Natural and Synthetic Polymers Modified with Acid Blue 113 for Removal of Cr3+, Zn2+ and Mn2+
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Equipment
2.3. Preparing the MS for AB 113 Adsorption Experiments
2.4. Preparing the XAD7HP Resin for AB 113 Adsorption Experiments
2.5. Procedure for Determining the Dose of Adsorbent Material
2.6. Batch Adsorption Experiments for AB 113 Removal
2.7. Procedure for Recovery AB 113 from MS and XAD7HP
2.8. Characterization of Materials Loaded in the Presence of Acidic Solutions
2.9. Metal Ions Removal Procedures
2.10. Procedure for Recovery of MX+ from the MS-AB 113 and XAD7HP-AB 113
2.11. Preparation of Metal Ions Solutions to Establish the Linearity of the Atomic Absorption Spectrometric Method (AAS)
2.12. Spectrometric Determination of AB 113
2.13. Determination of LD and LQ for UV-VIS Spectrometric Method
2.14. Determination of LD and LQ for AAS Method
3. Results and Discussion
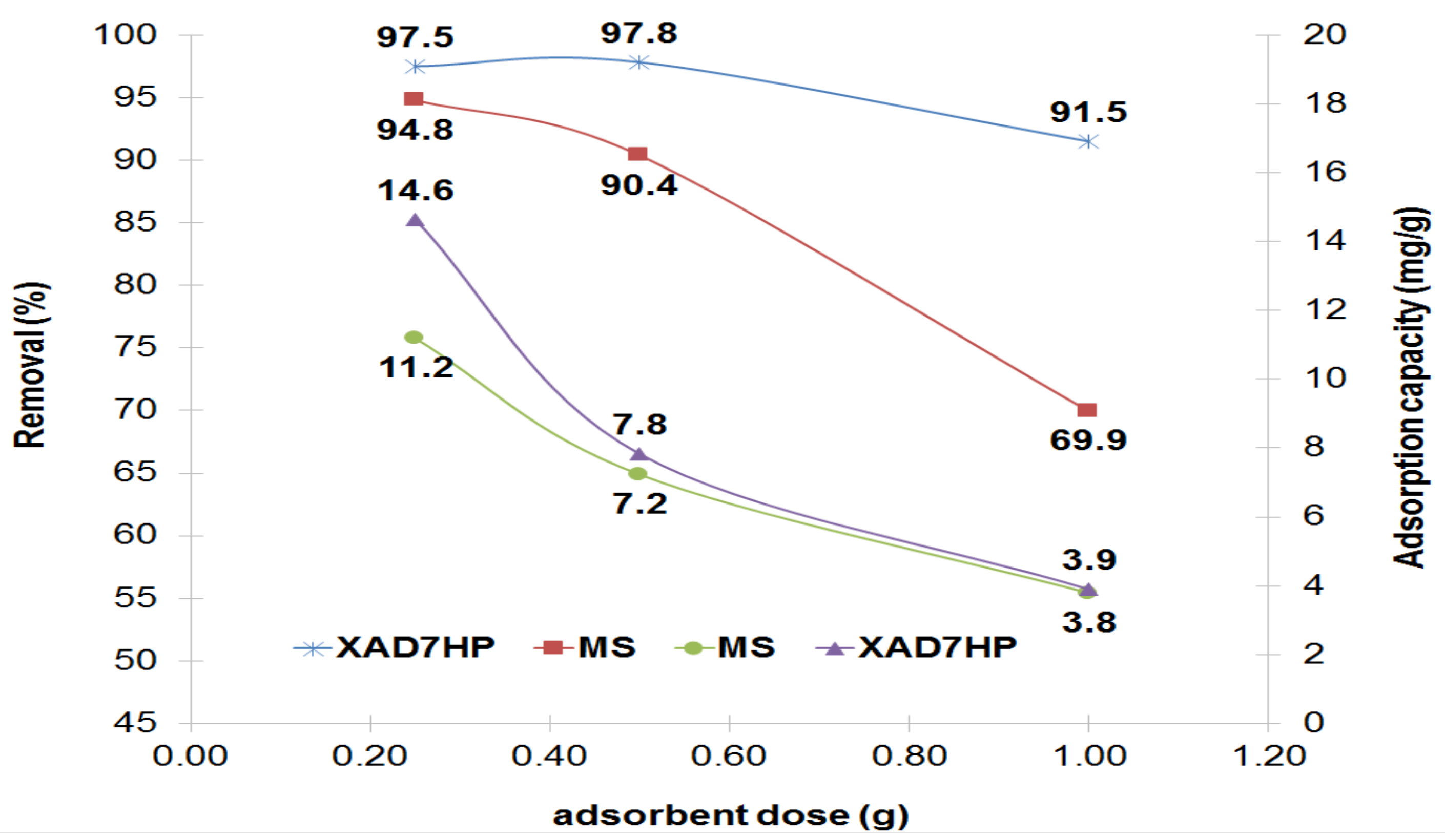
3.1. Influence of MS and XAD7HP Dosage
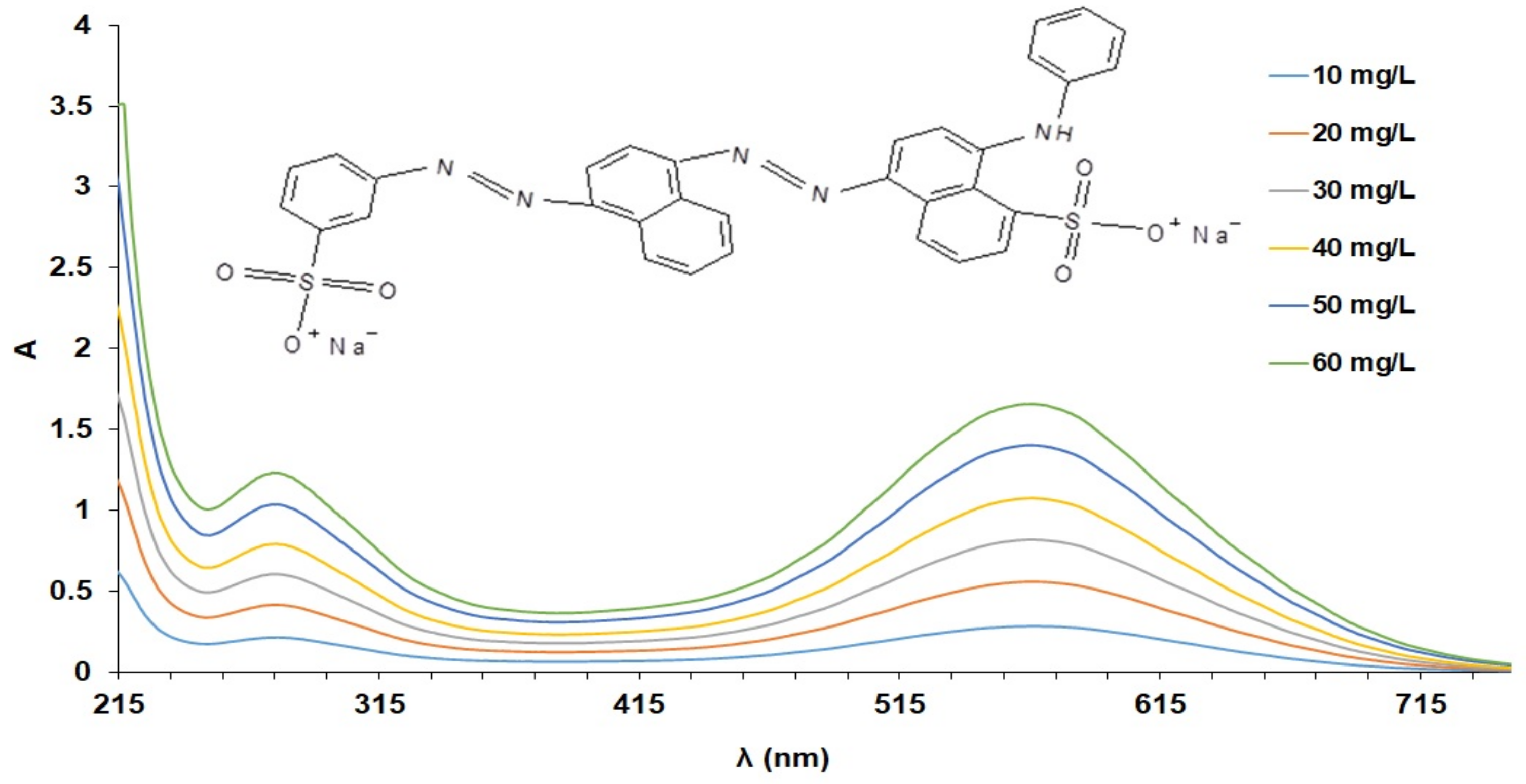
3.2. Influence of AB 113 Concentrations on the MS and XAD7HP Resin
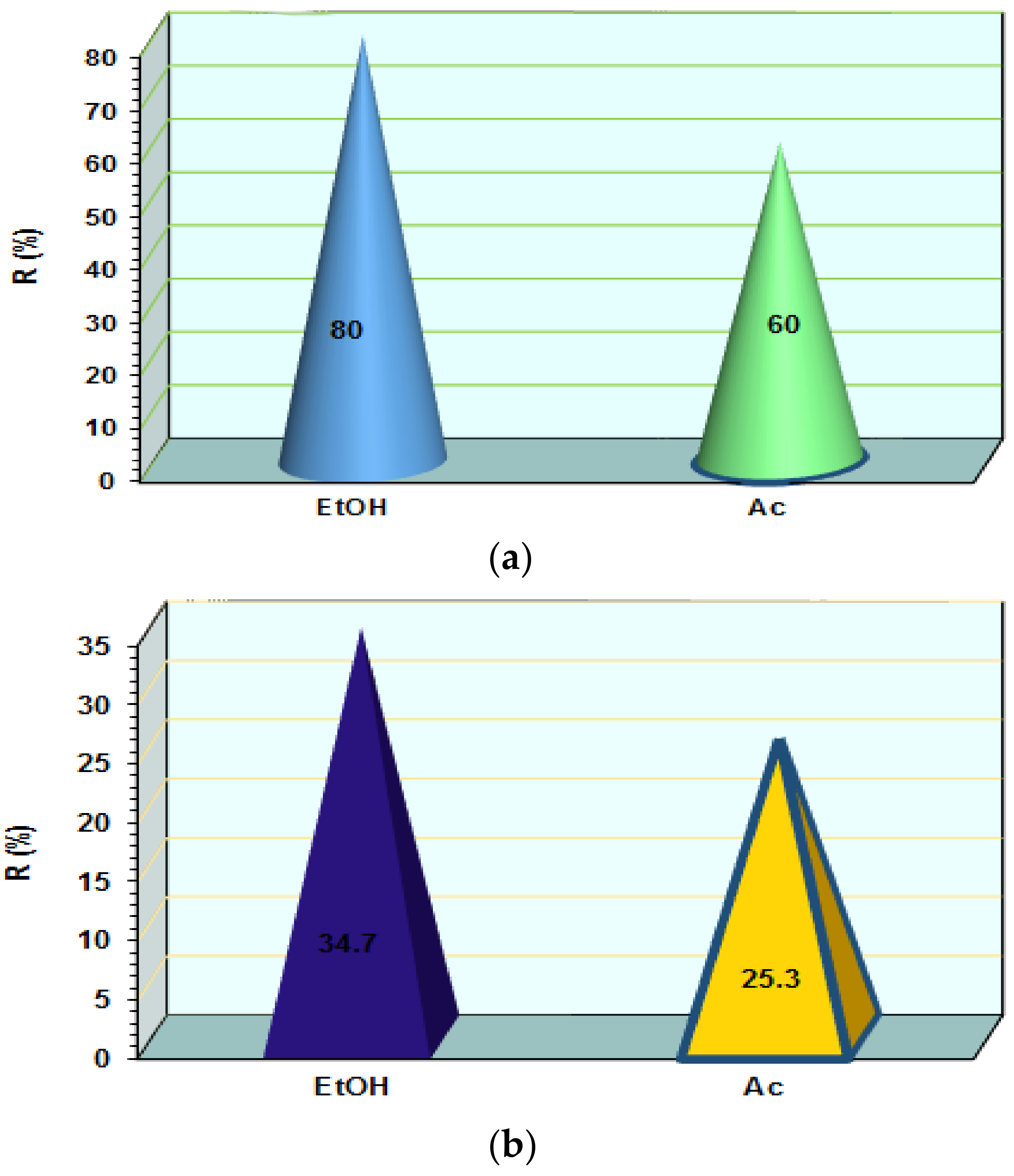
3.3. Influence of Organic Agent on AB 113 Desorption
3.4. Influence of Acid Medium on AB 113 Desorption
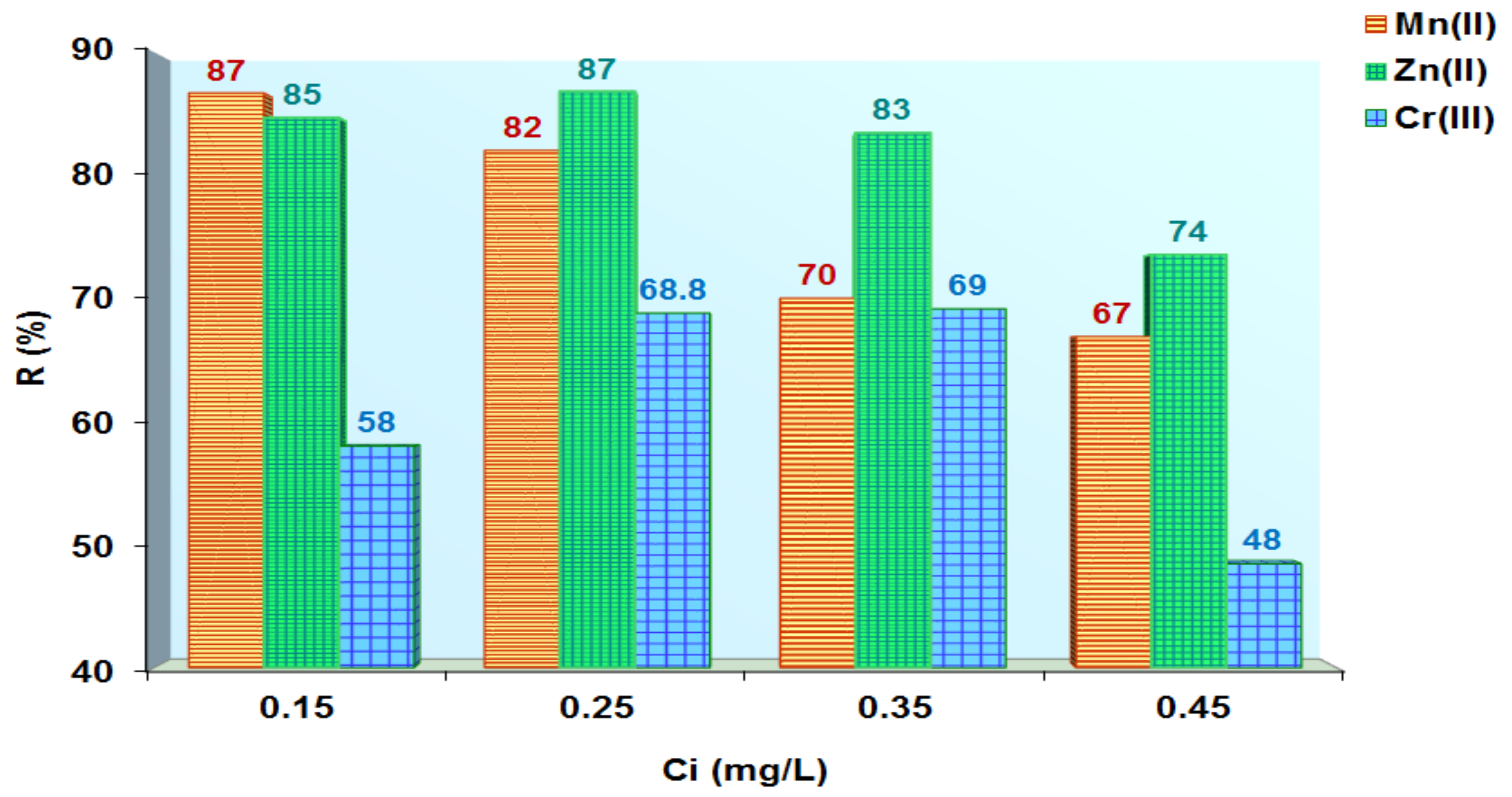
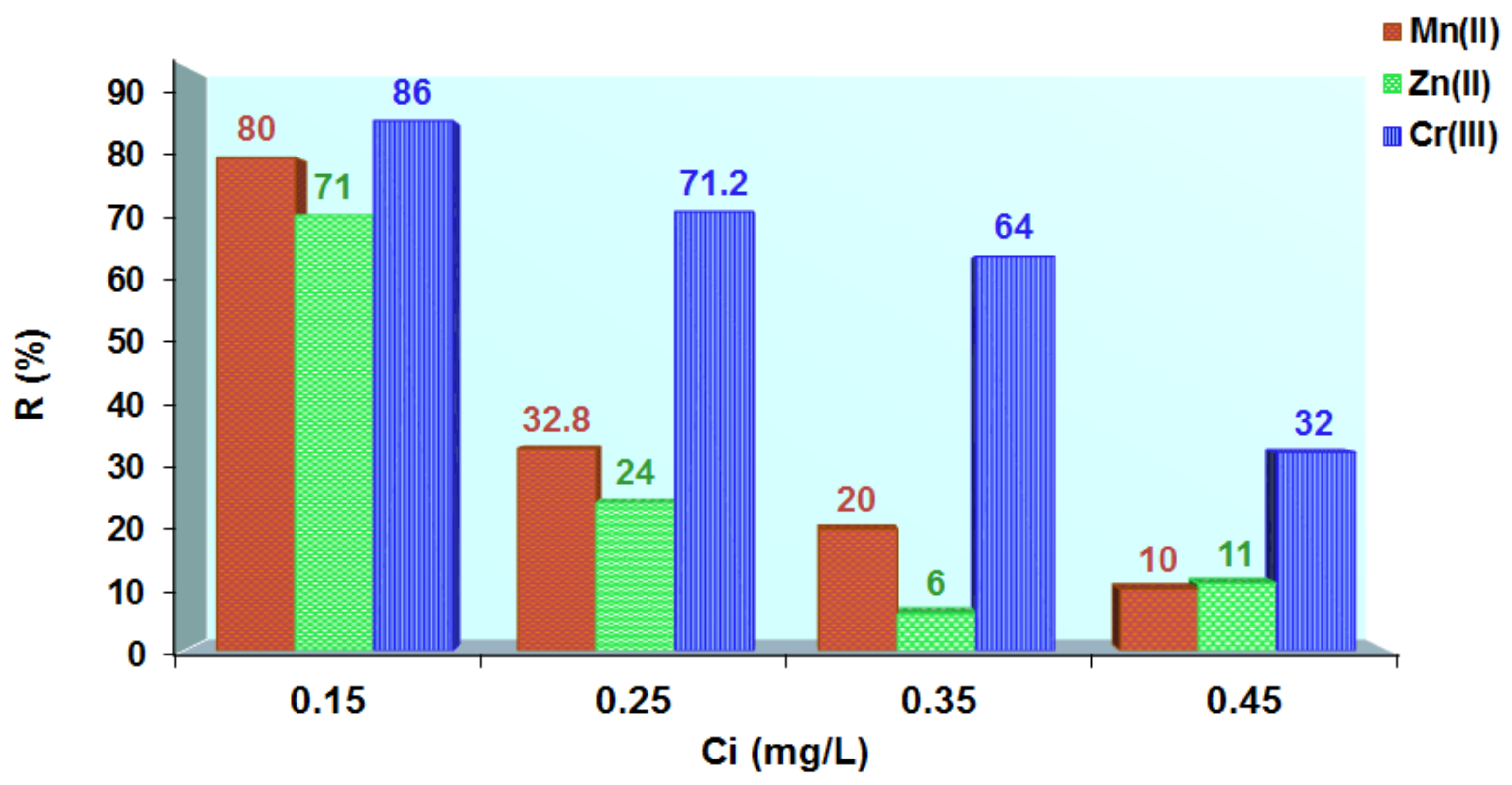
3.5. Applications of Loaded Polymers for Aqueous Samples Depollution with Cr3+, Zn2+ and Mn2+
3.6. Desorption of Metal Ions from the Loaded Polymers
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jally, B.; François, M.; Kessler, M.; Laubie, B.; Simonnot, M.-O. Recovery of nickel from strongly acidic bio-ore leachate using a bispicolylamine-based chelating resin. Sep. Purif. Technol. 2022, 293, 121126. [Google Scholar] [CrossRef]
