Effect of Copper (II) Sulfate on the Properties of Urea Formaldehyde Adhesive
Abstract
:1. Introduction
- (1)
- (2)
- Surface sealing method. Pibiri [14] showed that formaldehyde release can be reduced by 70% by applying a sealant coating to particleboard after 900 h. The advantage of such surface sealing is that it reduces the formaldehyde release during a certain period, but can only slow down the formaldehyde emission rate, the overall formaldehyde content in the board remains the same.
- (3)
- Using a formaldehyde adsorbent or scavenger. Inorganic formaldehyde adsorbents, such as alumina [15], attapulgite [16], and melamine [17], can physically adsorb formaldehyde and retard its release, but they do not change the total amount of formaldehyde within the material. Organic formaldehyde scavengers, such as tannin [18], montmorillonite [19], and methylamine, or ethylamine [10], react chemically with free formaldehyde, thereby reducing its content in an adhesive, and hence its release from UF products. A study by Ghani et al. [10] revealed that the addition of 1% propylamine to UF resin reduced the formaldehyde release from particleboard by 56%, but it also had a detrimental effect on the mechanical properties of the product.
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Experimental Methods
2.3.1. Preparation of UF Resin Adhesive
2.3.2. Preparation of Copper (II) Sulfate/UF Resin Adhesive
2.3.3. Preparation of Plywood
2.4. Determination of Free Formaldehyde in the UF Resin
2.5. Performance Tests of Plywood
2.5.1. Bonding Strength Test of Plywood
2.5.2. Test of Formaldehyde Release from Plywood
2.6. FTIR Characterization of UF Resin
2.7. TG Analysis of UF Resin
3. Results and Discussion
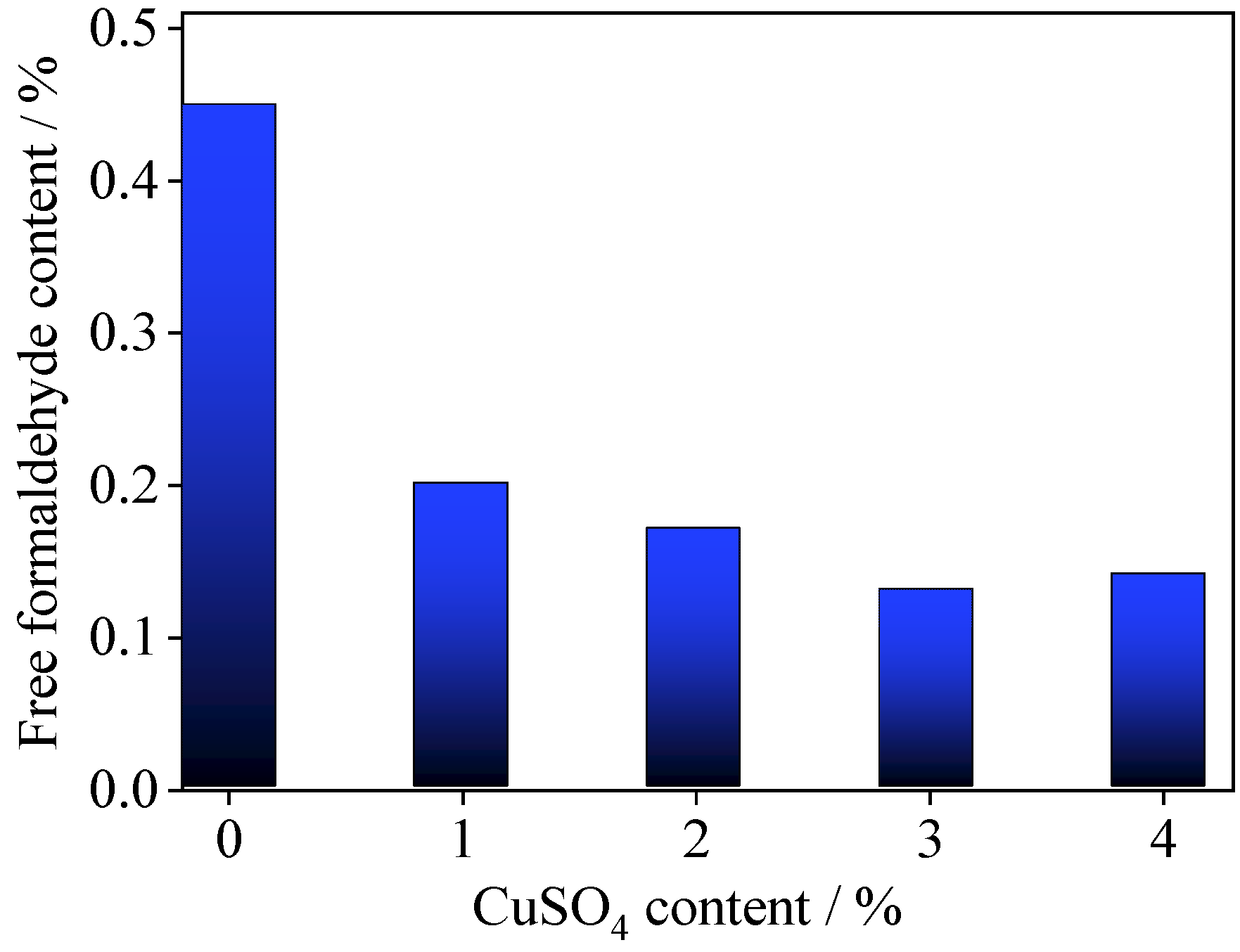
