Strength Enhancement of Regenerated Cellulose Fibers by Adjustment of Hydrogen Bond Distribution in Ionic Liquid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Molecular Simulation
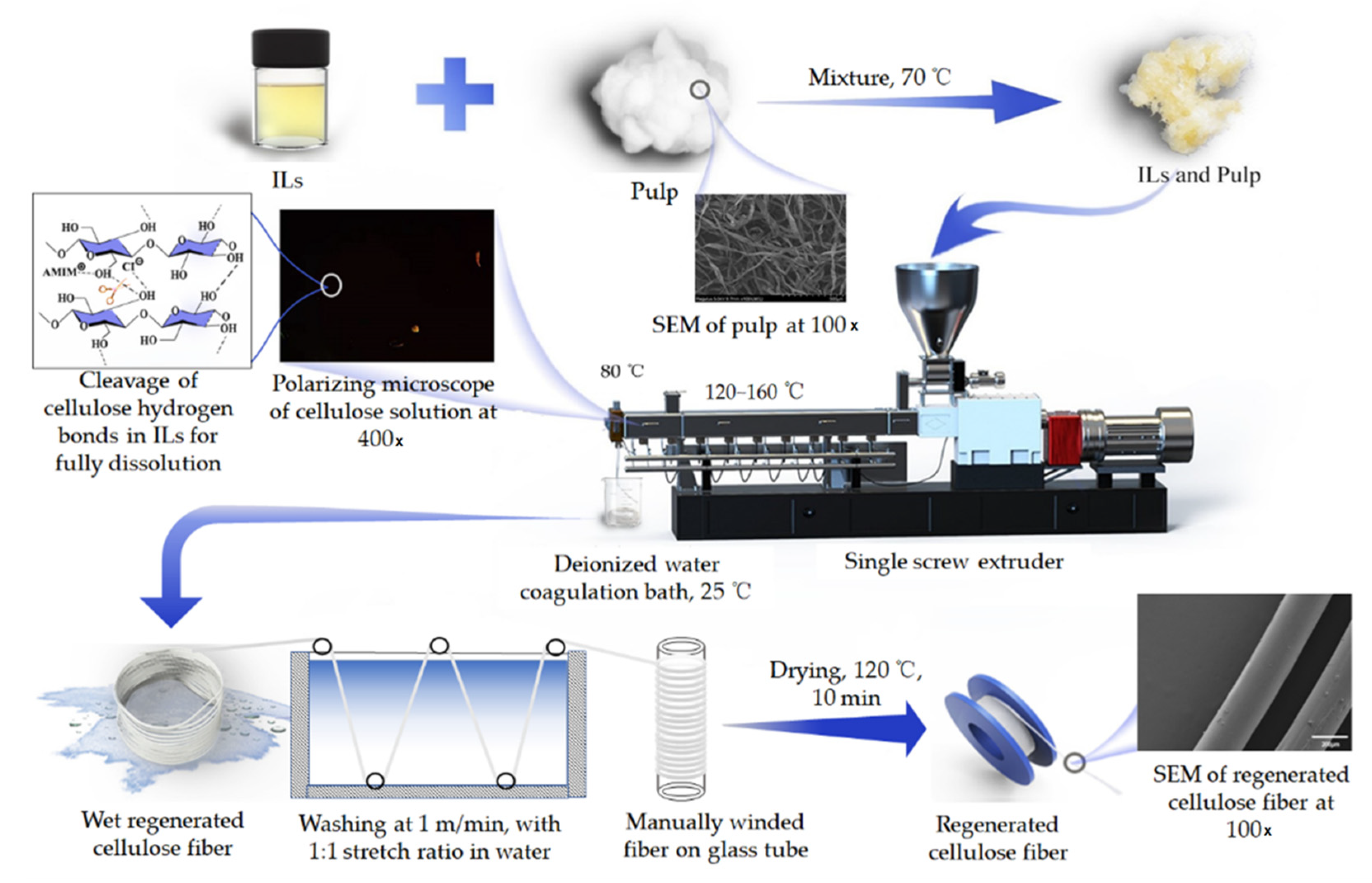
2.3. Preparation of Regenerated Cellulose Fibers
2.4. Characterization of Regenerated Fibers
2.4.1. The Mechanical Properties of the Regenerated Cellulose Fibers
2.4.2. X-ray Diffraction (XRD) Analysis
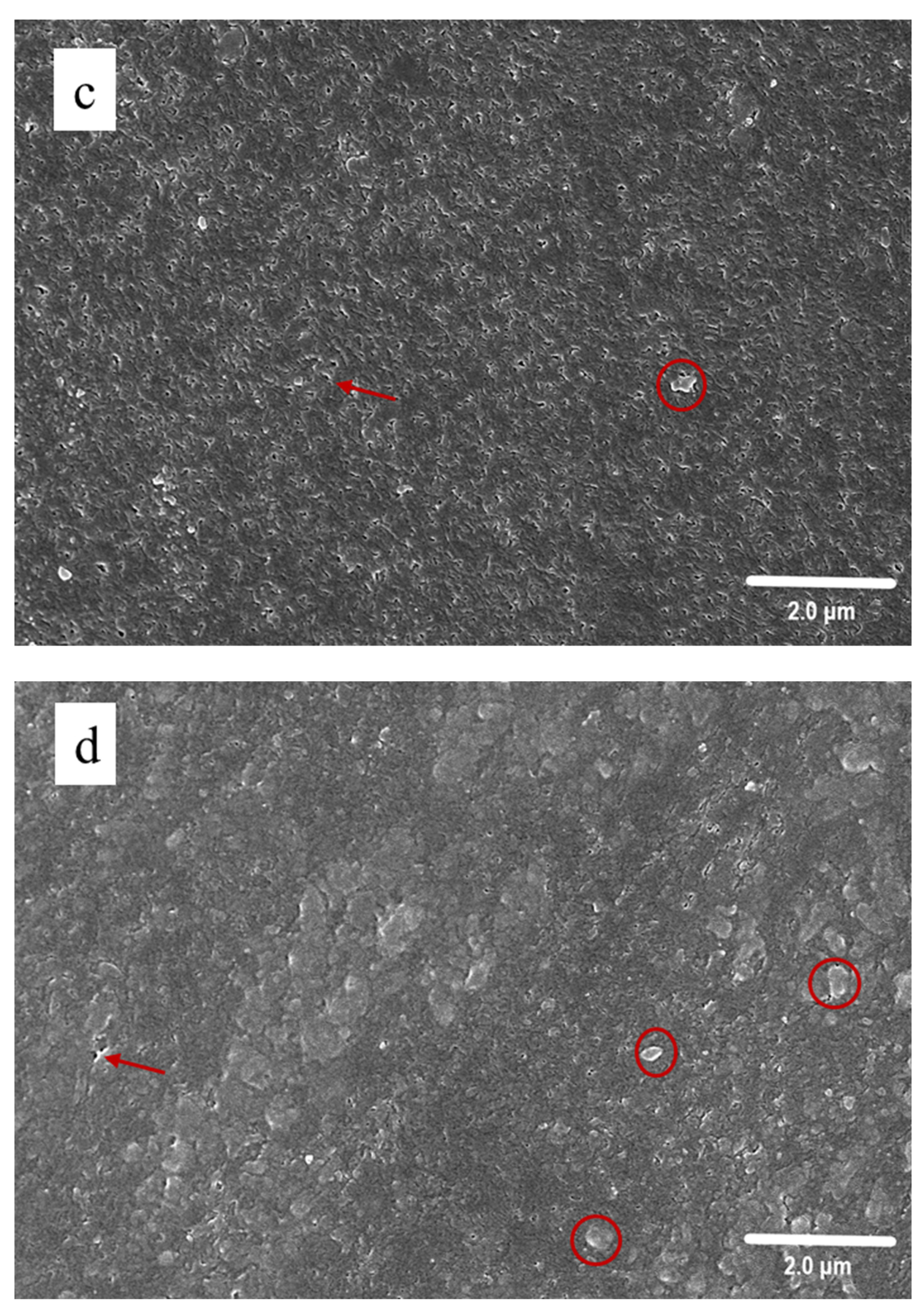
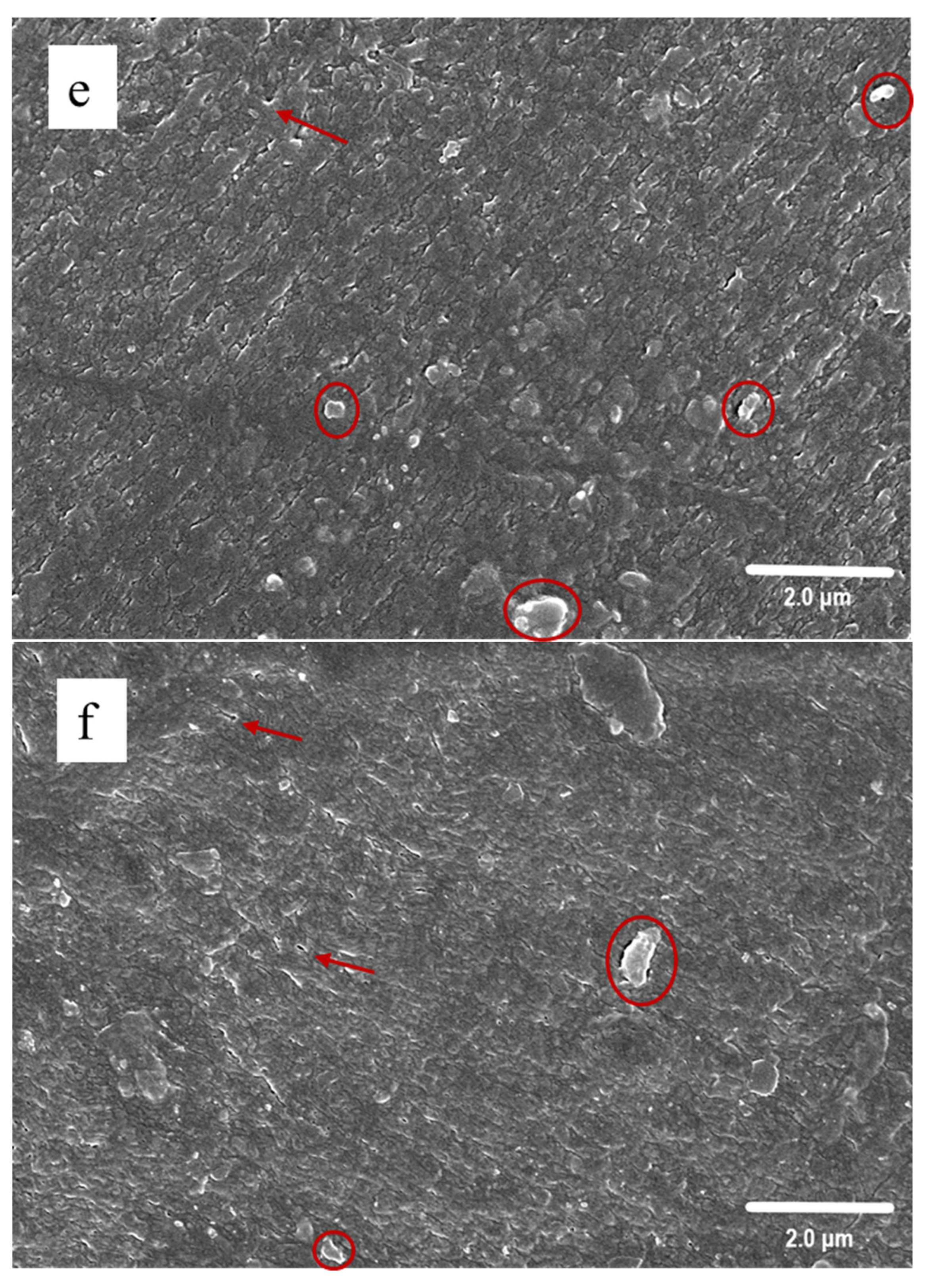
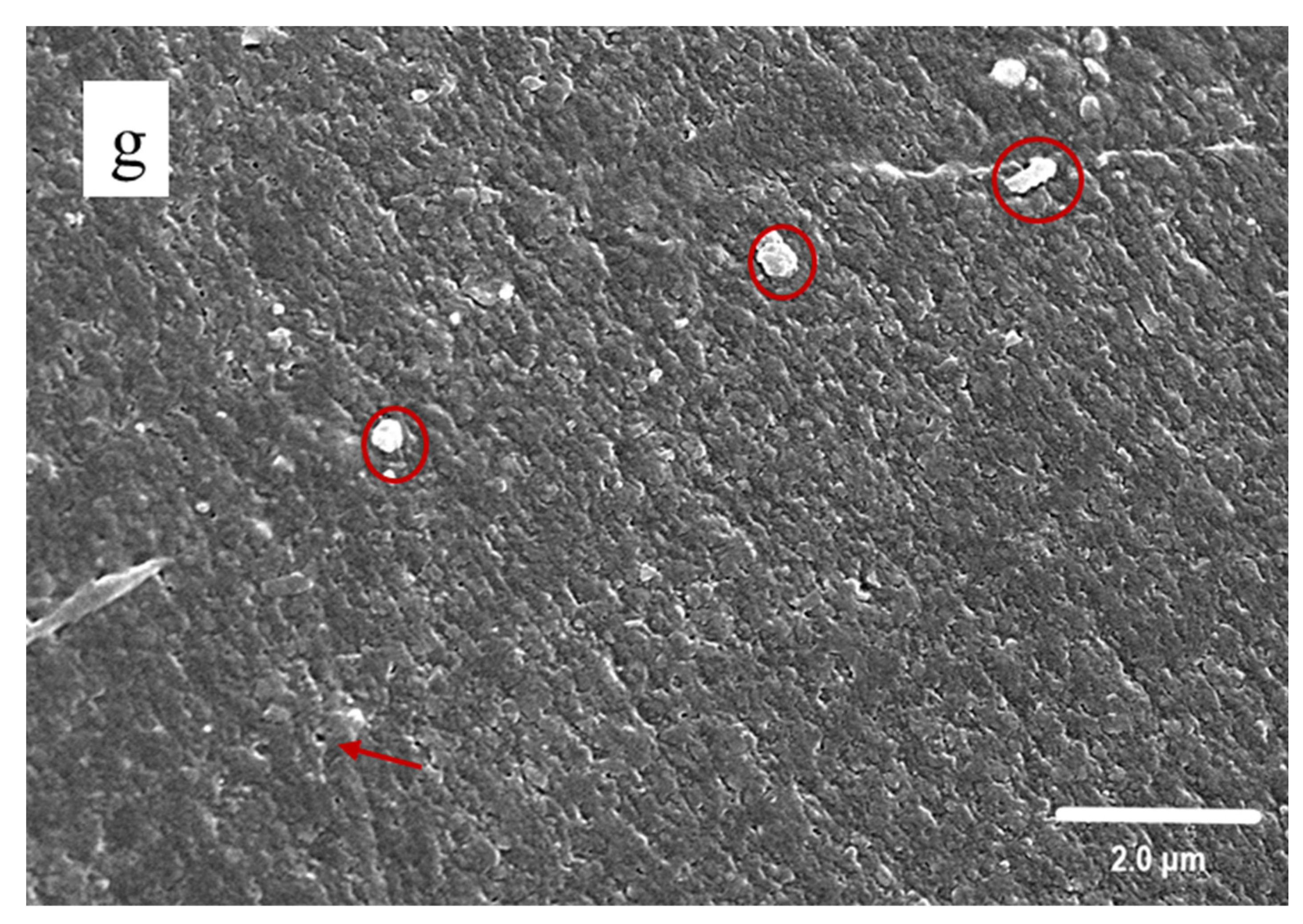
2.4.3. Scanning Electron Microscopy (SEM)
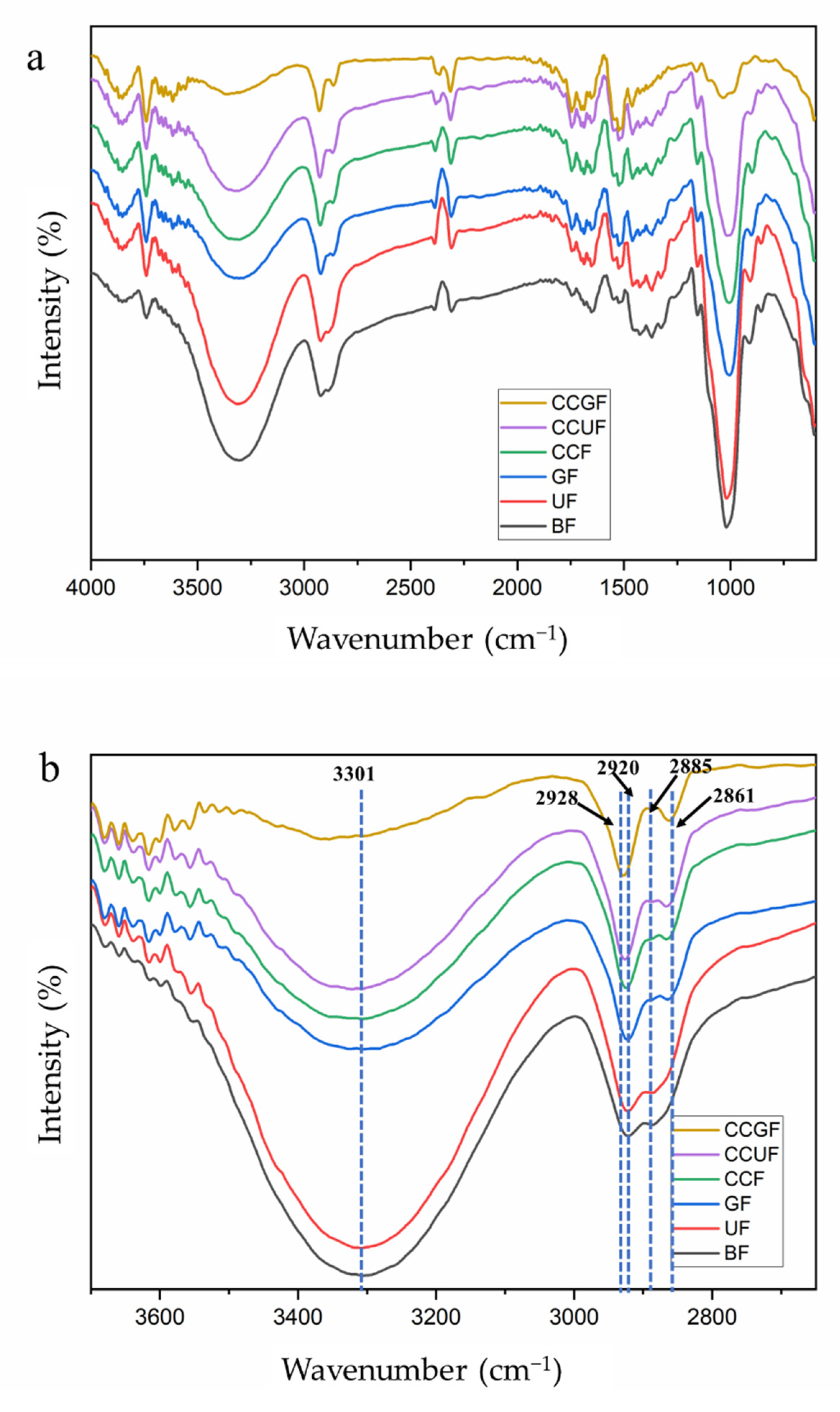
2.4.4. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.4.5. Thermal Analysis
2.4.6. Dissolution Analysis
2.4.7. Rheological Measurements
3. Results
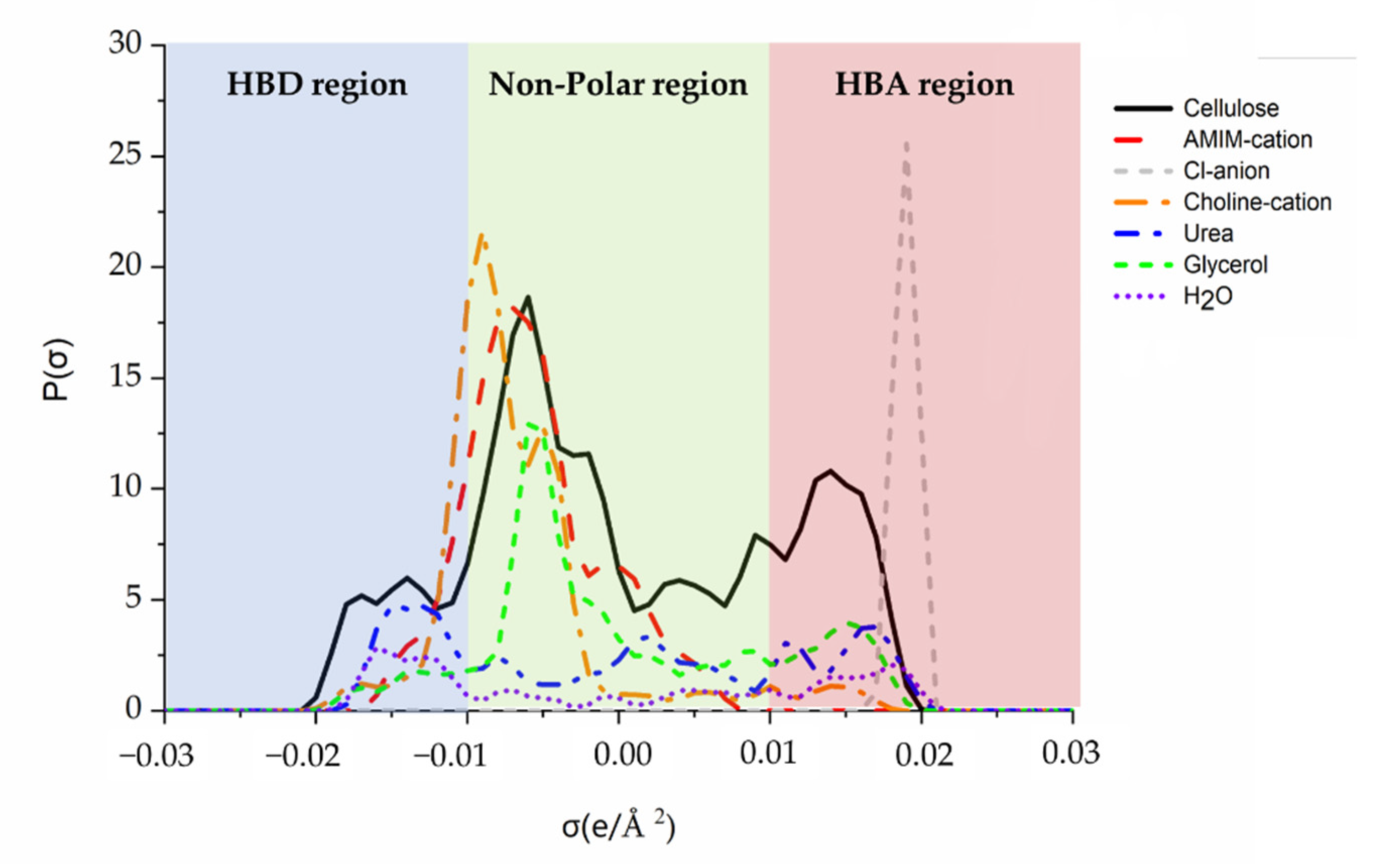
3.1. The Sigma Profiles Analyze
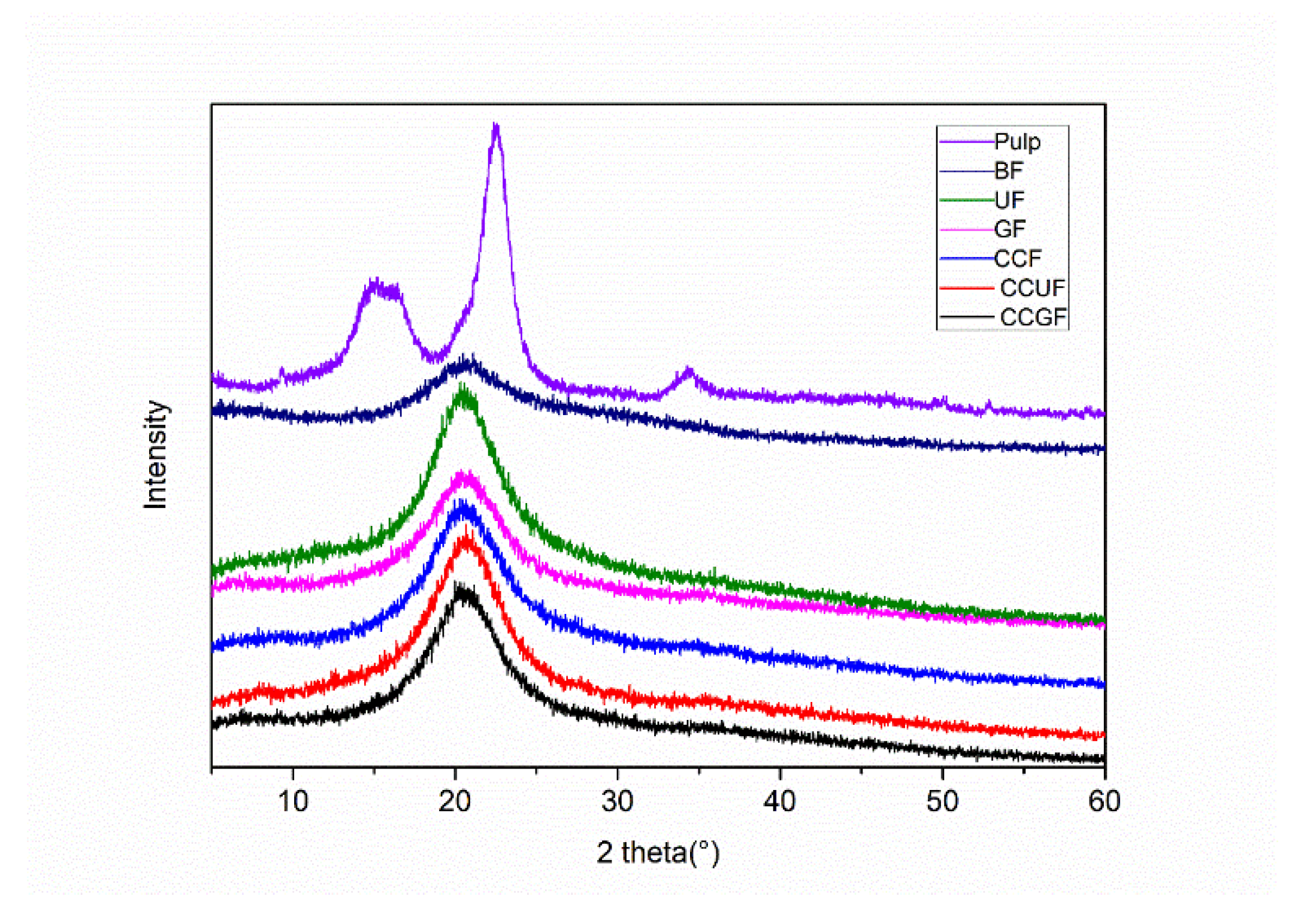
3.2. Tensile Properties and XRD Analysis
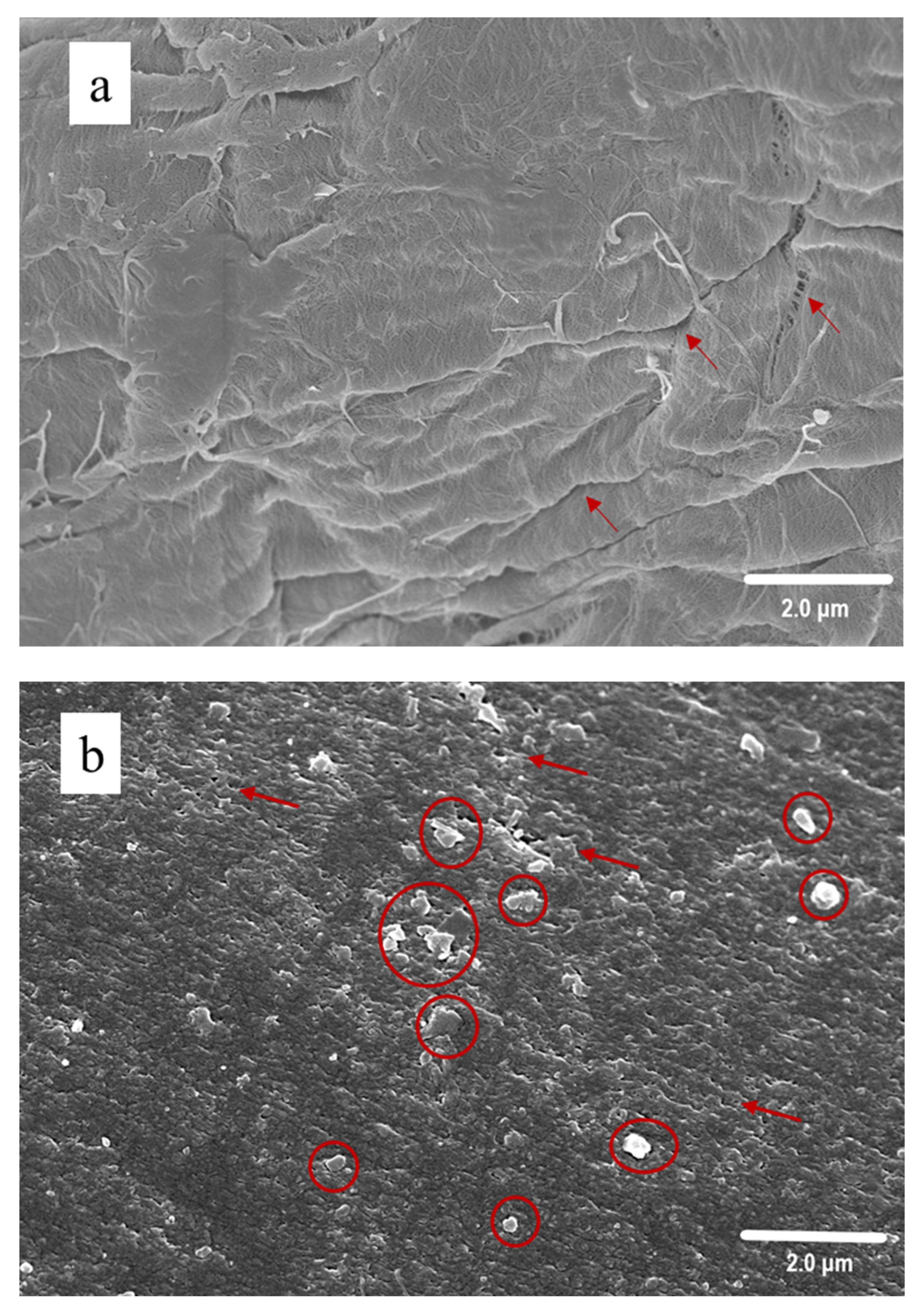
3.3. SEM Observation
3.4. FT-IR Spectroscopy Analysis
3.5. Thermal Analysis and Differential Scanning Calorimetry Analysis
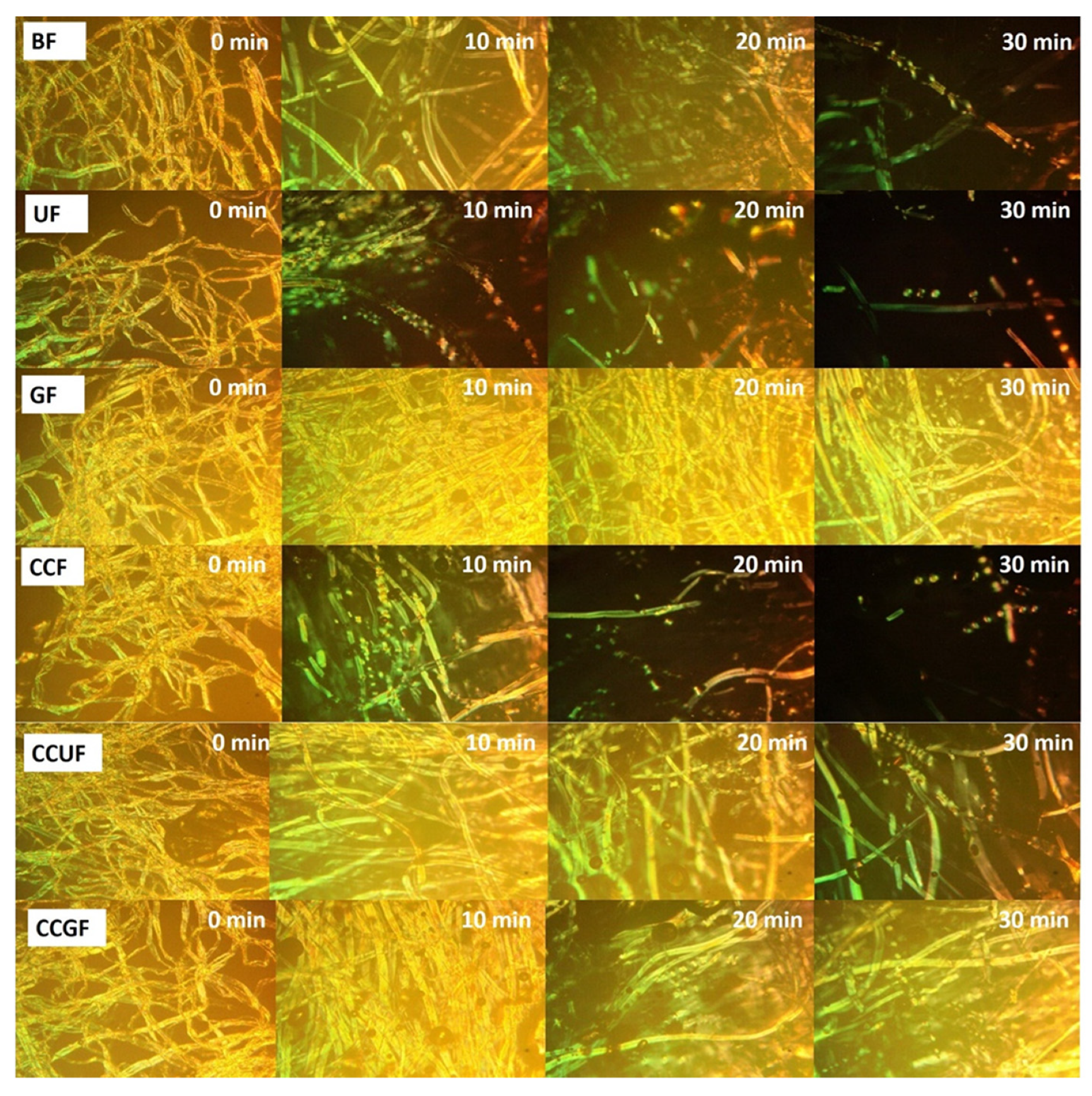
3.6. Cellulose Dissolution Analysis
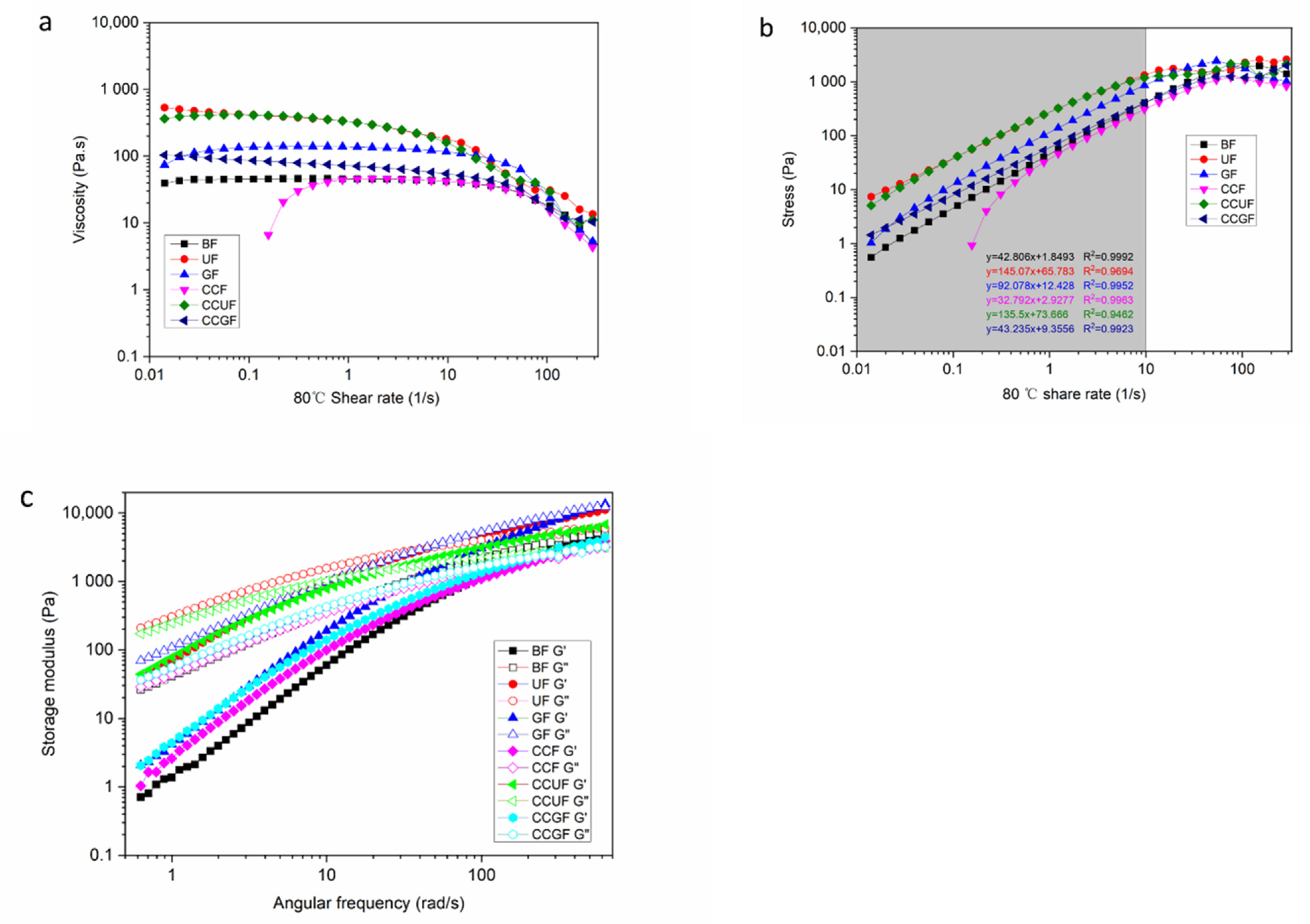
3.7. Rheological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rojas, O.J. Cellulose chemistry and properties: Fibers, nanocelluloses and advanced materials preface. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Rojas, O.J., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 271, pp. 3–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-allyl-3-methylimidazolium chloride room temperature ionic liquid a new and powerful nonderivatizing solvent for cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Tian, W.G.; Gao, X.X.; Zhang, J.M.; Yu, J.; Zhang, J. Cellulose nanosphere: Preparation and applications of the novel nanocellulose. Carbohydr. Polym. 2022, 277, 118863. [Google Scholar] [CrossRef] [PubMed]
- Kostag, M.; El Seoud, O.A. Dependence of cellulose dissolution in quaternary ammonium-based ionic liquids/DMSO on the molecular structure of the electrolyte. Carbohydr. Polym. 2019, 205, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Babicka, M.; Wozniak, M.; Szentner, K.; Bartkowiak, M.; Peplinska, B.; Dwiecki, K.; Borysiak, S.; Ratajczak, I. Nanocellulose production using ionic liquids with enzymatic pretreatment. Materials 2021, 14, 3264. [Google Scholar] [CrossRef]
