New Insights into Properties of Methanol Transport in Sulfonated Polysulfone Composite Membranes for Direct Methanol Fuel Cells
Abstract
1. Introduction
2. Experimental
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goor, M.; Menkin, S.; Peled, E. High power direct methanol fuel cell for mobility and portable applications. Int. J. Hydrogen Energy 2019, 44, 3138–3143. [Google Scholar] [CrossRef]
- Hardman, S.; Chandan, A.; Steinberger-Wilckens, R. Fuel cell added value for early market applications. J. Power Sources 2015, 287, 297–306. [Google Scholar] [CrossRef]
- Baglio, V.; Stassi, A.; Matera, F.V.; Antonucci, V.; Aricò, A.S. Investigation of passive DMFC mini-stacks at ambient temperature. Electrochim. Acta 2009, 54, 2004–2009. [Google Scholar] [CrossRef]
- Aricò, A.S.; Baglio, V.; Antonucci, V. Direct methanol fuel cells: History, status and perspectives. In Electrocatalysis of Direct Methanol Fuel Cells: From Fundamentals to Applications; Zhang, T.J., Liu, H.S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; Volume 1, pp. 1–78. [Google Scholar]
- Agnolucci, P. Economics and market prospects of portable fuel cells. Int. J. Hydrogen Energy 2007, 32, 4319–4328. [Google Scholar] [CrossRef]
- Mehmood, A.; Scibioh, M.A.; Prabhuram, J.; An, M.-G.; Ha, H.Y. A review on durability issues and restoration techniques in long-term operations of direct methanol fuel cells. J. Power Sources 2015, 297, 224–241. [Google Scholar] [CrossRef]
- Kim, Y.S.; Zelenay, P. Direct methanol fuel cell durability. In Polymer Electrolyte Fuel Cell Durability; Buchi, F.N., Inaba, M., Schmidt, T.J., Eds.; Springer: New York, NY, USA, 2009; pp. 223–240. [Google Scholar]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent approaches to improve Nafion performance for fuel cell applications: A