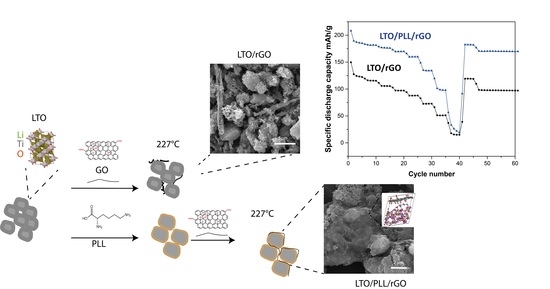
Application of Poly-L-Lysine for Tailoring Graphene Oxide Mediated Contact Formation between Lithium Titanium Oxide LTO Surfaces for Batteries
Abstract
1. Introduction
2. Experimental Section
2.1. Computational Details
2.2. Materials
2.2.1. Synthesis of Li4Ti5O12 (LTO) Nanostructured Material
2.2.2. TiO2 and LTO Functionalization
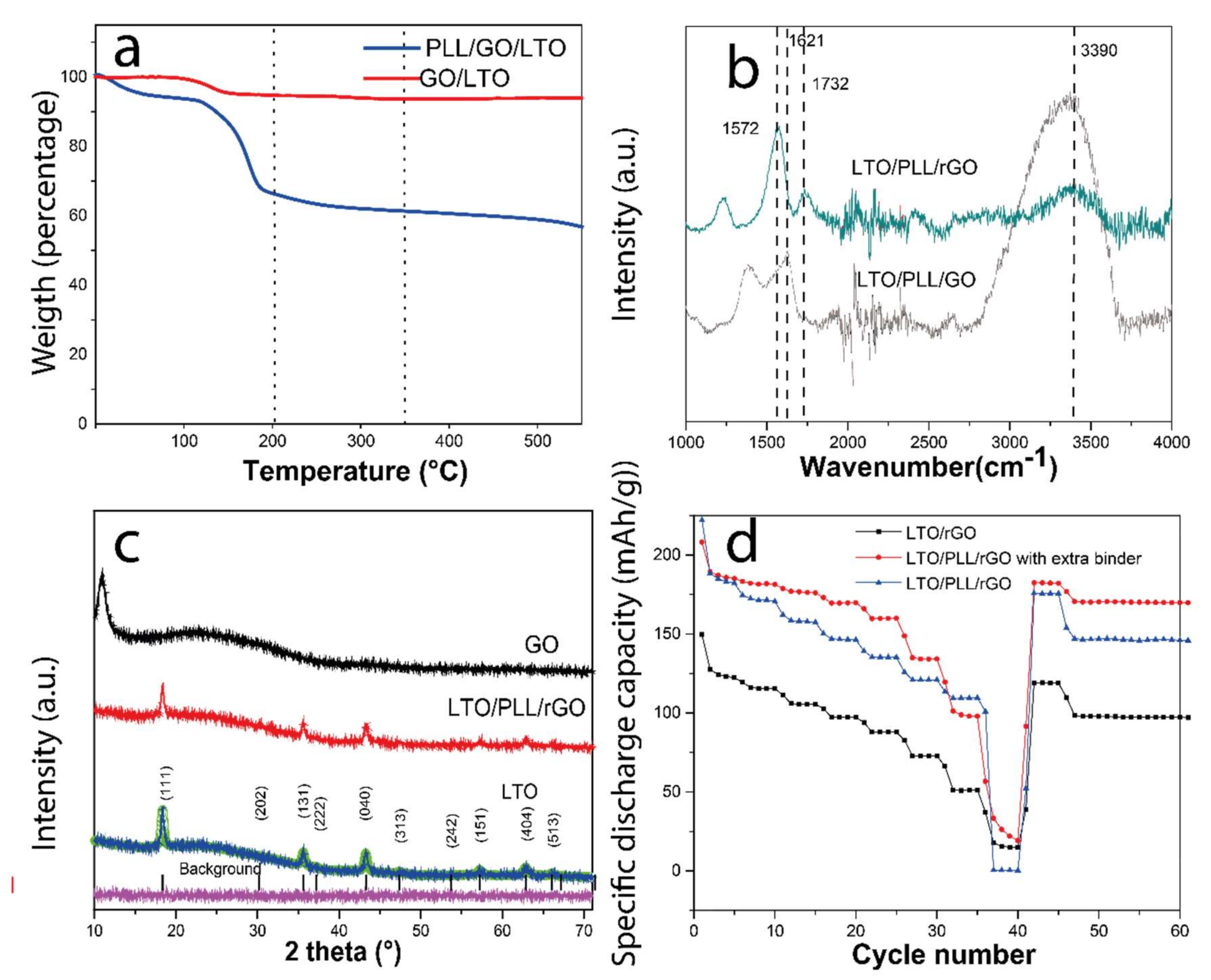
2.2.3. X-ray Diffraction Technique
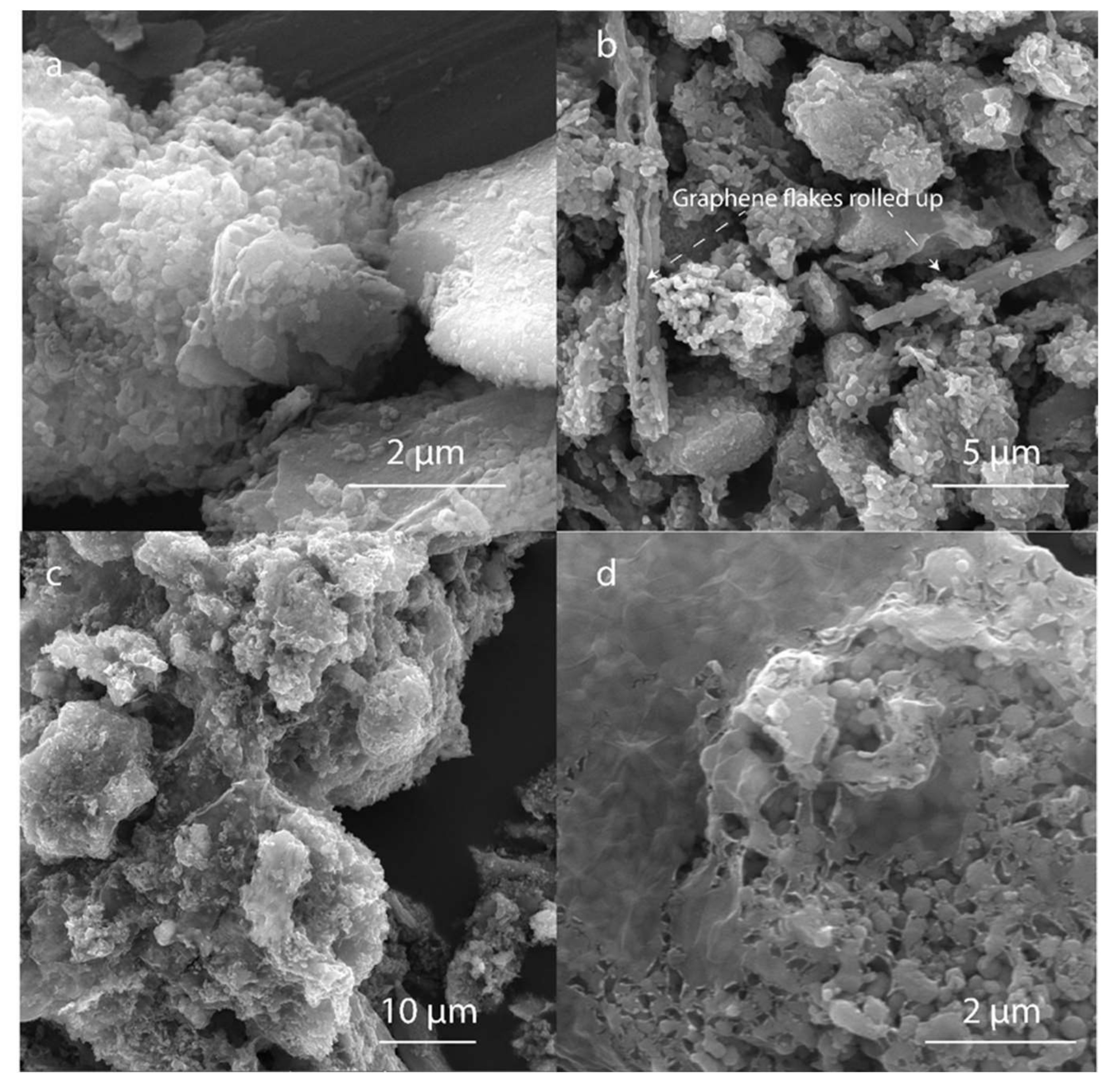
2.2.4. Scanning Electron Microscopy (SEM)
2.2.5. Thermogravimetric Analysis (TGA)
2.2.6. Attenuated Total Reflectance (ATR-FTIR)
2.2.7. X-ray Photoelectron Spectroscopy (XPS)
2.2.8. Atomic Force Microscopy (AFM)
2.2.9. Electrochemical Properties of Mesoporous LTO Microspheres
3. Results
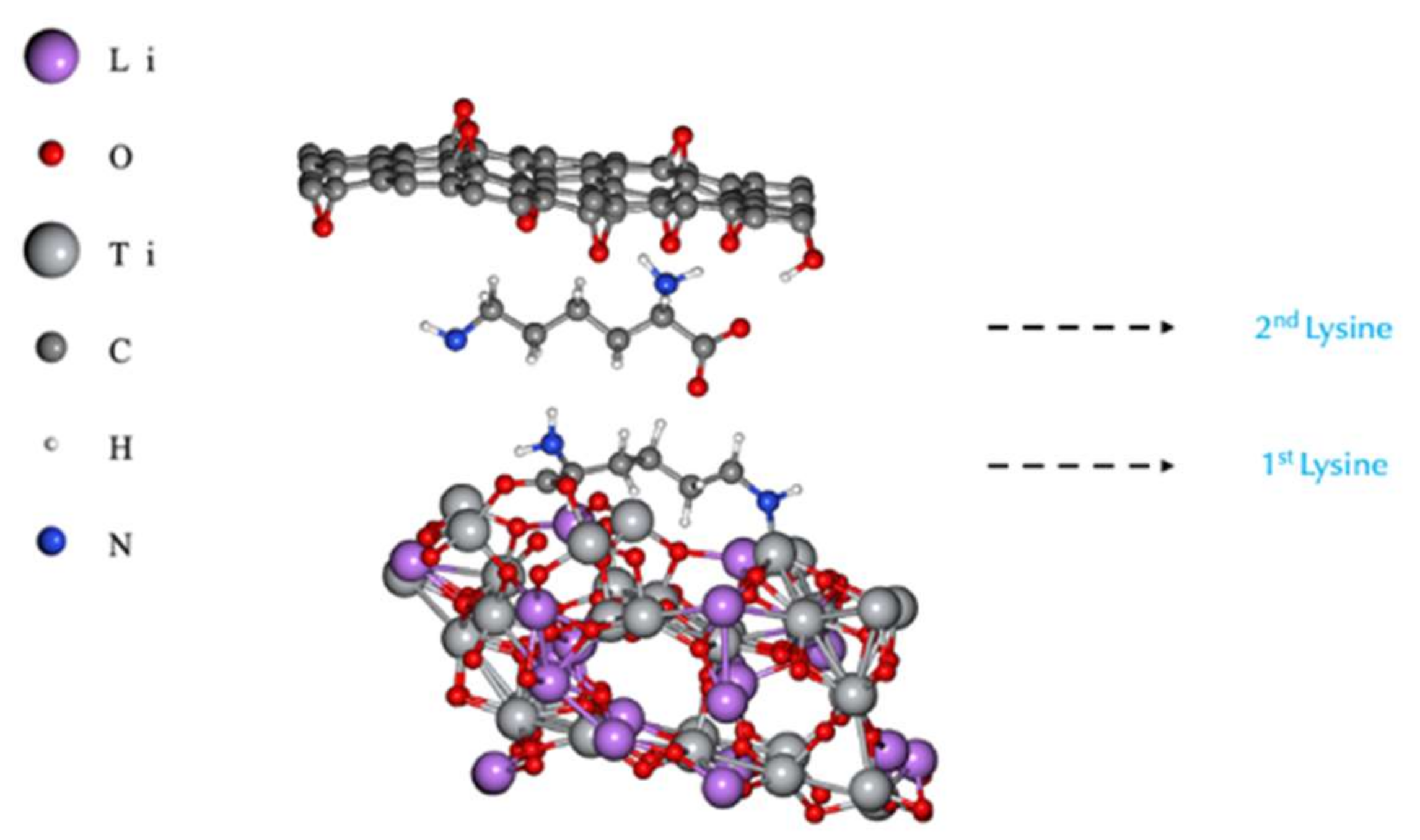
Conductivity Modelling: LTO/Lysine/rGO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, S.; Bleken, F.; Stier, S.; Flores, E.; Welzel, C.; Andersen, C.W.; Marcinek, M.; Szczesna-Chrzan, A.; Gaberscek, M.; Palacin, R.; et al. Toward a Unified Description of Battery Data. Adv. Energy Mater. 2021, 12, 2102702. [Google Scholar] [CrossRef]
- Greaves, M.; Barg, S.; Bissett, M. MXene-Based Anodes for Metal-Ion Batteries. Batter. Supercaps 2019, 3, 214–235. [Google Scholar] [CrossRef]
- Zhang, F.; Meng, T.; Gao, A.; Shu, D.; Chen, H.; Cheng, H.; Zhou, X. In Situ Supramolecular self-assembly assisted synthesis of Li4Ti5O12−carbon-reduced graphene oxide microspheres for Lithium-Ion batteries. ACS Sustain. Chem. Eng. 2019, 7, 916–924. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, J.; Usuelli, M.; Zhang, X.; Liu, B.; Mezzenga, R. Biomass vs inorganic and plastic-based aerogels: Structural design, functional tailoring, resource-efficient applications and sustainability analysis. Prog. Mater. Sci. 2021, 125, 100915. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Amine, K. Li4Ti5O12 spinel anodes. Nat. Energy 2021, 6, 683. [Google Scholar] [CrossRef]
- Chiou, Y.-C.; Olukan, T.A.; AlMahri, M.A.; Apostoleris, H.; Chiu, C.H.; Lai, C.-Y.; Lu, C.; Santos, S.; Almansouri, I.; Chiesa, M. Direct Measurement of the Magnitude of the van der Waals Interaction of Single and Multilayer Graphene. Langmuir 2018, 34, 12335–12343. [Google Scholar] [CrossRef]
