, and PE-loaded PCL/GEL electrospun nanofibers were identified by attenuated total reflection Fourier-transform infrared (ATR-FTIR) spectroscopy (RA_nity-1S, Shimadzu, Duisburg, Germany). The spectra were measured in a wavenumber range between 4000 and 400 cm−1 using a spectral resolution of 4 cm−1 and 32 scans.
The total phenol content (TPC) of the PE-loaded PCL/GEL electrospun nanofibers was determined by a Folin–Ciocalteu assay [
27] with modifications. In this assay, 2 mL of methanol was added to 10 mg of nanofiber for 24 h. After that time, 2 mL of Folin–Ciocalteu reagent (diluted in deionized water in a ratio of 1:9) and 4 mL of sodium carbonate (7.5%
wt.) were added to the above solution. The mixture was stored for 1.5 h in a dark room. Then, the absorbance of each sample and the blank (deionized water) was measured at 765 nm in a UV-Vis spectrophotometer (Specord 40, Analytik Jena GmbH, Jena, Germany). The measurements were performed in triplicate and the results were expressed in units of milligrams of equivalent gallic acid per milligram of fiber.
The wettability of the electrospun nanofibers was analyzed by contact angle measurements using a sessile drop method in a contact angle meter (Drop Shape Analyzer, DSA 30, CA Measurement setup, Kruess GmbH, Hamburg, Germany). The electrospun nanofibers were placed on a glass slide before testing, and a drop of deionized water was dropped onto their surface. Three measurements were performed at different points on the surface of the same electrospun nanofiber, and the average value was determined.
2.5.1. Mechanical Properties of Electrospun Nanofibers
A uniaxial tensile test was performed with the electrospun nanofibers to evaluate their mechanical properties using a universal testing machine Instron 5967 (Instron GmbH, Darmstadt, Germany). In this test, the electrospun nanofibers were cut in a rectangular shape (0.5 cm width and 4 cm length) and fixed in a suitable paper square framework. The measurements were performed at a 1 mm/min crosshead speed using a 100 N load cell. Mechanical properties such as Young’s modulus, tensile strength, and elongation at break were calculated from the tensile stress–strain curves. These measurements were performed six times for each electrospun nanofiber, and an average value was reported.
2.5.2. In Vitro Degradation Test
An in vitro degradation test was performed with the electrospun nanofibers as described previously [
10]. Briefly, each electrospun nanofiber was cut into 3 × 3 cm
2 pieces and immersed in 10 mL of PBS (1×, pH = 7.4). Then, the samples were incubated at 37 °C and 110 rpm for 1, 3, 7, 14, 21, 28, and 35 days. After each time interval, the samples were taken out from the PBS, rinsed with Milli-Q water, and dried at 37 °C for 3 days before the measurement. The weight loss of each electrospun nanofiber was calculated according to Equation (1):
where
Winitial and
Wdry are the initial and dry weight of fibers, respectively. This test was performed in triplicate for each sample. In addition, the chemical bonds of the electrospun nanofibers after drying were analyzed by ATR-FTIR spectroscopy.
2.5.3. Determination of PE Composition
The polyphenol composition of PE was identified and quantified using a reversed-phase high-performance chromatography (RP-HPLC)–mass spectrometry (MS) system coupled with a diode array detector (DAD). This analysis was performed following the protocol proposed by Bocalandro et al. [
24]. Briefly, 10 µL of the sample was filtered with Phenex-RC 15 mm syringe filters. Then 0.2 µm was injected three times (Phenomenex, Torrance, USA) using a mobile phase composed of 1% acetic acid (phase A) and acetonitrile (phase B) at a flow rate of 0.8 mL/min. The following program was used for the mobile phase: 0.8% to 2.4% phase B, 0–4.5 min; 2.4% to 4% phase B, 4.5–6 min; 4% to 6.8% phase B, 6–7.5 min; 6.8% to 14.4% phase B, 7.5–13 min; 14.4% to 15.4% phase B, 13–14 min; 15.4% to 24% phase B, 14–19 min; 24% to 40% phase B, 19–24 min; 40% phase B, 24–34 min; and 40% to 0.8% phase B, 34–40 min. The separation was carried out under room temperature and 150 bar pressure conditions. The detection was carried out in the wavelength range between 210 and 600 nm. Three wavelengths (240 nm, 280 nm, and 330 nm) were used for data analysis. Each extracted compound was identified by analysis of UV and MS data and quantified by DAD. For this quantification, a calibration curve of epigallocatechin (0.03–0.5 g/L), (−)-catechin hydrate (0.1–1.0 g/L), proanthocyanidin B-2 (0.03–1.0 g/L) taxifolin (0.06–1.0 g/L), 3,4 dihydroxybenzoic acid (0.12–1.2 g/L), (+)-epicatechin (0.1–1.06 g/L), quercetin (0.1–1.0 g/L), syringic acid (0.10–1.0 g/L), 3,4-dihydroxyphenyl acetic acid (0.10–1.0 g/L),
p-hydroxybenzoic acid (0.11–1.06 g/L), and 2-(4-hydroxy-3-methoxy-phenyl) acetic acid (0.10–1.02 g/L) dissolved in methanol and filtered with a 0.2 µm filter was used.
2.5.4. Release Study
A release study was performed with the PE-loaded PCL/GEL electrospun nanofibers. In this test, each electrospun nanofiber mat was weighted accurately (3.0 mg) and incubated with 10 mL of PBS (1×, pH = 7.4) at 37 °C for 1, 3, 6, 24, 48, 72, 120, 168, 336, 540, and 840 h. After each time point, 500 µL of the sample was taken out to measure its absorbance using a UV-Vis spectrophotometer (Spectroquant Prove 600 spectrometer, Merck, Germany) at 281 nm based on a calibration curve (
R2 = 0.9984). Then, 500 µL of fresh PBS was added to each nanofiber to ensure a constant volume during the assay. In addition, a blank absorbance (
Ao) and the maximum absorbance (
Absmax) equivalent to the pure PE concentration loaded into the electrospun nanofibers were also measured. Finally, the content of PE released was calculated according to Equation (2):
This test was performed in quintuplicate with reproducible results.
