Ecotoxicity and Biodegradation of Sustainable Environment-Friendly Bone-Glue-Based Adhesive Suitable for Insulation Materials
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Chemical Analyses and pH Values
3.2. Ecotoxicity
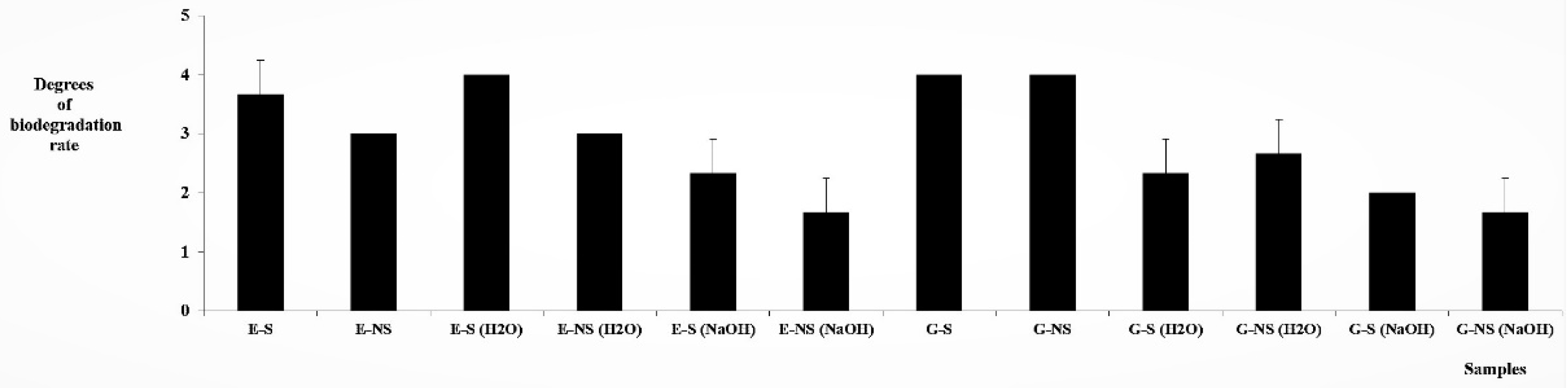
3.3. Biodegradation Tests with Molds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD. Available online: https://www.oecd.org/ (accessed on 23 May 2022).
- ISO. Available online: https://www.iso.org/home.html (accessed on 23 May 2022).
- ASTM. Available online: https://www.astm.org/ (accessed on 23 May 2022).
- REACH. Available online: https://europa.eu/youreurope/business/product-requirements/chemicals/registering-chemicals-reach/index_cs.htm (accessed on 23 May 2022).
- Pacheco-Torgal, F.; Jalali, S. Toxicity of building materials: A key issue in sustainable construction. Int. J. Sustain. Eng. 2019, 4, 281–287. [Google Scholar] [CrossRef]
- Krüger, O.; Kalbe, U.; Richter, E.; Egeler, P.; Römbke, J.; Berger, W. New approach to the ecotoxicological risk assessment of artificial outdoor sporting grounds. Environ. Pollut. 2013, 175, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Baderna, D.; Lomazzi, E.; Passoni, A.; Pogliaghi, A.; Petoumenou, M.I.; Bagnati, R.; Lodia, M.; Viarengo, A.; Sforzini, S.; Benfenati, E.; et al. Chemical characterization and ecotoxicity of three soil foaming agents used in mechanized tunnelling. J. Hazard. Mater. 2015, 296, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Gartiser, S.; Heisterkamp, I.; Schoknecht, U.; Bandow, N.; Burkhardt, N.M.; Ratte, M.; Ilvonen, O. Recommendation for a test battery for the ecotoxicological evaluation of the environmental safety of construction products. Chemosphere 2017, 171, 580–587. [Google Scholar] [CrossRef]
- Sickels, L.B. Organic additives in mortars. Edinb. Univ. Res. J. Architect. 1980, 8, 7–20. [Google Scholar]
- Loaiza, A.; Garcia, E.; Colorado, E.A. Evaluation of asphalt binder blended with coconut coir dust and residual coconut fibers for structural applications. Rev. Constr. 2019, 17, 542–554. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.; Kim, S.; Kim, J.T. Characteristics of Particleboards Using Tannin Resin as Novel Environment-Friendly Adhesion System. Indoor Built Environ. 2013, 22, 61–67. [Google Scholar] [CrossRef]
- Protano, C.; Buomprisco, G.; Cammalleri, V.; Poceni, R.N.; Marotta, D.; Simonazzi, S.; Cardoni, F.; Petyx, M.; Iavicoli, S.; Vitali, M. The Carcinogenic Effects of Formaldehyde Occupational Exposure: A Systematic Review. Cancers 2021, 14, 165. [Google Scholar] [CrossRef]
- Wi, S.; Park, J.H.; Kim, J.U.; Kim, S. Evaluation of environmental impact on the formaldehyde emission and flame-retardant performance of thermal insulation materials. J. Hazard. Mater. 2021, 402, 123463. [Google Scholar] [CrossRef]