review. Int. J. Hydrogen Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Sebastián, D.; Serov, A.; Matanovic, I.; Artyushkova, K.; Atanassov, P.; Aricò, A.S.; Baglio, V. Insights on the extraordinary tolerance to alcohols of Fe-N-C cathode catalysts in highly performing direct alcohol fuel cells. Nano Energy 2017, 34, 195–204. [Google Scholar] [CrossRef]
- Lufrano, F.; Baglio, V.; Staiti, P.; Antonucci, V.; Arico’, A.S. Performance analysis of polymer electrolyte membranes for direct methanol fuel cells. J. Power Sources 2013, 243, 519–534. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Serov, A.; Romero, H.; Lubers, A.; Zulevi, B.; Aricò, A.S.; Baglio, V. Commercial platinum group metal-free cathodic electrocatalysts for highly performed direct methanol fuel cell applications. J. Power Sources 2019, 437, 226948. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Sebastián, D.; Lázaro, M.J.; Aricò, A.S.; Baglio, V. Methanol-tolerant M–N–C catalysts for oxygen reduction reactions in acidic media and their application in direct methanol fuel cells. Catalysts 2018, 8, 650. [Google Scholar] [CrossRef]
- He, G.; Li, Z.; Zhao, J.; Wang, S.; Wu, H.; Guiver, M.D.; Jiang, Z. Nanostructured Ion-Exchange Membranes for Fuel Cells: Recent Advances and Perspectives. Adv. Mater. 2015, 27, 5280–5295. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, W.; Manthiram, A. Sulfonated polysulfone with 1,3-1H-dibenzimidazole-benzene additive as a membrane for direct methanol fuel cells. J. Membrane Sci. 2008, 310, 262–267. [Google Scholar] [CrossRef]
- Yun, S.; Parrondo, J.; Ramani, V. Composite sulfonated polyether ether ketone (SPEEK) membranes with 3-(Trihydroxysilyl)-1-Propanesulfonic acid for a direct methanol fuel cell (DMFC). ECS Trans. 2013, 50, 1233–1245. [Google Scholar] [CrossRef]
