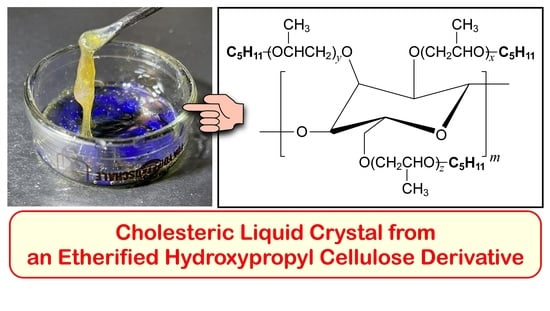
Rheological Properties of Cholesteric Liquid Crystal with Visible Reflection from an Etherified Hydroxypropyl Cellulose Derivative
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
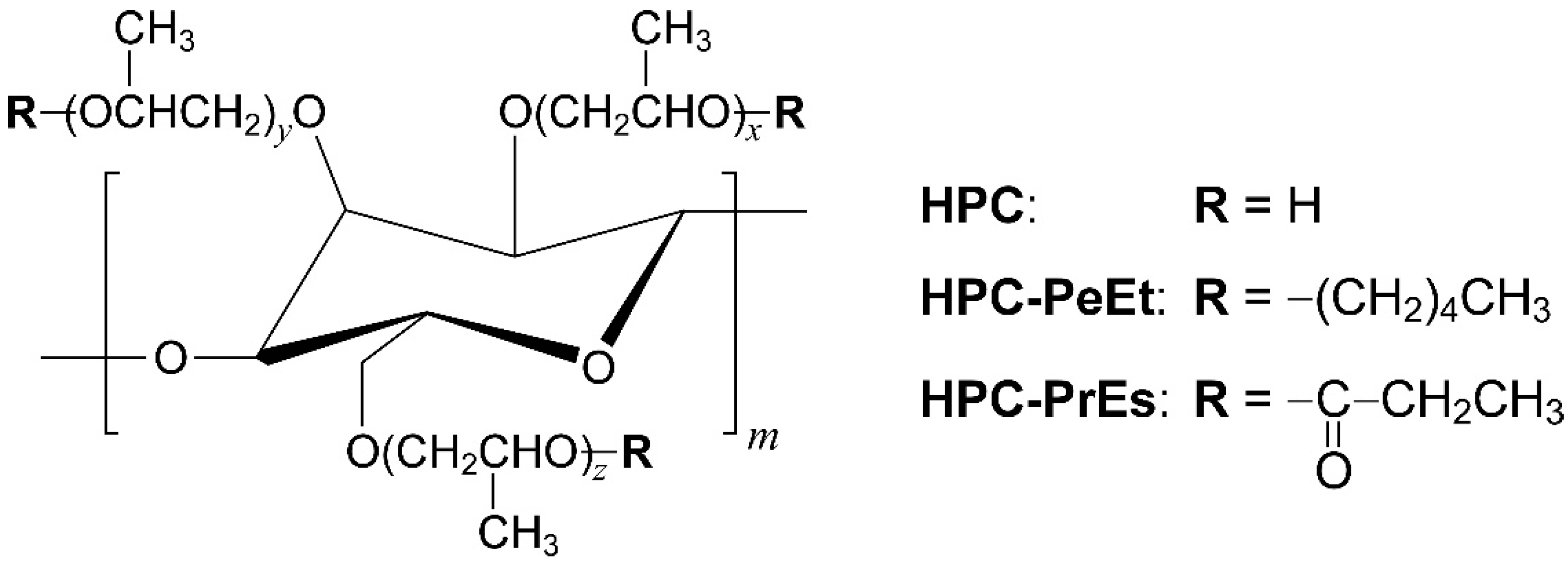
3.1. Characterization of HPC-PeEt
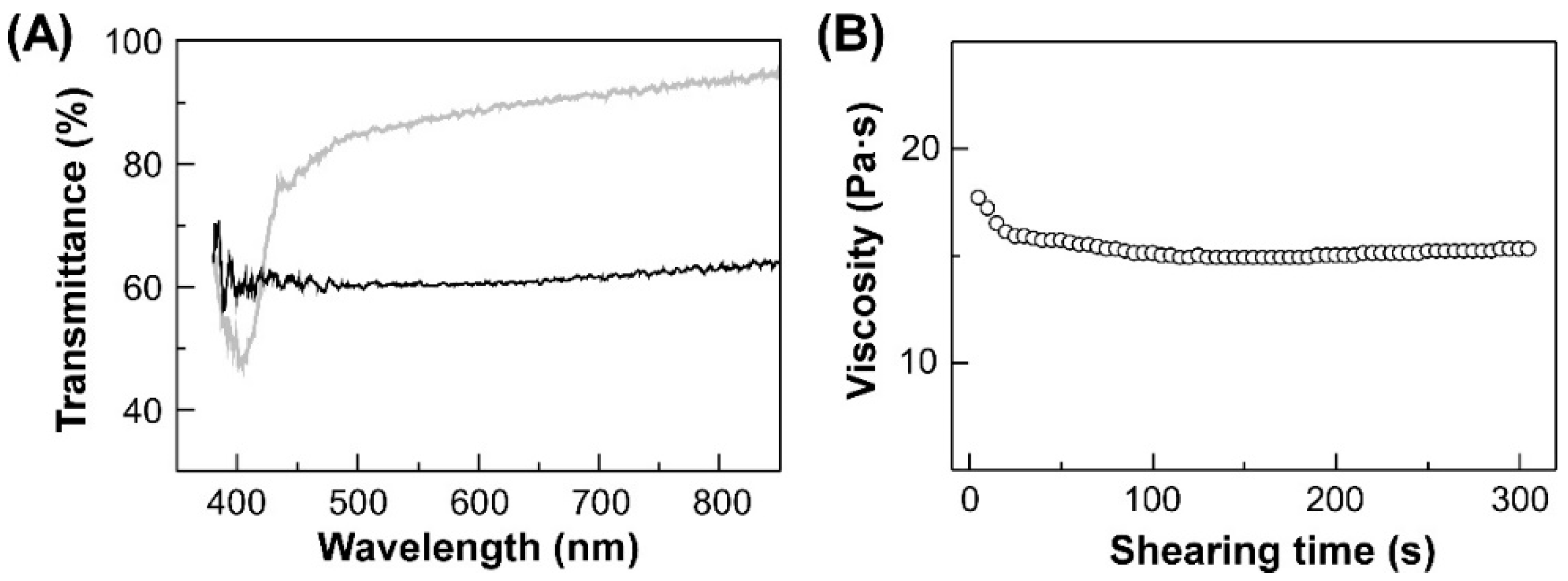
3.2. Optical Properties of HPC-PeEt
3.3. Pre-Treatments of HPC-PeEt before Rheological Measurements
3.4. Rheological Properties of HPC-PeEt
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Mathematical Derivation of Equation (2)
References
- Ali, A.; Ahmed, S. Recent Advances in Edible Polymer Based Hydrogels as a Sustainable Alternative to Conventional Polymers. J. Agric. Food Chem. 2018, 66, 6940–6967. [Google Scholar] [CrossRef] [PubMed]
- Kamita, G.; Frka-Petesic, B.; Allard, A.; Dargaud, M.; King, K.; Dumanli, A.G.; Vignolini, S. Biocompatible and Sustainable Optical Strain Sensors for Large-Area Applications. Adv. Opt. Mater. 2016, 4, 1950–1954. [Google Scholar] [CrossRef]
- Sarode, A.; Wang, P.; Cote, C.; Worthen, D.R. Low-Viscosity Hydroxypropylcellulose (HPC) Grades SL and SSL: Versatile Pharmaceutical Polymers for Dissolution Enhancement, Controlled Release, and Pharmaceutical Processing. AAPS PharmSciTech 2013, 14, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.L.C.; Bay, M.M.; Jacucci, G.; Vadrucci, R.; Williams, C.A.; van de Kerkhof, G.T.; Parker, R.M.; Vynck, K.; Frka-Petesic, B.; Vignolini, S. Visual Appearance of Chiral Nematic Cellulose-Based Photonic Films: Angular and Polarization Independent Color Response with a Twist. Adv. Mater. 2019, 31, 1905151. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.P.C.; Canejo, J.P.; Fernandes, S.N.; Echeverria, C.; Almeida, P.L.; Godinho, M.H. Cellulose-Based Biomimetics and their Applications. Adv. Mater. 2018, 30, 1703655. [Google Scholar] [CrossRef]