2.6. Antibacterial Assays
The antibacterial activity of PCL/GEL, PCL/GEL/0.18%PE, and PCL/GEL/0.36%PE electrospun nanofibers against
S. aureus (Gram-positive) and
E. coli (Gram-negative) was evaluated by a direct method. Each bacterial strain was separately incubated in a lysogeny broth medium (LB-medium) at 37 °C for 24 h. After that time, the optical density (OD) of each bacteria population was determined (600 nm, Thermo Scientific GENESYS 30; Schwerte; Germany) until reaching 0.015, according to the turbidity measurement of bacteria culture. Then, 10 mg of each electrospun nanofiber (sterilized by UV radiation for 1 h before the experiment) was placed in a 15 mL falcon tube with 30 µL of bacteria suspension and 2 mL of LB-medium. Each electrospun nanofiber was incubated at 37 °C for 3, 6, and 24 h. After each incubation time, the OD value of the samples was measured at 600 nm. The LB-medium and the PCL/GEL electrospun nanofiber mat were used as blank and control in this assay, respectively. Each measurement was performed in triplicate. The relative bacterial viability was calculated according to Equation (3):
2.7. Cell Culture
HaCaT cells were cultured in a DMEM supplemented with 10% fetal bovine serum, 4.5 g L−1 of glucose, and 1% penicillin/streptomycin solution at 37 °C for 24 h in a humidified atmosphere of 95% air and 5% CO2. After cells were grown to confluency, the DMEM was removed entirely and the cells were washed with PBS (5 mL). Then, the PBS was removed, and the cells were detached by trypsinization and counted by trypan blue assay using a hemocytometer (Roth, Germany). In parallel, each electrospun nanofiber mat was fixed on CellCrown 24 inserts (ScaffdexOy, Tampere, Finland) and sterilized by UV radiation for 1 h. Finally, counted cells were seeded on the top of electrospun nanofiber mats at a density of 125,000 cells/well and incubated at 37 °C in a humidified incubator with 5% CO2.
2.7.1. Cell Viability Assay
The cell viability of the electrospun nanofibers after 1- and 7-day incubation was analyzed using a WST-8 cell counting assay kit as indicated by the manufacturer. The absorbance of the dyes was measured in a spectrophotometric plate reader (FLUOstar-Omega BMG Labtech, Ortenberg, Germany) at 450 nm. In this assay, the PCL/GEL sample and the WST-8 reagent were used as control and blank, respectively. The cell viability of each electrospun nanofiber mat was calculated according to Equation (4):
2.7.2. Cell Staining
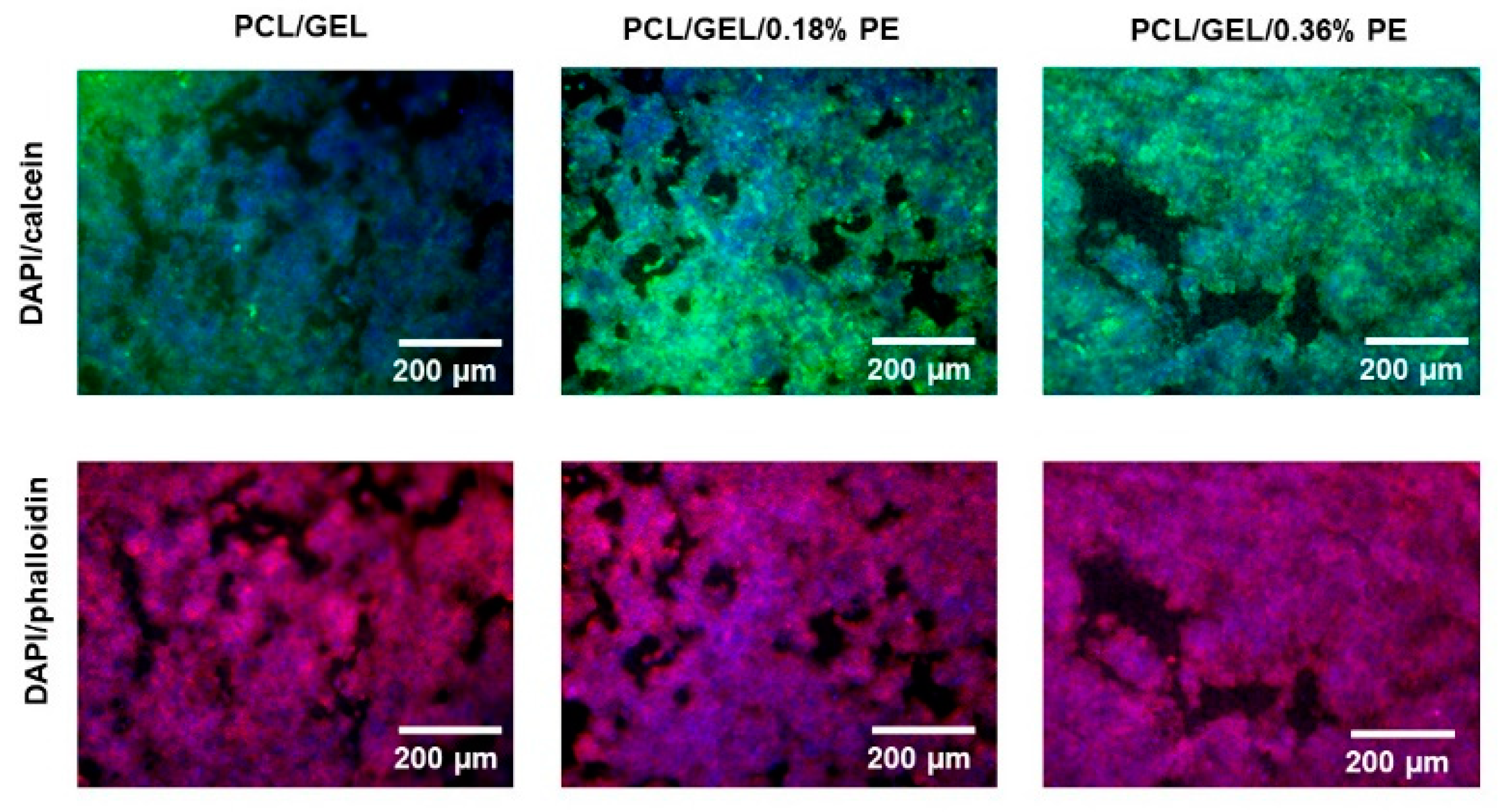
HaCaT cells were stained with calcein, DAPI, and rhodamine phalloidin dyes after cell viability assays for cell distribution and cytoskeleton analysis by fluorescence microscopy (Axio Scope A1, Carl-Zeiss, Jena, Germany). Firstly, HaCaT cells were washed with PBS, fixed with 1 mL of calcein (4 µg mL−1), and incubated at 37 °C for 45 min in a 5% CO2 atmosphere. Afterward, HaCaT cells were washed with PBS to remove the calcein, and 1 mL of Fluoro-Fix was added for 15 min. Then, the cells were washed with PBS, and 1 mL of a permeabilization buffer solution was added for 5 min. After removing this solution, 1 mL of rhodamine phalloidin reagent (8 µg mL−1) was added to the cells, which were incubated at 37 °C for 45 min in 5% CO2. HaCaT cells were again washed with PBS and 1 mL of DAPI (1 µg mL−1) was added for 5 min, which was removed. Finally, HaCaT cells were stored at 4 °C with 1 mL of PBS until further analysis.
