A Review of Biopolymers’ Utility as Emulsion Stabilizers
Abstract
:1. Introduction
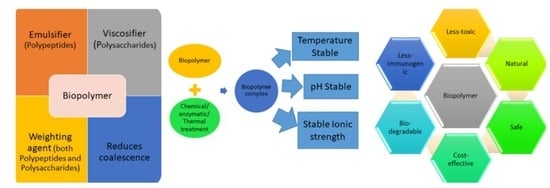
Application of Biopolymers
2. Emulsion and Emulsion Stabilizer
2.1. Emulsion and Emulsification
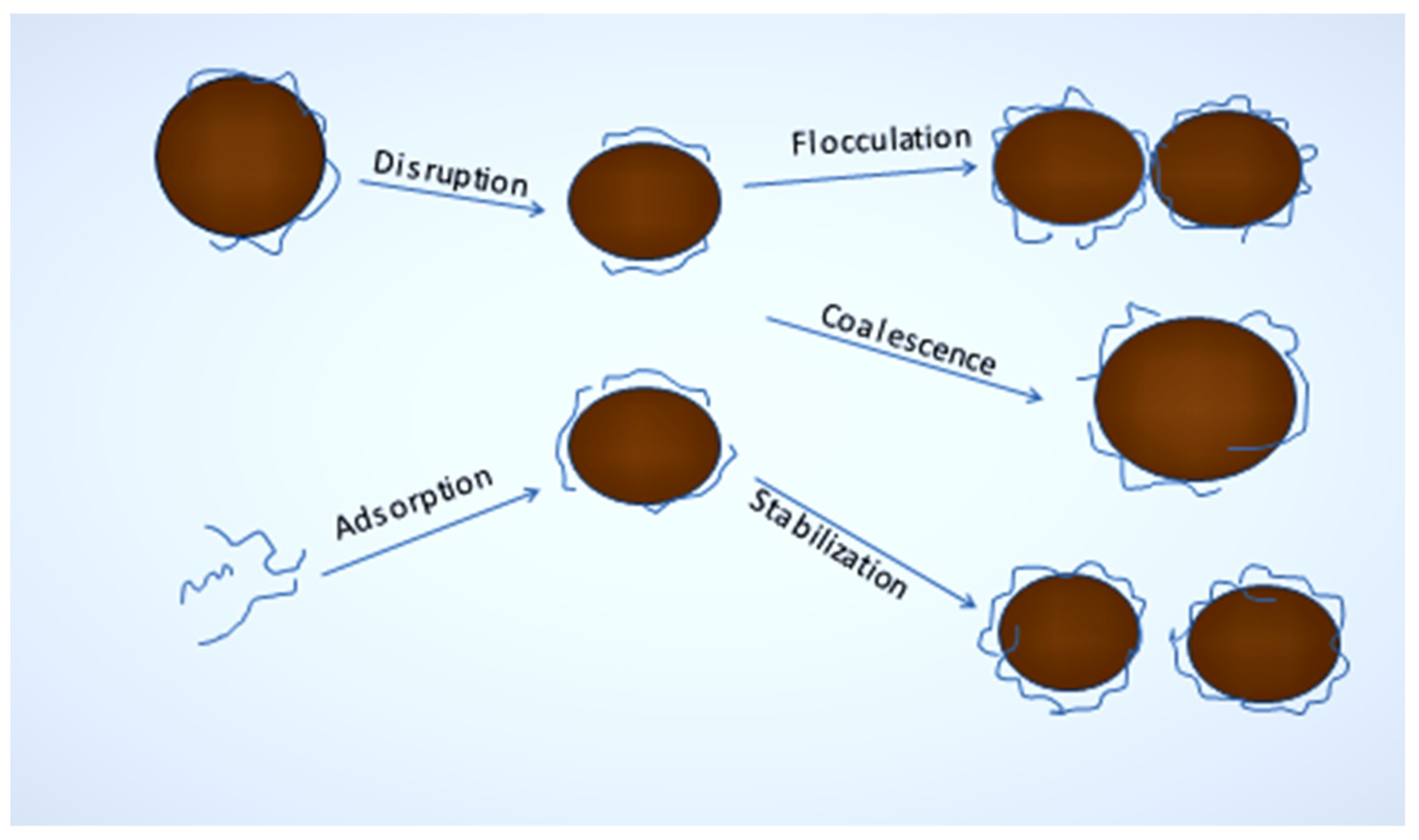
2.2. Stabilization and Destabilization of Nanoemulsion
2.3. Synthesis and Application of Nanoemulsion
3. Polysaccharides Chemical Structure and Their Properties
3.1. Synthesis of Polysaccharides Emulsion
3.2. Glycosyl with Polysaccharides
4. Food Protein and Food Protein Emulsions
4.1. Animal Protein and Plant Protein
4.2. Effectiveness of Plant Protein
4.3. Pea Protein Entrapment Efficiency
4.4. Other Plant Protein and Entrapment Efficiency
4.5. Modification of Protein into Functional Components
5. The Stability Factors of Proteins Nanoemulsions
5.1. Encapsulation and Encapsulation Efficiency
5.2. Emulsifying Properties of Proteins
- (a)
- Surface hydrophobicity: The percentage of hydrophobic proteins exposed on the surface of proteins measures how much of the protein can adsorb to the oil phase. The presence of hydrophobic sites buried inside proteins can be revealed by partial denaturation, which can increase their emulsifying ability [85].
- (b)
- The flexibility of proteins: It is a self-rearrangement property of proteins, when it is adsorbed at the oil–water (O/W) interface, most of the hydrophilic mass favors the watery parts and the hydrophobic mass favors the oily parts by reducing the attractive force between two liquids [86]. According to the composition of protein, hydrophilic loops of amino acids may enlarge away from the O/W interface in the form of waterish parts, slowing the reaction [86].
