A Short Review on Polymeric Biomaterials as Additives for Lubricants
Abstract
1. Introduction
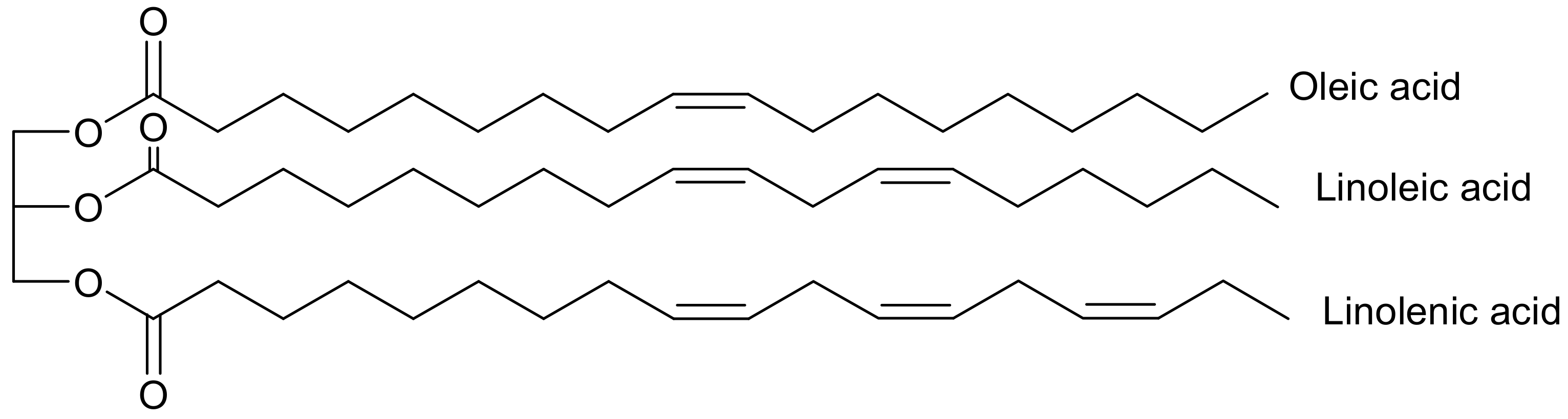
2. Vegetable Oils and Their Physicochemical Properties
3. Polymerization of Different Vegetable Oils/Derivatives, Their Characterization, and Application as Lubricant Additives
3.1. Polymers of Soybean Oil/Derivatives
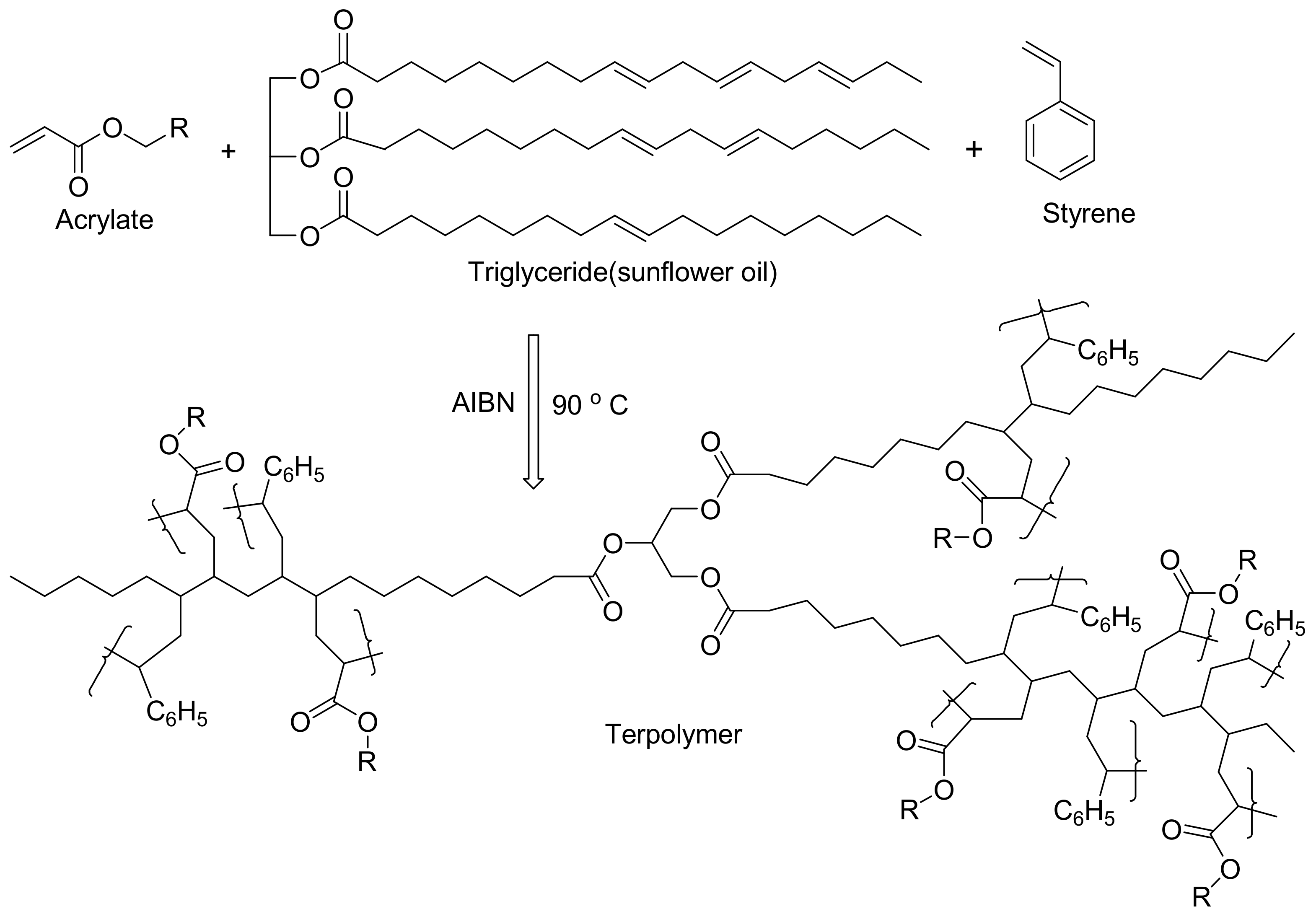
3.2. Polymers of Sunflower Oil/Derivatives
3.3. Polymers of Castor Oil/Derivatives
3.4. Rice Bran Oil-Based Polymer
3.5. Olive Oil-Based Polymers
3.6. Almond Oil-Based Polymers
3.7. Palm Oil-Based Polymers
3.8. Jojoba Oil-Based Polymers
3.9. Preparation of Vegetable Oil-Based Polymer Nanocomposites and Their Application as an Antifriction Additive for Biolubricants
4. Test of Biodegradability
4.1. Disc Diffusion (DD) Method
4.2. The Soil Burial Test (SBT)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta. Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef]
- Bähr, M.; Mülhaupt, R. Linseed and soybean oil-based polyurethanes prepared via the non-isocyanate route and catalytic carbon dioxide conversion. Green Chem. 2012, 14, 483–489. [Google Scholar] [CrossRef]
- Islam, M.R.; Beg, M.D.H.; Jamari, S.S. Development of Vegetable-Oil-Based Polymers. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Kayode, A.A. Review of vegetable oil-based polymers: Synthesis and applications. Open. J. Polym. Chem. 2015, 5, 34–40. [Google Scholar] [CrossRef]
- Florea, M.; Catrinoiu, D.; Paul, L.; Balliu, S. The influence of chemical composition on the pour-point depressant properties of methacrylate copolymers used as additives for lubricating oils. Lubr. Sci. 1999, 12, 31–44. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Naga, H.H.A.E.; Meneir, M.F.E. Multifunctional viscosity index improvers. Chem. Technol. Biotechnol. 1994, 60, 283–289. [Google Scholar] [CrossRef]
- Liu, W.; Hu, L.; Zhang, Z. Friction and wear of the film formed in the immersion test of oil containing antiwear and extreme pressure additives. Thin Solid Films 1995, 271, 88–91. [Google Scholar] [CrossRef]
- Spikes, H.A. Beyond ZDDP. Lubr. Sci. 2008, 20, 77–78. [Google Scholar] [CrossRef]
- Maleque, M.A.; Masjuki, H.H.; Sapuan, S.M. Vegetable-based biodegradable lubricating oil additives. Ind. Lubr. Tribol. 2003, 55, 137–143. [Google Scholar] [CrossRef]
- Maleque, M.A.; Masjuki, H.H.; Haseeb, A.S.M.A. Effect of mechanical factors on tribological properties of palm oil methyl ester blended lubricant. Wear 2000, 239, 117–125. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z.; Perez, J.M. Tribological studies of thermally and chemically modified vegetable oils for use as environmentally friendly lubricants. Wear 2004, 257, 359–367. [Google Scholar] [CrossRef]
- Biresaw, G.; Adhvaryu, A.; Erhan, S.Z. Friction properties of vegetable oils. J. Am. Oil. Chem. Soc. 2003, 80, 697. [Google Scholar] [CrossRef]
- Mobarak, H.M.; Niza Mohamad, E.; Masjuki, H.H.; Kalam, M.A.; Al Mahmud, K.A.H.; Habibullah, M.; Ashraful, A.M. The prospects of biolubricants as alternatives in automotive applications. Renew. Sustain. Energy. Rev. 2014, 33, 34–43. [Google Scholar] [CrossRef]
- Willing, A. Lubricants based on renewable resource—An environmentally compatible alternative to mineral oil products. Chemosphere 2001, 43, 89–98. [Google Scholar] [CrossRef]
