Strengthening Cellulose Nanopaper via Deep Eutectic Solvent and Ultrasound-Induced Surface Disordering of Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Bacterial Synthesis of Cellulose
2.2.2. Preparation of Deep Eutectic Solvents
2.2.3. Treatment of BC with DES and Preparation of BC-NF Suspension
2.2.4. Preparation of Cellulose Films
2.2.5. Infrared and Raman Spectroscopy
2.2.6. Wide-Angle X-ray Diffraction Study
2.2.7. Microscopic Investigation
2.2.8. Thermogravimetric Analysis (TGA)
2.2.9. Mechanical Measurements
3. Results
3.1. Preparation of Dispersions and Films
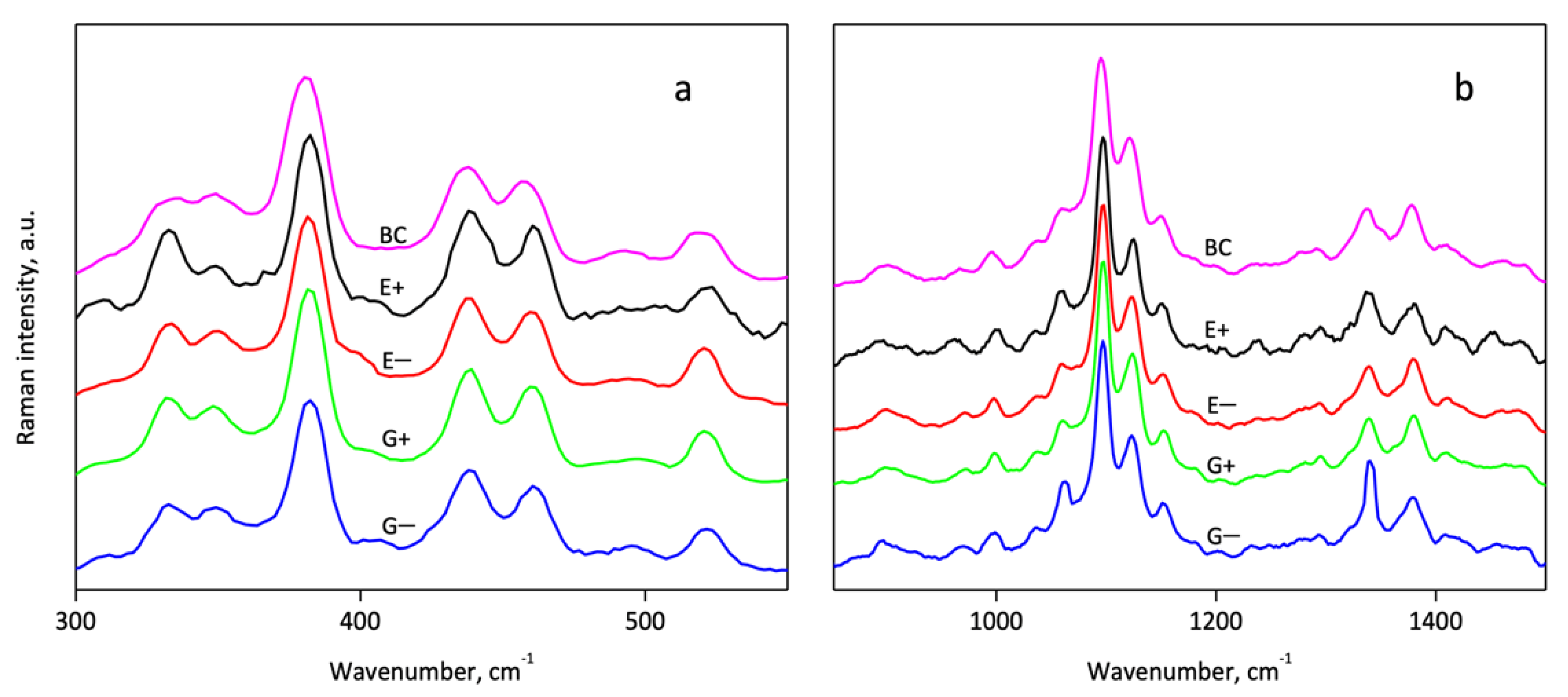
3.2. Infrared and Raman Spectroscopy
3.3. WAXD Data
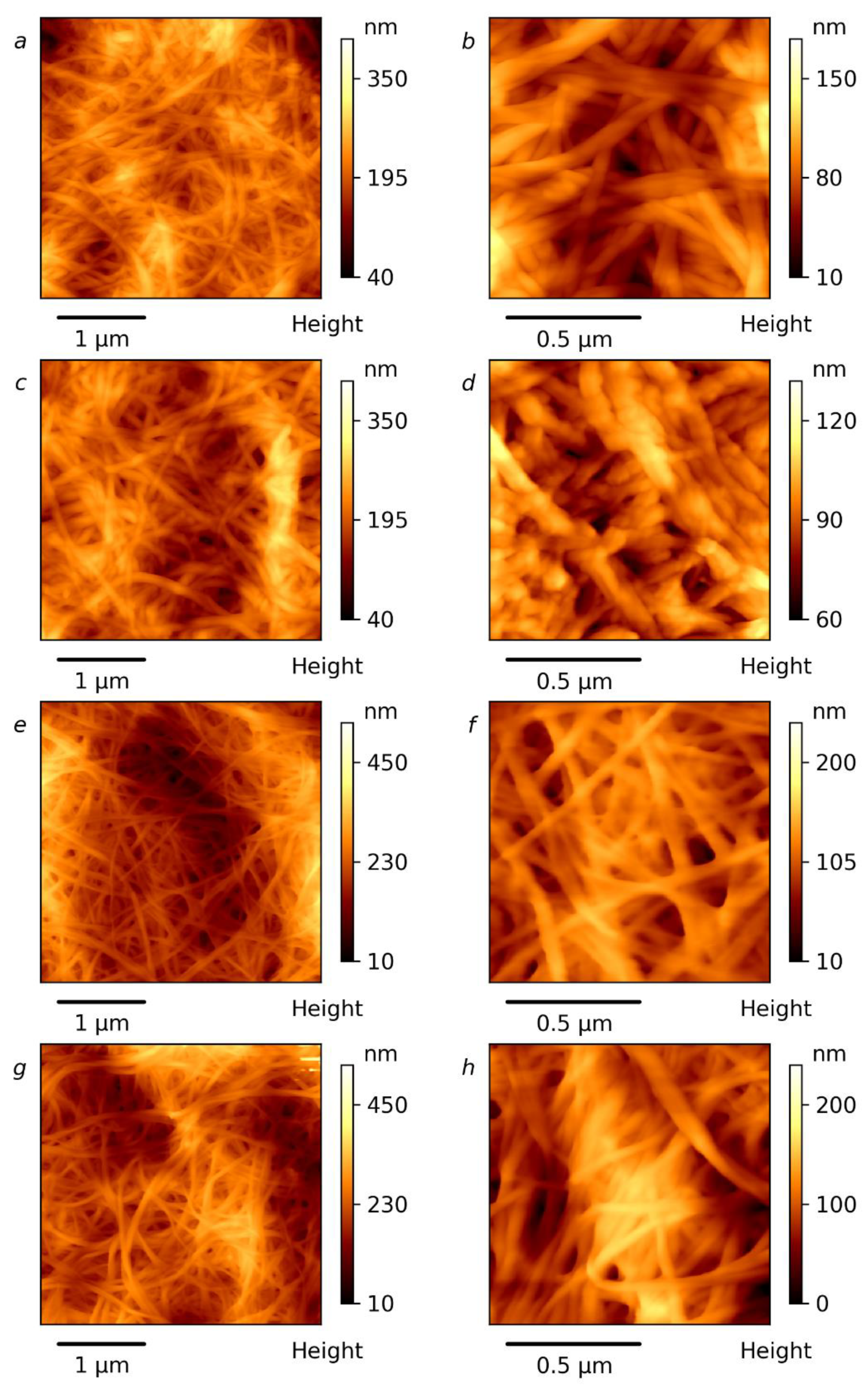
3.4. Microscopy Investigation
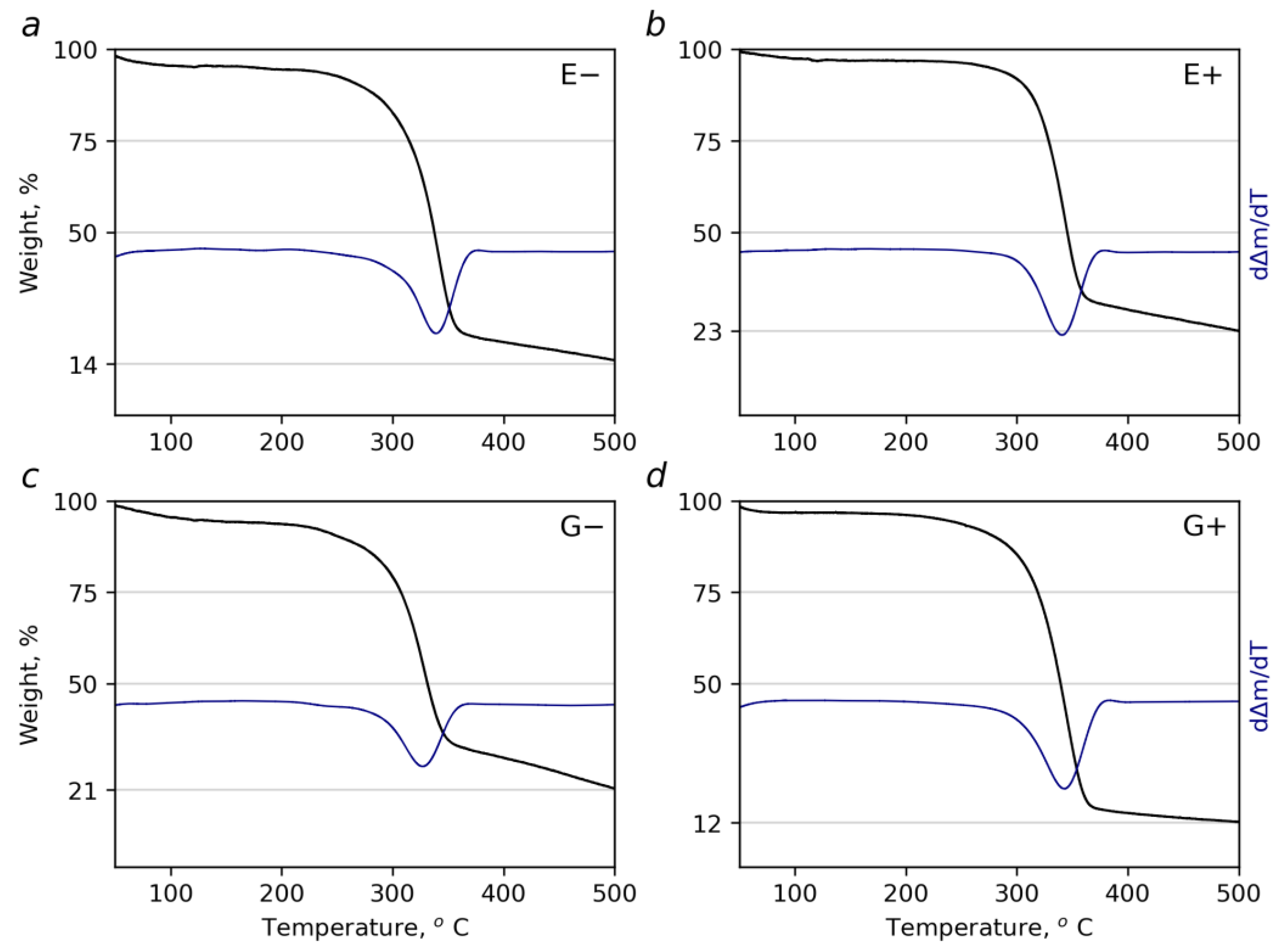
3.5. Thermogravimetric Analysis
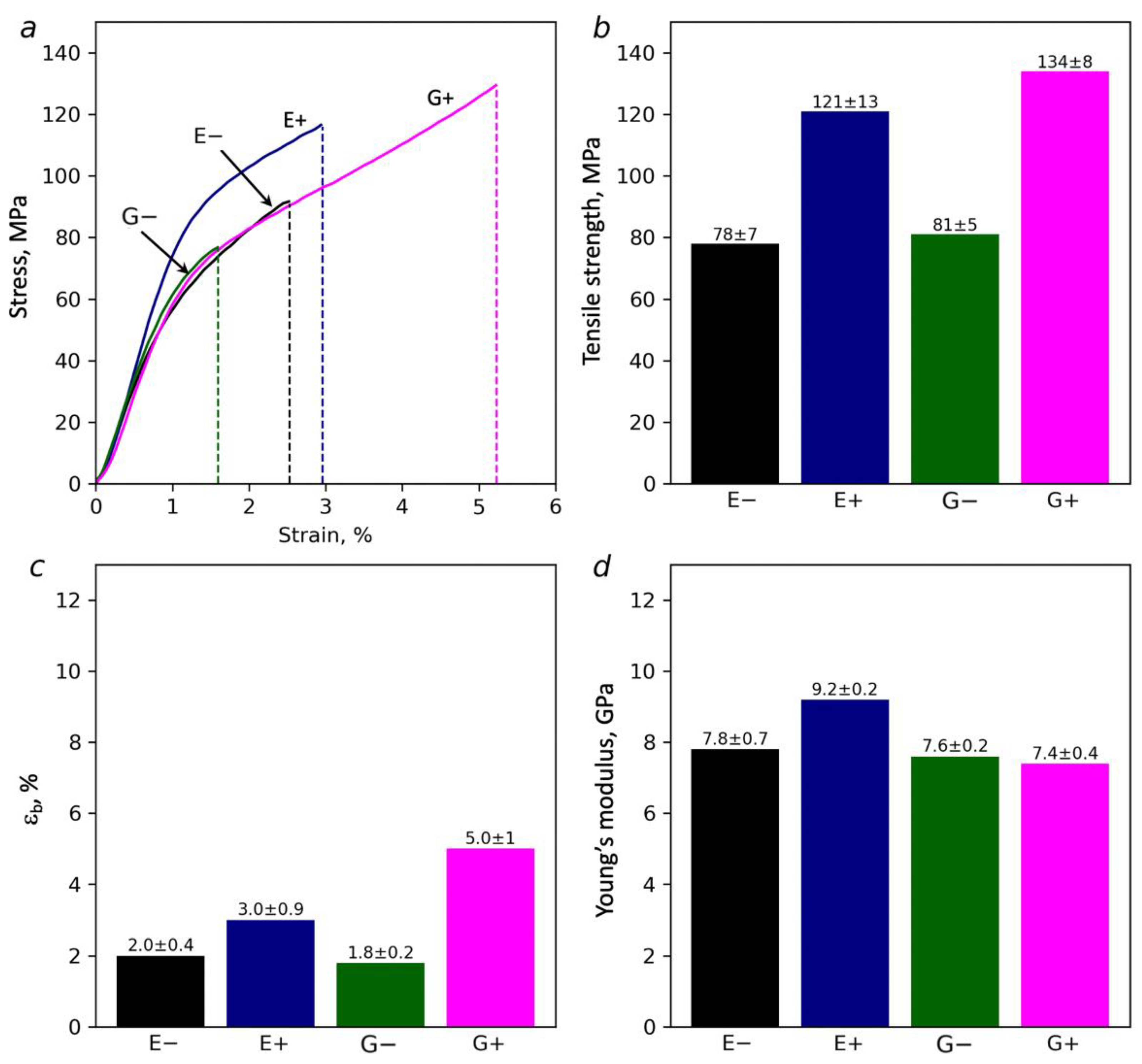
3.6. Mechanical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Höök, M.; Davidsson, S.; Johansson, S.; Tang, X. Decline and depletion rates of oil production: A comprehensive investigation. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20120448. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Jiang, X.; Bai, Y.; Chen, X.; Liu, W. A review on raw materials, commercial production and properties of lyocell fiber. J. Bioresour. Bioprod. 2020, 5, 16–25. [Google Scholar] [CrossRef]
- Calvino, C.; Macke, N.; Kato, R.; Rowan, S.J. Development, processing and applications of bio-sourced cellulose nanocrystal composites. Prog. Polym. Sci. 2020, 103, 101221. [Google Scholar] [CrossRef]
- Mondal, S. Preparation, properties and applications of nanocellulosic materials. Carbohydr. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Habibi, Y.; Adhikari, B. Surface modifications of nanocellulose: From synthesis to high-performance nanocomposites. Prog. Polym. Sci. 2021, 119, 101418. [Google Scholar] [CrossRef]
- Frone, A.N.; Berlioz, S.; Chailan, J.-F.; Panaitescu, D.M. Morphology and thermal properties of PLA–cellulose nanofibers composites. Carbohydr. Polym. 2013, 91, 377–384. [Google Scholar] [CrossRef]
- Narita, C.; Okahisa, Y.; Yamada, K. A novel technique in the preparation of environmentally friendly cellulose nanofiber/silk fibroin fiber composite films with improved thermal and mechanical properties. J. Clean. Prod. 2019, 234, 200–207. [Google Scholar] [CrossRef]
- Hasan, N.; Rahman, L.; Kim, S.-H.; Cao, J.; Arjuna, A.; Lallo, S.; Jhun, B.H.; Yoo, J.-W. Recent advances of nanocellulose in drug delivery systems. J. Pharm. Investig. 2020, 50, 553–572. [Google Scholar] [CrossRef]
