Lignocellulose Extraction from Sisal Fiber and Its Use in Green Emulsions: A Novel Method
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
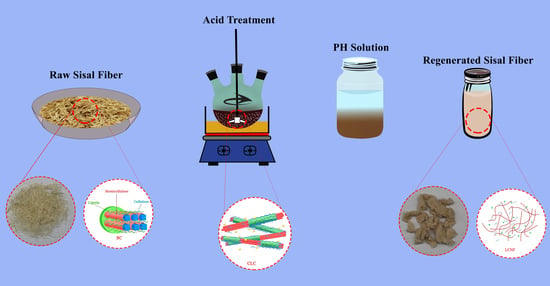
2.2. Preparation of RLCs
2.3. Preparation Pickering Emulsions
2.4. Characterization
3. Results and Discussion
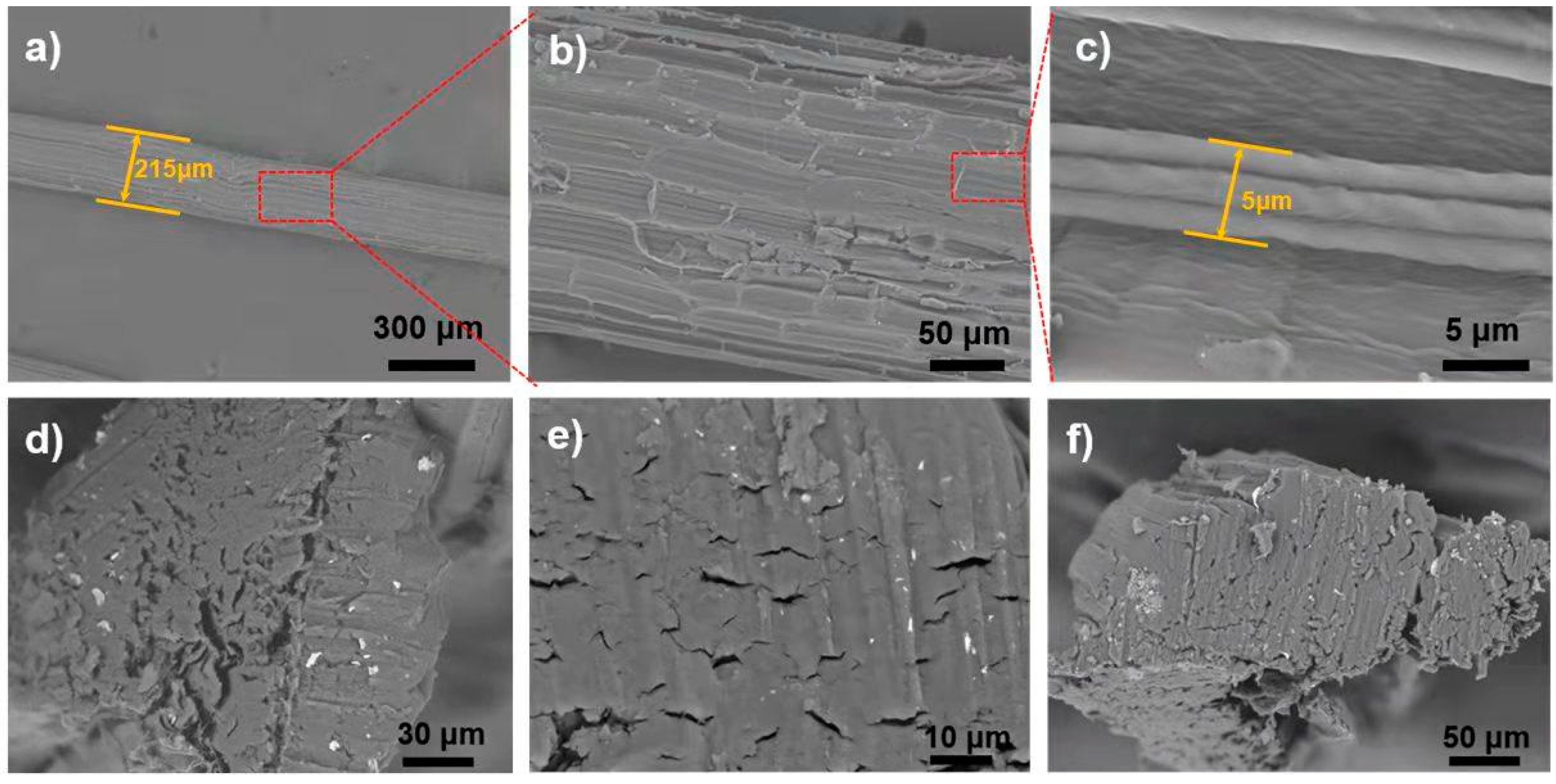
3.1. Morphology of Raw Sisal Fibers
3.2. Optical Microscopy of Raw Sisal Fiber Dissolution
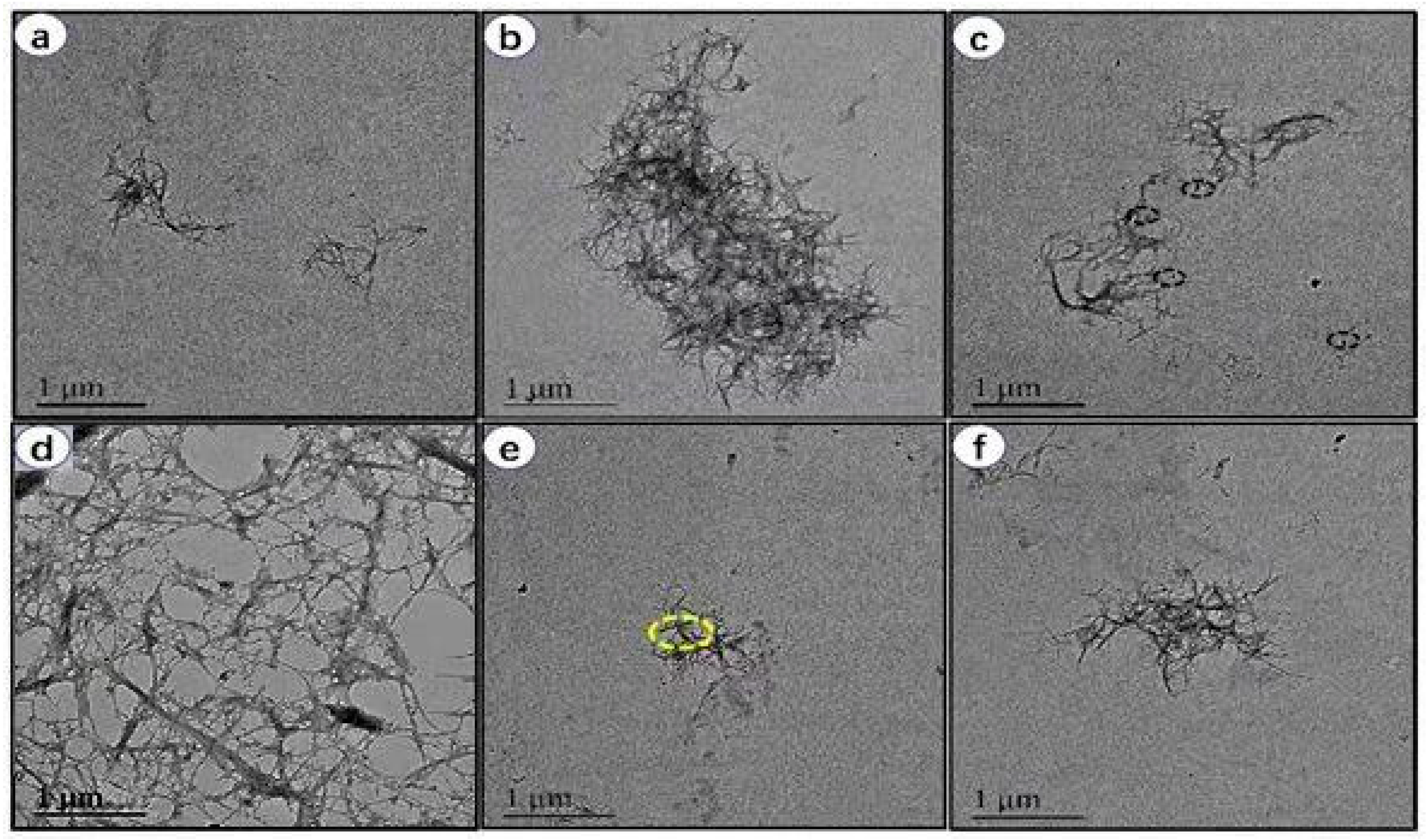
3.3. Morphology of RLCs
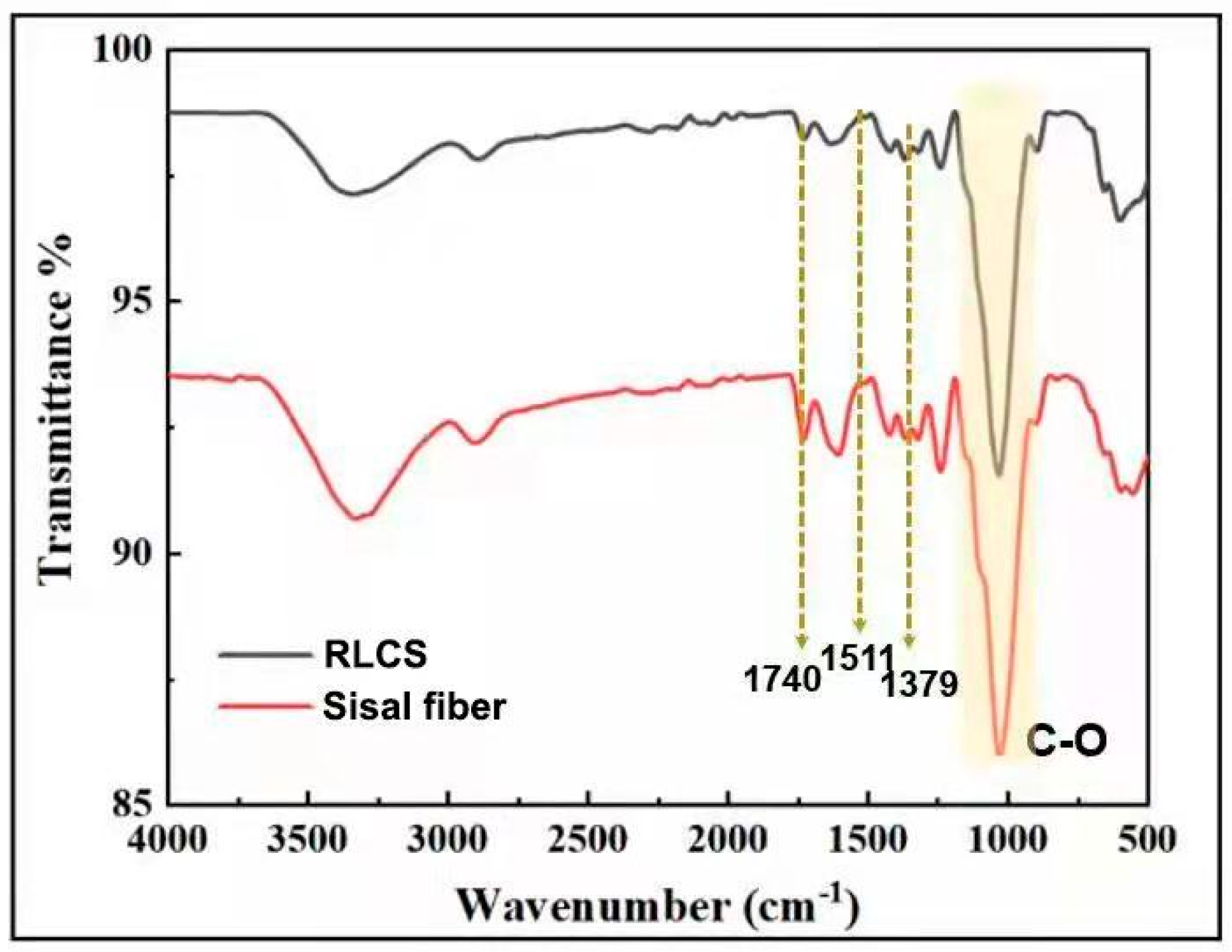
3.4. FT-IR Analysis
3.5. XRD
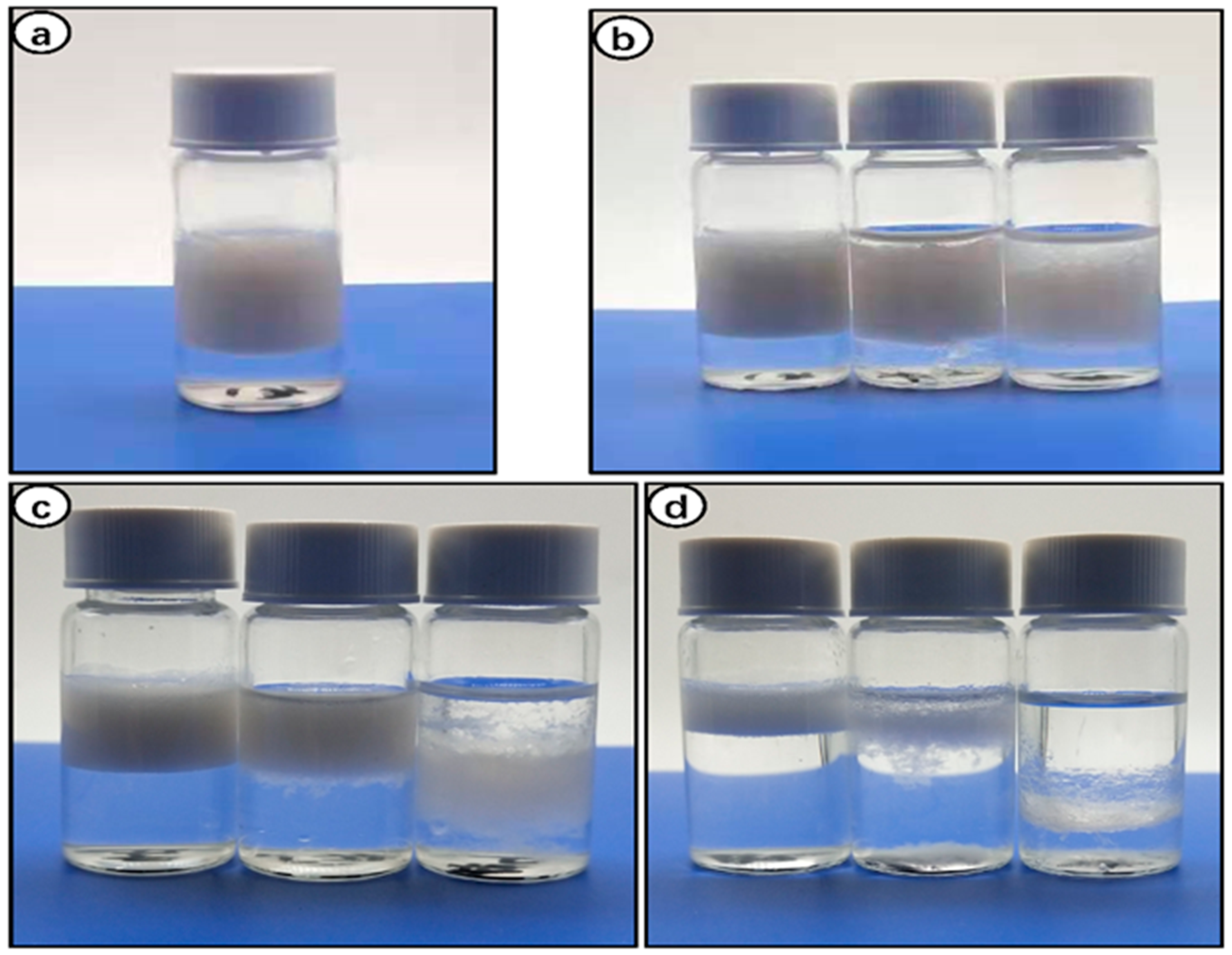
3.6. Stabilization of Pickering Emulsion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Wang, W.; Martin, J.C.; Oliphant, A.J.; Doerr, P.A.; Xu, J.F.; DeBorn, K.M.; Chen, C.; Sun, L. Extraction of Lignocellulose and Synthesis of Porous Silica Nanoparticles from Rice Husks: A Comprehensive Utilization of Rice Husk Biomass. ACS Sustain. Chem. Eng. 2012, 1, 254–259. [Google Scholar]
- de Aguiar, J.; Bondancia, T.J.; Claro, P.I.; Mattoso, L.H.; Farinas, C.S.; Marconcini, J.M. Enzymatic deconstruction of sugarcane bagasse and straw to obtain cellulose nanomaterials. ACS Sustain. Chem. Eng. 2020, 8, 2287–2299. [Google Scholar]
- Chen, Y.; Zhang, H.; Feng, X.; Ma, L.; Zhang, Y.; Dai, H. Lignocellulose nanocrystals from pineapple peel: Preparation, characterization and application as efficient Pickering emulsion stabilizers. Food Res. Int. 2021, 150, 110738. [Google Scholar] [PubMed]