- Suwannahong, K.; Sripirom, J.; Sirilamduan, C.; Thathong, V.; Kreetachart, T.; Panmuang, P.; Deepatana, A.; Punbut, S.; Wongcharee, S. Selective Chelating Resin for Copper Removal and Recovery in Aqueous Acidic Solution Generated from Synthetic Copper-Citrate Complexes from Bioleaching of E-waste. Adsorpt. Sci. Technol. 2022, 2022, 5009124. [Google Scholar] [CrossRef]
- Kaur, J.; Sengupta, P.; Mukhopadhyay, S. Critical Review of Bioadsorption on Modified Cellulose and Removal of Divalent Heavy Metals (Cd, Pb, and Cu). Ind. Eng. Chem. Res. 2022, 61, 1921–1954. [Google Scholar] [CrossRef]
- Chen, L.; Tang, J.; Wu, S.; Wang, S.; Ren, Z. Selective removal of Au(III) from wastewater by pyridine-modified chitosan. Carbohydr. Polym. 2022, 286, 119307. [Google Scholar] [CrossRef]
- Hassanzadeh-Afruzi, F.; Esmailzadeh, F.; Asgharnasl, S.; Ganjali, F.; Taheri-Ledari, R.; Maleki, A. Efficient removal of Pb(II)/Cu(II) from aqueous samples by a guanidine-functionalized SBA-15/Fe3O4. Sep. Purif. Technol. 2022, 291, 120956. [Google Scholar] [CrossRef]
- Hamidon, T.S.; Adnan, R.; Haafiz, M.K.M.; Hussin, M.H. Cellulose-based beads for the adsorptive removal of wastewater effluents: A review. Environ. Chem. Lett. 2022, 61, 1921–1954. [Google Scholar] [CrossRef]
- Khan, A.M.; Gautam, S.; Ganai, S.A. Sorption studies on cresol red modified Amberlite IR400 (Cl−) resin: Binary and selective separation of Hg2+ ions. Indian J. Chem. Technol. 2008, 15, 547–554. [Google Scholar]
- Roy, K.; Basu, S. Separation of Gold and Silver Using a Chelating Resin-Thiosemicarbazide Incorporated Amberlite IRC-50. Indian J. Chem. A 2005, 44, 531–534. [Google Scholar]
