Preparation, Physical Properties, and Applications of Water-Based Functional Polymer Inks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Techniques
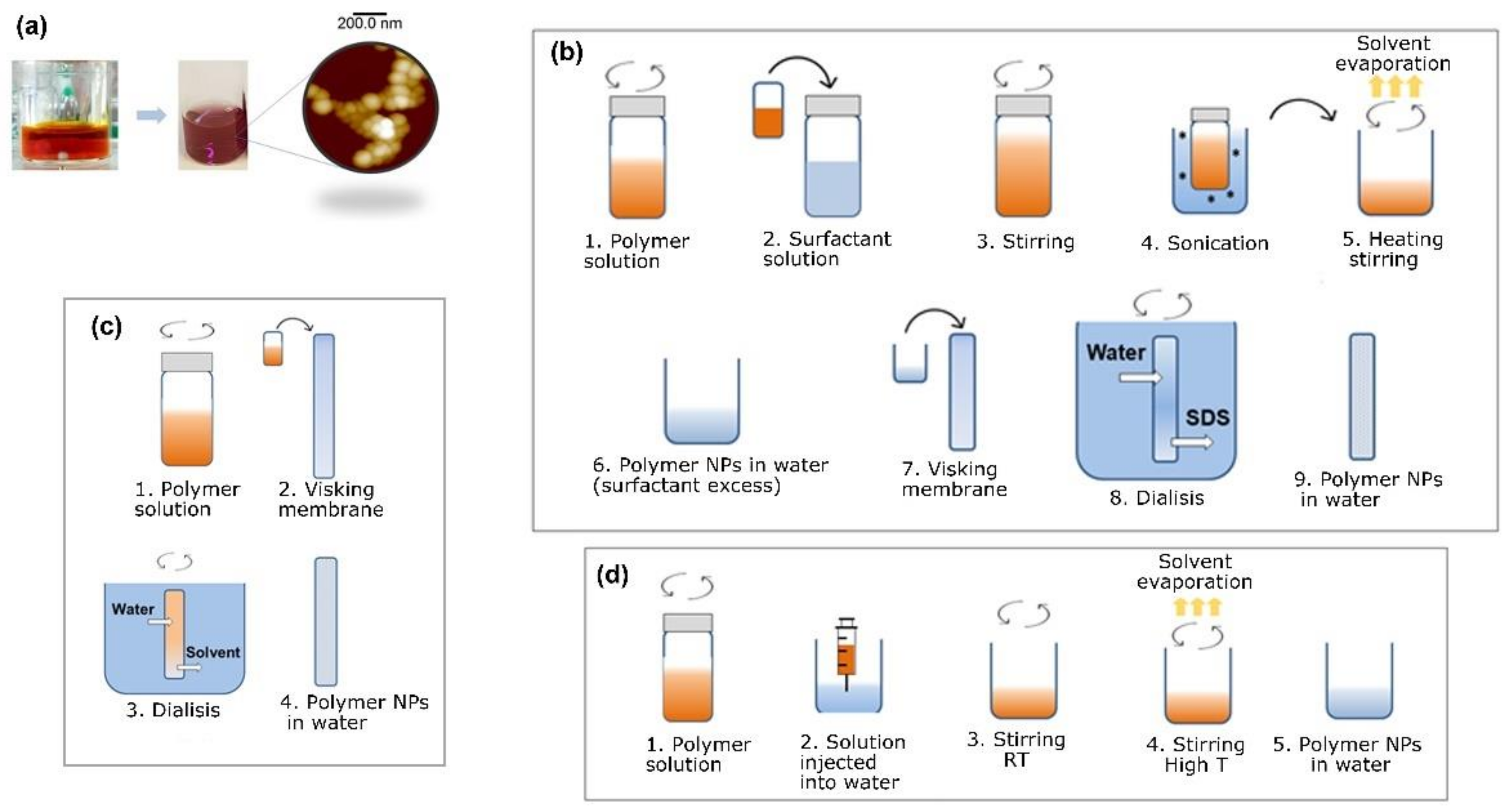
2.3. Description of the Techniques Used for the Preparation of Water-Based Inks
2.3.1. Water-Based Inks Prepared by Miniemulsion
2.3.2. Water-Based Inks: Surfactant-Free Methods
Flash Nanoprecipitation
Dialysis Nanoprecipitation
3. Results and Discussion
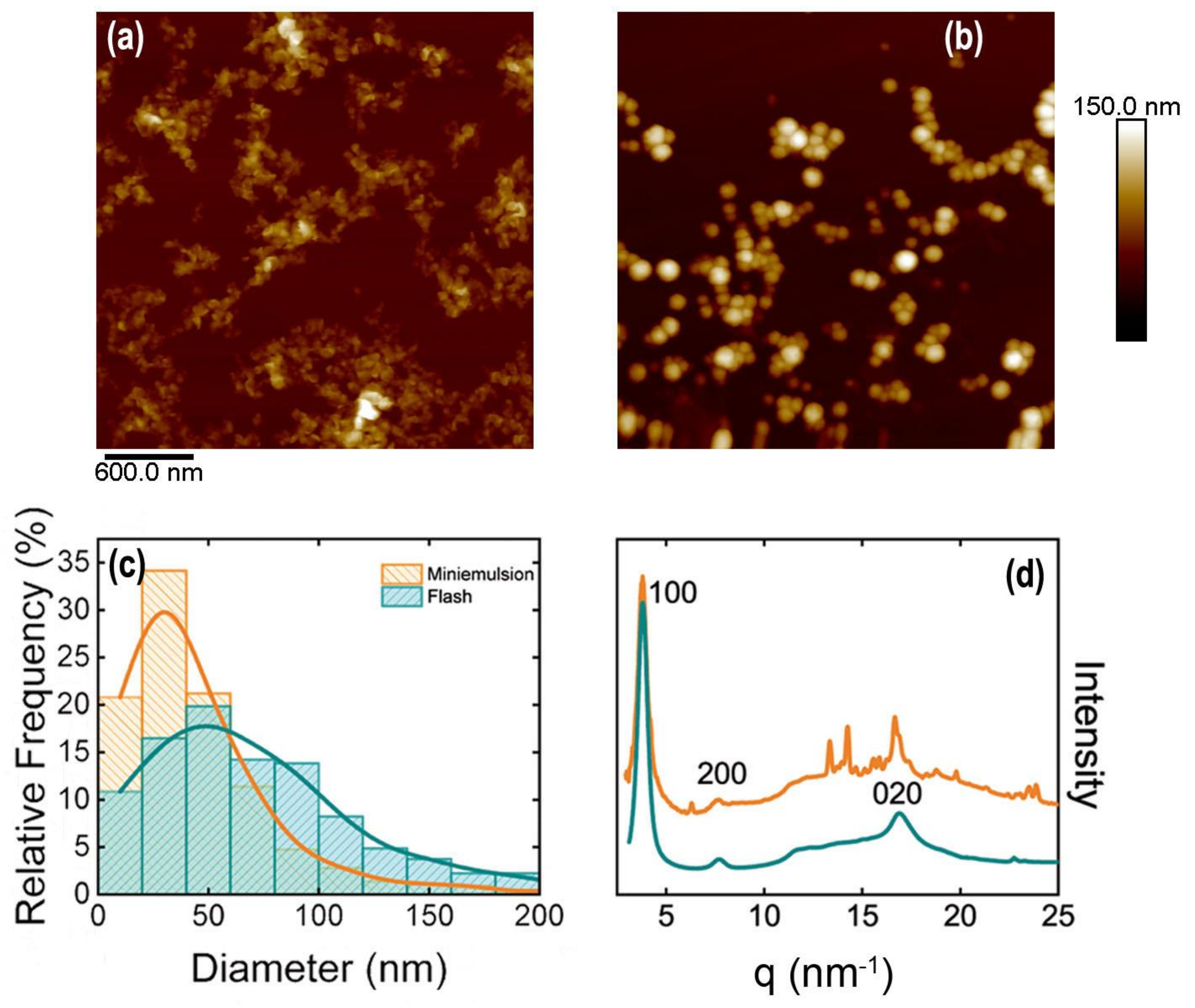
3.1. Semiconducting Polymer Water-Based Inks
Inks from P3HT
3.2. Ferroelectric Polymer Water-Based Inks
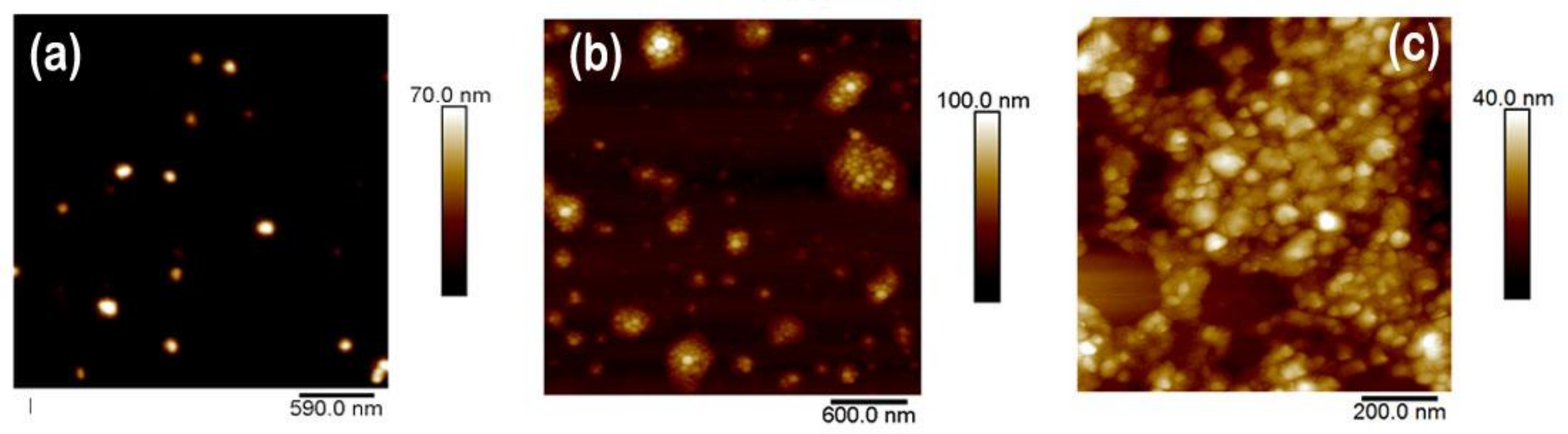
3.2.1. PVDF Nanoparticles
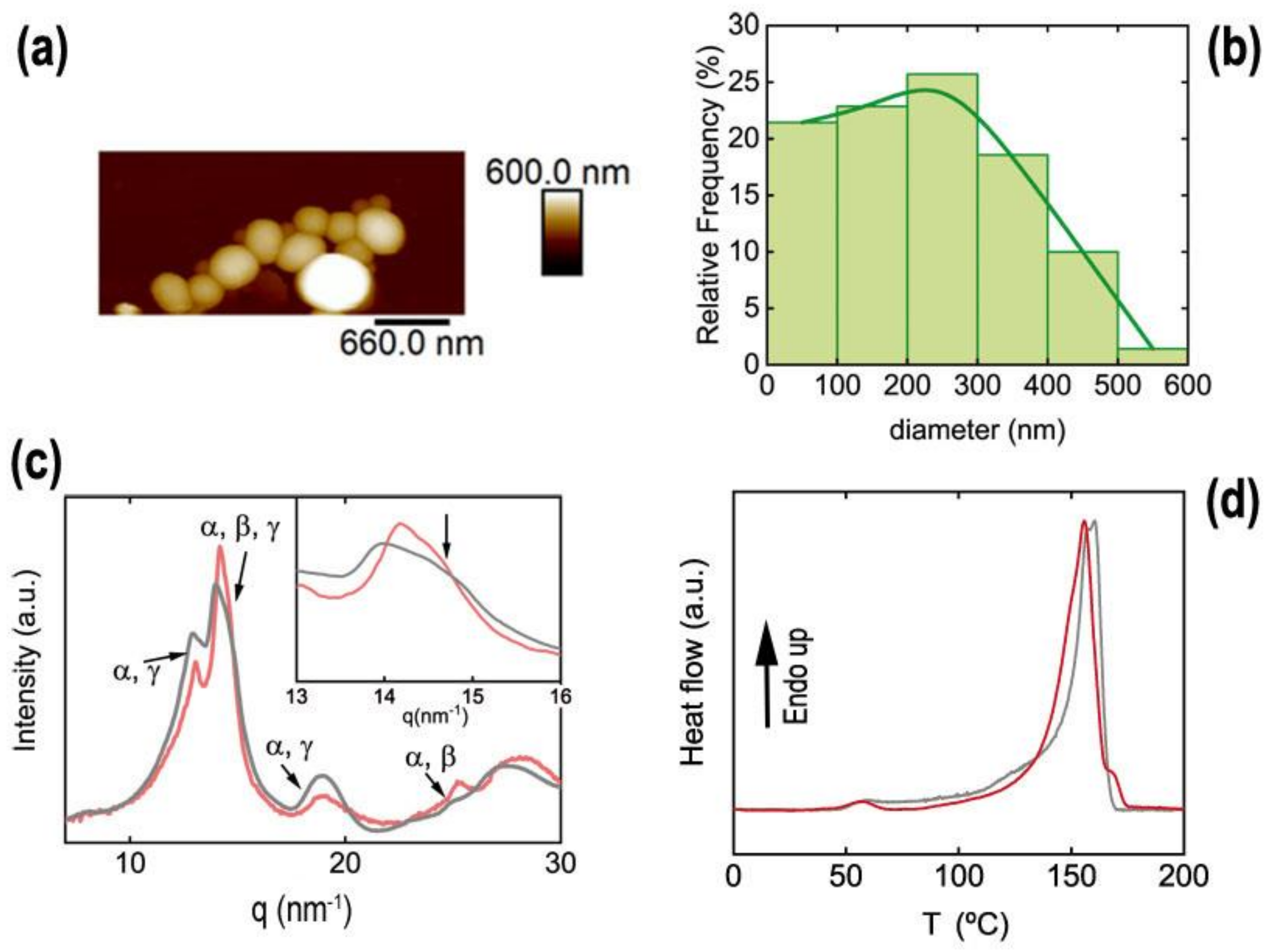
3.2.2. P(VDF-TrFE) Nanoparticles
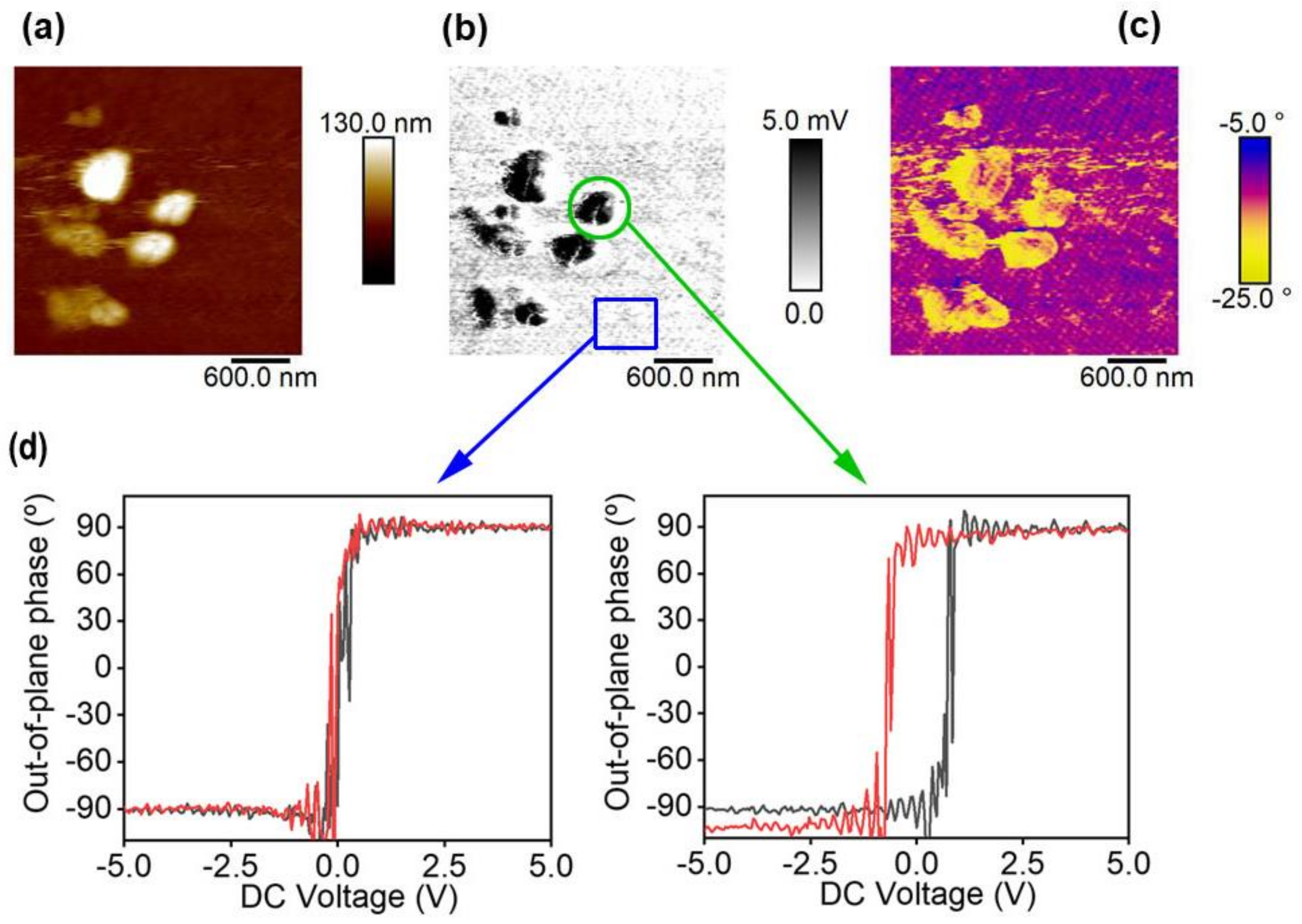
Ferroelectric Ink Functionality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asadi, K.; de Bruyn, P.; Blom, P.W.M.; de Leeuw, D.M. Origin of the efficiency enhancement in ferroelectric functionalized organic solar cells. Appl. Phys. Lett. 2011, 98, 183301. [Google Scholar] [CrossRef] [Green Version]
- Kleemann, H.; Krechan, K.; Fischer, A.; Leo, K. A Review of Vertical Organic Transistors. Adv. Funct. Mater. 2020, 30, 1907113. [Google Scholar] [CrossRef]
- Wu, J.; Meng, Y.; Guo, X.; Zhu, L.; Liu, F.; Zhang, M. All-polymer solar cells based on a novel narrow-bandgap polymer acceptor with power conversion efficiency over 10%. J. Mater. Chem. A 2019, 7, 16190–16196. [Google Scholar] [CrossRef]
- Fudouzi, H.; Xia, Y. Photonic Papers and Inks: Color Writing with Colorless Materials. Adv. Mater. 2003, 15, 892–896. [Google Scholar] [CrossRef]
- Reynolds, J.R. Pi-conjugated polymers: The importance of polymer synthesis. In Conjugated Polymers: A Practical Guide to Synthesis; The Royal Society of Chemistry: London, UK, 2014; pp. 1–11. [Google Scholar]
- Berger, P.R.; Kim, M. Polymer solar cells: P3HT:PCBM and beyond. J. Renew. Sustain. Energy 2018, 10, 013508. [Google Scholar] [CrossRef]
- Bhatnagar, P.K. Organic Light-Emitting Diodes—A Review; Springer: Singapore, 2018; pp. 261–287. [Google Scholar]