- Fu, X.; Zhong, W. Biomaterials for high-energy lithium-based batteries: Strategies, challenges, and perspectives. Adv. Energy Mater. 2019, 9, 1901774. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Minakshi, M.; Bahri, P.A.; Paul, S.; Kumari, P.; Manjunatha, K.N. Rational design on materials for developing next generation lithium-ion secondary battery. Prog. Solid State Chem. 2021, 62, 100298. [Google Scholar] [CrossRef]
- Mohsen, M.; Bahadorikhalili, S.; Lari, N.; Hadi, M.; Babaiee, M.; Eqra, R. The Effect of Crystalline microstructure of PVDF binder on mechanical and electrochemical performance of Lithium-Ion Batteries Cathode. Z. Für Phys. Chemie 2020, 234, 381–397. [Google Scholar]
- Kraytsberg, A.; Ein-Eli, Y. Conveying Advanced Li-ion Battery Materials into Practice The Impact of Electrode Slurry Preparation Skills. Adv. Energy Mater. 2016, 6, 1600655. [Google Scholar] [CrossRef]
- Richardson, J.J.; Cui, J.; Bjornmalm, M.; Braunger, J.A.; Ejima, H.; Caruso, F. Innovation in Layer-by-Layer Assembly. Chem. Rev. 2016, 116, 14828–14867. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent functionalization of gra-phene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Bostick, C.D.; Mukhopadhyay, S.; Pecht, I.; Sheves, M.; Cahen, D.; Lederman, D. Protein bioelectronics: A review of what we do and do not know. Rep. Prog. Phys. 2018, 81, 026601. [Google Scholar] [CrossRef] [PubMed]
- Brancolini, G.; Bellucci, L.; Maschio, M.C.; Di Felice, R.; Corni, S. The interaction of peptides and proteins with nanostructures surfaces: A challenge for nanoscience. Curr. Opin. Colloid Interface Sci. 2019, 41, 86–94. [Google Scholar] [CrossRef]
- Qiu, X.; Chiechi, R.C. Printable logic circuits comprising self-assembled protein complexes. Nat. Commun. 2022, 13, 1–10. [Google Scholar] [CrossRef]
- Tanaka, M. Physics of interactions at biological and biomaterial interfaces. Curr. Opin. Colloid Interface Sci. 2013, 18, 432–439. [Google Scholar] [CrossRef]