- Chen, Y.; Pan, B.; Zhang, S.; Li, H.; Lv, L.; Zhang, W. Immobilization of polyethylenimine nanoclusters onto a cation exchange resin through self-crosslinking for selective Cu(II) removal. J. Hazard. Mater. 2011, 190, 1037–1044. [Google Scholar] [CrossRef]
- Singh, B.; Maiti, B. Separation and preconcentration of U (VI) on XAD-4 modified with 8-hydroxy quinoline. Talanta 2006, 69, 393–396. [Google Scholar] [CrossRef]
- Leszczyńska-Wawrzkiewicz, M.; Hubicki, Z. Application of Amberlite IRA-402 Modified by Means of 2-(p-Sulphophenylazo)-1,8-dihydroxy-3,6-naphthalene Disulphonate for the Recovery of Cu (II), Co (II), Cd (II), Ni (II), Mn (II) and Fe (III) Ions. Adsorpt. Sci. Technol. 2008, 26, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Narin, I.; Soylak, M.; Kayakirilmaz, K.; Elci, L.; Dogan, M. Preparation of a chelating resin by immobilizing 1-(2-pyridylazo) 2-naphtol on amberlite XAD-16 and its application of solid phase extraction of Ni (II), Cd (II), Co (II), Cu (II), Pb (II), and Cr (III) in natural water samples. Anal. Lett. 2003, 36, 641–658. [Google Scholar] [CrossRef]
- Fatah, A.; Elashry, S.M. La (III) Separation by Tri Octyl Phosphine Oxide (Cyanex 921) Based on Amberlite Xad-4 Chelating Resin. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1–13. [Google Scholar] [CrossRef]
- Gao, W.; Roger Razanajatovo, M.; Song, Y.; Zhao, X.; Zhao, Z.; Peng, Q.; Jiao, T.; Liu, X.; Zhang, Q. Efficient heavy metal sequestration from water by Mussel-inspired polystyrene conjugated with polyethyleneimine (PEI). Chem. Eng. J. 2022, 429, 132599. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, Z.; Feng, L.; Luo, X.; Wu, P.; Cui, L.; Mao, X. Modified cellulose by polyethyleneimine and ethylenediamine with induced Cu(II) and Pb(II) adsorption potentialities. Carbohydr. Polym. 2018, 202, 470–478. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb (II), Cd (II) and Cu (II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef]