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef]
- Chen, X.; Han, X.; Shen, Q.-D. PVDF-Based Ferroelectric Polymers in Modern Flexible Electronics. Adv. Electron. Mater. 2017, 3, 1600460. [Google Scholar] [CrossRef] [Green Version]
- Zapsas, G.; Patil, Y.; Gnanou, Y.; Ameduri, B.; Hadjichristidis, N. Poly(vinylidene fluoride)-based complex macromolecular architectures: From synthesis to properties and applications. Prog. Polym. Sci. 2020, 104, 101231. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.; Han, S.; Zhou, Y. Ferroelectric polymers for non-volatile memory devices: A review. Polym. Int. 2020, 69, 533–544. [Google Scholar] [CrossRef]
- Bae, J.-H.; Chang, S.-H. PVDF-based ferroelectric polymers and dielectric elastomers for sensor and actuator applications: A review. Funct. Compos. Struct. 2019, 1, 012003. [Google Scholar] [CrossRef]
- Hu, Z.; Tian, M.; Nysten, B.; Jonas, A.M. Regular arrays of highly ordered ferroelectric polymer nanostructures for non-volatile low-voltage memories. Nat. Mater. 2009, 8, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, D.Y.; Kim, J.; Kim, J.H.; Kong, D.S.; Murillo, G.; Lee, G.; Park, J.Y.; Jung, J.H. Ferroelectric-Polymer-Enabled Contactless Electric Power Generation in Triboelectric Nanogenerators. Adv. Funct. Mater. 2019, 29, 1905816. [Google Scholar] [CrossRef]
- Whiter, R.A.; Boughey, C.; Smith, M.; Kar-Narayan, S. Mechanical Energy Harvesting Performance of Ferroelectric Polymer Nanowires Grown via Template-Wetting. Energy Technol. 2018, 6, 928–934. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Stingelin, N.; Michels, J.J.; Spijkman, M.-J.; Asadi, K.; Beerends, R.; Biscarini, F.; Blom, P.W.M.; de Leeuw, D.M. Processing and Low Voltage Switching of Organic Ferroelectric Phase-Separated Bistable Diodes. Adv. Funct. Mater. 2012, 22, 2750–2757. [Google Scholar] [CrossRef] [Green Version]