- Ng, L.Y.; Wong, T.J.; Ng, C.Y.; Amelia, C.K.M. A review on cellulose nanocrystals production and characterization methods from Elaeis guineensis empty fruit bunches. Arab. J. Chem. 2021, 14, 103339. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Rossi, B.R.; Pellegrini, V.O.; Cortez, A.A.; Chiromito, E.M.; Carvalho, A.J.; Pinto, L.O.; Rezende, C.A.; Mastelaro, V.R.; Polikarpov, I. Cellulose nanofibers production using a set of recombinant enzymes. Carbohydr. Polym. 2021, 256, 117510. [Google Scholar] [CrossRef]
- Ching, Y.C.; Rahman, A.; Ching, K.Y.; Sukiman, N.L.; Cheng, H.C. Preparation and Characterization of Polyvinyl Alcohol-Based Composite Reinforced with Nanocellulose and Nanosilica. Bioresources 2015, 10, 3364–3377. [Google Scholar] [CrossRef]
- Gwon, J.-G.; Cho, H.-J.; Chun, S.-J.; Lee, S.; Wu, Q.; Lee, S.-Y. Physiochemical, optical and mechanical properties of poly(lactic acid) nanocomposites filled with toluene diisocyanate grafted cellulose nanocrystals. RSC Adv. 2016, 6, 9438–9445. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Ávila, H.M.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose nanocomposites: Fabrication and biomedical applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Anirudhan, T.; Shainy, F. Effective removal of mercury(II) ions from chlor-alkali industrial wastewater using 2-mercaptobenzamide modified itaconic acid-grafted-magnetite nanocellulose composite. J. Colloid Interface Sci. 2015, 456, 22–31. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Pinjari, D.V.; Sonawane, S.H.; Bhanvase, B.A.; Sheikh, J.; Sillanpää, M. Cellulose-based nanomaterials for water and wastewater treatments: A review. J. Environ. Chem. Eng. 2021, 9, 106626. [Google Scholar] [CrossRef]
- Dou, C.; Li, Z.; Gong, J.; Li, Q.; Qiao, C.; Zhang, J. Bio-based poly (γ-glutamic acid) hydrogels reinforced with bacterial cellulose nanofibers exhibiting superior mechanical properties and cytocompatibility. Int. J. Biol. Macromol. 2021, 170, 354–365. [Google Scholar] [CrossRef]
- Sharma, A.; Mandal, T.; Goswami, S. Fabrication of cellulose acetate nanocomposite films with lignocelluosic nanofiber filler for superior effect on thermal, mechanical and optical properties. Nano-Struct. Nano-Objects 2021, 25, 100642. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose nanocrystals: Pretreatments, preparation strategies, and surface functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581. [Google Scholar] [CrossRef]
- Rantanen, J.; Maloney, T.C. Consolidation and dewatering of a microfibrillated cellulose fiber composite paper in wet pressing. Eur. Polym. J. 2015, 68, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Kargarzadeh, H.; Huang, J.; Lin, N.; Ahmad, I.; Mariano, M.; Dufresne, A.; Thomas, S.; Galeski, A. Recent developments in nanocellulose-based biodegradable polymers, thermoplastic polymers, and porous nanocomposites. Prog. Polym. Sci. 2018, 87, 197–227. [Google Scholar] [CrossRef]