- Asadauskas, S.; Perez, J.M.; Duda, J.L. Oxidative stability and anti-wear properties of high oleic vegetable oils. Lubr. Eng. 1996, 52, 877–882. [Google Scholar]
- Wagner, H. Lubricant base fluids based on renewable raw materials. Their catalytic manufacture and modification. Appl. Catal. A 2001, 221, 429–442. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Hupin, S.; Lecamp, L.; Vuluga, D.; Afonso, C.; Burel, F.; Loutelier-Bourhis, C. Thiol–ene chemistry of vegetable oils and their derivatives under UV and air: A model study by using infrared spectroscopy and mass spectrometry. RSC Adv. 2017, 7, 3343–3352. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Biolubricants: Raw materials, chemical modifications and environmental benefits. Eur. J. Lipid Sci. Technol. 2010, 112, 519–530. [Google Scholar] [CrossRef]
- Campanella, A.; Rusto, E.; Baldessari, A.; Baltanás, M.A. Lubricants from chemically modified vegetable oils. Bioresour. Technol. 2010, 101, 245–254. [Google Scholar] [CrossRef]
- Karmakar, G.; Ghosh, P.; Sharma, B.K. Chemically modifying vegetable oils to prepare green lubricants. Lubricants 2017, 5, 44. [Google Scholar] [CrossRef]
- Beran, E.; Łoś, M.; Kmiecik, A. Influence of thermo-oxidative degradation on the biodegradability of lubricant base oils. J. Synth. Lubr. 2008, 25, 75–83. [Google Scholar] [CrossRef]
- Karmakar, G.; Ghosh, P. Atom transfer radical polymerization of soybean oil and its evaluation as a biodegradable multifunctional additive in the formulation of eco-friendly lubricant. ACS Sustain. Chem. Eng. 2016, 4, 775–781. [Google Scholar] [CrossRef]
- Karmakar, G.; Ghosh, P. Green additives for lubricating oil. ACS Sustain. Chem. Eng. 2013, 1, 1364–1370. [Google Scholar] [CrossRef]
- Karmakar, G.; Ghosh, P. Soybean oil as a biocompatible multifunctional additive for lubricating oil. ACS Sustain. Chem. Eng. 2015, 3, 19–25. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Sharma, B.K.; Liu, Z.; Adhvaryu, A. Lubricant base stock potential of chemically modified vegetable oils. J. Agric. Food. Chem. 2008, 56, 8919–8925. [Google Scholar] [CrossRef]
- Quinchia, L.A.; Delgado, M.A.; Valencia, C.; Franco, J.M.; Gallegos, C. Natural and synthetic antioxidant additives for improving the performance of new biolubricant formulations. J. Agric. Food. Chem. 2011, 59, 12917–12924. [Google Scholar] [CrossRef]
- Ghosh, P.; Hoque, M.; Karmakar, G.; Yeasmin, S. Castor oil based multifunctional greener additives for lubricating oil. Curr. Environ. Eng. 2017, 4, 197–206. [Google Scholar] [CrossRef]
- Ghosh, P.; Karmakar, G. Evaluation of sunflower oil as a multifunctional lubricating oil additive. Int. J. Ind. Chem. 2014, 5, 1–10. [Google Scholar] [CrossRef]
- Ghosh, P.; Hoque, M.; Karmakar, G. Terpolymers based on sunflower oil/alkyl acrylate/styrene as sustainable lubricant additive. Polym. Bull. 2016, 74, 2685–2700. [Google Scholar] [CrossRef]
- Xia, Y.; Larock, R.C. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green. Chem. 2010, 12, 1893–1909. [Google Scholar] [CrossRef]
- Wool, R.P. Polymers and composite resins from plant oils. Bio-Based Polym. Compos. 2005, 56, 113. [Google Scholar] [CrossRef]
