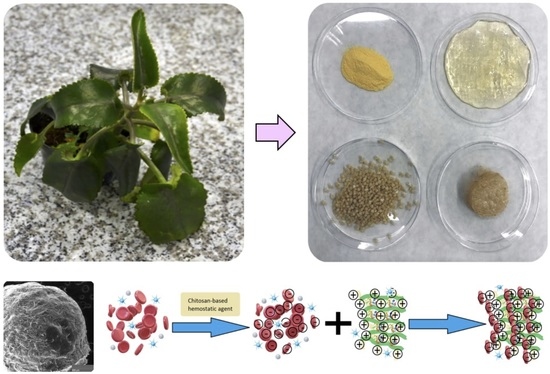
Fungal Chitosan-Derived Biomaterials Modified with Kalanchoe pinnata as Potential Hemostatic Agents—Development and Characterization
Abstract
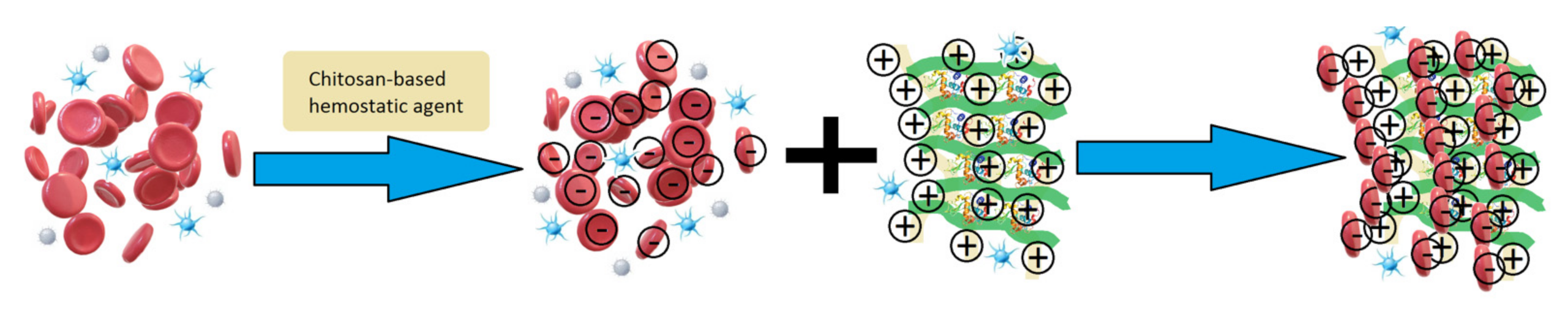
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
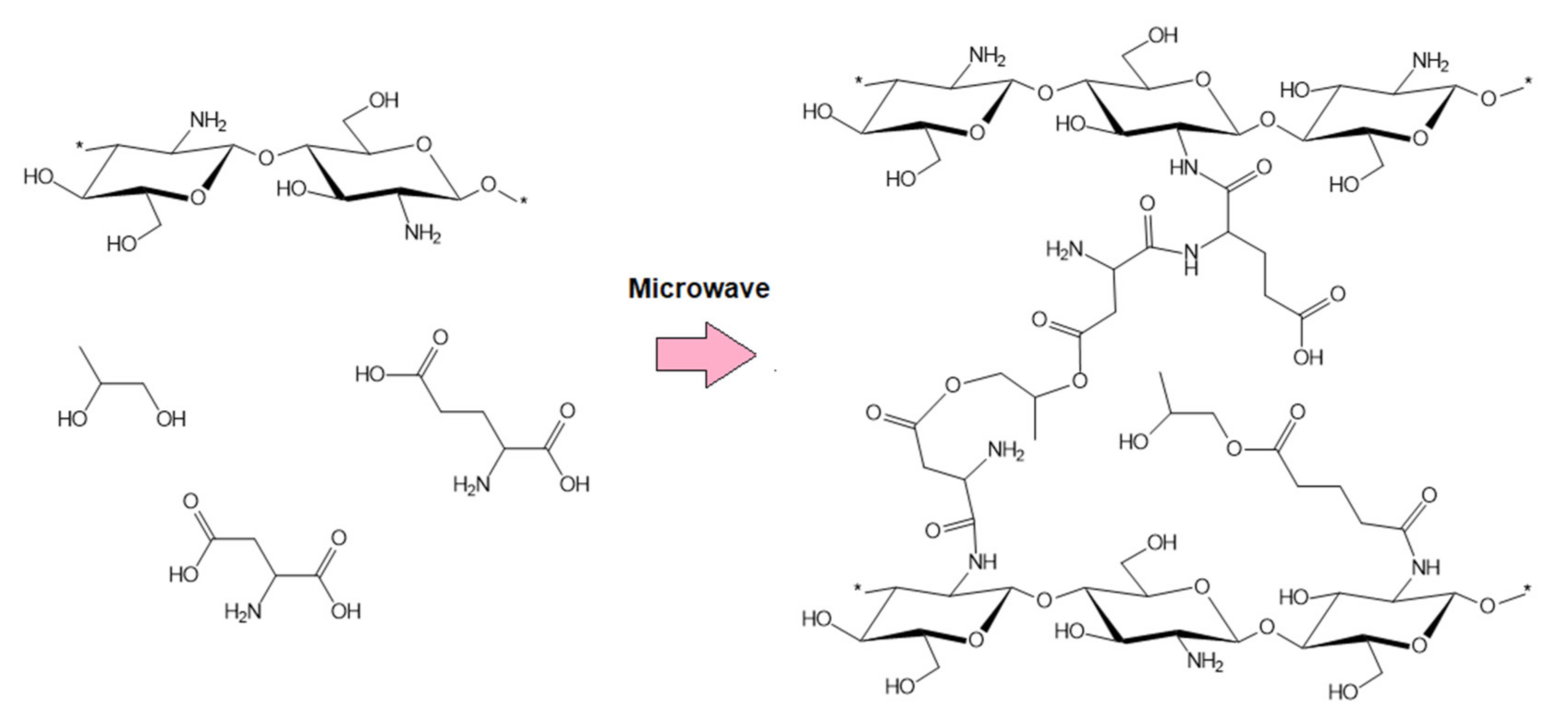
2.2.1. Samples’ Preparation
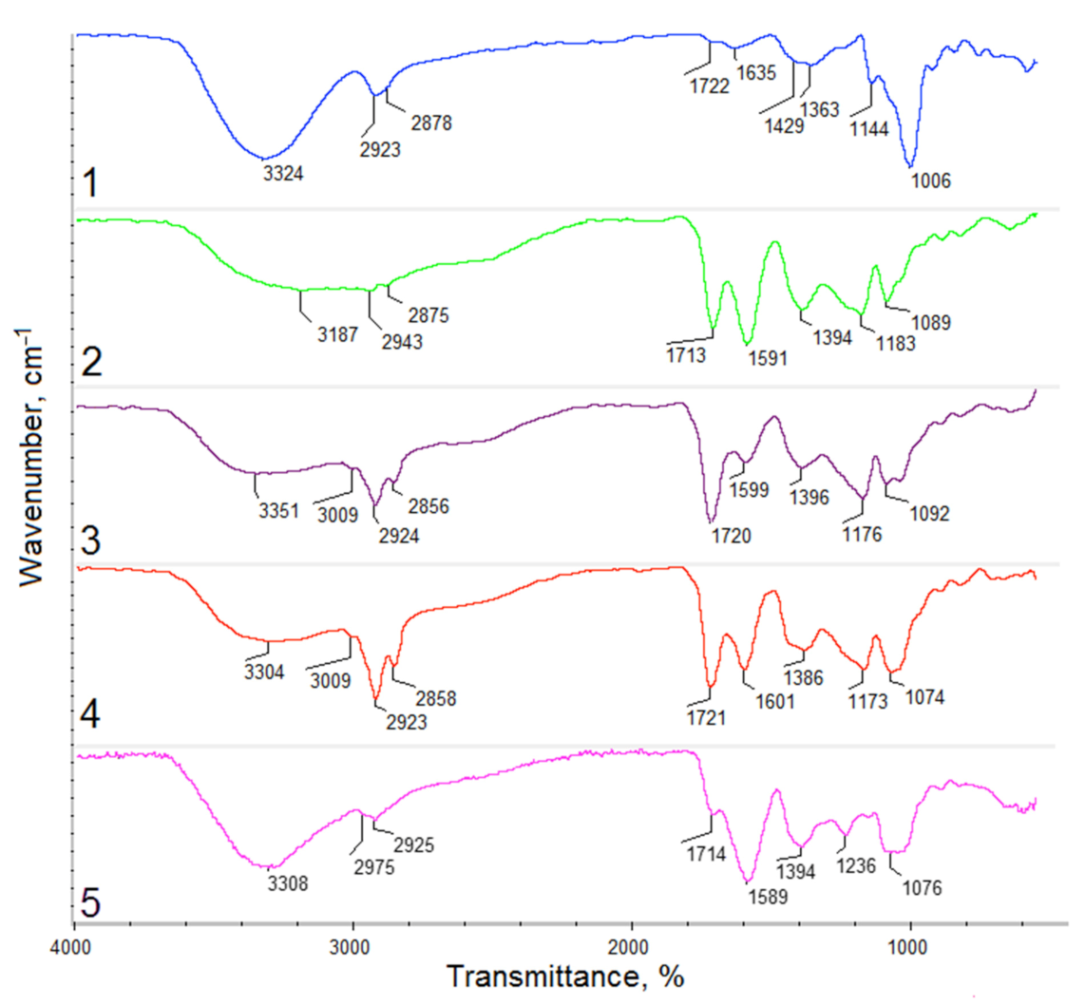