- Bandera, D.; Sapkota, J.; Josset, S.; Weder, C.; Tingaut, P.; Gao, X.; Foster, E.J.; Zimmermann, T. Influence of mechanical treatments on the properties of cellulose nanofibers isolated from microcrystalline cellulose. React. Funct. Polym. 2014, 85, 134–141. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Soeiro, V.S.; Tundisi, L.L.; Novaes, L.C.; Mazzola, P.G.; Aranha, N.; Grotto, D.; Júnior, J.M.; Komatsu, D.; Gama, F.M.; Chaud, M.V.; et al. Production of bacterial cellulose nanocrystals via enzymatic hydrolysis and evaluation of their coating on alginate particles formed by ionotropic gelation. Carbohydr. Polym. Technol. Appl. 2021, 2, 100155. [Google Scholar] [CrossRef]
- Haron, G.A.S.; Mahmood, H.; Noh, M.H.; Alam, Z.; Moniruzzaman, M. Ionic Liquids as a Sustainable Platform for Nanocellulose Processing from Bioresources: Overview and Current Status. ACS Sustain. Chem. Eng. 2021, 9, 1008–1034. [Google Scholar] [CrossRef]
- Xia, Z.; Li, J.; Zhang, J.; Zhang, X.; Zheng, X.; Zhang, J. Processing and valorization of cellulose, lignin and lignocellulose using ionic liquids. J. Bioresour. Bioprod. 2020, 5, 79–95. [Google Scholar] [CrossRef]
- Gonçalves, A.P.; Oliveira, E.; Mattedi, S.; José, N.M. Separation of cellulose nanowhiskers from microcrystalline cellulose with an aqueous protic ionic liquid based on ammonium and hydrogensulphate. Sep. Purif. Technol. 2018, 196, 200–207. [Google Scholar] [CrossRef]
- Pang, Z.; Wang, P.; Dong, C. Ultrasonic pretreatment of cellulose in ionic liquid for efficient preparation of cellulose nanocrystals. Cellulose 2018, 25, 7053–7064. [Google Scholar] [CrossRef]
- Long, S.; Feng, Y.; Liu, Y.; Zheng, L.; Gan, L.; Liu, J.; Zeng, X.; Long, M. Renewable and robust biomass carbon aerogel derived from deep eutectic solvents modified cellulose nanofiber under a low carbonization temperature for oil-water separation. Sep. Purif. Technol. 2021, 254, 117577. [Google Scholar] [CrossRef]
- Oh, Y.; Park, S.; Jung, D.; Oh, K.K.; Lee, S.H. Effect of hydrogen bond donor on the choline chloride-based deep eutectic solvent-mediated extraction of lignin from pine wood. Int. J. Biol. Macromol. 2020, 165, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, L.; Tian, H.; Zhang, L.; Lu, A. Strong, transparent cellulose film as gas barrier constructed via water evaporation induced dense packing. J. Membr. Sci. 2019, 585, 99–108. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Barron, J.C.; Ryder, K.; Wilson, D. Eutectic-Based Ionic Liquids with Metal-Containing Anions and Cations. Chem. — A Eur. J. 2007, 13, 6495–6501. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering—Promises and challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef]
