1. Introduction
Articular cartilage damage is a common joint disease that affects millions of people worldwide, especially sports people who suffer from trauma and knee injury [
1,
2]. Because of its lack of vasculature, lymphatic cells, and nerves, the self-healing ability of articular cartilage is limited. In addition, articular cartilage damage is a common cause of advanced osteoarthritis (OA), which shows severe pain, immobility, and dysfunction in the joint [
1,
3]. Current treatment to repair cartilage defects includes microfracture, autologous chondrocyte implantation, osteochondral autografts, and allograft-directed cartilage regeneration [
4]. Moreover, the standard treatment strategies exhibit limitations and drawbacks, including fibrocartilage regeneration, donor site morbidity, inferior mechanical properties to hyaline articular cartilage, etc. Therefore, the regeneration of the cartilage defect is still challenging in today’s world [
3,
4,
5].
Tissue engineering (TE) is a multifaceted field that combines material science and biology to replace or regenerate biological tissues [
5]. Cell source, scaffold, and signaling molecules are the fundamental element for TE. The three-dimensional (3D) porous scaffold serves as a template for tissue formation by providing a suitable environment for tissues or organs to grow [
5,
6]. Among them, hydrogels promise 3D polymeric networks with a high water and porosity content for nutrient and oxygen diffusion. However, hyaluronic acid (HA), gelatin, etc., natural hydrogels exhibit the composition and the structure of the native extracellular matrix, which shows a distinctive ability for regenerative and tissue engineering applications. It can be extensively used for 3D cell encapsulation and surface seeding to develop a biomimetic construct for cartilage tissue engineering [
7]. Regarding the cell source, the adipose-derived stem cells (ADSCs) have become the alternative source to bone marrow-derived mesenchymal stem cells (BM-MSCs). Compared to the BM-MSCs, ADSCs can proliferate rapidly and have a lower harvesting risk. ADSCs can be easily isolated and possess multilineage differentiation [
8].
The HA is an anionic biopolymer made up of D-N-acetylglucosamine and D-glucuronic acid repeating units and serves as a backbone to form proteoglycan complexes. HA is renowned as the main extracellular matrix (ECM) component in all connective tissues. HA is also predominantly found in cartilage tissue [
9]. A previous study stated that the cell surface receptors such as the cell adhesion molecule (CD44), named the homing cell adhesion molecule (HCAM), interact with HA and mediate the HA-receptor-mediated motility (HRAMM), therefore enhancing cell aggregation and instigating chondrogenesis and hyaline cartilage [
10,
11]. HA-based hydrogels for cartilage regeneration are becoming more common since they have demonstrated excellent biocompatibility and biological activity [
11]. However, some clinical problems include the HA hydrogel’s lack of good mechanical properties, 3D structure, and unsatisfactory degradation rate. Fortunately, the carboxyl and hydroxyl groups of HA can also be chemically modified by methacrylate to yield the formation of crosslinked HA methacryloyl (HAMA) hydrogels upon UV light irradiation, which improves their chemical and mechanical properties, providing larger rigidities, high levels of viscoelasticity after swelling resistance to enzymatic degradation compared with unmodified HA, and biocompatibility preservation [
11,
12,
13,
14]. In addition, the applicability of HA-based hydrogels is still limited by their poor cell adhesiveness, which hinders cell proliferation [
15]. Therefore, a potential strategy has been applied to overcome this challenge. The HAMA hybrid hydrogel has been formed by blending with other natural hydrogels enriched with ECM components, such as a gelatin-based hydrogel of gelatin methacryloyl (GelMA).
Gelatin is a natural hydrophilic polymer that hydrolyzes and denatures collagen with a lower immunogenicity because of the lower aromatic groups [
16]. Gelatin contains various bioactive amino acid motifs, including arginine–glycine–aspartic acid (RGD) sequences that support cell adhesion and the progress of different cell types, matrix metalloproteinase (MMP) sequences, cell remodeling, etc. [
17,
18]. However, gelatin’s thermostability and mechanical modulus are poor when temperatures exceed 37 °C. Therefore, gelatin can also be chemically modified; the amino groups and minor hydroxyl groups presenting on the side chains of gelatin are replaced by methacryloyl groups in methacrylic anhydride crosslinked by additive chain reaction exposure to UV light and forms the gelatin–methacryloyl (GelMA) hydrogel. Because the amount of the free amino groups of gelatin was just about 0.3184 mmole per gram [
17], meaning that even the amine group and hydroxyl group were replaced nearly wholly, the 100% degree of substitution (DS%) of the GelMA should contain less than 5.0 wt% of methacryloyl. This is why the GelMA structure also includes RGD sequences and MMP sequences of gelatin, which are not significantly affected. In that way, the cell adhesion property of the gelatin is retained in the GelMA hydrogel. Furthermore, GelMA hydrogels possess the better mechanical and tunable properties of most tissue engineering (TE) applications [
12]. Nevertheless, GelMA is still not suitable for clinical applications alone because of its fast degradability rate, which may be based on internal metalloproteinase and a low degree of crosslinking by the low percentage of the free amino groups within the gelatin [
12].
Some of the challenges in using the biological hydrogel for cartilage tissue engineering include that it will fail to mimic the mechanical and viscoelastic behavior of the native tissue and its excessive degradation rate. However, the development of a hybrid HAMA-GelMA (HG) hydrogel that incorporates several crosslinkers has emerged as a promising strategy for optimizing the physical and mechanical properties of the hydrogel so to make it suitable for cartilage regeneration or other biological applications [
19,
20]. For example, Shi et al. [
21] showed this HG hydrogel through a dynamic covalent bond between phenylboronic acid-grafted hyaluronic acid (HA-PBA) and poly(vinyl alcohol), which was further stabilized through a secondary crosslinking between the acrylate moiety on the HA-PBA and the free thiol group from the thiolated gelatin with the antireactive oxygen species’ potential ability to enhance cartilage tissue regeneration. In addition, interest in free radical photopolymerization with a photoinitiator (P.I.) for crosslinking hybrid hydrogels with improved and regulating physiological/physicochemical properties has increased significantly in recent years [
22]. Furthermore, lithium phenyl-2,4,6-trimethyl-benzoyl phosphinate (LAP) has been considered a promising P.I. that can be crosslinked via ultraviolet light and the blue light wavelength. The LAP has also shown promising results on cell viability in vitro [
23].