- Kristak, S.; Antov, P.; Bekhta, P.; Lubis, M.A.R.; Heri, I.A.; Réh, R.; Sedliacik, J.; Savov, V.; Taghiyari, H.R.; Papadopoulos, A.N.; et al. Recent Progress in Ultra-Low Formaldehyde Emitting Adhesive Systems and Formaldehyde Scavengers in Wood-Based Panels: A Review. Wood Mater. Sci. Eng. 2022, 17, 1–20. [Google Scholar] [CrossRef]
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, D.; Li, Z.; Li, Z.; Peng, X.; Liu, C.; Zhang, Y.; Zheng, P. Recent Developments in the Flame-Retardant System of Epoxy Resin. Materials 2020, 13, 2145. [Google Scholar] [CrossRef] [PubMed]
- ECHA. Available online: https://european-union.europa.eu/institutions-law-budget/institutions-and-bodies/institutions-and-bodies-profiles/echa_cs (accessed on 23 May 2022).
- Cosereanu, C.; Cerbu, C. Rape/wood particleboard. BioResources 2019, 14, 2903–2918. [Google Scholar]
- Mirski, R.; Derkowski, A.; Dziurka, D.; Wieruszewski, M.; Dukarska, D. Effects of chip type on the properties of chip-sawdust boards glued with polymeric diphenyl methane diisocyanate. Materials 2020, 13, 1329. [Google Scholar] [CrossRef]
- Mantanis, G.I.; Athanassiadou, E.T.; Barbu, M.C.; Wijnendaele, K. Adhesive systems used in the European particleboard, MDF and OSB industries. Wood Mater. Sci. Eng. 2017, 13, 104–116. [Google Scholar] [CrossRef]
- Sunardi, M.; Fawaid, R.; Lusiani, S.B.; Kesworo, A.; Widodo, T.D. The Effect of Wood Sawdust Mesh Combination on Mechanical Behaviour of Particle Board. IOP Conf. Ser. Mater. Sci. Eng. 2019, 494, 012089. [Google Scholar] [CrossRef]
- Budakci, M. The determination of adhesion strength of wood veneer and synthetic resin panel (laminate) adhesives. Wood Res. 2010, 55, 125–136. [Google Scholar]
- Berardi, U.; Iannace, G. Acoustic characterization of natural fibers for sound absorption applications. Energy Build. 2015, 94, 840–852. [Google Scholar] [CrossRef]
- Berardi, U.; Iannace, G. Predicting the sound absorption of natural materials: Best fit inverse laws for the acoustic impedance and the propagation constant. Appl. Acoust. 2017, 115, 131–138. [Google Scholar] [CrossRef]
- Richter, R.; Římovský, K. Organická Hnojiva, Jejich Výroba a Použití; Institut výchovy a vzdělávání Ministerstva zemědělství: Prague, Czech Republic, 1995; pp. 1–40. [Google Scholar]
- Strehler, A. Aufbereitung und Verfeuerung von Biomase als Festbrennstoff. In Energie aus Biomase, 1st ed.; Landtechnik: Bericht, Germany, 1994; pp. 171–192. [Google Scholar]
- Rahim, M.; Douzane, O.; Tran Le, A.D.; Promis, G.; Langlet, T. Characterization and comparison of hygric properties of rape straw concrete and hemp concrete. Constr. Build. Mater. 2016, 102, 679–687. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Chen, B. Influence of type of binder and size of plant aggregate on the hygrothermal properties of bio-concrete. Constr. Build. Mater. 2020, 251, 118981. [Google Scholar] [CrossRef]