- Basu, S.; Pacelli, S.; Paul, A. Self-healing DNA-based injectable hydrogels with reversible covalent linkages for controlled drug delivery. Acta Biomater. 2020, 105, 159–169. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Stamboroski, S.; Joshi, A.; Noeske, P.L.M.; Köppen, S.; Brüggemann, D. Principles of Fibrinogen Fiber Assembly In Vitro. Macromolecular Bioscience. 2021, 21, 2000412. [Google Scholar] [CrossRef]
- Stamboroski, S.; Boateng, K.; Lierath, J.; Kowalik, T.; Thiel, K.; Köppen, S.; Noeske, P.-L.M.; Brüggemann, D. Influence of Divalent Metal Ions on the Precipitation of the Plasma Protein Fibrinogen. Biomacromolecules 2021, 22, 4642–4658. [Google Scholar] [CrossRef]
- Dale-Evans, A.R.; Robinson, M.; Lloyd, H.; Gavaghan, D.; Bond, A.; Parkin, A.A. Voltammetric Perspective of Mul-ti-Electron and Proton Transfer in Protein Redox Chemistry: Insights from Computational Analysis of Escherichia coli HypD Fourier Transformed Alternating Current Voltammetry. Front. Chem. 2021, 9, 672831. [Google Scholar] [CrossRef] [PubMed]
- Toigo, C.; Arbizzani, C.; Pettinger, K.-H.; Biso, M. Study on Different Water-Based Binders for Li4Ti5O12 Electrodes. Molecules 2020, 25, 2443. [Google Scholar] [CrossRef] [PubMed]
- Liebel, C. Sustainable Battery Materials from Biomass. ChemSusChem 2020, 13, 2110–2141. [Google Scholar]
- Ramkumar, R.; Minakshi, M. Fabrication of ultrathin CoMoO4 nanosheets modified with chitosan and their improved performance in energy storage device. Dalton Trans. 2015, 44, 6158–6168. [Google Scholar] [CrossRef]
- Bargnesi, L.; Gigli, F.; Albanelli, N.; Toigo, C.; Arbizzani, C. Crosslinked Chitosan Binder for Sustainable Aqueous Bat-teries. Nanomaterials. 2022, 12. [Google Scholar] [CrossRef]
- Stamboroski, S.; Stachera, P.N.; Ureña, Y.R.C.; Hrycyna, G.H.; Neto, W.I.T.R.; De Azambuja, W.K.; Salz, D.; Ihde, J.; Noeske, P.-L.M.; Cavalcanti, W.L. Implementation of diverse non-centrosymmetric layer concepts for tuning the interface activity of a magnesium alloy. Appl. Adhes. Sci. 2016, 4, 37. [Google Scholar] [CrossRef][Green Version]