- Upadhyay, M.; Karmakar, G.; Kapur, G.S.; Ghosh, P. Multifunctional greener additives for lubricating oil. Polym. Eng. Sci. 2017. [Google Scholar] [CrossRef]
- Ghosh, P.; Upadhyay, M. Isodecyl acrylate-olive oil copolymers as potential biodegradable additive for lubricating oil. J. Polym. Res. 2016, 23, 100. [Google Scholar] [CrossRef]
- Talukdar, S.; Upadhyay, M.; Ghosh, P. Synthesis and performance evaluation of vegetable oil polymer as a multifunctional lube oil additive. Pet. Sci. Technol. 2018, 36, 1–8. [Google Scholar] [CrossRef]
- Biresaw, G.; Asadauskas, S.J.; McClure, T.G. Polysulfide and Biobased Extreme Pressure Additive Performance in Vegetable vs Paraffinic Base Oils. Ind. Eng. Chem. Res. 2012, 51, 262–273. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Adhvaryu, A.; Sharma, B.K. Poly(hydroxy thioether) Vegetable Oil Derivatives Useful as Lubricant Additives. U.S. Patent 7,279,448 B2, 9 October 2007. [Google Scholar]
- Landis, P.S. Telomerized Triglyceride Vegetable Oil for Lubricant Additives. U.S. Patent 5,229,023, 20 July 1993. [Google Scholar]
- Nassar, A.M.; Ahmed, N.S.; Nasser, R.M. Jojoba polymers as lubricating oil additives. Pet. Coal 2015, 57, 120–129. [Google Scholar]
- Samad, A.M.; Sinha, S.K. Nanocomposite UHMWPE–CNT Polymer Coatings for Boundary Lubrication on Aluminium Substrates. Tribol. Lett. 2010, 38, 301–311. [Google Scholar] [CrossRef]
- Samad, A.M.; Sinha, S.K. Dry sliding and boundary lubrication performance of a UHMWPE/CNTs nanocomposite coating on steel substrates at elevated temperatures. Wear 2011, 270, 395–402. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. Novel polymeric materials from vegetable oils and vinyl monomers: Preparation, properties, and applications. Chem. Sus. Chem. 2009, 2, 136–147. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Metzger, J.O.; Shubert, U.S. Plant Oil Renewable Resources as Green Alternatives in Polymer Science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Uyama, H.; Kobayashi, S. Green Nanocomposites from Renewable Resources: Biodegradable Plant Oil-Silica Hybrid Coatings. Macromol. Rapid. Commun. 2003, 24, 711–714. [Google Scholar] [CrossRef]
- Fernández-Gutiérrez, M.; Pérez-Köhler, B.; Benito-Martínez, S.; García-Moreno, F.; Gemma Pascual, L.; García-Fernández, M.; Rosa Aguilar, B.; Vázquez-Lasa, J.M.B. Development of Biocomposite Polymeric Systems Loaded with Antibacterial Nanoparticles for the Coating of Polypropylene Biomaterials. Polymers 2020, 12, 1829. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Gámez-Pérez, J.; Cabedo, L. Biocomposites of different lignocellulosic wastes for sustainable food packaging applications. Compos. B Eng. 2018, 145, 215–225. [Google Scholar] [CrossRef]
- Blayo, A.; Gandini, A.; Le Nest, J.F. Chemical and Rheological Characterizations of Some Vegetable Oils Derivates Commonly Used in Printing Inks. Ind. Crops. Prod. 2001, 14, 155–167. [Google Scholar] [CrossRef]
- Hill, K. Fats and Oils as Oleochemical Raw Materials. Pure Appl. Chem. 2000, 72, 1255–1264. [Google Scholar] [CrossRef]
- Soutis, C.; Yi, X.; Bachmann, J. How green composite materials could benefit aircraft construction. Sci. China Technol. Sci. 2019, 62, 1478–1480. [Google Scholar] [CrossRef]