- Li, X.; Li, J.; Kuang, Y.; Guo, S.; Mo, L.; Ni, Y. Stabilization of Pickering emulsions with cellulose nanofibers derived from oil palm fruit bunch. Cellulose 2019, 27, 839–851. [Google Scholar]
- Lu, H.; Zhang, L.; Liu, C.; He, Z.; Zhou, X.; Ni, Y. A novel method to prepare lignocellulose nanofibrils directly from bamboo chips. Cellulose 2018, 25, 7043–7051. [Google Scholar]
- Fan, F.; Zhu, M.; Fang, K.; Cao, E.; Yang, Y.; Xie, J.; Deng, Z.; Chen, Y.; Cao, X. Extraction and characterization of cellulose nanowhiskers from TEMPO oxidized sisal fibers. Cellulose 2021, 29, 213–222. [Google Scholar]
- Queiroz, B.G.; Ciol, H.; Inada, N.M.; Frollini, E. Hydrogel from all in all lignocellulosic sisal fibers macromolecular components. Int. J. Biol. Macromol. 2021, 181, 978–989. [Google Scholar]
- Veerasimman, A.; Shanmugam, V.; Rajendran, S.; Johnson, D.J.; Subbiah, A.; Koilpichai, J.; Marimuthu, U. Thermal Properties of Natural Fiber Sisal Based Hybrid Composites–A Brief Review. J. Nat. Fibers 2021, 1–11. [Google Scholar] [CrossRef]
- Guambo, M.P.R.; Spencer, L.; Vispo, N.S.; Vizuete, K.; Debut, A.; Whitehead, D.C.; Santos-Oliveira, R.; Alexis, F. Cellulose Fibers for Surgical Suture Applications. Polymers 2020, 12, 3042. [Google Scholar]
- Fednand, C.; Bigambo, P.; Mgani, Q. Modification of the Mechanical and Structural Properties of Sisal Fiber for Textile Applications. J. Nat. Fibers 2021, 1–12. [Google Scholar] [CrossRef]