- Ureña, Y.R.C.; Cavalcanti, W.L.; Soltau, M.; Villalobos, K.; Rischka, K.; Noeske, P.-L.M.; Brune, K.; Dieckhoff, S. Interfactant action of an amphiphilic polymer upon directing graphene oxide layer formation on sapphire substrates. Appl. Adhes. Sci. 2017, 5, 115. [Google Scholar] [CrossRef]
- Perez, F.M.; Ureña, Y.R.C.; Rischka, K.; Cavalcanti, W.L.; Noeske, P.-L.M.; Safari, A.A.; Wei, G.; Ciacchi, L.C. Bio-interfactants as double-sided tapes for graphene oxide. Nanoscale 2019, 11, 4236–4247. [Google Scholar] [CrossRef]
- Pignanelli, F.; Romero, M.; Faccio, R.; Fernandez, L.; Mombrú, A. Enhancement of Lithium-Ion Transport in Poly(acrylonitrile) with Hydrogen Titanate Nanotube Fillers as Solid Polymer Electrolytes for Lithium-Ion Battery Appli-cations. J. Phys. Chem. C 2018, 22, 1492–1499. [Google Scholar] [CrossRef]
- Deng, Z.; Mo, Y.; Ong, S.P. Computational studies of solid-state alkali conduction in rechargeable alkali-ion batteries. NPG Asia Mater. 2016, 8, e254. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Liu, D.; Gao, Y.; Wang, Y.; Bu, H.; Li, M.; Zhang, Y.; Gao, G.; Ding, S. A composite solid polymer electrolyte incorporating MnO2 nanosheets with reinforced mechanical properties and electrochemical stability for lithium metal batteries. J. Mater. Chem. A 2019, 8, 2021–2032. [Google Scholar] [CrossRef]
- Pereira-Pinheiro, M. Studies of Interphase Formation between Biomolecules and Metal and Oxide Surfaces in Aqueous Environment. Master’s Thesis, Universidad de Porto, Porto, Portugal, 2017. [Google Scholar]
- Nguyen, M.T.; Sutton, P.; Palumbo, A.; Fischer, M.G.; Hua, X.; Gunkel, I.; Steiner, U. Polymer-templated mesoporous lithium titanate microspheres for high-performance lithium batteries. Mater. Adv. 2021, 3, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Corso, A.D. Pseudopotentials periodic table: From H to Pu. Comput. Mater. Sci. 2014, 95, 337–350. [Google Scholar] [CrossRef]
- Wildberger, K.; Lang, P.; Zeller, R.; Dederichs, P.H. Fermi-Dirac distribution in ab initio Green’s-function calculations. Phys. Rev. B 1995, 52, 11502. [Google Scholar] [CrossRef]
- The Materials Project. Materials Data on Li4Ti5O12 by Materials Project. United States. Available online: https://www.osti.gov/dataexplorer/biblio/dataset/1284125 (accessed on 11 April 2022).
