A Review of the Recent Progress in the Development of Nanocomposites Based on Poly(ether-block-amide) Copolymers as Membranes for CO2 Separation
Abstract
:1. Introduction
2. Materials for the Analysed MMMs
2.1. Polymer Matrix
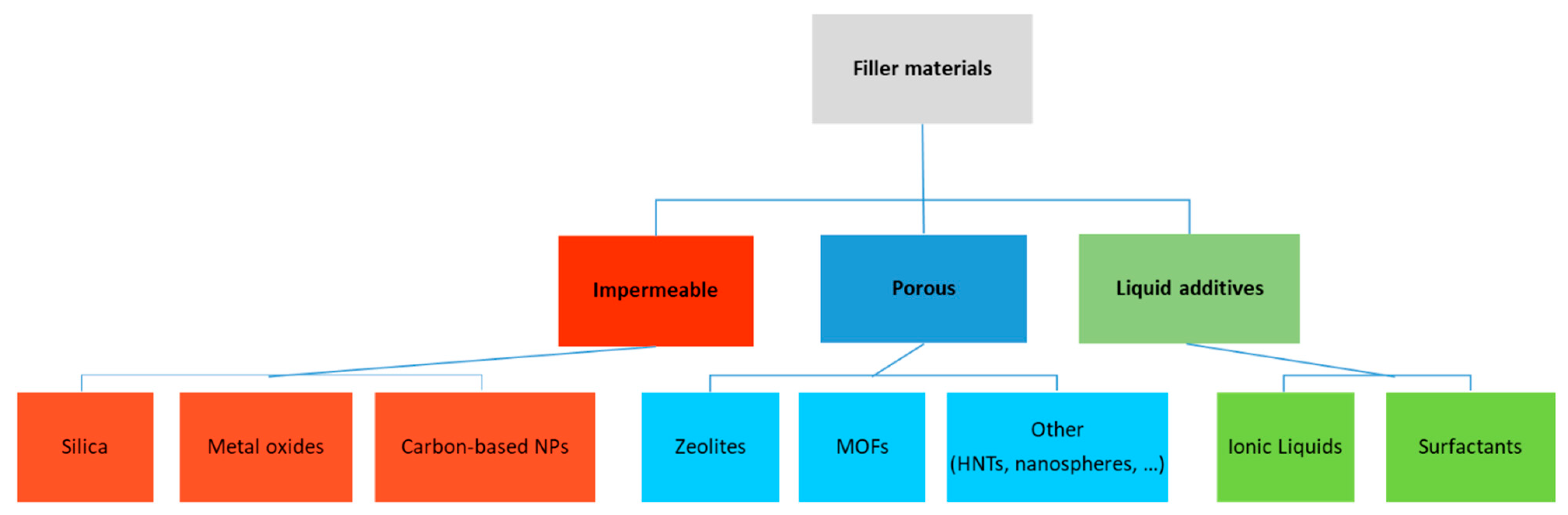
2.2. Filler Materials
2.2.1. Inorganic Impermeable Fillers
Silica (SiO2)
Clays
Metal Oxides
Carbon-Based Nanomaterials
Graphene and Graphene Oxide (GO)
2.2.2. Porous Fillers
Zeolites
Metal–Organic Frameworks (MOFs)
Two-Dimensional (2D) Fillers
2.2.3. Other Solid Fillers
2.2.4. Dual Fillers
2.2.5. Other Additives
3. Main Issues in MMMs Development
3.1. Particle/Polymer Interactions
3.2. Effect on Polymeric Chain Mobility
3.3. Effect on Polymer Crystallinity
3.4. Effect on Free Volume
3.5. Particle/Penetrant Interactions—Sorption Capacity for Specific Penetrants
3.6. Size Effect
3.7. Shape Effect
3.8. Loading Effect—Strong (Homogeneous Distribution) and Weak (Agglomeration) Points
3.9. Mechanical Properties
4. Gas Separation Performance
4.1. Transport Mechanisms Different from Those in Polymers
4.2. Defects—Causes and Countermeasures
4.3. Operation Conditions
5. Final Remarks
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Diverse Applications of Organic-Inorganic Nanocomposites: Emerging Research and Opportunities; Advances in Mechatronics and Mechanical Engineering Book Series; Clarizia, G.; Bernardo, P. (Eds.) IGI Global: Hershey, PA, USA, 2020; ISBN 13: 9781799815303. [Google Scholar]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, 1138–1148. [Google Scholar] [CrossRef] [Green Version]
- Kardani, R.; Asghari, M.; Mohammadi, T.; Afsari, M. Effects of nanofillers on the characteristics and performance of PEBA-based mixed matrix membranes. Rev. Chem. Eng. 2018, 34, 797–836. [Google Scholar] [CrossRef]
- Amirkhani, F.; Harami, H.R.; Asghari, M. CO2/CH4 mixed gas separation using poly(ether-b-amide)-ZnO nanocomposite membranes: Experimental and molecular dynamics study. Polym. Test. 2020, 86, 106464. [Google Scholar] [CrossRef]
- Reijerkerk, S.R.; Knoef, M.H.; Nijmeijer, K.; Wessling, M. Poly(ethylene glycol) and poly(dimethyl siloxane): Combining their advantages into efficient CO2 gas separation membranes. J. Membr. Sci. 2010, 352, 126. [Google Scholar] [CrossRef]
- Kojabad, M.E.; Babaluo, A.; Tavakoli, A. A novel semi-mobile carrier facilitated transport membrane containing aniline/poly (ether-block-amide) for CO2/N2 separation: Molecular simulation and experimental study. Separ. Purif. Tech. 2021, 266, 118494. [Google Scholar] [CrossRef]
- Monsalve-Bravo, G.M.; Bhatia, S.K. Modeling Permeation through Mixed-Matrix Membranes: A Review. Processes 2018, 6, 172. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, J.C. A Treatise on Electricity and Magnetism; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Merkel, T.C.; Freeman, B.D.; Spontak, R.J.; He, Z.; Pinnau, I.; Meakin, P.; Hill, A.J. Sorption, Transport, and Structural Evidence for Enhanced Free Volume in Poly(4-methyl-2-pentyne)/Fumed Silica Nanocomposite Membranes. Chem. Mater. 2003, 15, 109–123. [Google Scholar] [CrossRef]
- Fu, K.; Lü, F.; Xie, Q.; Ruan, H.; Yang, X.; Liang, S. The effects of shape and mass fraction of nano-SiO2 on thermomechanical properties of nano-SiO2/DGEBA/MTHPA composites: A molecular dynamics simulation study. AIP Adv. 2020, 10, 015339. [Google Scholar] [CrossRef] [Green Version]
- Azizi, N.; Azizi, S.; Homayoon, R. Experimental Study of CO2 and CH4 Permeability Values Through Pebax®-1074/Silica Mixed Matrix Membranes. Silicon 2019, 11, 2045–2057. [Google Scholar] [CrossRef]
- Azizi, N.; Mohammadi, T.; Behbahani, R.M. Comparison of permeability performance of PEBAX-1074/TiO2, PEBAX-1074/SiO2 and PEBAX-1074/Al2O3 nanocomposite membranes for CO2/CH4 separation. Chem. Eng. Res. Des. 2017, 117, 177–189. [Google Scholar] [CrossRef]
- Barzegar, T.; Hassanajili, S. Fabrication and characterization of dual layer PEBAX-SiO2/polyethersulfone nanocomposite membranes for separation of CO2/CH4 gases. Appl. Polym. Sci. 2021, 139, e51624. [Google Scholar] [CrossRef]
- Aghaei, Z.; Naji, L.; Asl, V.H.; Khanbabaei, G.; Dezhagah, F. The influence of fumed silica content and particle size in poly (amide 6-b-ethylene oxide) mixed matrix membranes for gas separation. Separ. Purif. Tech. 2018, 199, 47–56. [Google Scholar] [CrossRef]
- Wang, D.; Song, S.; Zhang, W.; He, Z.; Wang, Y.; Zheng, Y.; Yao, D.; Pan, Y.; Yang, Z.; Meng, Z.; et al. CO2 selective separation of Pebax-based mixed matrix membranes (MMMs) accelerated by silica nanoparticle organic hybrid materials (NOHMs). Separ. Purif. Tech. 2020, 241, 116708. [Google Scholar] [CrossRef]
- Maleh, M.S.; Raisi, A. Comparison of porous and nonporous filler effect on performance of poly (ether-block-amide) mixed matrix membranes for gas separation applications. Chem. Eng. Res. Des. 2019, 147, 545–560. [Google Scholar] [CrossRef]
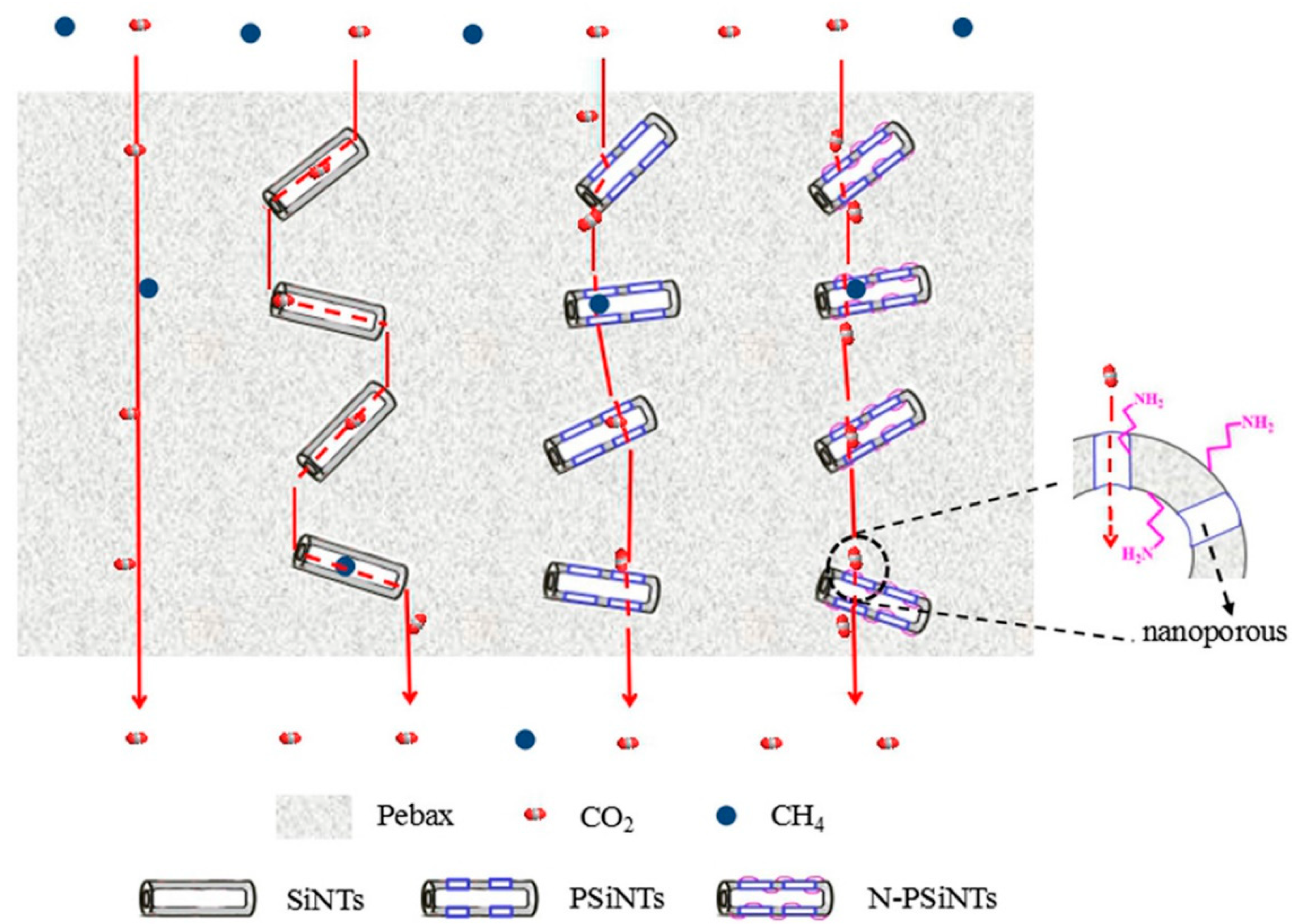
- Yang, L.; Zhang, S.; Wu, H.; Ye, C.; Liang, X.; Wang, S.; Wu, X.; Wu, Y.; Ren, Y.; Liu, Y.; et al. Porous organosilicon nanotubes in pebax-based mixed-matrix membranes for biogas purification. J. Membr. Sci. 2019, 573, 301–308. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Zulhairun, A.K.; Ismail, A.F.; Matsuura, T.; Abdullah, M.S.; Mustafa, A. Asymmetric mixed matrix membrane incorporating organically modified clay particle for gas separation. Chem. Eng. J. 2014, 241, 495–503. [Google Scholar] [CrossRef]
- Wang, Y.; Alhassan, S.M.; Yang, V.H.; Schiraldi, D.A. Polyether-block-amide copolymer/clay films prepared via a freeze-drying method. Compos. Part B Eng. 2013, 45, 625–630. [Google Scholar] [CrossRef]
- Behroozi, M.; Pakizeh, M. Study the effects of Cloisite15A nanoclay incorporation on the morphology and gas permeation properties of Pebax2533 polymer. J. Appl. Polym. Sci. 2017, 134, 45302. [Google Scholar] [CrossRef]
- Ahmad, S.; Lian, S.; Tan, Y.; Li, R.; Zhao, Q.; Song, C.; Liu, Q.; Lu, S. Solvent influence on the textural properties and CO2/N2 separation performance of novel Pebax-1657/attapulgite mixed matrix membranes. J. Environ. Chem. Eng. 2021, 9, 105806. [Google Scholar] [CrossRef]
