Dyeing Para-Aramid Textiles Pretreated with Soybean Oil and Nonthermal Plasma Using Cationic Dye
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
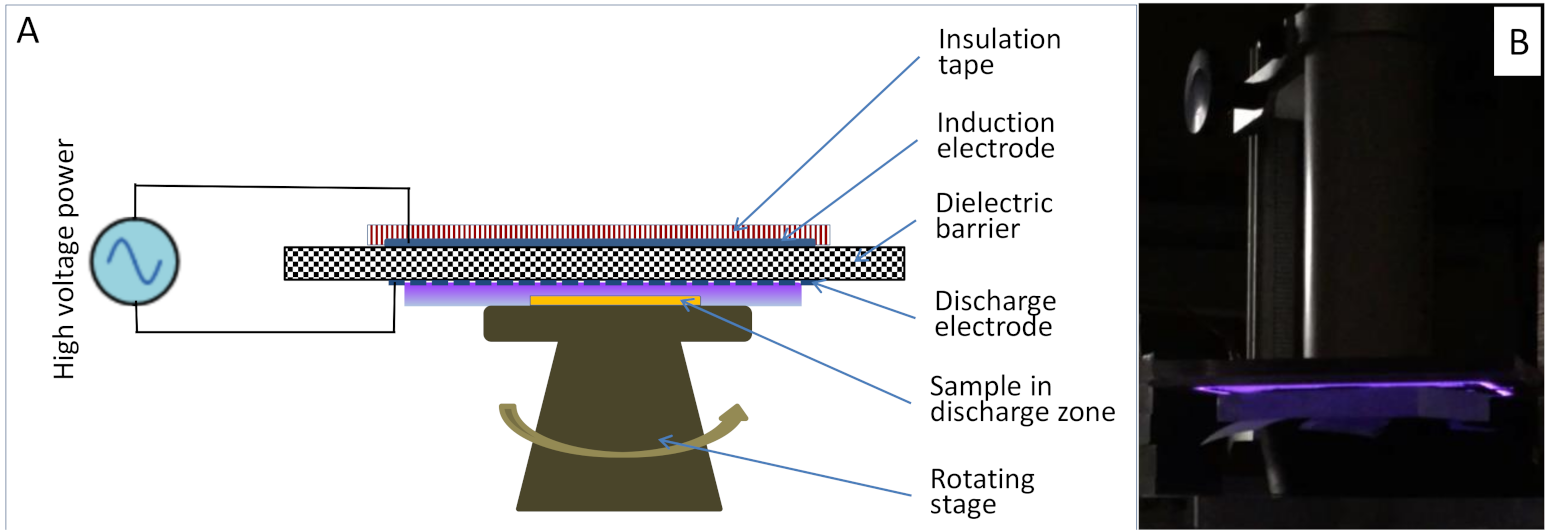
2.2. Experimental Methods
2.3. Pretreatment (Acetic Acid, Soybean Oil, NTP)
2.4. Dyeing Experimental Procedures
2.5. Color Strength Analysis
2.6. FTIR Analysis
3. Results and Discussion
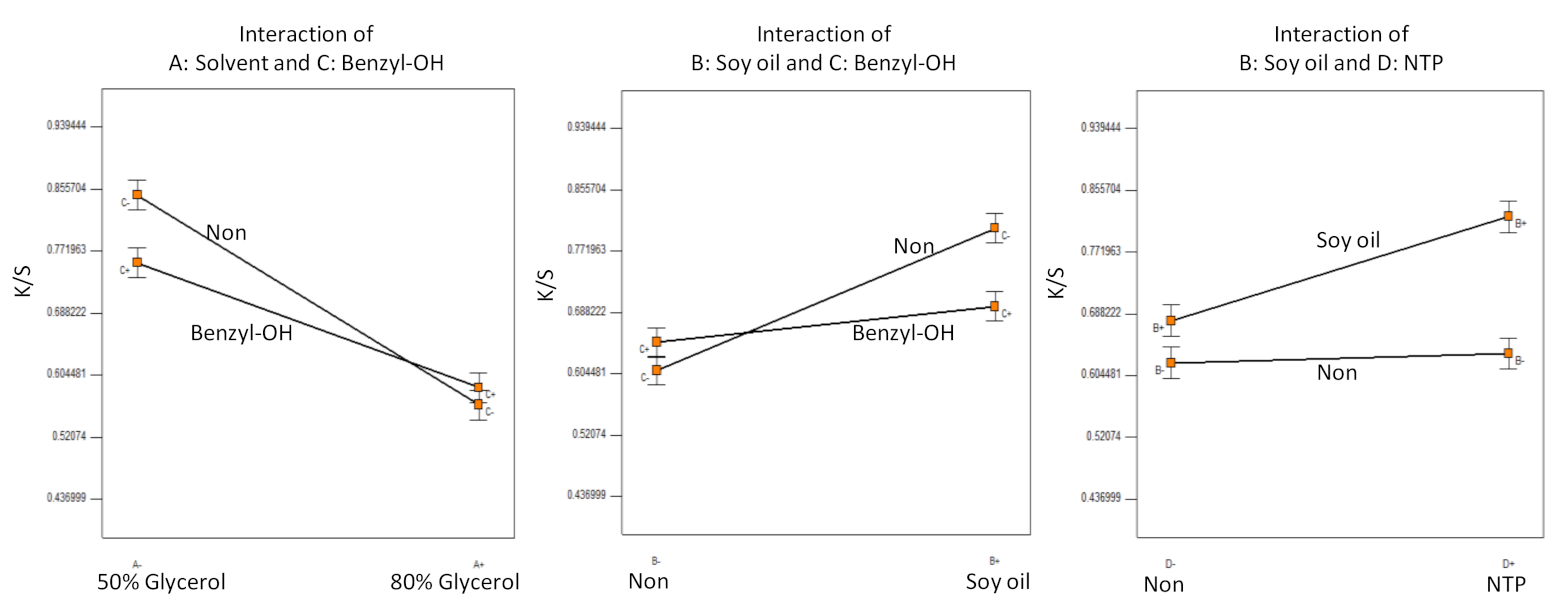
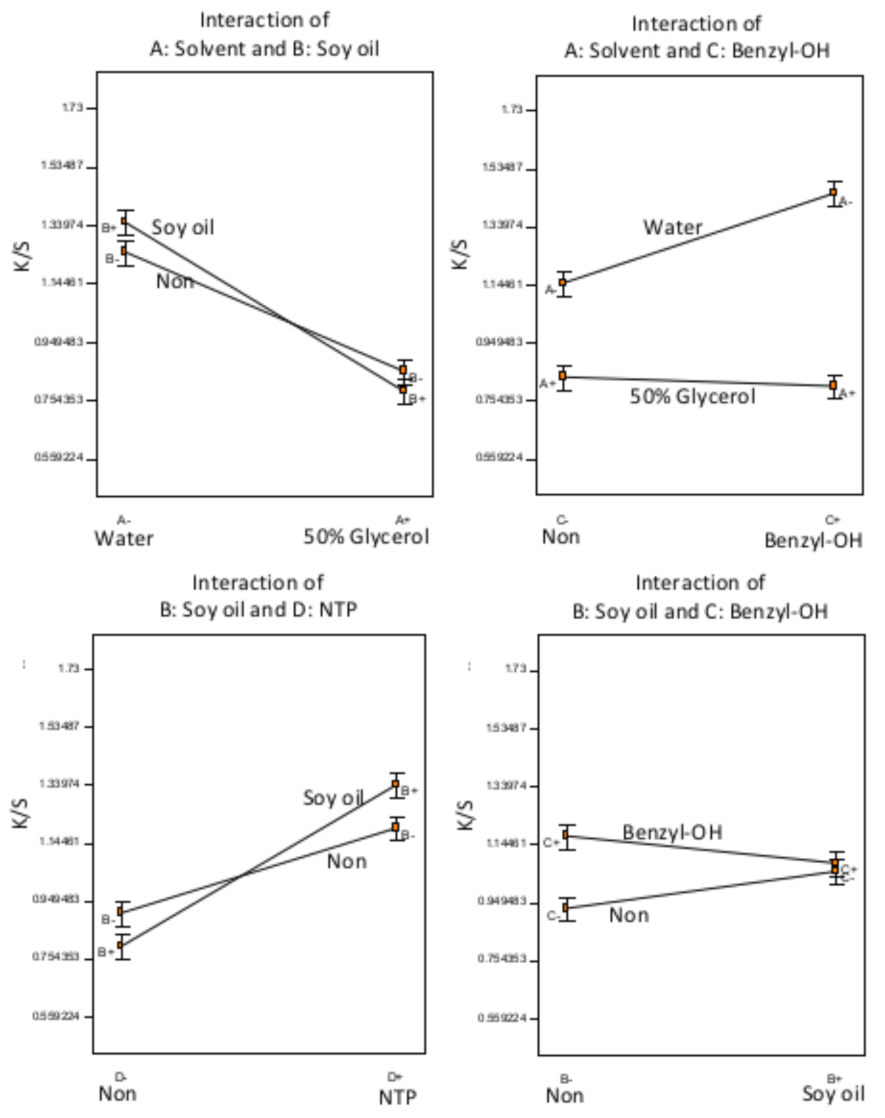
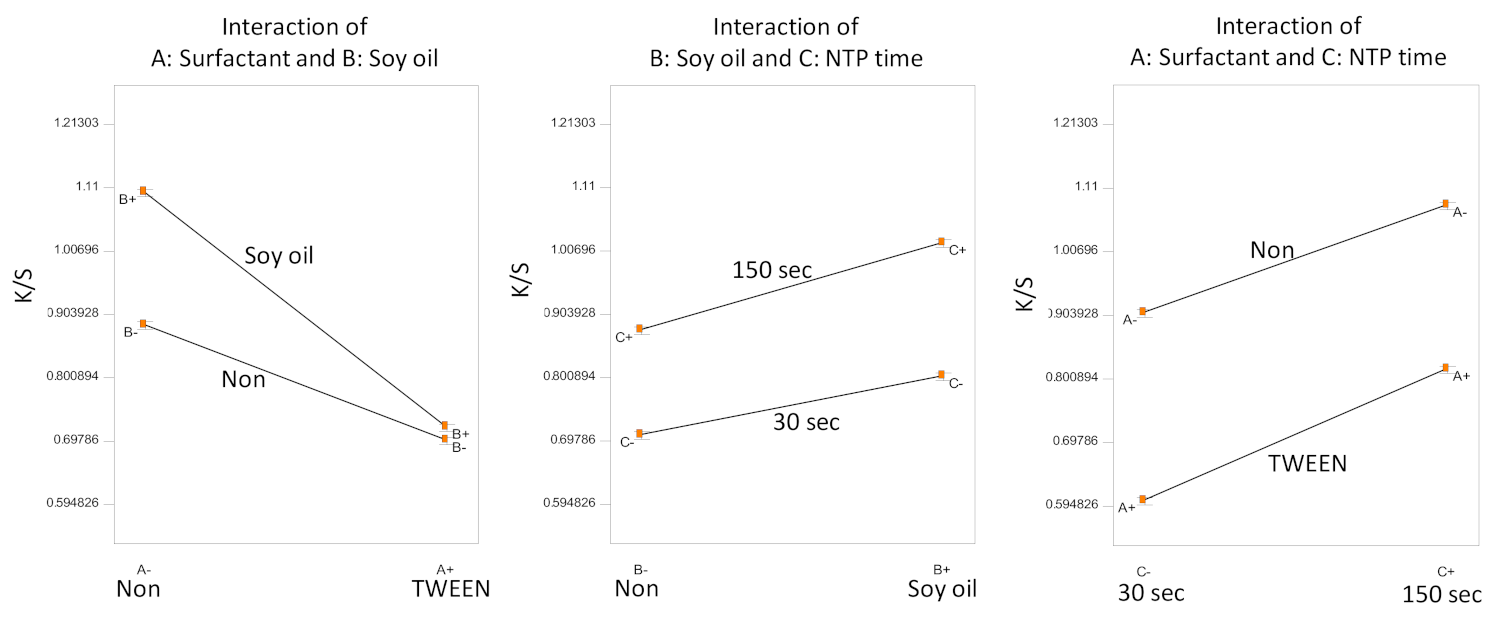
3.1. Experiments A–F: Analysis
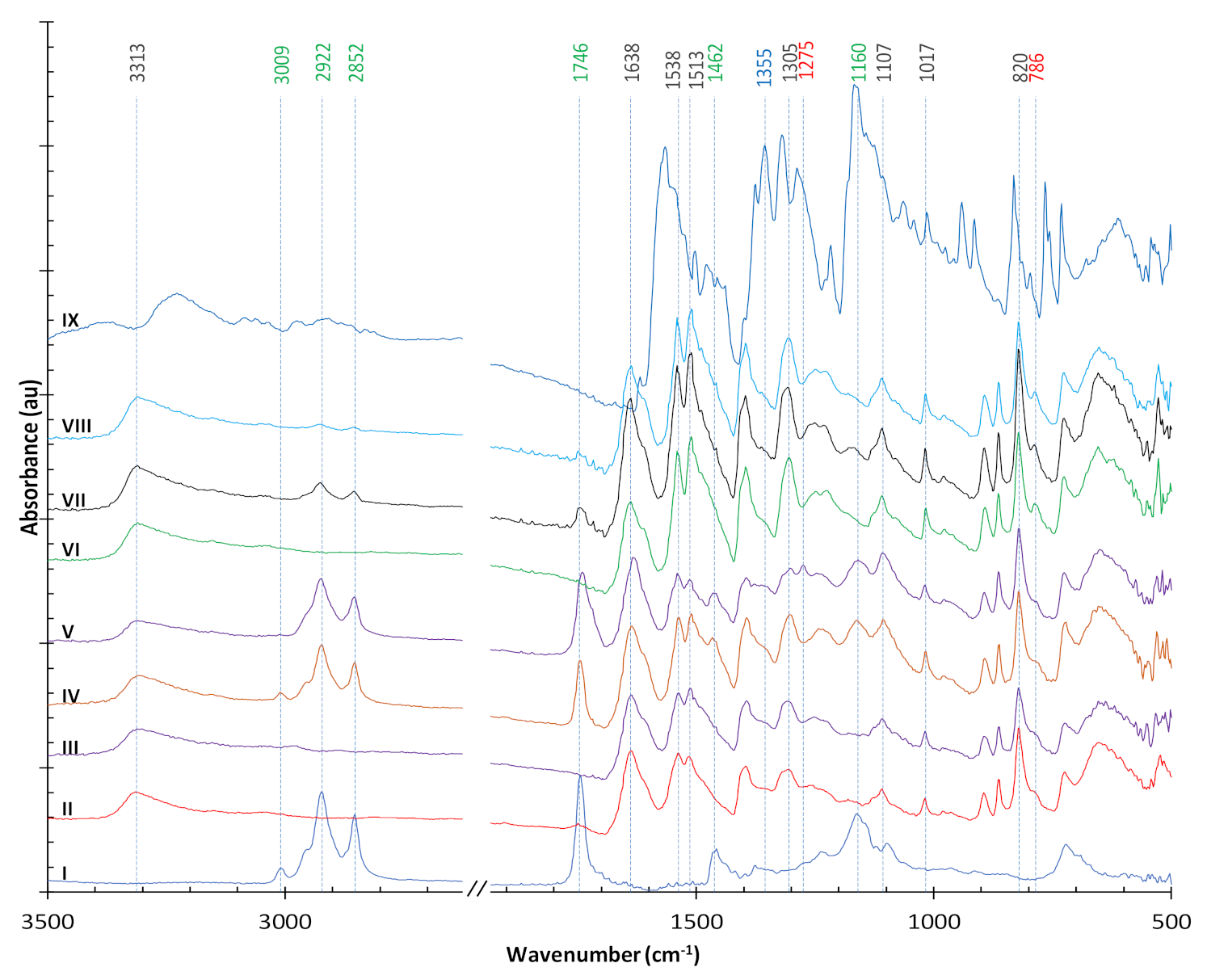
3.2. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roenbeck, M.R.; Sandoz-Rosado, E.J.; Cline, J.; Wu, V.; Moy, P.; Afshari, M.; Reichert, D.; Lustig, S.R.; Strawhecker, K.E. Probing the internal structures of Kevlar® fibers and their impacts on mechanical performance. Polymer 2017, 128, 200–210. [Google Scholar] [CrossRef]
- Zhao, J. Effect of surface treatment on the structure and properties of para-aramid fibers by phosphoric acid. Fibers Polym. 2013, 14, 59–64. [Google Scholar] [CrossRef]
- Xia, D.; Wang, L.J. Sulfuric Acid Treatment of Aramid Fiber for Improving the Cationic Dyeing Performance. Adv. Mater. Res. 2012, 627, 243–247. [Google Scholar] [CrossRef]
- Vassiliadis, A.A.; Roulia, M.; Boussias, C.M. Disperse Dyeing Systems for p-aramid Fibers. In Proceedings of the 37th International Symposium on Novelties in Textiles, Ljubljana, Slovenia, 15–17 June 2006. [Google Scholar]
- Dong, Y.; Jang, J. The enhanced cationic dyeability of ultraviolet/ozone-treated meta-aramid fabrics. Color. Technol. 2011, 127, 173–178. [Google Scholar] [CrossRef]
- Jelil, R.A. A review of low-temperature plasma treatment of textile materials. J. Mater. Sci. 2015, 50, 5913–5943. [Google Scholar] [CrossRef]