In short, the hybrid hydrogels of HAMA and GelMA are considered promising biomimetic material, which requires a P.I. and light for the in situ crosslinking reaction [
24]. For example, Hjortnaes et al. [
25] have reported that the HG hydrogel shows valvular interstitial cell phenotype differentiation. In another work, Camci-Unal et al. [
24] studied the HG hybrid hydrogel and showed increased hydrogel stiffness with a variety of different polymer concentrations. In addition, Teong et al. [
26] stated that the stiffness of the HG hybrid hydrogel could induce the chondrogenesis activity on hADSCs.
Despite all previous studies, the HG hybrid hydrogel’s environmental, mechanical, and biological properties have not yet been completely established for the clinical use of chondral defect regeneration. Recently, nano-silica (nSi) has demonstrated biosafety combined with proper cell labeling and visualization in histological sections [
27]. Some in the literature have also reported that nSi could enhance cell growth through ERK1/2 activation, improve osteoblast function, inhibit osteoclast function, and promote bone mineralization [
28]. Bunpetch et al. [
29] further reported that silicate-based bioceramic scaffolds could promote the osteogenesis of bone marrow stem cells (BMSCs) and contribute to maintaining a chondrocyte phenotype.
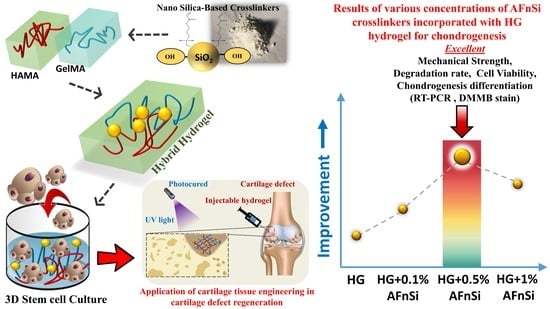
To the best of our knowledge, incorporating the acrylate-functionalized nSi nanoparticles into the HG hybrid hydrogels would be a promising solution for developing a 3D biomimetic scaffold for cartilage tissue engineering, which has not been reported thus far. The hypothesis is that the HG hybrid hydrogel incorporated with 3-acryloxypropyl silanetriol (APS)-functionalized nSi as a novel crosslinker (named for “acrylate functionalized nSi”; AFnSi) would be a better choice for tuning the physical, mechanical, and cellular activity and the increased chondrogenesis gene expression suitable for cartilage tissue engineering because the hydroxyl groups of the nSi surface can be grafted with multiple acrylates, which promotes the network crosslinking to form the C-C bond by the addition reaction within the HG hybrid hydrogels.
This study aimed to build a novel photocured hybrid hydrogel system comprising of HAMA, GelMA, and a minor AFnSi crosslinker to evaluate the cell viability and chondrogenic differentiation ability of human adipose-derived stromal cells (hADSCs). However, the lower cell toxicity of lithium phenyl-2,4,6-trimethyl-benzoyl phosphinate (LAP) is used as a photoinitiator.
Figure 1 shows the overall concept of this study, i.e., the hADSCs loaded with photocrosslinked hybrid hydrogels to depict the preparations, physical/chemistry properties, and chondrogenic differentiation evaluation.
Figure 1a–c shows the synthesis of the 2% (
w/
v) HAMA hydrogel, the 20% (
w/
v) GelMA hydrogel, and the hybrid hydrogels of the HG -AFnSi. The hybrid hydrogels of the HG -AFnSi system were photocured using LAP at 365 nm of UV light from
Figure 1d. The physicochemical characteristics of these hybrid hydrogels were exhibited, including the swelling behavior, morphological conformation, mechanical properties, and biodegradation of the properties of the hybrid hydrogels. To further investigate cell viability and chondrogenic differentiation, the hADSCs were employed as cells loaded with the 2% HAMA–20% GelMA hybrid hydrogel with 0–1.0 (
w/
v) AFnSi crosslinker to examine the process of optimal chondrogenic development (
Figure 1e), including in vitro cytotoxicity, chondrogenic differentiation gene expression, and sGAG, and the type II collagen results will explain whether or not this novel hybrid hydrogel design has excellent potential for cartilage tissue engineering applications in the future.
2. Materials and Methods
2.1. Materials
Hyaluronic acid (molecular weight of 2000 kDa) was purchased from Kikkoman (FCH-200, Tokyo, Japan). Gelatin from porcine skin (type B) and methacrylate anhydride (MA) (molecular weight of 154.16 Da) were purchased from Sigma-Aldrich, St. Louis, MO, USA). The P.I. of lithium phenyl-2,4,6-trimethyl-benzoyl phosphinate (LAP), nano-silica (SiO2; nSi), 3-acryloxypropyl trimethoxysilane (APMS) were also obtained from Sigma-Aldrich (St. Louis, MO, USA), and the sodium carbonate anhydrous (Na2CO3) and sodium bicarbonate (NaHCO3) were purchased from J.K Baker (Phillipsburg, NJ, USA). The other reagents, such as phosphate-buffered saline (PBS), Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Gibco BRL (Grandland, New York, NY, USA). All other solvents were purchased from Merck (Darmstadt, Germany), TEDIA (Fairfield, CT, USA), or J. T. Baker (Phillipsburg, NJ, USA). These chemicals were all analytical/reagent grade and were used without further purification.
2.2. Synthesis of HAMA Hydrogel
The hyaluronic acid methacryloyl (HAMA) was produced using the previously described method [
14,
26]. Briefly, a 1 wt% HA solution of 100 mL was prepared from a 2/1 ratio of deionized distilled water (DDW) and dimethylformamide (DMF), which was stirred until the solution was used, and dissolved at 37 °C. Then, the 8 mL of methacrylic anhydride (MAA) was added to the HA solution and maintained a pH of 8–9 with 3N of sodium hydroxide and stirred continuously for 24 h at 4 °C. The methacrylate-modified HA solution was dialyzed against DDW for three days to remove the unreacted methacrylate group for the purification process. The HAMA product was lyophilized and stored at 4 °C for further studies.
2.3. Synthesis of GelMA Hydrogel
The gelatin methacryloyl (GelMA) was also produced using the previously described method [
14,
17] and modified as follows: the 10 g of gelatin was dissolved in 100 mL of 0.25 M buffer solution (10
w/
v%) containing carbonate–bicarbonate (NaHCO
3 and Na
2CO
3) until the clear solution occurred at 50 °C. The initial pH adjustment at pH 9 by 5 M sodium hydroxide or 6 M hydrochloric acid. Subsequently, 1 mL of liquid MAA was added to a 10
w/
v% gelatin solution with a gelatin weight of 10 g under magnetic stirring at 50 °C under 500 rpm. The reaction proceeded for 3 h, and then the pH was readjusted to 7.4 to stop the reaction. After being filtered, dialyzed against the double-distilled water for 3 days, and lyophilized, the samples were stored at −80 °C until further use.