- Kohli, K.; Katuwal, S.; Biswas, A.; Sharma, B.K. Effective delignification of lignocellulosic biomass by microwave assisted deep eutectic solvents. Bioresour. Technol. 2020, 303, 122897. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Liu, K.; Liu, H.; Xie, H.; Si, C.; Pang, B.; Zhang, X. Sustainable preparation of cellulose nanofibrils via choline chloride-citric acid deep eutectic solvent pretreatment combined with high-pressure homogenization. Carbohydr. Polym. 2021, 267, 118220. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, R.; Nair, A.; Jayavel, A.; Sathiasivan, K.; Rajesh, M.; Ramaswamy, S.; Tamilarasan, K. Choline chloride-based deep eutectic solvents for efficient delignification of Bambusa bambos in bio-refinery applications. Chem. Pap. 2020, 74, 4533–4545. [Google Scholar] [CrossRef]
- Zhong, M.; Tang, Q.F.; Zhu, Y.W.; Chen, X.Y.; Zhang, Z.J. An alternative electrolyte of deep eutectic solvent by choline chloride and ethylene glycol for wide temperature range supercapacitors. J. Power Sources 2020, 452, 227847. [Google Scholar] [CrossRef]
- Ramakrishnan, A.; Ravishankar, K.; Dhamodharan, R. Preparation of nanofibrillated cellulose and nanocrystalline cellulose from surgical cotton and cellulose pulp in hot-glycerol medium. Cellulose 2019, 26, 3127–3141. [Google Scholar] [CrossRef]
- Pan, M.; Zhao, G.; Ding, C.; Wu, B.; Lian, Z.; Lian, H. Physicochemical transformation of rice straw after pretreatment with a deep eutectic solvent of choline chloride/urea. Carbohydr. Polym. 2017, 176, 307–314. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J. Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: A review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Hyypiö, K.; Asaadi, S.; Junka, K.; Liimatainen, H. High-strength cellulose nanofibers produced via swelling pretreatment based on a choline chloride–imidazole deep eutectic solvent. Green Chem. 2020, 22, 1763–1775. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Lang, J.; Lan, P.; Yang, H.; Lu, J.; Wang, Z. Study on the Dissolution Mechanism of Cellulose by ChCl-Based Deep Eutectic Solvents. Materials 2020, 13, 278. [Google Scholar] [CrossRef] [Green Version]
- Morais, E.S.; Lopes, A.M.D.C.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of Ionic Liquids and Deep Eutectic Solvents in Polysaccharides Dissolution and Extraction Processes towards Sustainable Biomass Valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, R.; Abbott, A. Solvation of carbohydrates in five choline chloride-based deep eutectic solvents and the implication for cellulose solubility. Green Chem. 2019, 21, 4673–4682. [Google Scholar] [CrossRef]