- Van Breemen, A.; Zaba, T.; Khikhlovskyi, V.; Michels, J.; Janssen, R.; Kemerink, M.; Gelinck, G. Surface Directed Phase Separation of Semiconductor Ferroelectric Polymer Blends and their Use in Non-Volatile Memories. Adv. Funct. Mater. 2015, 25, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Fernández, E.; Rebollar, E.; Cui, J.; Ezquerra, T.A.; Nogales, A. Morphology and Ferroelectric Properties of Semiconducting/Ferroelectric Polymer Bilayers. Macromolecules 2019, 52, 7396–7402. [Google Scholar] [CrossRef]
- Landfester, K.; Montenegro, R.; Scherf, U.; Güntner, R.; Asawapirom, U.; Patil, S.; Neher, D.; Kietzke, T. Semiconducting polymer nanospheres in aqueous dispersion prepared by a miniemulsion process. Adv. Mater. 2002, 14, 651–655. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, C.; Yuan, H.; Liu, L.; Lv, F.; Wang, S. Conjugated polymer nanoparticles: Preparation, properties, functionalization and biological applications. Chem. Soc. Rev. 2013, 42, 6620. [Google Scholar] [CrossRef]
- Martínez-Tong, D.E.; Cui, J.; Soccio, M.; García, C.; Ezquerra, T.A.; Nogales, A. Does the glass transition of polymers change upon 3D confinement? Macromol. Chem. Phys. 2014, 215, 1620–1624. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Tong, D.E.; Soccio, M.; Sanz, A.; García, C.; Ezquerra, T.A.; Nogales, A. Ferroelectricity and molecular dynamics of poly(vinylidenefluoride-trifluoroethylene) nanoparticles. Polymer 2015, 56, 428–434. [Google Scholar] [CrossRef]
- Rose, S.; Prevoteau, A.; Elzière, P.; Hourdet, D.; Marcellan, A.; Leibler, L. Nanoparticle solutions as adhesives for gels and biological tissues. Nature 2014, 505, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Xie, C.; Heumüller, T.; Gruber, W.; Tang, X.; Classen, A.; Schuldes, I.; Bidwell, M.; Späth, A.; Fink, R.H.; Unruh, T.; et al. Overcoming efficiency and stability limits in water-processing nanoparticular organic photovoltaics by minimizing microstructure defects. Nat. Commun. 2018, 9, 5335. [Google Scholar] [CrossRef] [Green Version]