- Lufrano, F.; Baglio, V.; Staiti, P.; Arico’, A.S.; Antonucci, V. Polymer electrolytes based on sulfonated polysulfone for direct methanol fuel cells. J. Power Sources 2008, 179, 34–41. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Shahi, V.K. Organic–inorganic nanocomposite polymer electrolyte membranes for fuel cell applications. Prog. Polym. Sci. 2011, 36, 945–979. [Google Scholar] [CrossRef]
- Lee, S.-H.; Choi, S.-H.; Gopalan, S.-A.; Lee, K.-P.; Anantha-Iyengar, G. Preparation of new self-humidifying composite membrane by incorporating graphene and phosphotungstic acid into sulfonated poly (ether-ether-ketone) film. Int. J. Hydrogen Energy 2014, 39, 17162–17177. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Zhai, Y.; Zhu, X.; Bi, C. Investigation of self-humidifying membranes based on sulfonated poly (ether ether ketone) hybrid with sulfated zirconia supported Pt catalyst for fuel cell applications. J. Power Sources 2007, 168, 323–329. [Google Scholar] [CrossRef]
- Son, D.-H.; Sharma, R.K.; Shul, Y.-G.; Kim, H. Preparation of Pt/zeolite-Nafion composite membranes for self-humidifying polymer electrolyte fuel cells. J. Power Sources 2007, 165, 733–738. [Google Scholar] [CrossRef]
- Liu, F.; Yi, B.; Xing, D.; Yu, J.; Hou, Z.; Fu, Y. Development of novel self-humidifying composite membranes for fuel cells. J. Power Sources 2003, 124, 81–89. [Google Scholar] [CrossRef]
- Watanabe, M.; Uchida, H.; Seki, Y.; Emori, M.; Stonehart, P. Self-humidifying polymer electrolyte membranes for fuel cells. J. Electrochem. Soc. 1996, 143, 3847–3852. [Google Scholar] [CrossRef]