- Ma, Y.; Xia, Q.; Liu, Y.; Chen, W.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Production of Nanocellulose Using Hydrated Deep Eutectic Solvent Combined with Ultrasonic Treatment. ACS Omega 2019, 4, 8539–8547. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, Y.; Jiang, F. Sustainable isolation of nanocellulose from cellulose and lignocellulosic feedstocks: Recent progress and perspectives. Carbohydr. Polym. 2021, 267, 118188. [Google Scholar] [CrossRef] [PubMed]
- Suopajärvi, T.; Sirviö, J.A.; Liimatainen, H. Nanofibrillation of deep eutectic solvent-treated paper and board cellulose pulps. Carbohydr. Polym. 2017, 169, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yuan, Y.; Li, P.; Zhang, K.; Lian, H.; Liimatainen, H. Enhancement of the nanofibrillation of birch cellulose pretreated with natural deep eutectic solvent. Ind. Crops Prod. 2020, 154, 112677. [Google Scholar] [CrossRef]
- Smirnov, M.A.; Sokolova, M.P.; Tolmachev, D.A.; Vorobiov, V.K.; Kasatkin, I.A.; Smirnov, N.N.; Klaving, A.V.; Bobrova, N.V.; Lukasheva, N.V.; Yakimansky, A.V. Green method for preparation of cellulose nanocrystals using deep eutectic solvent. Cellulose 2020, 27, 4305–4317. [Google Scholar] [CrossRef]
- Yang, X.; Xie, H.; Du, H.; Zhang, X.; Zou, Z.; Zou, Y.; Liu, W.; Lan, H.; Zhang, X.; Si, C. Facile Extraction of Thermally Stable and Dispersible Cellulose Nanocrystals with High Yield via a Green and Recyclable FeCl3-Catalyzed Deep Eutectic Solvent System. ACS Sustain. Chem. Eng. 2019, 7, 7200–7208. [Google Scholar] [CrossRef]
- Lakovaara, M.; Sirviö, J.A.; Ismail, M.Y.; Liimatainen, H.; Sliz, R. Hydrophobic modification of nanocellulose and all-cellulose composite films using deep eutectic solvent as a reaction medium. Cellulose 2021, 28, 5433–5447. [Google Scholar] [CrossRef]
- Yousefi, H.; Faezipour, M.; Hedjazi, S.; Mousavi, M.M.; Azusa, Y.; Heidari, A.H. Comparative study of paper and nanopaper properties prepared from bacterial cellulose nanofibers and fibers/ground cellulose nanofibers of canola straw. Ind. Crops Prod. 2013, 43, 732–737. [Google Scholar] [CrossRef]
- Fang, Z.; Hou, G.; Chen, C.; Hu, L. Nanocellulose-based films and their emerging applications. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100764. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Chen, C.; Kong, W.; Zhu, S.; Dai, J.; Diaz, A.J.; Hitz, E.; Solares, S.D.; Li, T.; et al. Transparent, Anisotropic Biofilm with Aligned Bacterial Cellulose Nanofibers. Adv. Funct. Mater. 2018, 28, 1707491. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Yao, S.; Chang, H.; Wang, Y.; Zhang, Z. UV-blocking, transparent and hazy cellulose nanopaper with superior strength based on varied components of poplar mechanical pulp. Cellulose 2020, 27, 6563–6576. [Google Scholar] [CrossRef]
- Chen, F.; Xiang, W.; Sawada, D.; Bai, L.; Hummel, M.; Sixta, H.; Budtova, T. Exploring Large Ductility in Cellulose Nanopaper Combining High Toughness and Strength. ACS Nano 2020, 14, 11150–11159. [Google Scholar] [CrossRef]
