Strontium Aluminate-Based Long Afterglow PP Composites: Phosphorescence, Thermal, and Mechanical Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Composites
2.2.2. Characterization of the Composites
3. Results
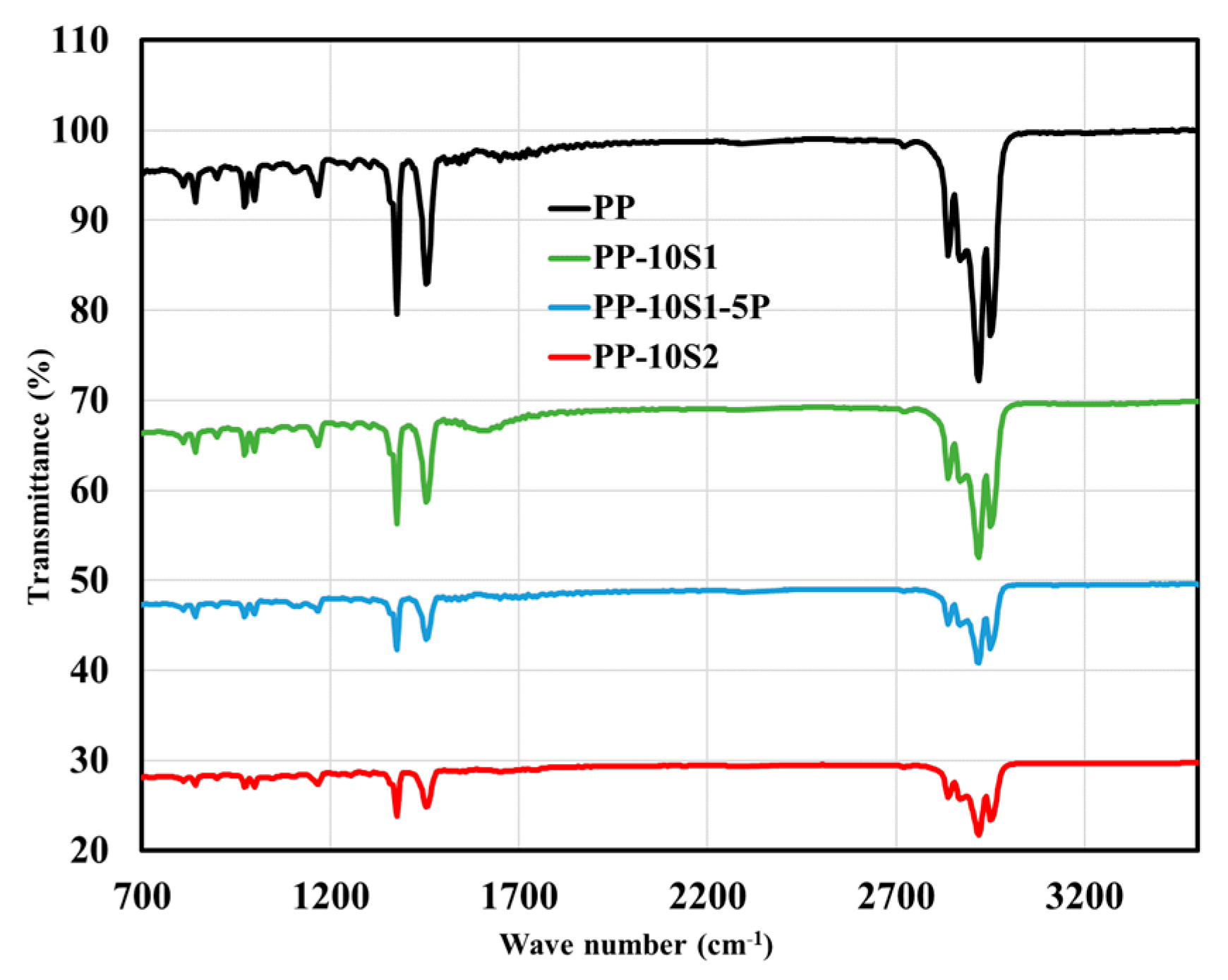
3.1. DSC and ATR-FTIR Data
3.2. X-ray Diffraction Studies of PP, PP/10S1, PP/10S2, and PP/10S1/5P
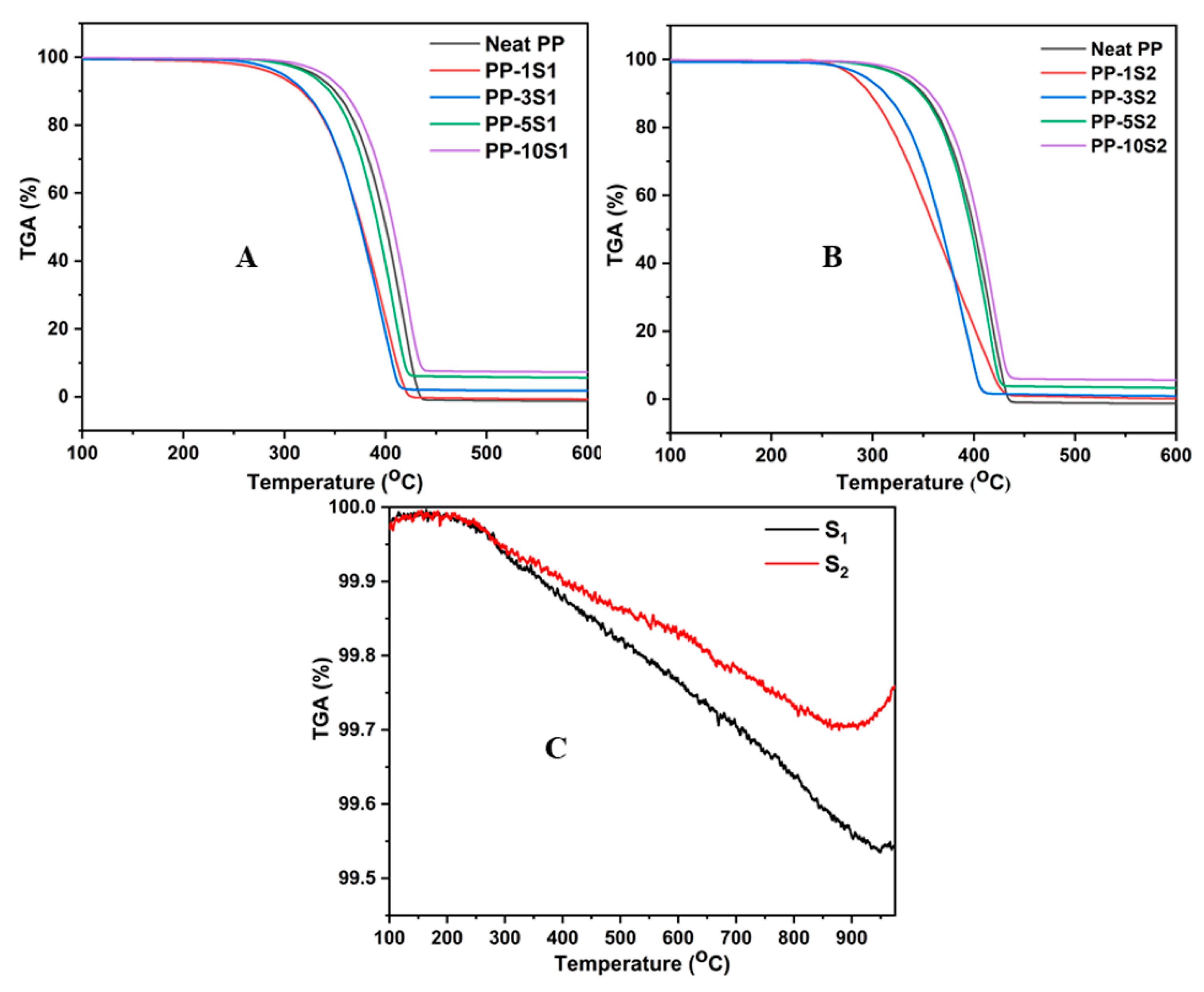
3.3. Thermal Gravimetric Analysis (TGA)
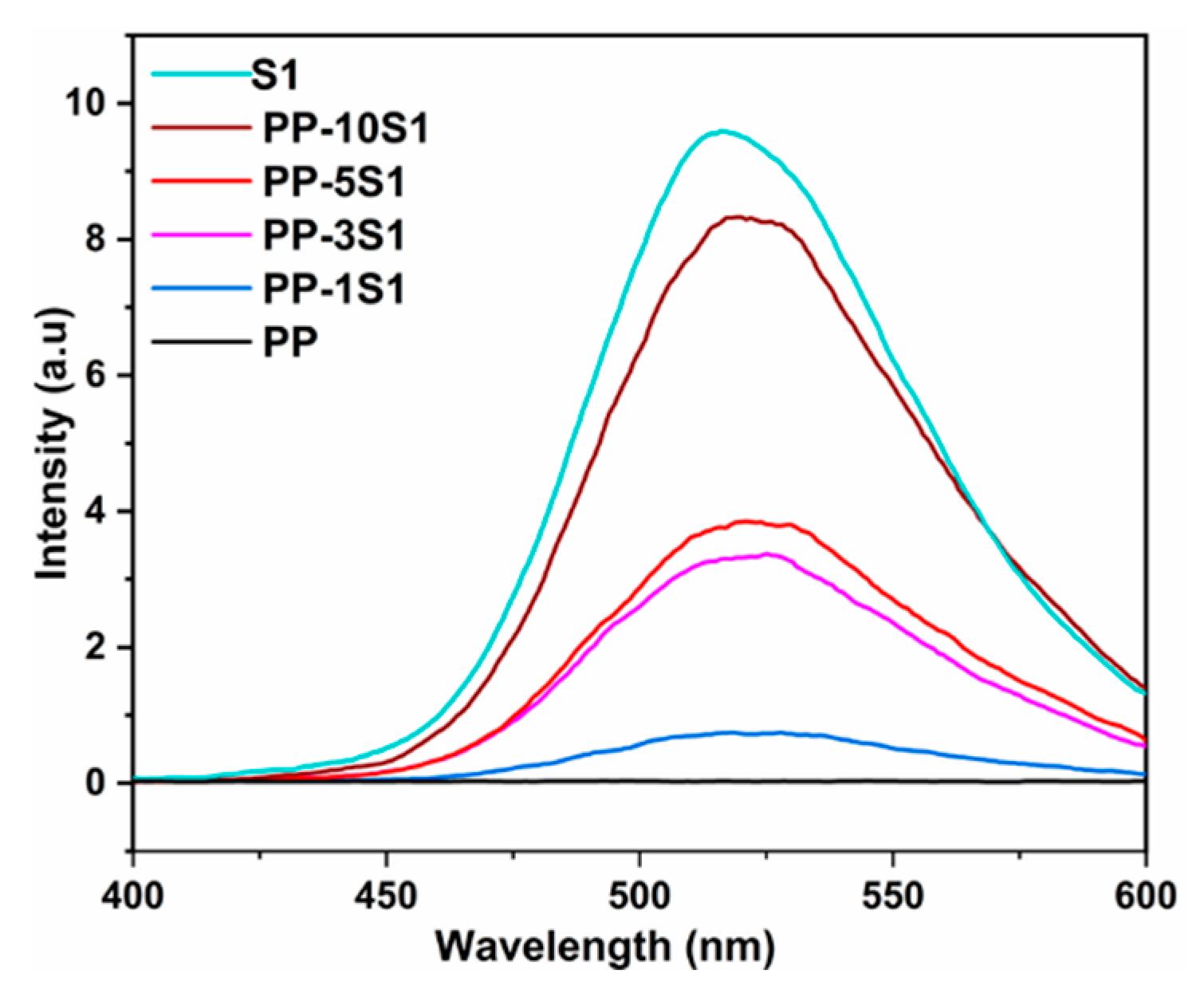
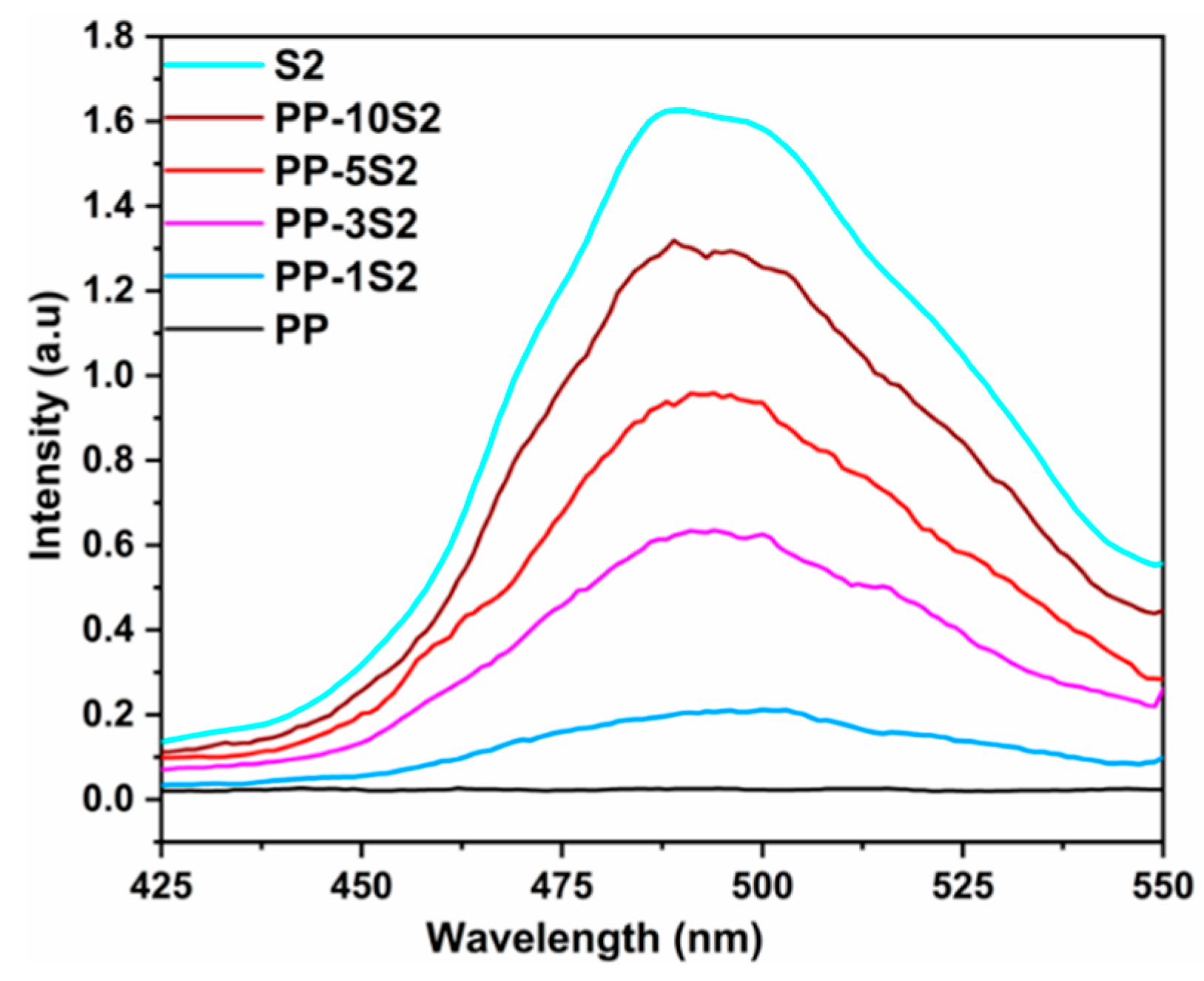
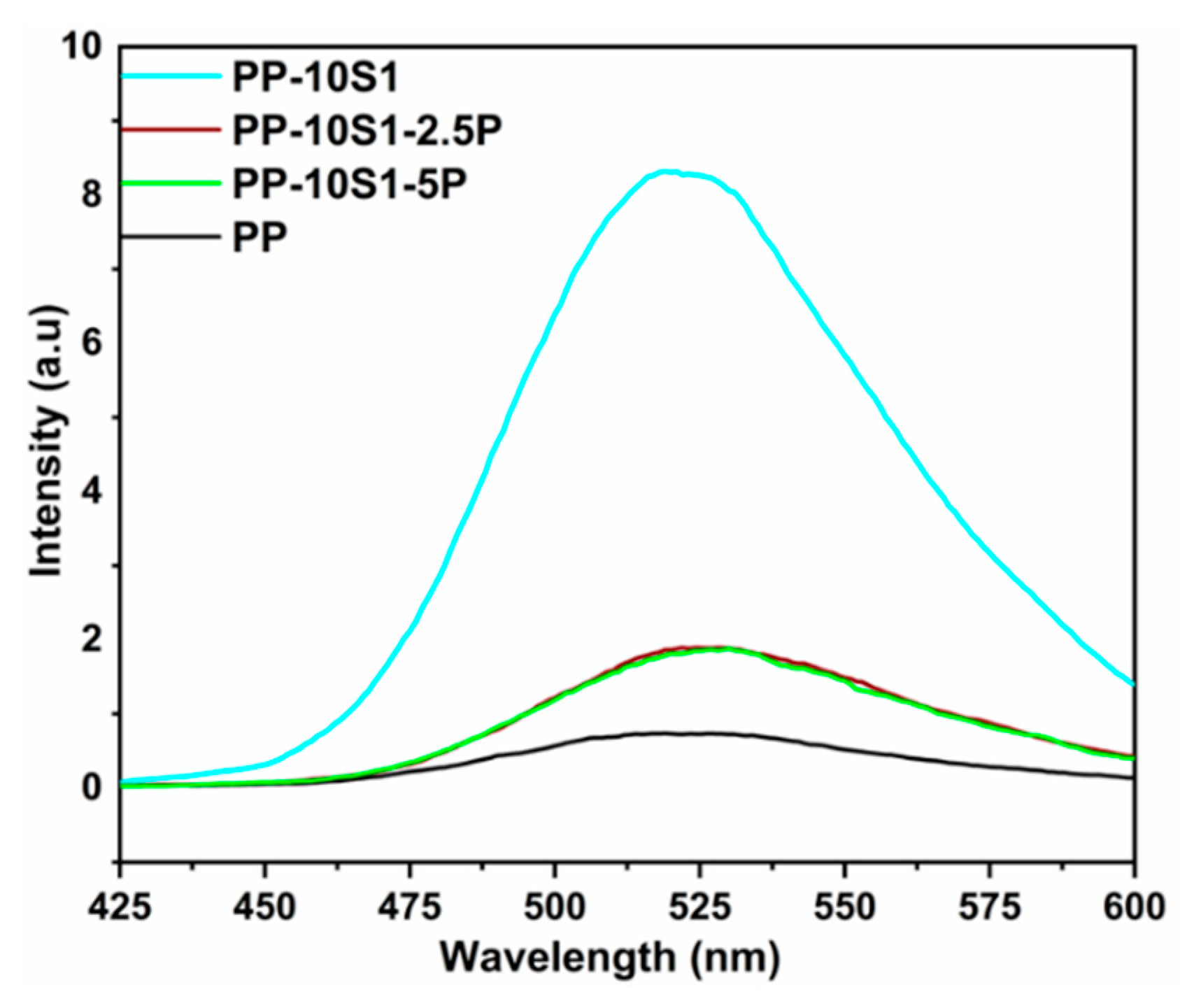
3.4. Phosphorescence Emission
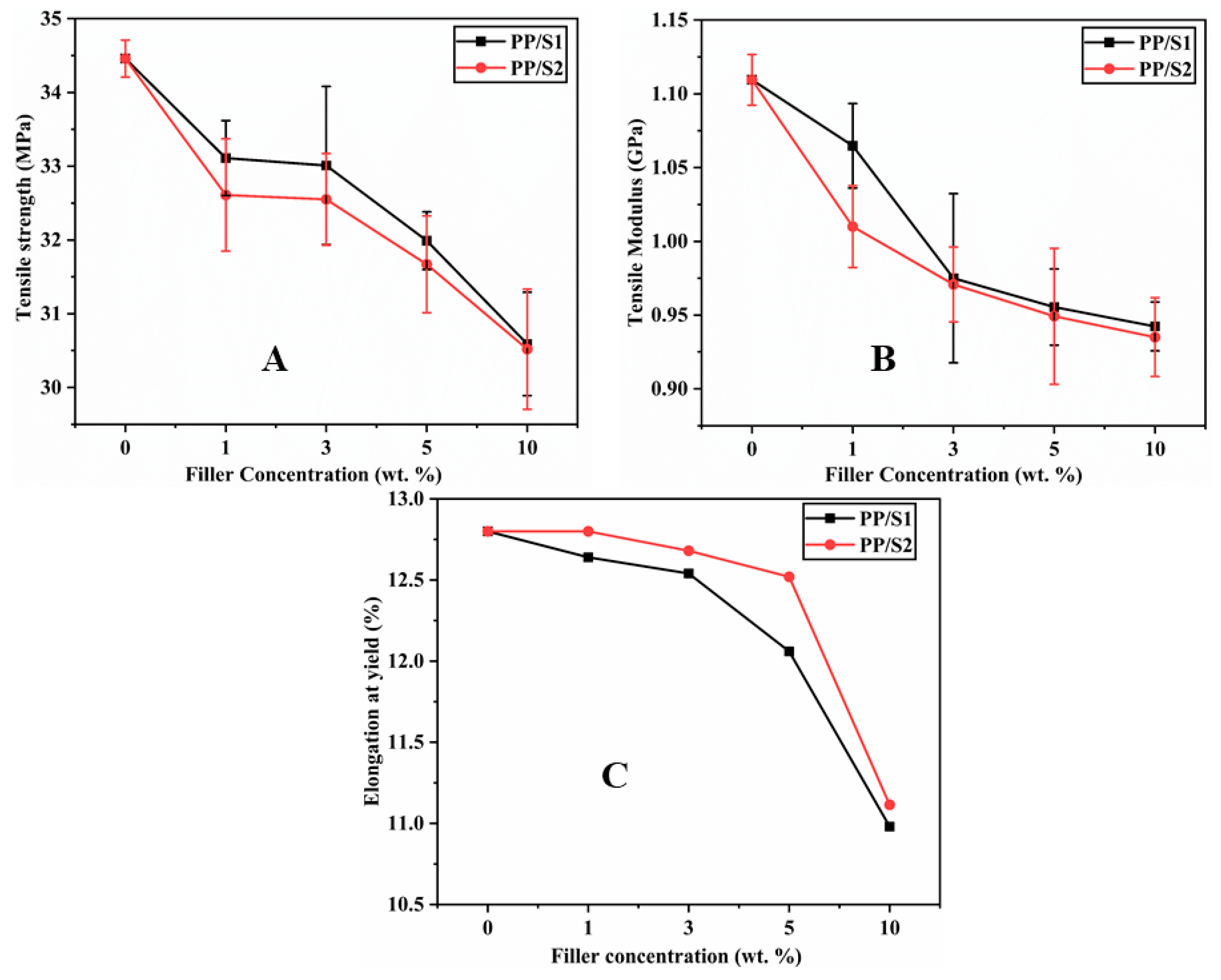
3.5. Mechanical Characteristics of PP/S1 and PP/S2 Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harvey, E.N. A History of Luminescence from the Earliest Times Until 1900; American Philosophical Society: Philadelphia, PA, USA, 1957. [Google Scholar] [CrossRef]
- Liu, X.; Tian, B.; Yu, C.; Tu, B.; Zhao, D. Microwave-assisted solvothermal synthesis of radial ZnS nanoribbons. Chem. Lett. 2004, 33, 522–523. [Google Scholar] [CrossRef]
- Wang, H.; Lu, X.; Zhao, Y.; Wang, C. Preparation and characterization of ZnS:Cu/PVA composite nanofibers via electrospinning. Mater. Lett. 2006, 60, 2480–2484. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Aoki, Y.; Takeuchi, N.; Murayama, Y. A new long phosphorescent phosphor with high brightness, SrAl2O4:Eu2+, Dy3+. J. Electrochem. Soc. 1996, 143, 2670–2673. [Google Scholar] [CrossRef]
- Palilla, F.C.; Levine, A.K.; Tomkus, M.R. Fluorescent properties of alkaline Earth aluminates of the type MAl2O4 activated by divalent europium. J. Electrochem. Soc. 1968, 115, 642–644. [Google Scholar] [CrossRef]
- Nakazawa, T.M.E. Traps in SrA12O4: Eu2+ phosphor with rare-Earth ion doping. J. Lumin. 1997, 72–74, 236–237. [Google Scholar] [CrossRef]