- Vu, N.; Michielsen, S. Near room temperature dyeing of m-aramid fabrics. J. Appl. Polym. Sci. 2019, 136, 48190. [Google Scholar] [CrossRef]
- Han, S.Y.; Jaung, J.Y. Acid dyeing properties of meta-aramid fiber pretreated with PEO45-MeDMA derived from [2-(methacryloyloxy)ethyl] trimethylammonium chloride. Fibers Polym. 2009, 10, 461–465. [Google Scholar] [CrossRef]
- Xi, M.; Li, Y.-L.; Shang, S.-Y.; Li, D.-H.; Yin, Y.-X.; Dai, X.-Y. Surface modification of aramid fiber by air DBD plasma at atmospheric pressure with continuous on-line processing. Surf. Coat. Technol. 2008, 202, 6029–6033. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, Q.; Chi, H.; Zhang, Y.; Shi, Y.; Fang, D.; Li, F. The application of gas plasma technologies in surface modification of aramid fiber. Fibers Polym. 2014, 15, 1–7. [Google Scholar] [CrossRef]
- Su, M.; Gu, A.; Liang, G.; Yuan, L. The effect of oxygen-plasma treatment on Kevlar fibers and the properties of Kevlar fibers/bismaleimide composites. Appl. Surf. Sci. 2011, 257, 3158–3167. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Eltaweel, F.; Elsisi, H. Water-free Dyeing of Polyester and Nylon 6 Fabrics with Novel 2-Oxoacetohydrazonoyl Cyanide Derivatives under a Supercritical Carbon Dioxide Medium. Fibers Polym. 2018, 19, 887–893. [Google Scholar] [CrossRef]
- Kaul, B.L. Mass pigmentation and solution dyeing of synthetic fibres. In Synthetic Fibre Dyeing; Hawkyard, C., Ed.; Society of Dyers and Colourists: Bradford, West Yorkshire, UK, 2004; p. 230. [Google Scholar]
- Kim, E.-M.; Choi, J.-H. Dyeing Properties and Color. Fastness of 100% meta-Aramid Fiber. Fibers Polym. 2011, 12, 484–490. [Google Scholar] [CrossRef]
- Ferrero, F.; Periolatto, M.; Rovero, G.; Giansetti, M. Alcohol-assisted dyeing processes: A chemical substitution study. J. Clean. Prod. 2011, 19, 1377–1384. [Google Scholar] [CrossRef]