- (c)
- (d)
- When encapsulating, a high solubility of proteins is preferable in order to allow higher movement in the O/W interface and higher continuous phase viscosities [85].
- (e)
- The factor influences in the protein solubility are the pH of the solvent, the ionic character, and the attractive or repulsive forces between closer globules showing emulsion instability or stability. When the solvent pH is not near the isoelectric point of proteins or when ionic conditions are low, charge repulsion can enhance emulsion stability [88].
5.3. Protein from Rice Bran as an Emulsifier
6. Phospholipids Nanoemulsion Stabilizer
6.1. Phospholipids
6.2. Type of Phospholipids and Application
6.3. Natural Phospholipids
7. Advantages and Limitations of Biopolymers over Synthetic Polymers
7.1. Challenges of Synthetic Polymers
7.2. Biopolymers
7.3. Comparison in Biodegradability
7.4. Comparative Life Cycle Assessment
8. Present Research Obstacles and Future Prospects
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kaplan, D.L. Introduction to biopolymers from renewable resources. In Biopolymers from Renewable Resources; Springer: Berlin/Heidelberg, Germany, 1998; pp. 1–29. [Google Scholar]
- Li, P.; Li, X.; Nisar, T.; Yang, X.; Sun, J.; Yang, X.; Guo, Y. Structural characteristics of binary biopolymers-based emulsion-filled gels: A case of mixed sodium caseinate/methyl cellulose emulsion gels. Food Struct. 2021, 30, 100233. [Google Scholar] [CrossRef]
- Osipow, L.; Snell, F.D.; York, W.C.; Finchler, A. Methods of Preparation Fatty Acid Esters of Sucrose. Ind. Eng.Chem. 1956, 48, 1459–1462. [Google Scholar] [CrossRef]
- Moreira, J.B.; de Morais, M.G.; de Morais, E.G.; da Silva Vaz, B.; Costa, J.A.V. Electrospun polymeric nanofibers in food packaging. In Impact of Nanoscience in the Food Industry; Academic Press: Cambridge, MA, USA, 2018; pp. 387–417. [Google Scholar]
- El Asjadi, S.; Nederpel, Q.A.; Cotiuga, I.M.; Picken, S.J.; Besseling, N.A.M.; Mendes, E.; Lommerts, B.J. Biopolymer scleroglucan as an emulsion stabilizer. Colloids Surf. A Physicochem. Eng. Aspects 2018, 546, 326–333. [Google Scholar] [CrossRef]
- Song, W.L.; Wang, P.; Cao, L.; Anderson, A.; Meziani, M.J.; Farr, A.J.; Sun, Y.P. Polymer/Boron Nitride Nanocomposite Materials for Superior Thermal Transport Performance. Angew. Chem. 2012, 51, 6498–6501. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G.; Parisi, O.I.; Iemma, F.; Cirillo, G.; Puoci, F.; Curcio, M.; Picci, N. Antioxidant-polysaccharide conjugates for food application by eco-friendly grafting procedure. Carbohydr. Polym. 2010, 79, 333–340. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, R.; Yao, P. Influence of Pea Protein Aggregates on the Structure and Stability of Pea Protein/Soybean Polysaccharide Complex Emulsions. Molecules 2015, 20, 5165–5183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maphosa, Y.; Jideani, V.A. Factors Affecting the Stability of Emulsions Stabilised by Biopolymers. Science and Technology Behind Nanoemulsions; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Ghashoghchi, R.A.; Hosseini, M.R.; Ahmadi, A. Effects of microbial cells and their associated extracellular polymeric substances on the bio-flocculation of kaolin and quartz. Appl. Clay Sci. 2017, 138, 81–88. [Google Scholar] [CrossRef]
- Nair, N.R.; Sekhar, V.C.; Nampoothiri, K.M.; Pandey, A. Biodegradation of biopolymers. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 739–755. [Google Scholar]
- Lambert, S. Biopolymers and Their Application as Biodegradable Plastics. Microbial Factories; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–9. [Google Scholar]
- Gao, Z.; Fang, Y.; Cao, Y.; Liao, H.; Nishinari, K.; Phillips, G.O. Hydrocolloid-food component interactions. Food Hydrocoll. 2017, 68, 149–156. [Google Scholar] [CrossRef]
- Kerkhofs, S.; Lipkens, H.; Velghe, F.; Verlooy, P.; Martens, J.A. Mayonnaise production in batch and continuous process exploiting magnetohydrodynamic force. J. Food Eng. 2011, 106, 35–39. [Google Scholar] [CrossRef]
- Pal, A.; Mondal, M.H.; Adhikari, A.; Bhattarai, A.; Saha, B. Scientific information about sugar-based emulsifiers: A comprehensive review. RSC Adv. 2021, 11, 33004–33016. [Google Scholar] [CrossRef]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion components screening and selection: A technical note. Aaps Pharmscitech. 2009, 10, 69–76. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll. 2015, 43, 547–556. [Google Scholar] [CrossRef]
- Zhang, Y.; Shang, Z.; Gao, C.; Du, M.; Xu, S.; Song, H.; Liu, T. Nanoemulsion for Solubilization, Stabilization, and In Vitro Release of Pterostilbene for Oral Delivery. Aaps Pharmscitech. 2014, 15, 1000–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.P.; He, S.N.; Li, Y.L.; Feng, D.L.; Lu, X.Y.; Du, Y.Z.; Yu, H.Y.; Hu, F.Q.; Yuan, H. Preparation and characteristics of lipid nanoemulsion formulations loaded with doxorubicin. Int. J. Nanomed. 2013, 8, 3141–3150. [Google Scholar] [CrossRef] [Green Version]
- Devalapally, H.; Zhou, F.; McDade, J.; Goloverda, G.; Owen, A.; Hidalgo, I.J.; Silchenko, S. Optimization of PEGylatednanoemulsions for improved pharmacokinetics of BCS class II compounds. Drug Deliv. 2013, 22, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Huang, Q. Improving the Oral Bioavailability of Curcumin Using Novel Organogel-Based Nanoemulsions. J. Agric. Food Chem. 2012, 60, 5373–5379. [Google Scholar] [CrossRef]
