Antimicrobial Activity of Various Disinfectants to Clean Thermoplastic Polymeric Appliances in Orthodontics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Search Strategy
2.3. Eligibility Criteria
- P: Population/Problem: Fabricated clear thermoplastic aligners or retainers
- I: Intervention: Cleaning and method of evaluation
- C: Controls: Negative control
- O: Outcomes: Amount of bacterial reduction
- S: Study designs: RCTs, Clinical Control Trials (CCTs), retrospective controlled cohort studies, retrospective uncontrolled cohort studies, case reports, and laboratory studies.
2.4. Risk of Bias in Individual Studies
3. Results
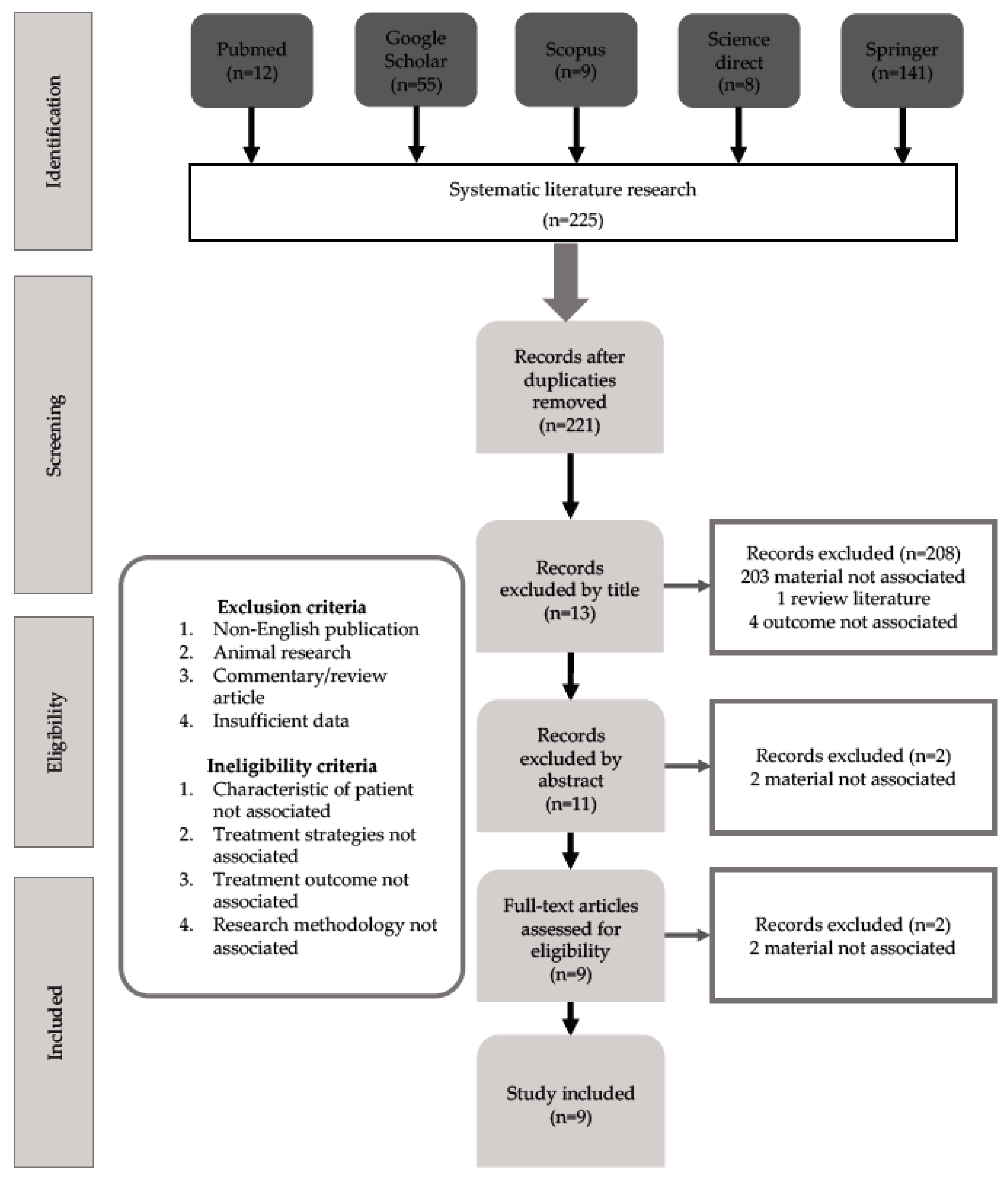
3.1. Study Selection and Identification
3.2. Study Characteristics
3.3. Thermoplastic Orthodontic Material
3.4. Chemical Disinfectant
3.5. Disinfection Protocol
3.6. Microorganism Reduction Evaluation
3.7. Effectiveness of Disinfectant
4. Discussion
4.1. Transparent Orthodontic Appliance Material
4.2. Changes in Physical Properties
4.3. Chemical Disinfectant
4.4. Microbial Reduction Evaluation
4.5. Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rokaya, D.; Kitisubkanchana, J.; Wonglamsam, A.; Santiwong, P.; Srithavaj, T.; Humagain, M. Nepalese esthetic dental (ned) proportion in nepalese population. Kathmandu Univ. Med. J. 2015, 13, 244–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirani, S.; Patel, U.; Patel, N. Invisible orthodontics—A review. IOSR J. Dent. Med. Sci. 2016, 15, 56–62. [Google Scholar]
- Harradine, N.W. Self-ligating brackets: Where are we now? J. Orthod. 2003, 30, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Lagravere, M.O.; Flores-Mir, C. The treatment effects of Invisalign orthodontic aligners: A systematic review. J. Am. Dent. Assoc. 2005, 136, 1724–1729. [Google Scholar] [CrossRef] [Green Version]
- Kalra, S.; Gupta, P.; Tripathi, T.; Rai, P. External apical root resorption in orthodontic patients: Molecular and genetic basis. J. Fam. Med. Prim. Care 2020, 9, 3872–3882. [Google Scholar] [CrossRef]
- Mai, W.; Meng, H.; Jiang, Y.; Huang, C.; Li, M.; Yuan, K.; Kang, N. Comparison of vacuum-formed and Hawley retainers: A systematic review. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 720–727. [Google Scholar] [CrossRef]
- Singh, P.; Grammati, S.; Kirschen, R. Orthodontic retention patterns in the United Kingdom. J. Orthod. 2009, 36, 115–121. [Google Scholar] [CrossRef]
- Hichens, L.; Rowland, H.; Williams, A.; Hollinghurst, S.; Ewings, P.; Clark, S.; Ireland, A.; Sandy, J. Cost-effectiveness and patient satisfaction: Hawley and vacuum-formed retainers. Eur. J. Orthod. 2007, 29, 372–378. [Google Scholar] [CrossRef]
- Littlewood, S.J.; Millett, D.T.; Doubleday, B.; Bearn, D.R.; Worthington, H.V. Orthodontic retention: A systematic review. J. Orthod. 2006, 33, 205–212. [Google Scholar] [CrossRef]
- Melrose, C.; Millett, D.T. Toward a perspective on orthodontic retention? Am. J. Orthod. Dentofac. Orthop. 1998, 113, 507–514. [Google Scholar] [CrossRef]
- Türköz, Ç.; Bavbek, N.C.; Varlik, S.K.; Akça, G. Influence of thermoplastic retainers on Streptococcus mutans and Lactobacillus adhesion. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Alshatti, H. Comparison of White Spot Lesions among Clear Aligners, Self-Ligating Brackets and Conventional Brackets—A Randomized Controlled Clinical Trial; University of Connecticut: Storrs, CT, USA, 2017. [Google Scholar]