2.2.2. Fourier Transform Infrared Spectroscopy (FTIR) Study
- DD—deacetylation degree, %.
- A1655—band absorption field at the wavenumber equal to 1635 cm−1.
- A2870—band absorption field at the wavenumber equal to 3324 cm−1.
2.2.3. Viscosity Study
2.2.4. Morphology Study
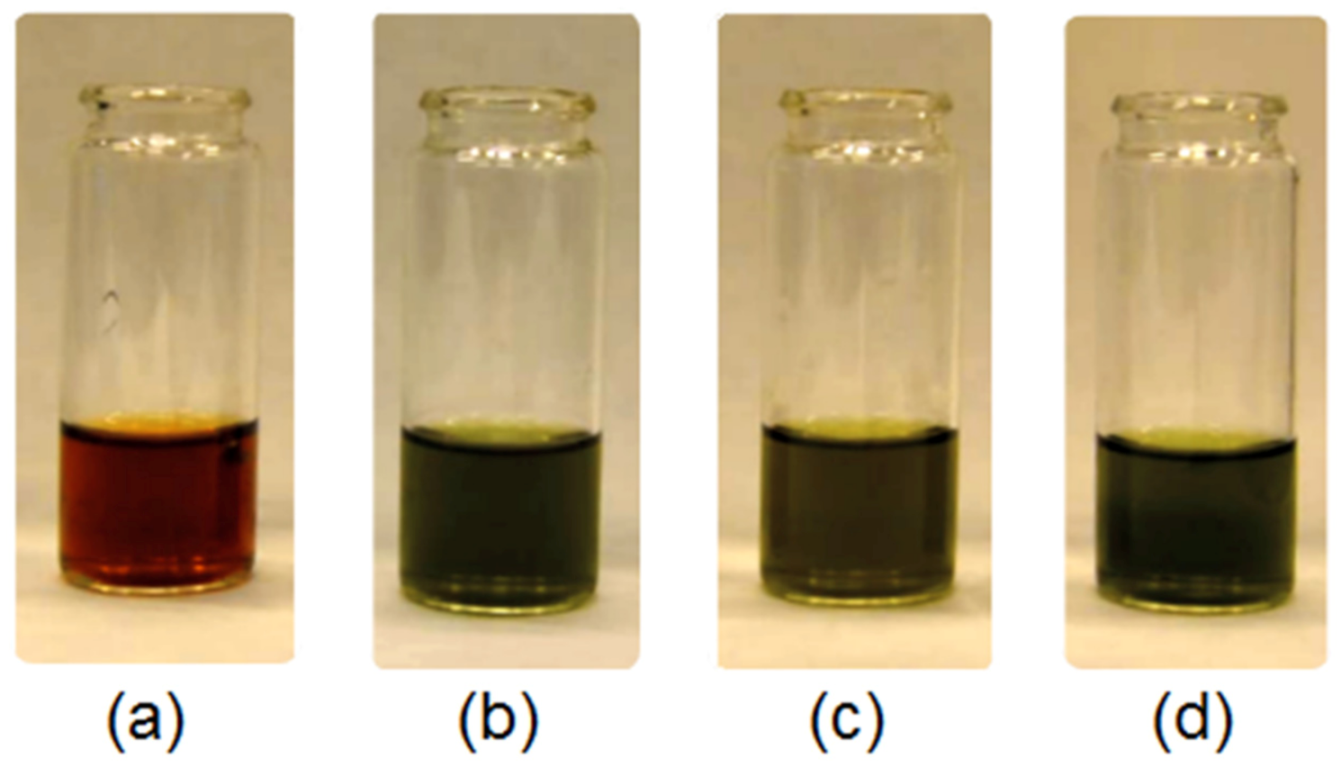
2.2.5. Natural Extracts’ Properties Study
2.2.6. Swelling Properties Study
- SD—swelling degree, %.