- Sun, W.; Ma, X.; Wei, X.; Xu, Y. Nano Composite Emulsion for Sustained Drug Release and Improved Bioavailability. Pharm. Res. 2014, 31, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Wang, Q.; Lu, R. Nanoemulsions as novel oral carriers of stiripentol: Insights into the protective effect and absorption enhancement. Int. J. Nanomed. 2015, 10, 4937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muheem, A.; Shakeel, F.; Jahangir, M.A.; Anwar, M.; Mallick, N.; Jain, G.K.; Warsi, M.H.; Ahmad, F.J. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm. J. 2016, 24, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef] [Green Version]
- Darwis, Y.; Ali Khan, A.; Mudassir, J.; Mohtar, N. Advanced drug delivery to the lymphatic system: Lipid-based nanoformulations. Int. J. Nanomed. 2013, 8, 2733. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.J.; McClements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Stanslas, J.; Basri, M.; Karjiban, R.A.; Kirby, B.; Sani, D.; Basri, H. Nanoemulsion-based Parenteral Drug Delivery System of Carbamazepine: Preparation, Characterization, Stability Evaluation and Blood-Brain Pharmacokinetics. Curr. Drug Deliv. 2015, 12, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Al-Edresi, S.; Baie, S. Formulation and stability of whitening VCO-in-water nano-cream. Int. J. Pharm. 2009, 373, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Makidon, P.E.; Nigavekar, S.S.; Bielinska, A.U.; Mank, N.; Shetty, A.M.; Suman, J.; Knowlton, J.; Myc, A.; Rook, T.; Baker, J.R. Characterization of Stability and Nasal Delivery Systems for Immunization with Nanoemulsion-Based Vaccines. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Lala, R.R.; Awari, N.G. Nanoemulsion-based gel formulations of COX-2 inhibitors for enhanced efficacy in inflammatory conditions. Appl. Nanosci. 2013, 4, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Samad, A.; Singh, S.K.; Ahsan, M.N.; Haque, M.W.; Faruk, A.; Ahmed, F.J. Nanoemulsion gel-based topical delivery of an antifungal drug: In vitro activity and in vivo evaluation. Drug Deliv. 2014, 23, 642–657. [Google Scholar] [CrossRef]
- Nasr, M.; Nawaz, S.; Elhissi, A. Amphotericin B lipid nanoemulsion aerosols for targeting peripheral respiratory airways via nebulization. Int. J. Pharm. 2012, 436, 611–616. [Google Scholar] [CrossRef]
- Amani, A.; York, P.; Chrystyn, H.; Clark, B.J. Evaluation of a Nanoemulsion-Based Formulation for Respiratory Delivery of Budesonide by Nebulizers. Aaps Pharmscitech 2010, 11, 1147–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mou, D.; Chen, H.; Du, D.; Mao, C.; Wan, J.; Xu, H.; Yang, X. Hydrogel-thickened nanoemulsion system for topical delivery of lipophilic drugs. Int. J. Pharm. 2008, 353, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Khani, S.; Keyhanfar, F.; Amani, A. Design and evaluation of oral nanoemulsion drug delivery system of mebudipine. Drug Deliv. 2015, 23, 2035–2043. [Google Scholar] [CrossRef] [Green Version]
- Pawar, V.K.; Panchal, S.B.; Singh, Y.; Meher, J.G.; Sharma, K.; Singh, P.; Bora, H.K.; Singh, A.; Datta, D.; Chourasia, M.K. Immunotherapeutic vitamin E nanoemulsion synergies the antiproliferative activity of paclitaxel in breast cancer cells via modulating Th1 and Th2 immune response. J. Control. Release 2014, 196, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.O.; Salama, H.A.; Ghorab, M.; Mahmoud, A.A. Nanoemulsion as a Potential Ophthalmic Delivery System for Dorzolamide Hydrochloride. Aaps Pharmscitech 2009, 10, 808–819. [Google Scholar] [CrossRef] [Green Version]
- Yukuyama, M.N.; Ghisleni, D.D.M.; Pinto, T.J.A.; Bou-Chacra, N.A. Nanoemulsion: Process selection and application in cosmetics—A review. Int. J. Cosmet. Sci. 2015, 38, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, X.; Zhang, G.; Dong, J.; Eastoe, J. Oil-in-water nanoemulsions for pesticide formulations. J. Colloid Interface Sci. 2007, 314, 230–235. [Google Scholar] [CrossRef]
- Nie, S.P.; Wang, C.; Cui, S.W.; Wang, Q.; Xie, M.Y.; Phillips, G.O. A further amendment to the classical core structure of gum arabic (Acacia senegal). FoodHydrocoll. 2013, 31, 42–48. [Google Scholar] [CrossRef]
- Enayati, M.; Gong, Y.; Goddard, J.M.; Abbaspourrad, A. Synthesis and characterization of lactose fatty acid ester biosurfactants using free and immobilized lipases in organic solvents. Food Chem. 2018, 266, 508–513. [Google Scholar] [CrossRef]
- Iglauer, S.; Wu, Y.; Shuler, P.; Tang, Y.; Goddard, W.A. Analysis of the Influence of Alkyl Polyglycoside Surfactant and Cosolvent Structure on Interfacial Tension in Aqueous Formulations versus n-Octane. Tenside Surf. Det. 2010, 47, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Goyal, A. Applications of Natural Polymer Gum Arabic: A Review. Int. J. Food Prop. 2015, 18, 986–998. [Google Scholar] [CrossRef]
- Tadros, T.F. Emulsion Formation and Stability; Wiley: Hoboken, NJ, USA, 2013. ISBN 978352764 7941. [CrossRef]
- Plat, T.; Linhardt, R.J. Syntheses and applications of sucrose-based esters. J. Surfactants Deterg. 2001, 4, 415–421. [Google Scholar] [CrossRef]
- Jun-xia, X.; Hai-yan, Y.; Jian, Y. Microencapsulation of sweet orange oil by complex coacervation with soybean protein isolate/gum Arabic. Food Chem. 2011, 125, 1267–1272. [Google Scholar] [CrossRef]
- Tang, C.H.; Li, X.R. Microencapsulation properties of soy protein isolate and storage stability of the correspondingly spray-dried emulsions. Food Res. Int. 2013, 52, 419–428. [Google Scholar] [CrossRef]