- Sakai, R.; Katsumata, T.; Komuro, S.; Morikawa, T. Effect of composition on the phosphorescence from BaAl2O4:Eu2+, Dy3+ crystals. J. Lumin. 1999, 85, 149–154. [Google Scholar] [CrossRef]
- Arellano-Tánori, O.; Meléndrez, R.; Pedroza-Montero, M.; Castañeda, B.; Chernov, V.; Yen, W.M.; Barboza-Flores, M. Persistent luminescence dosimetric properties of UV-irradiated SrAl2O4:Eu2+, Dy3+ phosphor. J. Lumin. 2008, 128, 173–184. [Google Scholar] [CrossRef]
- Smets, B.; Rutten, J.; Hoeks, G.; Verlijsdonk, J. 2SrO·3Al2O3:Eu2+ and 1.29 (Ba, Ca) O, 6Al2O3:Eu2+: Two new blue-emitting phosphors. J. Electrochem. Soc. 1989, 136, 2119–2123. [Google Scholar] [CrossRef]
- Nakazawa, E.; Murazaki, Y.; Saito, S. Mechanism of the persistent phosphorescence in Sr4Al14O25: Eu and SrAl2O4: Eu codoped with rare Earth ions. J. Appl. Phys. 2006, 100, 113113. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, M.; Zheng, Z.; Liu, L.; Li, L. Synthesis and characterization of europium-doped Sr3Al2O6 phosphors by sol–gel technique. J. Sol Gel Sci. Technol. 2007, 43, 59–64. [Google Scholar] [CrossRef]
- Toh, K.; Nagata, S.; Tsuchiya, B.; Shikama, T. Luminescence characteristics of Sr4Al14O25: Eu,Dy under proton irradiation. Nucl. Instrum. Methods Phys. Res. B 2006, 249, 209–212. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Xiong, Y.; Wang, D.; Yin, Q. SrAl2O4:Eu2+, Dy3+ phosphors derived from a new sol–gel route. Microelectron. J. 2004, 35, 379–382. [Google Scholar] [CrossRef]
- Katsumata, T.; Nabae, T.; Sasajima, K.; Komuro, S.; Morikawa, T. Effects of composition on the long phosphorescent SrAl2O4:Eu2+, Dy3+ phosphor crystals. J. Electrochem. Soc. 1997, 144, L243–L245. [Google Scholar] [CrossRef]