- Zhang, M.H.; Sisomphon, K.; Ng, T.S.; Sun, D.J. Effect of superplasticizers on workability retention and initial setting time of cement pastes. Constr. Build. Mater. 2010, 24, 1700–1707. [Google Scholar] [CrossRef]
- Tantawi, S.H.; Selim, I.Z. Role of some concrete admixtures on the resistivity of cement pastes and reinforced steel. Bull. Electrochem. 2004, 20, 175–182. [Google Scholar]
- Topcu, I.B.; Atesin, O. Effect of high dosage lignosulphonate and naphthalene sulphonate based plasticizer usage on micro concrete properties. Constr. Build. Mater. 2016, 120, 189–197. [Google Scholar] [CrossRef]
- Guo, M.H.; Wang, Y.; Liu, F. Performance Analysis of Ammonium Lignosulfonate/Urea Formaldehyde-free Fiberboards. Adv. Mater. Res. 2010, 113, 1774–1778. [Google Scholar] [CrossRef]
- Chupin, L.; Charrier, B.; Pizzi, A.; Perdomo, A.; Bouhtoury, C.E. Study of thermal durability properties of tannin–lignosulfonate adhesives. J. Therm. Anal. Calorim. 2015, 119, 1577–1585. [Google Scholar] [CrossRef]
- Antov, P.; Savov, V.; Trichkov, N.; Krišťák, L.; Réh, R.; Papadopoulos, A.N.; Takhiyari, H.R.; Pizzi, A.; Kunecová, D.; Pachikova, M. Properties of High-Density Fiberboard Bonded with Urea–Formaldehyde Resin and Ammonium Lignosulfonate as a Bio-Based Additive. Polymers 2021, 13, 2775. [Google Scholar] [CrossRef]
- Hemmila, V.; Adamopoulos, S.; Hosseinpourpia, R.; Ahmed, S.A. Ammonium Lignosulfonate Adhesives for Particleboards with pMDI and Furfuryl Alcohol as Crosslinkers. Polymers 2019, 11, 1633. [Google Scholar] [CrossRef]
- Antov, P.; Savov, V.; Mantanis, G.I.; Neykov, N. Medium-density fibreboards bonded with phenol-formaldehyde resin and calcium lignosulfonate as an eco-friendly additive. Wood Mater. Sci. Eng. 2020, 16, 42–48. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Grillet, A.C.; Diep, T.M.H.; Ha, T.C.N.; Woloszyn, M. Hygrothermal properties of bio-insulation building materials based on bamboo fibers and bio-glues. Constr. Build. Mater. 2017, 155, 852–866. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Grillet, A.C.; Bui, Q.B.; Diep, T.M.H.; Woloszyn, M. Building bio-insulation materials based on bamboo powder and bio-binders. Constr. Build. Mater. 2018, 186, 686–698. [Google Scholar] [CrossRef]
- Kunanopparat, T.; Menut, P.; Morel, M.H.; Guilbert, S. Improving wheat gluten materials properties by kraft lignin addition. J. Appl. Polym. Sci. 2012, 125, 1391–1399. [Google Scholar] [CrossRef]
- Dušek, J.; Jerman, M.; Podlena, M.; Böhm, M.; Černý, R. Sustainable composite material based on surface-modified rape straw and environment-friendly adhesive. Constr. Build. Mater. 2021, 300, 124036. [Google Scholar] [CrossRef]
- CSN EN 12457-4 (838005); Characterisation of Waste-Leaching-Compliance Test for Leaching of Granular Waste Materials and Sludges Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 I/kg for Materials with Particle Size Below 4 mm. Česká agentura pro standardizaci: Prague, Czech Republic, 2003.