3.1. Effects of Copper (II) Sulfate on the Properties of UF Resin Adhesive
3.2. Effect of Copper (II) Sulfate Content on the Properties of Plywood
3.2.1. Effect of Copper (II) Sulfate Content on the Bonding Strength of Plywood
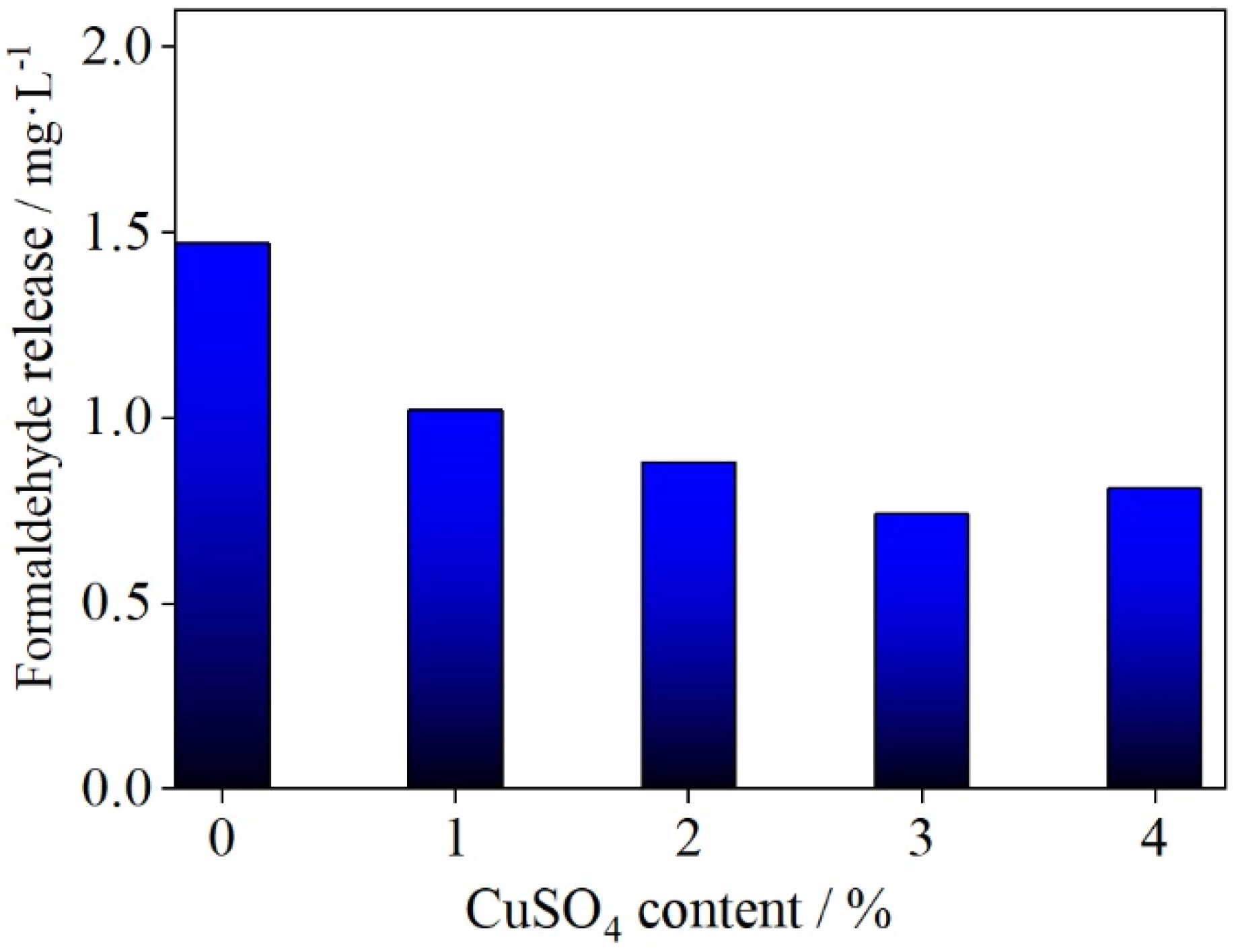
3.2.2. Effect of Copper (II) Sulfate Content on Formaldehyde Release from Plywood
3.3. ATR-IR Characterization of the UF Resin
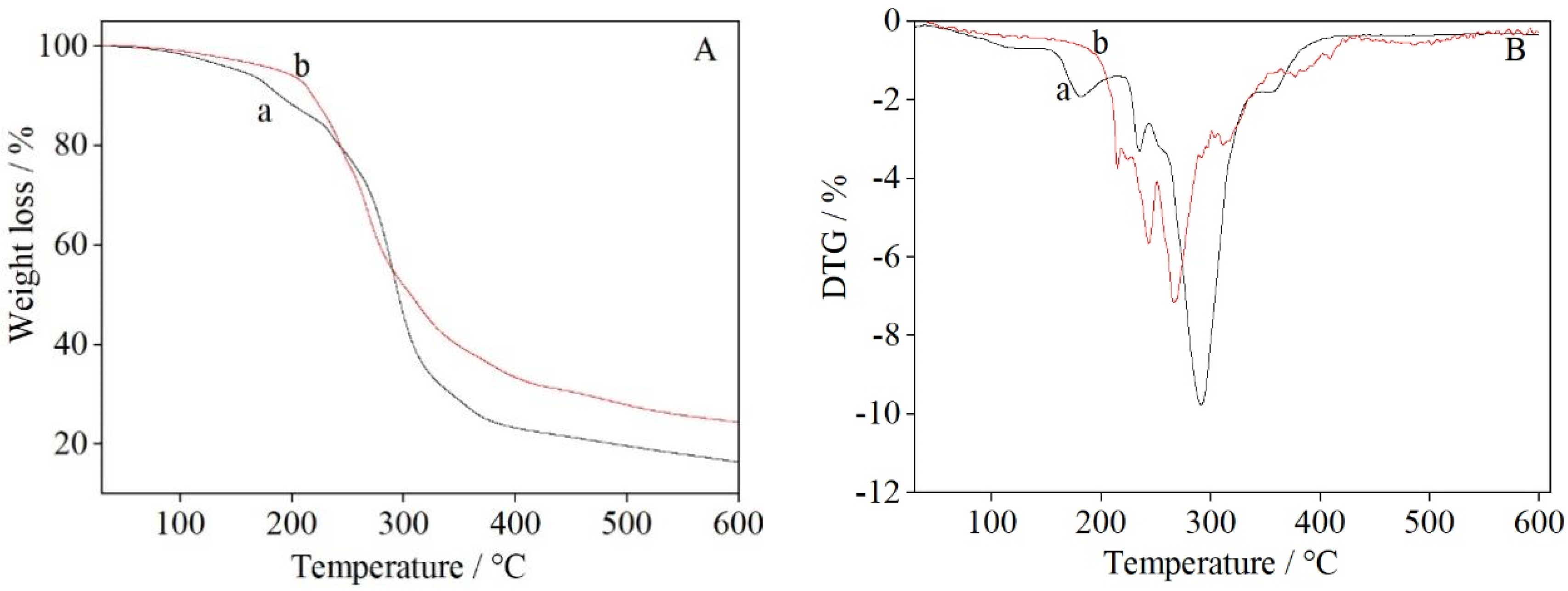
3.4. Thermogravimetric Analysis of UF Resin
- (1)
- The first process is the evaporation of residual water and the release of formaldehyde from the UF resin at 30–200 °C [39], which includes water and formaldehyde produced by the condensation reaction of hydroxymethyl and amine groups at high temperatures. The mass loss is about 12% (see Figure 5A(a)). Figure 5A(b) shows that copper (II) sulfate had a significant effect on the thermal behavior of the UF resin, with the mass loss from the UF/3% CuSO4 resin being only about 7% at 200 °C. The decrease in mass loss at 200 °C can be related to the low content of free formaldehyde in the modified UF resin, as well as the interaction between water and Cu2O, the reduction product of copper (II) sulfate, through hydrogen bonding [40]. It can be seen from the DTG traces in Figure 5B that UF resin displayed a small peak in the range 90–150 °C and a peak in the range 150–200 °C, whereas UF/3% CuSO4 resin showed no obvious peak in the range 90–200 °C. This indicated that the release of water and free formaldehyde from the UF/3% CuSO4 resin was significantly suppressed.
- (2)
- The second stage involves mass losses caused by the breaking and decomposition of the methylene ether, and methylene bonds, and the removal of other small molecules from the resin at 200–350 °C [41]. The cumulative weight losses from UF resin and UF/3% CuSO4 resin in this region were about 77% and 66%, respectively.