- Nance, J.; Sparks, T.D. From streetlights to phosphors: A review on the visibility of roadway markings. Prog. Org. Coat. 2020, 148, 105749. [Google Scholar] [CrossRef]
- Tan, H.; Wang, T.; Shao, Y.; Yu, C.; Hu, L. Crucial breakthrough of functional persistent luminescence materials for biomedical and information technological applications. Front. Chem. 2019, 7, 387. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, Z.; Zhang, Z. Preparation of long-afterglow Sr4Al14 O25-based luminescent material and its optical properties. Mater. Lett. 2001, 51, 14–18. [Google Scholar] [CrossRef]
- Zheng, R.; Xu, L.; Qin, W.; Chen, J.; Dong, B.; Zhang, L.; Song, H. Electrospinning preparation and photoluminescence properties of SrAl2O4:Ce3+ nanowires. J. Mater. Sci. 2011, 46, 7517–7524. [Google Scholar] [CrossRef]
- Sakirzanovas, S.; Katelnikovas, A.; Dutczak, D.; Kareiva, A.; Jüstel, T. Synthesis and Sm2+/Sm3+ doping effects on photoluminescence properties of Sr4Al14O25. J. Lumin. 2011, 131, 2255–2262. [Google Scholar] [CrossRef]
- Kim, S.J.; Won, H.I.; Hayk, N.; Won, C.W.; Jeon, D.Y.; Kirakosyan, A.G. Preparation and characterization of Sr4Al2O7:Eu3+, Eu2+ phosphors. Mater. Sci. Eng. B 2011, 176, 1521–1525. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, C.Y.; Lu, C.H. Preparation and photoluminescence properties of Sr4Al14O25:Eu2+ phosphors synthesized via the microemulsion route. J. Mater. Sci. Mater. Electron. 2013, 24, 1458–1462. [Google Scholar] [CrossRef]
- Katsumata, T.; Sasajima, K.; Nabae, T.; Komuro, S.; Morikawa, T. Characteristics of strontium aluminate crystals used for long-duration phosphors. J. Am. Ceram. Soc. 1998, 81, 413–416. [Google Scholar] [CrossRef]