- Calderín, L.; Karasiev, V.; Trickey, S.B. Kubo–Greenwood electrical conductivity formulation and implementation for projector augmented wave datasets. Comput. Phys. Commun. 2017, 221, 118–142. [Google Scholar] [CrossRef]
- Hoi, B.D.; Yarmohammadi, M. The Kubo-Greenwood spin-dependent electrical conductivity of 2D transition-metal dichalcogenides and group-IV materials: A Green’s function study. J. Magn. Magn. Mater. 2018, 451, 57–64. [Google Scholar] [CrossRef]
- Ureña, Y.R.C.; Lisboa-Filho, P.N.; Szardenings, M.; Gätjen, L.; Noeske, P.-L.M.; Rischka, K. Formation and composition of adsorbates on hydrophobic carbon surfaces from aqueous laccase-maltodextrin mixture suspension. Appl. Surf. Sci. 2016, 385, 216–224. [Google Scholar] [CrossRef]
- Gonçalves Dias, L.; Stamboroski, S.; Noeske, P.L.M.; Salz, D.; Rischka, K.; Pereira, R.; Mainardi, M.; Honorato, M.; Wiesing, M.; Bronze-Uhle, E.; et al. New details of assembling bioactive films from dispersions of am-phiphilic molecules on titania surfaces. RSC Adv. 2020, 10, 39854–39869. [Google Scholar] [CrossRef]
- Tian, R.; Alcala, N.; O’Neill, S.; Horvath, D.; Coelho, J.; Griffin, A.; Zhang, Y.; Nicolosi, V.; Colm O’Dwyer, C.; Coleman, J. Quantifying the Effect of Electronic Conductivity on the Rate Performance of Nanocomposite Battery Electrodes. ACS Appl. Energy Mater. 2020, 3, 2966–2974. [Google Scholar] [CrossRef]
- Mujica, V.; Kemp, M.; Roitberg, A.; Ratner, M. Current-voltage characteristics of molecular wires: Eigenvalue staircase, Coulomb blockade, and rectification. J. Chem. Phys. A 1996, 104, 7296. [Google Scholar] [CrossRef]
- Tian, W.; Datta, S.; Hong, S.; Reifenberger, R.; Henderson, J.I.; Kubiak, C.P. Conductance spectra of molecular wires. J. Chem. Phys. 1998, 109, 2874–2882. [Google Scholar] [CrossRef]
- Tomfohr, J.K.; Sankey, O.F. Complex band structure, decay lengths, and Fermi level alignment in simple molecular electronic systems. Phys. Rev. B 2002, 65, 245105. [Google Scholar] [CrossRef]
- Cui, X.D.; Primak, A.; Zarate, X.; Tomfohr, J.; Sankey, O.F.; Moore, A.L.; Moore, T.A.; Gust, D.; Harris, G.; Lindsay, S.M. Reproducible Measurement of Single-Molecule Conductivity. Science 2001, 294, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Beebe, J.M.; Engelkes, V.B.; Miller, L.L.; Frisbie, C.D. Contact Resistance in Metal−Molecule−Metal Junctions Based on Aliphatic SAMs: Effects of Surface Linker and Metal Work Function. J. Am. Chem. Soc. 2002, 124, 11268–11269. [Google Scholar] [CrossRef] [PubMed]