- Xiang, L.; Pan, Y.; Jiang, J.; Chen, Y.; Chen, J.; Zhang, L.; Wang, C. Thin poly(ether-block-amide)/attapulgite composite membranes with improved CO2 permeance and selectivity for CO2/N2 and CO2/CH4. Chem. Eng. Sci. 2017, 160, 236–244. [Google Scholar] [CrossRef]
- Roman, S.; Fujikawa, S. Molecular hybridization of polydimethylsiloxane with zirconia for highly gas permeable membranes. ACS Appl. Polym. Mater. 2019, 1, 1165–1174. [Google Scholar]
- Farashi, Z.; Azizi, N.; Homayoon, R. Applying Pebax-1657/ZnO mixed matrix membranes for CO2/CH4 separation. Pet. Sci. Tech. 2019, 37, 2412–2419. [Google Scholar] [CrossRef]
- Jazebizadeh, M.H.; Khazraei, S. Investigation of Methane and Carbon Dioxide Gases Permeability Through PEBAX/PEG/ZnO Nanoparticle Mixed Matrix Membrane. Silicon 2017, 9, 775–784. [Google Scholar] [CrossRef]
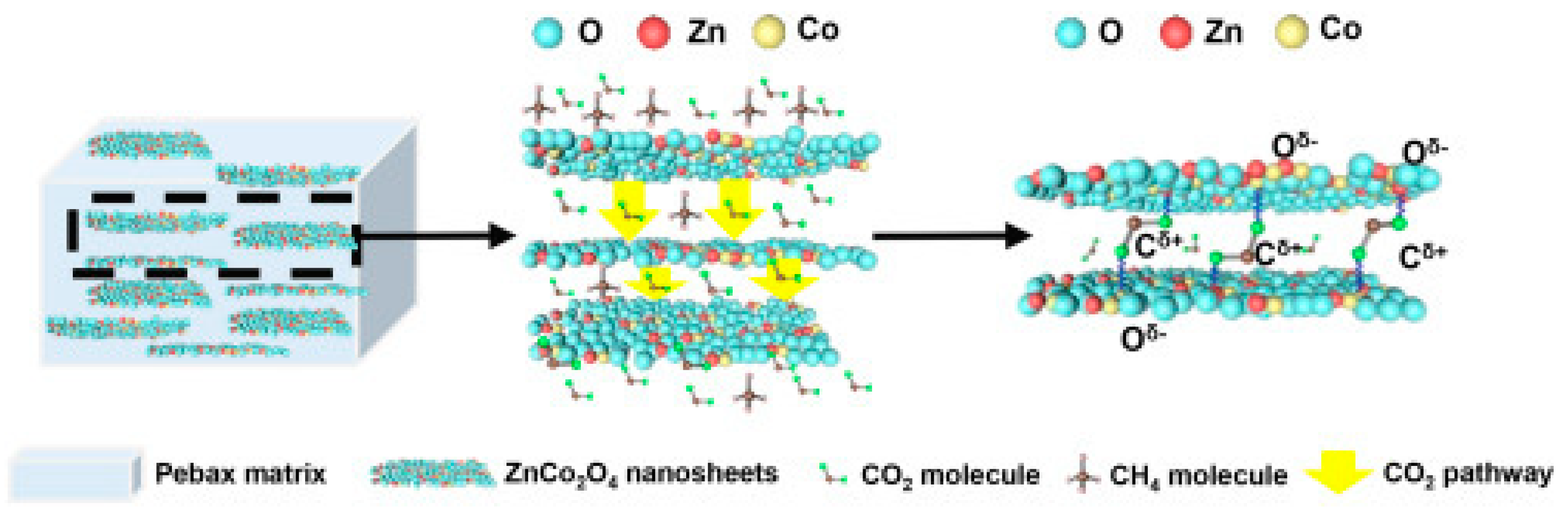
- Zhu, W.; Liu, F.; Gou, M.; Guo, R.; Li, X. Mixed matrix membrane containing metal oxide nanosheets for efficient CO2 separation. Green Chem. Eng. 2021, 2, 132–143. [Google Scholar] [CrossRef]
- Azizi, N.; Isanejad, M.; Mohammadi, T.; Behbahani, R.M. Effect of TiO2 loading on the morphology and CO2/CH4 separation performance of PEBAX-based membranes. Front. Chem. Sci. Eng. 2019, 13, 517–530. [Google Scholar] [CrossRef]
- Shamsabadi, A.A.; Seidi, F.; Salehi, E.; Nozari, M.; Rahimpoure, A.; Soroush, M. Efficient CO2-removal using novel mixed-matrix membranes with modified TiO2 nanoparticles. J. Mater. Chem. A 2017, 5, 4011–4025. [Google Scholar] [CrossRef]
- Farashi, Z.; Azizi, S.; Arzhandi, M.R.-D.; Noroozi, Z.; Azizi, N. Improving CO2/CH4 separation efficiency of Pebax-1657 membrane by adding Al2O3 nanoparticles in its matrix. J. Nat. Gas Sci. Eng. 2019, 72, 103019. [Google Scholar] [CrossRef]
- Kojabad, M.E.; Babaluo, A.A.; Tavakoli, A.; Sofla, R.L.M.; Kahnamouei, H.G. Comparison of acidic and basic ionic liquids effects on dispersion of alumina particles in Pebax composite membranes for CO2/N2 separation: Experimental study and molecular simulation. J. Environ. Chem. Eng. 2021, 9, 106116. [Google Scholar] [CrossRef]
- Harami, H.R.; Asghari, M. Magnetic nanoFe2O3-incorporated PEBA membranes for CO2/CH4 and CO2/N2 separation: Experimental study and grand canonical Monte Carlo and molecular dynamics simulations. Greenh. Gases Sci. Technol. 2019, 9, 306–330. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Li, Z.; Liu, M.; Kinloch, I.A.; Young, R.J. Mechanisms of mechanical reinforcement by graphene and carbon nanotubes in polymer nanocomposites. Nanoscale 2020, 12, 2228–2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamali, P.A.; Rahmani, M.; Kazemi, A.; Pourkhalil, M. Improved Gas Separation of PEBAX-CSWCNTs Mixed Matrix Membranes. J. Membr. Separ. Tech. 2017, 6, 55–70. [Google Scholar]
- Asghari, M.; Afsari, M. Effect of Ethylene Oxide Functional Groups in PEBA-CNT Membranes on CO2/CH4 Mixed Gas Separation. J. Membr. Sci. Res. 2018, 4, 34–40. [Google Scholar]
- Song, C.; Mujahid, M.; Li, R.; Ahmad, S.; Liu, Q.; Zhang, B.; Kitamura, Y. Pebax/MWCNTs-NH2 mixed matrix membranes for enhanced CO2/N2 separation. Greenh. Gas Sci. Technol. 2020, 10, 408–420. [Google Scholar] [CrossRef]
- Wang, D.; Yao, D.; Wang, Y.; Wang, F.; Xin, Y.; Song, S.; Zhang, Z.; Su, F.; Zheng, Y. Carbon nanotubes and graphene oxide-based solvent-free hybrid nanofluids functionalized mixed-matrix membranes for efficient CO2/N2 separation. Separ. Purif. Tech. 2019, 221, 421–432. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, W.; Yang, X.; Ning, M.; Li, X.; Xi, Y.; Yan, X.; Zhang, X.; Dai, Y.; Liu, H.; et al. Pebax-based mixed matrix membranes derived from microporous carbon nanospheres for permeable and selective CO2 separation. Separ. Purif. Tech. 2021, 274, 119015. [Google Scholar] [CrossRef]
- Vu, M.-T.; Monsalve-Bravo, G.M.; Lin, R.; Li, M.; Bhatia, S.K.; Smart, S. Mitigating the Agglomeration of Nanofiller in a Mixed Matrix Membrane by Incorporating an Interface Agent. Membranes 2021, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Althumayri, K.; Harrison, W.J.; Shin, Y.; Gardiner, J.M.; Casiraghi, C.; Budd, P.M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. The influence of graphene and other nanofillers on the gas permeability of the high-free-volume polymer PIM-1. Philos. Trans. R. Soc. A 2016, 374, 20150031. [Google Scholar] [CrossRef] [Green Version]
- Pazani, F.; Aroujalian, A. High-performance gas separation using mixed-matrix composite membranes containing graphene nanoplatelets. Polym. Bull. 2021, 78, 6847–6866. [Google Scholar] [CrossRef]
- Huang, T.-C.; Liu, Y.-C.; Lin, G.-S.; Lin, C.-H.; Liu, W.-R.; Tung, K.-L. Fabrication of pebax-1657-based mixed-matrix membranes incorporating N-doped few-layer graphene for carbon dioxide capture enhancement. J. Membr. Sci. 2020, 602, 117946. [Google Scholar] [CrossRef]
- Dimiev, A.M. Chapter 2—Mechanism of Formation and Chemical Structure of Graphene Oxide. In Graphene Oxide: Fundamentals and Applications; Dimiev, A.M., Eigler, S., Eds.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, M.; Liu, G.; Guan, K.; Jin, W. Size effects of graphene oxide on mixed matrix membranes for CO2 separation. AIChE J. 2016, 62, 2843–2852. [Google Scholar] [CrossRef]
- Shin, J.E.; Lee, S.K.; Cho, Y.H.; Park, H.B. Effect of PEG-MEA and graphene oxide additives on the performance of Pebax®1657 mixed matrix membranes for CO2 separation. J. Membr. Sci. 2019, 572, 300–308. [Google Scholar] [CrossRef]
- Casadei, R.; Baschetti, M.G.; Yoo, M.J.; Park, H.B.; Giorgini, L. Pebax® 2533/Graphene Oxide Nanocomposite Membranes for Carbon Capture. Membranes 2020, 10, 188. [Google Scholar] [CrossRef]
- Dai, Y.; Ruan, X.; Yan, Z.; Yang, K.; Yu, M.; Li, H.; Zhao, W.; He, G. Imidazole functionalized graphene oxide/PEBAX mixed matrix membranes for efficient CO2 capture. Separ. Purif. Tech. 2016, 166, 171–180. [Google Scholar] [CrossRef]
- Huang, G.; Isfahani, A.P.; Muchtar, A.; Sakurai, K.; Shrestha, B.B.; Qin, D.; Yamaguchi, D.; Sivaniah, E.; Ghalei, B. Pebax/ionic liquid modified graphene oxide mixed matrix membranes for enhanced CO2 capture. J. Membr. Sci. 2018, 565, 370–379. [Google Scholar] [CrossRef]