- Werbowyj, R.S.; Gray, D.G. Liquid Crystalline Structure in Aqueous Hydroxypropyl Cellulose Solutions. Mol. Cryst. Liq. Cryst. 1976, 34, 97–103. [Google Scholar] [CrossRef]
- Werbowyj, R.S.; Gray, D.G. Optical Properties of (Hydroxypropyl)cellulose Liquid Crystals. Cholesteric Pitch and Polymer Concentration. Macromolecules 1984, 17, 1512–1520. [Google Scholar] [CrossRef]
- Kosho, H.; Hiramatsu, S.; Nishi, T.; Tanaka, Y.; Kawauchi, S.; Watanabe, J. Thermotropic Cholesteric Liquid Crystals in Ester Derivatives of Hydroxypropylcellulose. High Perform. Polym. 1999, 11, 41–48. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Aoki, R.; Hayata, K.; Furukawa, M.; Fukawa, M.; Furumi, S. Fabrication of Human-Friendly Liquid Crystal Materials with α-Ionone. J. Photopolym. Sci. Technol. 2019, 32, 639–643. [Google Scholar] [CrossRef] [Green Version]
- Saito, S.; Hayata, K.; Furumi, S. Cholesteric Liquid Crystals from Cellulose Derivatives with Alkyl Ether Groups. J. Photopolym. Sci. Technol. 2020, 33, 461–465. [Google Scholar] [CrossRef]
- Baba, Y.; Saito, S.; Iwata, N.; Furumi, S. Synthesis and Optical Properties of Completely Etherified Hydroxypropyl Cellulose Derivatives. J. Photopolym. Sci. Technol. 2021, 34, 549–554. [Google Scholar]
- Yamagishi, T.; Sixou, P. Preparation and Characteristics of Cholesteric Gel from Pentyl Ether of Hydroxypropyl Cellulose. Polymer 1995, 36, 2315–2317. [Google Scholar] [CrossRef]
- Suto, S.; Hasegawa, S. Self-Colored Crosslinked Cholesteric Liquid Crystalline Solid Films of Hydroxypropyl Cellulose. J. Mater. Sci. 2002, 37, 4857–4863. [Google Scholar] [CrossRef]
- Yamagishi, T.; Guittard, F.; Godinho, M.H.; Martins, A.F.; Cambon, A.; Sixou, P. Comparison of Thermal and Cholesteric Mesophase Properties among the Three Kind of Hydroxypropylcellulose (HPC) Derivatives. Polym. Bull. 1994, 32, 47–54. [Google Scholar] [CrossRef]