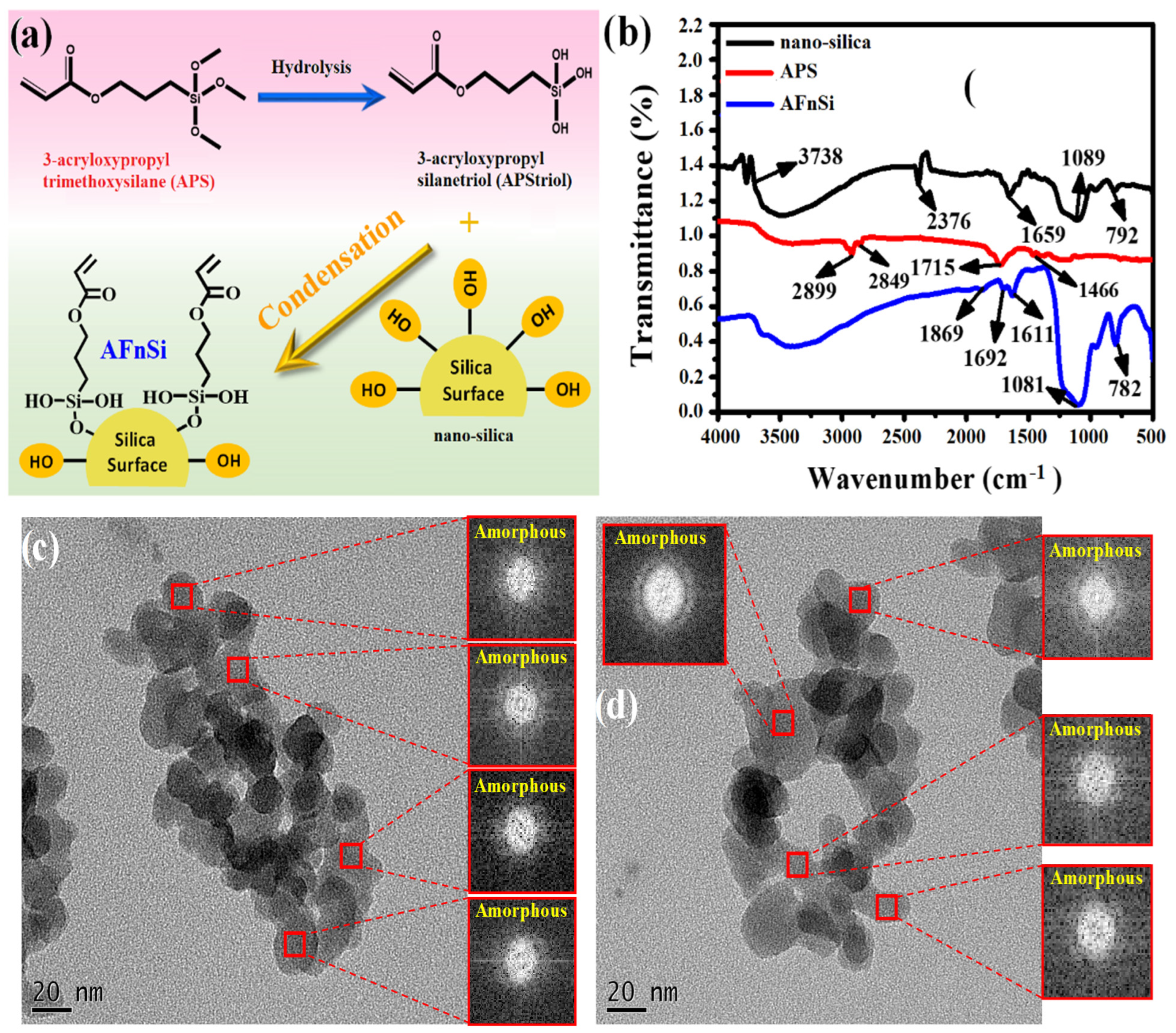
2.4. Synthesis of Acrylate Functionalized nSi Crosslinker (AFnSi)
To prevent nanoparticle aggregation, enhance dispersion stability, and act as a photocured coupling agent in an HG hybrid hydrogel network, this study used 3-acryloxypropyl silanetriol (APS)-functionalized nSi as reinforcing particles. Firstly, the 0.5% (w/v) of 3-acryloxypropyl trimethoxysilane (APMS) solution was prepared in 100 mL of 95% ethanol and stirred at 37 °C. The hydrolysis reaction changed the APMS into APS at pH 4 for 48 h. Finally, the pH was adjusted to 7 and the structure of APS was obtained and identified using Fourier transform infrared (FTIR) spectrometer. Subsequently, 10 g of nSi powder was added to the 100 mL of APS solution and stirred for 24 h to form the colloid solution ultimately. Then, the APS/nSi colloid solution was stirred in an oil bath at 100 °C for 4 h to carry out the condensation reaction between hydroxyl groups. Then, the 50 mL ethanol was added and centrifuged for 10 min at 8000 rpm. The sediment after centrifugation was collected and dried. The functional structure of acrylate-functionalized nSi (AFnSi) obtained was also identified by FTIR analysis.
2.5. Identification of the Synthesis of HAMA and GelMA
The proton NMR spectroscopy (Varian Gemini-200, Morgantown, PA, USA) was recorded for HA, HAMA, gelatin, and GelMA hydrogels to determine the incorporation of the methacrylate group. A total of 20 mg of each sample is completely dissolved in 1ml of deuterium oxide (D
2O)-containing 3-(trimethylsilyl) propionic-2,2,3,3-d
4 acid sodium (TMSP) salt serves as an internal standard. The degree of methacrylation (DoM) for HAMA was calculated by formulation (1). A brief explanation is as follows to determine the degree of methacrylation of HAMA is by
1H NMR analysis. The intensity of the signals of the vinyl groups (signals can typically be found around ~5.8 and 6.2 ppm) of the methacrylates are quantified and compared with a known peak in the spectrum. Sometimes the DoM is also quantified by quantifying the methacrylate methyl peak (at ~1.9 ppm) [
14,
29]. This study method looked at the sum of the methacrylate protons at δ 6.2 ppm and δ 5.8 ppm concerning the three protons on the methyl groups of the N-acetyl-glucosamine subunit at δ 2.0–2.1 ppm. The DoM calculation formula is shown in Equation (1), where the numerator and denominator are compared with the integral value of each proton. In addition, the number of free amine groups and methacryloyl groups in GelMA was also quantified by
1H-NMR assays and cited to the DoM (%) by the literature [
17]. The quantification of both methacrylamide and methacrylate groups in GelMA was done simultaneously. However, the DoM (%) of target 90–100% checked the signal of the free amino groups disappeared in gelatin (signals can typically be found around ~3.0 ppm).