- Liu, H.; Geng, S.; Hu, P.; Qin, Q.; Wei, C.; Lv, J. Study of Pickering emulsion stabilized by sulfonated cellulose nanowhiskers extracted from sisal fiber. Colloid Polym. Sci. 2014, 293, 963–974. [Google Scholar]
- Wang, H.; Innocent, M.T.; Memon, H.; Jin, X.; Zhu, F. Preparation and characterization of cellulose films from ficus natalensis bark cloth fibers. Wood Fiber Sci. 2021, 53, 62–68. [Google Scholar]
- Thomas, B.; Raj, M.C.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [PubMed]
- Nair, S.S.; Yan, N. Effect of high residual lignin on the thermal stability of nanofibrils and its enhanced mechanical performance in aqueous environments. Cellulose 2015, 22, 3137–3150. [Google Scholar]
- Chen, Y.; Fan, D.; Han, Y.; Lyu, S.; Lu, Y.; Li, G.; Jiang, F.; Wang, S. Effect of high residual lignin on the properties of cellulose nanofibrils/films. Cellulose 2018, 25, 6421–6431. [Google Scholar]
- Herrera, M.; Thitiwutthisakul, K.; Yang, X.; Rujitanaroj, P.O.; Rojas, R.; Berglund, L. Preparation and evaluation of high-lignin content cellulose nanofibrils from eucalyptus pulp. Cellulose 2018, 25, 3121–3133. [Google Scholar]
- Liu, K.; Du, H.; Zheng, T.; Liu, W.; Zhang, M.; Liu, H.; Zhang, X.; Si, C. Lignin-containing cellulose nanomaterials: Preparation and applications. Green Chem. 2021, 23, 9723–9746. [Google Scholar]
- Solala, I.; Iglesias, M.C.; Peresin, M.S. On the potential of lignin-containing cellulose nanofibrils (LCNFs): A review on properties and applications. Cellulose 2020, 27, 1853–1877. [Google Scholar]