- Krishnan, G.; Mohtar, S.S.; Aziz, F.; Jaafar, J.; Yusof, N.; Salleh, W.N.W.; Ismail, A.F. Mixed matrix composite membranes based on amination of reduced graphene oxide for CO2 separation: Effects of heating time and nanofiller loading. Korean J. Chem. Eng. 2020, 37, 2287–2294. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Nasir, A.M.; Aziz, F.; Kumar, G.; Sallehhudin, W.; Jaafar, J.; Lau, W.J.; Yusof, N.; Salleh, W.N.W.; Ismail, A.F. CO2/N2 selectivity enhancement of PEBAX MH 1657/Aminated partially reduced graphene oxide mixed matrix composite membrane. Separ. Purif. Tech. 2019, 223, 142–153. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Q.; Li, X.; Yun, M.; Xu, R.; Wang, S.; Li, Y.; Lin, L.; Ding, X.; Ye, H.; et al. Mixed matrix membranes comprising aminosilane-functionalized graphene oxide for enhanced CO2 separation. J. Membr. Sci. 2019, 570, 343–354. [Google Scholar] [CrossRef]
- Asghari, M.; Saadatmandi, S.; Parnian, M.J. Polypyrrole-aided surface decoration of graphene oxide nanosheets as fillers for poly(ether-b-amid) mixed matrix membranes to enhance CO2 capture. Int. J. Energy Res. 2021, 45, 10843–10857. [Google Scholar] [CrossRef]
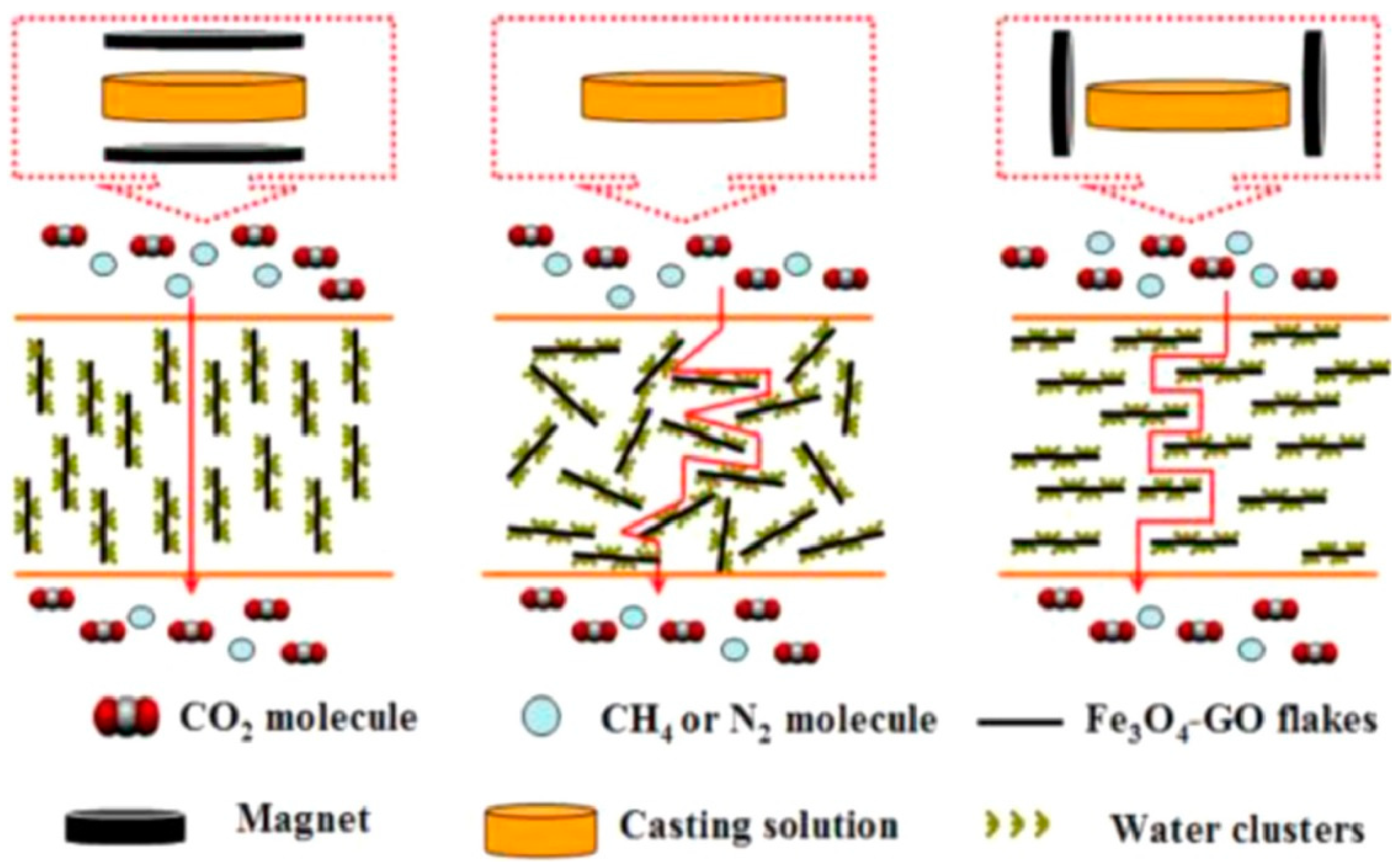
- Zhu, W.; Qin, Y.; Wang, Z.; Zhang, J.; Guo, R.; Li, X. Incorporating the magnetic alignment of GO composites into Pebax matrix for gas separation. J. Energy Chem. 2019, 31, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, Y.; Zhang, B.; Wang, Z. Preparation and characterization of CO2-selective Pebax/NaY mixed matrix membranes. J. Appl. Polym. Sci. 2020, 137, 48398. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, C.; Zheng, Y.; Wu, Y.; Song, C.; Liu, Q.; Wang, Z. Modification of CO2-selective mixed matrix membranes by a binary composition of poly(ethylene glycol)/NaY zeolite. J. Membr. Sci. 2021, 627, 119239. [Google Scholar] [CrossRef]
- Maleh, M.S.; Raisi, A. CO2-philic moderate selective layer mixed matrix membranes containing surface functionalized NaX towards highly-efficient CO2 capture. RSC Advances 2019, 9, 15542–15553. [Google Scholar] [CrossRef] [Green Version]
- Karamouz, F.; Maghsoudi, H.; Yegani, R. Synthesis of High-Performance Pebax®-1074/DD3R Mixed-Matrix Membranes for CO2/CH4 Separation. Chem. Eng. Tech. 2018, 41, 1767–1775. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, Y.; Wu, Y.; Zhang, B. Fabrication of Pebax/SAPO mixed matrix membranes for CO2/N2 separation. J. Appl. Polym. Sci. 2021, 138, 51336. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, M.; Liu, X.; Zhang, B. Pebax/two-dimensional MFI nanosheets mixed-matrix membranes for enhanced CO2 separation. J. Membr. Sci. 2021, 636, 119612. [Google Scholar] [CrossRef]
- Hassan, T.N.A.T.; Jusoh, N.; Yeong, Y.F. Synthesis and characterization of PEBAX 1657 and hierarchical Linde Type-T (h-LTT) zeolite for the fabrication of hybrid membranes. Mater. Today Proc. 2021, 47, 1263–1268. [Google Scholar] [CrossRef]
- Sánchez-Laínez, J.; Gracia-Guillén, I.; Zornoza, B.; Téllez, C.; Coronas, J. Thin supported MOF based mixed matrix membranes of Pebax® 1657 for biogas upgrade. New J. Chem. 2019, 43, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Ding, R.; Yang, K.; Dai, Y.; Yan, X.; He, G. ZIF-8 nanoparticles with tunable size for enhanced CO2 capture of Pebax based MMMs. Separ. Purif. Tech. 2019, 214, 111–119. [Google Scholar] [CrossRef]
- Ding, R.; Zheng, W.; Yang, K.; Dai, Y.; Ruan, X.; Yan, X.; He, G. Amino-functional ZIF-8 nanocrystals by microemulsion based mixed linker strategy and the enhanced CO2/N2 separation. Separ. Purif. Tech. 2020, 236, 116209. [Google Scholar] [CrossRef]
- Polak, D.; Sułkowska, J.; Szwast, M. The influence of surfactant pluronic P123 addition on the mixed matrix membrane PEBAX® 2533 – ZIF-8 separation properties. Desalination Water Treat. 2021, 214, 64–73. [Google Scholar] [CrossRef]
- Deng, J.; Dai, Z.; Hou, J.; Deng, L. Morphologically Tunable MOF Nanosheets in Mixed Matrix Membranes for CO2 Separation. Chem. Mater. 2020, 32, 4174–4184. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, Y.; Ruan, X.; Zheng, W.; Yan, X.; Li, X.; He, G. ZIF-8 hollow nanotubes based mixed matrix membranes with high-speed gas transmission channel to promote CO2/N2 separation. J. Membr. Sci. 2021, 630, 119323. [Google Scholar] [CrossRef]
- Zhu, W.; Li, X.; Sun, Y.; Guo, R.; Ding, S. Introducing hydrophilic ultra-thin ZIF-L into mixed matrix membranes for CO2/CH4 separation. RSC Adv. 2019, 9, 23390–23399. [Google Scholar] [CrossRef] [Green Version]
- Sabetghadam, A.; Liu, X.; Benzaqui, M.; Gkaniatsou, E.; Orsi, A.; Lozinska, M.M.; Sicard, C.; Johnson, T.; Steunou, N.; Wright, P.A.; et al. Influence of filler pore structure and polymer on the performance of MOF-based mixed matrix membranes for CO2 capture. Chem. A Eur. J. 2018, 24, 7949–7956. [Google Scholar] [CrossRef] [Green Version]
- Salahshoori, I.; Cacciotti, I.; Seyfaee, A.; Babapoor, A. Improvement efficiency of the of poly (ether-block-amide)-Cellulose acetate (Pebax-CA) blend by the addition of nanoparticles (MIL-53 and NH2-MIL-53): A molecular dynamics study. J. Polym. Res. 2021, 28, 223. [Google Scholar] [CrossRef]
- Fallahi, C.; Moradi, S.; Behbahani, R. The Synthesis and Implementation of Pebax/PEG 400/NH2-MIL125 Nanocomposite Membranes to Separate CO2/CH4. Iran. J. Oil Gas Sci. Technol. 2019, 8, 107–127. [Google Scholar]
- Habib, N.; Shamair, Z.; Tara, N.; Nizami, A.-S.; Akhtar, F.H.; Ahmad, N.M.; Gilani, M.A.; Bilad, M.R.; Khan, A.L. Development of highly permeable and selective mixed matrix membranes based on Pebax®1657 and NOTT-300 for CO2 capture. Separ. Purif. Tech. 2020, 234, 116101. [Google Scholar] [CrossRef]
- Lv, X.; Huang, L.; Ding, S.; Wang, J.; Li, L.; Liang, C.; Li, X. Mixed matrix membranes comprising dual-facilitated bio-inspired filler for enhancing CO2 separation. Separ. Purif. Tech. 2021, 276, 119347. [Google Scholar] [CrossRef]