- Kobetičová, K.; Fořt, J.; Černý, R. Interactions of superabsorbent polymers based on acrylamide substances with microorganisms occurring in human dwellings. Ecotoxicol. Environ. Saf. 2020, 195, 110522. [Google Scholar] [CrossRef] [PubMed]
- OECD 202; OECD Guidelines for the Testing of Chemicals. Daphnia sp. Acute Immobilisation Test. OECD: Geneva, Switzerland, 2004.
- EN ISO 846; Plastics. Evaluation of the Action of Microorganisms. International Organization: Geneva, Switzerland, 1997.
- Wopenka, B.; Pasteris, J.D. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Skinner, H.C.W. Biominerals. Mineral. Mag. 2005, 69, 621–641. [Google Scholar] [CrossRef]
- Vermeirssen, E.L.M.; Dietschweiler, C.; Werner, I.; Burkhardt, M. Corrosion protection products as a source of bisphenol A and toxicity to the aquatic environment. Water Res. 2017, 123, 586–593. [Google Scholar] [CrossRef]
- Pereira, E.O.A.; Labine, L.M.; Kleywegt, S.; Jobst, K.J.; Simpson, A.J.; Simpson, M.J. Metabolomics Reveals That Bisphenol Pollutants Impair Protein Synthesis-Related Pathways in Daphnia magna. Metabolites 2011, 11, 666. [Google Scholar] [CrossRef]
- Maisto, G.; Manzo, S.; De Nicola, F.; Carotenuto, R.; Rocco, A.; Alfani, A. Assessment of the effects of Cr, Cu, Ni and Pb soil contamination by ecotoxicological tests. J. Environ. Monit. 2011, 13, 3049–3056. [Google Scholar] [CrossRef]
- Baran, A.; Tarnawski, M.; Koniarz, T.; Szara, M. Content of nutrients, trace elements, and ecotoxicity of sediment cores from Ro(z) over dotnow reservoir (Southern Poland). Environ. Geochem. Health 2019, 41, 2929–2948. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, M.; Kaminski, A.; Pusz, A. Phytotoxicity of metal-contaminated soils. Przem. Chem. 2019, 98, 852–856. [Google Scholar]
- Plekhanova, I.O.; Zolotareva, O.A.; Tarasenko, I.D.; Yakovlev, A.S. Assessment of Ecotoxicity of Soils Contaminated by Heavy Metals. Eurasian Soil Sci. 2019, 52, 1274–1288. [Google Scholar] [CrossRef]
- Zoller, O.; Bruschweiler, B.J.; Magnin, R.; Reinhard, H.; Rhyn, P.; Rupp, H.; Zeltner, S.; Felleisen, R. Natural occurrence of bisphenol F in mustard. Food Addit. Contam. Part A-Chem. Anal. Control Expo. Risk Assess. 2016, 33, 137–146. [Google Scholar]
- Nakajima, N.; Ohshima, Y.; Serizawa, S.; Kouda, T.; Edmonds, J.S.; Shiraishi, F.; Aono, M.; Kubo, A.; Tamaoki, M.; Saji, H. Processing of bisphenol A by plant tissues: Glucosylation by cultured BY-2 cells and glucosylation/translocation by plants of Nicotiana tabacum. Plant Cell Physiol. 2002, 43, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Loffredo, E.; Senesi, N. Phytotoxic, clastogenic and bioaccumulation effects of the environmental endocrine disruptor bisphenol A in various crops grown hydroponically. Planta 2016, 223, 910–916. [Google Scholar] [CrossRef]