- Agarwal, U.P.; Ralph, S.A.; Reiner, R.S.; Hunt, C.G.; Baez, C.; Ibach, R.; Hirth, K.C. Production of high lignin-containing and lignin-free cellulose nanocrystals from wood. Cellulose 2018, 25, 5791–5805. [Google Scholar]
- Zheng, Q.; Zhou, T.; Wang, Y.; Cao, X.; Wu, S.; Zhao, M.; Wang, H.; Xu, M.; Zheng, B.; Zheng, J.; et al. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018, 8, 1321. [Google Scholar]
- Wen, Y.; Yuan, Z.; Liu, X.; Qu, J.; Yang, S.; Wang, A.; Wang, C.; Wei, B.; Xu, J.; Ni, Y. Preparation and characterization of lignin-containing cellulose nanofibril from poplar high-yield pulp via TEMPO-mediated oxidation and homogenization. ACS Sustain. Chem. Eng. 2019, 7, 6131–6139. [Google Scholar]
- Zhang, N.; Tao, P.; Lu, Y.; Nie, S. Effect of lignin on the thermal stability of cellulose nanofibrils produced from bagasse pulp. Cellulose 2019, 26, 7823–7835. [Google Scholar]
- Jordan, J.H.; Easson, M.W.; Thompson, S.; Wu, Q.; Condon, B.D. Lignin-containing cellulose nanofibers with gradient lignin content obtained from cotton gin motes and cotton gin trash. Cellulose 2021, 28, 757–773. [Google Scholar]
- Jia, X.; Chen, Y.; Shi, C.; Ye, Y.; Wang, P.; Zeng, X.; Wu, T. Preparation and characterization of cellulose regenerated from phosphoric acid. J. Agric. Food Chem. 2013, 61, 12405–12414. [Google Scholar] [PubMed]