- Suriyamurthy, N.; Panigrahi, B.S. Effects of non-stoichiometry and substitution on photoluminescence and afterglow luminescence of Sr4Al14O25:Eu2+, Dy3+ phosphor. J. Lumin. 2008, 128, 1809–1814. [Google Scholar] [CrossRef]
- Peng, T.; Huajun, L.; Yang, H.; Yan, C. Synthesis of SrAl2O4: Eu, Dy phosphor nanometer powders by sol–gel processes and its optical properties. Mater. Chem. Phys. 2004, 85, 68–72. [Google Scholar] [CrossRef]
- Yeşilay Kaya, S.; Karacaoglu, E.; Karasu, B. Effect of Al/Sr ratio on the luminescence properties of SrAl2O4:Eu2+, Dy3+ phosphors. Ceram. Int. 2012, 38, 3701–3706. [Google Scholar] [CrossRef]
- Chen, I.; Chen, T. Effect of host compositions on the afterglow properties of phosphorescent strontium aluminate phosphors derived from the sol–gel method. J. Mater. Res. 2001, 16, 1293–1300. [Google Scholar] [CrossRef][Green Version]
- Bem, D.B.; Swart, H.C.; Luyt, A.S.; Duvenhage, M.M.; Dejene, F.B. Characterization of luminescent and thermal properties of long afterglow SrAlxOy: Eu2+, Dy3+ phosphor synthesized by combustion method. Polym. Compos. 2011, 32, 219–226. [Google Scholar] [CrossRef]
- Xue, Z.; Deng, S.; Liu, Y. Synthesis and luminescence properties of SrAl2O4:Eu2+, Dy3+ nanosheets. Phys. B 2012, 407, 3808–3812. [Google Scholar] [CrossRef]
- Chang, C.; Yuan, Z.; Mao, D. Eu2+ activated long persistent strontium aluminate nano scaled phosphor prepared by precipitation method. J. Alloys Compd. 2006, 415, 220–224. [Google Scholar] [CrossRef]
- Ravichandran, D.; Johnson, S.T.; Erdei, S.; Roy, R.; White, W.B. Crystal chemistry and luminescence of the Eu2+-activated alkaline Earth aluminate phosphors. Displays 1999, 19, 197–203. [Google Scholar] [CrossRef]
- Pathak, P.K.; Kurchania, R. Synthesis and thermoluminescence properties of SrAl2O4 (EU) phosphor irradiated with cobalt-60, 6 MV and 16 MV photon beams. Radiat. Phys. Chem. 2015, 117, 48–53. [Google Scholar] [CrossRef]