- De Vries, H. Rotatory Power and Other Optical Properties of Certain Liquid Crystals. Acta Crystallogr. 1951, 4, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Furumi, S. Recent Progress in Chiral Photonic Band-Gap Liquid Crystals for Laser Applications. Chem. Rec. 2010, 10, 394–408. [Google Scholar] [CrossRef]
- Shimamura, K.; White, J.L.; Fellers, J.F. Hydroxypropylcellulose, a Thermotropic Liquid Crystal: Characteristics and Structure Development in Continuous Extrusion and Melt Spinning. J. Appl. Polym. Sci. 1981, 26, 2165–2180. [Google Scholar] [CrossRef]
- Onogi, S.; Asada, T. Rheology and Rheo-Optics of Polymer Liquid Crystals. In Rheology; Springer: Boston, MA, USA, 1980; Volume 10, pp. 127–147. [Google Scholar]
- Vshivkov, S.A.; Rusinova, E.V.; Saleh, A.S.A. Rheological Properties of Liquid Crystalline Solutions of Cellulose Derivatives. Polym. Sci. Ser. A 2021, 63, 363–368. [Google Scholar] [CrossRef]
- Ureña-Benavides, E.E.; Ao, G.; Davis, V.A.; Kitchens, C.L. Rheology and Phase Behavior of Lyotropic Cellulose Nanocrystal Suspensions. Macromolecules 2011, 44, 8990–8998. [Google Scholar] [CrossRef]
- Burghardt, W.R. Molecular Orientation and Rheology in Sheared Lyotropic Liquid Crystalline Polymers. Macromol. Chem. Phys. 1998, 199, 471–488. [Google Scholar] [CrossRef]
- Tseng, S.L.; Laivins, G.V.; Gray, D.G. Propanoate Ester of (2-Hydroxypropyl)cellulose: A Thermotropic Cholesteric Polymer that Reflects Visible Light at Ambient Temperatures. Macromolecules 1982, 15, 1262–1264. [Google Scholar] [CrossRef]
- Ogiwara, Y.; Iwata, N.; Furumi, S. Viscoelastic Properties of Cholesteric Liquid Crystals from Hydroxypropyl Cellulose Derivatives. J. Photopolym. Sci. Technol. 2021, 34, 537–542. [Google Scholar] [CrossRef]
- Ogiwara, Y.; Iwata, N.; Furumi, S. Unpublished results. 2022; manuscript in preparation. [Google Scholar]