- Crucho, C.I.C.C. Stimuli-Responsive Polymeric Nanoparticles for Nanomedicine. ChemMedChem 2015, 10, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.D.D.; Truong, N.P.; Gu, W.; Jia, Z.; Cooper, M.A.; Monteiro, M.J. Timed-Release Polymer Nanoparticles. Biomacromolecules 2013, 14, 495–502. [Google Scholar] [CrossRef]
- Xiao, Z.; Dong, Q.; Sharma, P.; Yuan, Y.; Mao, B.; Tian, W.; Gruverman, A.; Huang, J. Synthesis and Application of Ferroelectric P(VDF-TrFE) Nanoparticles in Organic Photovoltaic Devices for High Efficiency. Adv. Energy Mater. 2013, 3, 1581–1588. [Google Scholar] [CrossRef]
- Martínez-Tong, D.E.; Soccio, M.; Sanz, A.; García, C.; Ezquerra, T.A.; Nogales, A. Chain Arrangement and Glass Transition Temperature Variations in Polymer Nanoparticles under 3D-Confinement. Macromolecules 2013, 46, 4698–4705. [Google Scholar] [CrossRef]
- Gutiérrez-Fernández, E.; Ezquerra, T.A.; Rebollar, E.; Cui, J.; Marina, S.; Martín, J.; Nogales, A. Photophysical and structural modulation of poly(3-hexylthiophene) nanoparticles via surfactant-polymer interaction. Polymer 2021, 218, 123515. [Google Scholar] [CrossRef]
- Mastrorilli, P.; Nobile, C.F.; Suranna, G.P.; Corradi, A.; Leonelli, C.; Veronesi, P. Morphological characterization of poly(phenylacetylene) nanospheres prepared by homogeneous and heterogeneous catalysis. Appl. Organomet. Chem. 2003, 17, 711–716. [Google Scholar] [CrossRef]
- Asua, J.M. Miniemulsion polymerization. Prog. Polym. Sci. 2002, 27, 1283–1346. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Flory, P.; Volkenstein, M. Statistical Mechanics of Chain Molecules; Wiley: New York, NY, USA, 1969. [Google Scholar]
- Staff, R.H.; Schaeffel, D.; Turshatov, A.; Donadio, D.; Butt, H.-J.; Landfester, K.; Koynov, K.; Crespy, D. Particle Formation in the Emulsion-Solvent Evaporation Process. Small 2013, 9, 3514–3522. [Google Scholar] [CrossRef]
- Bag, M.; Gehan, T.S.; Algaier, D.D.; Liu, F.; Nagarjuna, G.; Lahti, P.M.; Russell, T.P.; Venkataraman, D. Efficient charge transport in assemblies of surfactant-stabilized semiconducting nanoparticles. Adv. Mater. 2013, 25, 6411–6415. [Google Scholar] [CrossRef]