2.7.3. SEM Analysis
After seven days of cell seeding, the morphology of HaCaT cells was analyzed by SEM, as mentioned in
Section 2.5. In this process, after the cell medium was removed from each well, the electrospun nanofiber mats were rinsed with PBS. Afterward, the cells were first fixed with 2.5% (
v/v) of glutaraldehyde solution for 2 h at room temperature, and then electrospun nanofibers were rinsed three times with PBS. Finally, the cell in the electrospun nanofibers was dehydrated in graded ethanol solutions (30, 50, 60, 70, 80, 90, 95, and 100%) and was dried with a critical point dryer (Leica EM CPD300, Istanbul, Turkey).
2.8. In Vitro Wound Healing Assay (Scratch Test)
An in vitro wound healing assay was performed according to previously described experimental procedures [
5,
11], with slight modifications. Briefly, HaCaT cells (500,000 cells/well) were seeded into a 24-well plate and incubated at 37 °C for 24 h in a humidified atmosphere with 5% CO
2. After that time, a vertical scratch was manually created in the middle of the HaCaT monolayer by using a 1000 µL sterile pipet tip. Then, each electrospun nanofiber mat was fixed on CellCrown 24 inserts (ScaffdexOy, Tampere, Finland) and placed on the 24-well plate without touching the surface. The wound closure rate and the cell migration were monitored over time (0, 4, 6, 24, 48, and 72 h) using a light microscope (Primo Vert, Carl Zeiss, Jena, Germany). Finally, the images were analyzed using ImageJ software. The wound closure rates were calculated according to Equation (5):
where
A0 is the initial wound area and
At is the wound area after each time interval. All measurements were performed in triplicate.
2.9. Statistical Analysis
Data analysis was performed using OriginPro8.5 software (Northampton, MA, USA), and ImageJ software was used to measure the average fiber diameters. Statgraphics Centurion XVII software (NIH, Bethesda, MD, USA) was used for one-way analysis of variance (ANOVA) and the analysis of multiple ranges (Duncan’s test). The level of significance was determined as p < 0.05, p < 0.01, and p < 0.001 in antibacterial activity and cell biocompatibility studies. The rest of the results were analyzed with a significance level of 95%. The data are presented as the mean ± SD, and the error bars are shown in each figure.
4. Discussion
The combination of natural compounds with engineered biomaterials to produce new composites is of increasing interest in the biomedical field. Among these,
Pinus radiata bark extracts, rich in phenolic compounds, particularly in flavonoids, possess interesting antioxidant and anti-inflammatory activities that could support their therapeutic use in biomedical applications. The combination of
Pinus radiata bark extracts with polymeric matrices has also proven to be a promising strategy to counteract potential drawbacks (mentioned earlier) of polyphenols [
5,
10,
15,
30], thus helping to preserve and protect their functionalities [
31]. In this context, the mixture of these materials could also provide ideal protection for PE against external factors such as light, heat, and humidity, thus preventing easy oxidation of its components and improving its controlled release. This study investigates the potential of PCL/GEL and PE-loaded PCL/GEL electrospun nanofibers fabricated via electrospinning for wound healing applications.
Electrospinning is a well-established technique widely used for the fabrication of nanofibrous mats for numerous biomedical applications [
19]. Several parameters such as concentration and viscosity of the polymer solution, electrical conductivity, temperature, humidity, voltage, and tip-to-collector distance influence the electrospinning process [
10,
32,
33]. In addition, decreasing the electrical conductivity or increasing the solution viscosity can increase the average fiber diameter [
5,
34,
35], thus modifying the electrospun sample’s morphology. The combined action of the above factors generates a direct effect on the surface morphology of the electrospun samples. Using SEM imaging, we observed that the addition of PE into PCL/GEL electrospun nanofibers did not affect the surface morphology or the average fiber diameter due to the limited PE concentration in the electrospun nanofibers. This finding is favorable for wound healing applications because it allows the development of a robust method for the reproducible fabrication of electrospun samples.
The influence of the electrospinning parameters on the morphological characteristics of PCL/GEL nanofibers loaded with different bioactive compounds has been previously investigated. Jiang et al. [
36] reported an increase in the average fiber diameter for PCL/GEL nanofibers loaded with palmatine compared to PCL/GEL nanofibers due to the decrease in the electrical conductivity of the spinning solution by adding palmatine. Similarly, Adeli-Sardou et al. [
37] found that the average fiber diameter of PCL/GEL nanofibers loaded with lawsone increased compared to that of PCL/GEL nanofibers. Unalan et al. [
5] reported as well that the increase in the fiber diameter of CLV-loaded PCL/GEL fiber mats compared to PCL/GEL fiber mats could be attributable to a reduction in solution electrical conductivity. These studies demonstrated that the morphological properties of electrospun samples are modified by loading natural compounds into the starting PCL/GEL solution. Therefore, future morphological studies should be performed in the PE-loaded PCL/GEL electrospun nanofibers using higher PE concentrations to examine possible morphological changes after adding PE.