- Ghosh, P.; Das, T.; Nandi, D.; Karmakar, G.; Mandal, A. Synthesis and Characterization of Biodegradable Polymer-Used as a Pour Point Depressant for Lubricating Oil. Int. J. Polym. Mater. 2010, 59, 1008–1017. [Google Scholar] [CrossRef]
- Ghosh, P.; Das, T.; Karmakar, G.; Das, M. Evaluation of acrylate-sunflower oil copolymer as viscosity index improvers for lube oils. J. Chem. Pharm. Res. 2011, 3, 547–556. [Google Scholar]
- Ogunniyi, D.S. Castor oil: A vital industrial raw material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Kunduru, K.R.; Basu, A.; Zada, M.H.; Domb, A.J. Castor oil-based biodegradable polyesters. Biomacromolecules 2015, 16, 2572–2587. [Google Scholar] [CrossRef]
- Mutlu, H.; Meier, M.A.R. Castor oil as a renewable resource for the chemical industry. Eur. J. Lipid. Sci. Technol. 2010, 112, 10–30. [Google Scholar] [CrossRef]
- Singh, A.K. Castor oil-based lubricants reduces smoke emission in two stroke engines. Ind. Crop. Prod. 2011, 33, 287–295. [Google Scholar] [CrossRef]
- Ghosh, P.; Hoque, M.; Karmakar, G. Castor oil as potential multifunctional additive in the formulation of eco-friendly lubricant. Polym. Bull. 2017. [Google Scholar] [CrossRef]
- Ghosh, P.; Dey, K.; Upadhyay, M.; Das, T. Multifunctional biodegradable lube oil additives: Synthesis, characterization, and performance evaluation. Pet. Sci. Technol. 2017, 35, 66–71. [Google Scholar] [CrossRef]
- Saha, D.K.; Upadhyay, M.; Ghosh, P. Dodecylmethacrylate-olive oil copolymers as potential biodegradable pour point depressant for lubricating oil. Pet. Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Saha, D.K.; Ghosh, P. Almond Oil as Potential Biodegradable Lube Oil Additive: A Green Alternative. J. Polym. Environ. 2017, 26, 2392–2400. [Google Scholar] [CrossRef]
- Liew, W.Y.H.; Dayou, S.; Dayou, J.; Siambun, N.J.; Ismail, M.A.B. The effectiveness of palm oil methyl ester as lubricant additive in milling and four-ball tests. Int. J. Surf. Sci. Eng. 2014, 8, 153–172. [Google Scholar] [CrossRef]
- Lee, K.Y.; Wong, L.L.C.; Blaker, J.J.; Hodgkinson, J.M.; Bismarck, A. Bio-based macroporous polymer nanocomposites made by mechanical frothing of acrylated epoxidised soybean oil. Green Chem. 2011, 13, 3117–3123. [Google Scholar] [CrossRef]
- Miyagawa, H.; Misra, M.; Drzal, L.T.; Mohanty, A.K. Novel biobased nanocomposites from functionalized vegetable oil and organically-modified layered silicate clay. Polymer 2005, 46, 445–453. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L.; Xie, G. A review on tribology of polymer composite coatings. Friction 2021, 9, 429–470. [Google Scholar] [CrossRef]
- Bhuyan, S.; Sundararajan, S.; Lu, Y.; Larock, R.C. A study of the physical and tribological properties of biobased polymer–clay nanocomposites at different clay concentrations. Wear 2010, 268, 797–802. [Google Scholar] [CrossRef]
- Bikiaris, D. Can nanoparticles really enhance thermal stability of polymers? Part II: An overview on thermal decomposition of polycondensation polymers. Thermochim. Acta. 2011, 523, 25–45. [Google Scholar] [CrossRef]
- Pagga, U.; Beimborn, D.B.; Yamamoto, M. Biodegradability and compostability of polymers—Test methods and criteria for evaluation. J. Environ. Polym. Degr. 1996, 4, 173–178. [Google Scholar] [CrossRef]
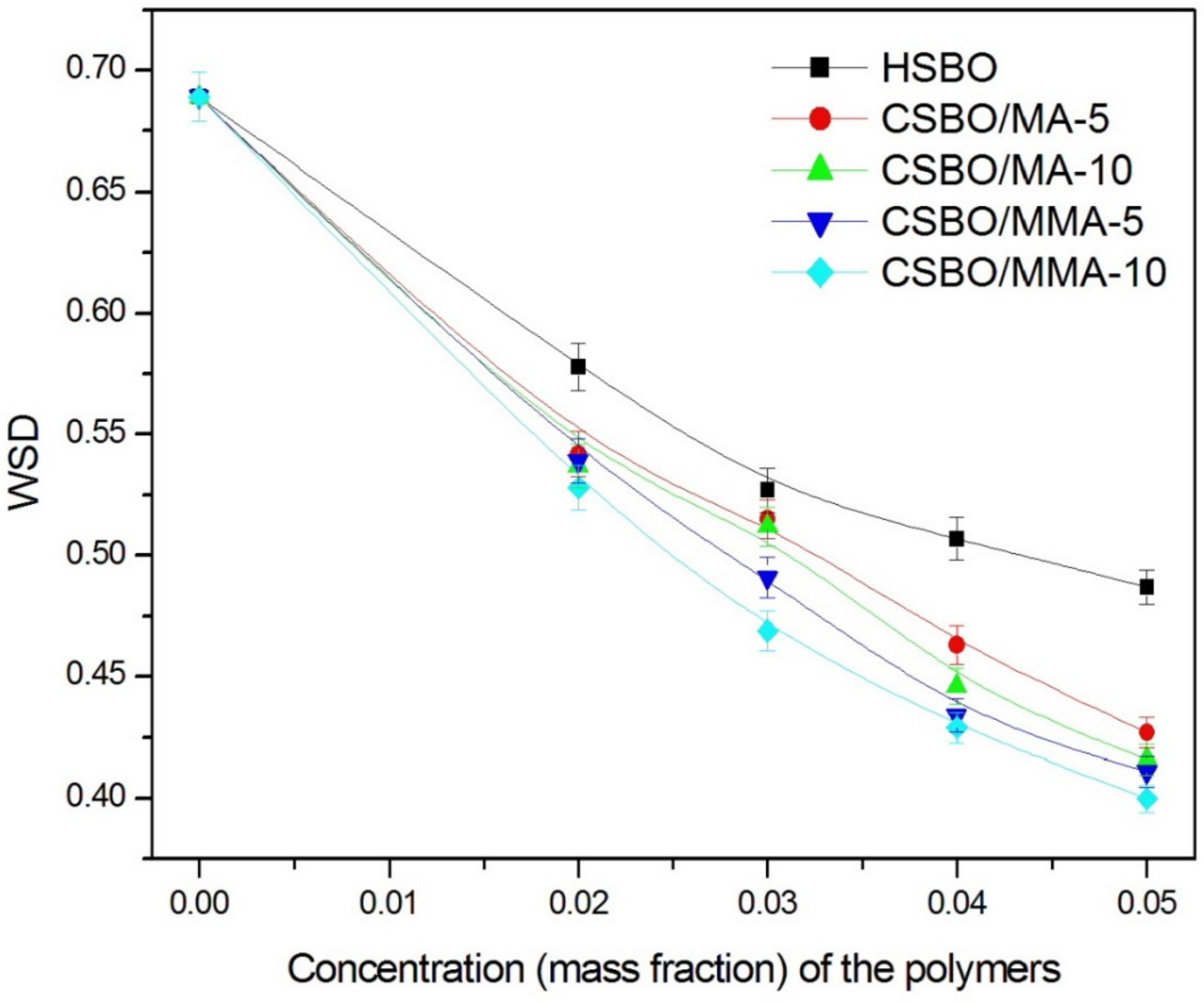
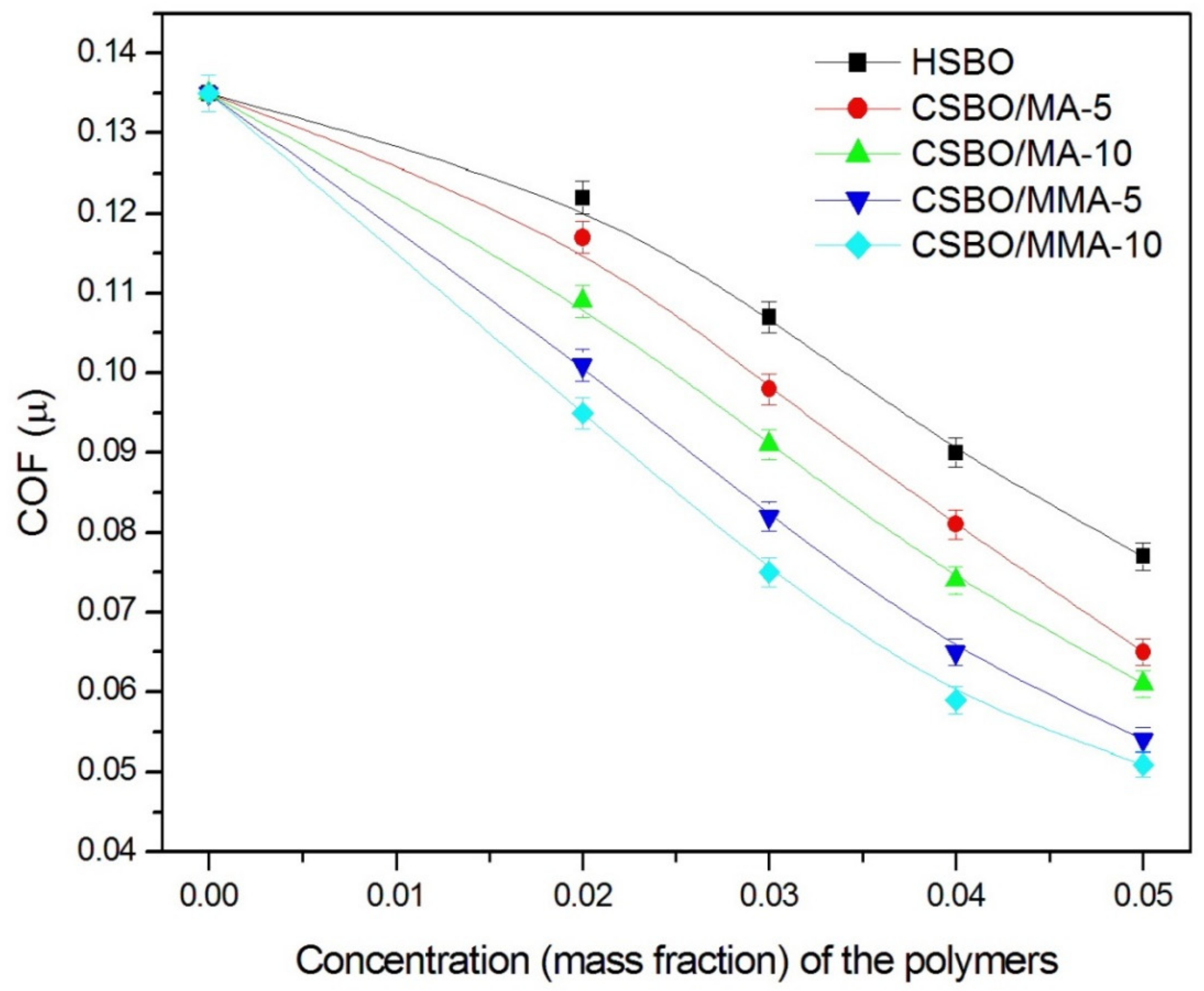
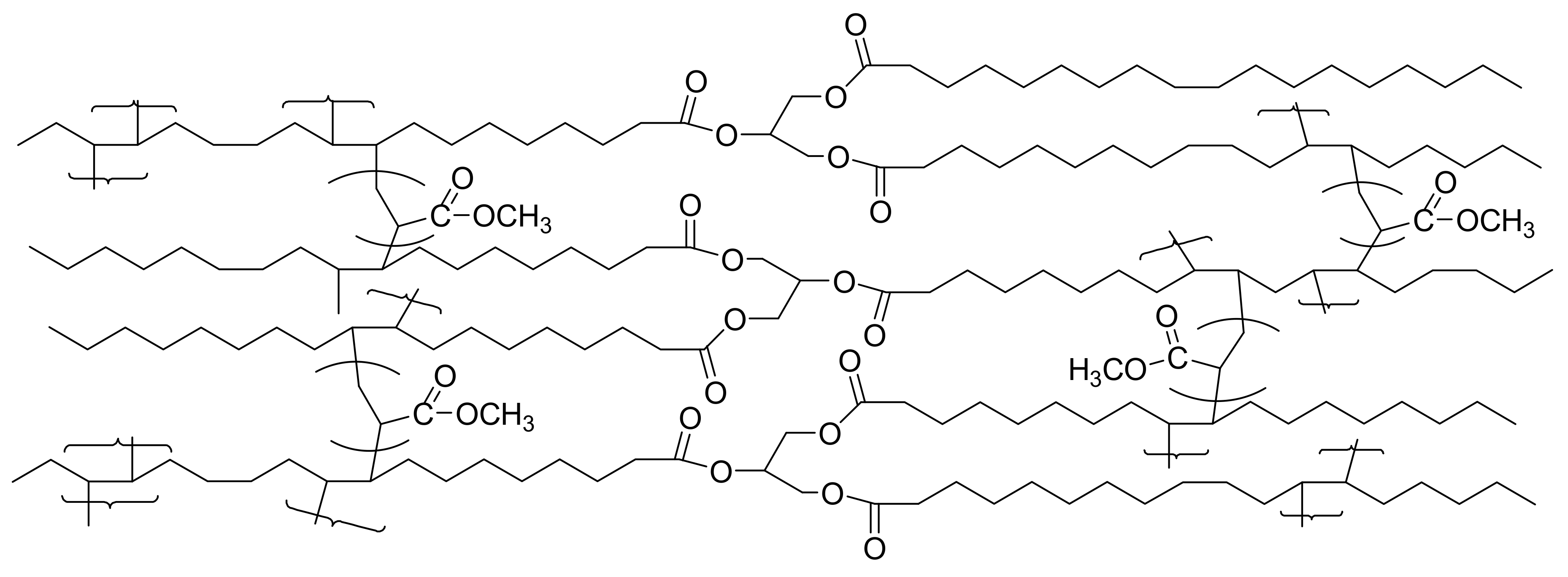