- Ducel, V.; Richard, J.; Saulnier, P.; Popineau, Y.; Boury, F. Evidence and characterization of complex coacervates containing plant proteins: Application to the microencapsulation of oil droplets. Colloids Surf. A Physicochem. Eng. Asp. 2004, 232, 239–247. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Saurel, R.; Chambin, O.; Cases, E.; Voilley, A.; Cayot, P. Utilisation of pectin coating to enhance spray-dry stability of pea protein-stabilised oil-in-water emulsions. Food Chem. 2010, 122, 447–454. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.H.; Nickerson, M.T. Potential use of plant proteins in the microencapsulation of lipophilic materials in foods. Trends Food Sci. Technol. 2015, 42, 5–12. [Google Scholar] [CrossRef]
- Xue, F.; Li, C.; Liu, Y.; Zhu, X.; Pan, S.; Wang, L. Encapsulation of tomato oleoresin with zein prepared from corn gluten meal. J. Food Eng. 2013, 119, 439–445. [Google Scholar] [CrossRef]
- Wang, R.; Tian, Z.; Chen, L. A novel process for microencapsulation of fish oil with barley protein. Food Res. Int. 2011, 44, 2735–2741. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, J.; Xiong, Y.L. Structural and Emulsifying Properties of Soy Protein Isolate Subjected to Acid and Alkaline pH-Shifting Processes. J. Agric. Food Chem. 2009, 57, 7576–7583. [Google Scholar] [CrossRef]
- Augustin, M.A.; Sanguansri, L.; Bode, O. Maillard reaction products as encapsulants for fish oil powders. J. Food Sci. 2006, 71, E25–E32. [Google Scholar] [CrossRef]
- Paraman, I.; Hettiarachchy, N.S.; Schaefer, C. Glycosylation and deamidation of rice endosperm protein for improved solubility and emulsifying properties. Cereal Chem. 2007, 84, 593–599. [Google Scholar] [CrossRef]
- Wong, B.T.; Day, L.; Augustin, M.A. Deamidated wheat protein-dextran Maillard conjugates: Effect of size and location of polysaccharide conjugated on steric stabilization of emulsions at acidic pH. Food Hydrocoll. 2011, 25, 1424–1432. [Google Scholar] [CrossRef]
- Tang, C.H.; Sun, X.; Foegeding, E.A. Modulation of physicochemical and conformational properties of kidney bean vicilin (phaseolin) by glycation with glucose: Implications for structure–function relationships of legume vicilins. J. Agric. Food Chem. 2011, 59, 10114–10123. [Google Scholar] [CrossRef]
- Jourdain, L.; Leser, M.E.; Schmitt, C.; Michel, M.; Dickinson, E. Stability of emulsions containing sodium caseinate and dextran sulfate: Relationship to complexation in solution. Food Hydrocoll. 2008, 22, 647–659. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Li, J.; Bai, Y.; Wang, W.; Tai, X.; Wang, G. Green Glucamine-Based Trisiloxane Surfactant: Surface Activity, Aggregate Behavior, and Superspreading on Hydrophobic Surfaces. ACS Sustain. Chem. Eng. 2019, 7, 4390–4398. [Google Scholar] [CrossRef]
- Teng, F.; He, M.; Xu, J.; Chen, F.; Wu, C.; Wang, Z.; Li, Y. Effect of ultrasonication on the stability and storage of a soy protein isolate-phosphatidylcholine nanoemulsions. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Xu, H.; Chang, C.; Yi, N.; Tao, P.; Song, C.; Wu, J.; Deng, T.; Shang, W. Coalescence, Spreading, and Rebound of Two Water Droplets with Different Temperatures on a Superhydrophobic Surface. ACS Omega 2019, 4, 17615–17622. [Google Scholar] [CrossRef]
- Carpenter, J.; Saharan, V.K. Ultrasonic assisted formation and stability of mustard oil in water nanoemulsion: Effect of process parameters and their optimization. Ultrason. Sonochemistry 2017, 35, 422–430. [Google Scholar] [CrossRef]
- Haahr, A.M.; Jacobsen, C. Emulsifier type, metal chelation and pH affect oxidative stability of n-3-enriched emulsions. Eur. J. Lipid Sci. Technol. 2008, 110, 949–961. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Wang, P.; Li, X.; Hu, X.; Hou, J.; Wang, L.; Zhang, F. Near-Infrared-Triggered Azobenzene-Liposome/Upconversion Nanoparticle Hybrid Vesicles for Remotely Controlled Drug Delivery to Overcome Cancer Multidrug Resistance. Adv. Mater. 2016, 28, 9341–9348. [Google Scholar] [CrossRef]
- Li, Y.; Maciel, D.; Rodrigues, J.; Shi, X.; Tomás, H. Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem. Rev. 2015, 115, 8564–8608. [Google Scholar] [CrossRef] [PubMed]
- Teranishi, R.; Matsuki, R.; Yuba, E.; Harada, A.; Kono, K. Doxorubicin Delivery Using pH and Redox Dual-Responsive Hollow Nanocapsules with a Cationic Electrostatic Barrier. Pharmaceutics 2016, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yang, Y.; Ma, M.; Xu, Y.; Sui, J.; Li, H.; Liang, J.; Sun, Y.; Fan, Y.; Zhang, X. Reductive responsive micelle overcoming multidrug resistance of breast cancer by co-delivery of DOX and specific antibiotic. J. Mater. Chem. B 2019, 7, 6075–6086. [Google Scholar] [CrossRef] [PubMed]
- Deepagan, V.; Kwon, S.; You, D.G.; Nguyen, V.Q.; Um, W.; Ko, H.; Lee, H.; Jo, D.G.; Kang, Y.M.; Park, J.H. In situ diselenide-crosslinked polymeric micelles for ROS-mediated anticancer drug delivery. Biomaterials 2016, 103, 56–66. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, C.; Pang, X.; Yu, Z.; Qi, Y.; Chen, X.; Liang, H.; Fang, X.; Sha, X. Adding Vitamin E-TPGS to the Formulation of Genexol-PM: Specially Mixed Micelles Improve Drug-Loading Ability and Cytotoxicity against Multidrug-Resistant Tumors Significantly. PLoS ONE 2015, 10, e0120129. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2012, 64, 37–48. [Google Scholar] [CrossRef]