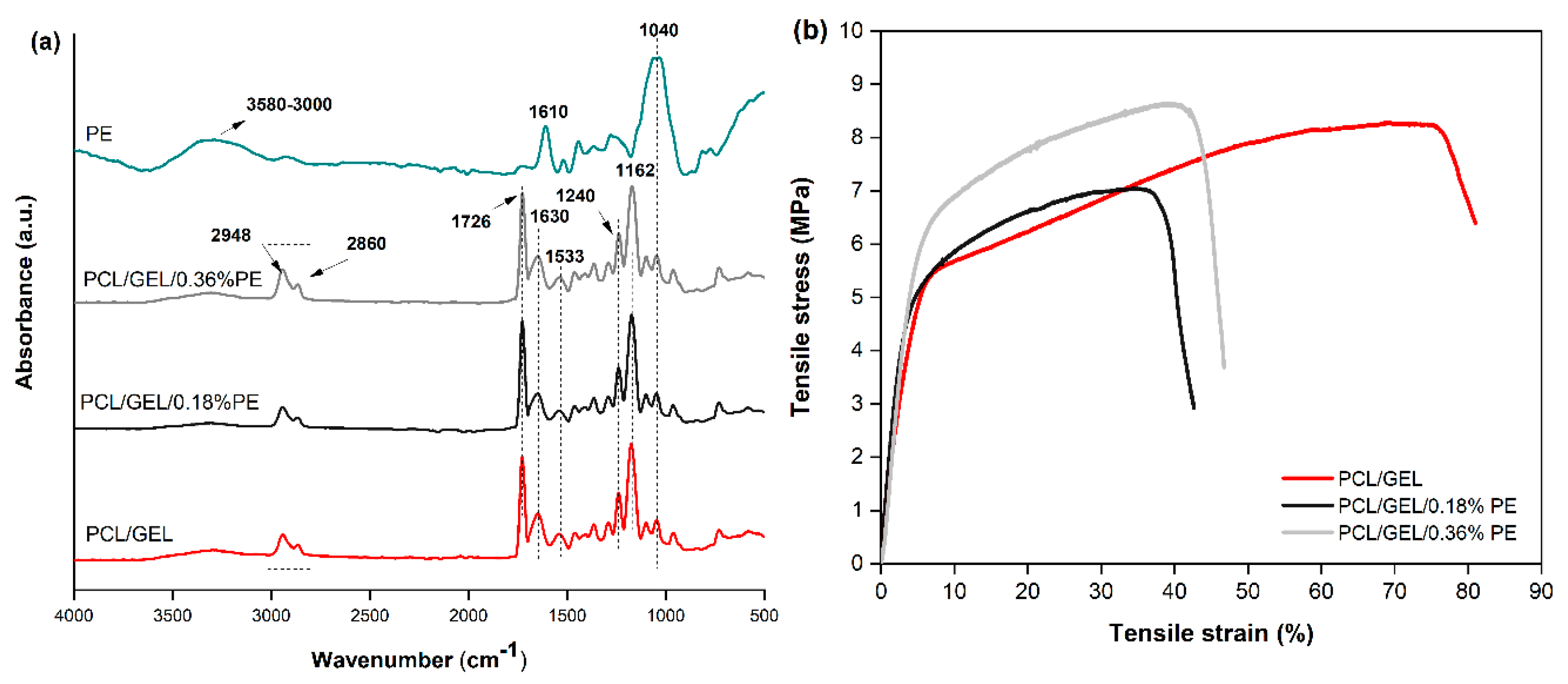
The chemical bonds of electrospun nanofibers and PE were confirmed through ATR-FTIR spectroscopy. The TPC was measured for the two PE-loaded electrospun nanofibers through a Folin-Ciocalteu assay. Both analyses confirmed the successful loading of PE into the PCL/GEL electrospun nanofibers.
The wettability of electrospun nanofiber mats is an essential property for biomedical applications since they should absorb wound exudates and maintain moisturized environments for wound healing [
5,
15,
38]. When the hydrophilicity was measured, it was found that the contact angle for the PCL/GEL/0.18%PE electrospun nanofiber significantly decreased compared to the PCL/GEL sample. This result could be attributed to the presence of -OH groups and hydrophilic chain portions contained in the PE’s chemical structure, as shown in the ATR-FTIR analysis. According to Ramalingam et al. [
20], the wettability of the PCL/GEL mats increased after adding
Gymnema sylvestre leaf extracts compared to the PCL/GEL nanofiber’s wettability due to the presence of multiple -OH groups in the extract’s structure. In another study, Unalan et al. [
5] ascribed the decrease in the contact angle for CLV-loaded PCL/GEL fiber mats compared to the PCL/GEL fiber mat’s wettability to the presence of polar and hydrophilic functional groups in the CLV structure. Both studies coincide in the fact that the presence of hydrophilic groups from new bioactive compounds increases the wettability of the electrospun samples, as demonstrated in the present study. Thus, PE-loaded electrospun nanofibers could be useful for wound healing applications due to their hydrophilic nature.
The mechanical properties of nanofibrous materials also play an essential role in wound healing applications since the nanofibers must be strong enough to withstand the mechanical stresses applied without causing large deformation or fracture during wound healing [
20,
37]. In the current study, the tensile strength values of fiber mats declined when the PE content loaded into the PCL/GEL electrospun nanofibers increased. Our results also agree with previous studies demonstrating that loading plant extracts in electrospun nanofibers reduces the mechanical performance of nanofibrous materials [
37,
38,
39]. Several explications have been attributed to the reduction in the mechanical properties of fibrous materials after loading natural compounds. Salehi et al. [
39] associated this behavior with the formation of random fibers instead of aligned fibers. On the other hand, Sardou et al. [
37] ascribed this reduction to the plasticizing effect of these materials. In contrast, Mohamadi et al. [
21] stated that after loading natural compounds into nanofibers, semi-interpenetrated systems are formed, leading to a decrease in the mechanical properties of the polymeric systems. In all these cases, there is a reduction in the cross-sectional area per unit area of the fibers, resulting in the formation of nanofiber networks capable of resisting external tensile forces due to their random distribution. In contrast, Young’s modulus and elongation at break were not affected after adding PE into electrospun nanofibers. Overall, the measured mechanical property values are within the ranges reported by other authors for wound dressing applications [