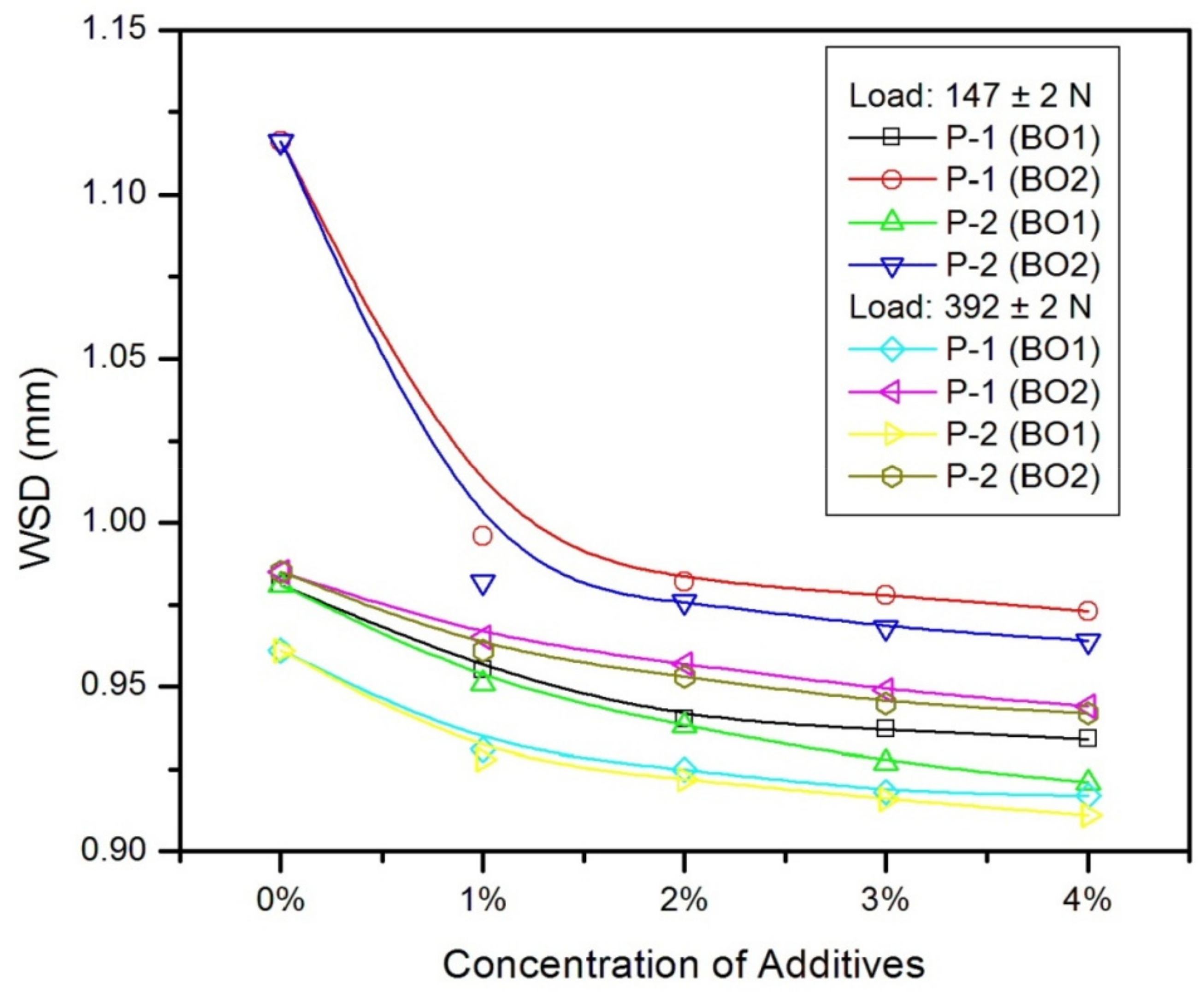

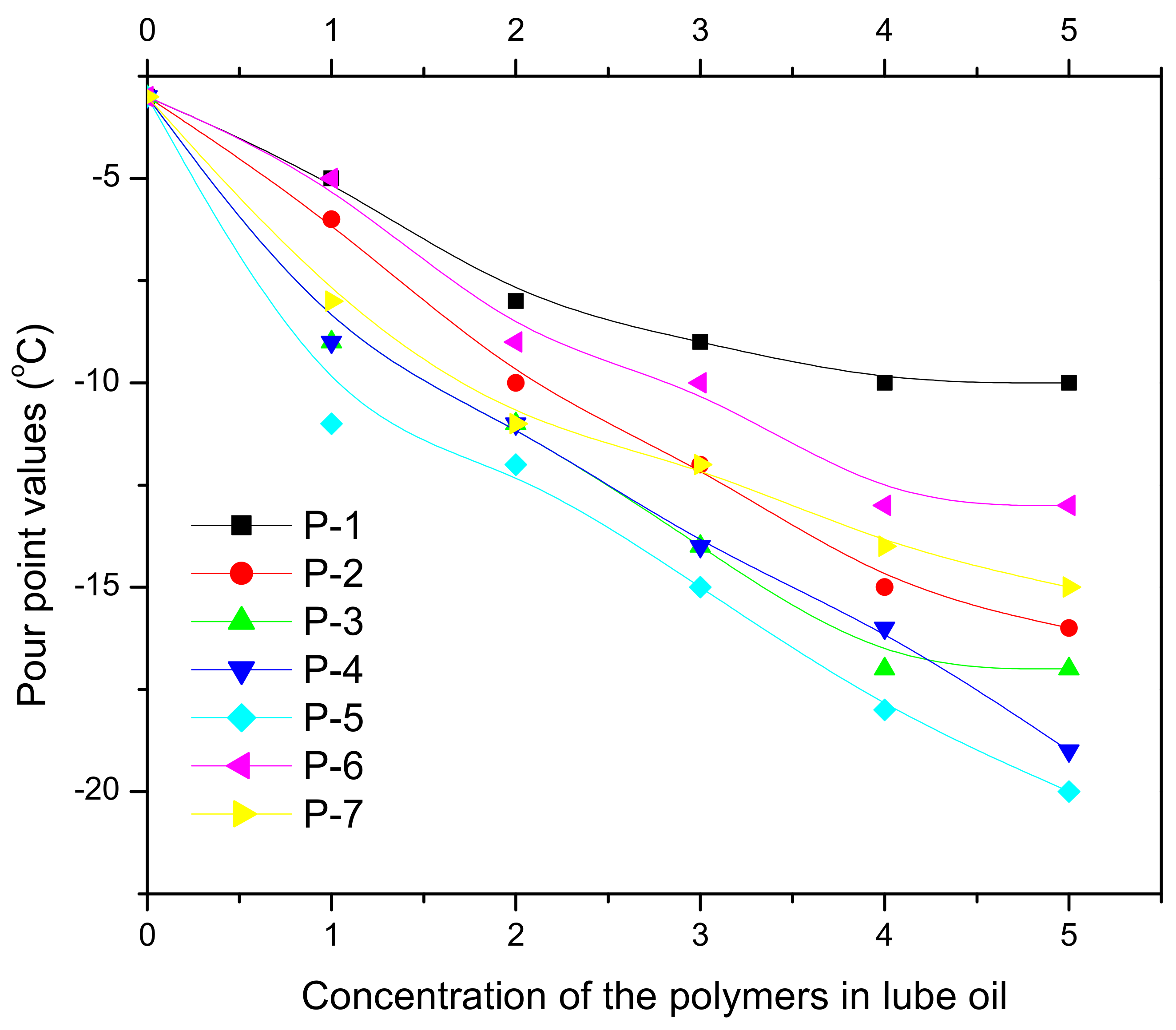
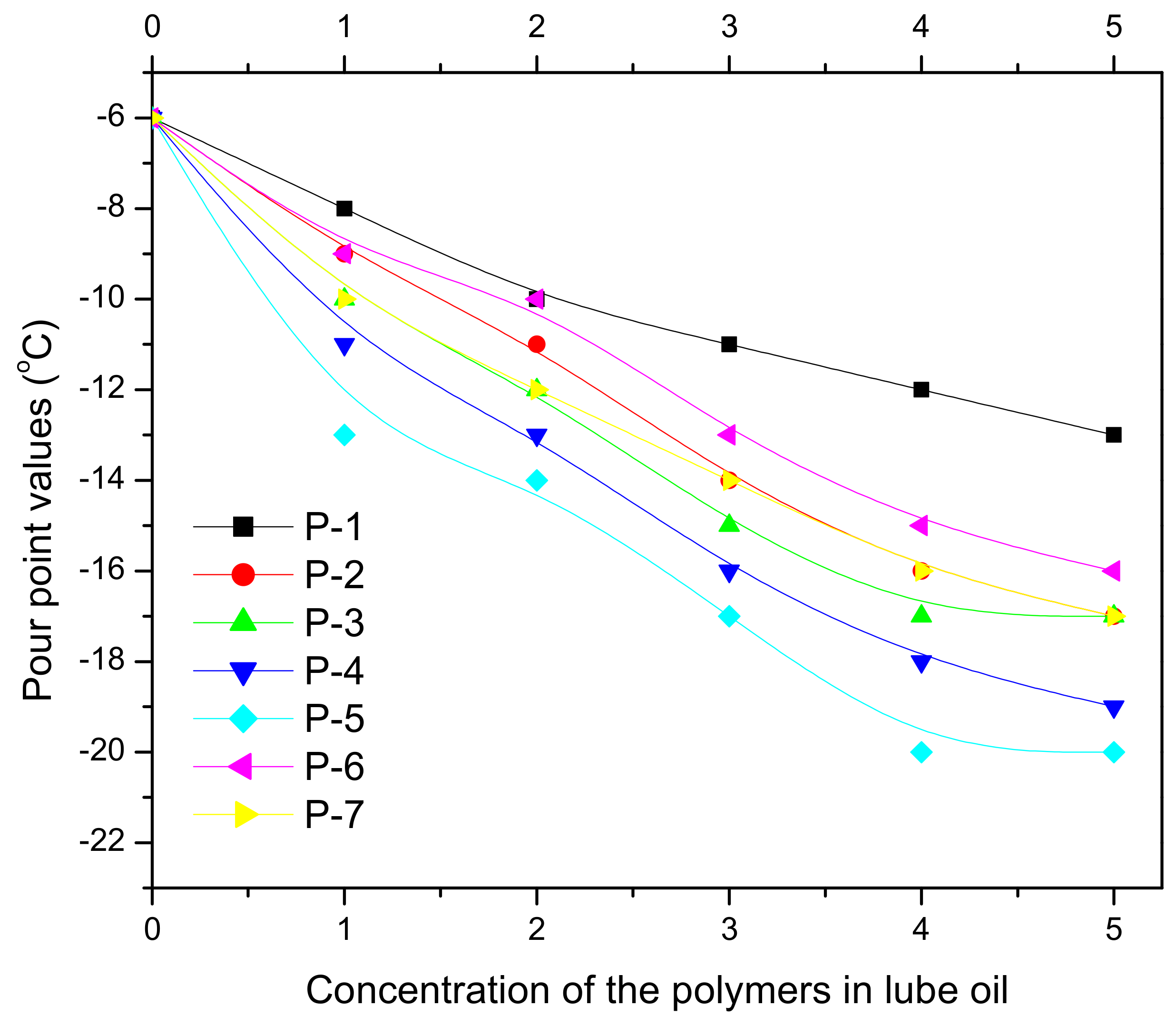
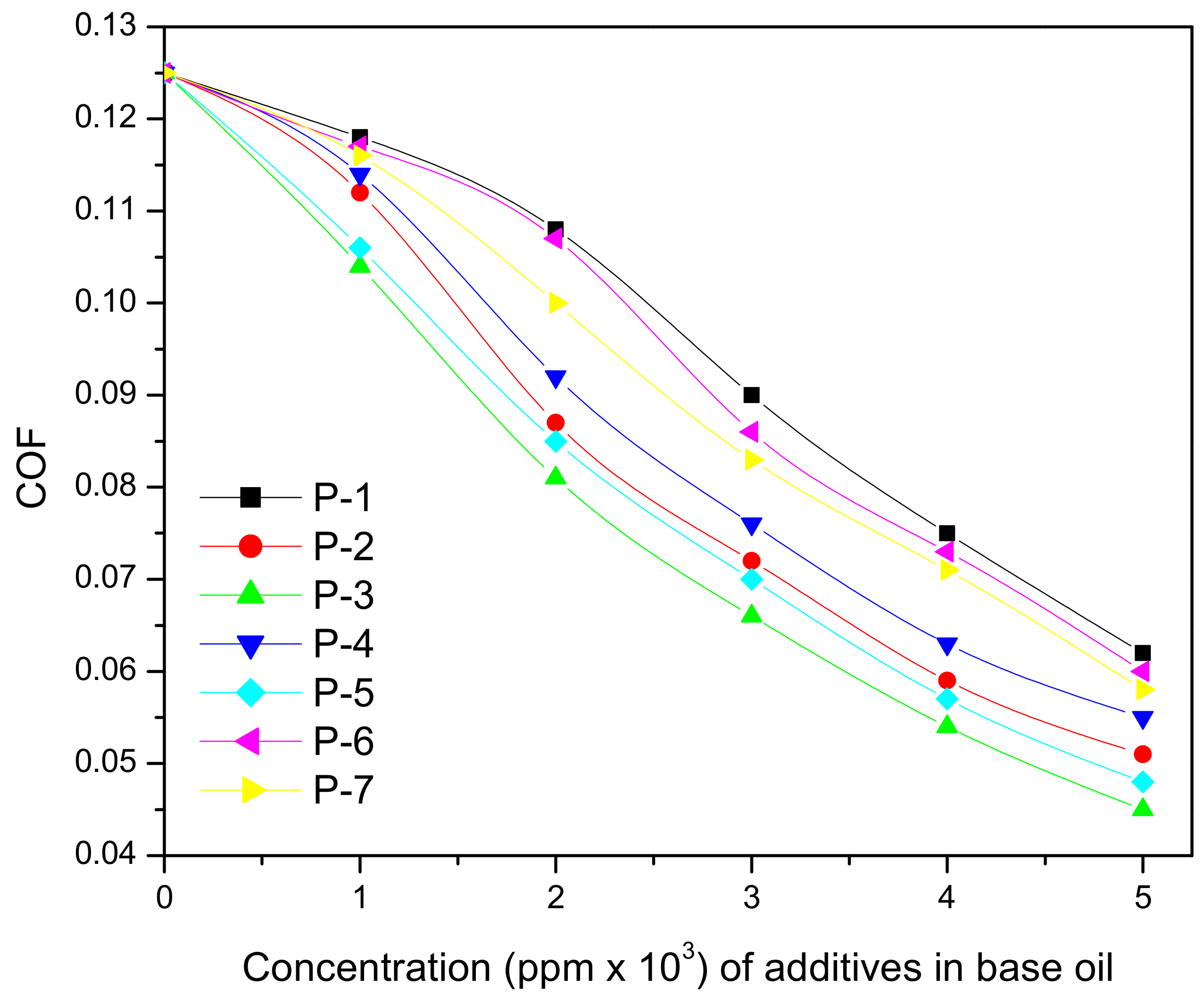
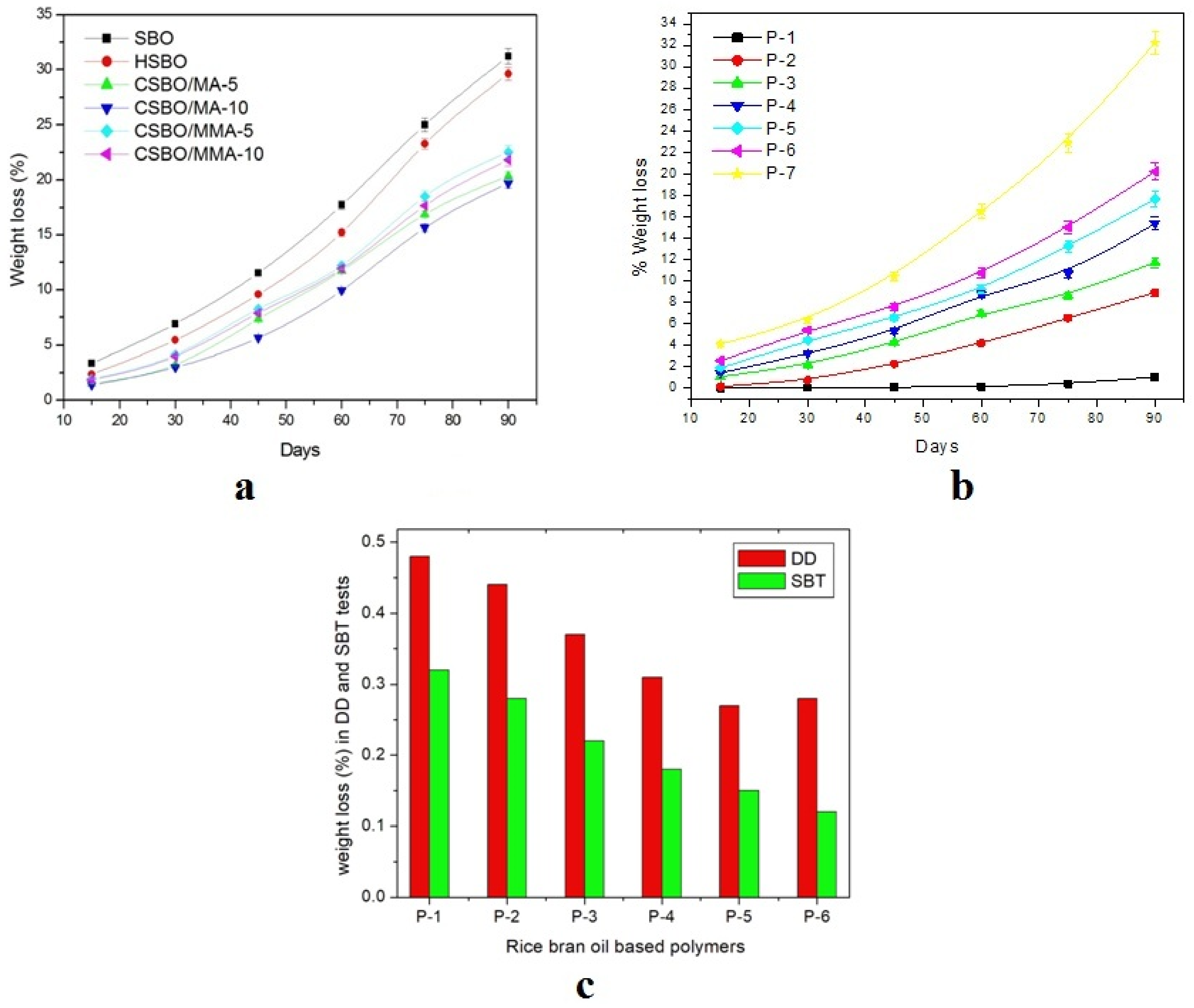
| Vegetable Oils | C12:0 | C14:0 | C16:0 | C18:0 | C16:1 | C18:1 | C18:2 | C18:3 | Others |
|---|---|---|---|---|---|---|---|---|---|
| Soybean oil | - | - | 11–12 | 3 | 0.2 | 24 | 53–55 | 6–7 | - |
| Sunflower oil | - | - | 7 | 5 | 0.3 | 20–25 | 63–68 | 0.2 | - |
| Rapeseed oil | - | - | 4–5 | 1–2 | 0.21 | 56–64 | 20–26 | 8–10 | 9.1 (20:1) |
| Palm oil | - | 1 | 37–41 | 3–6 | 0.4 | 40–45 | 8–10 | - | - |
| Rice bran oil | - | - | 20–22 | 2–3 | 0.19 | 42 | 31 | 1.1 | - |
| Cottonseed oil | - | 1 | 22–26 | 2–5 | 1.4 | 15–20 | 49–58 | - | - |
| Coconut oil | 44–52 | 13–19 | 8–11 | 1–3 | – | 5–8 | 0–1 | - | - |
| Corn (Maize) oil | - | - | 11–13 | 2–3 | 0.3 | 25–31 | 54–60 | 1 | - |
| Peanut/Groundnut | - | - | 10–11 | 2–3 | 0 | 48–50 | 39–40 | - | - |
| Sesame oil | - | - | 7–11 | 4–6 | 0.11 | 40–50 | 35–45 | - | - |
| Safflower oil | - | - | 5–7 | 1–4 | 0.08 | 13–21 | 73–79 | - | - |
| Karanja oil | - | - | 11–12 | 7–9 | – | 52 | 16–18 | - | - |
| Jatropha oil | - | 1.4 | 13–16 | 6–8 | – | 38–45 | 32–38 | - | - |
| Rubber seed oil | - | 2–3 | 10 | 9 | – | 25 | 40 | 16 | - |
| Mahua oil | - | - | 28 | 23 | – | 41–51 | 10–14 | - | - |
| Tung oil | - | - | 2.67 | 2.4 | – | 7.88 | 6.6 | 80.46 * | - |
| Neem oil | - | - | 18 | 18 | – | 45 | 18–20 | 0.5 | - |
| Castor oil | - | - | 0.5–1 | 0.5–1 | – | 4–5 | 2–4 | 0.5–1 | 83–85 # |
| Linseed oil | - | - | 4–5 | 2–4 | 0–0.5 | 19.1 | 12–18 | 56.6 | - |
| Olive oil | - | - | 13.7 | 2.5 | 1.8 | 71 | 10 | 0–1.5 | - |
| Vegetable Oils | Iodine Value | Pour Point (°C) | Cloud Point (°C) | Kinematic Viscosity at 40 °C (mm2/s) | Flash Point (°C) | Density at 15 °C (g/cm3) |
|---|---|---|---|---|---|---|
| Soybean oil | 138–143 | −12 | −4 | 29 | 254 | 0.914 |
| Sunflower oil | 125–140 | −15 | −9.5 | 36 | 274 | 0.916 |
| Rapeseed oil | 98–105 | −15 | −2 | 35 | 246 | 0.912 |
| Palm oil | 48–58 | 23.6 | 25.2 | 39.4 | 252 | 0.919 |
| Rice bran oil | 103 | 13 | 16 | 38.2 | 184 | 0.906 |
| Cotton seed oil | 90–119 | −4.5 | −0.5 | 34 | 234 | 0.918 |
| Coconut oil | 8–11 | 12.7 | 13.1 | 27 | 266 | 0.918 |
| Peanut/Ground nut/Arachis oil | 84–100 | −7 | 4.5 | 40 | 271 | 0.903 |
| Sesame oil | 104–116 | −11 | −8 | 36 | 260 | 0.918 |
| Karanja oil | 81–90 | −4 | 2 | 38.8 | 212 | 0.9358 |
| Jatropha oil | 82–98 | −6 | 11 | 34 | 225 | 0.94 |
| Rubber seed oil | 104 | 18 | 25 | 33.89 | 228 | 0.928 |
| Mahua oil | 58–70 | 11 | 20 | 37.18 | 238 | 0.945 |
| Neem oil | 81 | 7 | 13 | 35.8 | 200 | 0.918 |
| Castor oil | 83–86 | −21 | −18 | 251 | 229 | 0.960 |
| Linseed oil | 168–204 | −15 | 5 | 26–29 | 241 | 0.938 |
| Safflower oil | 145 | −7 | −2 | 28.3 | 260 | 0.914 |
| Olive oil | 75–94 | −14 | −11 | 39 | 177 | 0.918 |
| Polymers | FT-IR Peaks (cm−1) | NMR Peaks (δH, ppm) | Average Molecular Weights by GPC | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ester Carbonyl Group | C-O Stretching | Paraffinic C-H Stretching | C-H Bending | Ester Carbonyl Protons | Methyl Protons | Methylene Protons | Mn | Mw | PDI | ||
| Homopolymer of SBO | 1745.5 | 1164.9 | 2854.5–2923.9 | 724.2 | 4.117–4.315 | 0.858–1.607 | 2.002–2.769 | 46,400 | 48,500 | 1.05 | [23] |
