Meso- and Rac-[bis(3-phenyl-6-tert-butylinden-1-yl)dimethylsilyl]zirconium Dichloride: Precatalysts for the Production of Differentiated Polyethylene Products with Enhanced Properties
Abstract
:1. Introduction
2. Materials and Methods
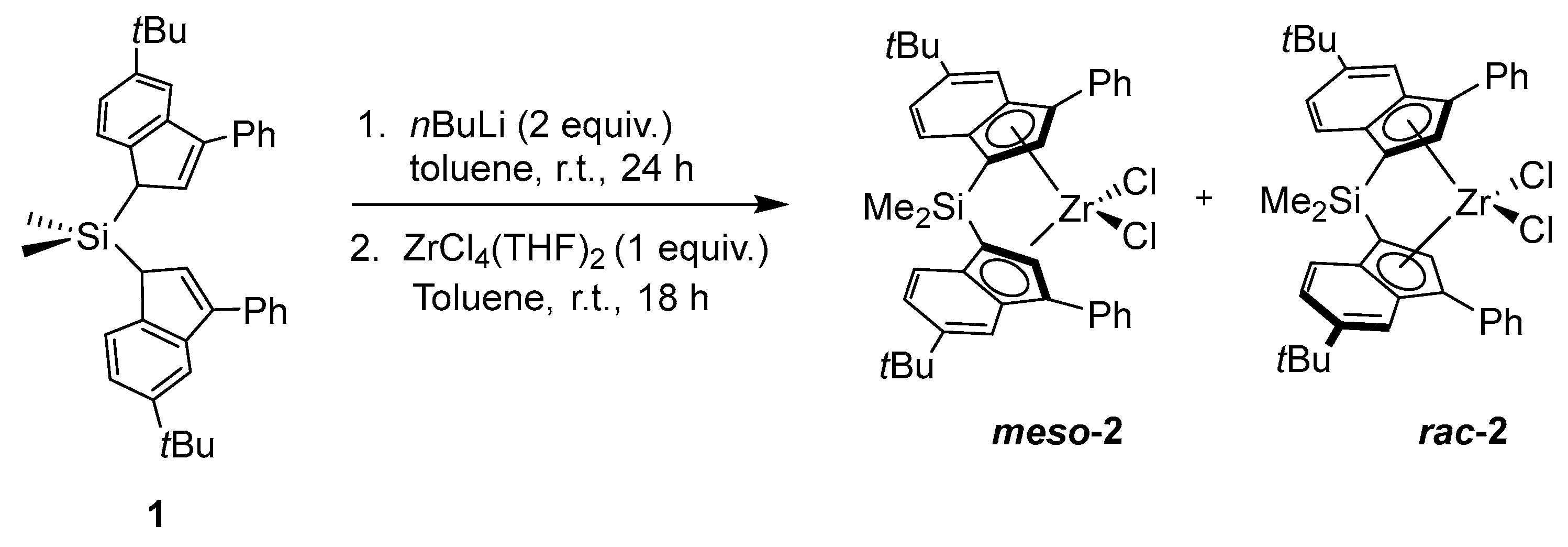
2.1. Synthesis of [Bis(3-phenyl-6-tert-butylinden-1-yl)dimethylsilyl]zirconium Dichloride (2)
2.2. Ethylene (Co)Polymerization Reactions
2.2.1. Homogeneous Conditions
2.2.2. Heterogeneous Conditions
3. Results and Discussion
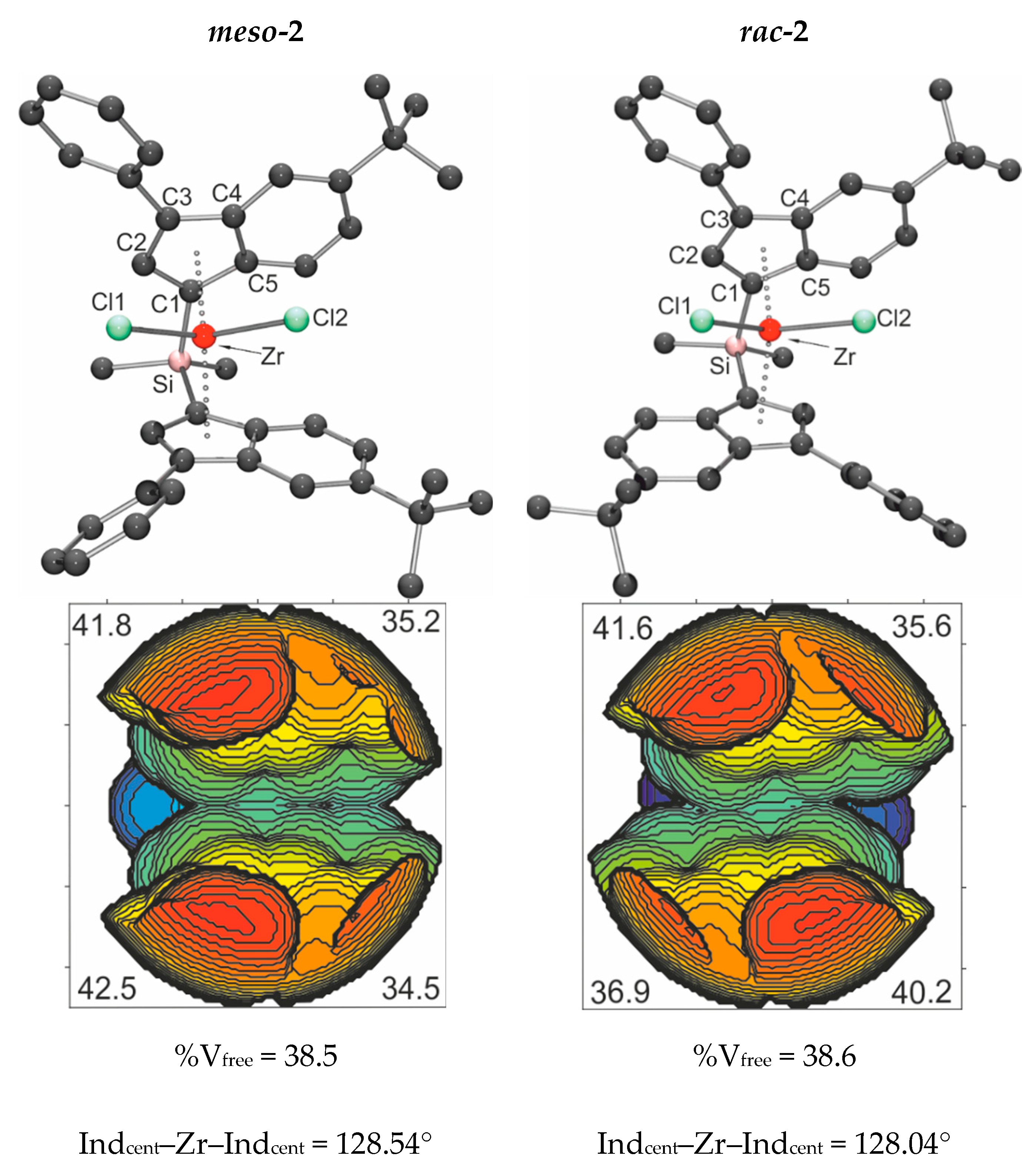
3.1. Metallocene Synthesis
3.2. Copolymerization of Ethylene and 1-Hexene Catalyzed by Rac- and Meso-2 in Homogeneous Conditions
3.3. Copolymerization of Ethylene with 1-Hexene Catalyzed by Heterogeneous Silica-Supported Rac- and Meso-2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Notes
- Tso, C.C.; DesLauriers, P.J. Comparison of methods for characterizing comonomer composition in ethylene 1-olefin copolymers: 3D-TREF vs. SEC-FTIR. Polymer 2004, 45, 2657–2663. [Google Scholar] [CrossRef]
- Li, C.; Shan, P.; Soares, J.B.P.; Penlidis, A. Mechanical properties of ethylene/1-hexene copolymers with tailored short chain branching distributions. Polymer 2002, 43, 767. [Google Scholar] [CrossRef]