- (3)
- The third mass loss stage occurs in the range 350–600 °C [41]. In this stage, volatile elements are removed, and the resin is carbonized. The cumulative weight losses from the UF and UF/3% CuSO4 resins at 600 °C were 83% and 75%, respectively. The lower mass loss from the UF resin incorporating CuSO4 may be attributed to the shielding effect caused by the presence of metal oxide particles or a greater residual mass, and does not necessarily represent an increase in thermal stability [15].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorieh, A.; Mahmoodi, N.O.; Mamaghani, M.; Pizzi, A.; Mohammadi Zeydi, M.; Moslemi, A. New in-sight into the use of latent catalysts for the synthesis of urea formaldehyde adhesives and the mechanical properties of medium density fiberboards bonded with them. Eur. Polym. J. 2019, 112, 195–205. [Google Scholar] [CrossRef]
- Liu, K.K.; Su, C.Q.; Ma, W.W.; Li, H.L.; Zeng, Z.; Li, L.Q. Free Formaldehyde Reduction in Urea-formaldehyde Resin Adhesive: Modifier Addition Effect and Physicochemical Property Characterization. Bioresources 2020, 15, 2339–2355. [Google Scholar] [CrossRef]
- Liu, M.; Thirumalai Rooban Venkatesh, K.G.; Wu, Y.Q.; Wan, H. Characterization of the crystalline regions of cured urea formaldehyde resin. RSC Adv. 2017, 7, 49536–49541. [Google Scholar] [CrossRef] [Green Version]
- Dorieh, A.; Mahmoodi, N.; Mamaghani, M.; Pizzi, A.; Zeydi, M.M.; Adhes, J. Comparison of the properties of urea-formaldehyde resins by the use of formalin or urea formaldehyde condensates. Sci. Technol. 2018, 32, 2537–2551. [Google Scholar] [CrossRef]