| Homopolymer of SFO | 1732 | 1167.8 | 2853.5–2942.8 | 721.3 | 4.135–4.173 | 0.857–1.607 | 2.001–2.362 | 33,000 | 35,100 | 1.06 | [23] |
| Copolymer of SBO with MA (10%) | 1739–1743 | 1175.6 | 2856.5–2926 | 725–727 | 4.058–4.262 | 0.857–1.608 | 2.020–2.337 | 29,520 | 39,570 | 1.34 | [22] |
| Copolymer of SBO with MMA (10%) | 1735–1744 | 1171.5 | 2849–2930 | 725–727 | 4.080–4.278 | 0.820–1.585 | 1.965–2.304 | 34,030 | 48,840 | 1.43 | [22] |
| Terpolymer of OA, SFO, Styrene (2:1:1) | 1744 | 1051–1270 | 2857–2931 | 695–810 | 4.084–4.147 | 0.876–1.627 | 2.035–2.297 | 13,611 | 17,003 | 1.25 | [29] |
| Terpolymer of DA, SFO, Styrene (2:1:1) | 1744 | 1051–1270 | 2857–2931 | 695–810 | 4.084–4.147 | 0.876–1.627 | 2.035–2.297 | 13,623 | 20,522 | 1.51 | [29] |
| Homopolymer of CO | 1732.1 | 1169.3 | 2857–2922 | 722 | 4.118–4.328 | 0.861–1.611 | 2.033–2.343 | 7928 | 10,022 | 1.26 | [27] |
| Copolymer of CO with MMA (10%) | 1744.4 | 1169.3 | 2852–2926 | 724.4 | 4.900–3.719 | 0.941–1.260 | 1.784–2.083 | 40,879 | 50,979 | 1.25 | [27] |
| Copolymer of CO with DDA (10%) | 1742 | 1169.3 | 2847–2926 | 723.3 | 4.031–4.147 | 0.883–1.618 | 2.168–2.673 | 17,579 | 28,736 | 1.63 | [27] |
| Sample | Base Oil | VI | Pour Point (−°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 2% | 3% | 4% | 5% | 0% | 2% | 3% | 4% | 5% | ||
| S-1 | SN1 | 80 | 132 | 170 | 211 | 227 | −3 | −3 | −7 | −8 | −12 |
| SN2 | 89 | 140 | 200 | 227 | 256 | −6 | −6 | −8 | −9 | −15 | |
| S-2 | SN1 | 80 | 113 | 127 | 135 | 138 | −3 | −6 | −9 | −12 | −18 |
| SN2 | 89 | 113 | 133 | 153 | 177 | −6 | −8 | −12 | −15 | −20 | |
| S-3 | SN1 | 80 | 116 | 134 | 145 | 160 | −3 | −6 | −8 | −9 | −15 |
| SN2 | 89 | 122 | 144 | 173 | 189 | −6 | −8 | −10 | −12 | −15 | |
| S-4 | SN1 | 80 | 156 | 199 | 232 | 240 | −3 | −3 | −7 | −9 | −9 |
| SN2 | 89 | 150 | 212 | 234 | 262 | −6 | −6 | −8 | −9 | −10 | |
| S-5 | SN1 | 80 | 142 | 184 | 218 | 232 | −3 | −3 | −7 | −9 | −9 |
| SN2 | 89 | 152 | 210 | 242 | 270 | −6 | −6 | −6 | −8 | −10 | |
| S-6 | SN1 | 80 | 162 | 201 | 232 | 242 | −3 | −3 | −6 | −9 | −12 |
| SN2 | 89 | 166 | 211 | 244 | 272 | −6 | −6 | −8 | −10 | −15 | |
| S-7 | SN1 | 80 | 152 | 192 | 217 | 236 | −3 | −5 | −7 | −8 | −12 |
| SN2 | 89 | 162 | 199 | 231 | 268 | −6 | −6 | −8 | −9 | −15 | |
| Sample | Base Oil | VI | Pour Points (−°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 2% | 3% | 4% | 5% | 0% | 2% | 3% | 4% | 5% | ||
| S-1 | SN70 | 80.05 | 102 | 135 | 189 | 207 | −3 | −6 | −7 | −8 | −9 |
| SN150 | 89.02 | 115 | 142 | 198 | 220 | −6 | −6 | −8 | −9 | −10 | |
| S-2 | SN70 | 80.05 | 120 | 147 | 205 | 221 | −3 | −6 | −9 | −12 | −18 |
| SN150 | 89.02 | 125 | 161 | 213 | 232 | −6 | −6 | −9 | −15 | −18 | |
| S-3 | SN70 | 80.05 | 128 | 169 | 211 | 229 | −3 | −6 | −8 | −12 | −15 |
| SN150 | 89.02 | 132 | 174 | 223 | 239 | −6 | −6 | −9 | −12 | −15 | |
| S-4 | SN70 | 80.05 | 136 | 184 | 218 | 232 | −3 | −6 | −6 | −9 | −12 |
| SN150 | 89.02 | 142 | 201 | 232 | 255 | −6 | −6 | −6 | −10 | −15 | |
| S-5 | SN70 | 80.05 | 140 | 199 | 232 | 240 | −3 | −6 | −7 | −8 | −12 |
| SN150 | 89.02 | 146 | 212 | 234 | 262 | −6 | −6 | −8 | −9 | −12 | |
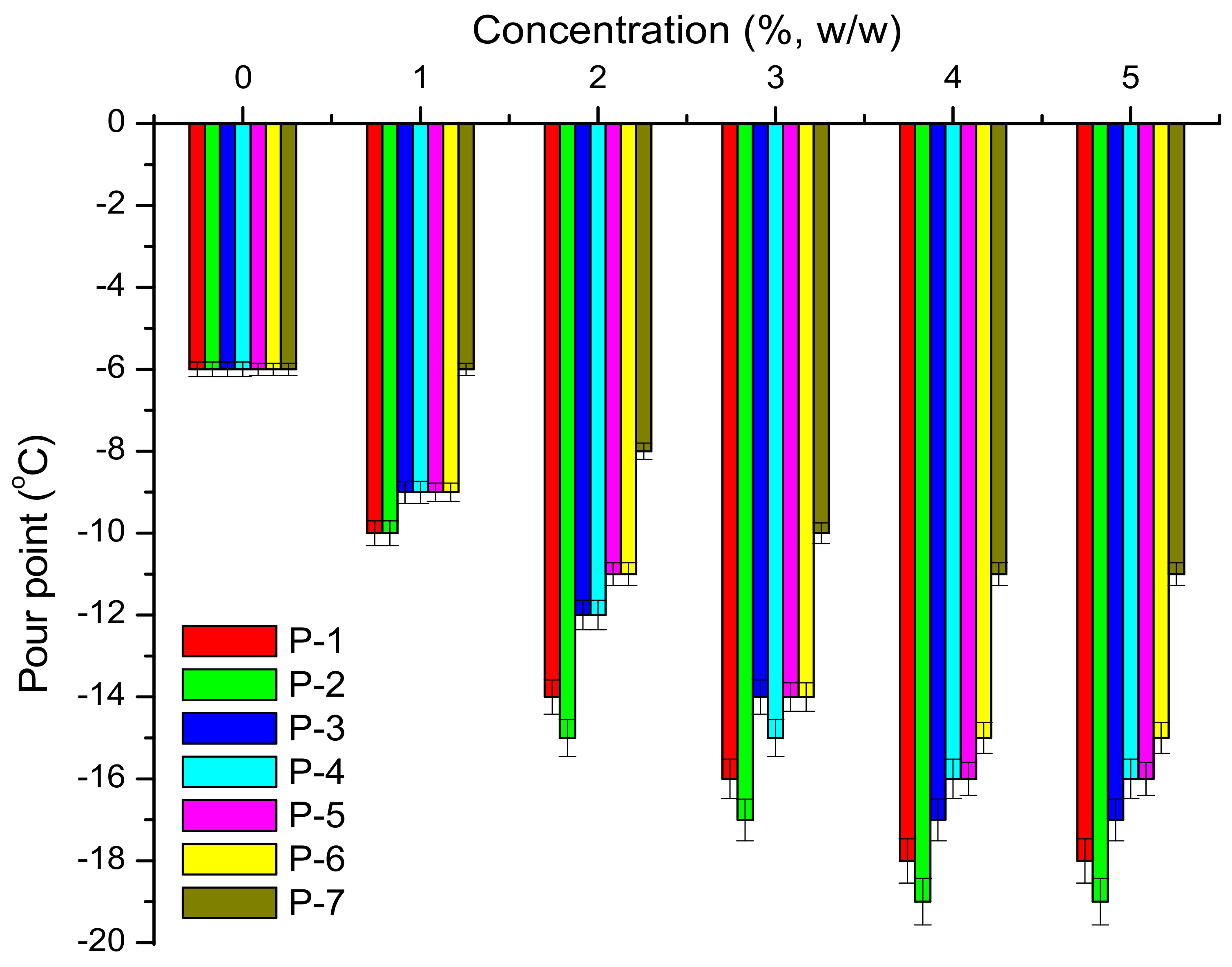
| Sample | Base Oils | Pour Points (°C) of Lubricants with Additives at Different Percentages | ||||