- Bilatto, S.; Marconcini, J.M.; Mattoso, L.H.; Farinas, C.S. Lignocellulose nanocrystals from sugarcane straw. Ind. Crops Prod. 2020, 157, 112938. [Google Scholar]
- Mikkonen, K.S. Strategies for structuring diverse emulsion systems by using wood lignocellulose-derived stabilizers. Green Chem. 2020, 22, 1019–1037. [Google Scholar]
- Cirelli, A.F.; Ojeda, C.; Castro, M.J.; Salgot, M. Surfactants in sludge-amended agricultural soils: A review. Org. Farming Pest Control. Remediat. Soil Pollut. 2009, 1, 227–251. [Google Scholar]
- Ramsden, W. Separation of solids in the surface-layers of solutions and ‘suspensions’ (observations on surface-membranes, bubbles, emulsions, and mechanical coagulation).—Preliminary account. Proc. R. Soc. Lond. 1904, 72, 156–164. [Google Scholar]
- Pickering, S.U. Cxcvi.—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef] [Green Version]
- Bao, C.; Chen, X.; Liu, C.; Liao, Y.; Huang, Y.; Hao, L.; Yan, H.; Lin, Q. Extraction of cellulose nanocrystals from microcrystalline cellulose for the stabilization of cetyltrimethylammonium bromide-enhanced Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125442. [Google Scholar]
- Xiao, J.; Li, Y.; Huang, Q. Recent advances on food-grade particles stabilized Pickering emulsions: Fabrication, characterization and research trends. Trends Food Sci. Technol. 2016, 55, 48–60. [Google Scholar]
