Dissipative Particle Dynamics Study on Interfacial Properties of Symmetric Ternary Polymeric Blends
Abstract
1. Introduction
2. Methods
2.1. Model
2.2. Simulation Details
3. Results and Discussion
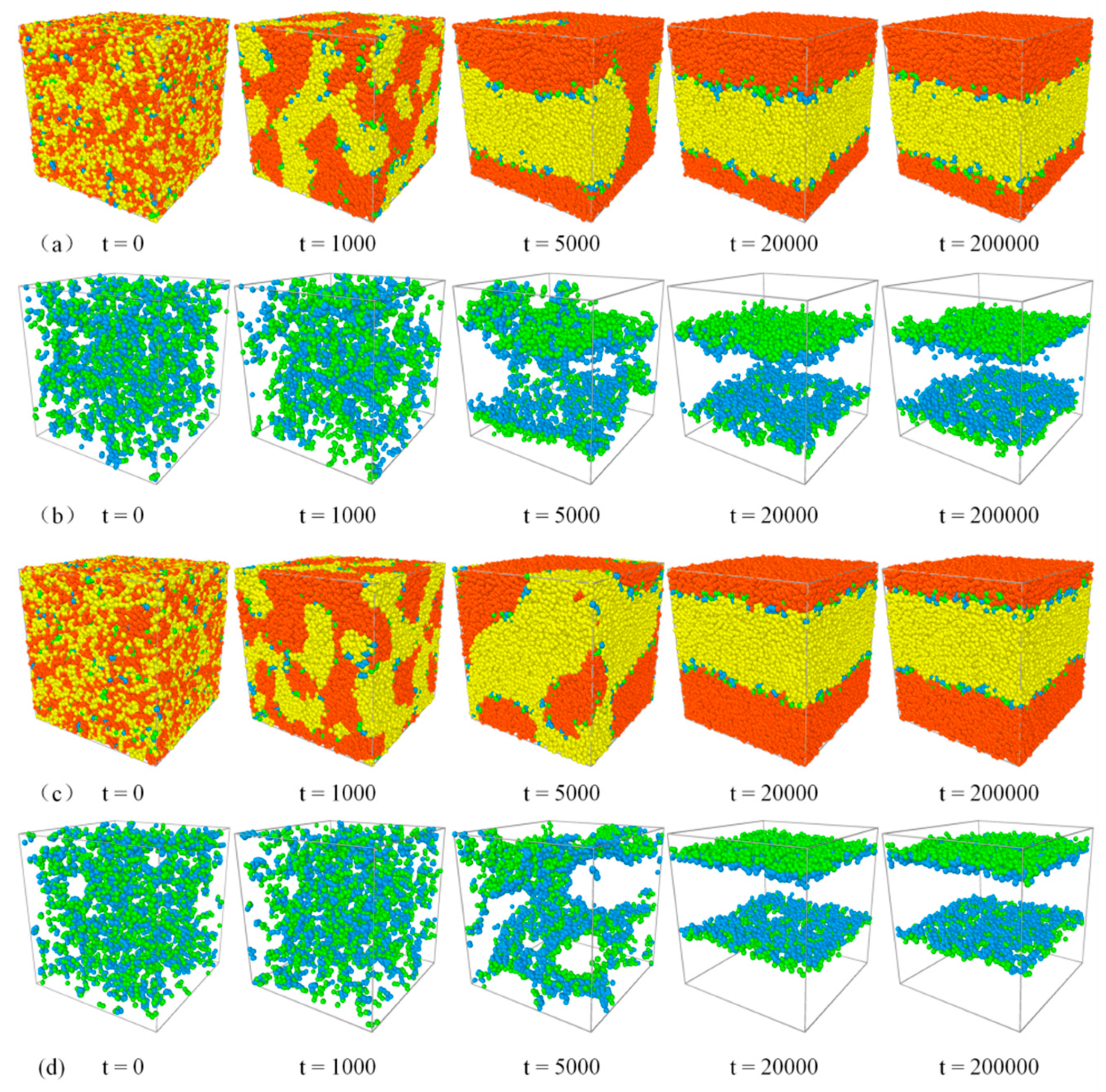
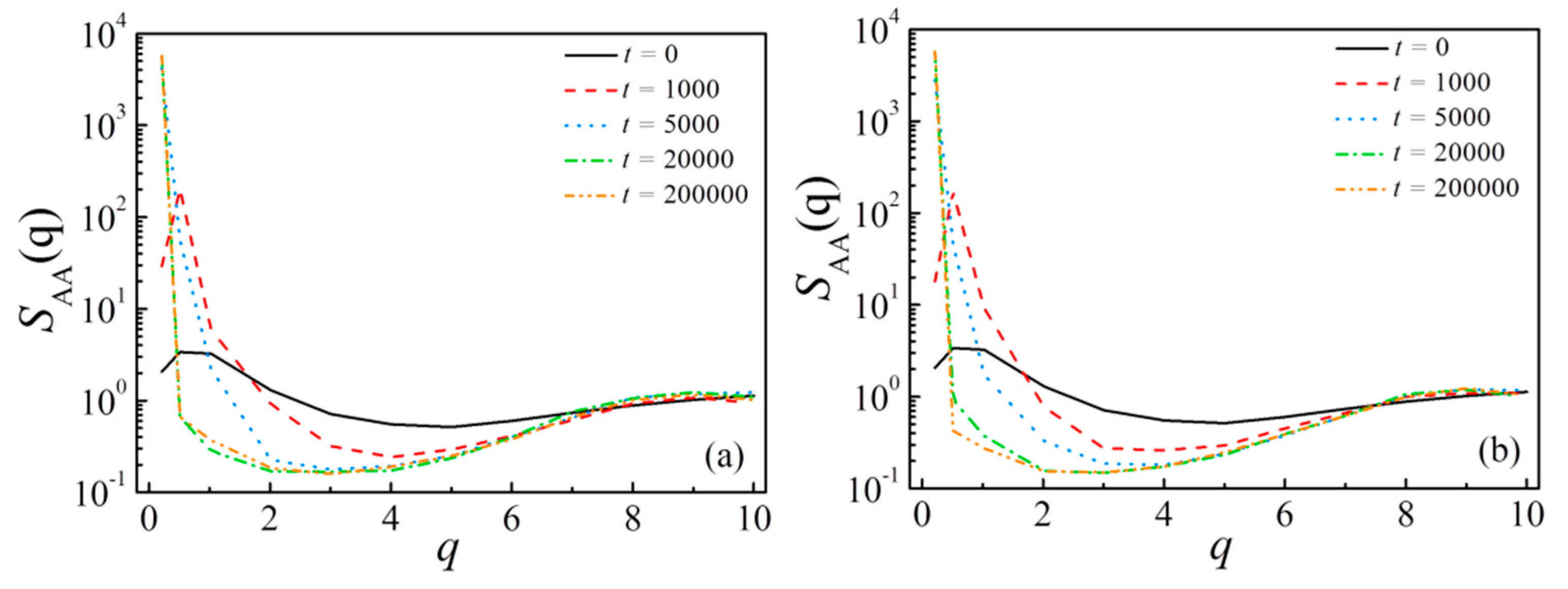
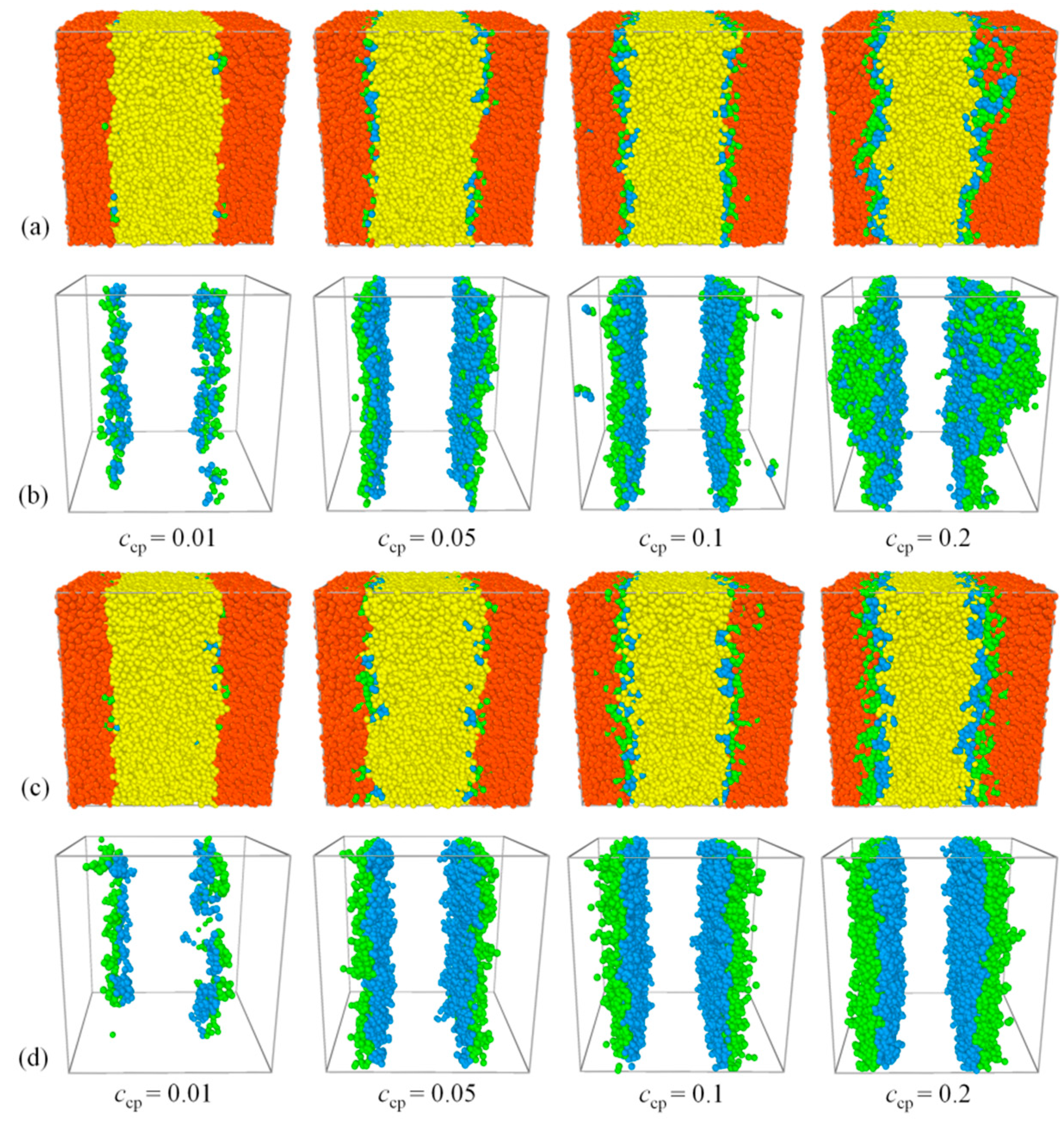
3.1. Evolution of Phase Separation
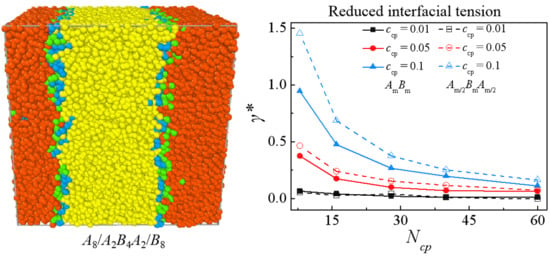
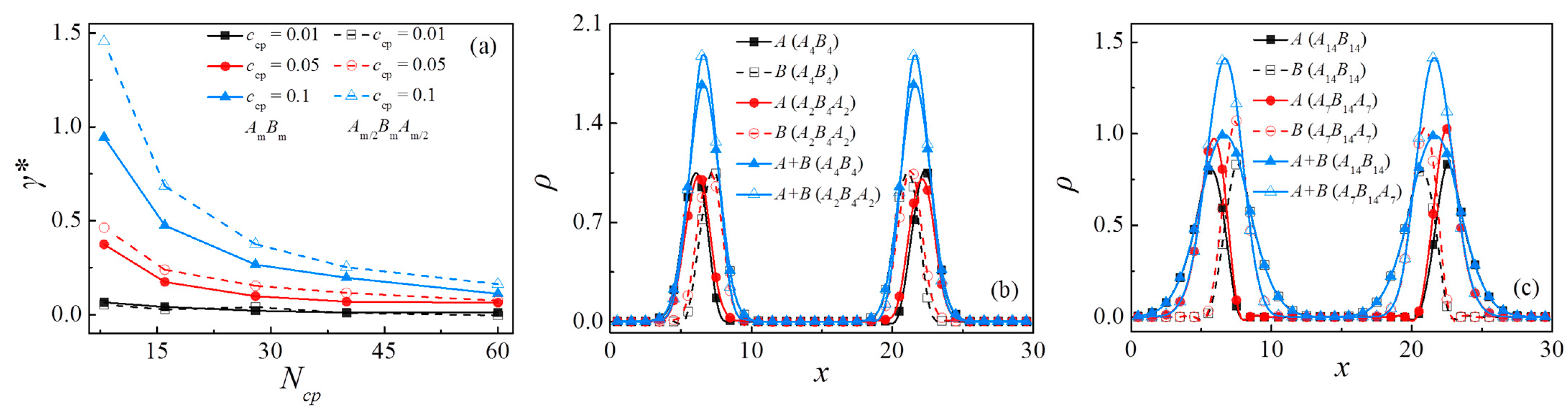
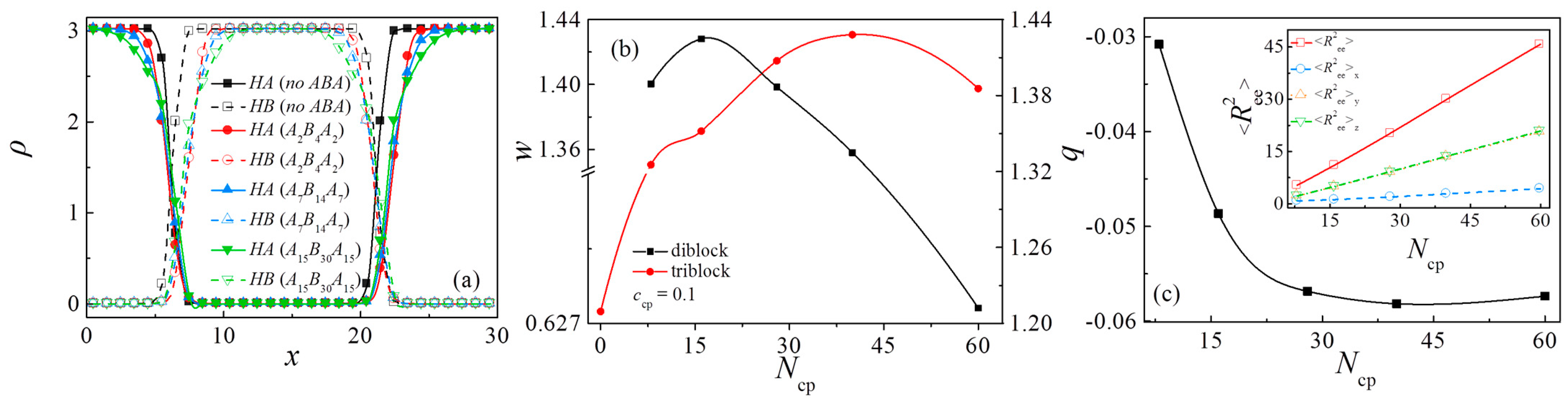
3.2. Comparison between An/AmBm/Bn and An/Am/2BmAm/2/Bn Blends
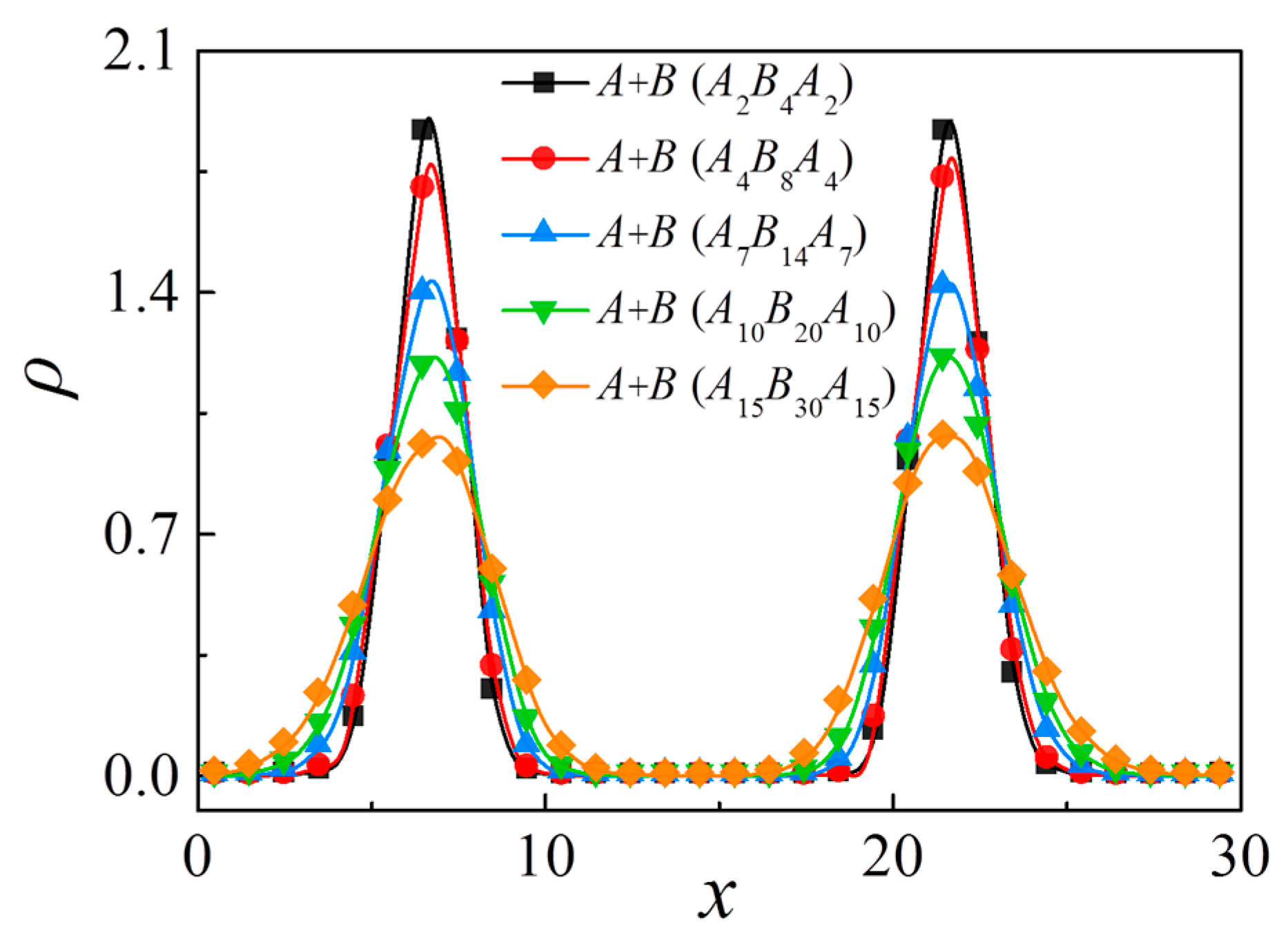
3.3. Effect of Chain Length of Triblock Copolymers
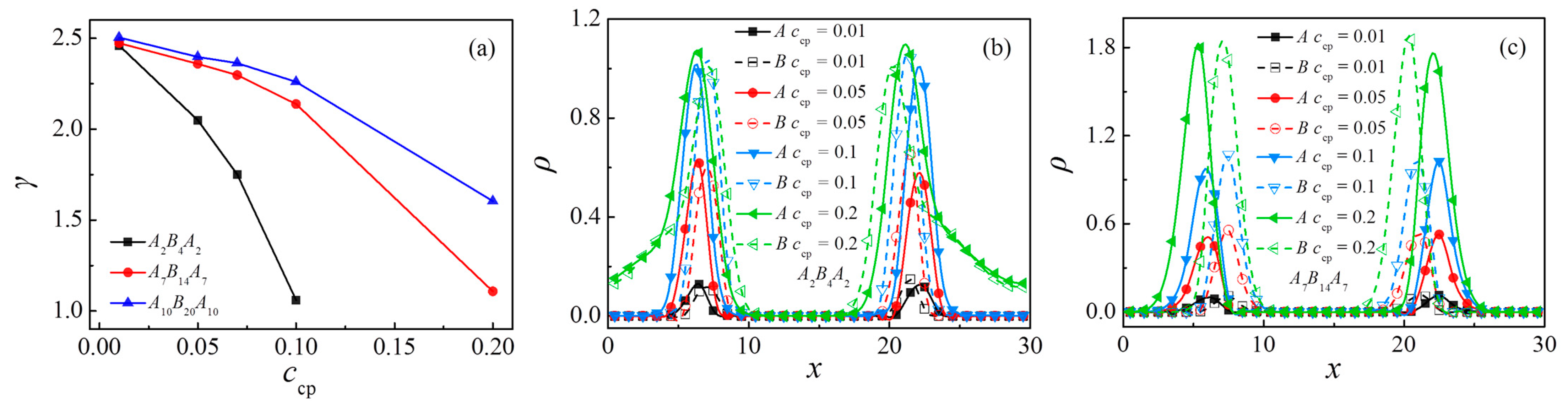
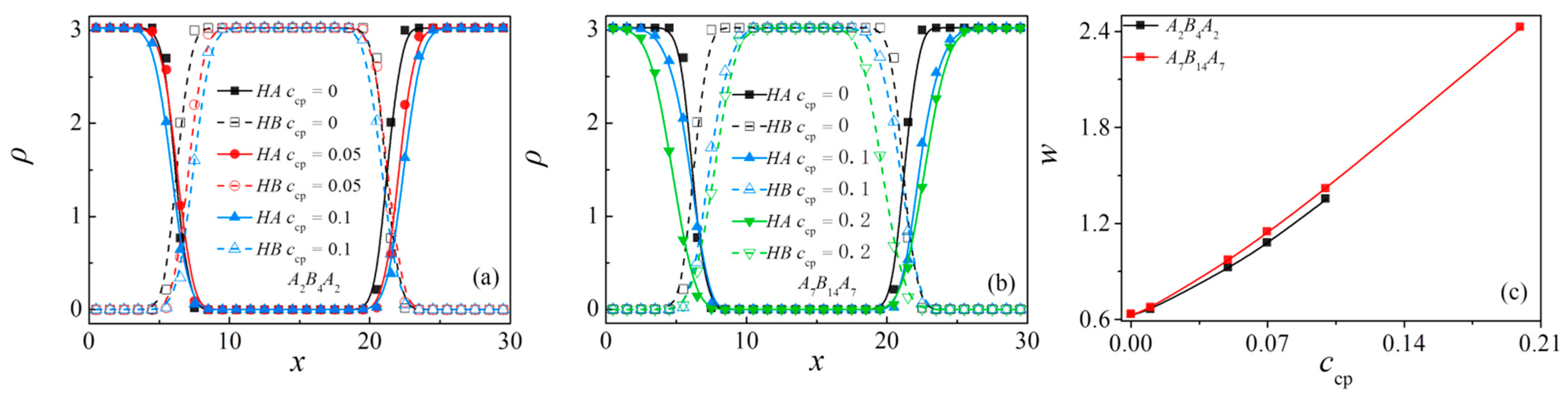
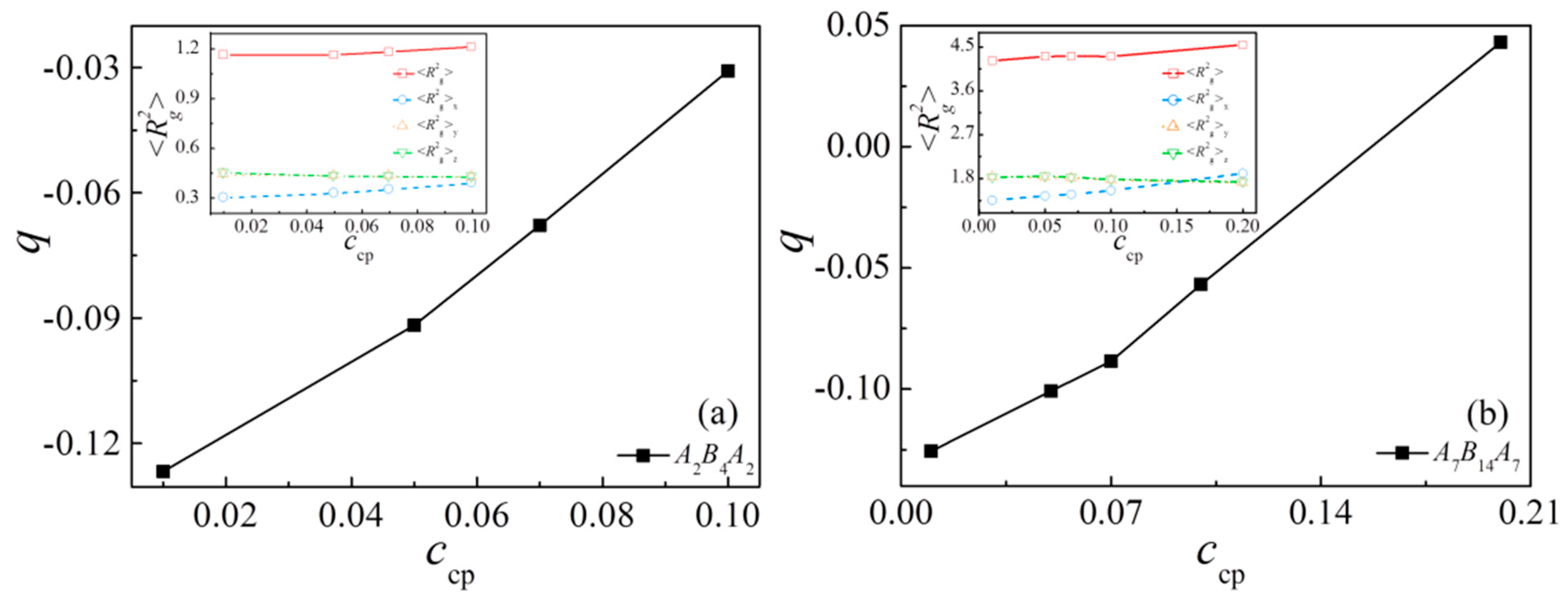
3.4. Effect of Concentration of Triblock Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.F.; Muller, M.; Wang, Z.G. Nucleation in A/B/AB blends: Interplay between microphase assembly and macrophase separation. J. Chem. Phys. 2009, 130, 154902. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Macosko, C.W.; Morse, D.C. Ultralow interfacial tensions of polymer/polymer interfaces with diblock copolymer surfactants. Macromolecules 2007, 40, 3819–3830. [Google Scholar] [CrossRef]
- Retsos, H.; Anastasiadis, S.; Pispas, S.; Mays, J.; Hadjichristidis, N. Interfacial Tension in Binary Polymer Blends in the Presence of Block Copolymers. 2. Effects of Additive Architecture and Composition. Macromolecules 2004, 37, 524–537. [Google Scholar] [CrossRef]