- Acharya, S.; Hu, Y.; Abidi, N. Mild condition dissolution of high molecular weight cotton cellulose in 1-butyl-3-methylimidazolium acetate/n,n-dimethylacetamide solvent system. J. Appl. Polym. Sci. 2018, 135, 45928. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhang, J.M.; Zhang, B.Q.; Zhang, X.Y.; Xu, L.L.; Zhang, J.; He, J.S.; Liu, C.Y. Determination of intrinsic viscosity-molecular weight relationship for cellulose in bmimac/dmso solutions. Cellulose 2016, 23, 2341–2348. [Google Scholar] [CrossRef]
- Bian, J.; Peng, F.; Peng, X.P.; Xiao, X.; Peng, P.; Xu, F.; Sun, R.C. Effect of emim ac pretreatment on the structure and enzymatic hydrolysis of sugarcane bagasse cellulose. Carbohydr. Polym. 2014, 100, 211–217. [Google Scholar] [CrossRef]
- Cao, Y.J.; Zhang, R.B.; Cheng, T.; Guo, J.; Xian, M.; Liu, H.Z. Imidazolium-based ionic liquids for cellulose pretreatment: Recent progresses and future perspectives. Appl. Microbiol. Biotechnol. 2017, 101, 521–532. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, S.; Shanti, R.; Morris, E. Plasticizing effect of 1-allyl-3-methylimidazolium chloride in cellulose acetate based polymer electrolytes. Carbohydr. Polym. 2012, 87, 2624–2629. [Google Scholar] [CrossRef]
- Edgar, K.J.; Zhang, H.H. Antibacterial modification of lyocell fiber: A review. Carbohydr. Polym. 2020, 250, 116932. [Google Scholar] [CrossRef]
- Minnick, D.L.; Flores, R.A.; DeStefano, M.R.; Scurto, A.M. Cellulose solubility in ionic liquid mixtures: Temperature, cosolvent, and antisolvent effects. J. Phys. Chem. B 2016, 120, 7906–7919. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R. Instantaneous dissolution of cellulose in organic electrolyte solutions. Chem. Commun. 2011, 47, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Qi, L.T.; Lin, Z.Y.; Yang, G.H.; He, M.; Chen, J.C. High-strength regenerated cellulose fiber reinforced with cellulose nanofibril and nanosilica. Nanomaterials 2021, 11, 2664. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.H.; Xiao, Y.F.; Huang, W.J.; Gong, P.X.; Peng, S.H.; He, J.P.; Fan, M.M.; Wang, K. An insight into the influence of hydrogen bond acceptors on cellulose/1-allyl-3-methyl imidazolium chloride solution. Carbohydr. Polym. 2017, 178, 295–301. [Google Scholar] [CrossRef]
- Trovatti, E.; Fernandes, S.C.M.; Rubatat, L.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Sustainable nanocomposite films based on bacterial cellulose and pullulan. Cellulose 2012, 19, 729–737. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Fang, C.Q.; Cheng, Y.L.; Chen, J.; Huang, Z.G.; Han, H.Z. Construction and properties of cellulose diacetate film derived from waste cigarette filters. Cellulose 2020, 27, 8899–8907. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhang, X.; You, T.T.; Xu, F. Deep eutectic solvents (DESs) for cellulose dissolution: A mini-review. Cellulose 2019, 26, 205–213. [Google Scholar] [CrossRef]