- Moshiri, M.; Eckhart, J.E.; Mcshane, P.; German, D.S. Consequences of poor oral hygiene during clear aligner therapy. J. Clin. Orthod. 2013, 8, 494–498. [Google Scholar]
- Apratim, A.; Shah, S.S.; Sinha, M.; Agrawal, M.; Chhaparia, N.; Abubakkar, A. Denture hygiene habits among elderly patients wearing complete dentures. J. Contemp. Dent. 2013, 14, 1161. [Google Scholar]
- Baran, I.; Nalçacı, R. Self-reported denture hygiene habits and oral tissue conditions of complete denture wearers. Arch. Gerontol. Geriatr. 2009, 49, 237–241. [Google Scholar] [CrossRef]
- Barreiro, D.M.; Scheid, P.A.; May, L.G.; Unfer, B.; Braun, K.O. Evaluation of procedures employed for the maintenance of removable dentures in elderly individuals. Oral Health Prev. Dent. 2009, 7, 243–249. [Google Scholar]
- Axe, A.S.; Varghese, R.; Bosma, M.; Kitson, N.; Bradshaw, D.J. Dental health professional recommendation and consumer habits in denture cleansing. J. Prosthet. Dent. 2016, 115, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Freitas, L.B.; Vassilakos, N.; Arnebrant, T. Interactions of chlorhexidine with salivary films adsorbed at solid/liquid and air/liquid interfaces. J. Periodontal Res. 1993, 28, 92–97. [Google Scholar] [CrossRef]
- Wible, E.; Agarwal, M.; Altun, S.; Ramir, T.; Viana, G.; Evans, C.; Lukic, H.; Megremis, S.; Atsawasuwan, P. Long-term effects of various cleaning methods on polypropylene/ethylene copolymer retainer material. Angle Orthod. 2019, 89, 432–437. [Google Scholar] [CrossRef] [Green Version]
- McGowan, M.J.; Shimoda, L.M.; Woolsey, G.D. Effects of sodium hypochlorite on denture base metals during immersion for short-term sterilization. J. Prosthet. Dent. 1988, 60, 212–218. [Google Scholar] [CrossRef]
- Sadaghiani, L.; Wilson, M.A.; Wilson, N.H. Effect of selected mouthwashes on the surface roughness of resin modified glass-ionomer restorative materials. Dent. Mater. 2007, 23, 325–334. [Google Scholar] [CrossRef]
- de Souza, R.F.; de Freitas Oliveira Paranhos, H.; da Silva, C.H.L.; Abu-Naba’a, L.; Fedorowicz, Z.; Gurgan, C.A. Interventions for cleaning dentures in adults. Cochrane Database Syst. Rev. 2009, Cd007395. [Google Scholar] [CrossRef] [PubMed]
- Charavet, C.; Gourdain, Z.; Graveline, L.; Lupi, L. Cleaning and disinfection protocols for clear orthodontic aligners: A systematic review. Healthcare 2022, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.; Korber, D.; Hoyle, B.; Costerton, J.W.; Caldwell, D. Optical sectioning of microbial biofilms. J. Bacteriol. 1991, 173, 6558–6567. [Google Scholar] [CrossRef] [Green Version]
- Barcot, O.; Boric, M.; Poklepovic Pericic, T.; Cavar, M.; Dosenovic, S.; Vuka, I.; Puljak, L. Risk of bias judgments for random sequence generation in Cochrane systematic reviews were frequently not in line with Cochrane Handbook. BMC Med. Res. Methodol. 2019, 19, 170. [Google Scholar] [CrossRef] [Green Version]