- Shamiri, A.; Chakrabarti, M.H.; Jahan, S.; Hussain, M.A.; Kaminsky, W.; Aravind, P.V.; Yehye, W.A. The Influence of Ziegler-Natta and Metallocene Catalysts on Polyolefin Structure, Properties, and Processing Ability. Materials 2014, 7, 5069–5108. [Google Scholar] [CrossRef] [PubMed]
- Cicmil, D.; Meeuwissen, J.; Vantomme, A.; Wang, J.; van Ravenhorst, I.K.; van der Bij, H.E.; Muñoz-Murillo, A.; Weckhuysen, B.M. Polyethylene with Reverse Co-monomer Incorporation: From an Industrial Serendipitous Discovery to Fundamental Understanding. Angew. Chem. Int. Ed. 2015, 54, 13073–13079. [Google Scholar] [CrossRef] [Green Version]
- Böhm, L.L.; Enderle, H.F.; Fleifßner, M. High-density polyethylene pipe resins. Adv. Mater. 1992, 4, 234–238. [Google Scholar] [CrossRef]
- Scheirs, J.; Böhm, L.L.; Boot, J.C.; Leevers, P.S. PE100 Resins for Pipe Applications: Continuing the Development into the 21st Century. Trends Polym. Sci. 1996, 4, 408–415. [Google Scholar]
- Arnold, T.A.Q.; Buffet, J.-C.; Turner, Z.R.; O’Hare, D. Synthesis, characterisation, and polymerisation studies of hexamethylindenyl zirconocenes and hafnocenes. J. Organomet. Chem. 2015, 792, 55–65. [Google Scholar] [CrossRef]