- Luo, M.; Gu, S.H.; Zhao, S.H.; Zhang, F.; Wu, N.H. Rice GTPase OsRacB: Potential accessory factor in plant salt-stress signaling. Acta Biochim. Biophys. Sin. 2006, 38, 393–402. [Google Scholar] [CrossRef][Green Version]
- Speranza, A.; Crosti, P.; Malerba, M.; Stocchi, O.; Scoccianti, V. The environmental endocrine disruptor, bisphenol A, affects germination, elicits stress response and alters steroid hormone production in kiwifruit pollen. Plant Biol. 2011, 13, 209–217. [Google Scholar] [CrossRef]
- Qiu, Z.Y.; Wang, L.H.; Zhou, Q. Effects of bisphenol A on growth, photosynthesis and chlorophyll fluorescence in above-ground organs of soybean seedlings. Chemosphere 2013, 90, 1274–1280. [Google Scholar] [CrossRef]
- Hu, H.; Wang, L.; Wang, Q.; Jiao, L.; Hua, W.; Zhu, Q.; Huang, X. Photosynthesis, chlorophyll fluorescence characteristics, and chlorophyll content of soybean seedlings under combined stress of bisphenol A and cadmium. Environ. Toxicol. Chem. 2014, 11, 2455–2462. [Google Scholar] [CrossRef]
- Nakajima, N.; Teramoto, T.; Kasai, F.; Sano, T.; Tamaoki, M.; Aono, M.; Kubo, A.; Kamada, H.; Azumi, Y.; Saji, H. Glycosylation of bisphenol A by freshwater microalgae. Chemosphere 2007, 69, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.Y.; Chen, Q.; Duan, S.S. Transcriptional Analysis of Chlorella pyrenoidosa Exposed to Bisphenol A. Int. J. Environ. Res. Public Health 2019, 16, 1374. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.M.; Baier, R.; Kocher, B.; Reifferscheid, G.; Buchinger, S.; Ternes, T. Ecotoxicological characterization of emissions from steel coatings in contact with water. Water Res. 2020, 173, 115525. [Google Scholar] [CrossRef]
- Kyrila, G.; Katsoulas, A.; Schoretsanati, V.; Rigopoulos, A.; Rizou, E.; Douůgeridou, S.; Sarli, V.; Samanidon, V.; Touraki, M. Bisphenol A removal and degradation pathways in microorganisms with probiotic properties. J. Hazard. Mater. 2021, 413, 125363. [Google Scholar] [CrossRef]
- Kwasniewska-Sip, P.; Cofta, G.; Nowak, P.B. Resistance of fungal growth on Scots pine treated with caffeine. Int. Biodeter. Biodegr. 2018, 132, 178–184. [Google Scholar] [CrossRef]
- Bae, B.; Jeong, J.H.; Lee, S.J. The quantification and characterization of endocrine disruptor bisphenol—A leaching from epoxy resin. Water. Sci. Technol. 2020, 46, 381–387. [Google Scholar] [CrossRef]


| Chemical | Composition |
|---|---|
| Bone glue | glutin and its fission products |
| Sodium lignosulfonate | sodium salts C14-16-alkanhydroxy, C14-16- alkene sulfonic acids, sodium hydroxide, 2-oktyl-2H-isothiazol-3-on |
| Epoxy resin | 4,4’-Isopropylidenediphenol, oxirane, mono[(C12-14-alkyloxy)methyl] derivates, 4-hyroxymethyl-1,3-dioxolan-2-one, benzyl alcohol, benzoic acid, 4[{(methylphenylamino) methylene} amino]-, ethyl ester |