- Albanna, R.H.; Farawanah, H.M.; Aldrees, A.M. Microbial evaluation of the effectiveness of different methods for cleansing clear orthodontic retainers: A randomized clinical trial. Angle Orthod. 2017, 87, 460–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akgün, F.A.; Şenışık, N.E.; Çetin, E.S. Evaluation of the efficacy of different cleaning methods for orthodontic thermoplastic retainers in terms of bacterial colonization. Turk. J. Orthod. 2019, 32, 219. [Google Scholar] [CrossRef]
- Shpack, N.; Greenstein, R.B.-N.; Gazit, D.; Sarig, R.; Vardimon, A.D. Efficacy of three hygienic protocols in reducing biofilm adherence to removable thermoplastic appliance. Angle Orthod. 2014, 84, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Levrini, L.; Novara, F.; Margherini, S.; Tenconi, C.; Raspanti, M. Scanning electron microscopy analysis of the growth of dental plaque on the surfaces of removable orthodontic aligners after the use of different cleaning methods. Clin. Cosmet. Investig. Dent. 2015, 7, 125. [Google Scholar]
- Meto, A.; Colombari, B.; Castagnoli, A.; Sarti, M.; Denti, L.; Blasi, E. Efficacy of a copper–calcium–hydroxide solution in reducing microbial plaque on orthodontic clear aligners: A case report. Eur. J. Dent. 2019, 13, 478–484. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.S.; Al-Awadi, S.; Ready, D.; Noar, J. An assessment of the effectiveness of mechanical and chemical cleaning of Essix orthodontic retainer. J. Orthod. 2014, 41, 110–117. [Google Scholar] [CrossRef]
- Levrini, L.; Mangano, A.; Margherini, S.; Tenconi, C.; Vigetti, D.; Muollo, R.; Marco Abbate, G. ATP bioluminometers analysis on the surfaces of removable orthodontic aligners after the use of different cleaning methods. Int. J. Dent. 2016, 2016, 5926941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilloni, J.A. Human Saliva Biofilm Reduction by Thermoplastic Retainer Cleaners: An In Vitro Study; State University of New York at Stony Brook: New York, NY, USA, 2019. [Google Scholar]
- Ismah, N.; Purwanegara, M.K.; Rahmawati, T. Efficacy of disinfectants against gram-positive bacteria found on thermoplastic retainers. Int. J. Appl. Pharm. 2019, 11, 130–133. [Google Scholar] [CrossRef]
- Low, B.; Lee, W.; Seneviratne, C.J.; Samaranayake, L.P.; Hägg, U. Ultrastructure and morphology of biofilms on thermoplastic orthodontic appliances in ‘fast’ and ‘slow’ plaque formers. Eur. J. Orthod. 2011, 33, 577–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.-L.; Sun, W.-T.; Liao, W.; Lu, W.-X.; Li, Q.-W.; Jeong, Y.; Liu, J.; Zhao, Z.-H. Colour stabilities of three types of orthodontic clear aligners exposed to staining agents. Int. J. Oral Sci. 2016, 8, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Bakdach, W.M.M.; Haiba, M.; Hadad, R. Changes in surface morphology, chemical and mechanical properties of clear aligners during intraoral usage: A systematic review and meta-analysis. Int. Orthod. 2022, 20, 100610. [Google Scholar] [CrossRef]
- Kiesow, A.; Sarembe, S.; Pizzey, R.L.; Axe, A.S.; Bradshaw, D.J. Material compatibility and antimicrobial activity of consumer products commonly used to clean dentures. J. Prosthet. Dent. 2016, 115, 189–198.e188. [Google Scholar] [CrossRef] [Green Version]
- Walter, A.; Gutknecht, J. Monocarboxylic acid permeation through lipid bilayer membranes. J. Membr. Biol. 1984, 77, 255–264. [Google Scholar] [CrossRef]