- Ferrero, F.; Periolatto, M. Glycerol in comparison with ethanol in alcohol-assisted dyeing. J. Clean. Prod. 2012, 33, 127–131. [Google Scholar] [CrossRef]
- Pawar, S.S.; Maiti, S.; Biranje, S.; Kulkarni, K.; Adivarekar, R.V. A novel green approach for dyeing polyester using glycerine based eutectic solvent as a dyeing medium. Heliyon 2019, 5, e01606. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Liu, L.; Ye, X.P. Acrolein Production from Crude Glycerol in Sub- and Super-Critical Water. J. Am. Oil Chem. Soc. 2013, 90, 601–610. [Google Scholar] [CrossRef]
- Liu, L.; Ye, X.P. Simultaneous production of lactic acid and propylene glycol from glycerol using solid catalysts without external hydrogen. Fuel Process. Technol. 2015, 137, 55–65. [Google Scholar] [CrossRef]
- Liu, L.; Ye, X.P.; Bozell, J.J. A Comparative Review of Petroleum-Based and Bio-Based Acrolein Production. ChemSusChem 2012, 5, 1162–1180. [Google Scholar] [CrossRef]
- Zou, B.; Ren, S.; Ye, X.P. Glycerol Dehydration to Acrolein Catalyzed by ZSM-5 Zeolite in Supercritical Carbon Dioxide Medium. ChemSusChem 2016, 9, 3268–3271. [Google Scholar] [CrossRef]
- Baliarsingh, S.; Jena, J.; Das, T.; Das, N.B. Role of cationic and anionic surfactants in textile dyeing with natural dyes extracted from waste plant materials and their potential antimicrobial properties. Ind. Crops Prod. 2013, 50, 618–624. [Google Scholar] [CrossRef]
- Gur–Arieh, Z.; Ingamells, W. The Role of Benzyl Alcohol in the Solvent-assisted Dyeing of Acrylic Fibres. J. Soc. Dye. Colour. 2008, 90, 8–11. [Google Scholar] [CrossRef]