- Mazzapioda, L.; Lo Vecchio, C.; Danyliv, O.; Baglio, V.; Martinelli, A.; Navarra, M.A. Composite Nafion-CaTiO3-δ membranes as electrolyte component for PEM fuel cells. Polymers 2020, 12, 2019. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Yao, J.; Zhou, S.; Zheng, J.; Li, S.; Zhang, S.; Qian, H. Enhancement of proton/methanol selectivity via the in-situ cross-linking of sulfonated poly (p-phenylene-co-aryl ether ketone) and graphene oxide (GO) nanosheets. J. Membrane Sci. 2020, 605, 118102. [Google Scholar] [CrossRef]
- Shukla, A.; Dhanasekaran, P.; Sasikala, S.; Nagaraju, N.; Bhat, S.D.; Pillai, V.K. Covalent grafting of polystyrene sulfonic acid on graphene oxide nanoplatelets to form a composite membrane electrolyte with sulfonated poly (ether ether ketone) for direct methanol fuel cells. J. Membrane Sci. 2020, 595, 117484. [Google Scholar] [CrossRef]
- Simari, C.; Baglio, V.; Lo Vecchio, C.; Aricò, A.S.; Agostino, R.G.; Coppola, L.; Oliviero Rossi, C.; Nicotera, I. Reduced methanol crossover and enhanced proton transport in nanocomposite membranes based on clay−CNTs hybrid materials for direct methanol fuel cells. Ionics 2017, 23, 2113–2123. [Google Scholar] [CrossRef]
- Lufrano, E.; Simari, C.; Lo Vecchio, C.; Aricò, A.S.; Baglio, V.; Nicotera, I. Barrier properties of sulfonated polysulfone/layered double hydroxides nanocomposite membrane for direct methanol fuel cell operating at high methanol concentrations. Int. J. Hydrogen Energy 2020, 45, 20647–20658. [Google Scholar] [CrossRef]
- Aricò, A.S.; Sebastian, D.; Schuster, M.; Bauer, B.; D’Urso, C.; Lufrano, F.; Baglio, V. Selectivity of direct methanol fuel cell membranes. Membranes 2015, 5, 793–809. [Google Scholar] [CrossRef]
- Laberty-Robert, C.; Vallé, K.; Pereira, F.; Sanchez, C. Design and properties of functional hybrid organic–inorganic membranes for fuel cells. Chem. Soc. Rev. 2011, 40, 961. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Capitani, D.; Donnadio, A.; Narducci, R.; Pica, M.; Sganappa, M. Novel Nafion-zirconium phosphate nanocomposite membranes with enhanced stability of proton conductivity at medium temperature and high relative humidity. Electrochim. Acta 2007, 52, 8125–8132. [Google Scholar] [CrossRef]