- Ling, Z.; Edwards, J.V.; Guo, Z.W.; Prevost, N.T.; Nam, S.; Wu, Q.L.; French, A.D.; Xu, F. Structural variations of cotton cellulose nanocrystals from deep eutectic solvent treatment: Micro and nano scale. Cellulose 2019, 26, 861–876. [Google Scholar] [CrossRef]
- Nguyen, H.V.D.; De Vries, R.; Stoyanov, S.D. Natural deep eutectics as a “green” cellulose cosolvent. ACS Sustain. Chem. Eng. 2020, 8, 14166–14178. [Google Scholar] [CrossRef]
- Khan, A.S.; Ibrahim, T.H.; Rashid, Z.; Khamis, M.I.; Nancarrow, P.; Jabbar, N.A. COSMO-RS based screening of ionic liquids for extraction of phenolic compounds from aqueous media. J. Mol. Liq. 2021, 328, 115387. [Google Scholar] [CrossRef]
- Muhammad, N.; Gonfa, G.; Rahim, A.; Ahmad, P.; Iqbal, F.; Sharif, F.; Khan, A.S.; Khan, F.U.; Khan, Z.U.; Rehman, F.; et al. Investigation of ionic liquids as a pretreatment solvent for extraction of collagen biopolymer from waste fish scales using cosmo-rs and experiment. J. Mol. Liq. 2017, 232, 258–264. [Google Scholar] [CrossRef]
- Jiang, G.S.; Yuan, Y.; Wang, B.C.; Yin, X.M.; Mukuze, K.S.; Huang, W.F.; Zhang, Y.M.; Wang, H.P. Analysis of regenerated cellulose fibers with ionic liquids as a solvent as spinning speed is increased. Cellulose 2012, 19, 1075–1083. [Google Scholar] [CrossRef]
- Ma, B.M.; Qin, A.W.; Li, X.; He, C.J. High tenacity regenerated chitosan fibers prepared by using the binary ionic liquid solvent (gly center dot hcl)- Bmim cl. Carbohydr. Polym. 2013, 97, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Akatan, K.; Kabdrakhmanova, S.; Kuanyshbekov, T.; Ibraeva, Z.; Battalova, A.; Joshy, K.S.; Thomas, S. Highly-efficient isolation of microcrystalline cellulose and nanocellulose from sunflower seed waste via environmentally benign method. Cellulose 2022, 29, 3787–3802. [Google Scholar] [CrossRef]
- French, A.D. Increment in evolution of cellulose crystallinity analysis. Cellulose 2020, 27, 5445–5448. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, T.; Makarem, M.; Cintron, M.S.; Cheng, H.N.; Kang, X.; Bacher, M.; Potthast, A.; Rosenau, T.; King, H.; et al. Effects of ball milling on the structure of cotton cellulose. Cellulose 2019, 26, 305–328. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of x-ray diffraction and ftir spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Duchemin, B.; Le Corre, D.; Leray, N.; Dufresne, A.; Staiger, M.P. All-cellulose composites based on microfibrillated cellulose and filter paper via a NaOH-urea solvent system. Cellulose 2016, 23, 593–609. [Google Scholar] [CrossRef]