|---|---|---|---|---|---|---|
| 0% | 2.5% | 5% | 10% | |||
| P-1 | BO1 | S1 | −3 | −6 | −6 | −6 |
| S2 | −3 | −3 | −3 | −3 | ||
| BO2 | S1 | −6 | −9 | −9 | −9 | |
| S2 | −6 | −9 | −9 | −9 | ||
| P-2 | BO1 | S1 | −3 | −12 | −12 | −12 |
| S2 | −3 | −12 | −15 | −15 | ||
| BO2 | S1 | −6 | −15 | −15 | −15 | |
| S2 | −6 | −15 | −15 | −15 | ||
| P-3 | BO1 | S1 | −3 | −12 | −12 | −12 |
| S2 | −3 | −15 | −15 | −12 | ||
| BO2 | S1 | −6 | −15 | −12 | −12 | |
| S2 | −6 | −15 | −12 | −12 | ||
| P-4 | BO1 | S1 | −3 | −15 | −12 | −12 |
| S2 | −3 | −15 | −12 | −12 | ||
| BO2 | S1 | −6 | −15 | −12 | −12 | |
| S2 | −6 | −15 | −12 | −12 | ||
| P-5 | BO1 | S1 | −3 | −15 | −18 | −15 |
| S2 | −3 | −15 | −18 | −21 | ||
| BO2 | S1 | −6 | −18 | −21 | −21 | |
| S2 | −6 | −18 | −21 | −24 | ||
| P-6 | BO1 | S1 | −3 | −9 | −9 | −9 |
| S2 | −3 | −9 | −9 | −9 | ||
| BO2 | S1 | −6 | −9 | −12 | −12 | |
| S2 | −6 | −9 | −12 | −12 | ||
| P-7 | BO1 | S1 | −3 | −9 | −9 | −9 |
| S2 | −3 | −9 | −9 | −9 | ||
| BO2 | S1 | −6 | −9 | −9 | −9 | |
| S2 | −6 | −9 | −9 | −9 | ||
| Sample | Base Oils | VI of Lubricants with Additives at Different Percentages | ||||
|---|---|---|---|---|---|---|
| 0% | 2% | 3% | 4% | 5% | ||
| S-1 | BO1 | 80 | 88 | 81 | 100 | 166 |
| BO2 | 89 | 100 | 116 | 139 | 197 | |
| BO3 | 96 | 96 | 108 | 113 | 148 | |
| S-2 | BO1 | 80 | 91 | 93 | 93 | 151 |
| BO2 | 89 | 108 | 116 | 133 | 197 | |
| BO3 | 96 | 101 | 111 | 113 | 145 | |
| S-3 | BO1 | 80 | 93 | 107 | 121 | 173 |
| BO2 | 89 | 89 | 127 | 139 | 212 | |
| BO3 | 96 | 100 | 113 | 122 | 152 | |
| S-4 | BO1 | 80 | 144 | 182 | 203 | 226 |
| BO2 | 89 | 95 | 120 | 133 | 210 | |
| BO3 | 96 | 101 | 109 | 112 | 148 | |
| S-5 | BO1 | 80 | 109 | 184 | 230 | 262 |
| BO2 | 89 | 97 | 108 | 118 | 215 | |
| BO3 | 96 | 98 | 114 | 121 | 137 | |
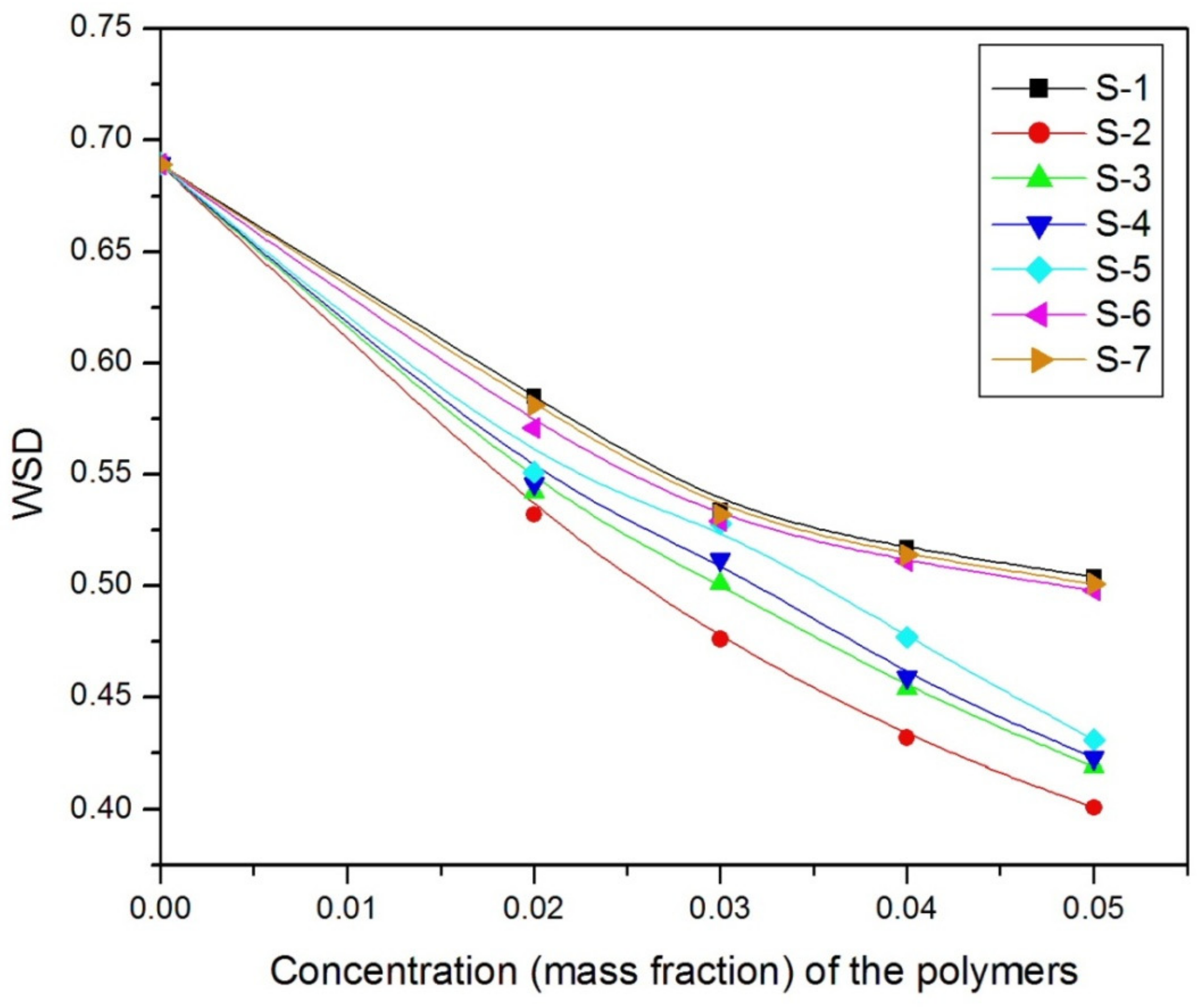
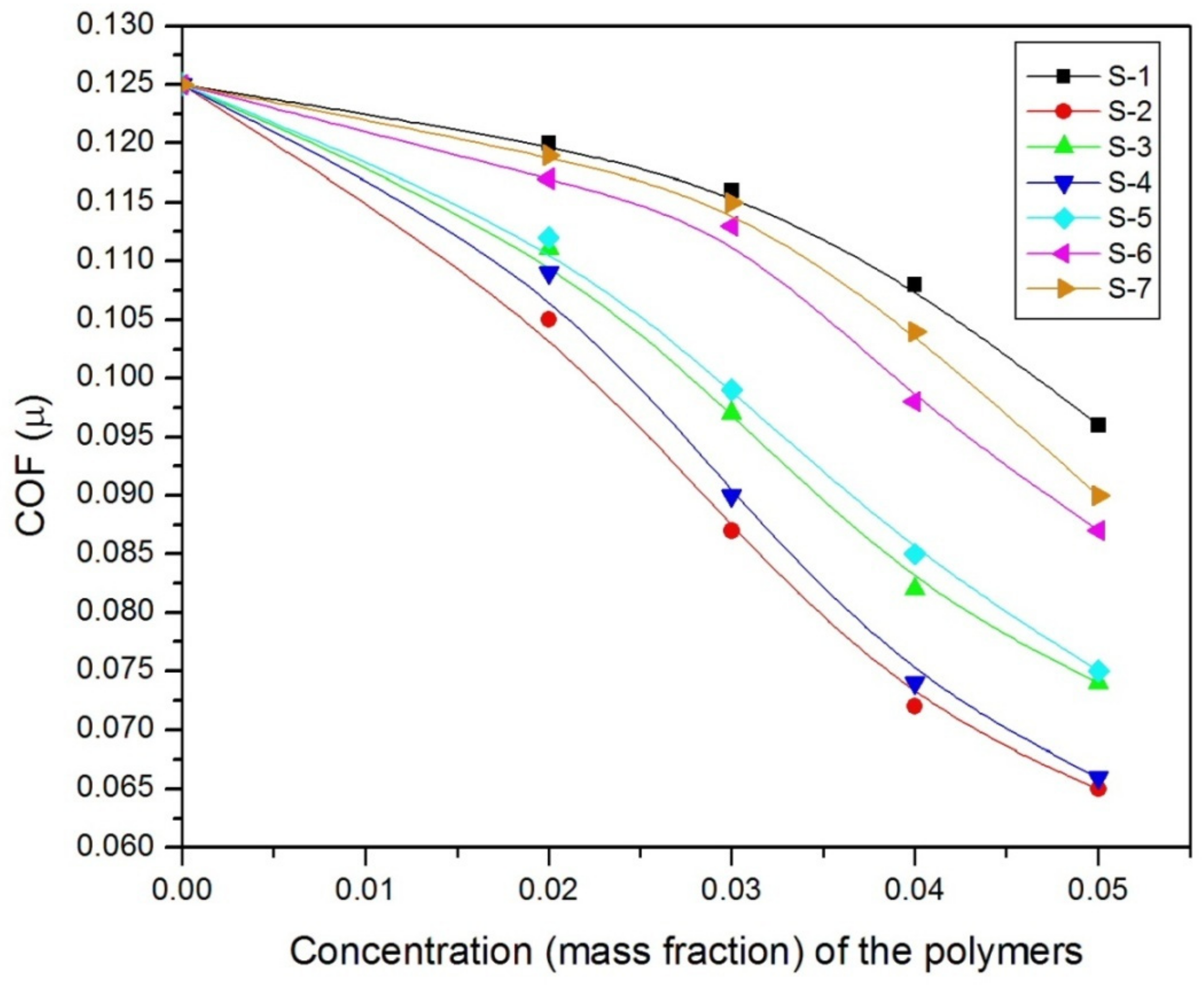
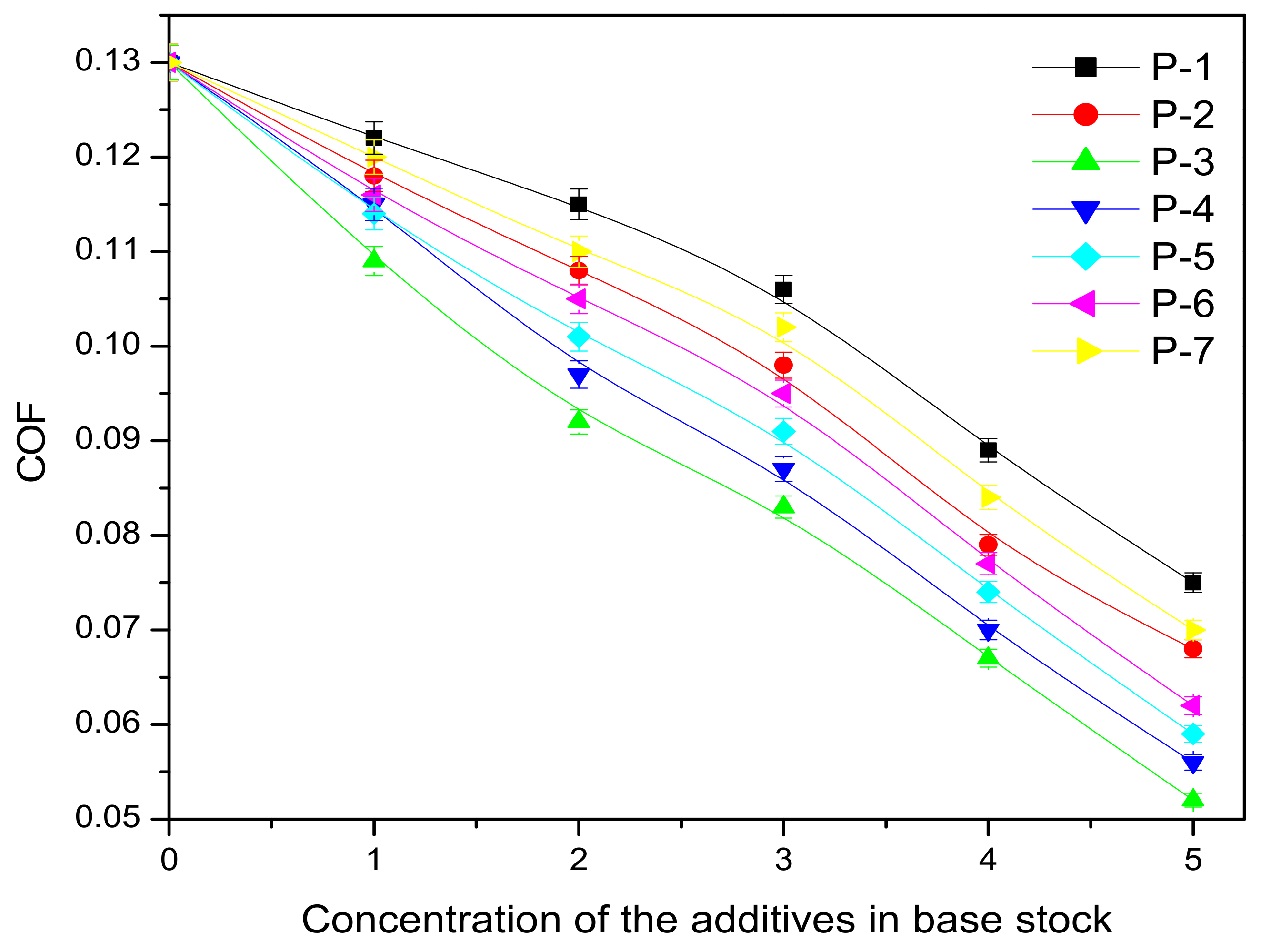
| Polymer Code | Base Fluids | WSD of Lubricant in mm at Different Additive Concentrations (% in w/w) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| A | BO1 | 0.982 | 0.965 | 0.955 | 0.93 | 0.891 | 0.846 |
| BO2 | 1.119 | 1.079 | 1.062 | 1.048 | 1.016 | 0.992 | |
| B | BO1 | 0.982 | 0.964 | 0.952 | 0.928 | 0.89 | 0.842 |
| BO2 | 1.119 | 1.078 | 1.061 | 1.046 | 1.012 | 0.984 | |
| C | BO1 | 0.982 | 0.951 | 0.944 | 0.919 | 0.875 | 0.833 |
| BO2 | 1.119 | 1.072 | 1.053 | 1.038 | 1.006 | 0.974 | |
| D | BO1 | 0.982 | 0.938 | 0.927 | 0.906 | 0.861 | 0.816 |
| BO2 | 1.119 | 1.061 | 1.048 | 1.03 | 0.991 | 0.962 | |
| E | BO1 | 0.982 | 0.919 | 0.908 | 0.885 | 0.844 | 0.802 |
| BO2 | 1.119 | 1.046 | 1.031 | 0.985 | 0.958 | 0.938 | |
| Biopolymers | Base Stocks | Parameters for Evaluating Performance of Different Lubricant Compositions | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VI | PPD (°C) | Antiwear Data [WSD (mm)] | ||||||||||||||||
| 0% | 1% | 2% | 3% | 4% | 5% | 0% | 1% | 2% | 3% | 4% | 5% | 0% | 1% | 2% | 4% | 5% | ||
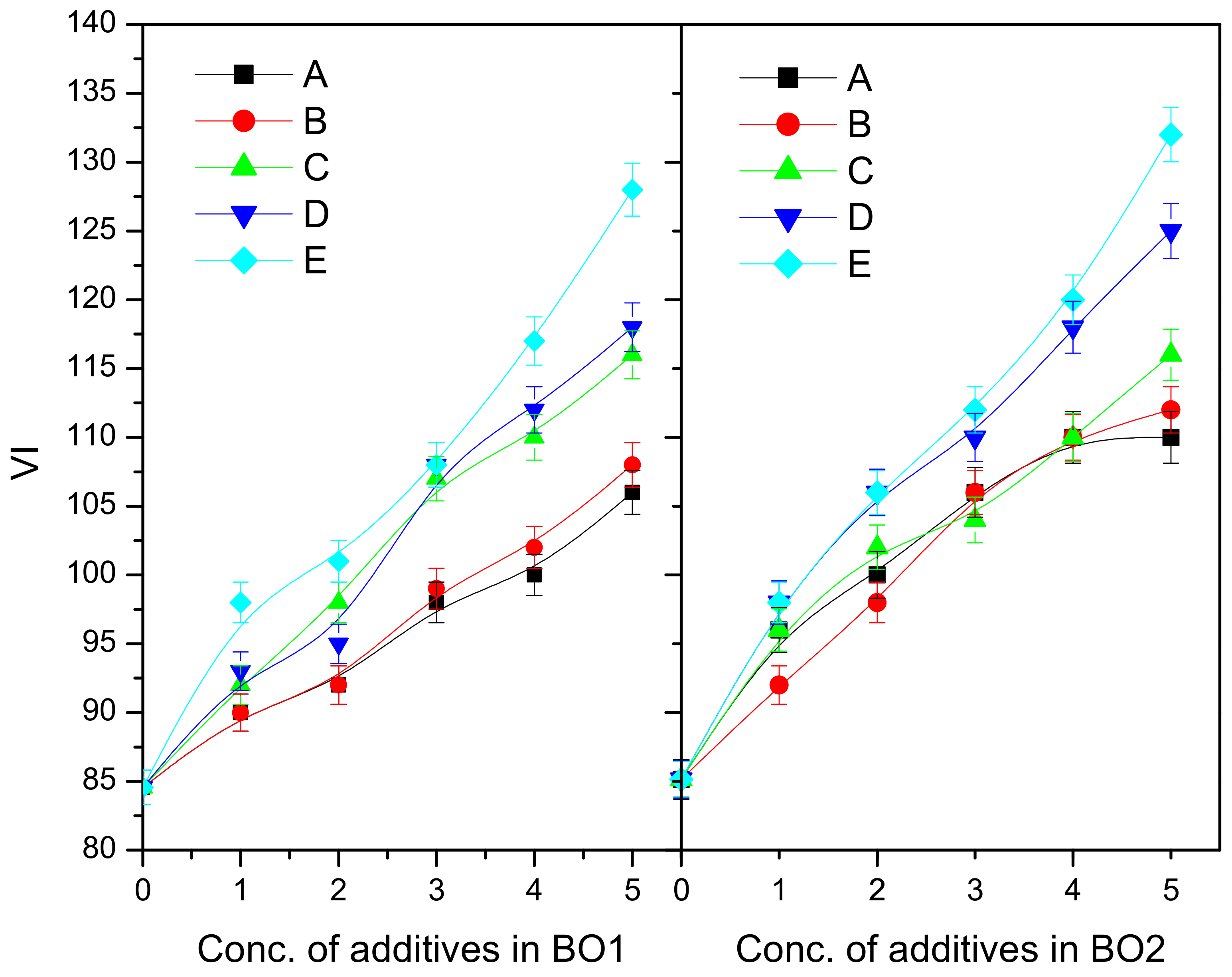
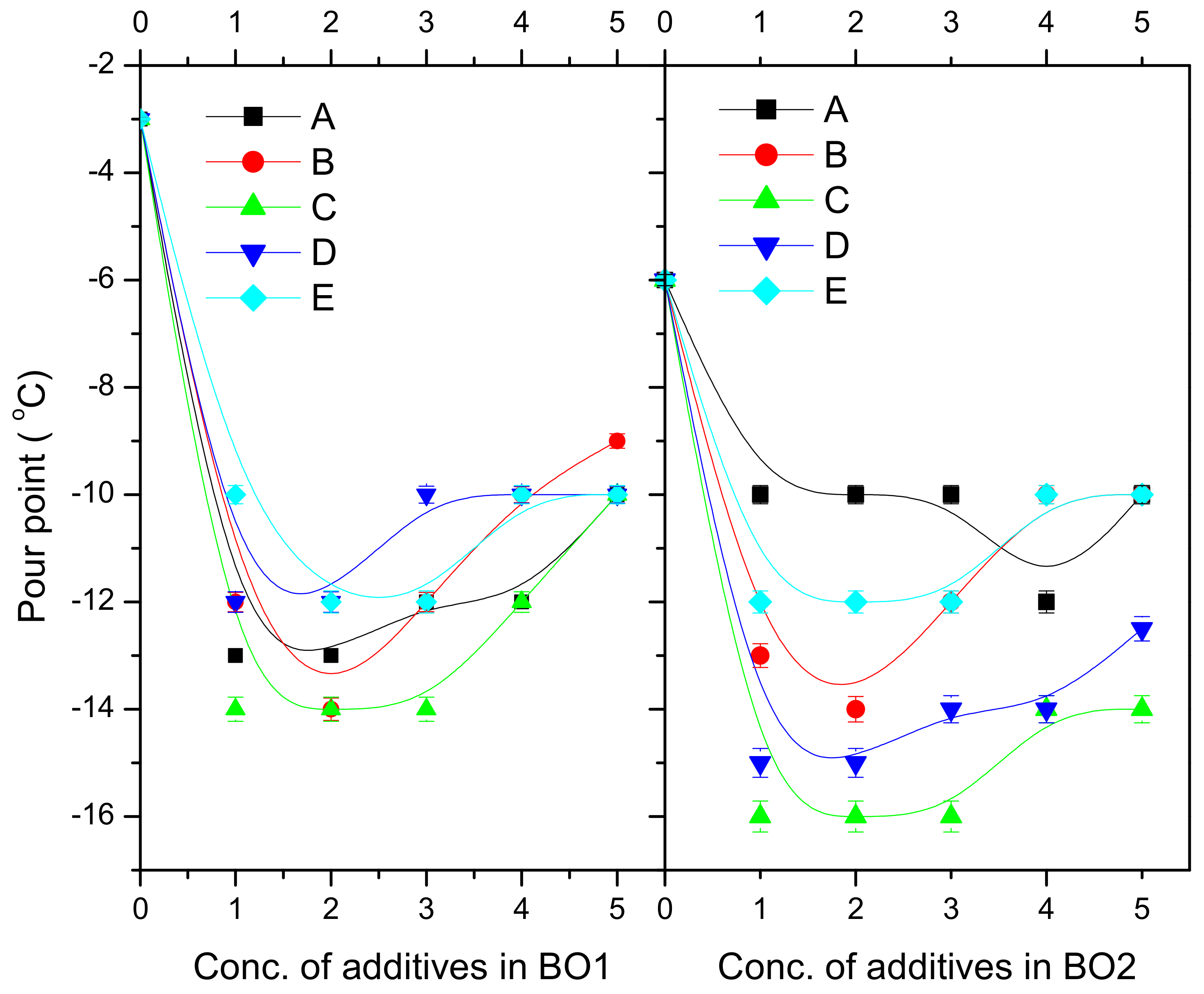
| P-1 | BO1 | 84.6 | 95 | 99.5 | 111 | 114 | 121 | −3 | −6 | −8 | −8 | −7 | −7 | 0.982 | 0.905 | 0.856 | 0.754 | 0.698 |
| BO2 | 85.2 | 90.5 | 95.8 | 102 | 114 | 118 | −6 | −9 | −10 | −11 | −10.5 | −10 | 1.119 | 0.989 | 0.925 | 0.798 | 0.719 | |