- Shao, D.; Xu, C.; Wang, H.; Du, J. Enhancing the Dyeability of Polyimide Fibers with the Assistance of Swelling Agents. Materials 2019, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Nechwatal, A.; Rossbach, V. The Carrier Effect in the m-Aramid Fiber/Cationic Dye/Benzyl Alcohol System. Text. Res. J. 1999, 69, 635–641. [Google Scholar] [CrossRef]
- Zheng, H.-D.; Zhang, J.; Yan, J.; Zheng, L.-J. Investigations on the effect of carriers on meta-aramid fabric dyeing properties in supercritical carbon dioxide. RSC Adv. 2017, 7, 3470–3479. [Google Scholar] [CrossRef]
- Morris, M.; Ye, X.P.; Doona, C.J. Soybean Oil and Nonthermal Plasma Pretreatment to Dye Para-Aramid Woven Fabrics with Disperse Dye Using a Glycerol-Based Dye Bath. J. Am. Oil Chem. Soc. 2021, 98, 463–473. [Google Scholar] [CrossRef]
- Standard, ISO 105-C10:2006. Colour Fastness to Washing with Soap or Soap and Soda. In Textiles—Tests for Colour Fastness. In Internaltional Standardisation Organisation; Internaltional Standardisation Organisation: Geneva, Switzerland, 2006.
- Etters, J.N.; Hurwitz, M.D. Opaque reflectance of translucent fabric. Text. Chem. Color. 1986, 18, 19–26. [Google Scholar]
- Lumakso, F.; Rohman, A.M.H.; Riyanto, S.; Yusof, F. Detection and quantification of soybean and corn oils as adulterants in avocado oil using fourier transform mid infrared (FT-MIR) spectroscopy aided with multivariate calibration. J. Teknol. 2015, 77, 251–255. [Google Scholar] [CrossRef]
- Man, Y.; Rohman, A. Analysis of Canola Oil in Virgin Coconut Oil Using FTIR Spectroscopy and Chemometrics. J. Food Pharm. Sci. 2013, 1, 5–9. [Google Scholar]
- Sienkiewicz, A.M.; Czub, P. The unique activity of catalyst in the epoxidation of soybean oil and following reaction of epoxidized product with bisphenol A. Ind. Crops Prod. 2016, 83, 755–773. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, J.; Tao, D.; Xu, X. Synthesis and Tribological Properties of Air Plasma Polymerized Soybean Oil with N-Containing Structures. J. Am. Oil Chem. Soc. 2014, 91, 827–837. [Google Scholar] [CrossRef]