- Rivera-Galletti, A.; Gough, C.R.; Kaleem, F.; Burch, M.; Ratcliffe, C.; Lu, P.; Salas-de La Cruz, D.; Hu, X. Silk-cellulose acetate biocomposite materials regenerated from ionic liquid. Polymers 2021, 13, 2911. [Google Scholar] [CrossRef]
- Lan, W.; Liu, C.F.; Yue, F.X.; Sun, R.C.; Kennedy, J.F. Ultrasound-assisted dissolution of cellulose in ionic liquid. Carbohydr. Polym. 2011, 86, 672–677. [Google Scholar] [CrossRef]
- Lin, J.Y.; Yu, L.B.; Tian, F.; Zhao, N.; Li, X.H.; Bian, F.G.; Wang, J. Cellulose nanofibrils aerogels generated from jute fibers. Carbohydr. Polym. 2014, 109, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Lemaoui, T.; Darwish, A.S.; Hammoudi, N.E.; Abu Hatab, F.; Attoui, A.; Alnashef, I.M.; Benguerba, Y. Prediction of electrical conductivity of deep eutectic solvents using COSMO-RS sigma profiles as molecular descriptors: A quantitative structure-property relationship study. Ind. Eng. Chem. Res. 2020, 59, 13343–13354. [Google Scholar] [CrossRef]
- Oliveira, G.; Farias, F.O.; Sosa, F.H.B.; Igarashi-Mafra, L.; Mafra, M.R. Green solvents to tune the biomolecules’ solubilization in aqueous media: An experimental and in silico approach by COSMO-RS. J. Mol. Liq. 2021, 341, 117314. [Google Scholar] [CrossRef]
- Li, J.Y.; Zhang, X.C.; Zhang, J.M.; Mi, Q.Y.; Jia, F.W.; Wu, J.; Yu, J.; Zhang, J. Direct and complete utilization of agricultural straw to fabricate all-biomass films with high-strength, high-haze and uv-shielding properties. Carbohydr. Polym. 2019, 223, 115057. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.O.; Maheswari, C.U.; Dhlamini, M.S.; Mothudi, B.M.; Zhang, J.M.; Zhang, J.; Nagarajan, R.; Rajulu, A.V. Preparation and characterization of regenerated cellulose films using borassus fruit fibers and an ionic liquid. Carbohydr. Polym. 2017, 160, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; Li, J.R.; Ma, L.Y.; Ma, X.J. Preparation and properties of biocomposite from poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) reinforced with regenerated cellulose. Cellulose 2019, 26, 5427–5436. [Google Scholar] [CrossRef]
- Yang, Y.P.; Zhang, Y.; Dawelbeit, A.; Deng, Y.; Lang, Y.X.; Yu, M.H. Structure and properties of regenerated cellulose fibers from aqueous NaOH/thiourea/urea solution. Cellulose 2017, 24, 4123–4137. [Google Scholar] [CrossRef]