- Wd—weight of the dried sample, g.
- Ws—weight of the swollen sample, g.
2.2.7. Mechanical Properties Study
2.2.8. Cytotoxicity Study
3. Results
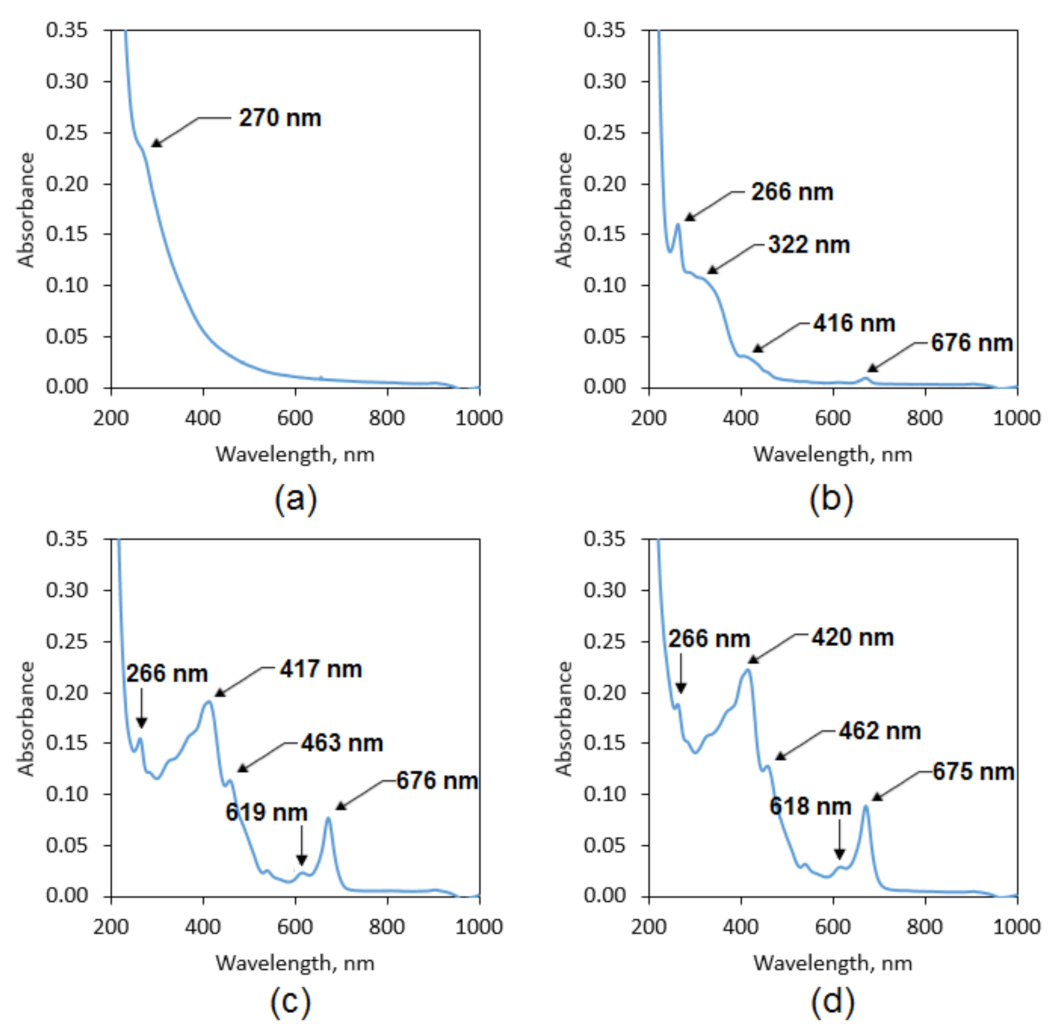
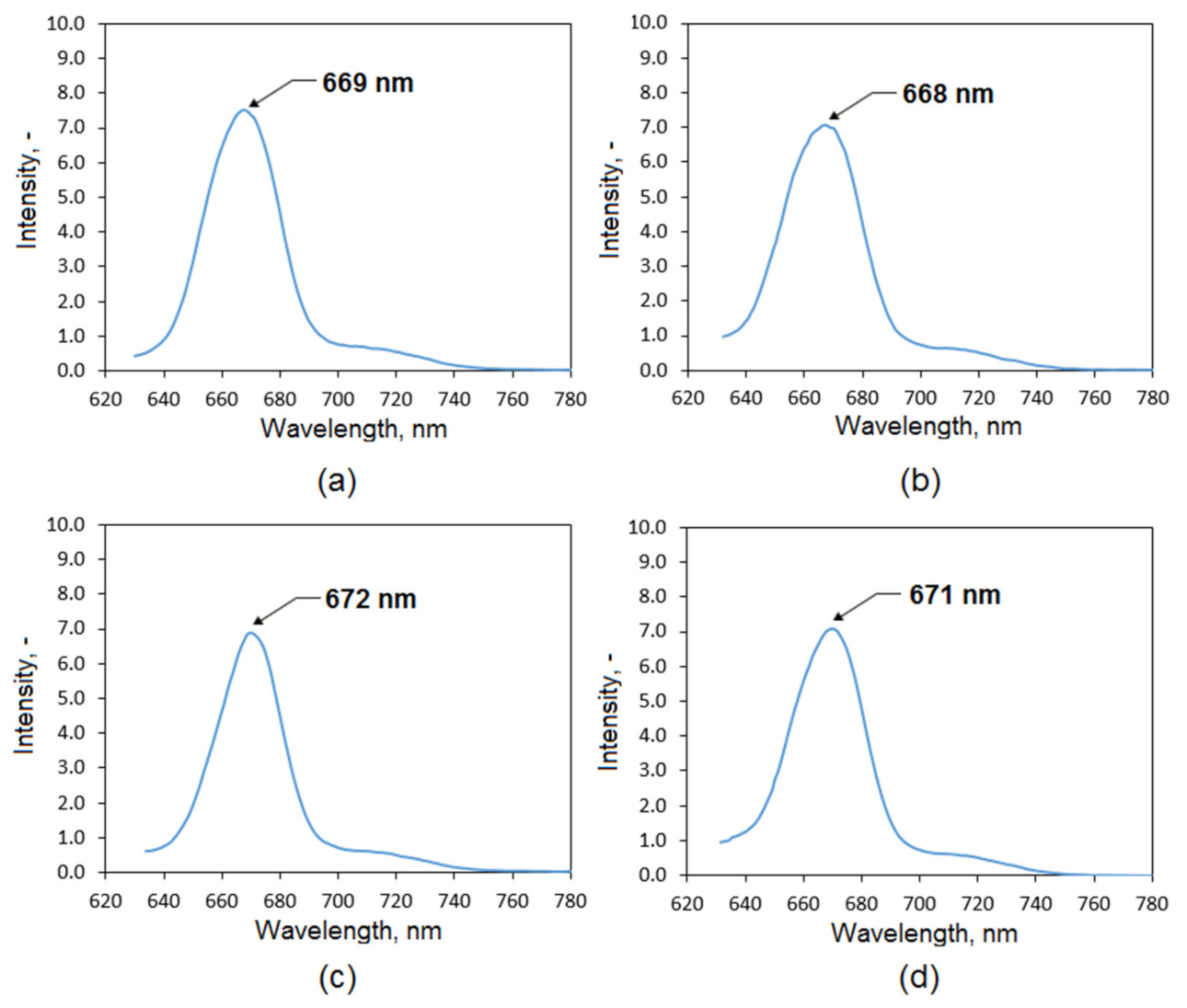
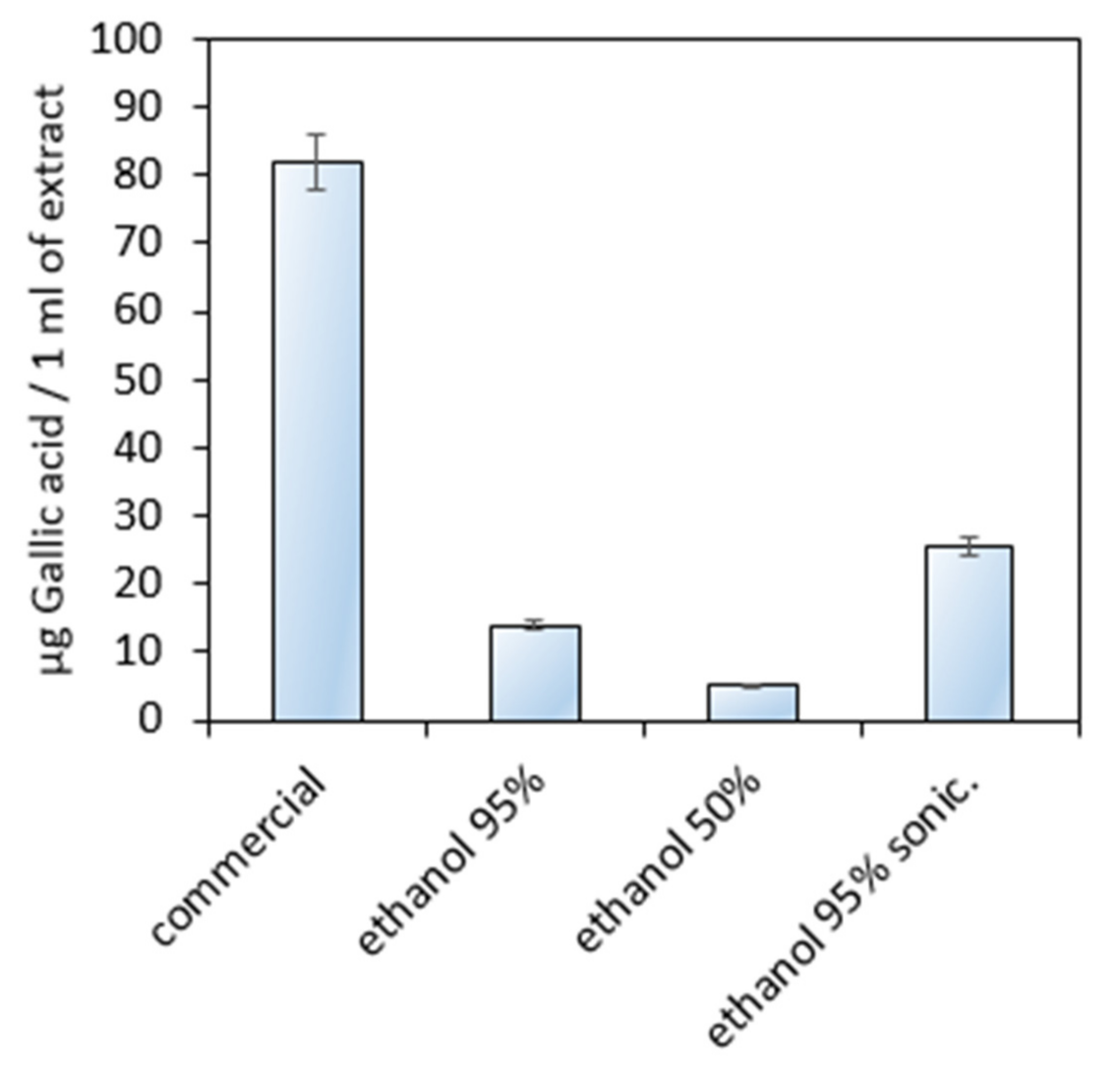