- Sarmadi, R.; Salimi, M.; Pirouzfar, V. The assessment of honeycomb structure UiO-66 and amino functionalized UiO-66 metal–organic frameworks to modify the morphology and performance of Pebax®1657-based gas separation membranes for CO2 capture applications. Environ. Sci. Pollut. Res. 2020, 27, 40618–40632. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Q.; Chen, G.; Duan, J.; Liu, G.; Jin, W. MOF-801 incorporated PEBA mixed-matrix composite membranes for CO2 capture. Separ. Purif. Tech. 2019, 217, 229–239. [Google Scholar] [CrossRef]
- Erfani, A.; Asghari, M. Comparison of different MOF fillers on CO2 removal performance of supported PEBA mixed matrix membranes. Greenh. Gases Sci. Technol. 2021, 11, 128–143. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Hou, J.; Li, H.; Zhang, Y.; Chen, V. Improved operational stability of Pebax-based gas separation membranes with ZIF-8: A comparative study of flat sheet and composite hollow fibre membranes. J. Membr. Sci. 2017, 524, 266–279. [Google Scholar] [CrossRef]
- Li, G.; Kujawski, W.; Knozowska, K.; Kujawa, J. Thin Film Mixed Matrix Hollow Fiber Membrane Fabricated by Incorporation of Amine Functionalized Metal-Organic Framework for CO2/N2 Separation. Materials 2021, 14, 3366. [Google Scholar] [CrossRef]
- Shinde, P.V.; Singh, M.K. Chapter 4—Synthesis, Characterization, and Properties of Graphene Analogs of 2D Material. In Woodhead Publishing Series in Electronic and Optical Materials, Fundamentals and Sensing Applications of 2D Materials; Hywel, M., Rout, C.S., Late, D.J., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 91–143. ISBN 9780081025772. [Google Scholar]
- Liu, G.; Cheng, L.; Chen, G.; Liang, F.; Liu, G.; Jin, W. Pebax-Based Membrane Filled with Two-Dimensional Mxene Nanosheets for Efficient CO2 Capture. Chem. Asian J. 2020, 15, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Sun, J.; Wang, J.; Liu, M.; Yan, Z.; Zhu, B.; Li, Y.; Cao, X. MXene versus graphene oxide: Investigation on the effects of 2D nanosheets in mixed matrix membranes for CO2 separation. J. Membr. Sci. 2021, 620, 118850. [Google Scholar] [CrossRef]
- Shi, F.; Sun, J.; Wang, J.; Liu, M.; Wang, S.; Cao, X.; Yan, Z.; Li, Y.; Nunes, S.P. Exploration of the synergy between 2D nanosheets and a non-2D filler in mixed matrix membranes for gas separation. Front. Chem. 2020, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, K.; Ellison, C.J. Chapter 20—Layered Double Hydroxides: An Emerging Class of Flame Retardants, Polymer Green Flame Retardants; Papaspyrides, C.D., Kiliaris, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 675–707. ISBN 9780444538086. [Google Scholar]
- Ding, S.; Li, X.; Ding, S.; Zhang, W.; Guo, R.; Zhang, J. Ionic liquid-decorated nanocages for cooperative CO2 transport in mixed matrix membranes. Separ. Purif. Tech. 2020, 239, 116539. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, N.; Wu, H.; Ren, Y.; Yang, L.; Wang, X.; Wu, Y.; Liu, Y.; Zhao, R.; Jiang, Z. Exfoliation-free layered double hydroxides laminates intercalated with amino acids for enhanced CO2 separation of mixed matrix membrane. J. Membr. Sci. 2021, 618, 118691. [Google Scholar] [CrossRef]
- Duan, K.; Wang, J.; Zhang, Y.; Liu, J. Covalent organic frameworks (COFs) functionalized mixed matrix membrane for effective CO2/N2 separation. J. Membr. Sci. 2019, 572, 588–595. [Google Scholar] [CrossRef]
- Afshoun, H.R.; Chenar, M.P.; Moradi, M.R.; Ismail, A.F.; Matsuura, T. Effects of halloysite nanotubes on the morphology and CO2/CH4 separation performance of Pebax/polyetherimide thin-film composite membranes. J. Appl. Polym. Sci. 2020, 137, 48860. [Google Scholar] [CrossRef]
- Ahmadi, S.M.A.; Mohammadi, T.; Azizi, N. Superior Pebax-1657/amine-modified halloysite nanotubes mixed-matrix membranes to improve the CO2/CH4 separation efficiency. J. Appl. Polym. Sci. 2021, 138, 50749. [Google Scholar] [CrossRef]
- Wang, X.; Ding, X.; Zhao, H.; Fu, J.; Xin, Q.; Zhang, Y. Pebax-based mixed matrix membranes containing hollow polypyrrole nanospheres with mesoporous shells for enhanced gas permeation performance. J. Membr. Sci. 2020, 602, 117968. [Google Scholar] [CrossRef]
- Liu, N.; Cheng, J.; Hu, L.; Hou, W.; Yang, X.; Luo, M.; Zhang, H.; Ye, B.; Zhou, J. Boosting CO2 transport of poly (ethylene oxide) membranes by hollow Rubik-like “expressway” channels with anion pillared hybrid ultramicroporous materials. Chem. Eng. J. 2022, 427, 130845. [Google Scholar] [CrossRef]
- Zhao, H.; Xie, Q.; Ding, X.; Cai, R.; Tan, X.; Zhang, Y. Advanced mixed matrix membranes of Pebax embedded with amino acid ionic liquids@PIM core-shell composite nanoparticles for CO2 separation. Sep. Purif. Technol. 2021, 263, 118350. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Ahmadi, R.; Amooghin, A.E.; Ghanbari, D. A novel ternary mixed matrix membrane containing glycerol-modified poly(ether-block-amide) (Pebax 1657)/copper nanoparticles for CO2 separation. J. Membr. Sci. 2019, 573, 234–246. [Google Scholar] [CrossRef]
- Ghazali, A.A.; Rahman, N.A.; Samah, R.A. Pebax 1657 Nanocomposite Membranes Incorporated with Nanoadsorbent Derived from Oil Palm Frond for CO2/CH4 Separation. Mater. Sci. Forum 2020, 1007, 52–57. [Google Scholar] [CrossRef]
- Valero, M.; Zornoza, B.; Téllez, C.; Coronas, J. Mixed matrix membranes for gas separation by combination of silica MCM-41 and MOF NH2-MIL-53(Al) in glassy polymers. Microporous Mesoporous Mater. 2014, 192, 23–28. [Google Scholar] [CrossRef]
- Zhang, Y.; Tong, Y.; Li, X.; Guo, S.; Zhang, H.; Chen, X.; Cai, K.; Cheng, L.; He, W. Pebax Mixed-Matrix Membrane with Highly Dispersed ZIF-8@CNTs to Enhance CO2/N2 Separation. ACS Omega 2021, 6, 18566–18575. [Google Scholar] [CrossRef]
- Li, X.; Yu, S.; Li, K.; Ma, C.; Zhang, J.; Li, H.; Chang, X.; Zhu, L.; Xue, Q. Enhanced gas separation performance of Pebax mixed matrix membranes by incorporating ZIF-8 in situ inserted by multiwalled carbon nanotubes. Separ. Purif. Tech. 2020, 248, 117080. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, H.; Li, F.; Dong, S.; Yang, L.; Ren, Y.; Wu, Y.; Wu, X.; Jiang, Z.; Cao, X. Heterostructured filler in mixed matrix membranes to coordinate physical and chemical selectivities for enhanced CO2 separation. J. Membr. Sci. 2018, 567, 272–280. [Google Scholar] [CrossRef]