- Ahn, Y.; Song, Y.; Kwak, S.Y.; Kim, H. Highly ordered cellulose ii crystalline regenerated from cellulose hydrolyzed by 1-butyl-3-methylimidazolium chloride. Carbohydr. Polym. 2016, 137, 321–327. [Google Scholar] [CrossRef]
- Rajeev, A.; Deshpande, A.P.; Basavaraj, M.G. Rheology and microstructure of concentrated microcrystalline cellulose (MCC)/1-allyl-3-methylimidazolium chloride (Amimcl)/water mixtures. Soft Matter 2018, 14, 7615–7624. [Google Scholar] [CrossRef]
- Astruc, J.; Nagalakshmaiah, M.; Laroche, G.; Grandbois, M.; Elkoun, S.; Robert, M. Isolation of cellulose-ii nanospheres from flax stems and their physical and morphological properties. Carbohydr. Polym. 2017, 178, 352–359. [Google Scholar] [CrossRef]
- Huang, Z.L.; Liu, C.; Feng, X.Y.; Wu, M.Y.; Tang, Y.J.; Li, B. Effect of regeneration solvent on the characteristics of regenerated cellulose from lithium bromide trihydrate molten salt. Cellulose 2020, 27, 9243–9256. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; You, T.; Wang, K.; Xu, F. Effects of polymorphs on dissolution of cellulose in NaOH/urea aqueous solution. Carbohydr. Polym. 2015, 125, 85–91. [Google Scholar] [CrossRef] [PubMed]
- French, A.D.; Cintron, M.S. Cellulose polymorphy, crystallite size, and the segal crystallinity index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- Cai, T.; Zhang, H.H.; Guo, Q.H.; Shao, H.L.; Hu, X.C. Structure and properties of cellulose fibers from ionic liquids. J. Appl. Polym. Sci. 2010, 115, 1047–1053. [Google Scholar] [CrossRef]
- Chen, J.H.; Guan, Y.; Wang, K.; Xu, F.; Sun, R.C. Regenerated cellulose fibers prepared from wheat straw with different solvents. Macromol. Mater. Eng. 2015, 300, 793–801. [Google Scholar] [CrossRef]
- Yang, Y.P.; Zhang, Y.; Lang, Y.X.; Yu, M.H. Structure development in the condensed state of cellulose fiber regenerated from alkali complex solution. Cellulose 2018, 25, 1555–1569. [Google Scholar] [CrossRef]
- Liu, X.; Pang, J.H.; Zhang, X.M.; Wu, Y.Y.; Sun, R.C. Regenerated cellulose film with enhanced tensile strength prepared with ionic liquid 1-ethyl-3-methylimidazolium acetate (EmimAc). Cellulose 2013, 20, 1391–1399. [Google Scholar] [CrossRef]
- Rohaeti, E.; Fx, E.W.L.; Rakhmawati, A. Mechanical properties and antibacterial activity of cellulose composite based coconut water with addition of glycerol, chitosan, and silver nanoparticle. Orient. J. Chem. 2018, 34, 1341–1349. [Google Scholar] [CrossRef]