- Haijuan, K.; Hui, S.; Jin, C.; Haiquan, D.; Xiaoma, D.; Mengmeng, Q.; Muhuo, Y.; Youfeng, Z. Improvement of adhesion of kevlar fabrics to epoxy by surface modification with acetic anhydride in supercritical carbon dioxide. Polym. Compos. 2019, 40, E920–E927. [Google Scholar] [CrossRef]
- Mukherjee, M.; Kumar, S.; Bose, S.; Das, C.; Kharitonov, A. Study on the Mechanical, Rheological, and Morphological Properties of Short Kevlar™ Fiber/s-PS Composites. Polym. Plast. Technol. Eng. 2008, 47, 623–629. [Google Scholar] [CrossRef]
- Kašparová, M.; Šašková, J.; Gregr, J.; Wiener, J. Using of DSCBD plasma for treatment of Kevlar and Nomex fibers. Chem. Listy 2008, 102, 1515–1518. [Google Scholar]
- Ximena, V.Y. Characterization and Analysis of High Voltage Atmospheric Cold Plasma Treatment of Soilbean Oil. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 2020. [Google Scholar]
- Van Durme, J.; Nikiforov, A.; Vandamme, J.; Leys, C.; De Winne, A. Accelerated lipid oxidation using non-thermal plasma technology: Evaluation of volatile compounds. Food Res. Int. 2014, 62, 868–876. [Google Scholar] [CrossRef]
- Zou, X.; Xu, M.; Pan, S.; Gan, L.; Zhang, S.; Chen, H.; Liu, D.; Lu, X.; Ostrikov, K.K. Plasma Activated Oil: Fast Production, Reactivity, Stability, and Wound Healing Application. ACS Biomater. Sci. Eng. 2019, 5, 1611–1622. [Google Scholar] [CrossRef] [PubMed]

| Sample | Dye Solvent | Pretreatment | NTP Time (s) | Swelling Agent | K/S | K/S Std * |
|---|---|---|---|---|---|---|
| A1 | Water | None | 60 | None | 1.26 | 0.009 |
| A2 | Water | None | 0 | None | 1.20 | 0.054 |
| A3 | Water | Acetic acid | 60 | None | 1.22 | 0.011 |