- Zhang, C.; Chung, J.W.; Priestley, R.D. Dialysis Nanoprecipitation of Polystyrene Nanoparticles. Macromol. Rapid Commun. 2012, 33, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Holmes, N.P.; Marks, M.; Cave, J.M.; Feron, K.; Barr, M.G.; Fahy, A.; Sharma, A.; Pan, X.; Kilcoyne, D.A.L.; Zhou, X.; et al. Engineering Two-Phase and Three-Phase Microstructures from Water-Based Dispersions of Nanoparticles for Eco-Friendly Polymer Solar Cell Applications. Chem. Mater. 2018, 30, 6521–6531. [Google Scholar] [CrossRef] [Green Version]
- Kietzke, T. Recent Advances in Organic Solar Cells. Adv. Optoelectron. 2007, 2007, 15. [Google Scholar] [CrossRef] [Green Version]
- Holmes, N.P.; Burke, K.B.; Sista, P.; Barr, M.; Magurudeniya, H.D.; Stefan, M.C.; Kilcoyne, A.L.D.; Zhou, X.; Dastoor, P.C.; Belcher, W.J. Nano-domain behaviour in P3HT:PCBM nanoparticles, relating material properties to morphological changes. Sol. Energy Mater. Sol. Cells 2013, 117, 437–445. [Google Scholar] [CrossRef]
- Ulum, S.; Holmes, N.; Darwis, D.; Burke, K.; David Kilcoyne, A.L.; Zhou, X.; Belcher, W.; Dastoor, P. Determining the structural motif of P3HT:PCBM nanoparticulate organic photovoltaic devices. Sol. Energy Mater. Sol. Cells 2013, 110, 43–48. [Google Scholar] [CrossRef]
- Ameri, M.; Al-Mudhaffer, M.F.; Almyahi, F.; Fardell, G.C.; Marks, M.; Al-Ahmad, A.; Fahy, A.; Andersen, T.; Elkington, D.C.; Feron, K.; et al. Role of Stabilizing Surfactants on Capacitance, Charge, and Ion Transport in Organic Nanoparticle-Based Electronic Devices. ACS Appl. Mater. Interfaces 2019, 11, 10074–10088. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Tang, X.; Berlinghof, M.; Langner, S.; Chen, S.; Späth, A.; Li, N.; Fink, R.H.; Unruh, T.; Brabec, C.J. Robot-Based High-Throughput Engineering of Alcoholic Polymer: Fullerene Nanoparticle Inks for an Eco-Friendly Processing of Organic Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 23225–23234. [Google Scholar] [CrossRef]