- Guo, H.X.; Cruz, M.O. A computer simulation study of the segregation of amphiphiles in binary immiscible matrices: Short asymmetric copolymers in short homopolymers. J. Chem. Phys. 2005, 123, 17490. [Google Scholar] [CrossRef]
- Qian, H.J.; Lu, Z.Y.; Chen, L.J.; Li, Z.S.; Sun, C.C. Dissipative particle dynamics study on the interfaces in incompatible A/B homopolymer blends and with their block copolymers. J. Chem. Phys. 2005, 122, 184907. [Google Scholar] [CrossRef] [PubMed]
- Kancharla, S.; Zoyhofski, N.A.; Bufalini, L.; Chatelais, B.F.; Alexandridis, P. Association between Nonionic Amphiphilic Polymer and Ionic Suifactant in Aqueous Solutions: Effect of Polymer Hydrophobicity and Micellization. Polymers 2020, 12, 1831. [Google Scholar] [CrossRef] [PubMed]
- Lemos, T.; Abreu, C.; Pinto, J.C. DPD Simulations of Homopolymer-Copolymer-Homopolymer Mixtures: Effects of Copolymer Structure and Concentration. Macromol. Theory Simul. 2020, 29. [Google Scholar] [CrossRef]
- Russell, T.P.; Mayes, A.M.; Deline, V.R.; Chung, T.C. Hairpin configurations of triblock copolymers at homopolymer interfaces. Macromolecules 1992, 25, 5783–5789. [Google Scholar] [CrossRef]
- Wagner, M.; Wolf, B.A. Effect of block copolymers on the interfacial-tension between 2. Immiscible/homopolymers. Polymer 1993, 34, 1460–1464. [Google Scholar] [CrossRef]
- Jorzik, U.; Wagner, M.; Wolf, B.A. Effect of block copolymer architecture on the interfacial tension between immiscible polymers. Prog. Colloid. Polym. Sci. 1996, 101, 170–171. [Google Scholar]
- Welge, I.; Wolf, B.A. Reduction of the interfacial tension between ‘immiscible’ polymers: To which phase one should add a compatibilizer. Polymer 2001, 42, 3467–3473. [Google Scholar] [CrossRef]