- Kutty, R.V.; Feng, S.S. Cetuximab conjugated vitamin E TPGS micelles for targeted delivery of docetaxel for treatment of triple negative breast cancers. Biomaterials 2013, 34, 10160–10171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Chu, M.; Tan, S.; Zhao, S.; Liu, H.; Otieno, B.O.; Yang, X.; Xu, C.; Zhang, Z. Chitosan-g-TPGS Nanoparticles for Anticancer Drug Delivery and Overcoming Multidrug Resistance. Mol. Pharm. 2013, 11, 59–70. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Huang, L.; Qiao, J.; Wang, N.; Yu, D.; Zhang, G.; Yu, S.; Guan, Q. Synergistic effects of antitumor efficacy via mixed nano-size micelles of multifunctional Bletilla striata polysaccharide-based copolymer and D-α-tocopheryl polyethylene glycol succinate. Int. J. Biol. Macromol. 2020, 154, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G.H.; Jin Park, H. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Augustin, M.A.; Hemar, Y. Nano- and micro-structured assemblies for encapsulation of food ingredients. Chem. Soc. Rev. 2009, 38, 902–912. [Google Scholar] [CrossRef]
- McClements, D.; Decker, E.; Weiss, J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef]
- Subirade, M.; Chen, L. Food-Protein-Derived Materials and Their Use as Carriers and Delivery Systems for Active Food Components. Delivery and Controlled Release of Bioactives in Foods and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2008; pp. 251–278. [Google Scholar]
- Sikorski, Z.E. Functional Properties of Proteins in Food Systems. Chemical and Functional Properties of Food Proteins; Routledge: Oxfordshire, UK, 2001; pp. 113–135. [Google Scholar]
- Damodaran, S. Protein-Stabilized Foams and Emulsions. Food Proteins and Their Applications; CRC Press: Boca Raton, FL, USA, 2017; pp. 57–110. [Google Scholar]
- Luyten, H.; Vereijken, J.; Buecking, M. Using proteins as additives in foods: An introduction. In Proteins in Food Processing; Elsevier: Amsterdam, The Netherlands, 2004; pp. 421–441. [Google Scholar]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Zhang, S.; Li, J.; Zheng, H.; Jin, H.; Xu, J. Comparison of different protein emulsifiers on physicochemical properties of β-carotene-loaded nanoemulsion: Effect on formation, stability, and in vitro digestion. Nanomaterials 2021, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ju, X.; Aluko, R.E.; Zou, Y.; Wang, Z.; Liu, M.; He, R. Rice bran protein-based nanoemulsion carrier for improving stability and bioavailability of quercetin. Food Hydrocoll. 2020, 108, 106042. [Google Scholar] [CrossRef]
- Allegretti, C.; Denuccio, F.; Rossato, L.; D’Arrigo, P. Polar Head Modified Phospholipids by Phospholipase D-Catalyzed Transformations of Natural Phosphatidylcholine for Targeted Applications: An Overview. Catalysts 2020, 10, 997. [Google Scholar] [CrossRef]
- van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [Green Version]
- van Nieuwenhuyzen, W.; Tomás, M.C. Update on vegetable lecithin and phospholipid technologies. Eur. J. Lipid Sci. Technol. 2008, 110, 472–486. [Google Scholar] [CrossRef]
- Vertzoni, M.; Markopoulos, C.; Symillides, M.; Goumas, C.; Imanidis, G.; Reppas, C. Luminal Lipid Phases after Administration of a Triglyceride Solution of Danazol in the Fed State and Their Contribution to the Flux of Danazol Across Caco-2 Cell Monolayers. Mol. Pharm. 2012, 9, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Hanin, I.; Pepeu, G. Phospholipids: Biochemical, Pharmaceutical, and Analytical Considerations; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Merolli, A.; Santin, M. Role of Phosphatidyl-Serine in Bone Repair and Its Technological Exploitation. Molecules 2009, 14, 5367–5381. [Google Scholar] [CrossRef]
- Devitt, A.; Pierce, S.; Oldreive, C.; Shingler, W.H.; Gregory, C.D. CD14-dependent clearance of apoptotic cells by human macrophages: The role of phosphatidylserine. Cell Death Differ. 2003, 10, 371–382. [Google Scholar] [CrossRef]
- Lentz, B.R. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog. Lipid Res. 2003, 42, 423–438. [Google Scholar] [CrossRef]
- Komath, S.; Garg, A.; Wahajuddin, M. Development and evaluation of Chrysin-Phospholipid complex loaded solid lipid nanoparticles-storage stability and in vitro anti-cancer activity. J. Microencapsul. 2018, 35, 600–617. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, P.X.; Rogers, M.A.; Wettig, S.D. Investigating the phospholipid effect on the bioaccessibility of rosmarinic acid-phospholipid complex through a dynamic gastrointestinal in vitro model. Pharmaceutics 2019, 11, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nafee, N.; Gaber, D.M.; Elzoghby, A.O.; Helmy, M.W.; Abdallah, O.Y. Promoted antitumor activity of myricetin against lung carcinoma via nanoencapsulated phospholipid complex in respirable microparticles. Pharm. Res. 2020, 37, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Hottle, T.A.; Bilec, M.M.; Landis, A.E. Sustainability assessments of bio-based polymers. Polym. Degrad. Stab. 2013, 98, 1898–1907. [Google Scholar] [CrossRef]
- Gironi, F.; Piemonte, V. Bioplastics and Petroleum-based Plastics: Strengths and Weaknesses. Energy Sources Part. A Recovery Util. Environ. Eff. 2011, 33, 1949–1959. [Google Scholar] [CrossRef]
- Plastics: Material-Specific Data. US EPA. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data (accessed on 30 September 2021).