- Ho, F.F.L.; Kohler, R.R.; Ward, G.A. Determination of Molar Substitution and Degree of Substitution of Hydroxypropyl Cellulose by Nuclear Magnetic Resonance Spectrometry. Anal. Chem. 1972, 44, 178–181. [Google Scholar] [CrossRef]
- Hou, H.; Reuning, A.; Wendorff, J.H.; Greiner, A. Tuning of the Pitch Height of Thermotropic Cellulose Esters. Macromol. Chem. Phys. 2000, 201, 2050–2054. [Google Scholar] [CrossRef]
- Laivins, G.V.; Gray, D.G. Optical Properties of (Acetoxypropyl)cellulose Mesophases: Factors Influencing the Cholesteric Pitch. Polymer 1985, 26, 1435–1442. [Google Scholar] [CrossRef]
- Ishizaki, T.; Uenuma, S.; Furumi, S. Thermotropic Properties of Cholesteric Liquid Crystal from Hydroxypropyl Cellulose Mixed Esters. Kobunshi Ronbunshu 2015, 72, 737–745. [Google Scholar] [CrossRef]



| Sample | Mn (× 104) | Mw (× 104) | Mw/Mn |
|---|---|---|---|
| Pristine HPC | 2.68 | 6.37 | 2.38 |
| HPC-PeEt | 1.65 | 4.89 | 2.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, K.; Ogiwara, Y.; Iwata, N.; Furumi, S. Rheological Properties of Cholesteric Liquid Crystal with Visible Reflection from an Etherified Hydroxypropyl Cellulose Derivative. Polymers 2022, 14, 2059. https://doi.org/10.3390/polym14102059
Matsumoto K, Ogiwara Y, Iwata N, Furumi S. Rheological Properties of Cholesteric Liquid Crystal with Visible Reflection from an Etherified Hydroxypropyl Cellulose Derivative. Polymers. 2022; 14(10):2059. https://doi.org/10.3390/polym14102059
Chicago/Turabian StyleMatsumoto, Kazuma, Yuki Ogiwara, Naoto Iwata, and Seiichi Furumi. 2022. "Rheological Properties of Cholesteric Liquid Crystal with Visible Reflection from an Etherified Hydroxypropyl Cellulose Derivative" Polymers 14, no. 10: 2059. https://doi.org/10.3390/polym14102059
APA StyleMatsumoto, K., Ogiwara, Y., Iwata, N., & Furumi, S. (2022). Rheological Properties of Cholesteric Liquid Crystal with Visible Reflection from an Etherified Hydroxypropyl Cellulose Derivative. Polymers, 14(10), 2059. https://doi.org/10.3390/polym14102059