| A4 | Water | Acetic acid | 0 | None | 1.12 | 0.018 |
| A5 | Water | None | 60 | Benzyl–OH | 1.44 | 0.003 |
| A6 | Water | None | 0 | Benzyl–OH | 1.56 | 0.026 |
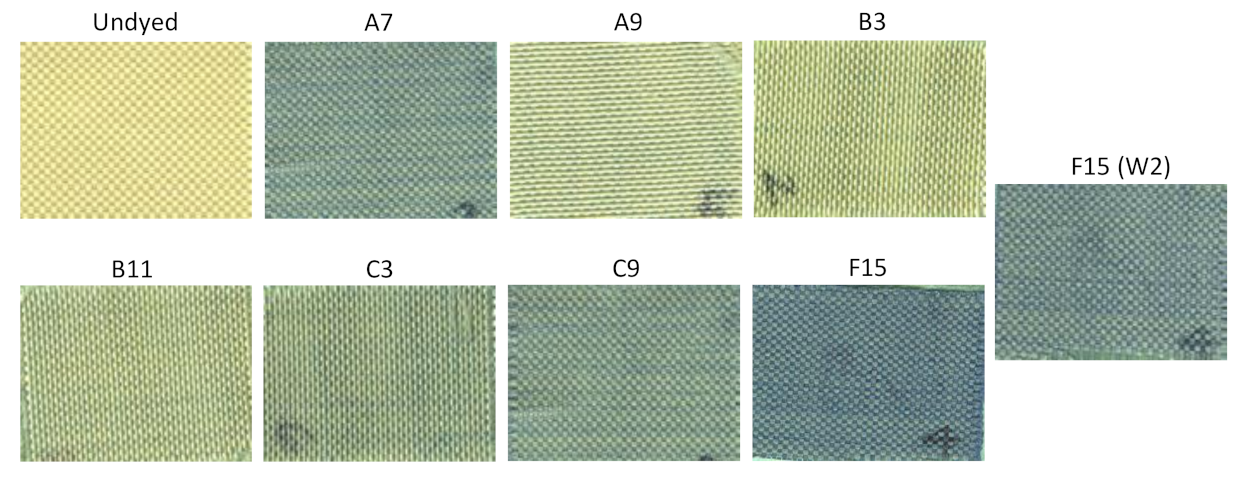
| A7 | Water | Acetic acid | 60 | Benzyl–OH | 1.74 | 0.021 |
| A8 | Water | Acetic acid | 0 | Benzyl–OH | 1.59 | 0.016 |
| A9 | Glycerol | None | 60 | None | 0.42 | 0.005 |
| A10 | Glycerol | None | 0 | None | 0.39 | 0.009 |
| A11 | Glycerol | Acetic acid | 60 | None | 0.45 | 0.004 |
| A12 | Glycerol | Acetic acid | 0 | None | 0.40 | 0.005 |
| A13 | Glycerol | None | 60 | Benzyl–OH | 0.41 | 0.002 |
| A14 | Glycerol | None | 0 | Benzyl–OH | 0.42 | 0.003 |
| A15 | Glycerol | Acetic acid | 60 | Benzyl–OH | 0.48 | 0.019 |
| A16 | Glycerol | Acetic acid | 0 | Benzyl–OH | 0.47 | 0.004 |
| Sample | Dye Solvent | Pretreatment | Swelling Agent | NTP Time (s) | K/S | K/S Std * |
|---|---|---|---|---|---|---|
| B1 | 80% Glycerol | Soy oil | Benzyl–OH | 60 | 0.70 | 0.015 |
| B2 | 80% Glycerol | Soy oil | Benzyl–OH | 0 | 0.57 | 0.012 |
| B3 | 80% Glycerol | Soy oil | None | 60 | 0.79 | 0.014 |
| B4 | 80% Glycerol | Soy oil | None | 0 | 0.56 | 0.004 |
| B5 | 80% Glycerol | None | Benzyl–OH | 60 | 0.55 | 0.003 |
| B6 | 80% Glycerol | None | Benzyl–OH | 0 | 0.53 | 0.004 |
| B7 | 80% Glycerol | None | None | 60 | 0.46 | 0.017 |
| B8 | 80% Glycerol | None | None | 0 | 0.44 | 0.013 |
| B9 | 50% Glycerol | Soy oil | Benzyl–OH | 60 | 0.84 | 0.005 |
| B10 | 50% Glycerol | Soy oil | Benzyl–OH | 0 | 0.67 | 0.009 |
| B11 | 50% Glycerol | Soy oil | None | 60 | 0.94 | 0.001 |
| B12 | 50% Glycerol | Soy oil | None | 0 | 0.92 | 0.005 |
| B13 | 50% Glycerol | None | Benzyl–OH | 60 | 0.81 | 0.014 |
| B14 | 50% Glycerol | None | Benzyl–OH | 0 | 0.70 | 0.010 |
| B15 | 50% Glycerol | None | None | 60 | 0.72 | 0.006 |