- Dorenbos, P. Mechanism of persistent luminescence in Eu2+ and Dy3+ codoped aluminate and silicate compounds. J. Electrochem. Soc. 2005, 152, H107–H110. [Google Scholar] [CrossRef]
- Lü, X.; Zhong, M.; Shu, W.; Yu, Q.; Xiong, X.; Wang, R. Alumina encapsulated SrAl2O4:Eu2+, Dy3+ phosphors. Powder Technol. 2007, 177, 83–86. [Google Scholar] [CrossRef]
- Guo, C.; Luan, L.; Huang, D.; Su, Q.; Lv, Y. Study on the stability of phosphor SrAl2O4:Eu2+, Dy3+ in water and method to improve Its moisture resistance. Mater. Chem. Phys. 2007, 106, 268–272. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, J.; Li, W.; Xu, L.; Guan, Q.; Liu, Y. Encapsulation of strontium aluminate phosphors to enhance water resistance and luminescence. Appl. Surf. Sci. 2009, 255, 7580–7585. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, S.; Liu, Y.; Yang, J. The organic ligands coordinated long afterglow phosphor. Mater. Lett. 2007, 61, 3185–3188. [Google Scholar] [CrossRef]
- Tian, S.; Wen, J.; Fan, H.; Chen, Y.; Yan, J.; Zhang, P. Sunlight-activated long persistent luminescent polyurethane incorporated with amino-functionalized SrAl2O4:Eu2+, Dy3+ phosphor. Polym. Int. 2016, 65, 1238–1244. [Google Scholar] [CrossRef]
- Bem, D.B.; Luyt, A.S.; Dejene, F.B.; Botha, J.R.; Swart, H.C. Structural, luminescent and thermal properties of blue SrAl2O4:Eu2+, Dy3+ phosphor filled low-density polyethylene composites. Phys. B 2009, 404, 4504–4508. [Google Scholar] [CrossRef]
- Bem, D.B.; Swart, H.C.; Luyt, A.S.; Coetzee, E.; Dejene, F.B. Properties of green SrAl2O4 phosphor in LDPE and PMMA polymers. J. Appl. Polym. Sci. 2010, 117, 2635–2640. [Google Scholar] [CrossRef]