- Hogger, E.M.; van Herwijnen, H.W.G.; Moser, J.; Kantner, W.; Konnerth, J. influence of cold tack properties on bond performance of cured urea-formaldehyde resin. Eur. J. Wood. Wood. Prod. 2019, 77, 487–490. [Google Scholar] [CrossRef]
- Mazaheri, M.; Moghimi, H.; Taheri, R.A. Urea impregnated multiwalled carbon nanotubes; a formaldehyde scavenger for urea formaldehyde adhesives and medium density fiberboards bonded with them. J. Appl. Polym. Sci. 2021, 139, 51445. [Google Scholar] [CrossRef]
- Suresh, S.; Bandosz, T.J. Removal of formaldehyde on carbon-based materials: A review of the recent approaches and findings. Carbon 2018, 137, 207–221. [Google Scholar] [CrossRef]
- Sun, S.J.; Zhao, Z.Y.; Shen, J. Effects of the Manufacturing Conditions on the VOCs Emissions of Particleboard. BioResources 2020, 15, 1074–1084. [Google Scholar] [CrossRef]
- Indoor Decorating and Refurbishing Materials Limit of Formal Dehyde Emission of Wood Based Panels and Finishing Products; GB 18580-2001; China Standard Press: Beijing, China, 2001.
- Ghani, A.; Ashaari, Z.; Bawon, P.; Lee, S.H. Reducing formaldehyde emission of urea formaldehyde-bonded particleboard by addition of amines as formaldehyde scavenger. Build. Enviorn. 2018, 142, 188–194. [Google Scholar] [CrossRef]
- Lu, L.; Wang, Y.; Li, T.H.; Wang, S.P.; Yang, S.L.; Qing, Y.; Li, X.G.; Wu, Y.Q.; Liu, M. Calcium carbonate modified urea-formaldehyde resin adhesive for strength enhanced medium density fiberboard production. RSC Adv. 2021, 11, 25010–25017. [Google Scholar] [CrossRef]
- Wang, H.; Cao, M.; Li, T.H.; Yang, L.; Duan, Z.G.; Zhou, X.J.; Du, G.B. Characterization of the Low Molar Ratio Urea-Formaldehyde Resin with C-13 NMR and ESI-MS: Negative Effects of the Post-Added Urea on the Urea-Formaldehyde Polymers. Polymers 2018, 10, 602. [Google Scholar] [CrossRef] [Green Version]