- Slowinski, K.; Fong, H.K.Y.; Majda, M.J. Mercury−Mercury Tunneling Junctions. 1. Electron Tunneling Across Symmetric and Asymmetric Alkanethiolate Bilayers. J. Am. Chem. Soc. 1999, 121, 7257–7261. [Google Scholar] [CrossRef]
- Cui, X.; Zarate, X.; Tomfohr, J.; Sankey, O.F.; Primak, A.; Moore, A.L.; Moore, T.A.; Gust, D.; Harris, G.; Lindsay, S.M. Making electrical contacts to molecular monolayers. Nanotechnology 2001, 13, 5–14. [Google Scholar] [CrossRef]
- Haubner, K.; Murawski, J.; Olk, P.; Eng, L.M.; Ziegler, C.; Adolphi, B.; Jaehne, E. The Route to Functional Graphene Oxide. ChemPhysChem 2010, 11, 2131–2139. [Google Scholar] [CrossRef]
- Acik, M.; Lee, G.; Mattevi, C.; Pirkle, A.; Wallace, R.; Chhowalla, M.; Cho, K.; Chabal, Y. The Role of Oxygen during Thermal Reduction of Graphene Oxide Studied by Infrared Absorption Spectroscopy. J. Phys. Chem. C. 2011, 115, 40–19761. [Google Scholar] [CrossRef]
- Marsden, A.; Papageorgiou, D.; Vallés, C.; Liscio, A.; Palermo, V.; Bissett, M.; Young, R.; Kinloch, I. Electrical percolation in graphene–polymer composites. 2D Mater. 2018, 5, 032003. [Google Scholar] [CrossRef]
- Melitz, W.; Shen, J.; Kummel, A.C.; Lee, S. Kelvin probe force microscopy and its application. Surf. Sci. Rep. 2011, 66, 1–27. [Google Scholar] [CrossRef]
- Salerno, M.; Dante, S. Scanning Kelvin Probe Microscopy: Challenges and Perspectives towards Increased Application on Biomaterials and Biological Samples. Materials 2018, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Birkenhauer, E.; Neethirajan, S. Surface Potential Measurement of Bacteria Using Kelvin Probe Force Microscopy. J. Vis. Exp. 2014, 93, e52327. [Google Scholar] [CrossRef]
- Chang, K.; Jun, H.; Hee, Z.; Hzeon, J.; Lee, T.; Won, H.; Kwak, K.; Park, K.; Zoung, S. Effect of Amine-Based Organic Compounds on the Work-Function decrease of Graphene. J. Phys. Chem. C 2016, 120, 1309–1316. [Google Scholar]
- Zhou, Y.; Fuentes-Hernandez, C.; Shim, J.; Meyer, J.; Giordano, A.; Li, H.; Winget, P.; Papadopoulos, T.; Cheun, H.; Kippelen, B. A Universal Method to Produce Low-Work Function Electrodes. Science 2012, 336, 327. [Google Scholar] [CrossRef]