3.1. Natural Extracts Investigation
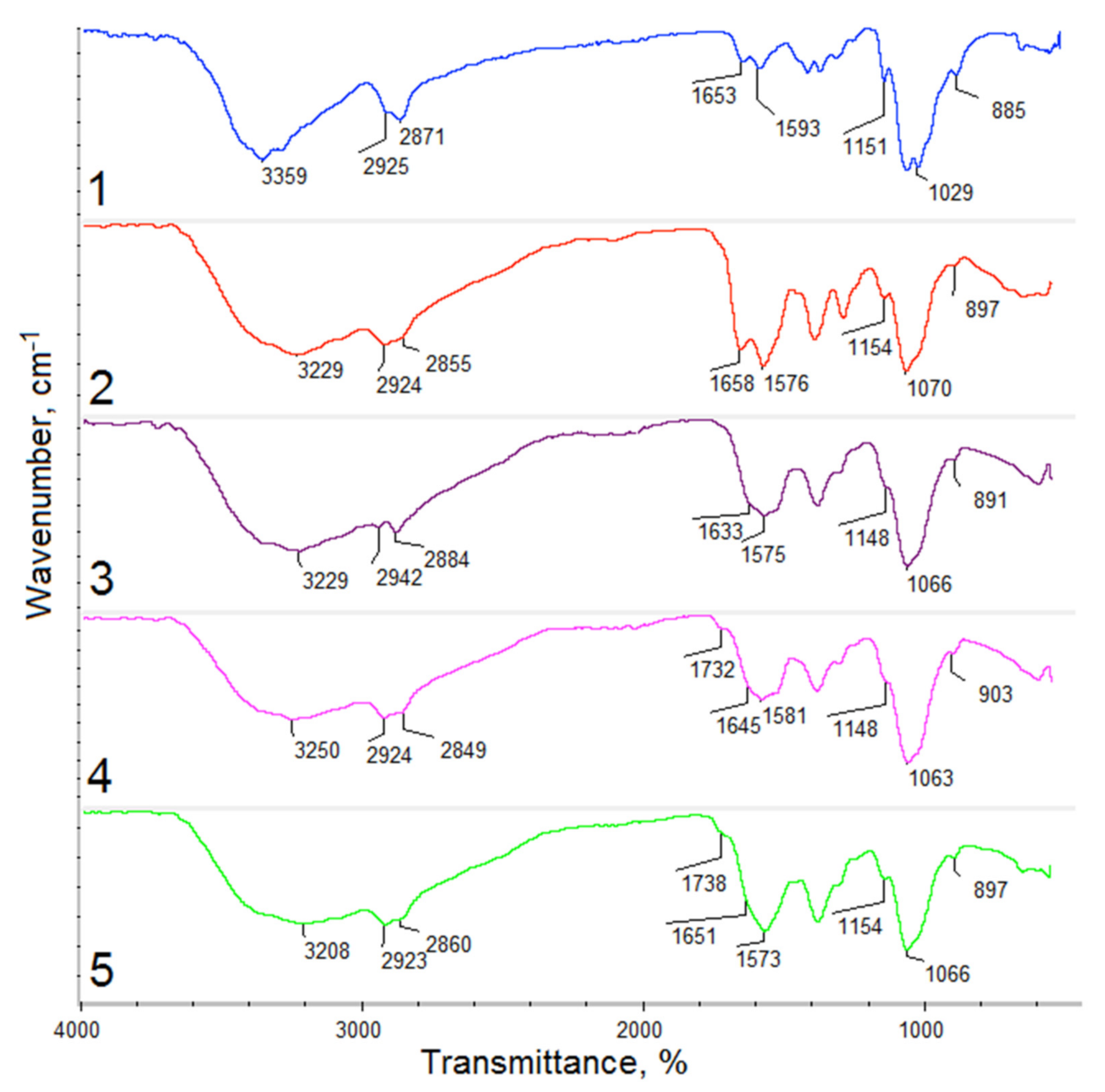
3.2. Hemostatic Agents FTIR Analysis