37,
40].
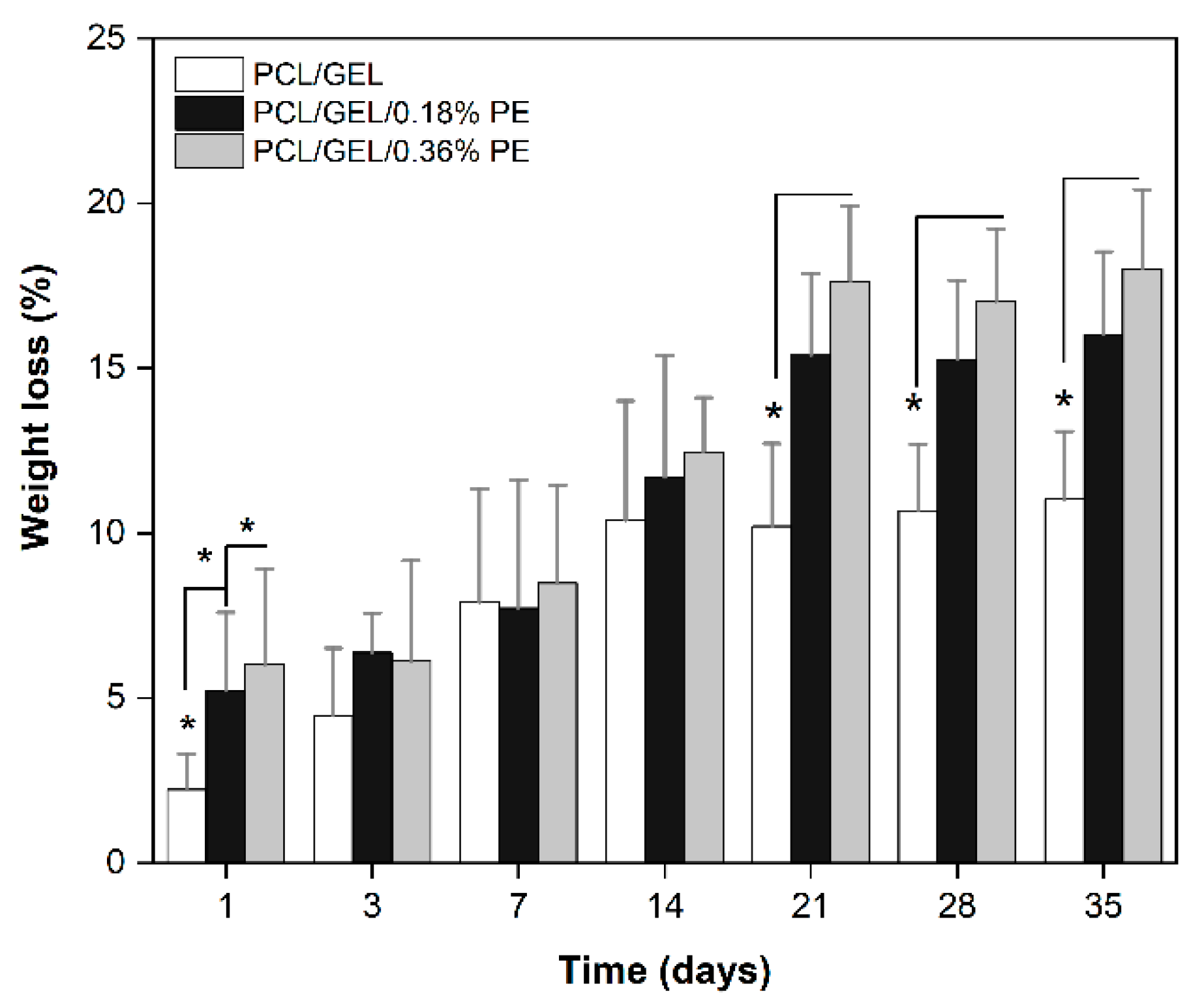
The biodegradability of materials is a critical requirement that must be evaluated for wound healing applications. In the present study, we found that PE-loaded electrospun nanofibers exhibited higher degradation rates over time compared to the PCL/GEL nanofiber. This degradation behavior could be mainly due to the presence of hydrophilic chain portions and -OH groups in the PE structure, enhancing the affinity of PE-loaded electrospun nanofibers for water molecules. These results can be further explained by the PE release, which will be discussed below. These findings also agree with our wettability data. By monitoring the electrospun nanofiber’s functional groups during the degradation assay, slight changes in the -OH band intensity were detected. This result might be attributable to the intermolecular interactions occurring between the electrospun nanofibers and PBS, which are enhanced for PE-loaded electrospun nanofibers due to the presence of PE hydroxylic groups. In contrast, no noticeable changes in the main functional groups of GEL and PCL were detected during degradation. Due to the chemical complexity of these molecules, the knowledge about the nature of the degradation products is still limited. To our knowledge, there are no previous studies in which the degradation products of Pinus radiata bark extracts have been isolated and characterized the mechanism of degradation successfully elucidated.
The degradation behavior of natural extracts loaded into PCL/GEL electrospun nanofibers has been investigated in previous studies, showing that the incorporation of natural compounds into PCL/GEL nanofibers increases the degradation rates over time [
10,
15,
22,
36,
37], which agrees with our degradation results. In addition, two of these studies support the idea that the degradation rate increases due to lowering intermolecular forces between PCL/GEL nanofibers after the loading of extracts [
10,
37]. Based on our degradation results, PE-loaded electrospun nanofibers could be beneficial for wound healing applications because of their combination of biodegradable and hydrophilic properties.
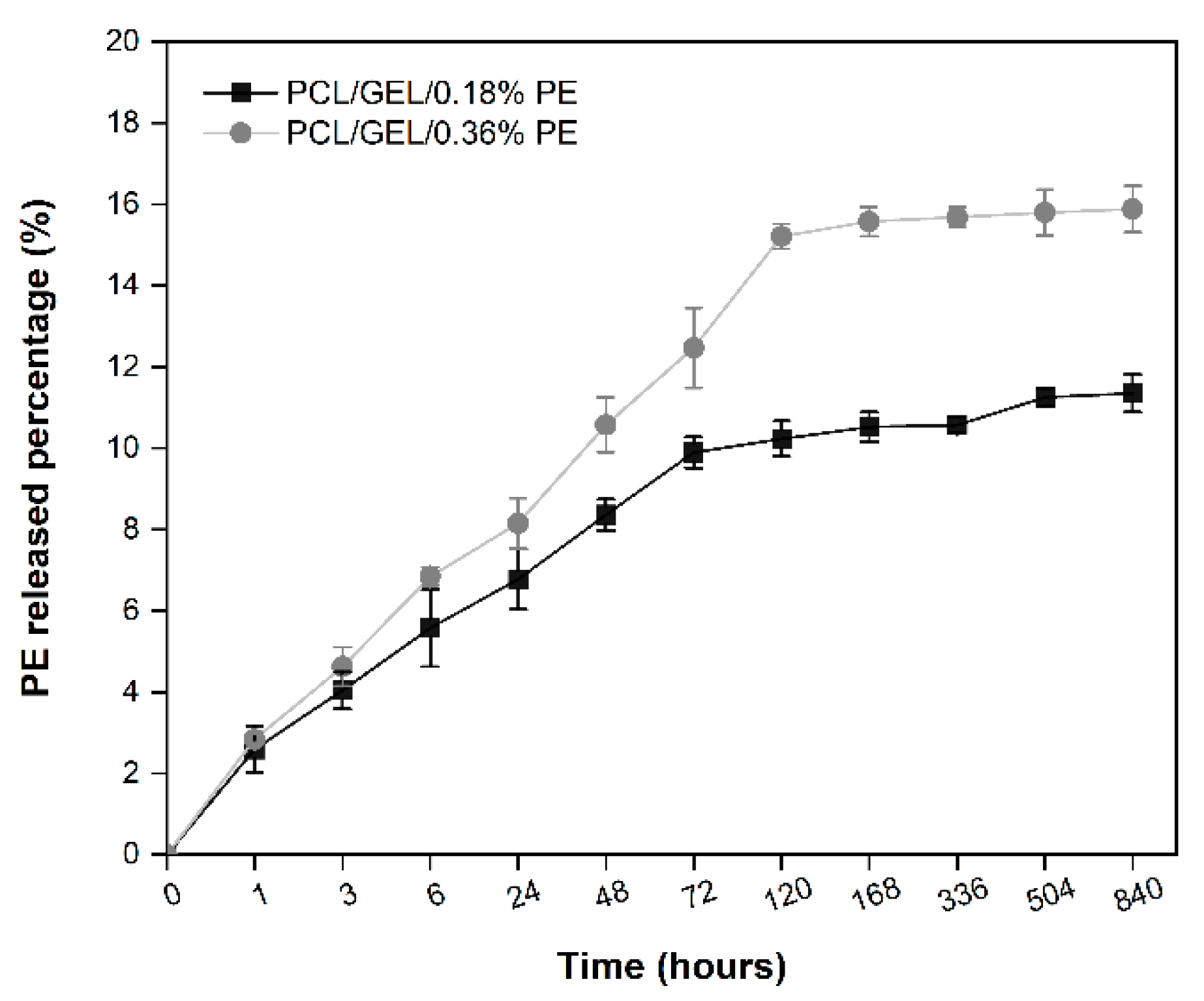
In vitro drug release testing is required to evaluate a material’s applicability in treating different wounds. In the present study, the process of releasing PE from PE-loaded electrospun nanofibers exhibited a sustained and controlled release kinetics, which is favorable for wound healing applications. The low release percentages achieved by both PE-loaded electrospun nanofibers over time could be explained, firstly, by the limited PE concentration loaded into the PCL/GEL electrospun nanofiber, which is consistent with the TPC results. Secondly, the low PE release may be due to its own nature, including its complex chemical structure that consists of several compounds, as summarized in
Table S1. In addition, these results are directly related to the degradation behavior of our electrospun nanofibers since, due to the high susceptibility of phenolic compounds to degradation by many environmental factors [
41,
42], it is difficult to identify which small molecules are diffusing out and releasing into the medium. Consequently, different release and degradation rates are expected for each of the compounds present in PE.