- Amberlite™ XAD7HP (XAD7)—Product Information Sheet. Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/236/853/xad7pis.pdf (accessed on 19 April 2022).
- Marin, N.M.; Stanculescu, I. Removal of procainamide and lidocaine on Amberlite XAD7HP resin and of As(V), Pb(II) and Cd(II) on the impregnated resin for water treatment. Mater. Chem. Phys. 2022, 277, 125582. [Google Scholar] [CrossRef]
- Marin, N.M.; Dinu, L.; Stanculescu, I.; Cristea, N.I.; Ionescu, A.I. Maize stalk material for on-site treatment of highly polluted leachate and mine wastewater. Materials 2021, 14, 956. [Google Scholar] [CrossRef]
- Călinescu, O.; Marin, N.M.; Ioniță, D.; Pascu, L.F.; Tudorache, A.; Surpățeanue, G.; Badea, I.A.; Aboul-Enein, H.Y. Selective removal of sulfate ion from different drinking waters. Environ. Nanotechnol. Monit. Manag. 2016, 6, 164–168. [Google Scholar] [CrossRef]
- Kosugi, M.; Mizuna, K.; Sazawa, K.; Okazaki, T.; Kuramitz, H.; Taguchi, S.; Hata, N. Organic Ion-Associate Phase Microextraction/Back-Microextraction for Preconcentration: Determination of Nickel in Environmental Water Using 2-Thenoyltrifluoroacetone via GF-AAS. AppliedChem 2021, 1, 130–141. [Google Scholar] [CrossRef]
- Indrayanto, G. Chapter Five—Validation of Chromatographic Methods of Analysis: Application for Drugs That Derived from Herbs. Profiles Drug Subst. Excip. Relat. Methodol. 2018, 43, 359–392. [Google Scholar] [CrossRef]
- Ezeh, K.; Ogbu, I.; Akpomie, K.; Ojukwu, N.; Ibe, J. Utilizing the Sorption Capacity of Local Nigerian Sawdust for Attenuation of Heavy Metals from Solution: Isotherm, Kinetic, and Thermodynamic Investigations. Pac. J. Sci. Technol. 2017, 18, 251–264. [Google Scholar]