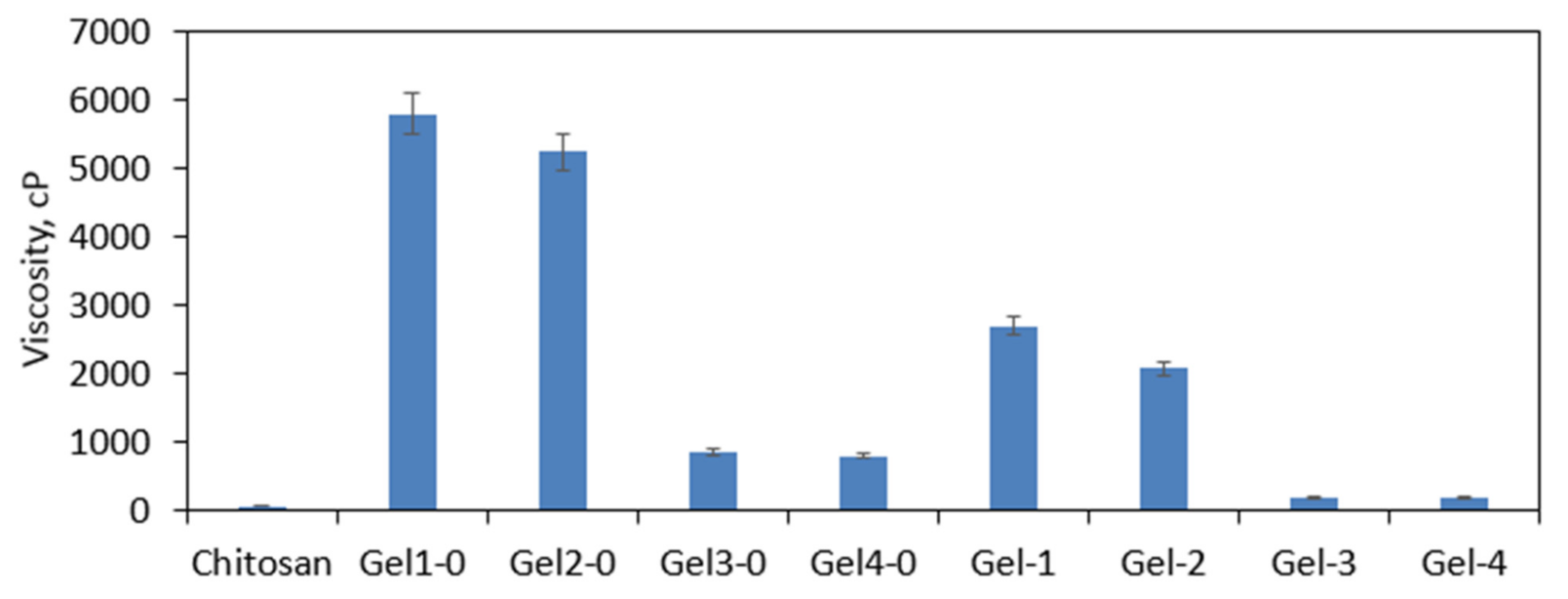
3.3. Viscosity Study
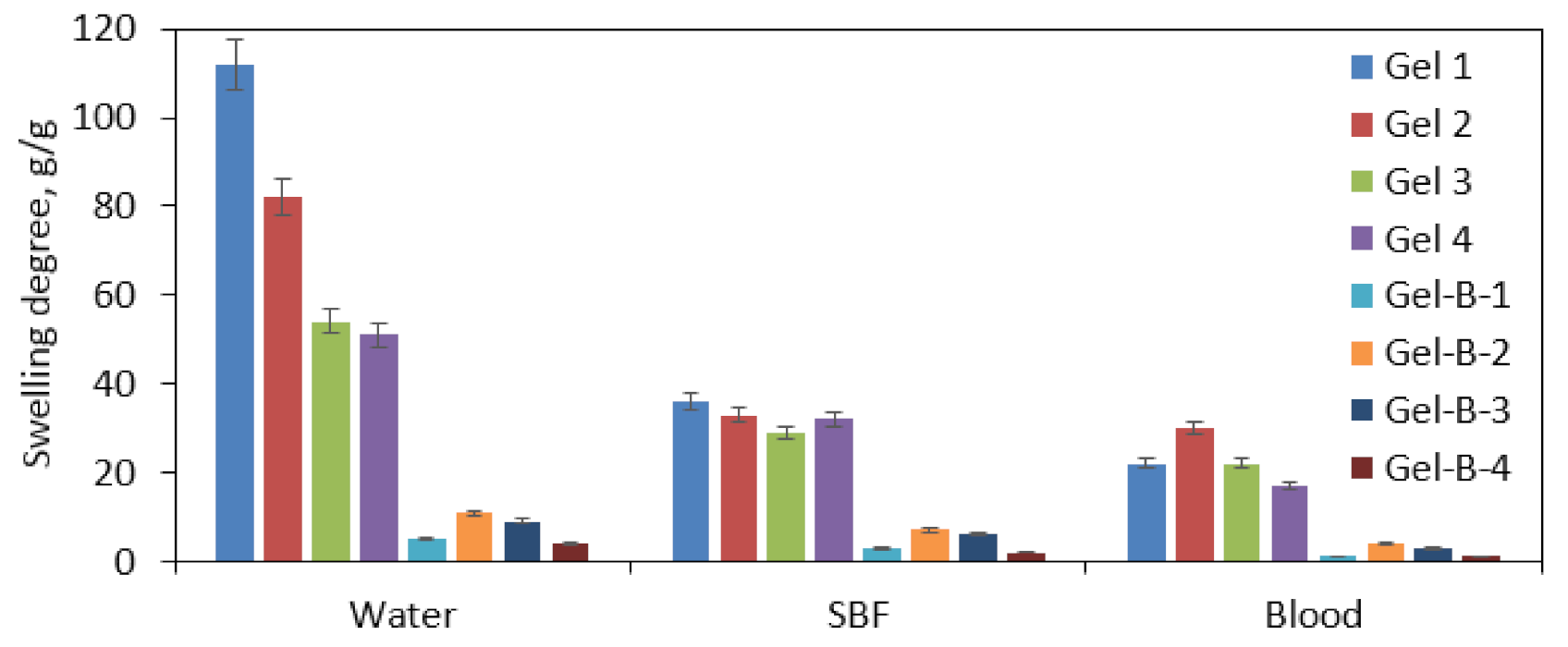
3.4. Swelling Properties Study