- Yu, W.; Wang, C.; Yi, Y.; Wang, H.; Yang, Y.; Zeng, L.; Tan, Z. Direct pretreatment of raw ramie fibers using an acidic deep eutectic solvent to produce cellulose nanofibrils in high purity. Cellulose 2021, 28, 175–188. [Google Scholar] [CrossRef]
- Brown, A.J. XLIII.—On an acetic ferment which forms cellulose. J. Chem. Soc. Trans. 1886, 49, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Chen, S.; Chen, Y.; Wang, B.; Pei, Q.; Wang, H. Macrofibers with High Mechanical Performance Based on Aligned Bacterial Cellulose Nanofibers. ACS Appl. Mater. Interfaces 2017, 9, 20330–20339. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, X.; Ng, P.F.; I Lee, K.; Fei, B.; Xin, J.H.; Wu, J.-Y. Polyethylenimine coated bacterial cellulose nanofiber membrane and application as adsorbent and catalyst. J. Colloid Interface Sci. 2015, 440, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Göktürk, I.; Tamahkar, E.; Yılmaz, F.; Denizli, A. Protein depletion with bacterial cellulose nanofibers. J. Chromatogr. B 2018, 1099, 1–9. [Google Scholar] [CrossRef]
- Meneguin, A.B.; Barud, H.D.S.; Sábio, R.M.; de Sousa, P.Z.; Manieri, K.F.; de Freitas, L.A.P.; Pacheco, G.; Alonso, J.D.; Chorilli, M. Spray-dried bacterial cellulose nanofibers: A new generation of pharmaceutical excipient intended for intestinal drug delivery. Carbohydr. Polym. 2020, 249, 116838. [Google Scholar] [CrossRef]
- Zhai, X.; Lin, D.; Li, W.; Yang, X. Improved characterization of nanofibers from bacterial cellulose and its potential application in fresh-cut apples. Int. J. Biol. Macromol. 2020, 149, 178–186. [Google Scholar] [CrossRef]
- Huang, L.; Du, X.; Fan, S.; Yang, G.; Shao, H.; Li, D.; Cao, C.; Zhu, Y.; Zhu, M.; Zhang, Y. Bacterial cellulose nanofibers promote stress and fidelity of 3D-printed silk based hydrogel scaffold with hierarchical pores. Carbohydr. Polym. 2019, 221, 146–156. [Google Scholar] [CrossRef]
- Parit, M.; Du, H.; Zhang, X.; Prather, C.; Adams, M.; Jiang, Z. Polypyrrole and cellulose nanofiber based composite films with improved physical and electrical properties for electromagnetic shielding applications. Carbohydr. Polym. 2020, 240, 116304. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M.; Ukkola, J.; Liimatainen, H. Effect of plasticizers on the mechanical and thermomechanical properties of cellulose-based biocomposite films. Ind. Crops Prod. 2018, 122, 513–521. [Google Scholar] [CrossRef]
- Valtasaari, L. The configuration of cellulose dissolved iron-sodiumtartrate. Macromol. Chem. Phys. 1971, 150, 117–126. [Google Scholar] [CrossRef]