- Shirai, M.; Grossmann, M.; Mali, S.; Yamashita, F.; Garcia, P.; Müller, C. Development of biodegradable flexible films of starch and poly (lactic acid) plasticized with adipate or citrate esters. Carbohydr. Polym. 2013, 92, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages and applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef] [PubMed]
- Flieger, M.; Kantorová, M.; Prell, A.; Řezanka, T.; Votruba, J. Biodegradable plastics from renewable sources. Folia Microbiol. 2003, 48, 27–44. [Google Scholar] [CrossRef]
- Landis, A.E.; Miller, S.A.; Theis, T.L. Life Cycle of the Corn−Soybean Agroecosystem for Biobased Production. Environ. Sci. Technol. 2007, 41, 1457–1464. [Google Scholar] [CrossRef]
- Luo, Y.; Hu, Q. Food-derived biopolymers for nutrient delivery. In Nutrient Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 251–291. [Google Scholar] [CrossRef]
- Gowthaman, N.; Lim, H.; Sreeraj, T.; Amalraj, A.; Gopi, S. Advantages of Biopolymers over Synthetic Polymers. Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 351–372. [Google Scholar] [CrossRef]
- Miller, S.A.; Landis, A.E.; Theis, T.L. Feature: Environmental Trade-Offs of Biobased Production; ACS Publications: Washington, DC, USA, 2007. [Google Scholar]
- Cao, V.; Margni, M.; Favis, B.D.; Deschênes, L. Aggregated indicator to assess land use impacts in life cycle assessment (LCA) based on the economic value of ecosystem services. J. Clean. Prod. 2015, 94, 56–66. [Google Scholar] [CrossRef]
- Pathak, S.; Sneha, C.L.R.; Mathew, B.B. Bioplastics: Its timeline based scenario & challenges. J. Polym. Biopolym. Phys. Chem. 2014, 2, 84–90. [Google Scholar]
- Ibrahim, M.S.; Sani, N.; Adamu, M.; Abubakar, M.K. Biodegradable polymers for sustainable environmental and economic development. MOJ Bioorganic Org. Chem. 2018, 2, 192–194. [Google Scholar] [CrossRef] [Green Version]
- Yates, M.R.; Barlow, C.Y. Life cycle assessments of biodegradable, commercial biopolymers—A critical review. Resour. Conserv. Recycl. 2013, 78, 54–66. [Google Scholar] [CrossRef]
- Burzic, I.; Pretschuh, C.; Kaineder, D.; Eder, G.; Smilek, J.; Másilko, J.; Kateryna, W. Impact modification of PLA using biobased biodegradable PHA biopolymers. Eur. Polym. J. 2019, 114, 32–38. [Google Scholar] [CrossRef]
- Peelman, N.; Ragaert, P.; Ragaert, K.; de Meulenaer, B.; Devlieghere, F.; Cardon, L. Heat resistance of new biobased polymeric materials, focusing on starch, cellulose, PLA, and PHA. J. Appl. Polym. Sci. 2015, 132, 42305. [Google Scholar] [CrossRef]
- Khan, B.; Bilal Khan Niazi, M.; Samin, G.; Jahan, Z. Thermoplastic Starch: A Possible Biodegradable Food Packaging Material—A Review. J. Food Process. Eng. 2016, 40, e12447. [Google Scholar] [CrossRef]
- Fazeli, M.; Florez, J.P.; Simão, R.A. Improvement in adhesion of cellulose fibers to the thermoplastic starch matrix by plasma treatment modification. Compos. Part. B Eng. 2019, 163, 207–216. [Google Scholar] [CrossRef]
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2016, 181, 1314–1337. [Google Scholar] [CrossRef] [PubMed]
- Aminabhavi, T.M.; Balundgi, R.H.; Cassidy, P.E. A Review on Biodegradable Plastics. Polym. Plast. Technol. Eng. 1990, 29, 235–262. [Google Scholar] [CrossRef]
- Chau, H.; Yu, P. Production of biodegradable plastics from chemical wastewater—A novel method to reduce excess activated sludge generated from industrial wastewater treatment. Water Sci. Technol. 1999, 39, 273–280. [Google Scholar] [CrossRef]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J. Enhanced biological stability of collagen porous scaffolds by using amino acids as novel cross-linking bridges. Biomaterials 2004, 25, 2997–3004. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.; Strand, B.L.; Formo, K.; Alsberg, E.; Christensen, B.E. Alginates as Biomaterials in Tissue Engineering. Carbohydrate Chemistry; The Royal Society of Chemistry Press: London, UK, 2011. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Campoccia, D.; Doherty, P.; Williams, R.; Benedetti, L.; Williams, D. Biodegradation of hyaluronic acid derivatives by hyaluronidase. Biomaterials 1994, 15, 359–365. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Gunatillake, P. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Chen, G.Q.; Jiang, X.R. Engineering bacteria for enhanced polyhydroxyalkanoates (PHA) biosynthesis. Synth. Syst. Biotechnol. 2017, 2, 192–197. [Google Scholar] [CrossRef]
- Kuppens, T.; Cornelissen, T.; Carleer, R.; Yperman, J.; Schreurs, S.; Jans, M.; Thewys, T. Economic assessment of flash co-pyrolysis of short rotation coppice and biopolymer waste streams. J. Environ. Manag. 2010, 91, 2736–2747. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Woodley, J.M. Bioprocesses: Modeling needs for process evaluation and sustainability assessment. Comput. Chem. Eng. 2010, 34, 1009–1017. [Google Scholar] [CrossRef]
- Barker, M.; Safford, R. Industrial Uses for Crops: Markets for Bioplastics; (No. PR450); HGCA: London, UK, 2001; Available online: https://ahdb.org.uk/industrial-uses-for-crops-markets-for-bioplastics (accessed on 30 September 2021).