2.6. Identification of the Synthesis AFnSi Crosslinker
The infrared spectra (IR) of the nSi, 3-acryloxypropyl trimethoxysilane (APMS), and acrylate-functionalized nSi (AFnSi) were recorded for functional groups of the chemical structure using FTIR spectroscopy (system 2000 FT-IR, Perkin Elmer, Waltham, MA, USA) in the attenuated reflection (ATR) mode. The transmittance readings of the samples were measured by accumulating 32 scans at a resolution of 4 cm−1 in the spectral region of 4000–400 cm−1. The X-ray powder diffraction (XRD) was used for phase identification of crystalline material for the nSi and AFnSi samples and performed on a Bruker D8 advance diffractometer (Germany). The following parameters were set for the apparatus: Cu-K radiation using a graphite monochromator, a voltage of 40 kV, an electric current of 40 mA, and a scan range from 10° to 60° at a scanning speed of 2°/min. Transmission electron microscopy (TEM) was also used to examine the size and morphology of these nanoparticles. That means that the nSi and AFnSi suspension samples were dropped onto a copper grid coated with a carbon/formvar support film using a Pasteur pipette for TEM observation. After 15 s, the excess sample was blotted with filter paper and dried at room temperature. The grid was placed in a specimen holder and inserted into a 200 kV Joel JEM-2100 TEM for observation.
2.7. Fabrication of HG Hybrid Hydrogels
The HAMA hydrogel solutions were prepared in the concentration of 1% (w/v) and GelMA in the concentration of 10% (w/v), respectively. Then, the hybrid hydrogel by 2:1 volume ratio of HG solution was mixed with 0.3% (w/v) LAP P.I. at different concentrations of AFnSi crosslinker 0.1, 0.5, and 1% (w/v), respectively. The hybrid hydrogel made without AFnSi crosslinker was also as a control group. The hybrid hydrogel solutions were vortexed for homogenous mixing and photocured under UV light using the UV curing chamber from XYZ printing (USA) at a 280–400 nm wavelength for 120 s. However, these hybrid hydrogels were denoted as HAMA-GelMA (HG), HAMA-GelMA + 0.1% (w/v) AFnSi (HG+0.1% AFnSi), HAMA-GelMA + 0.5% (w/v) AFnSi (HG+0.5% AFnSi), and HAMA-GelMA + 1% (w/v) AFnSi (HG+1% AFnSi).
2.8. Characteristics of HG Hybrid Hydrogels
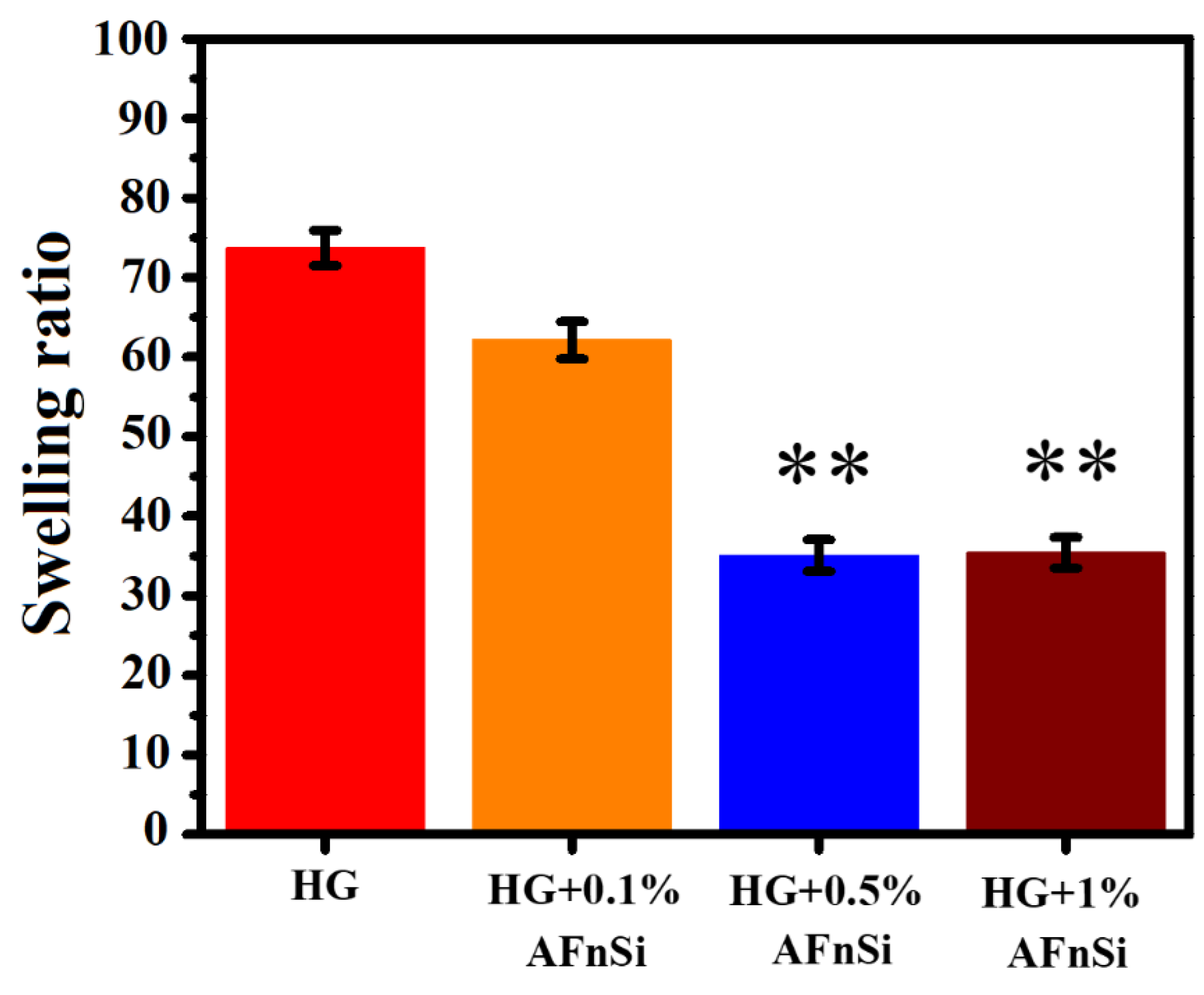
2.8.1. Swelling Ratio Evaluation
To prepare the hybrid hydrogel samples for the swelling ratio measurement, a 300 µL of the hybrid hydrogel by 2:1 volume ratio of HG solution, each of different concentrations of AFnSi crosslinker, such as 0, 0.1, 0.5 and 1.0% (
w/
v) with 0.3% (
w/
v) P.I. of LAP, was added to the cylindrical plastic mold. These hybrid hydrogel solutions of each group were then exposed to UV light at 280–400 nm for 120 s. After the photopolymerization procedure, each hybrid hydrogel sample containing different concentrations of AFnSi crosslinker was placed in an Eppendorf tube with 2 mL of PBS for 24 h to maintain the equilibrium. After 24 h, the swollen hydrogels were weighed, and gently removed the excess water by blotting with kimwipes. Four replicates were used for each hydrogel sample. The swelling ratio of the hydrogel sample was calculated using the following formula:
Ww: wet weight of the hydrogel sample;
W0: dry weight of the hydrogel sample.