- Harrats, C.; Fayt, R.; Jérme, R.; Blacher, S. Stabilization of a cocontinuous phase morphology by a tapered diblock or triblock copolymer in polystyrene-rich low-density polyethylene/polystyrene blends. J. Polym. Sci. Pt. B Polym. Phys. 2003, 41, 202–216. [Google Scholar] [CrossRef]
- Fayt, R.; Jerome, R.; Teyssie, P. Characterization and control of interfaces in emulsified incompatible polymer blends. Polym. Eng. Sci. 1987, 27, 328–334. [Google Scholar] [CrossRef]
- Dai, K.H.; Washiyama, J.; Kramer, E.J. Segregation study of a bab triblock copolymer at the a/b homopolymer interface. Macromolecules 1994, 27, 4544–4553. [Google Scholar] [CrossRef]
- Hlavata, D.; Horak, Z.; Fort, V. Localization of styrene-butadiene block copolymers in polystyrene/polypropylene blends. Polym. Eng. Sci. 1996, 6, 15–19. [Google Scholar]
- Xu, Y.; Thurber, C.M.; Macosko, C.W.; Lodge, T.P.; Hillmyer, M.A. Poly(methyl methacrylate)-block-polyethylene-block-poly(methyl methacrylate) Triblock Copolymers as Compatibilizers for Polyethylene/Poly(methyl methacrylate) Blends. Ind. Eng. Chem. Res. 2014, 53, 4718–4725. [Google Scholar] [CrossRef]
- Sun, Z.Q.; Zhang, B.; Bian, X.C. Synergistic effect of PLA-PBAT-PLA tri-block copolymers with two molecular weights as compatibilizers on the mechanical and rheological properties of PLA/PBAT blends. RSC Adv. 2015, 5, 73842–73849. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Huang, Y.J.; Kong, M.Q.; Li, G.X. Assessment of compatibilization efficiency of SEBS in the PP/PS blend. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Peter, C.; Kremer, K. Multiscale simulation of soft matter systems. Faraday Discuss. 2010, 144, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.C.; Li, B.H. Self-assembly of diblock copolymers under confinement. Soft Matter 2013, 9, 1398–1413. [Google Scholar] [CrossRef]
- Balazs, A.C.; Siemasko, C.P.; Lantman, C.W. Monte-Carlo simulations for the behavior of multiblock copolymers at a penetrable interface. J. Chem. Phys. 1991, 94, 1653–1663. [Google Scholar] [CrossRef]
- Wang, Y.M.; Li, Y.; Mattice, W.L. Simulation of the adsorption of unsymmetric diblock copolymers at the interface between the 2 monomeric homopolymers. J. Chem. Phys. 1993, 99, 4068–4075. [Google Scholar] [CrossRef]
- Schmid, F.; Müller, M. Quantitative comparison of self-consistent-field theories for polymers near interfaces with monte-carlo simulations. Macromolecules 1995, 28, 8639–8645. [Google Scholar] [CrossRef]
- Müller, M.; Schick, M. Bulk and interfacial thermodynamics of a symmetric, ternary homopolymer-copolymer mixture: A Monte Carlo study. J. Chem. Phys. 1996, 105, 8885–8901. [Google Scholar] [CrossRef]
- Werner, A.; Schmid, F.; Binder, K.; Müller, M. Diblock copolymers at a homopolymer-homopolymer interface: A Monte Carlo simulation. Macromolecules 1996, 29, 8241–8248. [Google Scholar] [CrossRef]
- Liu, D.M.; Duan, X.Z.; Shi, T.F.; Jiang, F.; Zhang, H.Z. Monte Carlo Simulation of Effects of Homopolymer Chain Length on Interfacial Properties of A/AB/B Ternary Polymer Blends. Chem. J. Chin. Univ. Chin. 2015, 36, 2532–2539. [Google Scholar]