- Ransom, P.; Ashley, A.E.; Brown, N.D.; Thompson, A.L.; O’Hare, D. Synthesis, Characterization, and Polymerization Studies of Ethylenebis(hexamethylindenyl) Complexes of Zirconium and Hafnium. Organometallics 2011, 30, 800–814. [Google Scholar] [CrossRef]
- Cirriez, V.; Welle, A.; Vantomme, A. Dual Catalyst Composition. Patent WO/2019/025528, 2 August 2018. [Google Scholar]
- Brintzinger, H.H.; Fischer, D.; Mülhaupt, R.; Rieger, B.; Waymouth, R.M. Stereospecific Olefin Polymerization with Chiral Metallocene Catalysts. Angew. Chem. Int. Ed. Engl. 1995, 34, 1143–1170. [Google Scholar] [CrossRef] [Green Version]
- Severn, J.R.; Chadwick, J.C.; Duchateau, R.; Friederichs, N. “Bound but Not Gagged” Immobilizing Single-Site α-Olefin Polymerization Catalysts. Chem. Rev. 2005, 105, 4073–4147. [Google Scholar] [CrossRef]
- Kaminsky, W. The discovery of metallocene catalysts and their present state of the art. J. Polym. Sci. A Polym. Chem. 2004, 42, 3911–3921. [Google Scholar] [CrossRef]
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in Propene Polymerization with Metallocene Catalysts. Chem. Rev. 2000, 100, 1253–1346. [Google Scholar] [CrossRef]
- Attempts towards rac- and meso-selective synthesis were also carried-out. See the Supplementary Materials.