2.8.2. The Microstructure Morphology Analysis
The morphological characteristics of these hybrid hydrogels were observed after photocuring with the HG hybrid hydrogel with different concentrations of AFnSi crosslinkers of 0, 0.1, 0.5, and 1.0% (w/v). However, these hybrid hydrogels were prepared as previously described for the swelling ratio samples and the cross-section area obtained after the freeze-dried method. Micrographs or the element mapping analysis of all the hydrogel samples were taken using a scanning electron microscope (SEM, JEOL, Tokyo, Japan) after these samples were coated with gold using a sputter coater under an ambient temperature. The average diameters of pores in HG hybrid hydrogel with different concentrations of 0, 0.1, 0.5, and 1.0% (w/v) AFnSi crosslinkers were evaluated using Image-J software (Media Cybernetics Inc., Rockville, MD, USA).
2.8.3. Mechanical Properties Evaluation
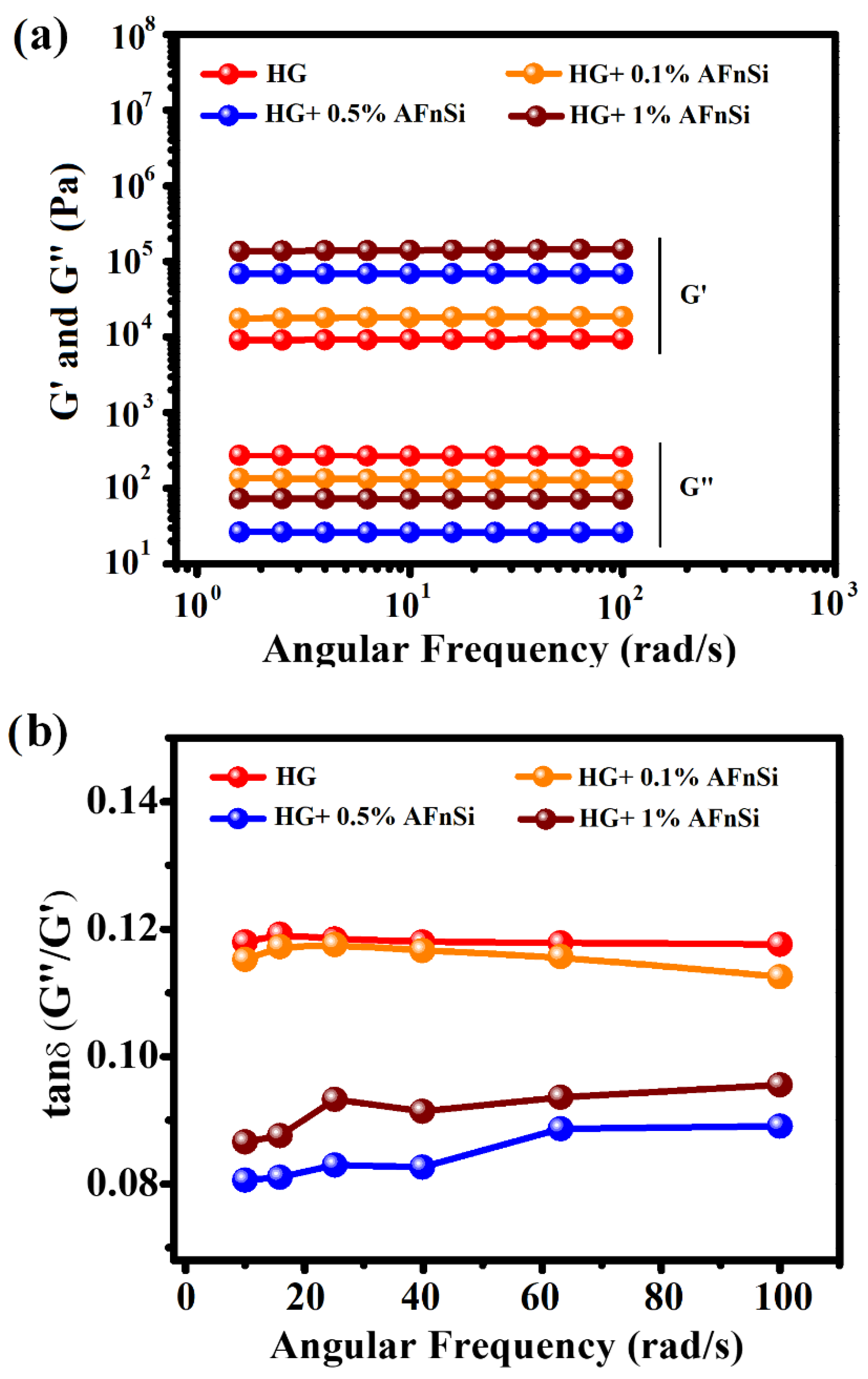
These HG hybrid hydrogels with different concentrations of AFnSi crosslinkers of 0, 0.1, 0.5, and 1.0% (w/v) after photocuring were also measured for their compression strength and rheological behavior. The universal mechanical compression testing machine (Instron 5567, Noorwood, MA, USA) was used for compressive mechanical testing. The speed of the crosshead was 4 mm/s, and the loading cell was 200 N. The compression strength was calculated as stress when the compressive strain in the hydrogel reached 60%. The compression test was repeated for five samples of each hybrid hydrogel. In addition, the storage modulus (G′) and loss modulus (G″), and loss factor (Tan δ), as a function of the angular frequency (rad/s) behavior of these hybrid hydrogels was tested using HR-2 Discovery Hybrid Rheometer-2 (TA Instruments, New Castle, DE, USA) with the attachment of the 20 mm parallel plate. Each hybrid hydrogel was added to the 0.5 mm gap between the plates and waited until the normal force became zero. Firstly, frequency sweeps (ω; rad/s) were tested at a constant 1% strain at 37 °C to check the elasticity properties of the hybrid hydrogel. Furthermore, the storage modulus (G′) as a function of the temperature and time sweep was also carried out by increasing the temperature with 0.5 °C/min heating rate from 10 to 60 °C at a frequency of 1 rad/s.