- Ewulonu, C.M.; Liu, X.; Wu, M.; Yong, H. Lignin-Containing Cellulose Nanomaterials: A Promising New Nanomaterial for Numerous Applications. J. Bioresour. Bioprod. 2019, 4, 3–10. [Google Scholar]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2007, 15, 149–159. [Google Scholar]
- Trifol, J.; Sillard, C.; Plackett, D.; Szabo, P.; Bras, J.; Daugaard, A.E. Chemically extracted nanocellulose from sisal fibres by a simple and industrially relevant process. Cellulose 2017, 24, 107–118. [Google Scholar]
- Camarero Espinosa, S.; Kuhnt, T.; Foster, E.J.; Weder, C. Isolation of thermally stable cellulose nanocrystals by phosphoric acid hydrolysis. Biomacromolecules 2013, 14, 1223–1230. [Google Scholar]
- Guo, S.; Li, X.; Kuang, Y.; Liao, J.; Liu, K.; Li, J.; Mo, L.; He, S.; Zhu, W.; Song, J.; et al. Residual lignin in cellulose nanofibrils enhances the interfacial stabilization of Pickering emulsions. Carbohydr. Polym. 2021, 253, 117223. [Google Scholar]
- Sain, M.; Panthapulakkal, S. Bioprocess preparation of wheat straw fibers and their characterization. Ind. Crops Prod. 2006, 23, 1–8. [Google Scholar]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar]
- Dai, H.; Wu, J.; Zhang, H.; Chen, Y.; Ma, L.; Huang, H.; Huang, Y.; Zhang, Y. Recent advances on cellulose nanocrystals for Pickering emulsions: Development and challenge. Trends Food Sci. Technol. 2020, 102, 16–29. [Google Scholar]
- Jiménez Saelices, C.; Capron, I. Design of Pickering micro-and nanoemulsions based on the structural characteristics of nanocelluloses. Biomacromolecules 2018, 19, 460–469. [Google Scholar] [PubMed]