- D’Urso, C.; Oldani, C.; Baglio, V.; Merlo, L.; Aricò, A.S. Towards fuel cell membranes with improved lifetime: Aquivion® Perfluorosulfonic Acid membranes containing immobilized radical scavengers. J. Power Sources 2014, 272, 753–758. [Google Scholar] [CrossRef]
- Baglio, V.; Aricò, A.S.; Di Blasi, A.; Antonucci, V.; Antonucci, P.L.; Licoccia, S.; Traversa, E.; Fiory, F.S. Nafion-TiO2 composite DMFC membranes: Physico-chemical properties of the filler versus electrochemical performance. Electrochim. Acta 2005, 50, 1241–1246. [Google Scholar] [CrossRef]
- Zakil, F.A.; Kamarudin, S.K.; Basri, S. Modified Nafion membranes for direct alcohol fuel cells: An overview. Renew. Sustain. Energy Rev. 2016, 65, 841–852. [Google Scholar] [CrossRef]
- Al-Batty, S.; Dawson, C.; Shanmukham, S.P.; Roberts, E.P.L.; Holmes, S.M. Improvement of direct methanol fuel cell performance using a novel mordenite barrier layer. J. Mater. Chem. A 2016, 4, 10850–10857. [Google Scholar] [CrossRef]
- Ru, C.; Li, Z.; Zhao, C.; Duan, Y.; Zhuang, Z.; Bu, F.; Na, H. Enhanced proton conductivity of sulfonated hybrid poly (arylene ether ketone) membranes by incorporating an amino–sulfo bifunctionalized metal–organic framework for direct methanol fuel cells. ACS Appl. Mater. Interfaces 2018, 10, 7963–7973. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi, S.; Sahu, A.K.; Bhat, S.D.; Sridhar, P.; Pitchumani, S.; Shukla, A.K. Mesostructured-aluminosilicate-Nafion hybrid membranes for direct methanol fuel cells. Electrochim. Acta 2013, 89, 35–44. [Google Scholar] [CrossRef]