- Mishra, S.B.; Mishra, A.K.; Luyt, A.S.; Revaprasadu, N.; Hillie, K.T.; vdM Steyn, W.J.; Coetsee, E.; Swart, H.C. Ethylvinyl acetate copolymer-SrAl2O4: Eu,Dy and Sr4Al14O25:Eu,Dy phosphor-based composites: Preparation and material properties. J. Appl. Polym. Sci. 2010, 115, 579–587. [Google Scholar] [CrossRef]
- Mishra, S.B.; Mishra, A.K.; Revaprasadu, N.; Hillie, K.T.; Steyn, W.V.; Coetsee, E.; Swart, H.C. Strontium aluminate/polymer composites: Morphology, luminescent properties, and durability. J. Appl. Polym. Sci. 2009, 112, 3347–3354. [Google Scholar] [CrossRef]
- Nance, J.; Sparks, T.D. Comparison of coatings for SrAl2O4:Eu2+, Dy3+ powder in waterborne road striping paint under wet conditions. Prog. Org. Coat. 2020, 144, 105637. [Google Scholar] [CrossRef]
- Wan, M.; Jiang, X.; Nie, J.; Cao, Q.; Zheng, W.; Dong, X.; Fan, Z.H.; Zhou, W. Phosphor powders-incorporated polylactic acid polymeric composite used as 3D printing filaments with green luminescence properties. J. Appl. Polym. Sci. 2020, 137, 48644. [Google Scholar] [CrossRef]
- Piramidowicz, R.; Jusza, A.; Lipińska, L.; Gil, M.; Mergo, P. Re3+: LaAlO3 doped luminescent polymer composites. Opt. Mater. 2019, 87, 35–41. [Google Scholar] [CrossRef]
- Barletta, M.; Puopolo, M.; Trovalusci, F.; Vesco, S. High-density polyethylene/SrAl2O4:Eu2+, Dy3+ photoluminescent pigments: Material design, melt processing, and characterization. Polym. Plast. Technol. Eng. 2017, 56, 400–410. [Google Scholar] [CrossRef]
- Paukkeri, R.; Lehtinen, A. Thermal behaviour of polypropylene fractions: 1. influence tacticity mol. weight crystallization melting behaviour. Polymer 1993, 34, 4075–4082. [Google Scholar] [CrossRef]