- Dong, L.-L.; Zhang, C.-F.; Zhang, Y.-Y.; Bai, Y.-X.; Gu, J.; Sun, Y.-P.; Chen, M.-Q. Improving CO2/N2 separation performance using nonionic surfactant Tween containing polymeric gel membranes. RSC Adv. 2015, 5, 4947–4957. [Google Scholar] [CrossRef]
- Bernardo, P.; Clarizia, G. Enhancing Gas Permeation Properties of Pebax® 1657 Membranes via Polysorbate Nonionic Surfactants Doping. Polymers 2020, 12, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simari, C.; Nicotera, I.; Perrotta, I.; Clarizia, G.; Bernardo, P. Investigation of self-diffusion and gas transport properties in Pebax®1657 loaded with nonionic surfactants. Polymer 2020, 209, 122949. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Wu, H.; Xin, Q.; Wang, S.; Liu, Y.; Tian, Z.; Zhou, T.; Jiang, Z.; Tian, H.; et al. Anionic surfactant-doped Pebax membrane with optimal free volume characteristics for efficient CO2 separation. J. Membr. Sci. 2015, 493, 460–469. [Google Scholar] [CrossRef]
- Lee, H.J.; Kang, S.W. Activated potassium ions as CO2 carriers for PEBAX-5513/KBF4 composite membranes. Separ. Purif. Tech. 2021, 258, 117971. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, D.; Ren, J.; Qiu, Y.; Feng, Y.; Deng, M. Effect of triglyceride on the microstructure and gas permeation performance of Pebax-based blend membranes. Separ. Purif. Tech. 2021, 256, 117824. [Google Scholar] [CrossRef]
- Thanakkasaranee, S.; Kim, D.; Seo, J. Preparation and Characterization of Poly(ether-block-amide)/Polyethylene Glycol Composite Films with Temperature Dependent Permeation. Polymers 2018, 10, 225. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Liu, G.; Huang, K.; Jin, W.; Lee, K.-R.; Xu, N. Membranes with fast and selective gas-transport channels of laminar graphene oxide for efficient CO2 capture. Angew. Chem. Int. Ed. 2015, 54, 578–582. [Google Scholar]
- Sharma, P.; Kim, Y.-J.; Kim, M.-Z.; Alam, S.F.; Cho, C.H. A stable polymeric chain configuration producing high performance PEBAX-1657 membranes for CO2 separation. Nanoscale Adv. 2019, 1, 2633–2644. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.H.; Turnbull, D. Molecular transport in liquids and glasses. J. Chem. Phys. 1959, 31, 1164. [Google Scholar] [CrossRef] [Green Version]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Satyapal, S.; Filburn, T.; Trela, J.; Strange, J. Performance and properties of a solid amine sorbent for carbon dioxide removal in space life support applications. Energy Fuels 2001, 15, 250–255. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.-V. PEG modified poly(amide-b-ethylene oxide) membranes for CO2 separatio. J. Membr. Sci. 2008, 307, 88–95. [Google Scholar] [CrossRef]
- Feng, S.; Ren, J.; Zhao, D.; Li, H.; Hua, K.; Li, X.; Deng, M. Effect of poly(ethylene glycol) molecular weight on CO2/N2 separation performance of poly(amide-12-b-ethylene oxide)/poly(ethylene glycol) blend membranes. J. Energy Chem. 2019, 28, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, P.; Jansen, J.C.; Bazzarelli, F.; Tasselli, F.; Fuoco, A.; Friess, K.; Izák, P.; Jarmarová, V.; Kačírková, M.; Clarizia, G. Gas transport properties of Pebax®/room temperature ionic liquid gel membranes. Separ. Purif. Tech. 2012, 97, 73–82. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Li, S.; Ji, P.; Jiang, C. Preparation of composite poly(ether block amide) membrane for CO2 capture. J. Energy Chem. 2014, 23, 717–725. [Google Scholar] [CrossRef]

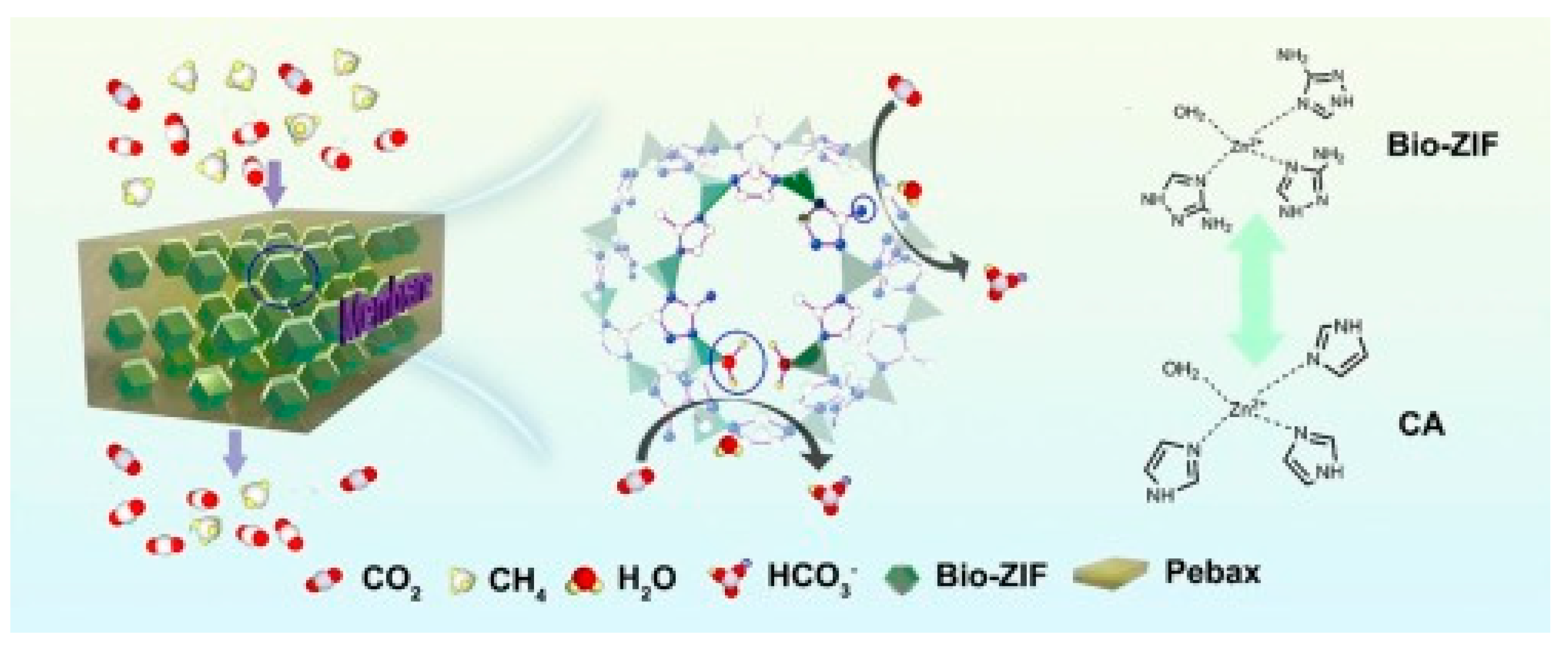



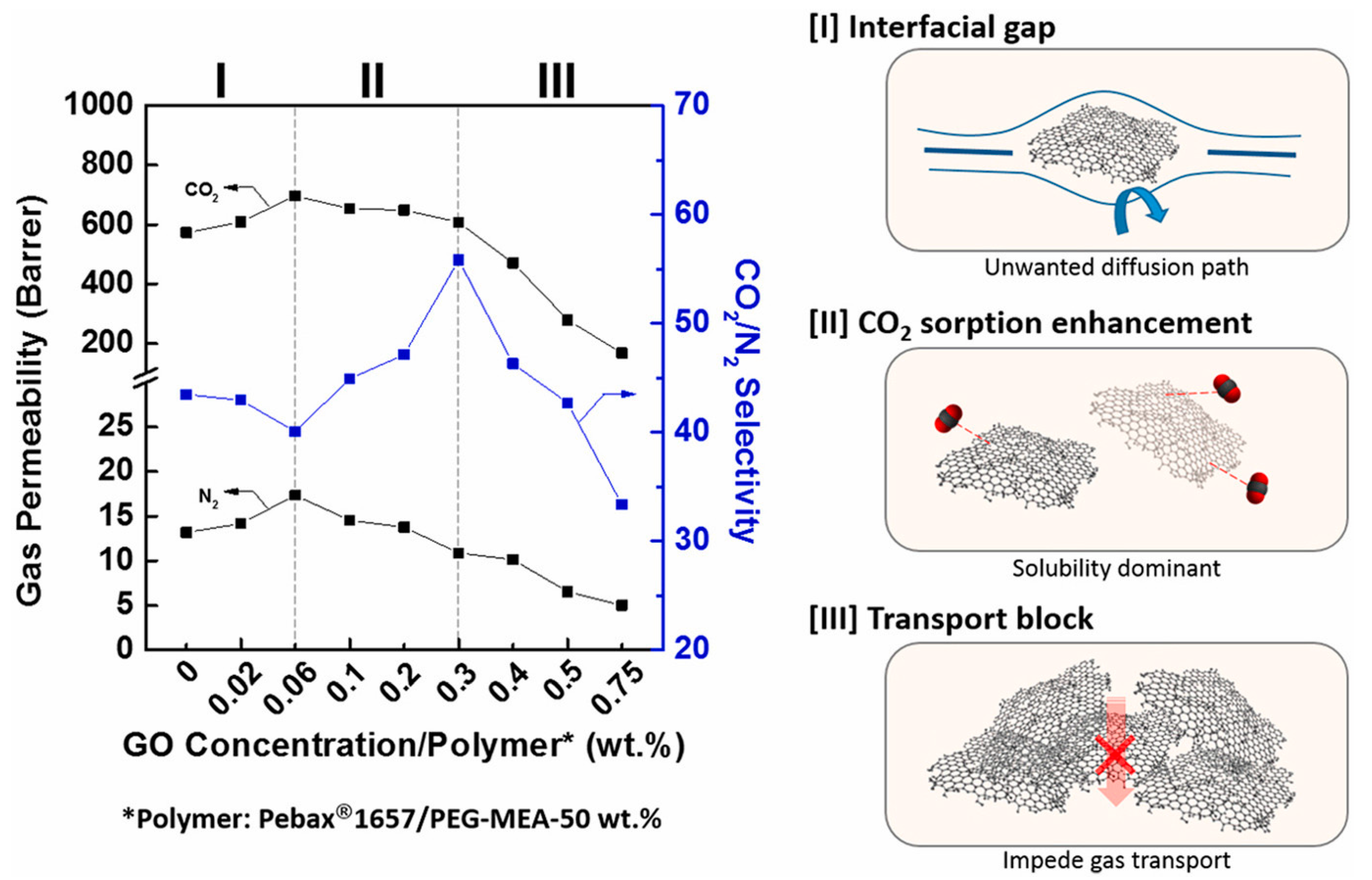
| Pebax Grade | Soft Polyether, wt% | Rigid Polyamide, wt% |
|---|---|---|
| 1657 | 40 | 60 |
| 1074 | 55 | 45 |
| 5513 | 60 | 40 |
| 2533 | 80 | 20 |
| Pebax Type | Filler Type | Filler Amount (wt%) | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | Ref. |
|---|---|---|---|---|---|---|
| 1657 | - | - | 0.103 | 107 | [5] | |
| ZIF-8 | 5 | 0.124 | 134 | |||
| ZIF-8@CNT | 5 | 0.136 | 214 | |||
| 1657 | - | - | 11.49 | 20.36 | 983 | [28] |
| 2D zinc cobaltate nanosheets (ZnCo2O4) with a thickness of about 60 nm | 0.5 | 22.57 | 15.25 | 925 | ||
| 1 | 27.39 | 13.00 | 779 | |||
| 1.5 | 41.94 | 12.84 | 702 | |||
| 2 | 57.88 | 15.04 | 757 | |||
| 2.5 | 62.51 | 13.42 | 810 | |||
| 3 | 76.35 | 17.65 | 893 | |||
| 1657 | - | - | 8.47 | 363 | [49] | |
| Imidazole-functionalized graphene oxide (ImGO) 0.5–1 μm | 0.2 | 387 | ||||
| 0.5 | 451 | |||||
| 0.8 | 13.53 | 451 | ||||
| 1657 | - | - | 201.5 | 22.6 | [50] | |
| GO | 0.2 | 231.0 | 14.1 | |||
| GO-IL | 0.2 | 214.7 | 17.3 | |||
| 1657 | - | - | 76.26 | 35.66 | 1410 | [55] |
| Fe3O4–GO, random alignment | 49.26 | 29.26 | 1090 | |||
| Fe3O4–GO, magnetic alignment/vertical | 3 | 48.31 | 28.89 | 1060 | ||
| Fe3O4–GO, magnetic alignment/horizontal | 64.2 | 31.12 | 1290 | |||
| 1657 | - | - | 44 | 7.4 | 395 | [83] |
| GO | 1 | 96 | 7.5 | 387 | ||
| 2 | 104 | 8.9 | 221 | |||
| 5 | 91 | 8.2 | 132 | |||
| MXene | 1 | 105 | 9.0 | 386 | ||
| 2 | 185 | 9.9 | 376 | |||
| 5 | 155 | 9.6 | 382 | |||
| 10 | 114 | 9.3 | 389 | |||
| 20 | 97 | 8.7 | 391 | |||
| 1657 | - | 59.42 | 18.50 | 483 | [86] | |
| LDHN | 6 | 52.17 | 18.21 | 430 | ||
| [Hmim][NTf2]@LDHN | 6 | 92.69 | 24.89 | 1262 | ||
| 1657 | - | - | 126.4 | 17.8 | 863 | [91] |
| Hollow polypyrrole (PPy) nanospheres | 0.5 | 109.5 | 12.9 | 725 | ||
| 1 | 106.2 | 12.4 | 661 | |||
| 2 | 102.1 | 12.1 | 631 | |||
| 7 | 97.9 | 11.3 | 495 | |||
| 1657 | - | 4.70 | 19.2 | [98] | ||
| ZIF-8 | 8 | 5.70 | 31.4 | |||
| ZIF-8 particles inserted in situ using multiwalled carbon tubes (MWCNTs@ZIF) | 8 | 8.25 | 74.2 | |||
| 1657 | - | 107 (dry) 80 (humid) | 8.4 (dry) 7.0 (humid) | 167 (dry) 185 (humid) | [103] | |
| Pebax–CaLS (60:1) | 102 (dry) 61 (humid) | 7.6 (dry) 5.3 (humid) | 159 (dry) 174 (humid) | |||
| Pebax–CaLS (30:1) | 95 (dry) 57 (humid) | 7.2 (dry) 5.0 (humid) | 155 (dry) 168 (humid) | |||
| Pebax–CaLS (15:1) | 92 (dry) 60 (humid) | 7.6 (dry) 5.3 (humid) | 144 (dry) 152 (humid) | |||
| Pebax–CaLS (7.5:1) | 94 (dry) 63 (humid) | 7.4 (dry) 5.0 (humid) | 129 (dry) 140 (humid) | |||
| 2533 | - | - | 7.7 | 3.5 | [105] | |
| Triglyceride (TPP) | 20 | 4.7 | 4.4 | |||
| 1657 | - | - | 234.1 | 40.6 | 488 | [7] |