- Lufrano, F.; Baglio, V.; Di Blasi, O.; Staiti, P.; Antonucci, V.; Aricò, A.S. Design of efficient methanol impermeable membranes for fuel cell applications. Phys. Chem. Chem. Phys. 2012, 14, 2718. [Google Scholar] [CrossRef]
- Lufrano, F.; Baglio, V.; Di Blasi, O.; Staiti, P.; Antonucci, V.; Aricò, A.S. Solid polymer electrolyte based on sulfonated polysulfone membranes and acidic silica for direct methanol fuel cells. Solid State Ionics 2012, 216, 90–94. [Google Scholar] [CrossRef]
- Zolfigol, M.A. Silica sulfuric acid/NaNO2 as a novel heterogeneous system for production of thionitrites and disulfides under mild conditions. Tetrahedron 2001, 57, 9509. [Google Scholar] [CrossRef]
- Lufrano, F.; Baglio, V.; Staiti, P.; Stassi, A.; Aricò, A.S.; Antonucci, V. Investigation of sulfonated polysulfone membranes as electrolyte in a passive-mode direct methanol fuel cell mini-stack. J. Power Sources 2010, 195, 7727–7733. [Google Scholar] [CrossRef]
- Simari, C.; Vecchio, C.L.; Enotiadis, A.; Davoli, M.; Baglio, V.; Nicotera, I. Toward optimization of a robust low-cost sulfonated polyethersulfone containing layered double hydroxide for PEM fuel cells. J. Appl. Polym. Sci. 2019, 136, 47884. [Google Scholar] [CrossRef]
- Simari, C.; Enotiadis, A.; Lo Vecchio, C.; Baglio, V.; Coppola, L.; Nicotera, I. Advances in hybrid composite membranes engineering for high-performance direct methanol fuel cells by alignment of 2D nanostructures and a dual-layer aqpproach. J. Membrane Sci. 2020, 599, 117858. [Google Scholar] [CrossRef]
- Qi, Z.; Kaufman, A. Open circuit voltage and methanol crossover in DMFCs. J. Power Sources 2002, 110, 177–185. [Google Scholar] [CrossRef]
- Fu, Y.-Z.; Manthiram, A. Synthesis and characterization of sulfonated polysulfone membranes for direct methanol fuel cells. J. Power Sources 2006, 157, 222–225. [Google Scholar] [CrossRef]