- Algaier, D.D. Impact of Fabrication Parameters on the Internal Structure of Poly(3-hexylthiophene) Nanoparticles. Ph.D. Thesis, University of Massachusetts, Amherst, MA, USA, 2015. [Google Scholar]
- Brinkmann, M.; Wittmann, J.C. Orientation of Regioregular Poly(3-hexylthiophene) by Directional Solidification: A Simple Method to Reveal the Semicrystalline Structure of a Conjugated Polymer. Adv. Mater. 2006, 18, 860–863. [Google Scholar] [CrossRef]
- Tan, B.; Li, Y.; Palacios, M.F.; Therrien, J.; Sobkowicz, M.J. Effect of surfactant conjugation on structure and properties of poly(3-hexylthiophene) colloids and field effect transistors. Colloids Surf. A Physicochem. Eng. Asp. 2016, 488, 7–14. [Google Scholar] [CrossRef]
- Holmes, A.; Deniau, E.; Lartigau-Dagron, C.; Bousquet, A.; Chambon, S.; Holmes, N.P. Review of Waterborne Organic Semiconductor Colloids for Photovoltaics. ACS Nano 2021, 15, 3927–3959. [Google Scholar] [CrossRef] [PubMed]
- Visaveliya, N.; Köhler, J.M. Control of Shape and Size of Polymer Nanoparticles Aggregates in a Single-Step Microcontinuous Flow Process: A Case of Flower and Spherical Shapes. Langmuir 2014, 30, 12180–12189. [Google Scholar] [CrossRef] [PubMed]
- Satapathi, S.; Gill, H.S.; Li, L.; Samuelson, L.; Kumar, J.; Mosurkal, R. Synthesis of nanoparticles of P3HT and PCBM for optimizing morphology in polymeric solar cells. In Proceedings of the Applied Surface Science; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 323, pp. 13–18. [Google Scholar]
- Richards, J.J.; Whittle, C.L.; Shao, G.; Pozzo, L.D. Correlating structure and photocurrent for composite semiconducting nanoparticles with contrast variation small-Angle neutron scattering and photoconductive atomic force microscopy. ACS Nano 2014, 8, 4313–4324. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A critical analysis of the α, β and γ phases in poly (vinylidene fluoride) using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef] [Green Version]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Esterly, D.M.; Love, B.J. Phase transformation to beta-poly(vinylidenefluoride) by milling. J. Polym. Sci. Part B 2003, 42, 91–97. [Google Scholar] [CrossRef]