- Lubis, M.A.R.; Park, B.D. Enhancing the performance of low molar ratio urea-formaldehyde resin adhesives via in-situ modification with intercalated nanoclay. J. Adhes. 2021, 97, 1271–1290. [Google Scholar] [CrossRef]
- Zhang, J.J.; Song, F.F.; Tao, J.; Zhang, Z.F.; Shi, S.Q. Research Progress on Formaldehyde Emission of Wood-Based Panel. Int. J. Polym. Sci. 2018, 2018, 9349721. [Google Scholar] [CrossRef]
- De Cademartori, P.H.G.; Artner, M.A.; de Freitas, R.A.; Magalhaes, W.L.E. Alumina nanoparticles as formaldehyde scavenger for urea-formaldehyde resin: Rheological and in-situ cure performance. Compos. B Eng. 2019, 176, 107281. [Google Scholar] [CrossRef]
- Shao, H.X.; Tang, Q.G.; Liang, J.S.; Ding, Y. Effect of Mineral Composite Fillers on Properties of Man-Made Plywood. Adv. Mat. Res. 2010, 178, 97–102. [Google Scholar] [CrossRef]
- Cavdar, A.D. Effect of zeolite as filler in medium density fiberboards bonded with urea formaldehyde and melamine formaldehyde resins. J. Build. Eng. 2020, 27, 101000. [Google Scholar] [CrossRef]
- Boran, S.; Usta, M.; Ondaral, S.; Gumuskaya, E. The efficiency of tannin as a formaldehyde scavenger chemical in medium density fiberboard. Compos. B Eng. 2012, 43, 2487–2491. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, M.; Zheng, Y.M.; Tong, D.S.; Zhou, C.H.; Yu, W.H. Effect of a novel environmentally friendly additive of polyaspartic acid on the properties of urea formaldehyde resins/montmorillonite. J. Appl. Polym. Sci. 2019, 136, 48038. [Google Scholar] [CrossRef]
- Shanaghi, A.; Amiri, A.; Kazazi, M.; Souri, A.R.; Moradi, H.; Jaffari, A. Effects of processing parameters on phase, morphology, mechanical and corrosion properties of W-Cu nanocomposite powder prepared by electroless copper plating. Appl. Phys. A Mater. Sci. Process. 2020, 126, 18. [Google Scholar] [CrossRef]