- Dreyer, L.C.; Hauschild, M.Z.; Schierbeck, J. Characterisation of social impacts in LCA. Part 2: Implementation in six company case studies. Int. J. Life Cycle Assess. 2010, 15, 385–402. [Google Scholar] [CrossRef]
- Weidema, B.P. The Integration of Economic and Social Aspects in Life Cycle Impact Assessment. Int. J. Life Cycle Assess. 2005, 11, 89–96. [Google Scholar] [CrossRef]
- Álvarez-Chávez, C.R.; Edwards, S.; Moure-Eraso, R.; Geiser, K. Sustainability of bio-based plastics: General comparative analysis and recommendations for improvement. J. Clean. Prod. 2012, 23, 47–56. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; Cabezudo, S.; Caba, K.D.L. Environmental assessment of chitosan-based films. J. Clean. Prod. 2013, 41, 312–318. [Google Scholar] [CrossRef]
- Department of environment and regional planning of the Basque Government. The Eco Design of Industrial Products Engineering—EcodesignIhobe; Sociedad publica de gestio´nambiental: Bilbao, Spain, 2007.
- Goedkoop, M.; Spriensma, R. A Damage Oriented Method for Life Cycle Impact Assessment; The Eco-Indicator 99: Amersfoort, The Netherlands, 1999. [Google Scholar]
- Geetha, D.; Tyagi, R. Alkyl Poly Glucosides (APGs) Surfactants and Their Properties: A Review. Tenside Surfactants Deterg. 2012, 49, 417–427. [Google Scholar] [CrossRef]
- Pian, V.; Le Hen-Ferrenbach, C.; Beuché, M.; Roussel, M. New Sucrose Polystearate Effective Emulsifier Combining Skin Touch with Moisturizing Properties; ISFCC: Florence, Italy, 2005. [Google Scholar]

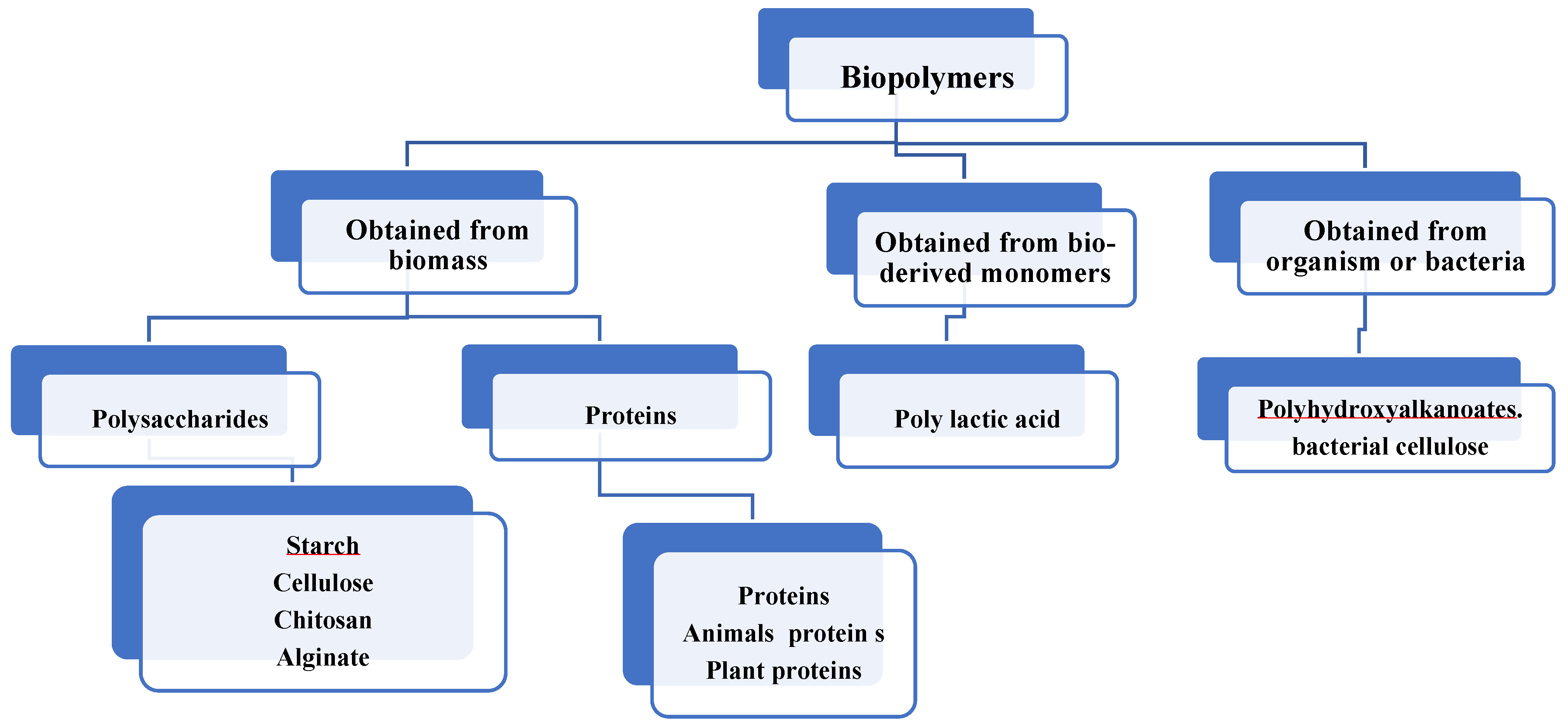

| S. N | Biopolymers | Degradation | Sources |
|---|---|---|---|
| 1 | Polysaccharides | Degradation by enzymes | Starch (wheat, potato, maize), Ligno-cellulosic products (wood, straw), Chitosan/Chitin. |
| 2 | Proteins and Lipids | Degradation by enzymes | Animals (casein, whey, gelation, collagen), Plants (zein, soy, etc.) |
| 3 | Polyhydroxyalkanoates (PHA) | Degradation by hydrolysis | Polyhydroxybutanoate (PHB), Polyhydroxy butyrate co-hydroxyvalerate (PHBV) |
| 4 | Polylactic Acid | Degradation by hydrolysis | Polylactic Acid |
| 5 | Petrochemical Polymers | Degradation by hydrolysis | Polycaprolactone (PCL), Polyester Amides (PEA) |
| Properties | Emulsions | Nanoemulsions | References |
|---|---|---|---|
| Droplet size | Lager than nanoemulsions | 20–200 nm | [16] |
| Stability | Thermodynamically unstable | Thermodynamically stable | [17] |
| Formation | By high shear homogenization methods | Micro-fluidization of emulsions | [18] |
| Viscosity | Higher viscosity than nanoemulsions | Lower viscosity than emulsions | [18] |