| Aniline molecules | 25 | 379.1 | 43.3 | 416 | ||
| 50 | 261.7 | 28.7 | 291 | |||
| 75 | 149.2 | 25.33 | 344 |
| Pebax Type | Additive | Additive Amount (wt%) | Test Conditions | CO2 Permeability (Barrer) | CO2/N2 Selectivity (-) | CO2/CH4 Selectivity (-) | Ref. |
|---|---|---|---|---|---|---|---|
| 3000 | - | - | 25 °C, 6 bar | 39.7 | 23.3 | 13.2 | [35] |
| 1657 | 25 °C, 1 bar | 66.5 | 57.8 | 19.5 | [101] | ||
| 1657 | - | - | 25 °C, 10 bar | 65.71 | 81.9 | [94] | |
| 1657 | - | - | 30 °C, 2 bar | 106 | 41 | [84] | |
| 1657 | - | - | 30 °C, 1 bar Mixed CO2/CH4 (30/70 vol%) | 91 (Dry) 456 (Humid) | 17.2 (Dry) 20.9 (Humid) | [99] | |
| 1657 | - | - | 30 °C, 2 bar Mixed CO2/CH4 (30/70 vol%) | 95 (Dry) 470 (Humid) | 17.4 (Dry) 20.5 (Humid) | [87] | |
| 1657 | - | - | 30 °C, 2 bar Mixed CO2/CH4 (30/70 vol%) N2 as sweep gas | 90 (Dry) 490 (Humid) | 17.5 (Dry) 17 (Humid) | [18] | |
| 1657 | - | - | 30 °C, 2 bar (dry) 30 °C, 2 bar (humidified) | 89 210 | 53 38 | [83] | |
| 1657 | - | 35 °C, 5 bar | 104 | 38 | [97] | ||
| 1657 | - | - | 35 °C, 1 bar | 83 | 43 | [47] | |
| 1657 | PEG-MEA | 50 | 35 °C, 1 bar | 572 | 43 | [47] | |
| 1657 | Glycerol (Gl) | 15 | 25 °C, 10 bar | 50.42 (−23%) | 222.7 | [94] | |
| 1074 | - | - | 25 °C, 3 bar | 110.67 | 11.09 | [13] | |
| 1074 | - | - | 30 °C, 1.5 bar | 145.3 (single) 98.6 (mixed) | 19.4 (single) 33.3 (mixed) | [58] | |
| 1074 | - | - | 30 °C, 1.5 bar | 145.3 | 19.2 | [61] | |
| 2533 | - | - | 35 °C, 1 bar | 364.61 | 23.80 | [48] | |
| 2533 | - | - | 35 °C, 5 bar | 298 | 24 | [105] |
| 0 | Filler Type | Filler Amount (wt%) | Test Conditions | CO2 (Barrer 1) | Selectivity (-) | Ref. |
|---|---|---|---|---|---|---|
| 1074 | SiO2 nanoparticles | 10 | 25 °C, 3 bar | 105.94 | 26.09 (CO2/CH4) | [12] |
| 1074 | SiO2 nanoparticles (particle size 20 nm) | 8 | 25 °C, 3 bar | 152.10 | 13.28 (CO2/CH4) | [13] |
| 1657 | Non-porous SiO2 | 1 | 25 °C, 4 bar | 73.65 (+44%) | 81.82 | [17] |
| 1657 | Fumed silica (FS) (7 nm) | 10 | 25 °C, 12 bar | 72.91 | 113.92 (CO2/N2) 28.04 (CO2/CH4) | [15] |
| 1657 | Silica nanoparticle organic hybrid materials (NOHMs) Liquid-like nanoparticle (120 nm) Canopy: polyetheramine M2070 (P-NOHMs-120-(15)) | 15 | 25 °C, 2 bar dry feed gas | 246.7 | 66.4 (CO2/N2) | [16] |
| 1657 | Non-porous organosilicon nanotubes (SiNTs) | 0.5 | 30 °C, 2 bar Mixed CO2/CH4 (30/70 vol%) N2 as sweep gas | 130 (Dry) 750 (Humid) | 21 (Dry) 24 (Humid) (CO2/CH4) | [18] |
| Porous organosilicon nanotubes (PSiNTs) | 0.5 | 150 (Dry) 900 (Humid) | 23 (Dry) 25 (Humid) (CO2/CH4) | |||
| Porous organosilicon nanotubes amino-modified (N-PSiNTs) | 0.5 | 160 (Dry) 972 (Humid) | 24 (Dry) 29.2 (Humid) (CO2/CH4) | |||
| 1657 | ZnO nanoparticles | 0.5 | 25 °C, 14 bar | 140 (sim. 132.29) | 95 (sim. 96.56) (CO2/N2) 30 (sim. 29.13) (CO2/CH4) | [5] |
| 1657 | ZnO nanoparticles | 10.0 | 30 °C, 3 bar | 149.8 (+13%) | 24 (CO2/CH4) (+21%) | [26] |
| 1657/PEG400 (40 wt%) | ZnO | 4 | 25 °C, 7 bar | 94.49 | 31.58 (CO2/CH4) | [27] |
| 1657 | 2D nanosheet zinc cobaltate (ZnCo2O4) (thickness of about 60 nm) | 0.5 | 25 °C, 2 bar Mixed CO2/CH4 (10/90 vol%) | 139.10 pure (+165.67%) 118.6 mixed | 15.38 pure (CO2/CH4) (+75.57%) 32.46 mixed | [28] |
| 1657 | 2D nanosheet zinc cobaltate (ZnCo2O4) | 1 | Mixed gas, Wet | 415.96 | 31.29 (CO2/CH4) | |
| 1074 | TiO2 nanoparticles (21 nm) | 8 | 25 °C, 3 bar | 150.31 | 13.18 (CO2/CH4) | [13] |
| 1657 | TiO2 | 8 | 30 °C, 3 bar | 172.32 | 24.79 (CO2/CH4) | [29] |
| 1657 | TiO2 modified by silane grafting (AS-TiO2) | 3 | 25 °C, 20 bar | 188.6 | 84.9 (CO2/N2) | [30] |
| TiO2 modified by grafting with carboxymethyl chitosan (CMC-TiO2) | 3 | 25 °C, 20 bar | 194.6 | 82.4 (CO2/N2) | ||
| 1657 | Al2O3 | 8 | 25 °C, 3 bar | 159.27 | 24.73 (CO2/CH4) | [31] |
| 1074 | γ-Al2O3 nanoparticles (20 nm) | 8 | 25 °C, 3 bar | 163.87 | 14.24 (CO2/CH4) | [13] |
| 1657 | γ-Al2O3/ILs acidic IL-modified particles (0.5 µm) | 10 | 25 °C, 7 bar | 126 (+47%) | 101 (CO2/N2) (+ 124%) | [32] |
| γ-Al2O3/ILs basic IL-modified particles (0.5 µm) | 10 | 25 °C, 7 bar | 108 | 78 (CO2/N2) | ||
| 1657 | Fe2O3 magnetic | 1.5 | 14 bar | 165.6 | 157.25 (CO2/N2) 55.95 (CO2/CH4) | [33] |
| Pebax Type | Filler Type | Filler Amount (wt%) | Test Conditions | CO2 (Barrer 1) | Selectivity (-) | Ref. |
|---|---|---|---|---|---|---|
| 3000 | Carboxyl-functionalized single-wall carbon nanotubes (CSWCNTs) (length 30 nm; outer d 1–2 nm; inner d 0.8–1.6 nm) | 10 | 25 °C, 6 bar | 53.2 | 106.4 (CO2/N2) 31.3 (CO2/CH4) | [35] |
| 1657/PEG200 | CNT | 8 CNT 50 PEG | 25 °C, 14 bar mixed CO2/CH4 (50/50 vol%) 25 °C, 12 bar 40 °C, 12 bar | 302 (pure) 138 (mixed) 193 (mixed) | 45 CO2/CH4 (pure) 19 CO2/CH4 mixed 15.7 CO2/CH4 mixed | [36] |
| 1657 | MWCNT-NH2 (outer diameter 8–15 nm, length ∼50 µm, 0.45 wt% NH2) NMP as solvent | 6 | 30 °C, 3.5 bar | 174 | 32 (CO2/N2) | [37] |
| 6 | 45 °C, 3.5 bar | 285 | 57 (CO2/N2) | |||
| 6 | 60 °C, 3.5 bar | 405 | 51 (CO2/N2) | |||
| 1657 | Carbon nanospheres (CNs-600) (650 nm) | 0.5 | 25 °C, 4 bar mixed CO2/N2 (10/90 vol%) | 100 (pure gas) 97 (mixed gas) | 76 (pure gas) 64 (mixed gas) | [39] |
| 1657 | Nanodiamonds (ND) 5–10 nm | 0.5 | 35 °C, 2 bar feed pressure and 0.015 bar downstream | 46 | 35.5 | [40] |
| Nanodiamonds (ND) decorated with polyethyleneimine (PEI) (5–10 nm) | 0.5 | 35 °C, 2 bar feed pressure and 0.015 bar downstream | 50 | 51 | ||
| 1657 on PES support | Graphene nanoplatelets (GNP) | 0.7 | 25 °C and 4 bar | 45 (+68%) | 112 CO2/N2 (+50%), 9.9 O2/N2 (+28%) | [43] |
| 1657 on PVDF support | N-doped few-layer graphene (N-FLG) (thickness ~4 nm, ~10 layers) | 4 | Room T, 1–2 bar | 239.8 | 95.5 (CO2/N2) | [44] |
| 1657/ PEG-MEA | Graphene oxide (GO) | 0.3 GO 50 PEG-MEA | 35 °C, 1 bar | 600 | 55.8 (CO2/N2) | [47] |
| 1657 | GO sheets Medium-lateral sized (GO-M) (1–2 μm, d-spacing of 0.8 nm) | 0.1 | single: 25 °C, 3 bar mixed: 25 °C, 1 bar CO2/N2 (50/50 vol%) | 95 Single gas 75 Mixed dry 110 Mixed humid | 85 Single gas (CO2/N2) 72 Mixed dry 80 Mixed humid | [46] |
| 1657 | GO (sheet size: 500–1000 nm, thickness: 1.5–2.0 nm) | 1 | 30 °C, 2 bar (dry) | 110 | 67 (CO2/N2) | [83] |