- Reinprecht, L.; Vidholdová, Z.; Iždinský, J. Particleboards prepared with addition of copper sulphate—Part 1: Biological resistance. Acta Facu. Xylologiae 2017, 59, 53–60. [Google Scholar]
- Iždinský, J.; Reinprecht, L. Particleboards prepared with addition of copper sulphate—Part 2: Moisture and strength properties. Acta Facu. Xylologiae 2017, 59, 61–66. [Google Scholar]
- Gao, S.S.; Cheng, Z.H.; Zhou, X.; Liu, Y.P.; Chen, R.Q.; Wang, J.F.; Wang, C.P.; Chu, F.X.; Xu, F.; Zhang, D.H. Unexpected role of amphiphilic lignosulfonate to improve the storage stability of urea formaldehyde resin and its application as adhesives. Int. J. Biol. Macromol. 2020, 161, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Bekhta, P.; Sedliačik, J. Environmentally-Friendly High-Density Polyethylene-Bonded Plywood Panels. Polymers 2019, 11, 1166. [Google Scholar] [CrossRef] [Green Version]
- Ordinary Plywood; GB/T 9846-2015; China Standard Press: Beijing, China, 2015.
- Test Method for Physical and Chemical Properties of Wood-Based Panels and Facing Wood-Based Panels; GB/T 17657-2013; China Standard Press: Beijing, China, 2013.
- Dehchar, C.; Chikouche, I.; Kherrat, R.; Sahari, A.; Zouaoui, A.; Merati, A. Electroless copper deposition on epoxy glass substrate for electrocatalysis of formaldehyde. Mater. Lett. 2018, 228, 439–442. [Google Scholar] [CrossRef]
- Shacham-Diamand, Y.; Dubin, V.; Angyal, M. Electroless copper deposition for ULSI. Thin Solid Films 1995, 262, 93–103. [Google Scholar] [CrossRef]
- Inspection Method of Adhesives and Resins for Wood Industry; GB/T 14074-2017; China Standard Press: Beijing, China, 2017.