In addition, it is worth mentioning that the release processes of molecules loaded into nanofibers are influenced by the hydrophilic/hydrophobic nature of the drug, the morphology, the pore size of the nanofibers, and the diffusive processes involved during the release, among other factors [
15,
43]. The combined action of such variables directly influences the release of compounds loaded into electrospun fibrous samples.
The release of phenolic compounds loaded into nanofibrous materials has been previously investigated by other authors. Lin et al. [
43] reported that grape seed extract (GSE)-loaded silk fibroin(SF)/polyethylene oxide (PEO) nanofibers released 65% of the GSE content after 350 h in PBS. In a similar study, Locilento et al. [
15] found that polylactic acid (PLA)/PEO and PLA nanofibrous membranes loaded separately with GSE released about 40% and 70% of their GSE content after 700 h of testing in a PBS solution, respectively. Both studies agreed on loading GSE with a chemical composition similar to PE into nanofibrous matrices. Therefore, the differences in the released extract contents from nanofibers could be attributed to the extract’s content loaded into the nanofiber. Due to the structural complexity of PE, it is difficult to measure its release accurately; therefore, further studies in this area are required.
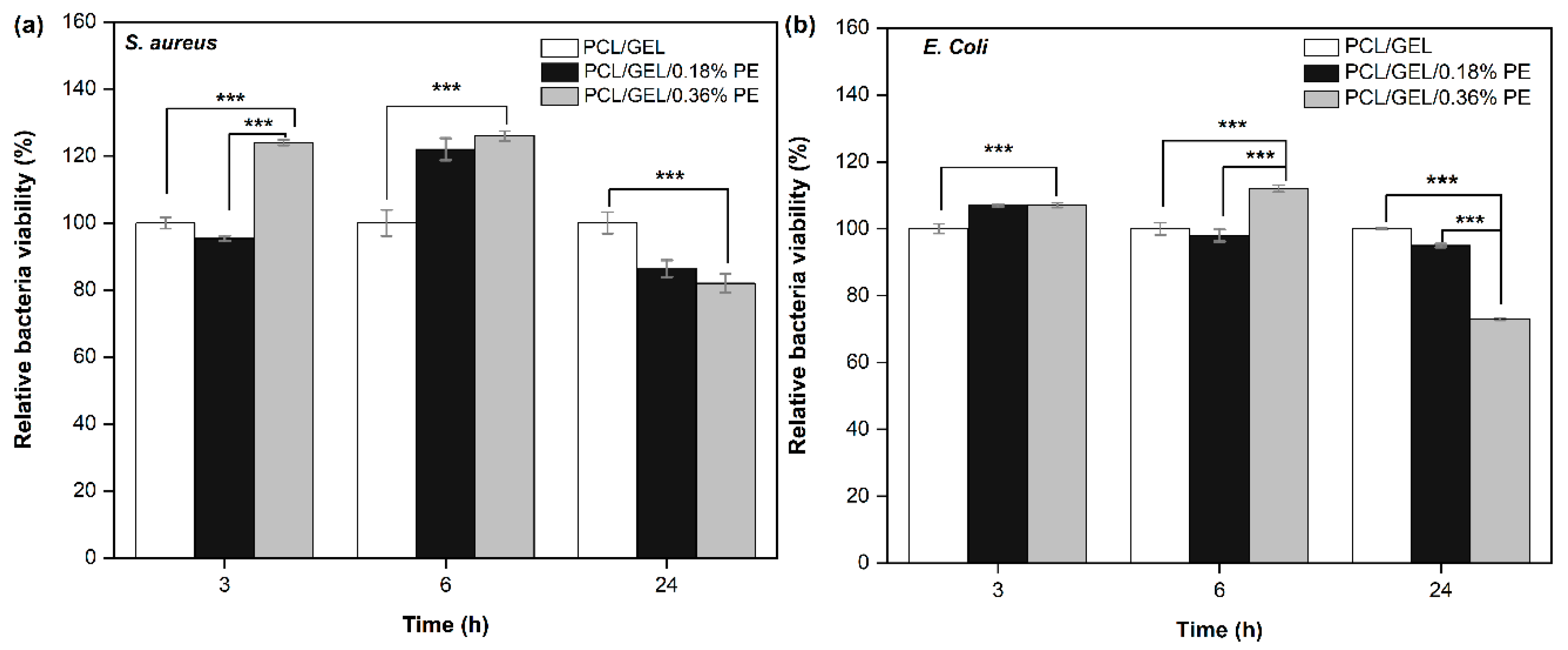
Antibacterial activity of wound dressing materials plays a crucial role in avoiding bacterial contamination in wound healing processes [
37]. Among the microorganisms involved in such processes are
S. aureus bacteria, which appear at an early stage of healing, and
E. coli bacteria, which are more related to chronic wounds [
11]. In previous studies, catechin and taxifolin, as the main compounds of PE, were investigated in terms of their antibacterial activity. For instance, Díaz-Gómez et al. [
44] reported that the inhibition zone was 5 mm for 7.5 mg catechin, and the inhibition zone increased with increasing catechin concentration, indicating the inhibitory effect against
E. coli. In another study, Ahamad et al. [
45] investigated the antibacterial activity of taxifolin toward
S. mutants and
L. acidophilus. Their results revealed that the inhibition zone was 18–22 mm for
S. mutans and 7–13 mm for
L. acidophilus at concentrations in the range of 1.5–2.5 mg/mL. The mentioned studies thus confirmed that PE has the potential to be used as antibacterial material. In the present study, the antibacterial activity of PE-loaded PCL/GEL electrospun nanofibers was tested on
S. aureus and
E. coli bacteria. A slight bacterial viability reduction in both bacteria strains was observed after 24 h incubation, being higher for the PCL/GEL/0.36%PE electrospun nanofiber against
E. coli bacteria. This finding is related to a higher content of PE released by the PCL/GEL/0.36%PE nanofiber at 24 h and could be also ascribed to the different characteristics and morphology of each bacteria strain. Gram-negative bacteria have a higher resistance to being penetrated due to their double-layer cell membrane compared to the single membrane of Gram-positive bacteria [
5,
46]. In addition, the antibacterial properties of phenolic compounds such as GSE, with a chemical structure similar to PE, have been previously investigated using various bacteria strains. GSEs have shown suitable antibacterial properties against
A. actinomycetemcomitans,
S. mutans, and
E. faecalis [
47,
48]. In addition, they have exhibited inhibitory effects on
E. coli and
S. aureus [
49]. The GSE antibacterial effects have been attributed by some authors to the general mechanism of polyphenols acting on the bacterial cell membrane [
48]. However, other authors have associated it with the presence of gallic acid that had shown inhibitory effects on
E. coli and
S. enteritidis [
50]. Based on these results, future investigations by using higher PE concentrations should be performed to verify the antibacterial activity of PE-loaded PCL/GEL electrospun nanofibers.