- Liu, D.M.; Dai, L.J.; Duan, X.Z.; Shi, T.F.; Zhang, H.Z. Monte Carlo Simulation of Interfacial Properties in Homopolymer/Diblock Copolymer/Homopolymer Ternary Polymer Blends. Chem. J. Chin. Univ. Chin. 2015, 36, 1752–1758. [Google Scholar]
- Chen, L.; Xiao, S.; Zhu, H.; Wang, L.; Liang, H. Shape-dependent internalization kinetics of nanoparticles by membranes. Soft Matter 2016, 12, 2632–2641. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, D.; He, L.; Zhang, L. Entropic Interactions in Semiflexible Polymer Nanocomposite Melts. J. Phys. Chem. B 2016, 120, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Li, Z.Z.; Gu, X.P.; Feng, L.F.; Zhang, C.L.; Hu, G.H. A dissipative particle dynamics study on the compatibilizing process of immiscible polymer blends with graft copolymers. Polymer 2012, 53, 4448–4454. [Google Scholar] [CrossRef]
- Anastasiadis, S.H.; Gancarz, I.; Koberstein, J.T. Interfacial-tension of immiscible polymer blends - temperature and molecular-weight dependence. Macromolecules 1988, 21, 2980–2987. [Google Scholar] [CrossRef]
- Zhu, P.F.; Li, Y.; Li, Q.W. Mesoscopic Simulation of the Interfacial Behavior of Biosurfactant Rhamnolipids and the Synergistic Systems. Acta. Chim. Sin. 2011, 69, 2420–2426. [Google Scholar]
- Catarino Centeno, R.; Bustamante-Rendon, R.A.; Hernandez-Fragoso, J.S.; Arroyo-Ordonez, I.; Perez, E.; Alas, S.J.; Gama Goicochea, A. Surfactant chain length and concentration influence on the interfacial tension of two immiscible model liquids: A coarse-grained approach. J. Mol. Model. 2017, 23, 306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Xu, J.B.; He, X.F. Effect of surfactants on the deformation of single droplet in shear flow studied by dissipative particle dynamics. Mol. Phys. 2018, 116, 1851–1861. [Google Scholar] [CrossRef]