| 10 | 30 °C, 2 bar (humidified) | 420 | 64 (CO2/N2) | |||
| 1657 | Imidazole-functionalized graphene oxide (ImGO) (0.5–1 μm) | 0.8 | 25 °C, 8 bar | 76.2 | 105.5 (CO2/N2) (+46.0%) | [49] |
| 1657 on PVDF support | Ionic-Liquid-functionalized graphene oxide (GO-IL) | 0.2 | 25 °C, 4 bar | 143 | 79.4 (CO2/N2) 13.8 (CO2/H2) | [50] |
| 1657 on PSf support | Aminated partially reduced GO nanofiller (A-prGO) | 0.1 | Room T, 4 bar | 47.5 | 105.6 (CO2/N2) 23.75 (CO2/CH4) | [52] |
| 1657 | Aminosilane-functionalized GO Nanosheets (f-GO) | 0.9 | 35 °C, 2 bar, humidified mixed CO2/N2 (20/80 vol%) mixed CO2/CH4 (30/70 vol%) | 934.3 | 71.1 (CO2/N2) 40.9 (CO2/CH4) | [53] |
| 1657 | GO nanosheets | 0.1 | 25 °C | 107 (4 bar) 117 (10 bar) | 104 (4 bar), 77 (10 bar) (CO2/N2) 22 (4 bar), 26 (10 bar) CO2/CH4 | [54] |
| 1657 | GO nanosheets modified by polypyrrole (GO-PPy) (100 to 200 nm) | 0.1 | 25 °C | 100 (4 bar) 122 (10 bar) | 107 (4 bar), 123 (10 bar) CO2/N2 (+62%) 22.5 (4 bar), 30 (10 bar) CO2/CH4 (+51%) | [54] |
| 1657 | GO nanosheets modified by polypyrrole and zinc cations (GO-PPy-Zn) | 0.1 | 25 °C | 118 (4 bar) 131 (10 bar) | 83 (4 bar), 119 (10 bar) CO2/N2 (+58%) 24 (4 bar), 30 (10 bar) CO2/CH4 (+56%) | [54] |
| 1657 | Covalently grafted polyetheramine (M2070)-carbon nanotube solvent-free hybrid nanofluids (CNTs NF) | 30 | 25 °C, 1.0 bar Dry mixed CO2/N2 (20/80 vol%) | 225 (pure 2 bar) 180 (mixed 2 bar) 332 (mixed 1 bar) (+442%) | 61 (pure 2 bar) 60 (mixed 2 bar) 72 (mixed 1 bar) (+77%) CO2/N2 | [38] |
| Covalently grafted polyetheramine (M2070)-graphene oxide solvent-free hybrid nanofluids (GO NF) | 15 | 25 °C, 1.0 bar Dry mixed CO2/N2 (20/80 vol%) | 150 (pure 2 bar) 140 (mixed 2 bar) 248 (mixed 1 bar) | 52 (pure 2 bar) 48 (mixed 2 bar) 56 (mixed 1 bar) CO2/N2 | ||
| 1657 | Graphite oxide flakes functionalized with iron oxide (Fe3O4–GO) magnetic alignment/vertical | 3 | 25 °C, 2 bar Mixed gas CO2/CH4 or CO2/N2 (10/90 vol%) | 538 | 75 (CO2/N2) 47 (CO2/CH4) | [55] |
| 2533 | GO | 0.02 | 35 °C, 1 bar | 371.39 | 24.00 (CO2/N2) | [48] |
| Porous (PGO) | 0.02 | 35 °C, 1 bar | 397.35 | 23.75 (CO2/N2) | ||
| Polyetheramine-functionalized graphene oxide (PEAGO) | 0.02 | 35 °C, 1 bar | 380.44 | 24.19 (CO2/N2) |
| Pebax Type | Filler Type | Filler Amount (wt%) | Test Conditions | CO2 (Barrer 1) | Selectivity (-) | Ref. |
|---|---|---|---|---|---|---|
| 1657 | MFI nanosheets | 5 | 25 °C, 2 bar | 188.9 (+63.5%) | 29.9 (CO2/CH4) (+76.4%) | [62] |
| 5 | 25 °C, 2 bar Mixed CO2/CH4 (50/50 vol%) | 159.1 (+63.5%) | 27.4 (CO2/CH4) (+76.4%) | |||
| 1657 | NaX (mean particle size 55 nm) | 2 | 25 °C, 4 bar | 50.70 | from 61.53 to 107.13 (CO2/N2) 6.06 (O2/N2) | [17] |
| 1657 | NaY | 40 | 30 °C, 2 bar | 131.8 | 130.8 (CO2/N2) | [57] |
| 1657 on PES support | NaX (40–90 nm) | 1.5 | 25 °C, 6 bar | 95 | 100 (CO2/N2), 32 (CO2/CH4) 3 (N2/CH4) | [59] |
| NaX-COOH (40–90 nm) | 1.5 | 25 °C, 6 bar | 187.76 | 288.86 (CO2/N2), 57.41 (CO2/CH4) 5.03 (N2/CH4) | ||
| 1074/PEG | NaY (1.7 μm) | 30 NaY and 20 PEG | 30 °C, 1.5 bar Mixed CO2/N2 (15/85 vol%) | 172.6 (single) 140.1 (mixed) | 107.9 (single) 166.7 (mixed) CO2/N2 | [58] |
| 1074 | DD3R (ca. 10 μm) | 5 | 30 °C, 5 barg | 120 | 31 (CO2/CH4) | [60] |
| 1074 | SAPO (0.45 μm) | 5 | 30 °C, 1.5 bar | 98.2 | 72.0 (CO2/N2) | [61] |
| Pebax Type | Filler Type | Filler Amount (wt%) | Test Conditions | CO2 (Barrer 1) | Selectivity (-) | Ref. |
|---|---|---|---|---|---|---|
| 1657 on PAN support | CuBTC (range size distribution 60–500 nm) | 35 | 25 °C, 12 bar Mixed CO2/CH4 (10/90 vol%) | 94.4 | 18.84 (CO2/CH4) | [78] |
| ZIF-67 (mean size around 400 nm) | 15 | 25 °C, 12 bar Mixed CO2/CH4 (10/90 vol%) | 42.2 | 17.36 (CO2/CH4) | ||
| ZIF-8 (mean size < 100 nm) | 35 | 25 °C, 12 bar Mixed CO2/CH4 (10/90 vol%) | 94.4 | 20.35 (CO2/CH4) | ||
| 1657 | ZIF-8 (90 nm) | 5 | 20 °C, 1 bar | 99.7 (+25%) | 59.6 (CO2/N2) (+25%) | [65] |
| 1657 | ZIF-8 (mean particle size 65 nm) | 2 | 25 °C, 4 bar | 112.65 (+120%) | 108.20 | [17] |
| 1657 | ZIF-8 | 5 | 35 °C, 5 bar | 165 | 44 (CO2/N2) | [97] |
| 1657 | ZIF-8 | 8 | 35 °C, 5 bar | 175 | 55 (CO2/N2) | [98] |
| 1657 | ZIF-8 | 2 | 30 °C, 1 bar Mixed CO2/CH4 (30/70 vol%) | 102 (Dry) 949 (Humid) | 17.3 (Dry) 24.1 (Humid) (CO2/CH4) | [99] |
| 1657 | NH2-ZIF-8(10) | 6 | 25 °C, 1 bar | 163.8 (+107.6%) | 62 (CO2/N2) (+27%) | [66] |
| 2533/ Pluronic P123 (surfactant) | ZIF-8 (Basolite® Z1200, 1300–1800 m2/g; diameter (D50) = 4.9 μm) | 5 ZIF-8 2.5 P123 | 45 °C, 4 bar | 328 | 19.5 (CO2/N2) | [67] |
| 1657 | Zeolitic imidazolate framework cuboid (ZIF-C) nanosheets (thickest ZIF-C nanosheet, 170 nm) | 20 | 25 °C, 2 bar Mixed CO2/N2 (10/90 vol%) wet (RH = 100%) | 387.2 | 47.1 (CO2/N2) | [68] |
| 1657 | 2D imidazole framework hydrophilically modified (hZIF-L) (leaf-like shapes, 5.9 × 2.4 nm) | 5 | 25 °C, 2 bar Mixed gas, wet | 502.44 | 33.82 (CO2/CH4) | [70] |
| 1657 | 2-D MIL-96(Al) (150 nm) | 25 | 25 °C, 2 bar Mixed CO2/N2 (15/85 vol%) | 55 (+25%) | 67.5 (CO2/N2) (+18%) | [71] |
| 1657 | 3-D ZIF-94 | 25 | 25 °C, 2 bar Mixed CO2/N2 (15/85 vol%) | 58.5 (+33%) | 63 (CO2/N2) | |
| 1657/ PEG 400 | NH2-MIL125 | 12 MOF 40 PEG | 25 °C, 2 bar Mixed CO2/CH4 (10/90 vol%) | 190.03 (pure) 183.11 (mixed) | 24.84 (pure) 17.05 (mixed) (CO2/CH4) | [73] |
| NH2-MIL125 | 12 MOF 40 PEG | 25 °C, 8 bar Mixed CO2/CH4 (10/90 vol%) | 304.76 (pure) 285.45 (mixed) | 32.84 (pure) 23.17 (mixed) (CO2/CH4) | ||
| 1657 | Bio-ZIF-12 | 12 | 25 °C, 2 bar Mixed CO2/CH4 (20/80 vol%), wet | 542 | 40 (CO2/CH4) | [75] |
| 1657 | Honeycomb-structured UiO-66 (15 nm) | 10 | 20 °C, 3 bar | 97.5 (+44.7%) | 79.2 (CO2/N2) 22.1 (CO2/CH4) | [76] |
| Honeycomb-structured amino-functionalized MOF UiO-66-NH2 (15 nm) | 10 | 20 °C, 3 bar | 118.3 (+49.4%) | 56.6 (+71.7%) (CO2/N2) 30.5 (+34.5%) (CO2/CH4) | ||
| 1657 | NOTT-300 (800 nm–1 µm) | 40 | 25 °C, 10 bar | 395 (+380%) single 356 (mixed CO2/N2) 340 (mixed CO2/CH4) | 61.2 CO2/N2 (+26%) 36.3 CO2/CH4 (+68%) single 58.36 CO2/N2 (mixed) 33.24 CO2/CH4 (mixed) | [74] |
| Pebax Type | Filler Type | Filler Amount (wt%) | Test Conditions | CO2 (Barrer 1) | Selectivity (-) | Ref. |
|---|---|---|---|---|---|---|