- Slonczewski, J.L.; Rosen, B.P.; Alger, J.R.; Macnab, R.M. pH homeostasis in Escherichia coli: Measurement by 31p nuclear magnetic resonance of methylphosphonate and phosphate. Proc. Natl. Acad. Sci. USA 1981, 78, 6271–6275. [Google Scholar] [CrossRef] [Green Version]
- Slonczewski, J.L.; Fujisawa, M.; Dopson, M.; Krulwich, T.A. Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv. Microb. Physiol. 2009, 55, 1–79, 317. [Google Scholar]
- Roe, A.J.; McLaggan, D.; Davidson, I.; O’Byrne, C.; Booth, I.R. Perturbation of anion balance during inhibition of growth of Escherichia coli by weak acids. J. Bacteriol. 1998, 180, 767–772. [Google Scholar] [CrossRef] [Green Version]
- Hirshfield, I.N.; Terzulli, S.; O’Byrne, C. Weak organic acids: A panoply of effects on bacteria. Sci. Prog. 2003, 86, 245–270. [Google Scholar] [CrossRef] [PubMed]
- Salmond, C.V.; Kroll, R.G.; Booth, I.R. The effect of food preservatives on pH homeostasis in Escherichia coli. Microbiology 1984, 130, 2845–2850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, H.J.; Greener, E.H. Characterization of some denture cleansers. J. Prosthet. Dent. 1980, 43, 491–496. [Google Scholar] [CrossRef]
- Manoil, D.; Filieri, A.; Gameiro, C.; Lange, N.; Schrenzel, J.; Wataha, J.C.; Bouillaguet, S. Flow cytometric assessment of Streptococcus mutans viability after exposure to blue light-activated curcumin. Photodiagnosis Photodyn. Ther. 2014, 11, 372–379. [Google Scholar] [CrossRef]
- Pantanella, F.; Valenti, P.; Natalizi, T.; Passeri, D.; Berlutti, F. Analytical techniques to study microbial biofilm on abiotic surfaces: Pros and cons of the main techniques currently in use. Ann. Ig. 2013, 25, 31–42. [Google Scholar]
- Li, X.; Yan, Z.; Xu, J. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology 2003, 149, 353–362. [Google Scholar] [CrossRef] [Green Version]
| Database | Search Term |
|---|---|
| Pubmed | (‘‘Disinfection’’ OR ‘‘Cleaning’’ OR ‘‘Antimicroorganism’’ OR ‘‘Killing’’ OR “Decolonization”) AND (“Microbial” OR “Bacteria” OR ‘‘Microorganism’’ OR “Plaque” OR “Biofilm”) AND (“Vacuum forming” OR “Clear” OR “Invisible” OR “Thermoplastic” OR “Transparent”) AND (“Orthodontic”) AND (“Appliance” OR “Retainer” OR “Aligner”) |
| Google Scholar | (cleaning) and (retainer or aligner or clear orthodontic appliance) |
| Scopus | ALL (cleaning AND clear AND orthodontic AND appliance) |
| ScienceDirect | (cleaning) and (retainer or aligner or clear orthodontic appliance) |
| Springer | “cleaning” AND “clear” AND “orthodontic” AND “appliance” |
| Inclusion Criteria | Exclusion Criteria | Ineligibility Criteria |
|---|---|---|
|
|
|
| Effectiveness of Disinfectant | Method of Microorganism Reduction Evaluation | Microorganism | Frequency | Disinfection Time | Cleaning Protocol | Chemical Disinfectant | Appliance Material | Type of Study | Author/Year |
|---|---|---|---|---|---|---|---|---|---|
| No significant differences between cleaning tablets and mechanical cleaning only | Bacterial Quantification with AlamarBlue® Assay | Total bacteria | Once a day before bedtime | 15 min | Step 1: one minute brushing with toothbrush and water before bedtime Step 2: Soaking in dissolved cleaning tablet in 150 mL tap water Step 3: Washing with tap water | 1. Corega® (GlaxoSmithKline, Dublin, Ireland) 2. Kukis® (Procter & Gamble Technical Center Ltd., Egham, UK) 3. Retainer Brite® (DENTSPLY, Bradenton, Fla) | Essix material (Invisacryl A, 0.030-inch, round, 0.75 mm/125 mm) | Randomized clinical trial (Split mouth study) | Albanna et al. (2016) [26] |