- Liang, X.P.; Wu, J.Q.; Yang, X.G. Investigation of oil-in-water emulsion stability with relevant interfacial characteristics simulated by dissipative particle dynamics. Colloid Surf. A-Physicochem. Eng. Asp. 2018, 546, 107–114. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yang, S.W.; Wang, R.C. Dissipative particle dynamics study on the temperature dependent interfacial tension in surfactant-oil-water mixtures. J. Pet. Sci. Eng. 2018, 169, 81–95. [Google Scholar] [CrossRef]
- Goodarzi, F.; Zendehboudi, S. Effects of Salt and Surfactant on Interfacial Characteristics of Water/Oil Systems: Molecular Dynamic Simulations and Dissipative Particle Dynamics. Ind. Eng. Chem. Res. 2019, 58, 8817–8834. [Google Scholar] [CrossRef]
- Goodarzi, F.; Kondori, J.; Rezaei, N.; Zendehboudi, S. Meso- and molecular-scale modeling to provide new insights into interfacial and structural properties of hydrocarbon/water/surfactant systems. J. Mol. Liq. 2019, 295, 111357. [Google Scholar] [CrossRef]
- Huo, J.H.; Jiang, H.; Chen, Z.; Zhou, J. Homoporous polymer membrane via forced surface segregation: A computer simulation study. Chem. Eng. Sci. 2018, 191, 490–499. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, T.L.; Chen, Z.; Huo, J.H.; Zhang, L.Z.; Zhou, J. Computer Simulations on Double Hydrophobic PS-b-PMMA Porous Membrane by Non-solvent Induced Phase Separation. Fluid Phase Equilib. 2020, 523, 112784. [Google Scholar] [CrossRef]