- Lei, T.; Cai, X.; Wang, X.; Yu, L.; Hu, X.; Zheng, G.; Lv, W.; Wang, L.; Wu, D.; Sun, D.; et al. Spectroscopic evidence for a high fraction of ferroelectric phase induced in electrospun polyvinylidene fluoride fibers. RSC Adv. 2013, 3, 24952–24958. [Google Scholar] [CrossRef]
- Gregorio, R. Determination of the α, β, and γ crystalline phases of poly(vinylidene fluoride) films prepared at different conditions. J. Appl. Polym. Sci. 2006, 100, 3272–3279. [Google Scholar] [CrossRef]
- Merlini, C.; Barra, G.M.O.; Medeiros Araujo, T.; Pegoretti, A. Electrically pressure sensitive poly(vinylidene fluoride)/polypyrrole electrospun mats. RSC Adv. 2014, 4, 15749–15758. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. Conformational changes and phase transformation mechanisms in PVDF solution-cast films. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3487–3495. [Google Scholar] [CrossRef]
- Horibe, H.; Sasaki, Y.; Oshiro, H.; Hosokawa, Y.; Kono, A.; Takahashi, S.; Nishiyama, T. Quantification of the solvent evaporation rate during the production of three PVDF crystalline structure types by solvent casting. Polym. J. 2014, 46, 104–110. [Google Scholar] [CrossRef]
- Calleja, F.J.B.; Arche, A.G.; Ezquerra, T.A.; Santa Cruz, C.; Batallan, F.; Frick, B.; Cabarcos, E.L. Structure and properties of ferroelectric copolymers of poly (vinylidene fluoride). In Structure in Polymers with Special Properties; Springer: Berlin/Heidelberg, Germany, 1993; pp. 1–48. [Google Scholar]
- Kang, S.J.; Bae, I.; Shin, Y.J.; Park, Y.J.; Huh, J.; Park, S.-M.; Kim, H.-C.; Park, C. Nonvolatile Polymer Memory with Nanoconfinement of Ferroelectric Crystals. Nano Lett. 2011, 11, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.C.; Huang, S.Y.; Shieh, J.; Chen, S.H. Enhanced piezoelectricity of nanoimprinted sub-20 nm poly(vinylidene fluoride-trifluoroethylene) copolymer nanograss. Macromolecules 2012, 45, 1580–1586. [Google Scholar] [CrossRef]