| B16 | 50% Glycerol | None | None | 0 | 0.82 | 0.013 |
| Sample | Dye Solvent | Pretreatment | Swelling Agent | NTP Time (s) | K/S | K/S Std * |
|---|---|---|---|---|---|---|
| C1 | 50% Glycerol | Soy oil | Benzyl–OH | 90 | 0.91 | 0.021 |
| C2 | 50% Glycerol | Soy oil | Benzyl–OH | 0 | 0.60 | 0.002 |
| C3 | 50% Glycerol | Soy oil | None | 90 | 1.06 | 0.045 |
| C4 | 50% Glycerol | Soy oil | None | 0 | 0.57 | 0.006 |
| C5 | 50% Glycerol | None | Benzyl–OH | 90 | 0.96 | 0.009 |
| C6 | 50% Glycerol | None | Benzyl–OH | 0 | 0.74 | 0.015 |
| C7 | 50% Glycerol | None | None | 90 | 1.04 | 0.027 |
| C8 | 50% Glycerol | None | None | 0 | 0.66 | 0.013 |
| C9 | Water | Soy oil | Benzyl–OH | 90 | 1.66 | 0.262 |
| C10 | Water | Soy oil | Benzyl–OH | 0 | 1.15 | 0.008 |
| C11 | Water | Soy oil | None | 90 | 1.72 | 0.072 |
| C12 | Water | Soy oil | None | 0 | 0.86 | 0.008 |
| C13 | Water | None | Benzyl–OH | 90 | 1.68 | 0.024 |
| C14 | Water | None | Benzyl–OH | 0 | 1.31 | 0.104 |
| C15 | Water | None | None | 90 | 1.09 | 0.034 |
| C16 | Water | None | None | 0 | 0.92 | 0.012 |
| Sample | Surfactant | Pretreatment | NTP Time (s) | K/S | K/S Std * |
|---|---|---|---|---|---|
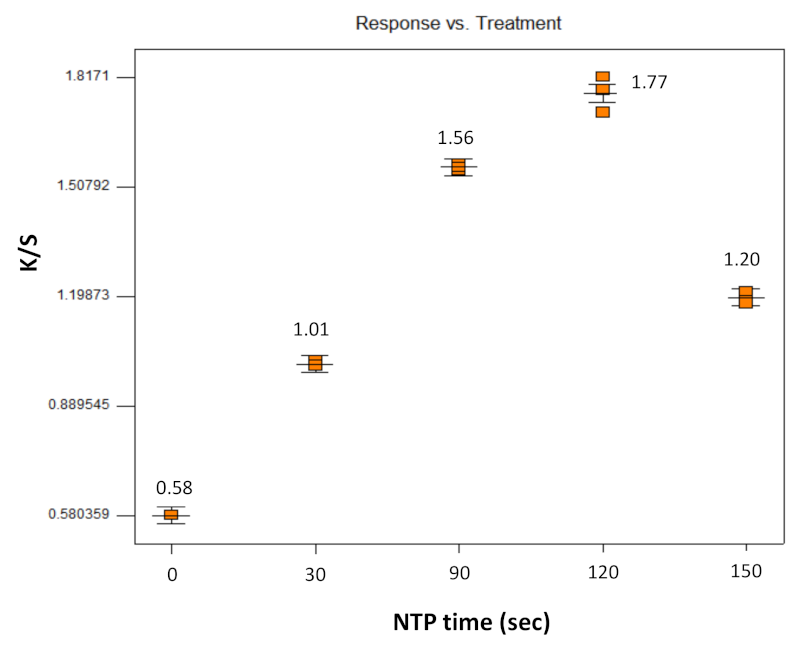
| D1 | None | None | 30 | 0.81 | 0.006 |
| D2 | None | Soy oil | 30 | 1.01 | 0.008 |
| D3 | None | None | 150 | 0.97 | 0.008 |
| D4 | None | Soy oil | 150 | 1.20 | 0.017 |
| D5 | TWEEN | None | 30 | 0.61 | 0.010 |
| D6 | TWEEN | Soy oil | 30 | 0.60 | 0.002 |
| D7 | TWEEN | None | 150 | 0.79 | 0.003 |
| D8 | TWEEN | Soy oil | 150 | 0.84 | 0.005 |
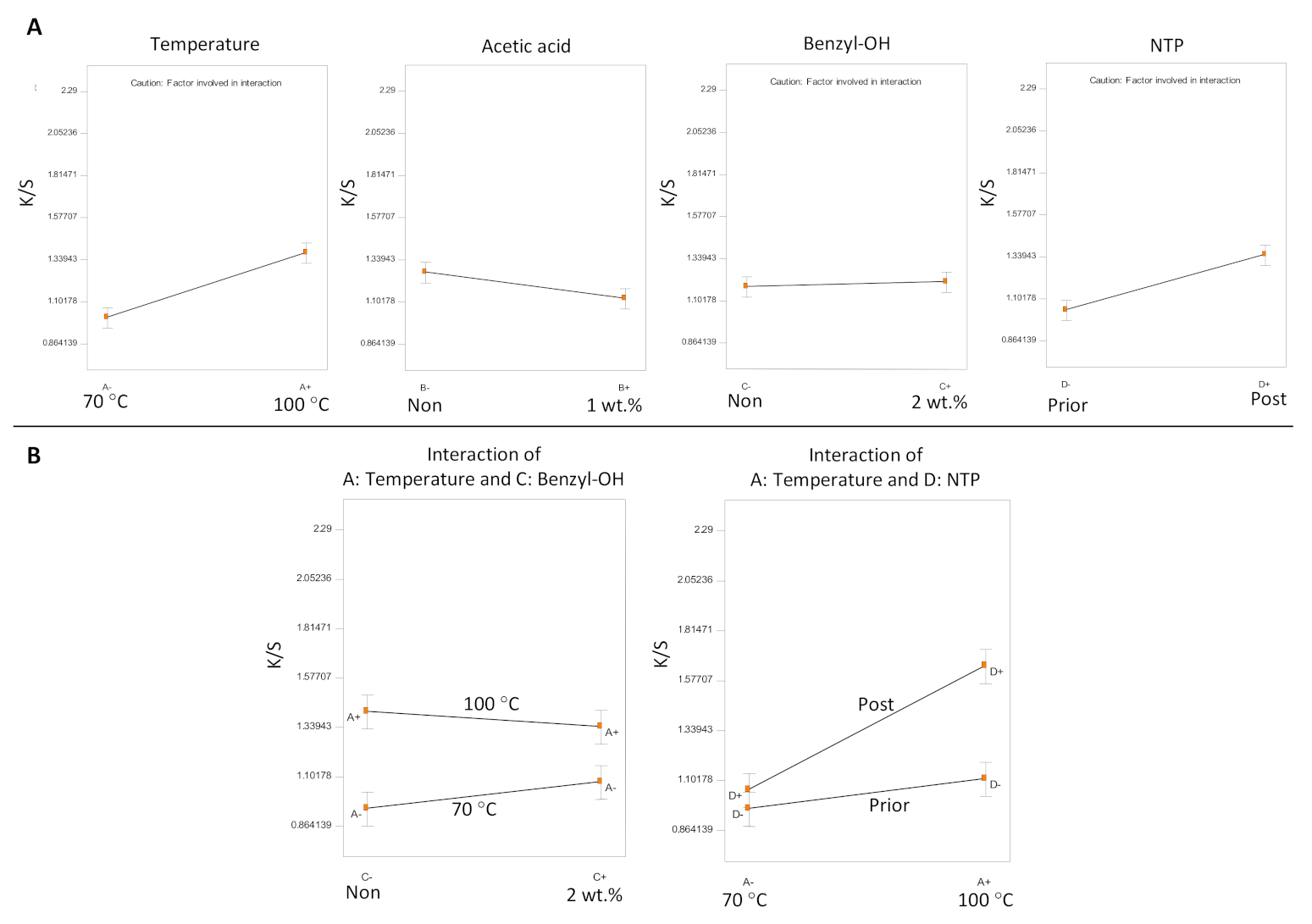
| Sample | Temperature | Acetic Acid | Swelling Agent | NTP | K/S | K/S Std * | ΔK/S # |
|---|---|---|---|---|---|---|---|
| F1 | 70 °C | Yes | Benzyl–OH | Post | 1.08 | 0.016 | −0.08 |