- Poulose, A.M.; Elnour, A.Y.; Kumar, N.S.; Alhamidi, A.; George, J.; Al-Ghurabi, E.H.; Boumaza, M.; Al-Zahrani, S. Utilization of polyethylene terephthalate waste as a carbon filler in polypropylene matrix: Investigation of mechanical, rheological, and thermal properties. J. Appl. Polym. Sci. 2021, 138, 50292. [Google Scholar] [CrossRef]
- Vidović, E.; Faraguna, F.; Jukić, A. Influence of inorganic fillers on PLA crystallinity and thermal properties. J. Therm. Anal. Calorim. 2017, 127, 371–380. [Google Scholar] [CrossRef]
- Chiu, F.C.; Chu, P.H. Characterization of solution-mixed polypropylene/clay nanocomposites without compatibilizers. J. Polym. Res. 2006, 13, 73–78. [Google Scholar] [CrossRef]
- Luitel, H.N.; Watari, T.; Torikai, T.; Yada, M. Luminescent properties of Cr3+ doped Sr4Al14O25: Eu/Dy blue–green and red phosphor. Opt. Mater. 2009, 31, 1200–1204. [Google Scholar] [CrossRef]
- Aitasalo, T.; Hölsä, J.; Jungner, H.; Lastusaari, M.; Niittykoski, J. Mechanisms of persistent luminescence in Eu2+, Re3+ doped alkaline Earth aluminates. J. Lumin. 2001, 94–95, 59–63. [Google Scholar] [CrossRef]
- Clabau, F.; Rocquefelte, X.; Jobic, S.; Deniard, P.; Whangbo, M.H.; Garcia, A.; Le Mercier, T. Mechanism of phosphorescence appropriate for the long-lasting phosphors Eu2+-doped SrAl2O4 with codopants Dy3+ and B3+. Chem. Mater. 2005, 17, 3904–3912. [Google Scholar] [CrossRef]
- Das, I.; Gupta, S.K. Polyethylene glycol degradation by UV irradiation. Indian J. Chem. 2005, 44a, 1355–1358. [Google Scholar]
- Mammeri, F.; Bourhis, E.L.; Rozes, L.; Sanchez, C. Mechanical properties of hybrid organic–inorganic materials. J. Mater. Chem. 2005, 15, 3787–3811. [Google Scholar] [CrossRef]




| Material | Tc (°C) | Tm (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|
| PP | 121.9 | 164.6 | 87.0 | 42.0 |