| P-2 | BO1 | 84.6 | 98.4 | 106 | 118 | 125 | 136 | −3 | −11 | −13 | −13.5 | −12 | −12 | 0.982 | 0.804 | 0.756 | 0.655 | 0.59 |
| BO2 | 85.2 | 96.4 | 99 | 112 | 121 | 134 | −6 | −15 | −16.5 | −17 | −17 | −16 | 1.119 | 0.936 | 0.856 | 0.696 | 0.616 | |
| P-3 | BO1 | 84.6 | 96.8 | 105 | 116 | 122 | 130 | −3 | −11 | −12 | −13 | −12 | −11 | 0.982 | 0.824 | 0.776 | 0.69 | 0.625 |
| BO2 | 85.2 | 95.8 | 100 | 109 | 116 | 128 | −6 | −14 | −16 | −16.5 | −16 | −15 | 1.119 | 0.951 | 0.882 | 0.722 | 0.631 | |
| P-4 | BO1 | 84.6 | 96 | 104 | 110 | 123 | 128 | −3 | −9 | −10 | −10 | −10 | −10 | 0.982 | 0.885 | 0.836 | 0.748 | 0.678 |
| BO2 | 85.2 | 95 | 101 | 108 | 114 | 125 | −6 | −12 | −14 | −14.5 | −14 | −13 | 1.119 | 0.985 | 0.915 | 0.775 | 0.705 | |
| P-5 | BO1 | 84.6 | 100 | 122 | 125 | 132 | 138 | −3 | −8 | −8 | −8 | −7 | −7 | 0.982 | 0.875 | 0.826 | 0.755 | 0.668 |
| BO2 | 85.2 | 99.4 | 108 | 121 | 128 | 138 | −6 | −10 | −12 | −12 | −12 | −11 | 1.119 | 0.978 | 0.902 | 0.736 | 0.692 | |
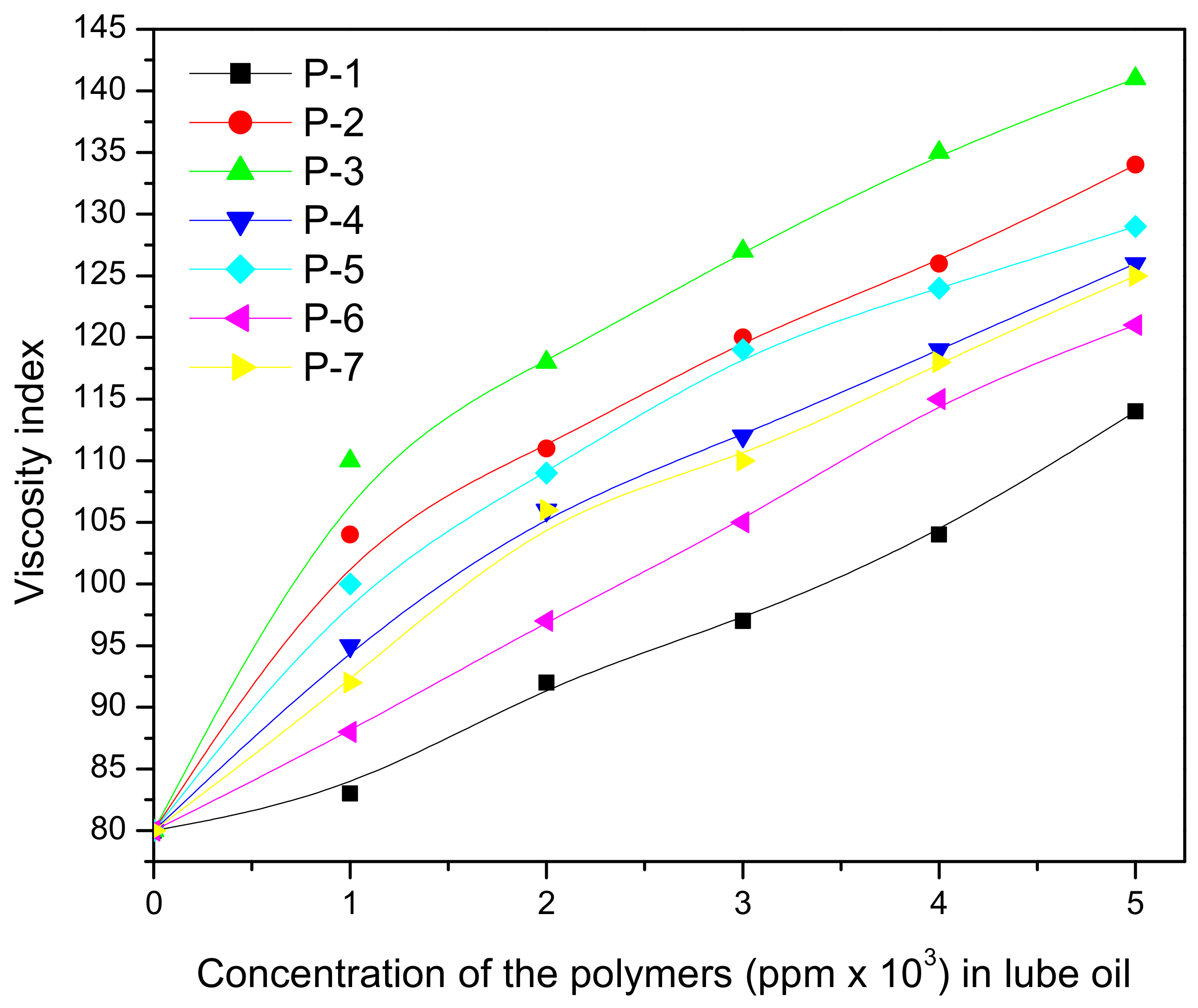
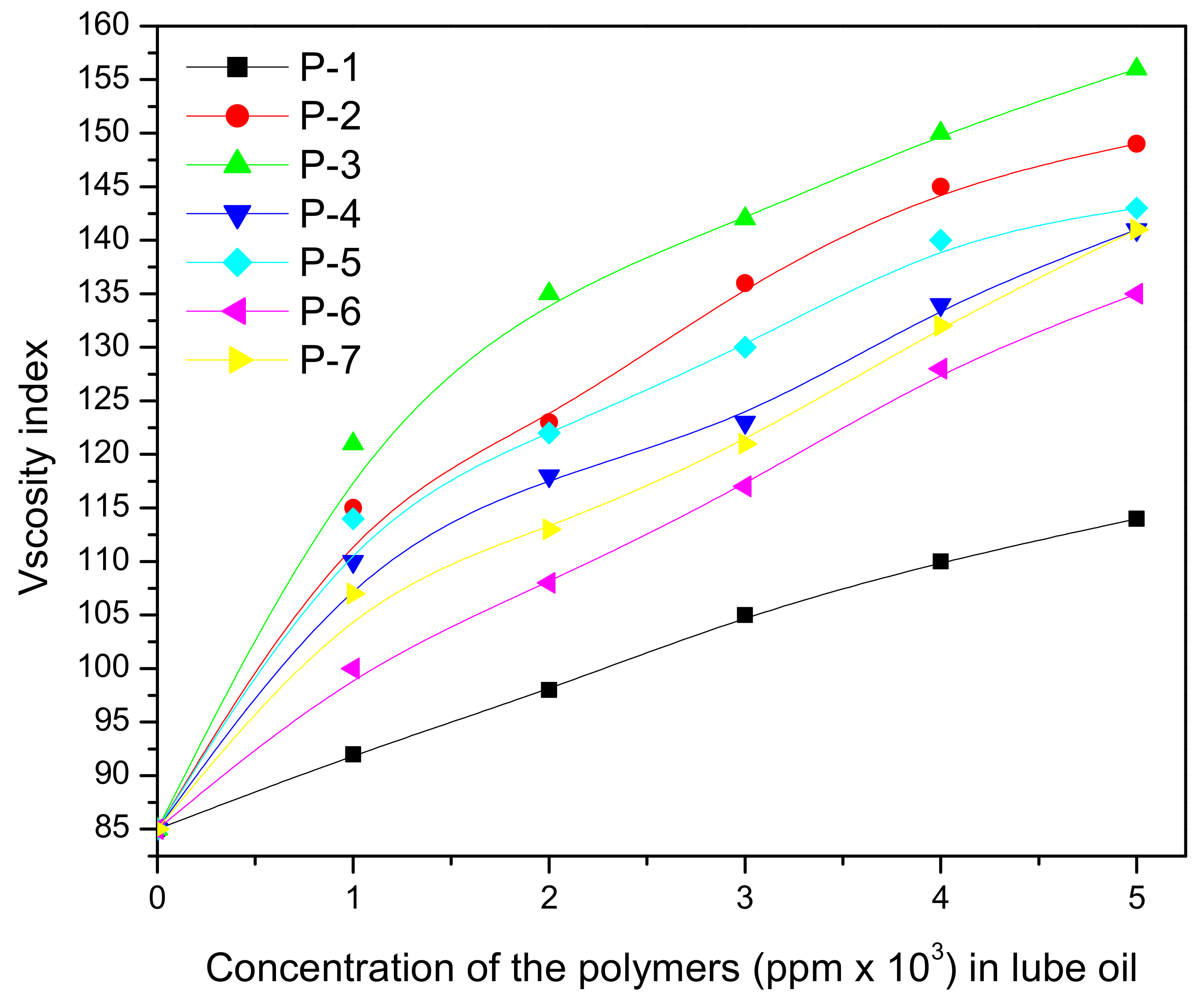
| Base Oil | Conc. (%) | Lubricants with Additives | ||||||
|---|---|---|---|---|---|---|---|---|
| P-1 | P-2 | P-3 | P-4 | P-5 | P-6 | |||
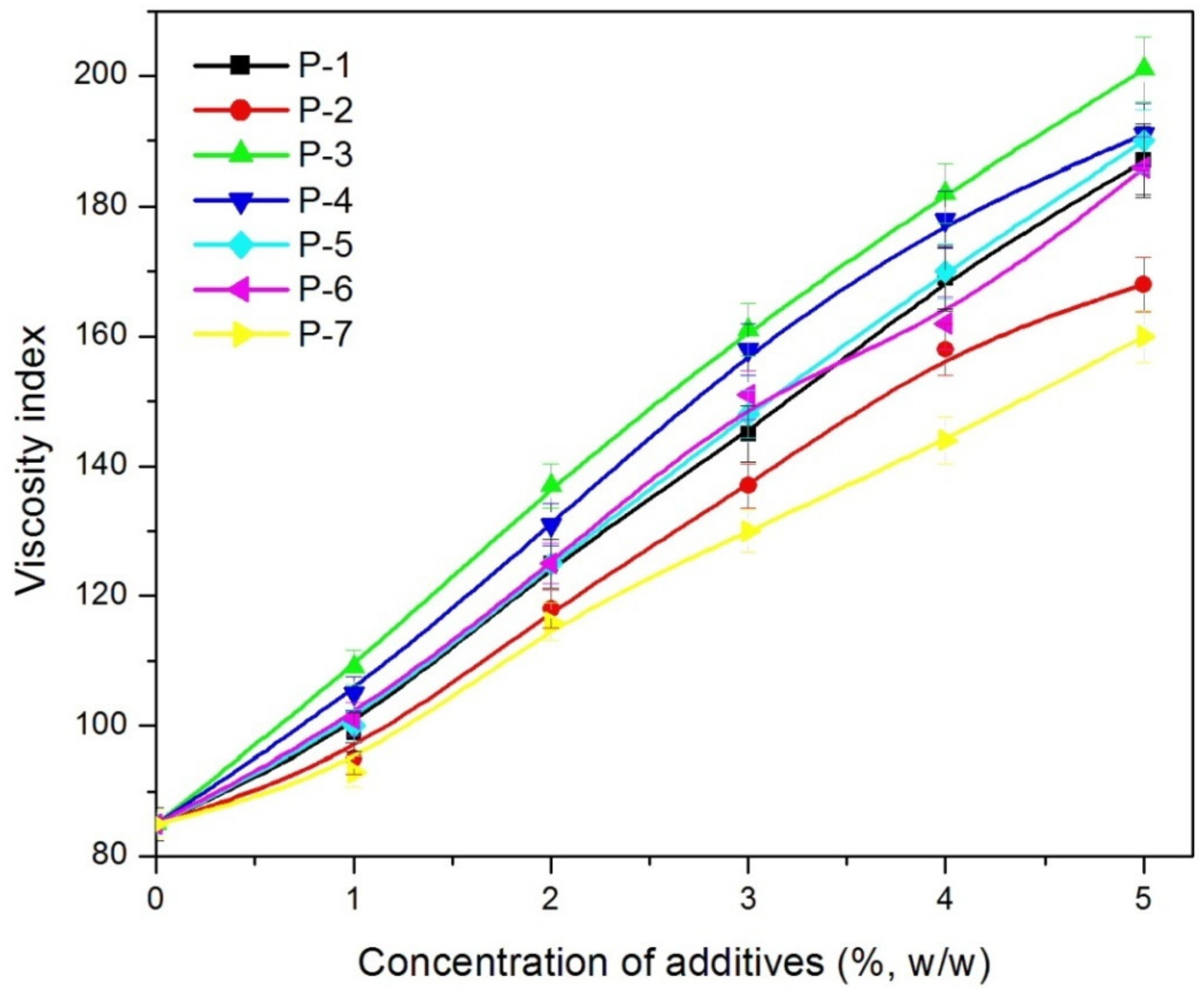
| VI | BO1 (85) | 1 | 112 | 116 | 119 | 100 | 110 | 118 |
| 2 | 112 | 118 | 125 | 104 | 112 | 122 | ||
| 3 | 125 | 130 | 132 | 106 | 114 | 128 | ||
| 4 | 125 | 135 | 138 | 118 | 126 | 135 | ||
| 5 | 128 | 136 | 145 | 121 | 128 | 138 | ||
| 6 | 134 | 136 | 146 | 125 | 129 | 137 | ||
| BO2 (80) | 1 | 95 | 98 | 101 | 98 | 100 | 104 | |
| 2 | 97 | 100 | 110 | 109 | 104 | 110 | ||
| 3 | 102 | 115 | 115 | 115 | 118 | 119 | ||
| 4 | 105 | 115 | 123 | 118 | 122 | 125 | ||
| 5 | 115 | 123 | 132 | 131 | 130 | 132 | ||
| 6 | 116 | 128 | 138 | 137 | 129 | 138 | ||
| PPD (°C) | BO1 (−3) | 1 | −9 | −15 | −15 | −9 | −9 | −12 |
| 2 | −12 | −18 | −21 | −9 | −12 | −12 | ||
| 3 | −15 | −21 | −24 | −12 | −15 | −15 | ||
| BO2 (−6) | 1 | −12 | −15 | −18 | −15 | −21 | −18 | |
| 2 | −15 | −18 | −21 | −18 | −24 | −24 | ||
| 3 | −15 | −24 | −27 | −21 | −18 | −24 | ||
| Lubrication Condition | Weld Load (N) | Average Wear Scar Diameter | ||
|---|---|---|---|---|
| 300 N | 600 N | 800 N | ||
| Mineral oil (without palm oil methyl ester) | 1200 | 0.28 | 1.97 | 2.6 |
| Mineral oil (with 5 vol% palm oil methyl ester) | 1450 | 0.29 | 1.79 | 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karmakar, G.; Dey, K.; Ghosh, P.; Sharma, B.K.; Erhan, S.Z. A Short Review on Polymeric Biomaterials as Additives for Lubricants. Polymers 2021, 13, 1333. https://doi.org/10.3390/polym13081333
Karmakar G, Dey K, Ghosh P, Sharma BK, Erhan SZ. A Short Review on Polymeric Biomaterials as Additives for Lubricants. Polymers. 2021; 13(8):1333. https://doi.org/10.3390/polym13081333
Chicago/Turabian StyleKarmakar, Gobinda, Koushik Dey, Pranab Ghosh, Brajendra K. Sharma, and Sevim Z. Erhan. 2021. "A Short Review on Polymeric Biomaterials as Additives for Lubricants" Polymers 13, no. 8: 1333. https://doi.org/10.3390/polym13081333
APA StyleKarmakar, G., Dey, K., Ghosh, P., Sharma, B. K., & Erhan, S. Z. (2021). A Short Review on Polymeric Biomaterials as Additives for Lubricants. Polymers, 13(8), 1333. https://doi.org/10.3390/polym13081333