- Bai, L.; Lv, S.; Xiang, W.; Huan, S.; McClements, D.J.; Rojas, O.J. Oil-in-water Pickering emulsions via microfluidization with cellulose nanocrystals: 1. Formation and stability. Food Hydrocoll. 2019, 96, 699–708. [Google Scholar]
- Zou, Y.; van Baalen, C.; Yang, X.; Scholten, E. Tuning hydrophobicity of zein nanoparticles to control rheological behavior of Pickering emulsions. Food Hydrocoll. 2018, 80, 130–140. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirah, S.; Wang, X.; Javed, M.; Simair, K.; Wang, B.; Sui, X.; Lu, C. Lignocellulose Extraction from Sisal Fiber and Its Use in Green Emulsions: A Novel Method. Polymers 2022, 14, 2299. https://doi.org/10.3390/polym14112299
Pirah S, Wang X, Javed M, Simair K, Wang B, Sui X, Lu C. Lignocellulose Extraction from Sisal Fiber and Its Use in Green Emulsions: A Novel Method. Polymers. 2022; 14(11):2299. https://doi.org/10.3390/polym14112299
Chicago/Turabian StylePirah, Sippi, Xiaodong Wang, Muhammad Javed, Keenjhar Simair, Bijia Wang, Xiaofeng Sui, and Changrui Lu. 2022. "Lignocellulose Extraction from Sisal Fiber and Its Use in Green Emulsions: A Novel Method" Polymers 14, no. 11: 2299. https://doi.org/10.3390/polym14112299
APA StylePirah, S., Wang, X., Javed, M., Simair, K., Wang, B., Sui, X., & Lu, C. (2022). Lignocellulose Extraction from Sisal Fiber and Its Use in Green Emulsions: A Novel Method. Polymers, 14(11), 2299. https://doi.org/10.3390/polym14112299