| Sources | Emulsification Techniques | Droplet Size | References |
|---|---|---|---|
| Fluids | Ultrasonic emulsification | 24.21 ± 0.11 nm | [30] |
| Pastes | Emulsion inversion point method | <300 nm | [31] |
| Fogs | High-pressure homogenization | 200–600 nm | [32] |
| Gels | Microfluidization | <100 nm | [33,34] |
| Fine liquid and solid particles in the air | Vertex mixing | 282 nm | [35,36] |
| Topical | High-pressure homogenization | 50–100 nm | [37] |
| Oral | Microfluidization | 22 ± 4.0 nm | [38] |
| Intravenous | High-pressure homogenization | 89.23 ± 7.2 nm | [39] |
| Intranasal, pulmonary, and ocular | High-pressure homogenization | 8.4 ± 12.7 nm | [40] |
| Cosmetic industry | Ultrasonic emulsification | 6–10 nm | [41] |
| Pesticide industry | Low-energy emulsification | ~30 nm | [42] |
| Polymer | Type | Lifespan/Degradation Time | Mechanism of Degradation | Reference |
|---|---|---|---|---|
| Collagen types I, II, III | Bio/ Semi-synthetic | 12 h | Enzymatic: collagenase | [124] |
| Cross-linked collagen | Semi-synthetic | >6 weeks | Enzymatic: collagenase | [125] |
| Alginate | Semi-synthetic | ~80 days | Hydrolytic disintegration | [126] |
| Cross-linked chitosan | Semi-synthetic | >20 weeks | Enzymatic: chitosanase and lysosome | [127] |
| Hyaluronan films | Biopolymer | 1 week to 4 months | Enzymatic: hyaluronidase | [128] |
| Braided silk | Biopolymer | 6 weeks | Proteolysis | [129] |
| Polycaprolactone (PCL) | Synthetic | >24 months | Hydrolytic | [130] |
| PLA | Synthetic | >24 months | Hydrolytic | [130] |
| PHA/PHB | Synthetic | >24 months | Bacterial fermentation | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamang, N.; Shrestha, P.; Khadka, B.; Mondal, M.H.; Saha, B.; Bhattarai, A. A Review of Biopolymers’ Utility as Emulsion Stabilizers. Polymers 2022, 14, 127. https://doi.org/10.3390/polym14010127
Tamang N, Shrestha P, Khadka B, Mondal MH, Saha B, Bhattarai A. A Review of Biopolymers’ Utility as Emulsion Stabilizers. Polymers. 2022; 14(1):127. https://doi.org/10.3390/polym14010127
Chicago/Turabian StyleTamang, Nirmala, Pooja Shrestha, Binita Khadka, Monohar Hossain Mondal, Bidyut Saha, and Ajaya Bhattarai. 2022. "A Review of Biopolymers’ Utility as Emulsion Stabilizers" Polymers 14, no. 1: 127. https://doi.org/10.3390/polym14010127
APA StyleTamang, N., Shrestha, P., Khadka, B., Mondal, M. H., Saha, B., & Bhattarai, A. (2022). A Review of Biopolymers’ Utility as Emulsion Stabilizers. Polymers, 14(1), 127. https://doi.org/10.3390/polym14010127