| No significant difference between cleaning tablets and vinegar but bacteria counts were statistically lower than in the control method | Colony count by colony-forming unit per 1 ml | Streptococcus mutans (SM) Lactobacillus (LB) | Once a day in the evening | 5 min | Step 1: Keep the retainer in the cleaning solution Step 2: Brush with a soft brush and rinse with running water | 1. Corega® (GlaxoSmithKline, Brentford, Middlesex, United Kingdom) (First 2 weeks) 2. Water (control) (Next 2 weeks) 3. 5% white vinegar (Ferfresh, Fersan, Izmir, Turkey) (Last 2 weeks) | DispoDent Sert Gece Plagi, Yagmur Dental | Prospective study with a cross-over design | Akgün et al. (2019) [27] |
| Maximal reduction in biofilm accumulation was obtained when immersed in CS with a vibrating bath | Photodensitometer with 1 % gentian violet staining | Total bacteria | Once a day | 15 min | Stage 1: Brush with 1400 ppm toothpaste (two aligners) Stage 2: Brush and immerse in CHX mouthwash and rinse water (70 days) Stage 3: Immerse in vibrating bath with CS and rinse with water | 1. 0.12% chlorhexidine (CHX) mouthwash (Laser Co., Barcelona, Spain) 2. Invisalign Cleaning-Crystal solution (CS) (Align Technology, Santa Clara, CA, USA) Santa Clara, Calif | Thermoplastic material (Align Technology, Santa Clara, CA, USA) | Prospective study | Shpack et al. (2013) [28] |
| Effervescent tablets with brushing can reduce bacterial accumulation significantly | Scanning Electron microscope analysis | TotalScanningia | Once a day | Water group: 15 s Tablet group: 30 min Brushing group: 30 s | Stage 1: Rinse in running water for 15 s at least twice a day (two weeks) Stage 2: Soak in dissolved cleaning tablets, then brush with toothbrush and toothpaste at least 30 s Stage 3: Brush with toothpaste at least 30 s | 1. Cold running water (control) 2. Effervescent tablets containing sodium carbonate and sulfate (Invisalign® Cleaning System, Align Technology, San Joe, CA, USA) 3. Toothbrush and toothpaste, Fla) | Unknown | Prospective study | Levrini et al. (2015) [29] |
| No bacteria and fungal were found in the Cupral group | Colony count by colony-forming unit | Total bacteria and fungal load | 1 time | 1 h | Dissolve in saline buffer or 1.25% Cupral at room temperature | 1. Saline buffer (control) 2. 1.25% Cupral (Humanchemie GmbH, Alfeld, Germany) | Novula (Rome, Italy) | Case report | Meto et al. (2019) [30] |
| All three cleaning methods removed 99% of microorganisms from the Essix orthodontic retainers | Colony count by colony forming unit | Methicillin-resistant Staphylococcus aureus-16(MRSA-16) Streptococcus sanguinis Candida albicans Actinomyces naeslundii | 1 time | Group 1: 30 s Group 2: 30 s Group 3: 10 min Group 4: 10 min | Group 1: Brush with toothpaste Group 2: Brush with CHX Group 3: Immerse in CHX solution Group 4: Immerse in phosphate-buffered saline | 1. Colgate cavity protection fluoride toothpaste (Group 1) 2. CHX gluconade gel (Corsodyl Dental Gel) (Group 2) 3. CHX solution (Corsodyl Alcohol-Free Mint Mouthwash) (Group 3) 4.Phosphate-buffered saline (Control group) | Essix ACETM (Dentsply Sirona, Charlotte, NC, USA) | In vitro laboratory study | Chang et al. (2013) [31] |