- Cheong, K.Y.; Tayeb, I.A.; Zhao, F.; Abdullah, J.M. Review on resistive switching mechanisms of bio-organic thin film for non-volatile memory application. Nanotechnol. Rev. 2021, 10, 680–709. [Google Scholar] [CrossRef]
- Chauhan, A.; Asylbekov, E.; Kespe, S.; Nirschl, H. Influence of carbon binder domain on the performance of lithium-ion batteries: Impact of size and fractal dimension. Electrochem. Sci. Adv. 2022, e2100151. [Google Scholar] [CrossRef]
- Sun, X.; Radovanovic, P.V.; Cui, B. ChemInform Abstract: Advances in Spinel Li4Ti5O12 Anode Materials for Lithium-Ion Batteries. ChemInform 2016, 46, 38–63. [Google Scholar] [CrossRef]
- Ahmad, M.; Han, S.A.; Tien, D.H.; Jung, J.; Seo, Y. Local conductance measurement of graphene layer using conductive atomic force microscopy. J. Appl. Phys. 2011, 110, 054307. [Google Scholar] [CrossRef]
- Kim, J.-B.; Premkumar, T.; Giani, O.; Robin, J.-J.; Schue, F.; Geckeler, K.E. Nanocomposites of poly(L-lysine) and single-walled carbon nanotubes. Polym. Int. 2008, 57, 311–315. [Google Scholar] [CrossRef]
- Huang, H.-H.; De Silva, K.K.H.; Kumara, G.R.A.; Yoshimura, M. Structural Evolution of Hydrothermally Derived Reduced Graphene Oxide. Sci. Rep. 2018, 8, 4836. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, Z.; Huang, J.; He, X.; Chen, Y.; Zhang, R.; Lin, R.; Li, Y.; Yu, S.; Xing, X.; et al. Elucidating the Limit of Li Insertion into the Spinel Li4Ti5O12. ACS Mater. Lett. 2019, 1, 96–102. [Google Scholar] [CrossRef]
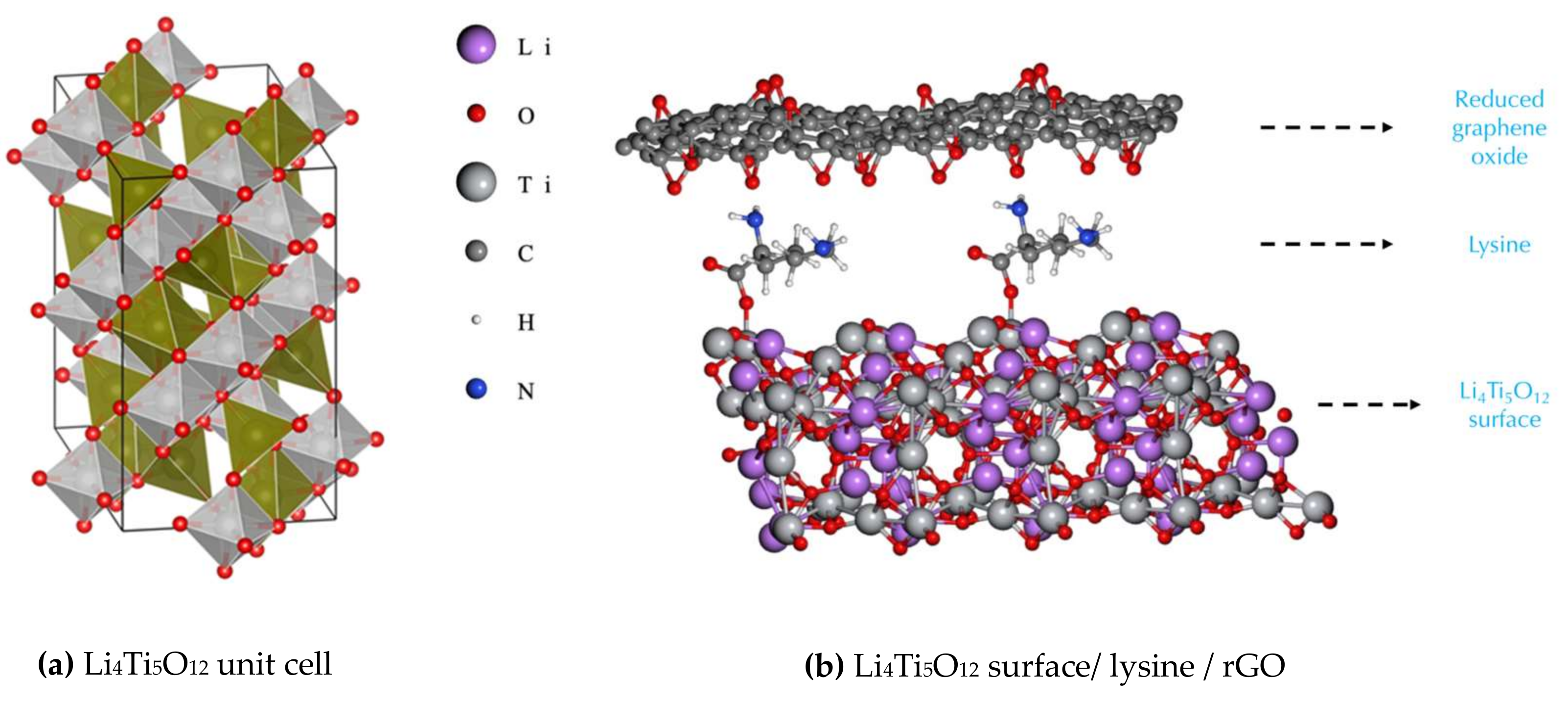
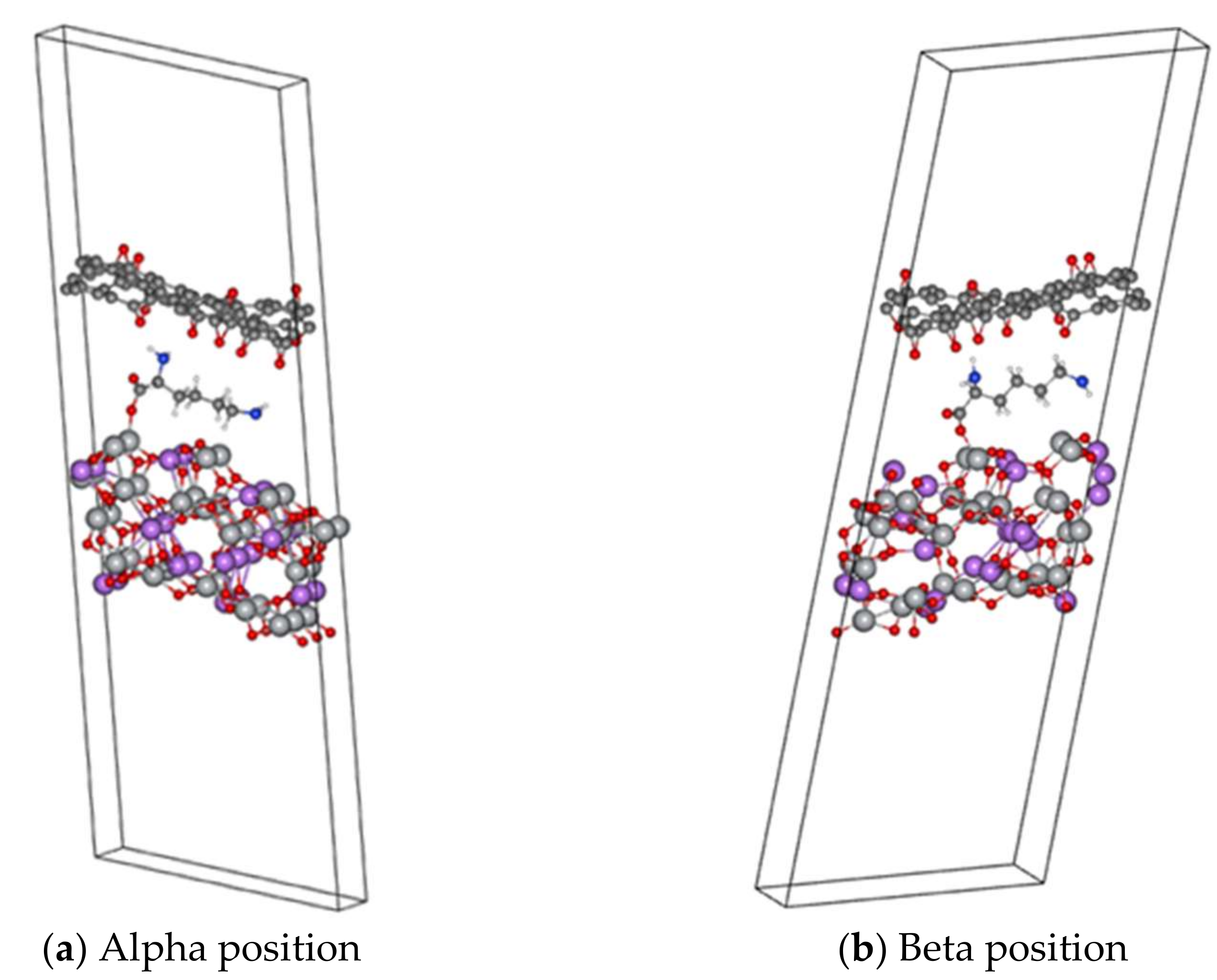
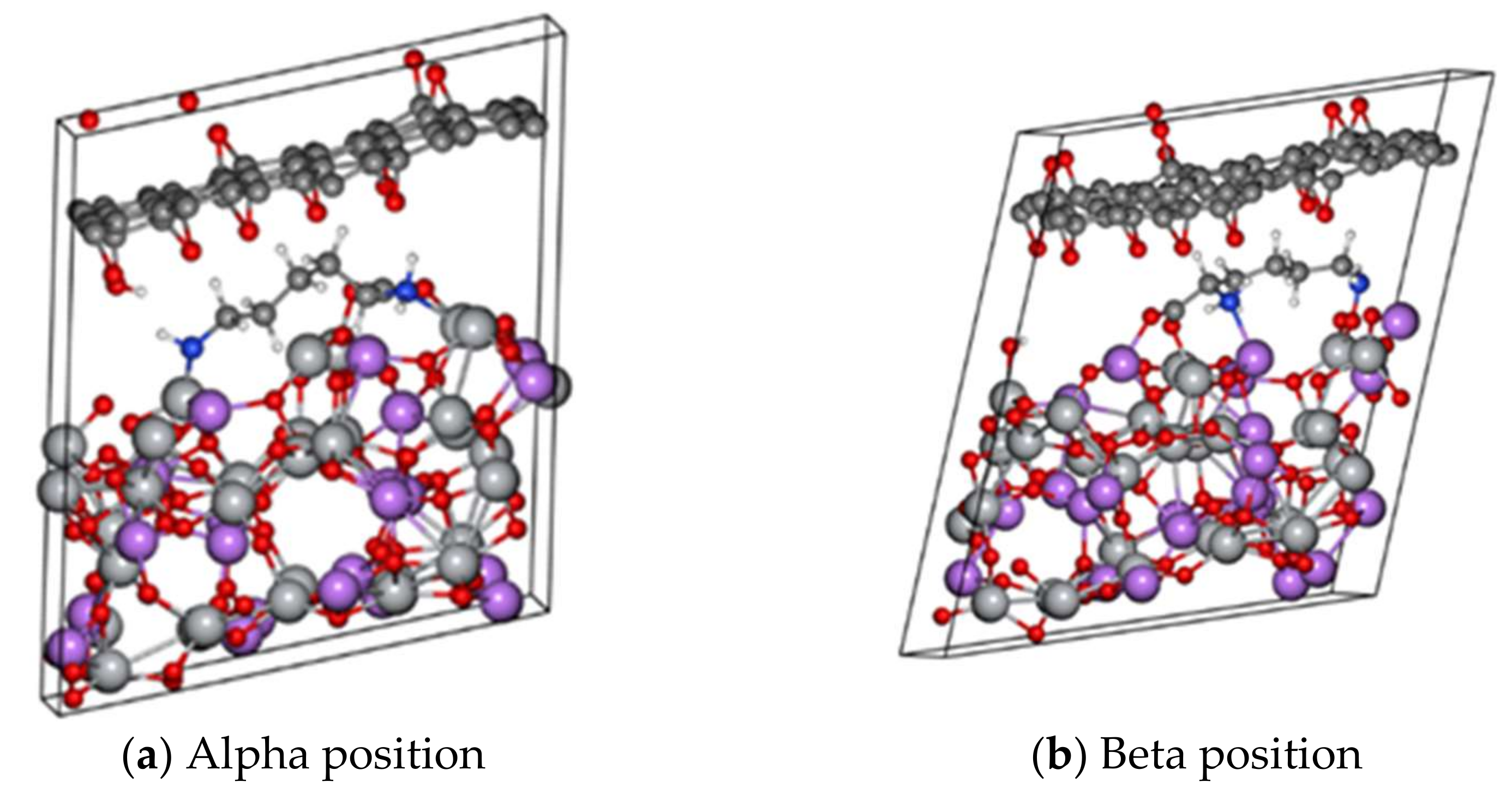

| Li4Ti5O12 Surface | Electrical Conductivity (S/m) | Energy per Atom (kcal/atom) |
|---|---|---|
| (000) | 32.984 | 7376.343 |
| (100) | 49.591 | 838.227 |
| (010) | 78.365 | 844.188 |
| (001) | 639.987 | 0.062 |
| (101) | 47.258 | 658.885 |
| (111) | 656.584 | 0.0000 |
| Li4Ti5O12 Surface | Electrical Conductivity (S/m) | Energy per Atom (kcal/atom) |
|---|---|---|
| (000) | 30.045 | 7485.435 |
| (100) | 139.888 | 1826.711 |
| (010) | 1002.756 | 1830.539 |
| (001) | 510.639 | 128.796 |
| (101) | 66.756 | 7003.100 |
| (111)α | 282.465 | 126.129 |
| (111)β | 1794.006 | 0.0000 |
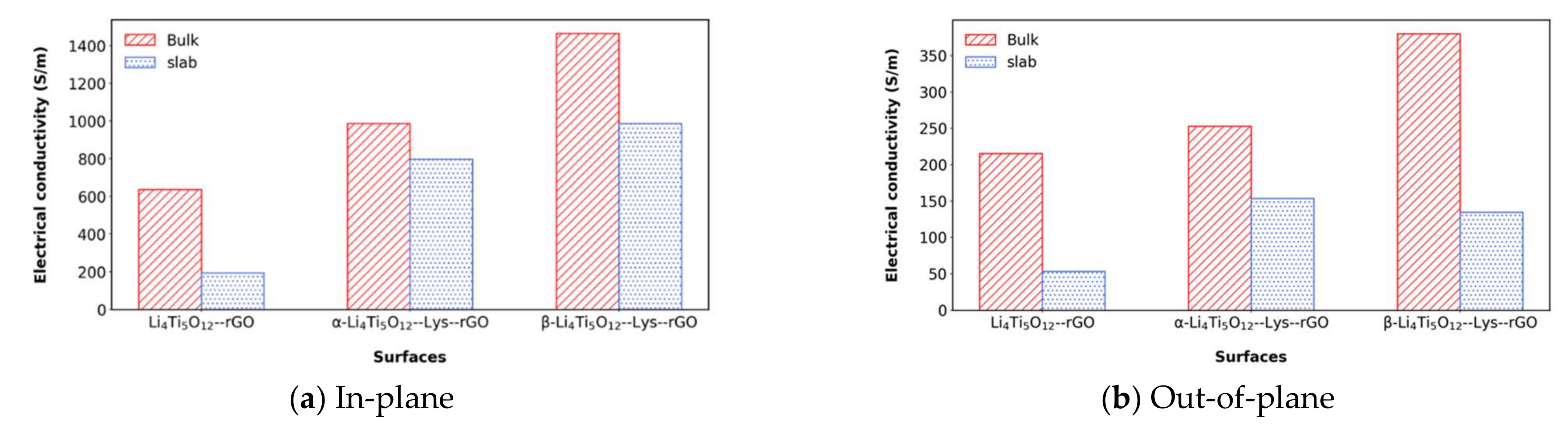
| Interphase | Slab (In-Plane) | Bulk (In-Plane) | Slab (Out-of Plane) | Bulk (Out-of-Plane) |
|---|---|---|---|---|