- Simari, C.; Lo Vecchio, C.; Baglio, V.; Nicotera, I. Sulfonated polyethersulfone/polyetheretherketone blend as high performing and cost-effective electrolyte membrane for direct methanol fuel cells. Renew. Energy 2020, 159, 336–345. [Google Scholar] [CrossRef]
- MacMillan, B.; Sharp, A.R.; Armstrong, R.L. An NMR investigation of the dynamical characteristics of water absorbed in Nafion. Polymer 1999, 40, 2471–2480. [Google Scholar] [CrossRef]
- Pereira, F.; Vallé, K.; Belleville, P.; Morin, A.; Lambert, S.; Sanchez, C. Advanced mesostructured hybrid silica−Nafion membranes for high-performance PEM fuel cell. Chem. Mater. 2008, 20, 1710–1718. [Google Scholar] [CrossRef]
- Nicotera, I.; Angjeli, K.; Coppola, L.; Aricò, A.S.; Baglio, V. NMR and electrochemical investigation of the transport properties of methanol and water in Nafion and clay-nanocomposites membranes for DMFCs. Membranes 2012, 2, 325–345. [Google Scholar] [CrossRef]
- Sebastián, D.; Baglio, V.; Aricò, A.S.; Serov, A.; Atanassov, P. Performance Analysis of a non-Platinum group metal catalyst based on Iron-Aminoantipyrine for direct methanol fuel cells. Appl. Catal. B Environ. 2016, 182, 297–305. [Google Scholar] [CrossRef]
- Seo, S.H.; Lee, C.S. Impedance characteristics of the direct methanol fuel cell under various operating conditions. Energy Fuels 2008, 22, 1204–1211. [Google Scholar] [CrossRef]
- Oedegaard, A. Characterisation of direct methanol fuel cells under near-ambient conditions. J. Power Sources 2006, 157, 244–252. [Google Scholar] [CrossRef]
- Baglio, V.; Stassi, A.; Matera, F.V.; Kim, H.; Antonucci, V.; Aricò, A.S. AC-Impedance investigation of different MEA configurations for passive-mode DMFC mini-stack applications. Fuel Cells 2010, 10, 124–131. [Google Scholar] [CrossRef]