- The SambVca 2.1 software was used to calculate %Vbur and generate steric maps:Falivene, L.; Cao, Z.; Petta, A.; Serra, L.; Poater, A.; Oliva, R.; Scarano, V.; Cavallo, L. Towards the online computer-aided design of catalytic pockets. Nat. Chem. 2019, 11, 872–879. [Google Scholar] [CrossRef] [Green Version]
- Galland, G.B.; Seferin, M.; Mauler, R.S.; Dos Santos, J.H.Z. Linear low-density polyethylene synthesis promoted by homogeneous and supported catalysts. Polym. Int. 1999, 48, 660–664. [Google Scholar] [CrossRef]
- Wu, Q.; García-Peñas, A.; Barranco-García, R.; Cerrada, M.L.; Benavente, R.; Pérez, E.; Gómez-Elvira, J.M. A New Insight into the Comonomer Effect through NMR Analysis in Metallocene Catalysed Propene–co–1-Nonene Copolymers. Polymers 2019, 11, 1266. [Google Scholar] [CrossRef] [Green Version]
- Melillo, G.; Izzo, L.; Zinna, M.; Tedesco, C.; Oliva, L. Branching Formation in the Ethylene Polymerization with Meso Ansa Metallocene-Based Catalysts. Macromolecules 2002, 35, 9256–9261. [Google Scholar] [CrossRef]
- Melillo, G.; Izzo, L.; Centore, R.; Tuzi, A.; Voskoboynikov, A.Z.; Oliva, L. meso-Me2Si(1-indenyl)2ZrCl2/methylalumoxane catalyzed polymerization of the ethylene to ethyl-branched polyethylene. J. Mol. Catal. A-Chem. 2005, 230, 29–33. [Google Scholar] [CrossRef]
- Caporaso, L.; Galdi, N.; Oliva, L.; Izzo, L. Tailoring the Metallocene Structure to Obtain LLDPE by Ethene Homopolymerization: An Experimental and Theoretical Study. Organometallics 2008, 27, 1367–1371. [Google Scholar] [CrossRef]
- Schwerdtfeger, E.D.; Irwin, L.J.; Miller, S.A. Highly Branched Polyethylene from Ethylene Alone via a Single Zirconium-Based Catalyst. Macromolecules 2008, 41, 1080–1085. [Google Scholar] [CrossRef]
- Izzo, L.; Puranen, A.T.; Repo, T.; Oliva, L. Comparison of the C1-symmetric diastereoisomers of a zirconocene-based catalyst in ethylene polymerization: A benzyl substituent as a regulator in branch formation. J. Polym. Sci. A 2006, 44, 3551–3555. [Google Scholar] [CrossRef]
- The amount of ethyl branches expressed in terms of wt% of 1-butene wt% is given strictly as a value for comparison to 1-hexene wt% incorporated, and it is not meant to infer that the generated ethyl branches are a result of in situ butene formation.
- Vathauer, M.; Kaminsky, W. Homopolymerizations of α-Olefins with Diastereomeric Metallocene/MAO Catalysts. Macromolecules 2000, 33, 1955–1959. [Google Scholar] [CrossRef]
- Schaverien, C.J.; Ernst, R.; Schut, P.; Skiff, W.M.A.; Resconi, L.; Barbassa, E.; Balboni, D.; Dubitsky, Y.A.; Orpen, A.G.; Mercandelli, P.; et al. New Class of Chiral Bridged Metallocene: Synthesis, Structure, and Olefin (Co)polymerization Behavior of rac- and meso-1,2-CH2CH2{4-(7-Me-indenyl)}2ZrCl2. J. Am. Chem. Soc. 1998, 120, 9945–9946. [Google Scholar] [CrossRef]
- Tisse, V.F.; Boisson, C.; McKenna, T.F.L. Activation and Deactivation of the Polymerization of Ethylene over rac-EtInd2ZrCl2 and (nBuCp)2ZrCl2 on an Activating Silica Support. Macromol. Chem. Phys. 2014, 215, 1358–1369. [Google Scholar] [CrossRef]
- Bochmann, M.; Lancaster, S.J. Monomer–Dimer Equilibria in Homo- and Heterodinuclear Cationic Alkylzirconium Complexes and Their Role in Polymerization Catalysis. Angew. Chem. Int. Ed. 1994, 33, 1634–1637. [Google Scholar] [CrossRef]
- Song, F.; Cannon, R.D.; Bochmann, M. Zirconocene-Catalyzed Propene Polymerization: A Quenched-Flow Kinetic Study. J. Am. Chem. Soc. 2003, 125, 7641–7653. [Google Scholar] [CrossRef]
- Albeit plausible, such hypothesis remains unlikely since such mechanism is commonly considered for the polymerization of higher olefines (1-butene and 1-pentene).