- Groot, R.D.; Warren, P.B. Dissipative particle dynamics: Bridging the gap between atomistic and mesoscopic simulation. J. Chem. Phys. 1997, 107, 4423–4435. [Google Scholar] [CrossRef]
- Lin, Y.; Boker, A.; He, J.B.; Sill, K.; Xiang, H.Q.; Abetz, C.; Li, X.F.; Wang, J.; Emrick, T.; Long, S.; et al. Self-directed self-assembly of nanoparticle/copolymer mixtures. Nature 2005, 434, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.H.; Xiao, N.; Li, L.; Xie, X.N. Investigation of nanoemulsion interfacial properties: A mesoscopic simulation. J. Food Eng. 2020, 276. [Google Scholar] [CrossRef]
- Zhang, J.W.; Chen, L.; Wang, A.L.; Yan, Z.C. Dissipative Particle Dynamics Simulation of Ionic Liquid-Based Microemulsion: Quantitative Properties and Emulsification Mechanism. Ind. Eng. Chem. Res 2020, 59, 763–773. [Google Scholar] [CrossRef]
- Hoogerbrugge, P.J.; Koelman, J. Simulating microscopic hydrodynamic phenomena with dissipative particle dynamics. Europhys. Lett. 1992, 19, 155–160. [Google Scholar] [CrossRef]
- Koelman, J.; Hoogerbrugge, P.J. Dynamic simulations of hard-sphere suspensions under steady shear. Europhys. Lett. 1993, 21, 363–368. [Google Scholar] [CrossRef]
- Jananl, S.; Lisa, M.H. Impact of ion content and electric field on mechanical properties of coarse-grained ionomers. J. Chem. Phys. 2018, 149, 163313. [Google Scholar]
- Irving, J.H.; Kirkwood, J.G. The statistical mechanical theory of transport processes .4. the equations of hydrodynamics. J. Chem. Phys. 1950, 18, 817–829. [Google Scholar] [CrossRef]