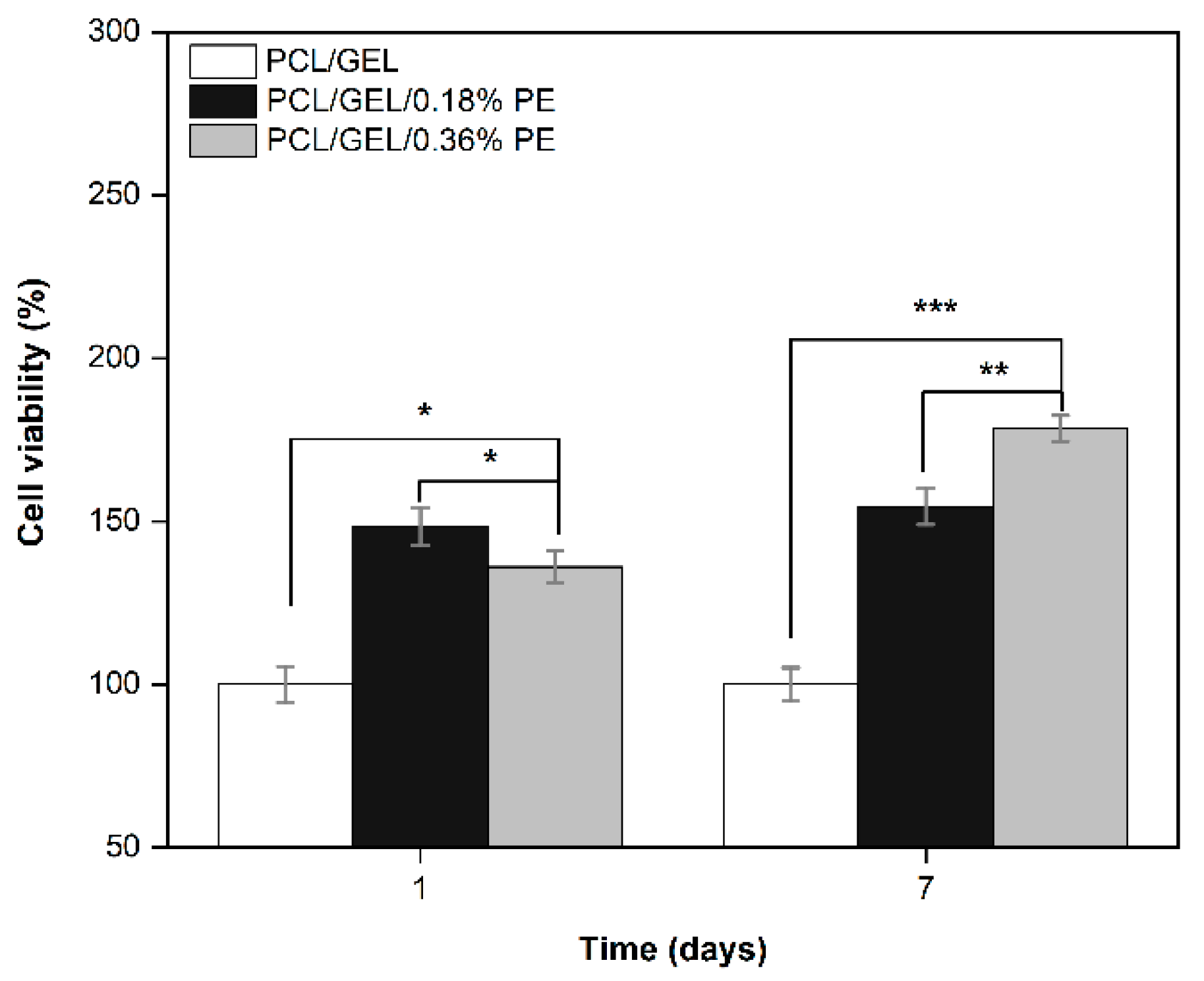
Keratinocyte cell lines from adult human skin (HaCaT cells) have been extensively used in scientific research as a reproducible model to characterize skin keratinocytes in vitro due to their high capacity to differentiate and proliferate in vitro [
51,
52,
53]. Therefore, HaCaT cells were used in this research as an in vitro model representative for human skin to evaluate the biocompatibility of the PE-loaded electrospun nanofibers. In this regard, an in vitro cell viability assay was performed by directly seeding the cells on the electrospun nanofibers to investigate cell adhesion, migration, and proliferation behavior for an incubation period of up to 7 days. HaCaT cells exhibited increased viability on electrospun nanofibers containing PE from the first day, which is correlated with the release profile of PE (see
Figure 4). Additionally, the viability of PCL/GEL/0.36%PE nanofibers significantly increased after seven days of incubation compared to day 1, indicating HaCaT cell proliferation. On the other hand, our experiments have shown that both samples were non-toxic for wound healing applications, and cell proliferation was induced by PCL/GEL/0.36%PE electrospun nanofibers after 7-day incubation. These results agree with the PE release profiles showing that PCL/GEL/0.36%PE nanofibers released more PE than PCL/GEL/0.18%PE nanofibers after incubation of up to 7 days, which could explain the higher cell viability in the PCL/GEL/0.36%PE electrospun nanofiber. In addition to cell viability investigation, the material’s biocompatibility is related to the cell adhesion ability on the material surface. HaCaT cells were well spread throughout the matrix of all electrospun nanofiber mats and attached to their surfaces, which is consistent with the wettability results because materials with high hydrophilicity provide better cell growth, attachment, and proliferation environments. All these findings can be explained, firstly, by the presence of GEL in the electrospun nanofibers, which contain the major components of the extracellular matrix that promote cell attachment and proliferation [
54]. Secondly, the PE incorporated into electrospun nanofibers improved the in vitro biocompatibility of HaCaT cells, which might be ascribed both to the high polyphenol concentration and to the presence of PAs in the chemical composition of PE, as reported in previous studies [
15,
55,
56]. In a related study, Locilento et al. [
15] demonstrated an enhancement in the activity of human foreskin fibroblast (HFF1) cells for GSE-loaded PLA/PEO nanofibrous membranes compared to a pristine PLA nanofiber. Their result was ascribed to the presence of PAs in the chemical composition of GSE, which is also present in PE’s chemical composition. In addition, the authors demonstrated that HFF1 cells were able to attach and grow on the nanofibrous membranes containing GSE [
15]. Herein, in vitro cell investigation demonstrated that PE-loaded PCL/GEL electrospun nanofibers improved cell biocompatibility, which suggests their promising potential for wound healing applications.