- Cazon, P.; Velazquez, G.; Vazquez, M. Novel composite films from regenerated cellulose-glycerol-polyvinyl alcohol: Mechanical and barrier properties. Food Hydrocoll. 2019, 89, 481–491. [Google Scholar] [CrossRef]
- Vanitjinda, G.; Nimchua, T.; Sukyai, P. Effect of xylanase-assisted pretreatment on the properties of cellulose and regenerated cellulose films from sugarcane bagasse. Int. J. Biol. Macromol. 2019, 122, 503–516. [Google Scholar] [CrossRef]
- Majdoub, M.; Essamlali, Y.; Amadine, O.; Ganetri, I.; Hafnaoui, A.; Khouloud, M.; Zahouily, M. Octadecylamine as chemical modifier for tuned hydrophobicity of surface modified cellulose: Toward organophilic cellulose nanocrystals. Cellulose 2021, 28, 7717–7734. [Google Scholar] [CrossRef]
- Angtika, R.S.; Widiyanti, P.; Aminatun. Bacterial cellulose-chitosan-glycerol biocomposite as artificial dura mater candidates for head trauma. J. Biomim. Biomater. Biomed. Eng. 2018, 36, 7–16. [Google Scholar] [CrossRef]
- Teng, J.; Yang, B.; Zhang, L.Q.; Lin, S.Q.; Xu, L.; Zhong, G.J.; Tang, J.H.; Li, Z.M. Ultra-high mechanical properties of porous composites based on regenerated cellulose and cross-linked poly(ethylene glycol). Carbohydr. Polym. 2018, 179, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Peng, X.; Zhong, L.; Jing, S.; Cao, X.; Lu, F.; Sun, R. Choline chloride/urea as an effective plasticizer for production of cellulose films. Carbohydr. Polym. 2015, 117, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Marechal, Y.; Chanzy, H. The hydrogen bond network in ib cellulose as observed by infrared spectrometry. J. Mol. Struct. 2000, 523, 183–196. [Google Scholar] [CrossRef]
- Carrillo, F.; Colom, X.; Suñol, J.J.; Saurina, J. Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Dissanayake, N.; Thalangamaarachchige, V.D.; Troxell, S.; Quitevis, E.L.; Abidi, N. Substituent effects on cellulose dissolution in imidazolium-based ionic liquids. Cellulose 2018, 25, 6887–6900. [Google Scholar] [CrossRef]
- Colom, X.; Carrillo, F. Crystallinity changes in lyocell and viscose-type fibres by caustic treatment. Eur. Polym. J. 2002, 38, 2225–2230. [Google Scholar] [CrossRef]
- Jia, X.J.; Chen, Y.W.; Shi, C.; Ye, Y.F.; Wang, P.; Zeng, X.X.; Wu, T. Preparation and characterization of cellulose regenerated from phosphoric acid. J. Agric. Food Chem. 2013, 61, 12405–12414. [Google Scholar] [CrossRef]
- Yang, S. Plant Fiber Chemistry; China Light Industry Press: Beijing, China, 2006. [Google Scholar]