| Li4Ti5O12/rGO (no lysine in between) | 193.7 | 636.0 | 52.6 | 215.4 |
| α-Li4Ti5O12/Lys/rGO | 798.3 | 985.8 | 153.8 | 253.1 |
| β-Li4Ti5O12/Lys/rGO | 987.3 | 1464.9 | 134.6 | 380.3 |
| β-Li4Ti5O12/Lys/Lys-rGO | 251.9 | 66.8 |
| Samples | [O] | [Ti] | [N] | [C] | Layer Thickness | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (at%) | (at%) | (at%) | (at%) | (nm) | ||||||
| as-dep. | annl. | as-dep. | annl. | as-dep. | annl. | as-dep. | annl. | as-dep. | annl. | |
| A | ||||||||||
| Pristine TiO2 | 47.8 | 53.1 | 21.2 | 23.0 | 0.4 | 0.5 | 28.6 | 21.1 | ||
| TiO2/PLL | 49.0 | 52.4 | 21.6 | 22.2 | 2.6 | 2.1 | 24.9 | 21.2 | 0.3 | 0.2 |
| TiO2/PLL/GO, sample A | 37.6 | 31.4 | 12.8 | 9.1 | 1.6 | 1.4 | 44.7 | 56.7 | 1.3 | 1.4 |
| TiO2/PLL/GO, sample B | 29.9 | 25.8 | 4.1 | 6.1 | 1.1 | 1.2 | 64 | 65.7 | 3.5 | 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borge-Durán, I.; Grinberg, I.; Vega-Baudrit, J.R.; Nguyen, M.T.; Pereira-Pinheiro, M.; Thiel, K.; Noeske, P.-L.M.; Rischka, K.; Corrales-Ureña, Y.R. Application of Poly-L-Lysine for Tailoring Graphene Oxide Mediated Contact Formation between Lithium Titanium Oxide LTO Surfaces for Batteries. Polymers 2022, 14, 2150. https://doi.org/10.3390/polym14112150
Borge-Durán I, Grinberg I, Vega-Baudrit JR, Nguyen MT, Pereira-Pinheiro M, Thiel K, Noeske P-LM, Rischka K, Corrales-Ureña YR. Application of Poly-L-Lysine for Tailoring Graphene Oxide Mediated Contact Formation between Lithium Titanium Oxide LTO Surfaces for Batteries. Polymers. 2022; 14(11):2150. https://doi.org/10.3390/polym14112150
Chicago/Turabian StyleBorge-Durán, Ignacio, Ilya Grinberg, José Roberto Vega-Baudrit, Minh Tri Nguyen, Marta Pereira-Pinheiro, Karsten Thiel, Paul-Ludwig Michael Noeske, Klaus Rischka, and Yendry Regina Corrales-Ureña. 2022. "Application of Poly-L-Lysine for Tailoring Graphene Oxide Mediated Contact Formation between Lithium Titanium Oxide LTO Surfaces for Batteries" Polymers 14, no. 11: 2150. https://doi.org/10.3390/polym14112150
APA StyleBorge-Durán, I., Grinberg, I., Vega-Baudrit, J. R., Nguyen, M. T., Pereira-Pinheiro, M., Thiel, K., Noeske, P.-L. M., Rischka, K., & Corrales-Ureña, Y. R. (2022). Application of Poly-L-Lysine for Tailoring Graphene Oxide Mediated Contact Formation between Lithium Titanium Oxide LTO Surfaces for Batteries. Polymers, 14(11), 2150. https://doi.org/10.3390/polym14112150