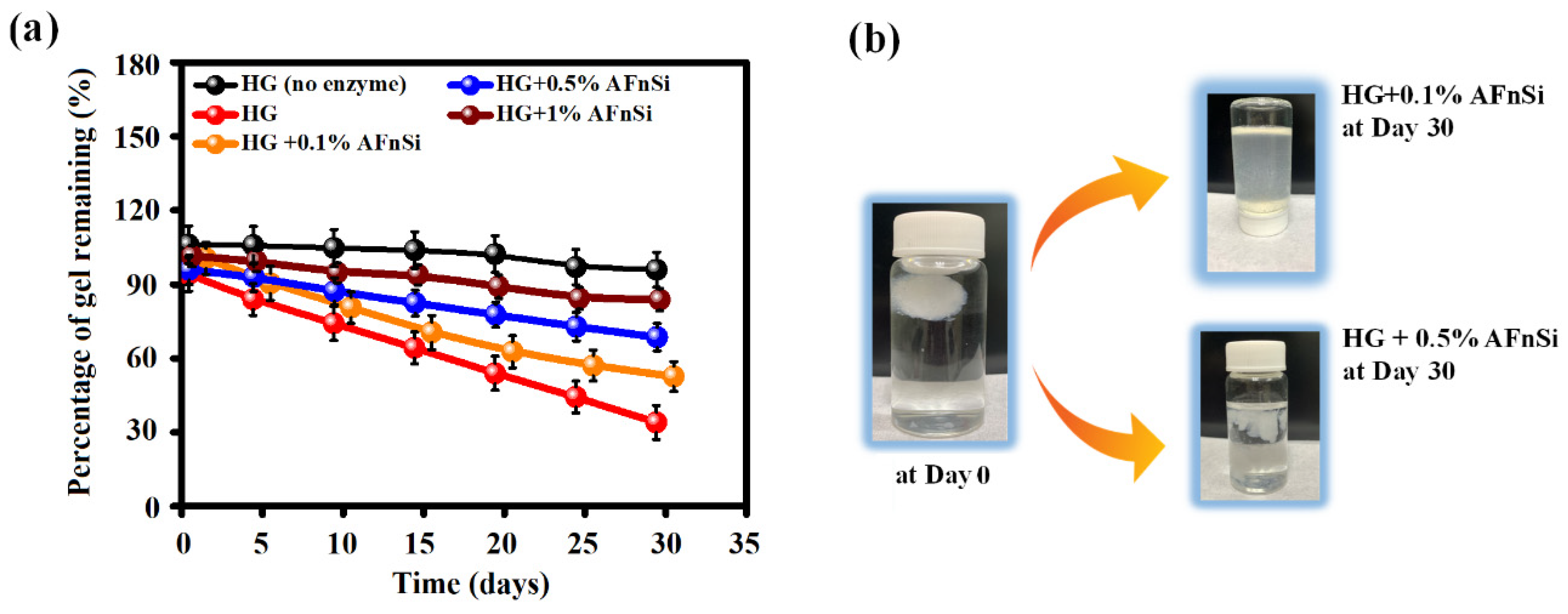
2.8.4. In Vitro Degradation Assay by Hyaluronidase
As mentioned previously in the swelling ratio experiment, these hybrid hydrogels were prepared and allowed to swell in PBS for 24 h to reach the swelling equilibrium first. Then, these hybrid hydrogels were incubated in 1 mL of PBS buffer containing 2.6 Uml−1 hyaluronidase (human plasma physiological concentration) at 37 °C. These hybrid hydrogels were collected every 5 days and weighed after being blotted for 30 days. However, the fresh hyaluronidase solution was replaced every day, and four replicates were used for each hybrid hydrogel. The degree of degradation of the hybrid hydrogels was calculated by normalizing the residual hydrogel wet weight and initial hydrogel wet weight.
2.9. Cell Viability and Chondrogenesis Assay of HG Hybrid Hydrogels
2.9.1. Isolation and Culturing of hADSCs
This study examined the isolation of hADSCs from human subcutaneous adipose tissue, which was done according to a previously described procedure [
11,
26]; the ADSCs were isolated from subcutaneous adipose tissue obtained from human patients during orthopedic surgery after obtaining informed consent from all the patients and approval from the Kaohsiung Medical University hospital ethics committee (KMUH-IRB-E(II)-20150193). Briefly, 3 g of human subcutaneous adipose tissue was extracted and cut into small pieces using scissors. The minced tissues were digested with 1 mg/mL of type Ⅰ collagenase at 37 °C under 5% CO
2 for 24 h. Then, centrifugation was performed at 1000 rpm for 5 min. The pellet was collected and washed with PBS twice. After that, the pellet was resuspended in a K-NAC medium, and the cells were counted and plated in a 100 mm culture dish. Subsequently, the ADSCs attached to the culture plate were maintained at 37 °C under a 5% CO
2 incubator. The K-NAC medium used in this study is suitable for the isolation and expansion of ADSCs described in the initial study; the K-NAC medium mainly contains Keratinocytes-SFM (Gibco BRL, Rockville, MD, USA) supplemented with 25 mg of bovine pituitary extract (BPE), 2.5 µg of human recombinant epidermal growth factor, 2 mM N-acetyl-l-cysteine, 0.2 mM L-ascorbic acid, and 5% FBS. The first medium change was performed after 24 h, and the unadhered ADSCs to the plate were washed off using PBS. Subsequently, the fresh medium was changed every two days. The cells could grow nearly 90% confluence and subculture for further cell study experiments.
2.9.2. Cell Viability Assay
The HG hybrid hydrogel with different concentrations of 0%, 0.5%, and 1.0% (w/v) of AFnSi crosslinker were prepared according to the protocol mentioned above. Briefly, 200 µL of each hydrogel sample was coated on the 24-well plates. Each hydrogel sample was crosslinked using UV light at 365 nm. To each crosslinked hydrogel sample, 1ml of PBS was added and incubated for 24 h at 37 °C under 5% CO2 to reach the swelling equilibrium rate. Afterwards, the PBS was removed gently. The samples can be further used to perform cell viability and differentiation analysis.