- Awasthi, A.; Datta, D. Application of Amberlite XAD-7HP resin impregnated with Aliquat 336 for the removal of Reactive Blue-13 dye: Batch and fixed-bed column studies. J. Environ. Chem. Eng. 2019, 7, 103502. [Google Scholar] [CrossRef]
- Mousa, K.M.; Taha, A.H. Adsorption of Reactive Blue dye onto natural and modified wheat straw. J. Chem. Eng. Process Technol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Padmavathy, K.; Madhu, G.; Haseena, P. A study on effects of pH, adsorbent dosage, time, initial concentration and adsorption isotherm study for the removal of hexavalent chromium (Cr (VI)) from wastewater by magnetite nanoparticles. Procedia Technol. 2016, 24, 585–594. [Google Scholar] [CrossRef]
- Kerkez, Ö.; Bayazit, Ş.S.; Uslu, H. A comperative study for adsorption of methylene blue from aqueous solutions by two kinds of amberlite resin materials. Desalin. Water Treat. 2012, 45, 206–214. [Google Scholar] [CrossRef]
- Marin, N.M.; Stanculescu, I. Application of amberlite IRA 402 resin adsorption and laccase treatment for acid blue 113 removal from aqueous media. Polymers 2021, 13, 3991. [Google Scholar] [CrossRef] [PubMed]
- Pourali, P.; Behzad, M.; Arfaeinia, H.; Ahmadfazeli, A.; Afshin, S.; Poureshgh, Y.; Rashtbari, Y. Removal of acid blue 113 from aqueous solutions using low-cost adsorbent: Adsorption isotherms, thermodynamics, kinetics and regeneration studies. Sep. Sci. Technol. 2021, 56, 3079–3091. [Google Scholar] [CrossRef]