- Hillmyer, M.A.; Maurer, W.W.; Lodge, T.P.; Bates, F.S.; Almdal, K. Model bicontinuous microemulsions in ternary homopolymer block copolymer blends. J. Phys. Chem. B 1999, 103, 4814–4824. [Google Scholar] [CrossRef]
- Stoykovich, M.P.; Edwards, E.W.; Solak, H.H.; Nealey, P.F. Phase behavior of symmetric ternary block copolymer-homopolymer blends in thin films and on chemically patterned surfaces. Phys. Rev. Lett. 2006, 97, 147802. [Google Scholar] [CrossRef]
- Liu, B.L.; Hu, B.; Du, J.; Cheng, D.M.; Zang, H.Y.; Ge, X.; Tan, H.Q.; Wang, Y.H.; Duan, X.Z.; Jin, Z.; et al. Precise Molecular-Level Modification of Nafion with Bismuth Oxide Clusters for High-performance Proton-Exchange Membranes. Angew. Chem.Int. Edit. 2021, 60, 6076–6085. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Gong, K.; Lin, Y.; Liu, T.; Liu, Y.; Duan, X. Dissipative Particle Dynamics Study on Interfacial Properties of Symmetric Ternary Polymeric Blends. Polymers 2021, 13, 1516. https://doi.org/10.3390/polym13091516
Liu D, Gong K, Lin Y, Liu T, Liu Y, Duan X. Dissipative Particle Dynamics Study on Interfacial Properties of Symmetric Ternary Polymeric Blends. Polymers. 2021; 13(9):1516. https://doi.org/10.3390/polym13091516
Chicago/Turabian StyleLiu, Dongmei, Kai Gong, Ye Lin, Tao Liu, Yu Liu, and Xiaozheng Duan. 2021. "Dissipative Particle Dynamics Study on Interfacial Properties of Symmetric Ternary Polymeric Blends" Polymers 13, no. 9: 1516. https://doi.org/10.3390/polym13091516
APA StyleLiu, D., Gong, K., Lin, Y., Liu, T., Liu, Y., & Duan, X. (2021). Dissipative Particle Dynamics Study on Interfacial Properties of Symmetric Ternary Polymeric Blends. Polymers, 13(9), 1516. https://doi.org/10.3390/polym13091516