| Sample | Al | B | Ba | Ca | Fe | K | Mg | Mn | Na | P | Si | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dist. water | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Rape straw (pure) | 0.00 | 0.00 | 0.00 | 32.2 | 0.03 | 41.7 | 4.00 | 0.10 | 5.21 | 0.00 | 0.20 | 0.02 |
| Sulfonate (pure) | 0.00 | 0.00 | 0.00 | 1.50 | 0.01 | 0.06 | 0.20 | 0.00 | 1.44 | 0.00 | 0.10 | 0.01 |
| Glue (pure) | 0.09 | 1.00 | 0.20 | 191 | 0.44 | >325 | 41.2 | 0.82 | >103 | 1.40 | 0.30 | 2.98 |
| Glue-LS-NaOH | 0.20 | 1.00 | 0.03 | 16.6 | 0.35 | 234 | 23.7 | 0.08 | >22 | 0.60 | 0.70 | 1.06 |
| Glue-LS-H2O | 0.13 | 0.70 | 0.06 | 127 | 0.52 | 227 | 24.3 | 0.34 | >66 | 0.40 | 2.60 | 2.51 |
| Epoxide (pure) | 0.02 | 1.40 | 0.15 | 186 | 0.11 | 288 | 35.5 | 0.71 | 87.9 | 2.30 | 0.00 | 0.17 |
| Epoxide-NaOH | 0.07 | 1.00 | 0.04 | 14.8 | 0.20 | 149 | 15.7 | 0.11 | >27 | 0.90 | 0.30 | 0.11 |
| Epoxide-H2O | 0.03 | 0.60 | 0.06 | 102 | 0.08 | 91.8 | 15.2 | 0.21 | 48.3 | 0.50 | 1.40 | 0.10 |
| Sample | pH Value |
|---|---|
| Distilled water | 6.9 |
| Rape straw (pure) | 6.5 |
| Glue (pure) | 6.5 |
| Glue-LS-NaOH | 9.5 |
| Glue-LS-H2O | 6.9 |
| Epoxide (pure) | 6.0 |
| Epoxide-NaOH | 9.5 |
| Epoxide-H2O | 7.6 |
| Inhibition | (%) | ||||
|---|---|---|---|---|---|
| Sample | Yeasts | Artemia | Artemia | Mustard | Algae |
| 24 h | 24 h | 48 h | 96 h | 72 h | |
| Growth Rate | Mortality | Mortality | Root Elongation | Growth Rate | |
| Control | 0 | 0 | 0 | 0 | 0 |
| Rape straw (pure) | −82 | 16 | 21 | −6 | −26 |
| Glue (pure) | −89 | 100 | 100 | 100 | 12 |
| Glue-LS-straw + NaOH | −34 | 74 | 100 | 100 | 0 |
| Glue-LS-straw + H2O | −21 | 100 | 100 | 100 | 10 |
| Epoxy (pure) | −100 | 100 | 100 | 100 | 0 |
| Epoxy-straw + NaOH | −37 | 5 | 100 | 100 | −16 |
| Epoxy-straw + H2O | −38 | 100 | 100 | 100 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobetičová, K.; Böhm, M.; Jerman, M.; Dušek, J.; Černý, R. Ecotoxicity and Biodegradation of Sustainable Environment-Friendly Bone-Glue-Based Adhesive Suitable for Insulation Materials. Polymers 2022, 14, 2209. https://doi.org/10.3390/polym14112209
Kobetičová K, Böhm M, Jerman M, Dušek J, Černý R. Ecotoxicity and Biodegradation of Sustainable Environment-Friendly Bone-Glue-Based Adhesive Suitable for Insulation Materials. Polymers. 2022; 14(11):2209. https://doi.org/10.3390/polym14112209
Chicago/Turabian StyleKobetičová, Klára, Martin Böhm, Miloš Jerman, Jaroslav Dušek, and Robert Černý. 2022. "Ecotoxicity and Biodegradation of Sustainable Environment-Friendly Bone-Glue-Based Adhesive Suitable for Insulation Materials" Polymers 14, no. 11: 2209. https://doi.org/10.3390/polym14112209
APA StyleKobetičová, K., Böhm, M., Jerman, M., Dušek, J., & Černý, R. (2022). Ecotoxicity and Biodegradation of Sustainable Environment-Friendly Bone-Glue-Based Adhesive Suitable for Insulation Materials. Polymers, 14(11), 2209. https://doi.org/10.3390/polym14112209