- Poletto, M.; Ornaghi, H.L.; Zattera, A.J. Native cellulose: Structure, characterization and thermal properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Zhang, Y.H.; Yan, D.P.; Zhang, C.Y.; Nie, S.X. Enzyme-assisted mechanical production of cellulose nanofibrils: Thermal stability. Cellulose 2018, 25, 5049–5061. [Google Scholar] [CrossRef]
- Zhu, C.C.; Koutsomitopoulou, A.F.; Eichhorn, S.J.; van Duijneveldt, J.S.; Richardson, R.M.; Nigmatullin, R.; Potter, K.D. High stiffness cellulose fibers from low molecular weight microcrystalline cellulose solutions using dmso as co-solvent with ionic liquid. Macromol. Mater. Eng. 2018, 303, 1800029. [Google Scholar] [CrossRef]
- Kim, T.; Song, Y.; Ahn, J.; Kim, M.; Ko, E.; Kim, H. Rheological interpretation of intermediate physical state of gel and liquid crystalline phases in cellulose solution and their synergetic effects on the mechanical property. Cellulose 2021, 28, 10863–10874. [Google Scholar] [CrossRef]
- Choe, D.; Kim, Y.M.; Nam, J.E.; Nam, K.; Shin, C.S.; Roh, Y.H. Synthesis of high-strength microcrystalline cellulose hydrogel by viscosity adjustment. Carbohydr. Polym. 2018, 180, 231–237. [Google Scholar] [CrossRef]
- Shakeel, A.; Mahmood, H.; Farooq, U.; Ullah, Z.; Yasin, S.; Iqbal, T.; Chassagne, C.; Moniruzzaman, M. Rheology of pure ionic liquids and their complex fluids: A review. Acs Sustain. Chem. Eng. 2019, 7, 13586–13626. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.B.; Xia, X.L.; Mukuze, K.S.; Zhang, Y.M.; Wang, H.P. Study on the temperature-induced sol-gel transition of cellulose/silk fibroin blends in 1-butyl-3-methylimidazolium chloride via rheological behavior. Cellulose 2014, 21, 3737–3743. [Google Scholar] [CrossRef]


| Sample | Named | AMIMCl (g) | Pulp (g) | Urea (g) | Glycerol (g) | Choline Chloride (g) |
|---|---|---|---|---|---|---|
| AMIMCl | BF | 100 | 10 | / | / | / |
| AMIMCl+ Urea | UF | 100 | 10 | 1.89 | / | / |
| AMIMCl+ Glycerol | GF | 100 | 10 | / | 2.9 | / |
| AMIMCl+ Choline Chloride | CCF | 100 | 10 | / | / | 4.4 |
| AMIMCl+ Urea+ Choline Chloride | CCUF | 100 | 10 | 1.26 | / | 1.46 |
| AMIMCl+ Glycerol + Choline Chloride | CCGF | 100 | 10 | / | 1.16 | 2.64 |
| Sample | Tensile Strength (MPa) | Initial Modulus (MPa) | Elongation at Break (%) | Crystallinity (%) |
|---|---|---|---|---|
| BF | 54.43 ± 2.74 | 382.33 ± 10.66 | 46.8 ± 5.32 | 60.06 ± 2.11 |
| UF | 88.57 ± 3.54 | 815.89 ± 9.57 | 33.24 ± 1.79 | 61.58 ± 2.36 |
| GF | 105.67 ± 4.50 | 2314.83 ± 26.56 | 13.34 ± 4.38 | 61.89 ± 1.76 |
| CCF | 67.29 ± 2.35 | 1323.40 ± 14.89 | 14.35 ± 3.59 | 62.13 ± 1.44 |