| F2 | 70 °C | Yes | Benzyl–OH | Prior | 0.91 | 0.022 | −0.13 |
| F3 | 70 °C | Yes | None | Post | 0.99 | 0.005 | −0.17 |
| F4 | 70 °C | Yes | None | Prior | 0.91 | 0.045 | −0.27 |
| F5 | 70 °C | No | Benzyl–OH | Post | 1.18 | 0.008 | −0.15 |
| F6 | 70 °C | No | Benzyl–OH | Prior | 1.12 | 0.025 | −0.27 |
| F7 | 70 °C | No | None | Post | 0.96 | 0.023 | −0.06 |
| F8 | 70 °C | No | None | Prior | 0.93 | 0.007 | −0.31 |
| F9 | 100 °C | Yes | Benzyl–OH | Post | 1.55 | 0.012 | −0.04 |
| F10 | 100 °C | Yes | Benzyl–OH | Prior | 1.15 | 0.016 | −0.20 |
| F11 | 100 °C | Yes | None | Post | 1.30 | 0.007 | −0.10 |
| F12 | 100 °C | Yes | None | Prior | 1.06 | 0.020 | −0.17 |
| F13 | 100 °C | No | Benzyl–OH | Post | 1.45 | 0.010 | −0.12 |
| F14 | 100 °C | No | Benzyl–OH | Prior | 1.21 | 0.068 | −0.16 |
| F15 | 100 °C | No | None | Post | 2.28 | 0.064 | −0.09 |
| F16 | 100 °C | No | None | Prior | 1.01 | 0.013 | −0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morris, M.; Ye, X.P.; Doona, C.J. Dyeing Para-Aramid Textiles Pretreated with Soybean Oil and Nonthermal Plasma Using Cationic Dye. Polymers 2021, 13, 1492. https://doi.org/10.3390/polym13091492
Morris M, Ye XP, Doona CJ. Dyeing Para-Aramid Textiles Pretreated with Soybean Oil and Nonthermal Plasma Using Cationic Dye. Polymers. 2021; 13(9):1492. https://doi.org/10.3390/polym13091492
Chicago/Turabian StyleMorris, Mary, Xiaofei Philip Ye, and Christopher J. Doona. 2021. "Dyeing Para-Aramid Textiles Pretreated with Soybean Oil and Nonthermal Plasma Using Cationic Dye" Polymers 13, no. 9: 1492. https://doi.org/10.3390/polym13091492
APA StyleMorris, M., Ye, X. P., & Doona, C. J. (2021). Dyeing Para-Aramid Textiles Pretreated with Soybean Oil and Nonthermal Plasma Using Cationic Dye. Polymers, 13(9), 1492. https://doi.org/10.3390/polym13091492