- Segal, L.G.J.M.A.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.; Isaksson, P.; Lindström, T.; Nishino, T. Cellulose Nanopaper Structures of High Toughness. Biomacromolecules 2008, 9, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, W.; Li, Y.; Guo, X.; Song, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H.; Zeng, J. Comparative study of the structure, mechanical and thermomechanical properties of cellulose nanopapers with different thickness. Cellulose 2016, 23, 1375–1382. [Google Scholar] [CrossRef]
- Hsieh, M.-C.; Koga, H.; Suganuma, K.; Nogi, M. Hazy Transparent Cellulose Nanopaper. Sci. Rep. 2017, 7, srep41590. [Google Scholar] [CrossRef] [PubMed]
- Łojewska, J.; Miskowiec, P.; Łojewski, T.; Proniewicz, L. Cellulose oxidative and hydrolytic degradation: In situ FTIR approach. Polym. Degrad. Stab. 2005, 88, 512–520. [Google Scholar] [CrossRef]
- Alves, A.P.P.; de Oliveira, L.P.; Castro, A.A.; Neumann, R.; de Oliveira, L.F.; Edwards, H.G.; Sant’Ana, A.C. The structure of different cellulosic fibres characterized by Raman spectroscopy. Vib. Spectrosc. 2016, 86, 324–330. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2013, 21, 885–896. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Mohite, L.V.; Deshmukh, N.A.; Pinjari, D.V. Effect of ultrasound treatment on swelling behavior of cellulose in aqueous N-methyl-morpholine-N-oxide solution. Ultrason. Sonochem. 2018, 49, 161–168. [Google Scholar] [CrossRef]
- Broido, A. A simple, sensitive graphical method of treating thermogravimetric analysis data. J. Polym. Sci. Part A-2 Polym. Phys. 1969, 7, 1761–1773. [Google Scholar] [CrossRef]
- Meng, Q.; Fu, S.; Lucia, L.A. The role of heteropolysaccharides in developing oxidized cellulose nanofibrils. Carbohydr. Polym. 2016, 144, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Yi, J.; Wu, Y.; Wu, Q.; Qing, Y.; Wang, L. Preparation of highly charged cellulose nanofibrils using high-pressure homogenization coupled with strong acid hydrolysis pretreatments. Carbohydr. Polym. 2016, 136, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, C.; Zhang, Y.; Yu, G.; Si, C.; Li, B. Preparation and characterization of functional cellulose nanofibrils via formic acid hydrolysis pretreatment and the followed high-pressure homogenization. Ind. Crops Prod. 2016, 94, 736–745. [Google Scholar] [CrossRef]
- Gofman, I.; Nikolaeva, A.; Khripunov, A.; Yakimansky, A.; Ivan’kova, E.; Romanov, D.; Ivanova, O.; Teplonogova, M.; Ivanov, V. Impact of nano-sized cerium oxide on physico-mechanical characteristics and thermal properties of the bacterial cellulose films. Nanosyst. Phys. Chem. Math. 2018, 9, 754–762. [Google Scholar] [CrossRef]
- Lan, W.; Liu, C.-F.; Yue, F.-X.; Sun, R.-C. Rapid Dissolution of Cellulose in Ionic Liquid with Different Methods. In Cellulose: Fundamental Aspects; IntechOpen: London, UK, 2013. [Google Scholar]