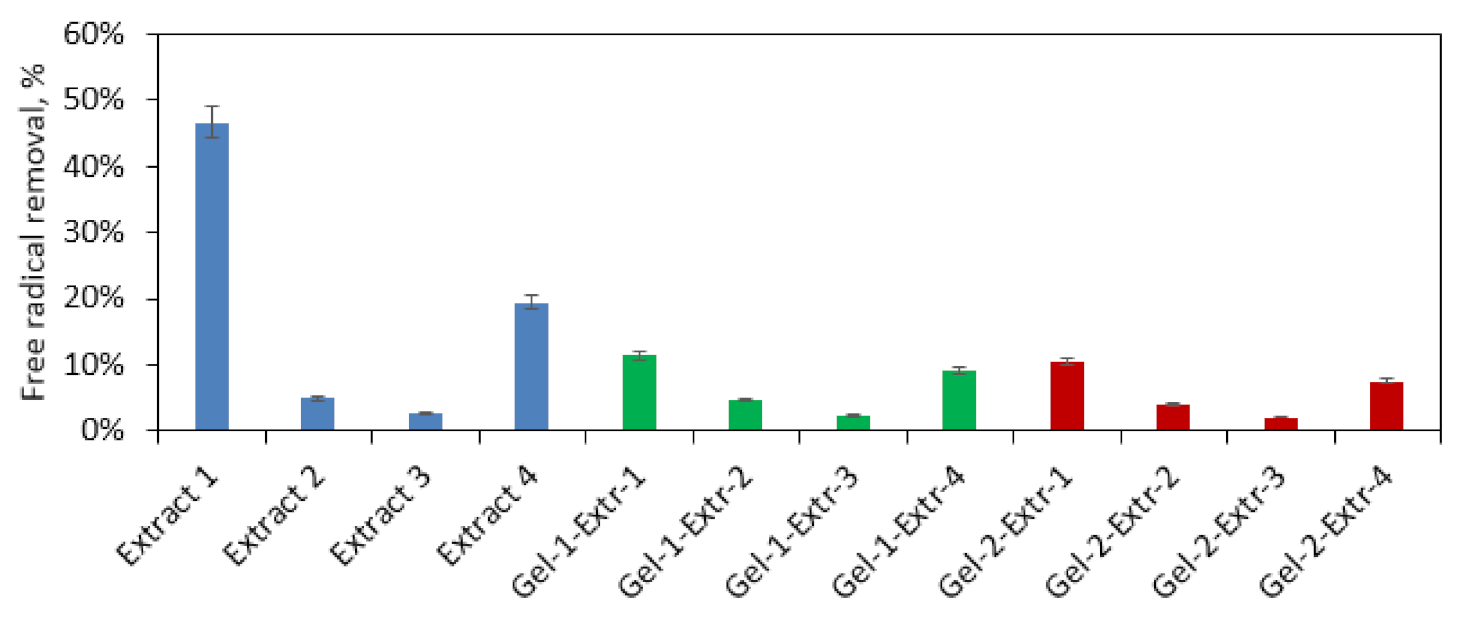
3.5. Antioxidant Activity Study
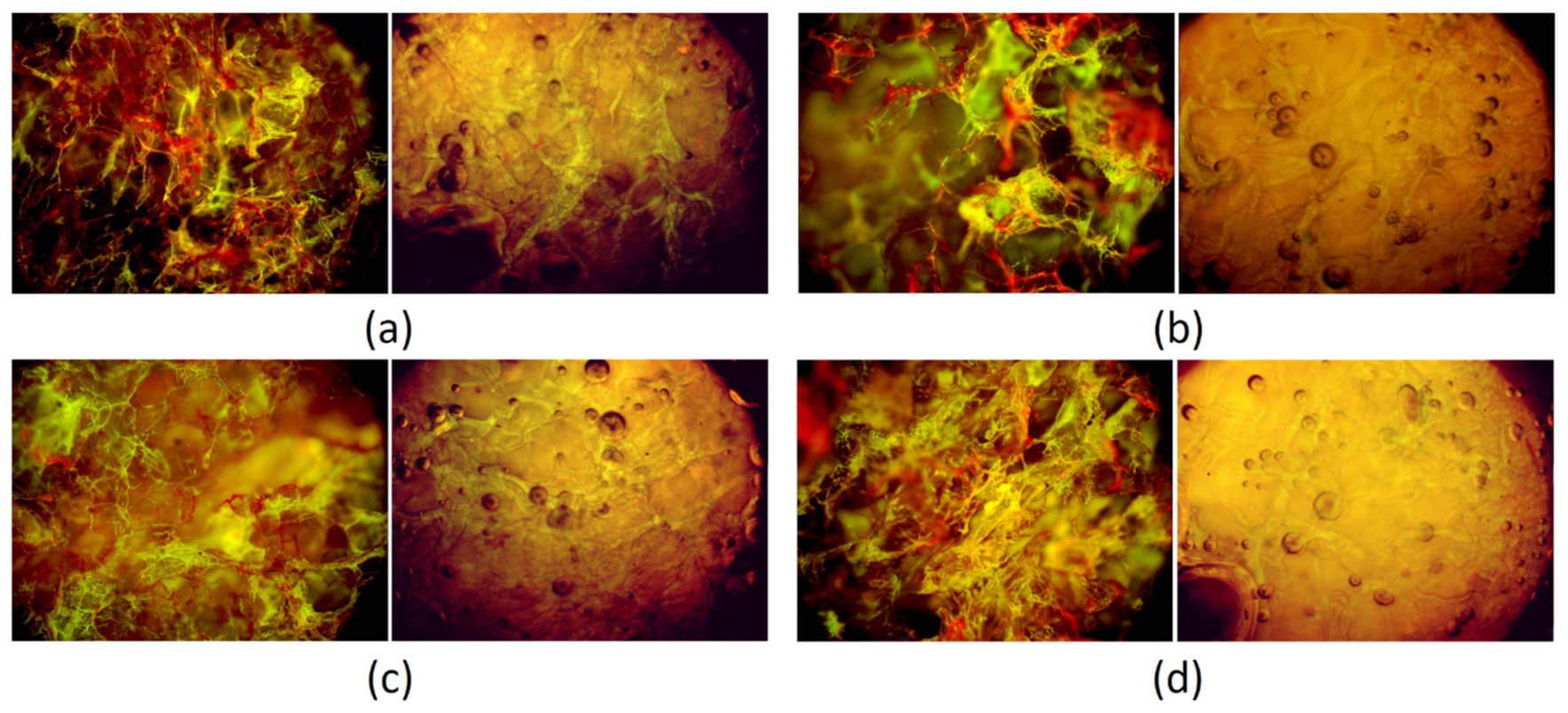