The CellTiter96® aqueous one-solution cell proliferation assay (MTS) kit was used to determine cell viability (Promega, Madison, WI, USA). In other words, the HG hybrid hydrogel with the different concentrations of 0%, 0.5%, and 1.0% (w/v) AFnSi crosslinkers and the HA hydrogel (control group) in hADSCs were tested by MTS assay; all the sample groups were cultured in DMEM medium with 1 × 106 cells for day 1, 3, and 5 at 37 °C under 5% CO2. Briefly, the culture media after incubation in each sample well at each time-indicated point was removed and replaced by 200 µL of fresh medium and 40 µL of MTS reagent to each sample well in a 24-well culture plate. The plates were incubated for 4 h at 37 °C under 5% CO2. Finally, 100 µL of the solution was pipetted out from each sample well plate and placed in a 96-well dish. The optical density (OD) at 490 nm was taken using a 96-well ELISA plate reader (Synergy H1, BioTek, Winooski, VT, USA).
Furthermore, the cell toxicity evaluation for the HG hybrid hydrogel with the different concentrations of 0%, 0.5%, and 1.0% (w/v) of AFnSi crosslinkers and HA hydrogel in hADSCs were evaluated using live/dead staining for day 1 and day 5. The live/dead staining assay was performed according to the manufacturer’s protocol. Briefly, 20 µL of ethidium homodimer-1 (EthD-1) and 5µL calcein-AM were mixed with 10 mL of PBS solution and vortexed. A total of 500 µL of working dye solution was added to each well containing the samples and incubated for 1 h at 37 °C under 5% CO2 conditions. Finally, the live and dead cells in the samples were observed using an inverted fluorescence microscope (Leica DMi8 Inverted Fluorescence Microscope).
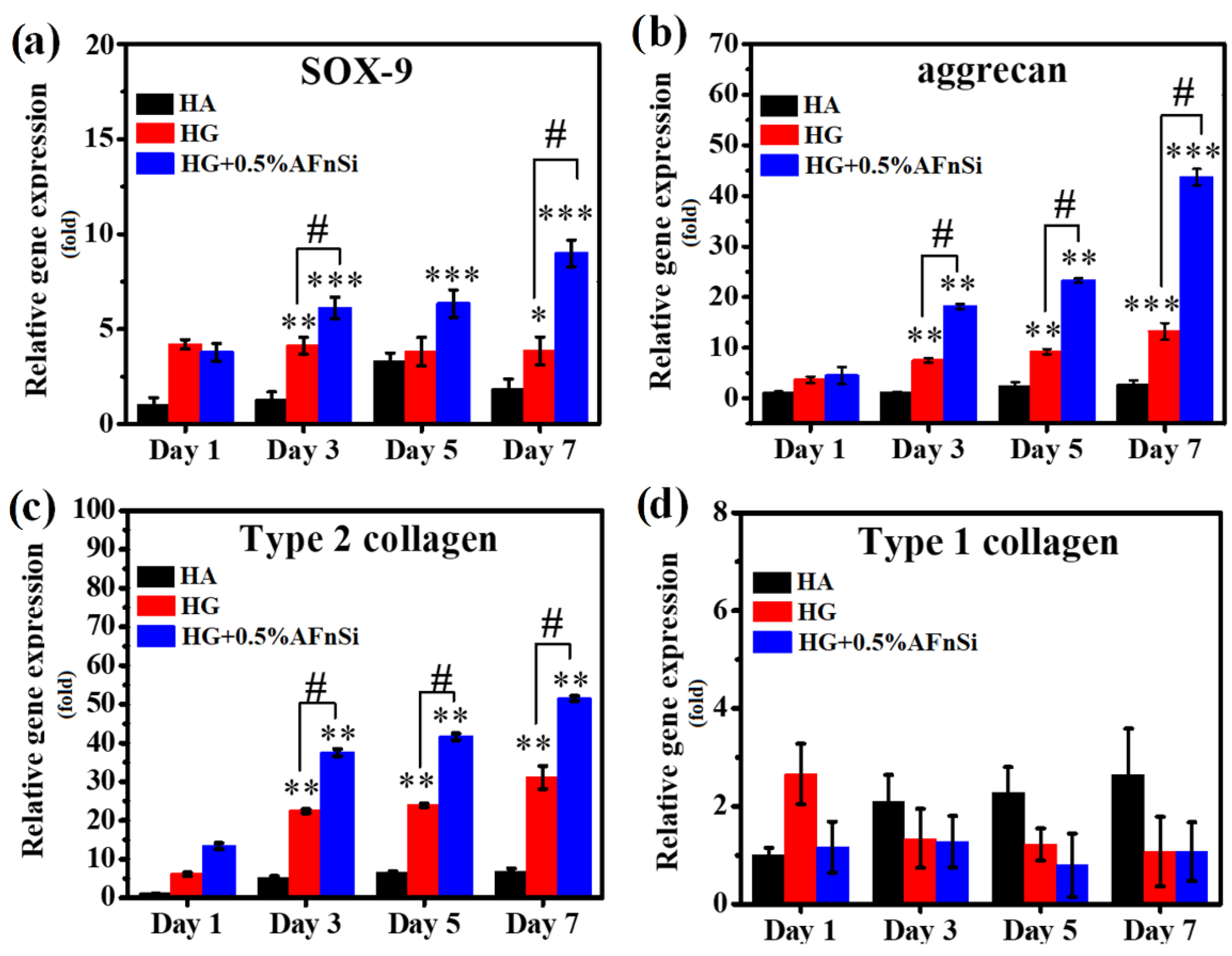
2.9.3. Chondrogenic Marker Gene Expression
The chondrogenic effect of the HG hybrid hydrogel with 0% and 0.5% (
w/
v) of AFnSi crosslinkers and HA hydrogel (control group) in hADSCs were examined for chondrogenic marker gene expression using quantitative real-time PCR assay. Each sample was cultured with 1 × 10
6 cells in 24-well plates in basal medium maintained at 37 °C under 5% CO
2 condition for days 1, 3, 5, and 7. At each time-indicated point, the HG hybrid hydrogel samples were collected. Total RNA extraction was performed using TRIzol reagent. The quality of the RNA was confirmed using a Thermo scientific NanoDrop
TM 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). RNA to cDNA was reverse-transcribed using the TOOLs easy Fast RT kit (Taipei, Taiwan). Real-time PCR was performed using IQ SYBR green supermix (Bio-Rad Laboratories, Hercules, CA, USA). The transcribed cDNA samples were analyzed for the genes of interest comprising SOX-9, aggrecan, type Ⅱ collagen, and type Ⅰ collagen. The primers used in this study are listed in
Table 1. After completing the real-time PCR, the dissociation curve was produced to check the specificity of each PCR product. All values of the gene of interest were normalized to the expression of glyceraldehyde-3–phosphate–dehydrogenase (GADPH) level with an average threshold cycle (Ct) value using the comparative method. The experiment was repeated three times at every time-indicated point.