- Wang, J.; Wang, Y.; Ma, Z.; Yan, L. Dissolution of highly molecular weight cellulose isolated from wheat straw in deep eutectic solvent of Choline/l-Lysine hydrochloride. Green Energy Environ. 2020, 5, 232–239. [Google Scholar] [CrossRef]
- Hervy, M.; Bock, F.; Lee, K.-Y. Thinner and better: (Ultra-)low grammage bacterial cellulose nanopaper-reinforced polylactide composite laminates. Compos. Sci. Technol. 2018, 167, 126–133. [Google Scholar] [CrossRef]




| Sample | Characteristic Viscosity, [η] (dL/g) | DP | Density of Paper (g/cm3) |
|---|---|---|---|
| BC | 9.1 ± 0.7 | 1800 ± 200 | – |
| E− | 4.5 ± 0.2 | 740 ± 50 | 1.18 ± 0.02 |
| E+ | 3.9 ± 0.1 | 610 ± 30 | 1.20 ± 0.03 |
| G− | 7.6 ± 0.3 | 1410 ± 70 | 1.22 ± 0.01 |
| G+ | 5.6 ± 0.5 | 970 ± 120 | 1.24 ± 0.03 |
| Sample | Tonset, °C | τ5, °C | Mass Loss at Low Temperature Step, % |
|---|---|---|---|
| E− | 309 ± 3 | 273 ± 16 | 5.3 ± 0.4 |
| E+ | 318 ± 3 | 300 ± 18 | 3.0 ± 0.2 |
| G− | 302 ± 3 | 270 ± 16 | 5.1 ± 0.4 |
| G+ | 312 ± 3 | 285 ± 17 | 3.3 ± 0.3 |
| BC | 319 ± 3 | 275 ± 17 | 4.0 ± 0.3 |
| TOCN TEMPO-mediated oxidation [82] | - | 200 | - |
| CNFs-H sulfuric acid hydrolysis [83] | 274 | - | - |
| F-CNF formic acid hydrolysis with high-pressure homogenization [84] | 291.24 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batishcheva, E.V.; Sokolova, D.N.; Fedotova, V.S.; Sokolova, M.P.; Nikolaeva, A.L.; Vakulyuk, A.Y.; Shakhbazova, C.Y.; Ribeiro, M.C.C.; Karttunen, M.; Smirnov, M.A. Strengthening Cellulose Nanopaper via Deep Eutectic Solvent and Ultrasound-Induced Surface Disordering of Nanofibers. Polymers 2022, 14, 78. https://doi.org/10.3390/polym14010078
Batishcheva EV, Sokolova DN, Fedotova VS, Sokolova MP, Nikolaeva AL, Vakulyuk AY, Shakhbazova CY, Ribeiro MCC, Karttunen M, Smirnov MA. Strengthening Cellulose Nanopaper via Deep Eutectic Solvent and Ultrasound-Induced Surface Disordering of Nanofibers. Polymers. 2022; 14(1):78. https://doi.org/10.3390/polym14010078
Chicago/Turabian StyleBatishcheva, Elizaveta V., Darya N. Sokolova, Veronika S. Fedotova, Maria P. Sokolova, Alexandra L. Nikolaeva, Alexey Y. Vakulyuk, Christina Y. Shakhbazova, Mauro Carlos Costa Ribeiro, Mikko Karttunen, and Michael A. Smirnov. 2022. "Strengthening Cellulose Nanopaper via Deep Eutectic Solvent and Ultrasound-Induced Surface Disordering of Nanofibers" Polymers 14, no. 1: 78. https://doi.org/10.3390/polym14010078
APA StyleBatishcheva, E. V., Sokolova, D. N., Fedotova, V. S., Sokolova, M. P., Nikolaeva, A. L., Vakulyuk, A. Y., Shakhbazova, C. Y., Ribeiro, M. C. C., Karttunen, M., & Smirnov, M. A. (2022). Strengthening Cellulose Nanopaper via Deep Eutectic Solvent and Ultrasound-Induced Surface Disordering of Nanofibers. Polymers, 14(1), 78. https://doi.org/10.3390/polym14010078