| The use of sodium carbonate and sulfate effervescent tablets combined with the mechanical debridement resulted in being the most effective method | Bioluminometer Microbiological Analysis | Total bacteria | Once a day | Group 1: 15 s Group 2: at least 30 s Group 3: 20 min | Group 1: Rinse in cold running water before eating Group 2: brush before eating Group 3: Soak in dissolved tablets and brush with toothpaste | 1. Running water (Group 1) 2. Toothpaste (Group 2) 3. Effervescent tablets containing sodium carbonate and sulfate (Invisalign® Cleaning System, Align Technology, San Joe, CA, USA) (Group 3) | Invisalign (Align Technology, Santa Clara, CA, USA) | Prospective study | Levrini et al. (2016) [32] |
| The effective cleaning tablets can be ordered as follow; Retainer Brite®, Smart guard®, Invisalign® Cleaning Crystals, and Fresh Guard® | Optical density by spectrophotometer | Total bacteria | 1 time | Group 1: 15 min Group 2: 5 min Group 3: 15–20 min Group 4: 20 min | Soak in cleaning tablet and follow the instruction | 1. Invisalign® Cleaning Crystals (Align Technology, Santa Clara, CA, USA) (Group 1) 2. Fresh Guard® (Efferdent®) (Group 2) 3. Retainer Brite® (Densply serona) (Group 3) 4.Smart guard® Retainer and Aligner Cleaner (Smart guard®) (Group 4) | Essix A+® Plastic (Dentsply Sirona Charlotte, NC, USA) | In vitro laboratory study | Pilloni (2019) [33] |
| Immersion in cleaning tablet once a day or in CHX solution twice per week will have the same efficacy for cleaning a thermoplastic retainer | Colony count by colony forming unit per millilitre | Streptococcus mutans | Group 1 and control group: once a day Group 2: once every 4 days | Group 1 and control group: 5 min Group 2: 10 min | Soak in cleaning solution | 1. Denture disinfectant tablet solution (Polident) (Group 1) 2. 0.1% CHX mouthwash (Minosep) (Group 2) 3. Aqua Dest water (Control group) | Essix C+® Plastic (Dentsply Sirona, Charlotte, NC, USA) | Clinical and laboratory experiment | Ismah et al. (2019) [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiatwarawut, K.; Rokaya, D.; Sirisoontorn, I. Antimicrobial Activity of Various Disinfectants to Clean Thermoplastic Polymeric Appliances in Orthodontics. Polymers 2022, 14, 2256. https://doi.org/10.3390/polym14112256
Kiatwarawut K, Rokaya D, Sirisoontorn I. Antimicrobial Activity of Various Disinfectants to Clean Thermoplastic Polymeric Appliances in Orthodontics. Polymers. 2022; 14(11):2256. https://doi.org/10.3390/polym14112256
Chicago/Turabian StyleKiatwarawut, Kanket, Dinesh Rokaya, and Irin Sirisoontorn. 2022. "Antimicrobial Activity of Various Disinfectants to Clean Thermoplastic Polymeric Appliances in Orthodontics" Polymers 14, no. 11: 2256. https://doi.org/10.3390/polym14112256
APA StyleKiatwarawut, K., Rokaya, D., & Sirisoontorn, I. (2022). Antimicrobial Activity of Various Disinfectants to Clean Thermoplastic Polymeric Appliances in Orthodontics. Polymers, 14(11), 2256. https://doi.org/10.3390/polym14112256