- Siracusano, S.; Stassi, A.; Baglio, V.; Aricò, A.S.; Capitanio, F.; Tavares, A.C. Investigation of carbon-supported Pt and PtCo catalysts for oxygen reduction in direct methanol fuel cells. Electrochim. Acta 2009, 54, 4844–4850. [Google Scholar] [CrossRef]
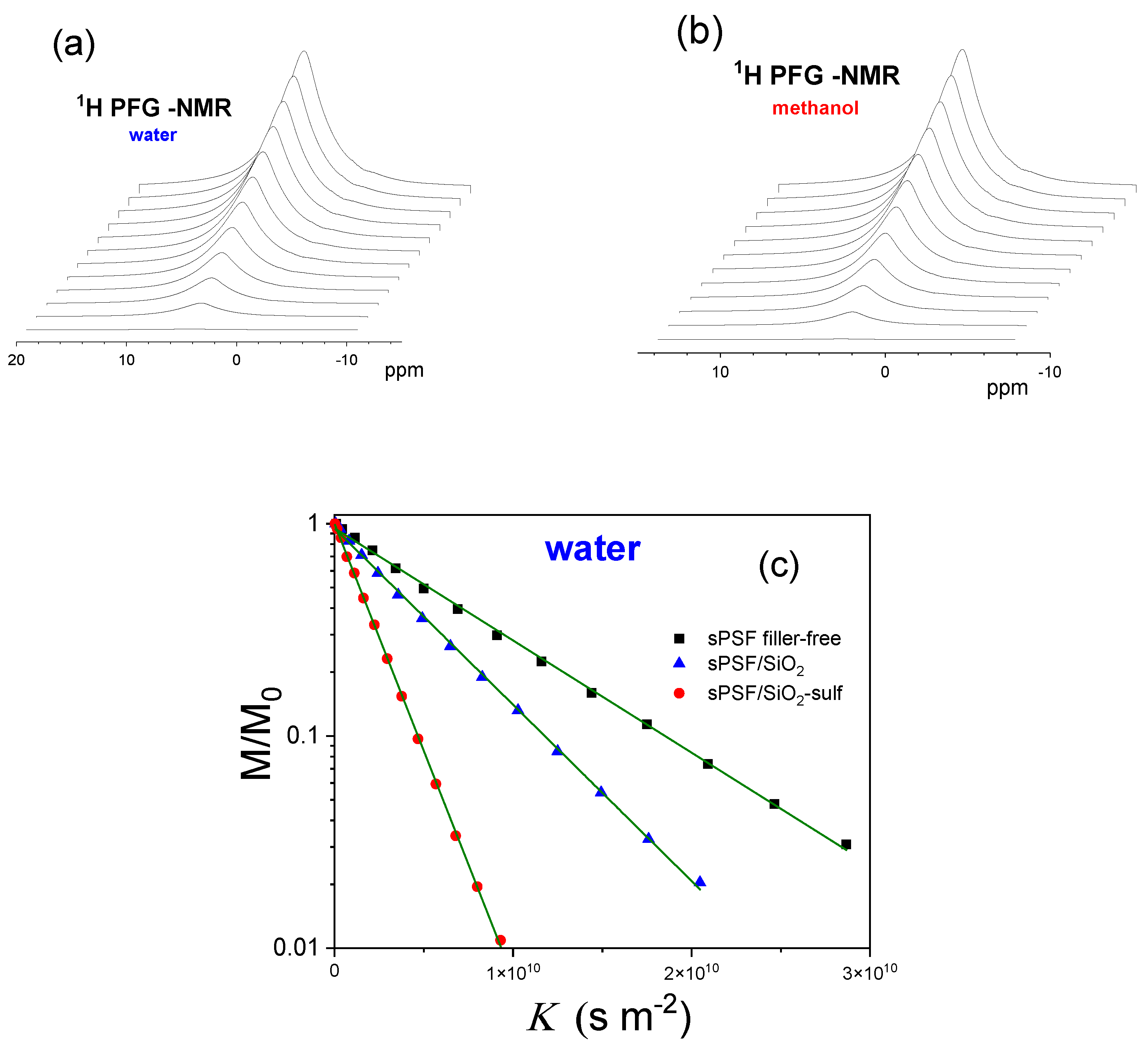
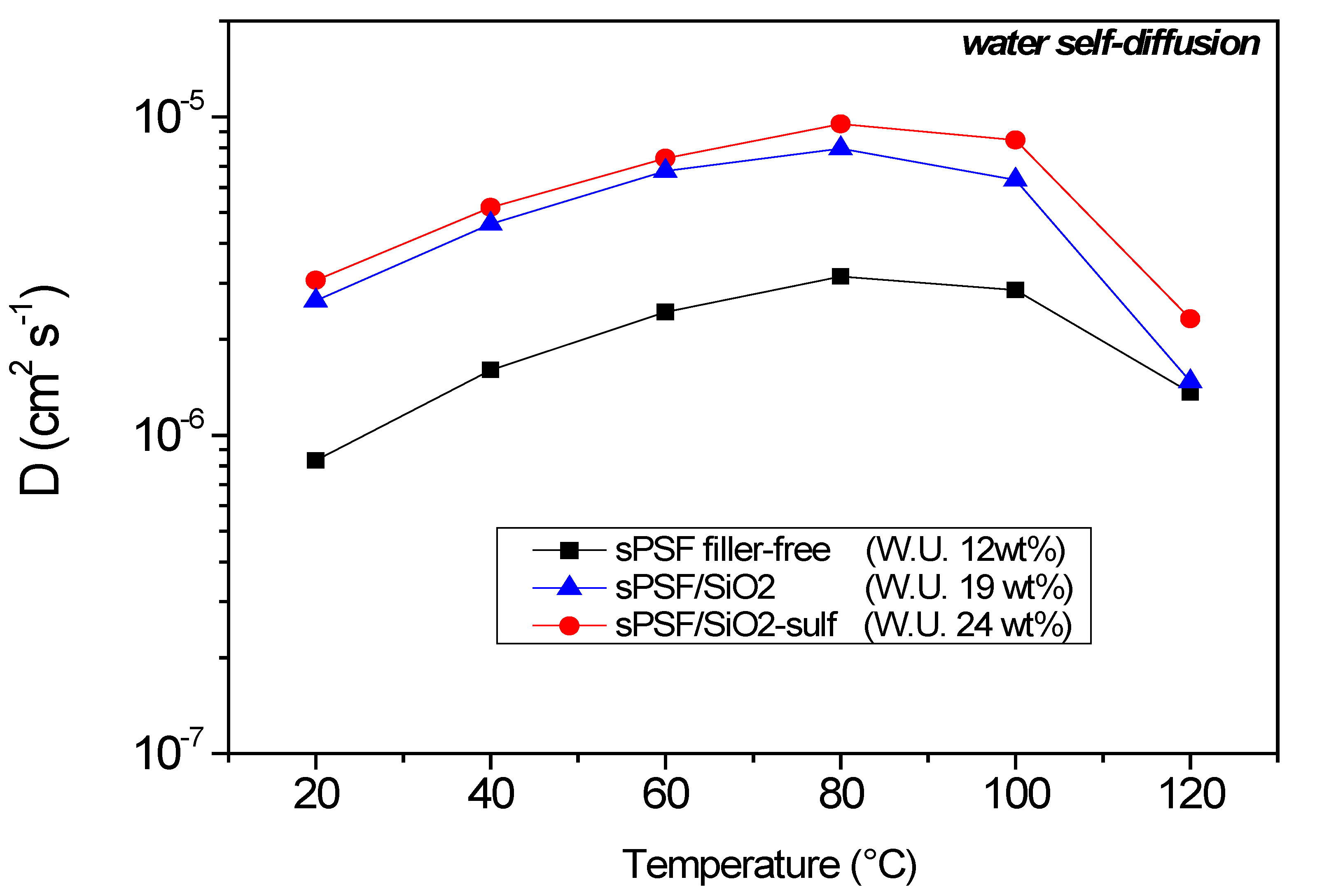
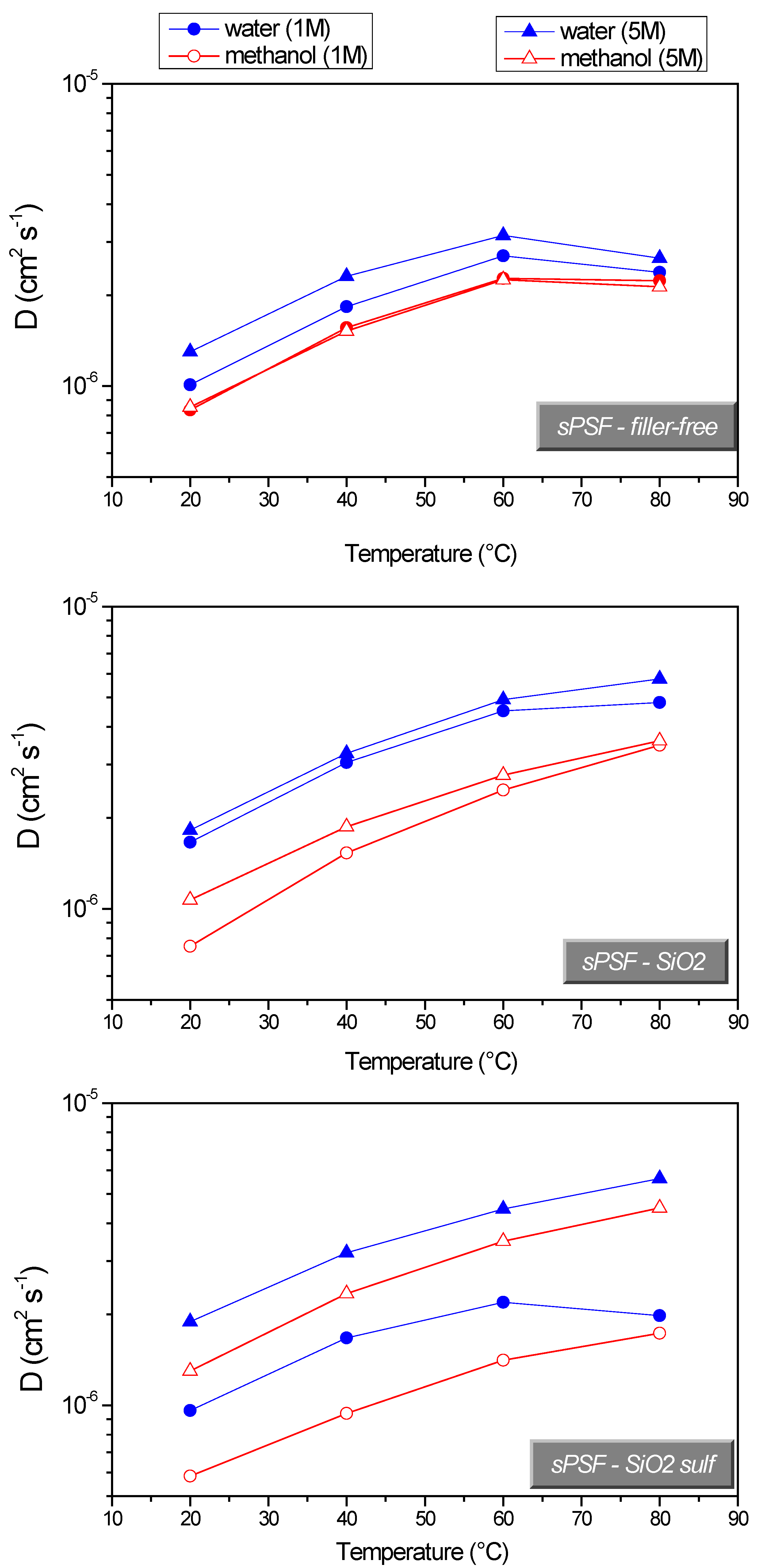
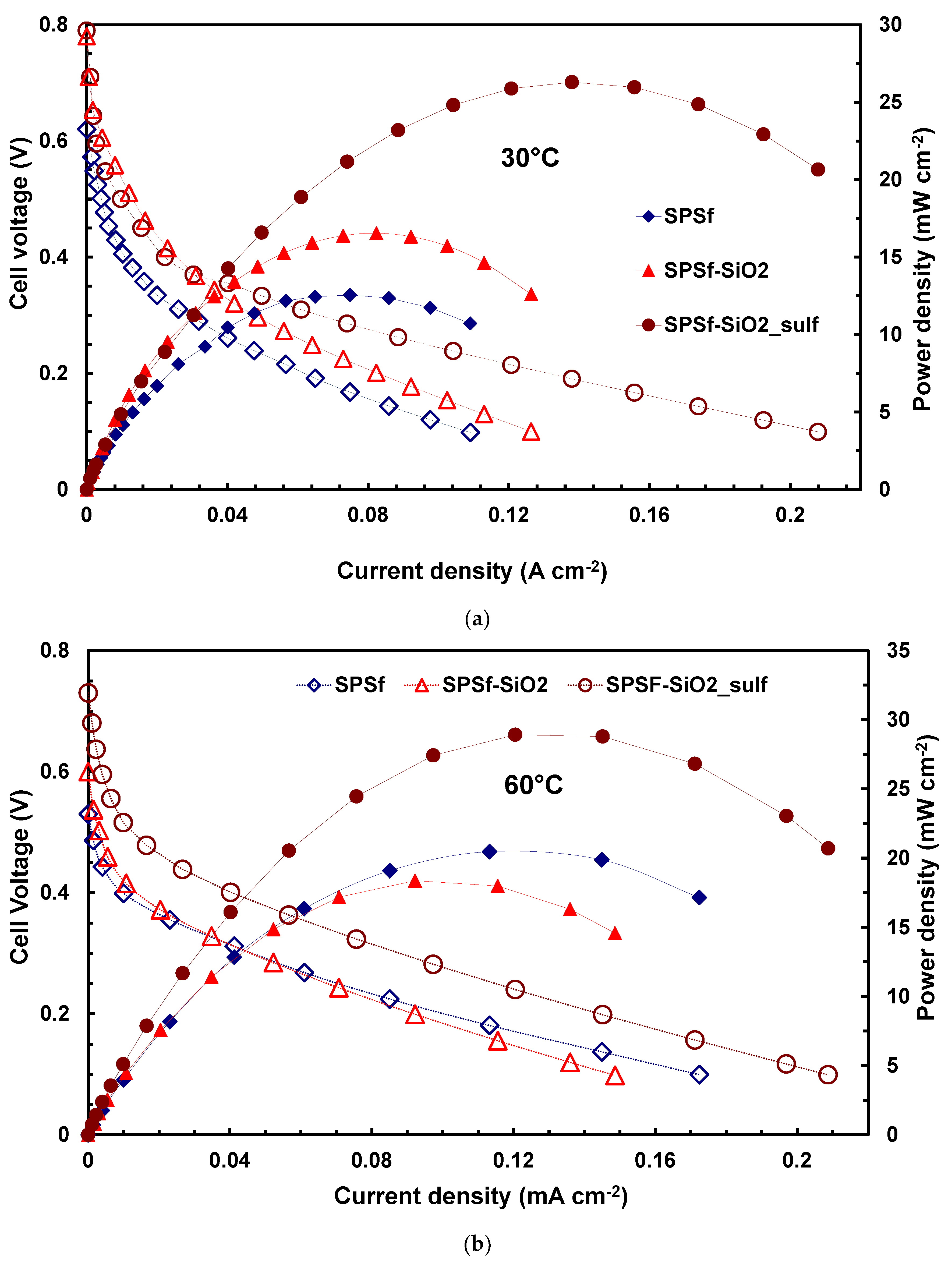
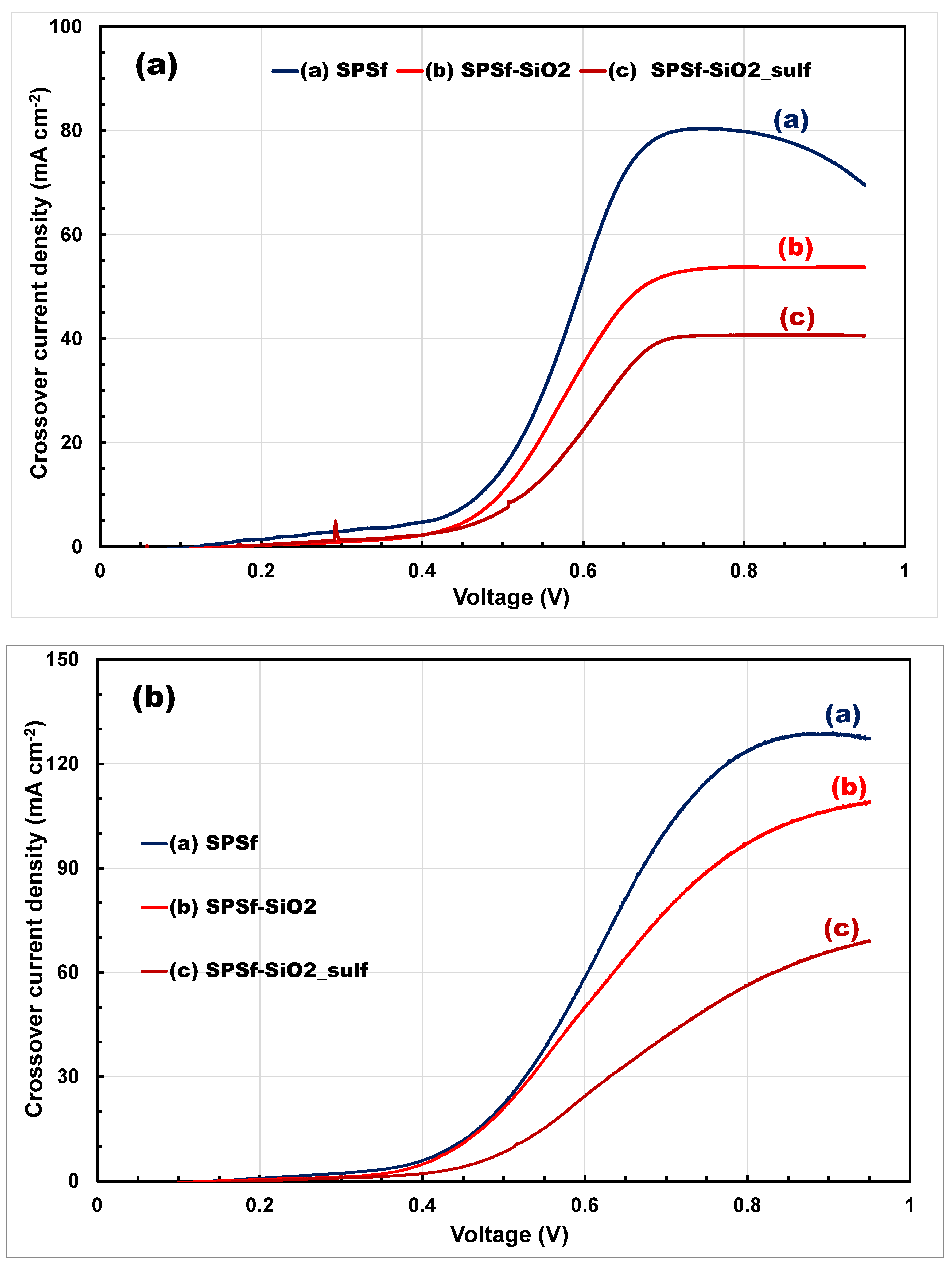

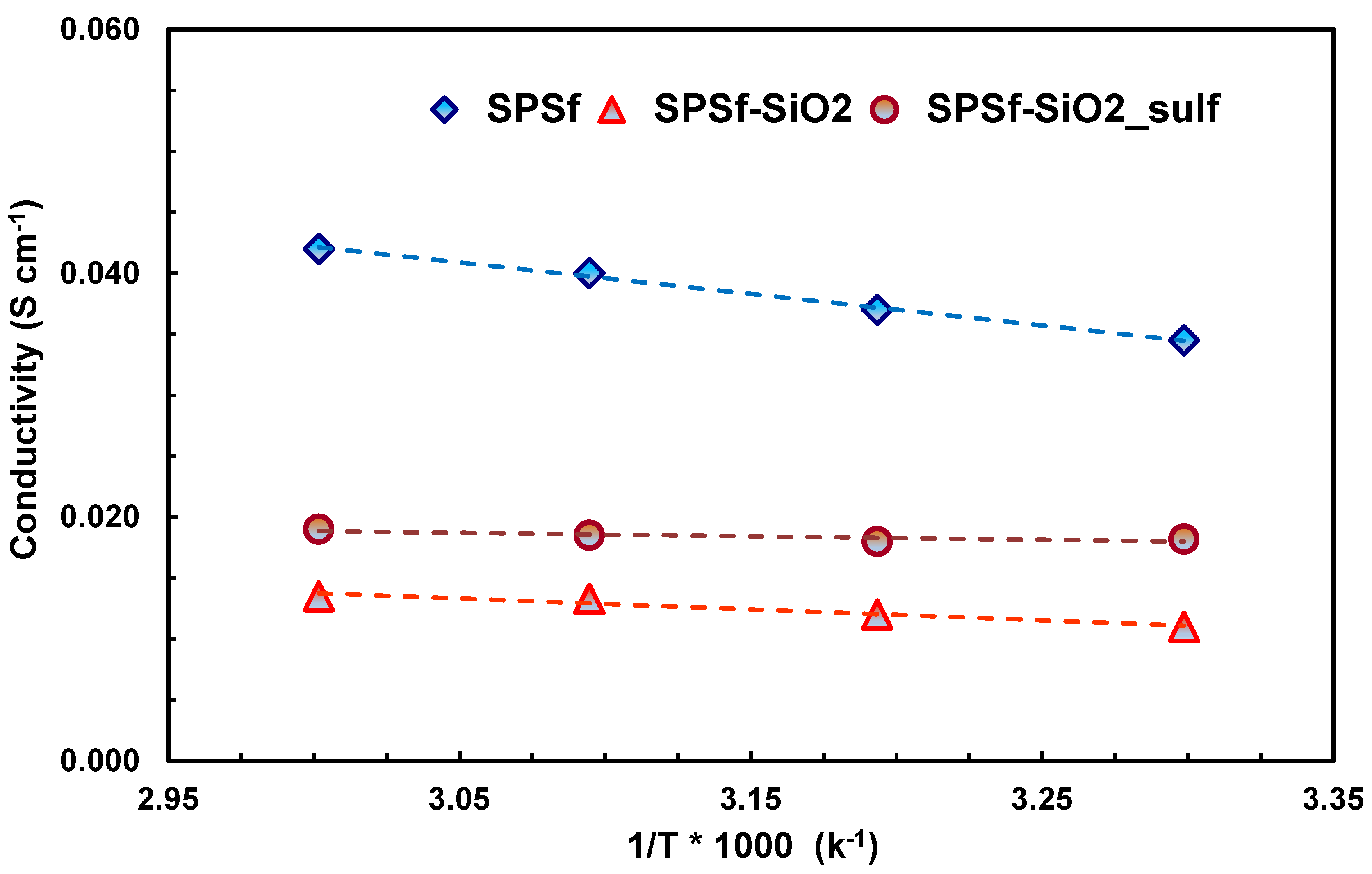
| Membranes | Uptake (wt%) 1 M MeOH Solution | Uptake (wt%) 5 M MeOH Solution |
|---|---|---|
| sPSf (filler-free) | 14 | 20 |
| sPSf-SiO2 | 24 | 30 |
| sPSf-SiO2_sulf | 27 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simari, C.; Nicotera, I.; Aricò, A.S.; Baglio, V.; Lufrano, F. New Insights into Properties of Methanol Transport in Sulfonated Polysulfone Composite Membranes for Direct Methanol Fuel Cells. Polymers 2021, 13, 1386. https://doi.org/10.3390/polym13091386
Simari C, Nicotera I, Aricò AS, Baglio V, Lufrano F. New Insights into Properties of Methanol Transport in Sulfonated Polysulfone Composite Membranes for Direct Methanol Fuel Cells. Polymers. 2021; 13(9):1386. https://doi.org/10.3390/polym13091386
Chicago/Turabian StyleSimari, Cataldo, Isabella Nicotera, Antonino Salvatore Aricò, Vincenzo Baglio, and Francesco Lufrano. 2021. "New Insights into Properties of Methanol Transport in Sulfonated Polysulfone Composite Membranes for Direct Methanol Fuel Cells" Polymers 13, no. 9: 1386. https://doi.org/10.3390/polym13091386
APA StyleSimari, C., Nicotera, I., Aricò, A. S., Baglio, V., & Lufrano, F. (2021). New Insights into Properties of Methanol Transport in Sulfonated Polysulfone Composite Membranes for Direct Methanol Fuel Cells. Polymers, 13(9), 1386. https://doi.org/10.3390/polym13091386