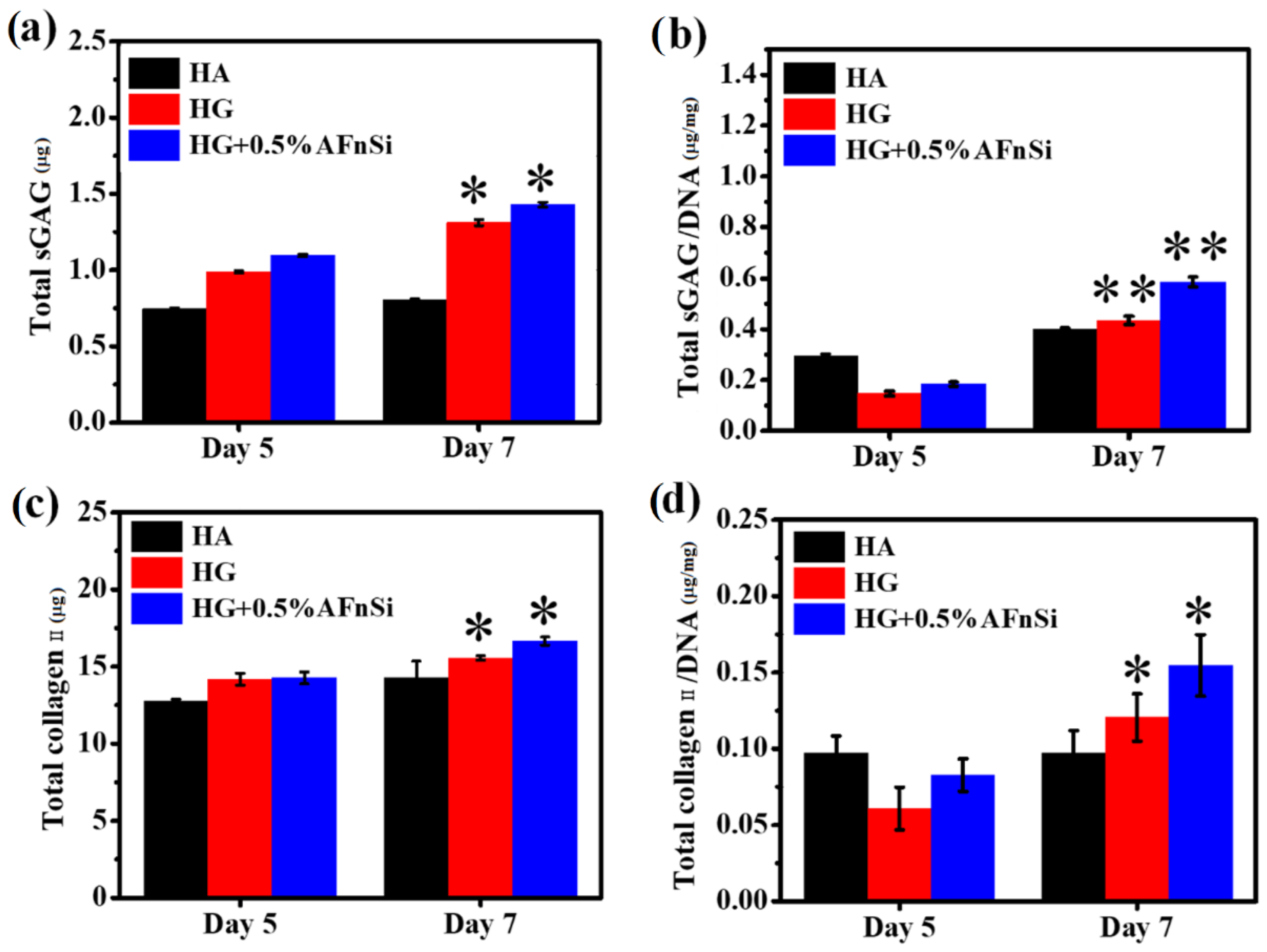
2.9.4. Quantification of DNA, sGAG Deposition, and Collagen Type Ⅱ Synthesis
The DNA content for each sample was measured using a Hoechst dye assay, and the calf thymus was used as a standard curve to measure the DNA content. The analysis of sulfated glycosaminoglycan’s (sGAG) assay protocol was performed with the BlyscanTM kit (Biocolor Ltd., Carrickfergus, Northern Ireland), and the chondroitin solution with different concentrations from 0 to 25 µg/µL was used as the standard for dimethylmethylene blue (DMMB) assay. Finally, the optical density measurement at 650 nm was taken using an ELISA plate reader (Synergy H1, Biotek, Winooski, VT, USA). The DMMB assay was performed to detect and quantify the amount of sGAG content in the HG hybrid hydrogel with 0% and 0.5% (w/v) of AFnSi crosslinkers and HA hydrogel in hADSC. These samples were cultured with 1 × 106 cells in 24-well plates for days 5 and 7 in a basal medium maintained at 37 °C under 5% CO2. These samples were harvested, washed with PBS, and digested at each indicated point sing a Papain solution for 15 h at 60 °C. Enzyme-linked immunosorbent assay (ELISA) was also used to quantify collagen type Ⅱ present in the HG hybrid hydrogel with 0% and 0.5% (w/v) of AFnSi crosslinkers and HA hydrogel in hADSCs. Each sample was cultured in a basal medium with 1 × 106 cells in 24-well plates for days 5 and 7, respectively. At each time-indicated point, these samples were harvested, and the type Ⅱ collagen content in each sample was measured using a type Ⅱ collagen detection kit (Chondrex, Redmond, WA, USA).
2.10. Statistical Analysis
The scoring data were statistically analyzed to express the mean ± SD (n = 3~6). A one-way ANOVA (or t-test method) was performed, and the * p < 0.05 and ** p < 0.001 were compared to the control or compared between treatment groups.