- Santoro, O.; Piola, L.; Mc Cabe, K.; Lhost, O.; Den Dauw, K.; Vantomme, A.; Welle, A.; Maron, L.; Carpentier, J.-F.; Kirillov, E. Al-alkenyl-induced formation of long-chain branched polyethylene via coordinative tandem insertion and chain-transfer polymerization using (nBuCp)2ZrCl2/MAO systems: An experimental and theoretical study. Eur. Polym. J. 2021, 154, 110567. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Ivchenko, P.V.; Bagrov, V.V.; Churakov, A.V.; Chevalier, R. Novel Effective Racemoselective Method for the Synthesis of ansa-Zirconocenes and Its Use for the Preparation of C2-Symmetric Complexes Based on 2-Methyl-4-aryltetrahydro(s)indacene as Catalysts for Isotactic Propylene Polymerization and Ethylen. Organometallics 2012, 31, 4340–4348. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Ivchenko, P.V.; Bagrov, V.V.; Churakov, A.V.; Mercandelli, P. Novel effective racemoselective method for the synthesis of ansa-zirconocenes and its use for the preparation of C2-symmetric complexes based on 2-methyl-4-aryltetrahydro(s)indacene as catalysts for isotactic propylene polymerization and ethylene-propylene copolymerization. Organometallics 2012, 31, 4962–4970. [Google Scholar]
- Chevalier, R.; Garcia, V.; Müller, P.; Sidot, C.; Tellier, C.; Delacray, L. Meso-Selective Synthesis of Ansa-Metallocenes. Patent WO2005058929A1, 15 December 2004. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Pittsburgh, PA, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approx- imation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry 0.3. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Marenich, A.V.; Cramer, C.J. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Castro, L.; Kirillov, E.; Miserque, O.; Welle, A.; Haspeslagh, L.; Carpentier, J.-F.; Maron, L. Are Solvent and Dispersion Effects Crucial in Olefin Polymerization DFT Calculations? Some Insights from Propylene Coordination and Insertion Reactions with Group 3 and 4 Metallocenes. ACS Catal. 2015, 5, 416–425. [Google Scholar] [CrossRef]
| Run | Complex | C6(add.) b [wt%] | Activity c [kg/g.h] | Tmd [°C] | Mne [kDa] | Mwe [kDa] | MWD e | C6(incorp.) f [wt%] | Ethyl Branches f,g [wt%] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | meso-2 | 0.0 | 140 | 125 | 7.0 | 37.8 | 5.7 | 0.0 | 5.9 |
| 2 h | 100 | 125 | 4.6 | 14.0 | 3.0 | 0.0 | 3.7 | ||
| 3 | rac-2 | 44 | 129 | 13.7 | 45.5 | 3.3 | 0.0 | 0.3 | |
| 4 h | 80 | 130 | 15.2 | 43.4 | 2.9 | 0.0 | 0.1 | ||
| 5 | meso-2 | 0.7 | 176 | 121 | 6.3 | 26.6 | 4.2 | 1.5 | 4.2 |
| 6 | rac-2 | 60 | 128 | 12.3 | 39.6 | 3.2 | 2.2 | 0.4 | |
| 7 | meso-2 | 1.3 | 188 | 120 | 6.0 | 21.9 | 3.6 | 2.8 | 4.1 |