- Bellet-Amalric, E.; Legrand, J.F. Crystalline structures and phase transition of the ferroelectric P(VDF-TrFE) copolymers, a neutron diffraction study. Eur. Phys. J. B 1998, 3, 225–236. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.-C.; Linares, A.; Martín-Fabiani, I.; Hernández, J.J.; Soccio, M.; Rueda, D.R.; Ezquerra, T.A.; Reynolds, M. Understanding crystallization features of P(VDF-TrFE) copolymers under confinement to optimize ferroelectricity in nanostructures. Nanoscale 2013, 5, 6006–6012. [Google Scholar] [CrossRef] [PubMed]



| Material | Preparation Method | Solvent | Concentration |
|---|---|---|---|
| P3HT | Miniemulsion | ClCH3 | 3 g/L |
| P3HT | Flash | THF | 3 g/L |
| PC71BM | Miniemulsion | ClCH3 | 3 g/L |
| PC71BM | Flash | THF | 3 g/L |
| P3HT/PC71BM blend | Miniemulsion | ClCH3 | 3 g/L |
| P(VDF-TrFE) | Flash | THF | 2 g/L |
| PVDF | Dialysis | DMA | 2 g/L |
| Solvent | Average Size | |
|---|---|---|
| PC71BM flash | THF (3 g/L) | 120 nm |
| PC71BM miniemulsion | ClCH3 (3 g/L) | 61 nm |
| P3HT/PC71BM 1:1 blend miniemulsion | ClCH3 (3 g/L) | 45 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Fernández, E.; Cui, J.; Martínez-Tong, D.E.; Nogales, A. Preparation, Physical Properties, and Applications of Water-Based Functional Polymer Inks. Polymers 2021, 13, 1419. https://doi.org/10.3390/polym13091419
Gutiérrez-Fernández E, Cui J, Martínez-Tong DE, Nogales A. Preparation, Physical Properties, and Applications of Water-Based Functional Polymer Inks. Polymers. 2021; 13(9):1419. https://doi.org/10.3390/polym13091419
Chicago/Turabian StyleGutiérrez-Fernández, Edgar, Jing Cui, Daniel E. Martínez-Tong, and Aurora Nogales. 2021. "Preparation, Physical Properties, and Applications of Water-Based Functional Polymer Inks" Polymers 13, no. 9: 1419. https://doi.org/10.3390/polym13091419
APA StyleGutiérrez-Fernández, E., Cui, J., Martínez-Tong, D. E., & Nogales, A. (2021). Preparation, Physical Properties, and Applications of Water-Based Functional Polymer Inks. Polymers, 13(9), 1419. https://doi.org/10.3390/polym13091419