- Mahrdt, E.; Pinkl, S.; Schmidberger, C.; van Herwijnen, H.W.G.; Veigel, S.; Gindl-Altmutter, W. Effect of addition of microfibrillated cellulose to urea-formaldehyde on selected adhesive characteristics and distribution in particle board. Cellulose 2016, 23, 571–580. [Google Scholar] [CrossRef]
- Ghani, A.; Bawon, P.; Ashaari, Z.; Wahab, M.W.; Hua, L.S.; Chen, L.W. Addition of propylamine as formaldehyde scavenger for urea formaldehyde-bonded particleboard. Wood Res. 2017, 62, 329–334. [Google Scholar]
- Costa, N.A.; Pereira, J.; Ferra, J.; Cruz, P.; Martins, J.; Magalhes, F.D.; Mendes, A.; Carvalho, L.H. Scavengers for achieving zero formaldehyde emission of wood-based panels. Wood Sci. Technol. 2013, 47, 1261–1272. [Google Scholar] [CrossRef]
- Park, B.D.; Kim, J.W. Dynamic Mechanical Analysis of Urea–Formaldehyde Resin Adhesives with Different Formaldehyde-to-Urea Molar Ratios. J. Appl. Polym. Sci. 2008, 108, 2045–2051. [Google Scholar] [CrossRef]
- Ong, H.R.; Khan, M.M.R.; Prasad, D.M.R.; Yousuf, A.; Chowdhury, M.N.K. Palm kernel meal as a melamine urea formaldehyde adhesive filler for plywood applications. Int. J. Adhes. Adhes. 2018, 85, 8–14. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.Z.; Yi, Z.; Yang, H.; Zhao, B.; Zhang, W.; Li, J.Z. Preparation and characterization of a novel environmentally friendly phenol-formaldehyde adhesive modified with tannin and urea. Int. J. Adhes. Adhes. 2016, 66, 26–32. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Wu, Y.Q.; He, Z.Q.; Wan, H.J. “Greener” adhesives composed of urea-formaldehyde resin and cottonseed meal for wood-based composites. J. Clean. Prod. 2018, 187, 361–371. [Google Scholar] [CrossRef]
- Luo, J.L.; Zhang, J.Z.; Luo, J.; Li, J.Z.; Gao, Q. Effect of Melamine Allocation Proportion on Chemical Structures and Properties of Melamine-Urea-Formaldehyde Resins. Bioresources 2015, 10, 3265–3276. [Google Scholar] [CrossRef] [Green Version]
- Terada, M. The Infrared Absorption Spectrum of Cuprous Oxide. Bull. Chem. Soc. Jpn. 1964, 37, 766–767. [Google Scholar] [CrossRef] [Green Version]
- Ullah, H.; Azizli, K.; Man, Z.B.; Ismail, M.B.C. Synthesis and Characterization of Urea-formaldehyde Microcapsules Containing Functionalized Polydimethylsiloxanes. Proced. Eng. 2016, 148, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Sacher, E.; Meunier, M. The effects of hydrogen bonds on the adhesion of inorganic oxide particles on hydrophilic silicon surfaces. J. Appl. Phys. 1999, 86, 1744–1748. [Google Scholar] [CrossRef]
- Lin, S.S.; Du, S.G.; Lu, Y.L. Preparation and Characterization of Submicron TiO2 Microspheres via Urea-formaldehyde Template. IOP Conf. Ser. Mater. Sci. Eng. 2020, 735, 012004. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Li, X.; Wang, X.; Meng, M.; Wang, X.; Huang, S.; Gan, W. Effect of Copper (II) Sulfate on the Properties of Urea Formaldehyde Adhesive. Polymers 2022, 14, 94. https://doi.org/10.3390/polym14010094
Zhao H, Li X, Wang X, Meng M, Wang X, Huang S, Gan W. Effect of Copper (II) Sulfate on the Properties of Urea Formaldehyde Adhesive. Polymers. 2022; 14(1):94. https://doi.org/10.3390/polym14010094
Chicago/Turabian StyleZhao, Hui, Xianzhen Li, Xi Wang, Mianwu Meng, Xiujian Wang, Siyu Huang, and Weixing Gan. 2022. "Effect of Copper (II) Sulfate on the Properties of Urea Formaldehyde Adhesive" Polymers 14, no. 1: 94. https://doi.org/10.3390/polym14010094
APA StyleZhao, H., Li, X., Wang, X., Meng, M., Wang, X., Huang, S., & Gan, W. (2022). Effect of Copper (II) Sulfate on the Properties of Urea Formaldehyde Adhesive. Polymers, 14(1), 94. https://doi.org/10.3390/polym14010094