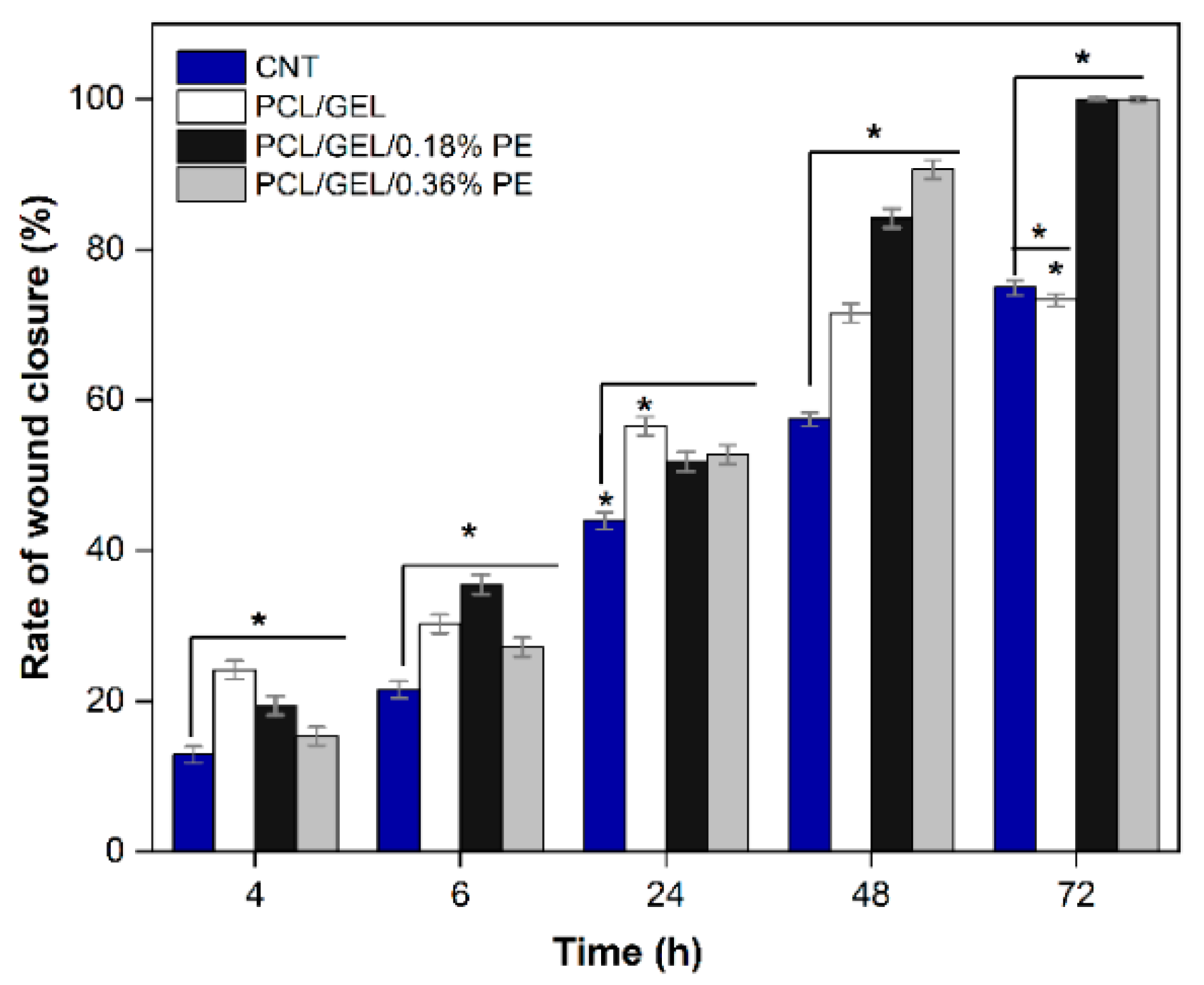
Wound healing is a complex process involving an initial inflammatory phase, a proliferative/repair phase, and a remodeling phase [
1]. In our study, the in vitro wound healing assay results showed that PE has a potential effect on wound healing considering that PE-loaded electrospun nanofibers favored the HaCaT cells’ migration to the scratch area after 72 h treatment, thus allowing the complete wound closure. These findings agree with the HaCaT cell viability results, demonstrating that the PE-loaded PCL/GEL electrospun nanofibers lead to improved cell biocompatibility. In the literature, studies conducted on evaluating the cell migration effect in natural compounds are limited. Among them, Schuhladen et al. [
11] demonstrated that adding Manuka honey and borate bioactive glass into PCL nanofibers improves HaCaT cell migration compared to neat PCL fibers. Similarly, Unalan et al. [
5] demonstrated that normal human dermal fibroblast (NHDF) cell migration and proliferation were reduced by increasing CLV concentration in PCL/GEL fiber mats, although no adverse effects on cell viability were observed. According to the authors’ knowledge, this is the first study to evaluate the HaCaT cell migration cultured in PE-loaded PCL/GEL electrospun nanofibers to determine the wound closure rate. Given the positive results obtained, further studies should be conducted to assess the biological activity of these electrospun nanofibers using higher PE concentrations to verify this effect, also for longer periods of time.
The combination of natural compounds with engineered biomaterials has emerged as a promising approach in the biomedical field [
57]. The complex chemical structures of such compounds, including their different main compounds and functional groups, directly influence their physicochemical and biological performance. In the present study, the formation of nanofibrous structures with outstanding cell biocompatibility and remarkable potential for usage in wound healing applications was highlighted.