- Shirzad-Siboni, M.; Jafari, S.J.; Giahi, O.; Kim, I.; Lee, S.-M.; Yang, J.-K. Removal of acid blue 113 and reactive black 5 dye from aqueous solutions by activated red mud. J. Ind. Eng. Chem. 2014, 20, 1432–1437. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Mengelizadeh, N.; Al Rawi, O.; Balarak, D. Capacity and modeling of acid blue 113 dye adsorption onto chitosan magnetized by Fe2O3 nanoparticles. J. Polym. Environ. 2022, 30, 344–359. [Google Scholar] [CrossRef]
- Soto, M.L.; Conde, E.; González-López, N.; Conde, M.J.; Moure, A.; Sineiro, J.; Falqué, E.; Domínguez, H.; Núñez, M.J.; Parajó, J.C. Recovery and concentration of antioxidants from winery wastes. Molecules 2012, 17, 3008–3024. [Google Scholar] [CrossRef] [PubMed]
- Marin, N.M.; Pascu, L.F.; Demba, A.; Nita-Lazar, M.; Badea, I.A.; Aboul-Enein, H. Removal of the Acid Orange 10 by ion exchange and microbiological methods. Int. J. Environ. Sci. Technol. 2019, 16, 6357–6366. [Google Scholar] [CrossRef]
- Marin, N.M.; Tiron, O.; Pascu, L.F.; Costache, M.; Nita-Lazar, M.; Badea, I.A. Synergistic methodology based on ion exchange and biodegradation mechanisms applied for metal complex dye removal from waste waters. Rev. Chim. 2018, 69, 38–44. [Google Scholar] [CrossRef]
- Ciopec, M.; Davidescu, C.M.; Negrea, A.; Grozav, I.; Lupa, L.; Negrea, P.; Popa, A. Adsorption studies of Cr (III) ions from aqueous solutions by DEHPA impregnated onto Amberlite XAD7–Factorial design analysis. Chem. Eng. Res. Des. 2012, 90, 1660–1670. [Google Scholar] [CrossRef]
- Davidescu, C.M.; Ciopec, M.; Negrea, A.; Popa, A.; Lupa, L.; Negrea, P.; Muntean, C.; Motoc, M. Use of di-(2-ethylhexyl) phosphoric acid (DEHPA) impregnated XAD7 copolymer resin for the removal of chromium (III) from water. Rev. Chim. 2011, 62, 712–717. [Google Scholar]
- Zheng, L.; Zhu, C.; Dang, Z.; Zhang, H.; Yi, X.; Liu, C. Preparation of cellulose derived from corn stalk and its application for cadmium ion adsorption from aqueous solution. Carbohydr. Polym. 2012, 90, 1008–1015. [Google Scholar] [CrossRef]