| CCUF | 117.36 ± 4.28 | 3166.16 ± 30.22 | 13.02 ± 4.31 | 62.50 ± 1.02 |
| CCGF | 139.62 ± 5.94 | 3611.72 ± 27.45 | 14.78 ± 2.54 | 62.79 ± 2.00 |
| Wavenumber (cm−1) | Bond and Functional Group | Reference |
|---|---|---|
| 3600~3100 | O-H stretching bond of hydrogen bonds of cellulose | [29,54,55,56] |
| 2940~2840 | -CH stretching bond of cellulose | [50,57] |
| 1650 | H-O-H bending of the water | [42,50] |
| 1500~1200 | -OH in plane bending of cellulose II | [57] |
| 1430 | -CH2 bending or scissoring motions in cellulose | [29,47] |
| 1315~1338 | O-H in-plane bending of the crystalline of cellulose II | [29] |
| 1135~1180 | deformations C-O-C groups of cellulose | [42,55] |
| 1050 | C-O stretching of internal hydrogen bonds between cellulose | [58] |
| 897 | β-glycosidic linkages between the sugar units of cellulose II | [42,57] |
| Sample | CI(IR) A (1427, 1425)/A (913, 897) | CI(IR) A (1368)/A (1262, 1260) | TCI A (1368)/A (2920) |
|---|---|---|---|
| BF | 99.94% | 99.04% | 101.47% |
| UF | 99.74% | 98.81% | 101.27% |
| GF | 100.05% | 99.18% | 100.97% |
| CCF | 100.07% | 99.19% | 101.23% |
| CCUF | 99.69% | 99.23% | 101.23% |
| CCGF | 99.24% | 99.59% | 100.52% |
| Sample | Ton 1 (°C) | Tmax 2 (°C) | Tg 3 (°C) |
|---|---|---|---|
| BF | 162.64 | 359.86 | 148.22 |
| UF | 186.70 | 362.76 | 149.21 |
| GF | 177.09 | 362.72 | 152.32 |
| CCF | 186.20 | 357.49 | 147.69 |
| CCUF | 184.80 | 361.62 | 157.26 |
| CCGF | 182.89 | 358.49 | 178.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Li, W.; Yang, G.; Lin, Z.; Qi, L.; Zhu, P.; Yu, J.; Chen, J. Strength Enhancement of Regenerated Cellulose Fibers by Adjustment of Hydrogen Bond Distribution in Ionic Liquid. Polymers 2022, 14, 2030. https://doi.org/10.3390/polym14102030
Xue Y, Li W, Yang G, Lin Z, Qi L, Zhu P, Yu J, Chen J. Strength Enhancement of Regenerated Cellulose Fibers by Adjustment of Hydrogen Bond Distribution in Ionic Liquid. Polymers. 2022; 14(10):2030. https://doi.org/10.3390/polym14102030
Chicago/Turabian StyleXue, Yu, Weidong Li, Guihua Yang, Zhaoyun Lin, Letian Qi, Peihua Zhu, Jinghua Yu, and Jiachuan Chen. 2022. "Strength Enhancement of Regenerated Cellulose Fibers by Adjustment of Hydrogen Bond Distribution in Ionic Liquid" Polymers 14, no. 10: 2030. https://doi.org/10.3390/polym14102030
APA StyleXue, Y., Li, W., Yang, G., Lin, Z., Qi, L., Zhu, P., Yu, J., & Chen, J. (2022). Strength Enhancement of Regenerated Cellulose Fibers by Adjustment of Hydrogen Bond Distribution in Ionic Liquid. Polymers, 14(10), 2030. https://doi.org/10.3390/polym14102030