| 8 | rac-2 | 64 | 126 | 11.1 | 35.7 | 3.2 | 3.4 | 0.4 | |
| 9 | meso-2 | 2.5 | 200 | 122 | 4.6 | 13.0 | 2.8 | 2.8 | 2.8 |
| 10 h | 112 | 123 | 4.1 | 11.4 | 2.8 | 2.7 | 2.2 | ||
| 11 | rac-2 | 72 | 124 | 10.3 | 34.5 | 3.3 | 4.9 | 0.4 | |
| 12 h | 88 | 126 | 11.4 | 33.0 | 2.9 | 1.4 | 0.1 | ||
| 13 | meso-2 | 3.0 | 208 | 118 | 5.6 | 17.7 | 3.1 | 5.5 | 4.8 |
| 14 | rac-2 | 72 | 123 | 9.8 | 34.4 | 3.5 | 7.9 | 0.5 |
| Entry | Supp-Cat | C6(added) b [wt%] | Activity c [kg/g.h] | Tmd [°C] | Mne [kDa] | Mwe [kDa] | MWD e | C6(incorp.) f [wt%] | Ethyl Branches f,g [wt%] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | meso-2 | 0 | 4.5 | 129 | 10.2 | 29.4 | 2.9 | 0 | 1.8 |
| 2 | rac-2 | 1.1 | 130 | 10.3 | 29.7 | 2.9 | 0 | 1.0 | |
| 3 | meso-2 | 0.7 | 4.3 | 129 | 10.1 | 29.0 | 2.9 | ≤0.1 | 1.9 |
| 4 | rac-2 | 1.3 | 127 | 10.2 | 27.7 | 2.7 | 1.0 | 1.0 | |
| 5 | meso-2 | 1.5 | 4.4 | 128 | 9.9 | 28.4 | 2.9 | 0.2 | 1.9 |
| 6 | rac-2 | 1.4 | 127 | 10.5 | 31.1 | 3.0 | 2.1 | 0.9 | |
| 7 | meso-2 | 2.3 | 4.3 | 129 | 9.9 | 28.6 | 2.9 | 0.2 | 1.9 |
| 8 | rac-2 | 1.2 | 126 | 10.5 | 31.1 | 3.0 | 2.1 | 0.9 | |
| 9 | meso-2 | 3.0 | 4.5 | 129 | 9.9 | 28.2 | 2.9 | 0.2 | 1.9 |
| 10 | rac-2 | 1.6 | 126 | 10.7 | 33.7 | 3.2 | 2.6 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giffin, K.A.; Cirriez, V.; Santoro, O.; Welle, A.; Kirillov, E.; Carpentier, J.-F. Meso- and Rac-[bis(3-phenyl-6-tert-butylinden-1-yl)dimethylsilyl]zirconium Dichloride: Precatalysts for the Production of Differentiated Polyethylene Products with Enhanced Properties. Polymers 2022, 14, 2217. https://doi.org/10.3390/polym14112217
Giffin KA, Cirriez V, Santoro O, Welle A, Kirillov E, Carpentier J-F. Meso- and Rac-[bis(3-phenyl-6-tert-butylinden-1-yl)dimethylsilyl]zirconium Dichloride: Precatalysts for the Production of Differentiated Polyethylene Products with Enhanced Properties. Polymers. 2022; 14(11):2217. https://doi.org/10.3390/polym14112217
Chicago/Turabian StyleGiffin, Kaitie A., Virginie Cirriez, Orlando Santoro, Alexandre Welle, Evgueni Kirillov, and Jean-François Carpentier. 2022. "Meso- and Rac-[bis(3-phenyl-6-tert-butylinden-1-yl)dimethylsilyl]zirconium Dichloride: Precatalysts for the Production of Differentiated Polyethylene Products with Enhanced Properties" Polymers 14, no. 11: 2217. https://doi.org/10.3390/polym14112217
APA StyleGiffin, K. A., Cirriez, V., Santoro, O., Welle, A., Kirillov, E., & Carpentier, J.-F. (2022). Meso- and Rac-[bis(3-phenyl-6-tert-butylinden-1-yl)dimethylsilyl]zirconium Dichloride: Precatalysts for the Production of Differentiated Polyethylene Products with Enhanced Properties. Polymers, 14(11), 2217. https://doi.org/10.3390/polym14112217