- Mittal, S.; Kumar, V.; Sharma, R.K. Solid phase extraction studies on cellulose based chelating resin for separation, pre-concentration and estimation of Cu2+ and Ni2+. J. Indian Chem. Soc. 2022, 99, 100481. [Google Scholar] [CrossRef]
- Marin, N.M.; Batrinescu, G.; Stanculescu, I.; Constantin, L.A.; Cristea, I.; Ionescu, I.; Catrina, G.A. Experimental Model for Cu (II) and Fe (III) Sorption from Synthetic Solutions Based on Maize Stalk. Rev. Chim. 2020, 71, 366–367. [Google Scholar] [CrossRef]
- Marin, N.M.; Pascu, L.F.; Stanculescu, I.; Iordache, O.; Jianu, D.; Petrescu, L.; Badea, I.A. Maize stalk as natural ion exchanger for hazardous pollutants. Rev. Chim. 2017, 68, 1726–1731. [Google Scholar] [CrossRef]



| Resin Type/Chemical Form/Particle Size | Chelating Agents | Metal Ion | Applications | References |
|---|---|---|---|---|
| Amberlite IRA 400, in Cl− form, 20–25 mesh (600–750 μm) | Cresol Red | Hg2+, Fe3+, Cu2+, Al3+ and Ni2+ | Hg2+ was separated by the Fe3+, Cu2+, Al3+ and Ni2 | Khan et al. [7] |
| Amberlite IRC-50, in COO− form, 16–50 mesh size | Thiosemicarbazone | Au3+ and Ag+ separation from binary mixtures | Au3+ and Ag+ were separated by the Cu2+ and Pb2+ | Roy et al. [8] |
| D001-strongly acidic cation exchange resin, in SO3− form 0.40 to 0.85 mm, | Polyethylenimine (PEI) and glutaraldehyde | Cu2+, Mg2+, Ca2+, Sr2+, | Cu2+ was separated in the presence of Mg2+, Ca2+, Sr2+ | Chen et al. [9] |
| Amberlite XAD-4, polystyrene divinyl benzene, 20–50 mesh | 8-hydroxy quinoline | U4+ | Synthetic solutions | Singh et al. [10] |