| 1657 | MXene (lateral dimension: 1–2 μm; thickness: 1–2 nm) | 1 | 30 °C, 2 bar (dry) | 148 | 63 (CO2/N2) | [83] |
| 10 | 30 °C, 2 bar (humidified) | 584 | 59 (CO2/N2) | |||
| 1657 | Layered double hydroxides (LDHs) | 2 | 30 °C, 1 bar Mixed CO2/CH4 (30/70 vol%) | 98.6 (Dry) 619 (Humid) | 18.5 (Dry) 28.2 (Humid) (CO2/CH4) | [99] |
| 1657 | Layered double hydroxide nanocage (LDHN) | 6 | Mixed CO2/CH4 (10/90 vol%) humidified | 426 | 18 | [86] |
| Ionic liquid-decorated layered double hydroxide nanocage ([Hmim][NTf2]@LDHN) | 6 | Mixed CO2/CH4 (10/90 vol%) humidified | 644 | 34 (CO2/CH4) | ||
| 1657 | LDHs (lateral dimension 150–200 nm) | 2 | 30 °C, 2 bar Mixed CO2/CH4 (30/70 vol%) | 104 (Dry) | 19.1 (Dry) 28 (Humid) (CO2/CH4) | [87] |
| 740 (Humid) | ||||||
| 1657 | Exfoliation-free laminates’ LDH intercalated with amino acids’ hydrophobic phenylalanine (Phe-LDH) (lateral dimension 100–150 nm) | 5 | 101 (Dry) 760 (Humid) | 20.1 (Dry) 36.1 (Humid) (CO2/CH4) | ||
| 1657 | Exfoliation-free laminates’ LDH intercalated with amino acids’ hydrophilic glutamic acid (Glu-LDH) (lateral dimension 100–150 nm) | 5 | 109 (Dry) 790 (Humid) | 19.8 (Dry) 37.7 (Humid) (CO2/CH4) |
| Pebax Type | Filler Type | Filler Amount (wt%) | Test Conditions | CO2 (Barrer 1) | Selectivity (-) | Ref. |
|---|---|---|---|---|---|---|
| 1657 | Nanoadsorbent from oil palm frond (OPF) waste | 5 | 25 °C, 2 bar | 1475.09 | 40.48 (CO2/CH4) | [95] |
| 1657 | Covalent organic frameworks (COFs) COF-5 | 0.4 | 30 °C, 1 bar | 493 | 49.3 (CO2/N2) | [88] |
| 1657 | Hollow polypyrrole (PPy) nanospheres | 1 | 35 °C, 2 bar | 274 | 40.1 (CO2/N2) 12.8 (CO2/CH4) | [91] |
| 1657/ PEGDME (50/50 wt/wt) | Anion-pillared hybrid ultramicroporous materials GEFSIX-2-Cu-i (Average pore size 3.60 Å; from 200 to 1000 nm) | 1 | 35 °C, 4 bar | 460 | 57 (CO2/N2) (+9.6%), 18 (CO2/CH4) (+24.1%) 17 (CO2/H2) (+12.2%) | [92] |
| 1657/Glycerol | Cu nanoparticles | Gl 15/ Cu 1.5 | 25 °C, 10 bar | 63.6 | 200 | [94] |
| 2533 | Amino acid ionic liquids @polymers of intrinsic microporosity (core-shell) composite nanoparticles (AAILs@PIM (core-shell) CNPs) (25–30 nm) | 25 | 65 °C, 2 bar | 400 | 33 (CO2/N2) | [93] |
| Pebax Type | Filler Type | Filler Amount (wt%) | Test Conditions | CO2 (Barrer 1) | Selectivity (-) | Ref. |
|---|---|---|---|---|---|---|
| 1657 | ZIF-8@CNT | 5 | 35 °C, 5 bar | 225.5 | 48.9 (CO2/N2) | [97] |
| 1657 | MWCNTs@ZIF | 8 | 35 °C, 5 bar | 158 | 49 (CO2/N2) | [98] |
| 1657 | ZIF-8 particles in-situ inserted by multiwalled carbon tubes (MWCNTs@ZIF) | 8 | 35 °C, 5 bar | 186.3 | 61.3 (CO2/N2) | [98] |
| 1657 | Heterostructured filler— in-situ growth of ZIF-8 on LDH surface (ZIF-8@LDH) | 2 | 30 °C, 1 bar Mixed CO2/CH4 (30/70 vol%) | 122 (Dry) 1307 (Humid) | 19.2 (Dry) 31.6 (Humid) (CO2/CH4) | [99] |
| 1657 | M-Xene/SiO2 | 0.2/0.8 | 30 °C, 2 bar | 216 (+104%) | 61 (CO2/N2) (+49%) | [84] |
| 1657 | M-Xene/HNTs | 0.2/0.8 | 168 | 51 (CO2/N2) | ||
| 1657 | GO/HNTs | 0.5/0.5 | 245 (+153%) | 71 (CO2/N2) (+72%) |
| Pebax Type | Filler Type | Filler Amount (wt%) | Test Conditions | CO2 (Barrer 1) | Selectivity (-) | Ref. |
|---|---|---|---|---|---|---|
| 2533 | Triglyceride (TPP) | 20 | 35 °C, 5 bar | 566 | 25 (CO2/N2) | [105] |
| 2533 | Tween21 | 65 | 25 °C and 0.6 atm | 221 | 32.0 (CO2/N2) | [100] |
| Tween20 | 65 | 25 °C and 0.6 atm | 267 | 36.6 (CO2/N2) | ||
| Tween80 | 65 | 25 °C and 0.6 atm | 289 | 40.70 (CO2/N2) | ||
| 1657 | Tween20 | 50 | 25 °C, 1 bar | 144 | 50.7 (CO2/N2) 14.1 (CO2/CH4) | [101] |
| Tween80 | 50 | 25 °C, 1 bar | 167 | 47.9 (CO2/N2) 13.8 (CO2/CH4) | ||
| 1657 | Calcium lignosulfonate (CaLS) | Pebax/CaLS(15:1) | 25 °C, 3 bar Dry | 133 | 69 (CO2/N2) 23 (CO2/CH4) | [103] |
| Pebax/CaLS(15:1) | 25 °C, 3 bar Humid | 3585 | 29 (CO2/CH4) 71 (CO2/N2) | |||
| Pebax/CaLS(15:1) | 85 °C, 3 bar Mixed CO2/N2 (10/90 vol%) humid | 7480 | 42 (CO2/N2) | |||
| 1657 | Aniline | 50 | 25 °C, 7 bar | 151 (+76%) | 92.5 (CO2/N2) (+101%) | [7] |
| 50 | 25 °C, 7 bar Mixed CO2/N2 (20/80 vol %) | 123.12 (+48%) | 68.34 (+262%) | |||
| 5513 | KBF4 | 0.0045 | 2 bar | 36.8 GPU (CO2 permeance) | 27.6 (CO2/N2) | [104] |
| Pebax Type | Filler Type | Filler Amount (wt%) | Test Conditions | CO2 Permeance (GPU 1) | Selectivity (-) | Ref. |
|---|---|---|---|---|---|---|
| 1657 on PAN support with amino-PDMS gutter layer | - | - | 20 °C, 5 barg | 350 | 50 (CO2/N2) | [115] |
| 1657/PEG-DME on PAN support with amino-PDMS gutter layer | - | - | 20 °C, 5 barg | 400 | 65 (CO2/N2) | |
| 1657 on PVDF support | Ionic-Liquid-functionalized graphene oxide (GO-IL) | 0.05 | 25 °C, 4 bar | 905 (+50%) | 44.8 (CO2/N2) (+ over 90%) 5.8 (CO2/H2) | [50] |
| 1657 on P84 support | UiO-66 | 10 | 35 °C, 5 bar Mixed CO2/CH4 (50/50 vol%) | 11.5 | 55.6 (CO2/CH4) | [64] |
| 1657 on PAN support | MOF-801 nanocrystal 400–500 nm (3 spin coating cycles) | 7.5 | 20 °C, 1 bar Mixed CO2/N2 (50/50 vol%) | 22.4 | 66 (CO2/N2) | [77] |
| 1657 on PAN support | 2D Mxene Nanosheets | 0.15 | 25 °C, 2 bar | 21.6 | 72.5 (CO2/N2) | [82] |
| 2533 on polypropylene (PP) hollow fiber supports | UiO-66-NH2 (dip coating) | 10 | 25 °C, 2 bar | 26 | 37 (CO2/N2) | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarizia, G.; Bernardo, P. A Review of the Recent Progress in the Development of Nanocomposites Based on Poly(ether-block-amide) Copolymers as Membranes for CO2 Separation. Polymers 2022, 14, 10. https://doi.org/10.3390/polym14010010
Clarizia G, Bernardo P. A Review of the Recent Progress in the Development of Nanocomposites Based on Poly(ether-block-amide) Copolymers as Membranes for CO2 Separation. Polymers. 2022; 14(1):10. https://doi.org/10.3390/polym14010010
Chicago/Turabian StyleClarizia, Gabriele, and Paola Bernardo. 2022. "A Review of the Recent Progress in the Development of Nanocomposites Based on Poly(ether-block-amide) Copolymers as Membranes for CO2 Separation" Polymers 14, no. 1: 10. https://doi.org/10.3390/polym14010010
APA StyleClarizia, G., & Bernardo, P. (2022). A Review of the Recent Progress in the Development of Nanocomposites Based on Poly(ether-block-amide) Copolymers as Membranes for CO2 Separation. Polymers, 14(1), 10. https://doi.org/10.3390/polym14010010