| PP/1S1 | 122.6 | 165.7 | 83.8 | 40.5 |
| PP/3S1 | 122.7 | 164.5 | 83.2 | 40.2 |
| PP/5S1 | 122.5 | 164.1 | 83.3 | 40.2 |
| PP/10S1 | 122.9 | 164.2 | 83.8 | 40.5 |
| PP/10S1/2.5P | 122.0 | 164.0 | 82.5 | 39.9 |
| PP10S1/5P | 121.6 | 163.9 | 76.6 | 37.0 |
| Material | Tc (°C) | Tm (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|
| PP | 121.9 | 164.6 | 87.0 | 42.0 |
| PP/1S2 | 120.9 | 163.5 | 74.9 | 36.2 |
| PP/3S2 | 120.0 | 164.4 | 71.4 | 34.5 |
| PP/5S2 | 120.1 | 164.6 | 68.6 | 33.1 |
| PP/10S2 | 120.7 | 164.9 | 66.9 | 32.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulose, A.M.; Anis, A.; Shaikh, H.; Alhamidi, A.; Siva Kumar, N.; Elnour, A.Y.; Al-Zahrani, S.M. Strontium Aluminate-Based Long Afterglow PP Composites: Phosphorescence, Thermal, and Mechanical Characteristics. Polymers 2021, 13, 1373. https://doi.org/10.3390/polym13091373
Poulose AM, Anis A, Shaikh H, Alhamidi A, Siva Kumar N, Elnour AY, Al-Zahrani SM. Strontium Aluminate-Based Long Afterglow PP Composites: Phosphorescence, Thermal, and Mechanical Characteristics. Polymers. 2021; 13(9):1373. https://doi.org/10.3390/polym13091373
Chicago/Turabian StylePoulose, Anesh Manjaly, Arfat Anis, Hamid Shaikh, Abdullah Alhamidi, Nadavala Siva Kumar, Ahmed Yagoub Elnour, and Saeed M. Al-Zahrani. 2021. "Strontium Aluminate-Based Long Afterglow PP Composites: Phosphorescence, Thermal, and Mechanical Characteristics" Polymers 13, no. 9: 1373. https://doi.org/10.3390/polym13091373
APA StylePoulose, A. M., Anis, A., Shaikh, H., Alhamidi, A., Siva Kumar, N., Elnour, A. Y., & Al-Zahrani, S. M. (2021). Strontium Aluminate-Based Long Afterglow PP Composites: Phosphorescence, Thermal, and Mechanical Characteristics. Polymers, 13(9), 1373. https://doi.org/10.3390/polym13091373