| Amberlite IRA 402, in Cl− form, 20–60 mesh | 2-(p-Sulfophenylazo)-1,8-dihydroxy-3,6-naphthalene disulfonate (SPADNS) | Cu2+, Co2+, Cd2+, Ni2+, Mn2+ and Fe3+ | Synthetic solutions | Wawrzkiewicz et al. [11] |
| Amberlite XAD-16, styrene divinylbenzene, 20–60 mesh | 1-(2-pyridylazo) 2-naphtol | Ni2+, Cd2+, Co2+, Cu2+, Pb2+ and Cr3+ | Natural water | Narin et al. [12] |
| Amberlite XAD-4, styrene divinylbenzene, 20–60 mesh | Tri Octyl Phosphine Oxide (Cyanex 921) | La3+ | Wastewater | Fatah et al. [13] |
| Sulfonated polystyrene (PSN) | Polyethyleneimine (PEI) and dopamine (PDA) | Pb2+, Fe3+, Cu2+ and Ni2+ | Wastewater | Gao et al. [14] |
| Cellulose acetate (CA) | Polyethyleneimine grafting (CAP) and then by ethylenediamine (CAPE) | Cu2+ and Pb2+ | Synthetic solutions | Huang et al. [15] |
| Biochar | Chitosan and pyromellitic dianhydride (PMDA) | Cd2+, Cu2+ and Pb2+ | Synthetic solutions | Deng et al. [16] |
| Parameter | Mn(II) | Zn(II) | Cr(III) |
|---|---|---|---|
| LD (µg/L) | 0.4 | 0.6 | 0.7 |
| LQ (µg/L) | 1.3 | 2.0 | 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, N.M. Natural and Synthetic Polymers Modified with Acid Blue 113 for Removal of Cr3+, Zn2+ and Mn2+. Polymers 2022, 14, 2139. https://doi.org/10.3390/polym14112139
Marin NM. Natural and Synthetic Polymers Modified with Acid Blue 113 for Removal of Cr3+, Zn2+ and Mn2+. Polymers. 2022; 14(11):2139. https://doi.org/10.3390/polym14112139
Chicago/Turabian StyleMarin, Nicoleta Mirela. 2022. "Natural and Synthetic Polymers Modified with Acid Blue 113 for Removal of Cr3+, Zn2+ and Mn2+" Polymers 14, no. 11: 2139. https://doi.org/10.3390/polym14112139
APA StyleMarin, N. M. (2022). Natural and Synthetic Polymers Modified with Acid Blue 113 for Removal of Cr3+, Zn2+ and Mn2+. Polymers, 14(11), 2139. https://doi.org/10.3390/polym14112139