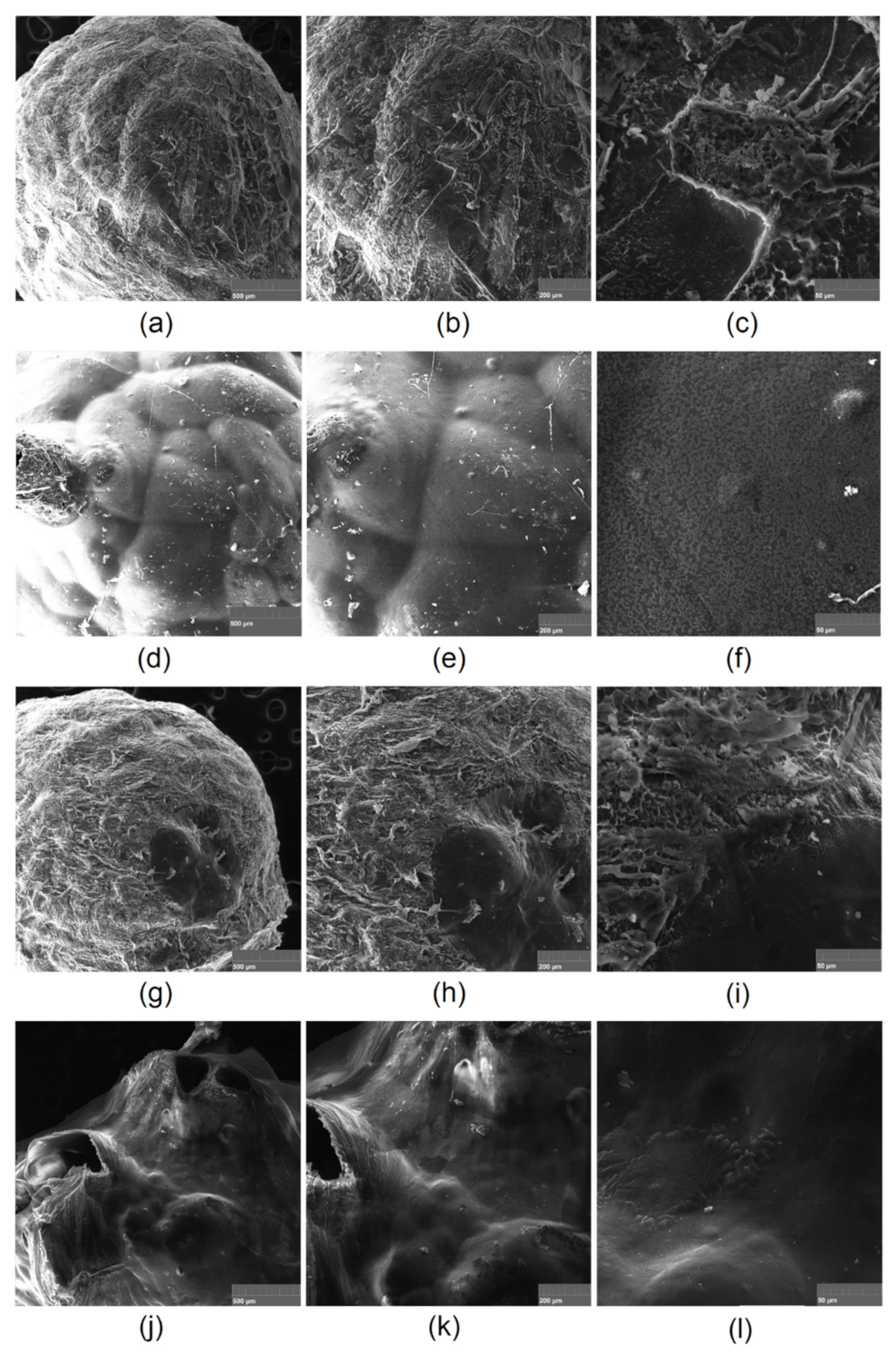
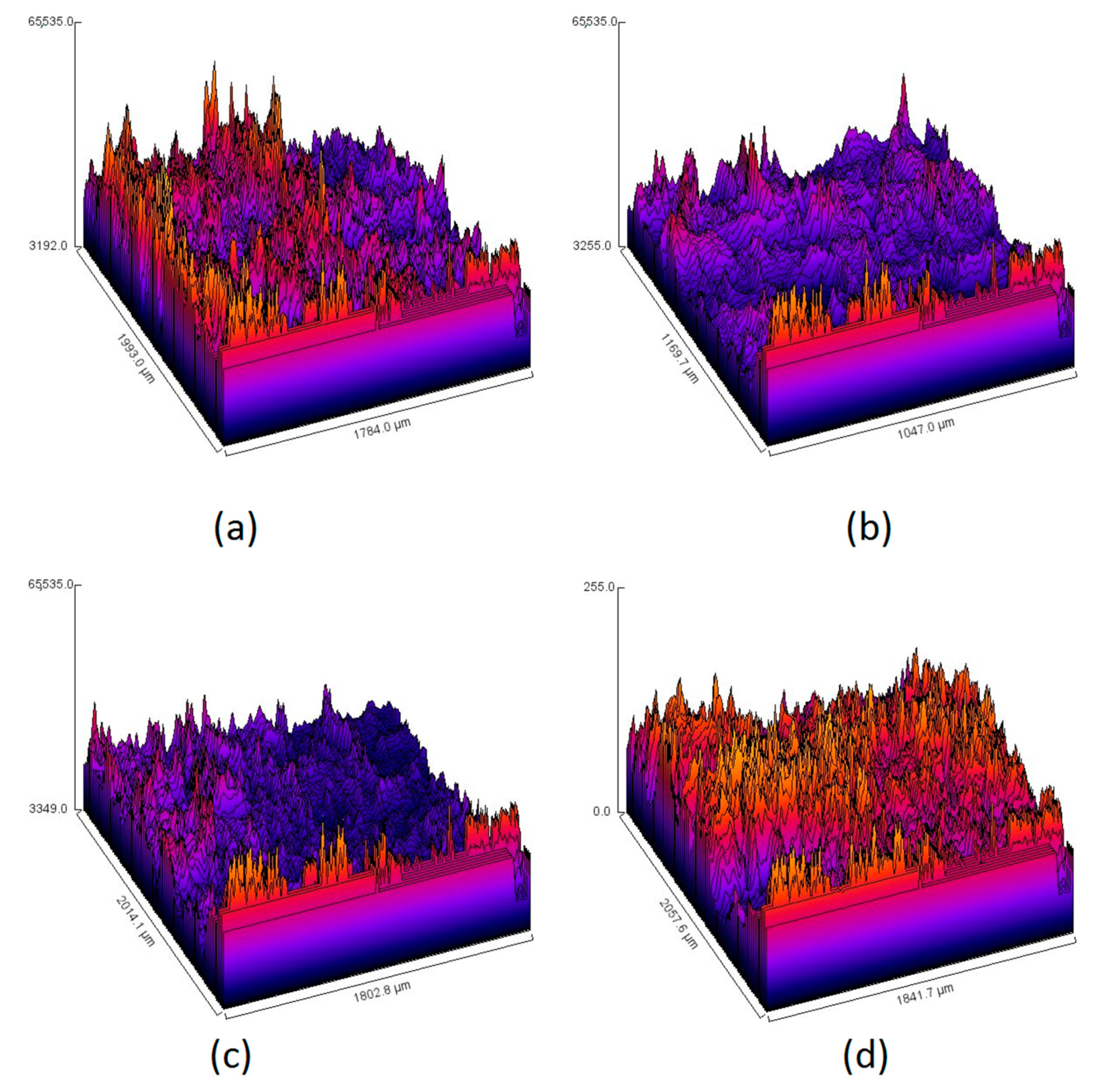
3.6. Morphology Study
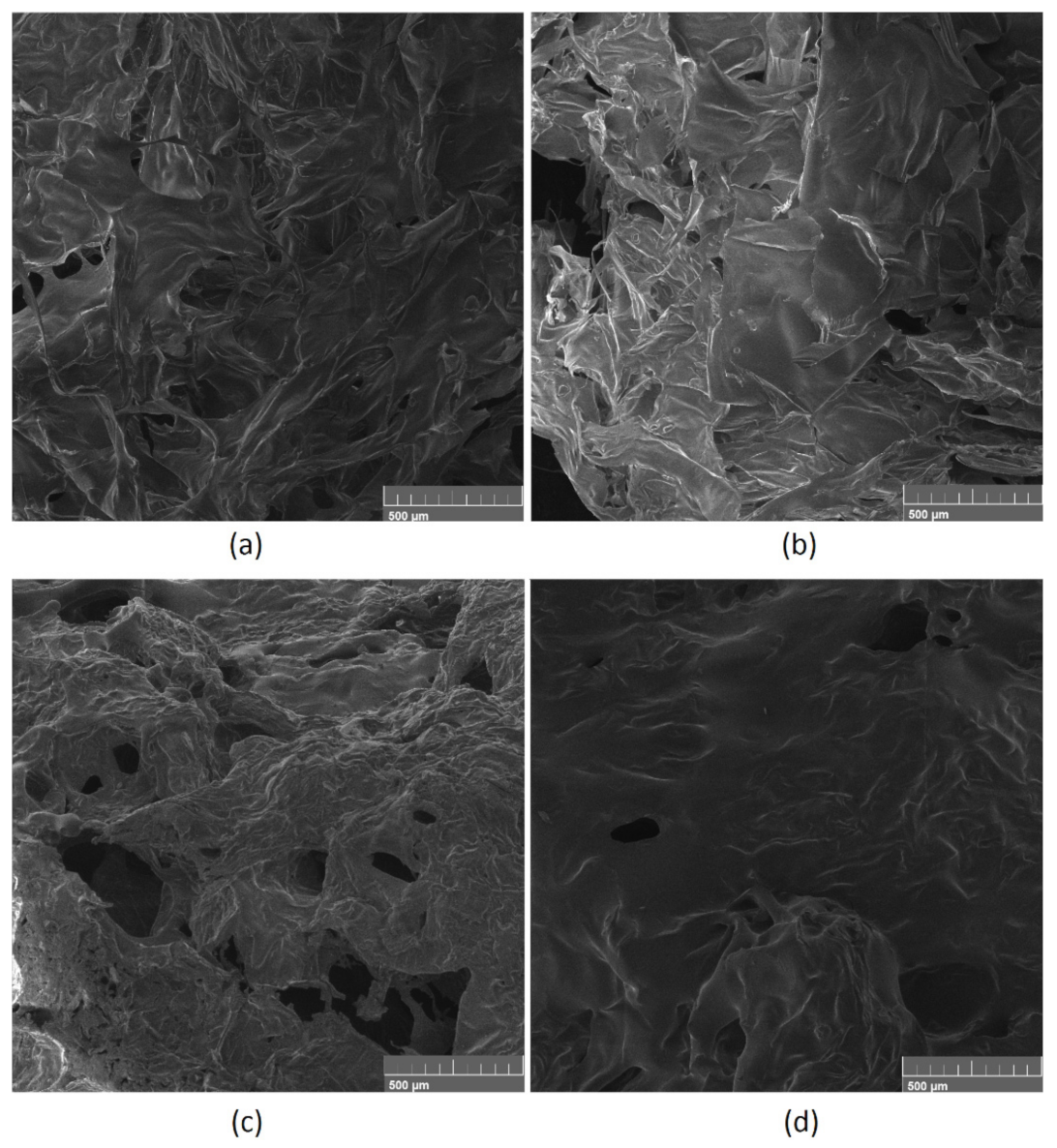
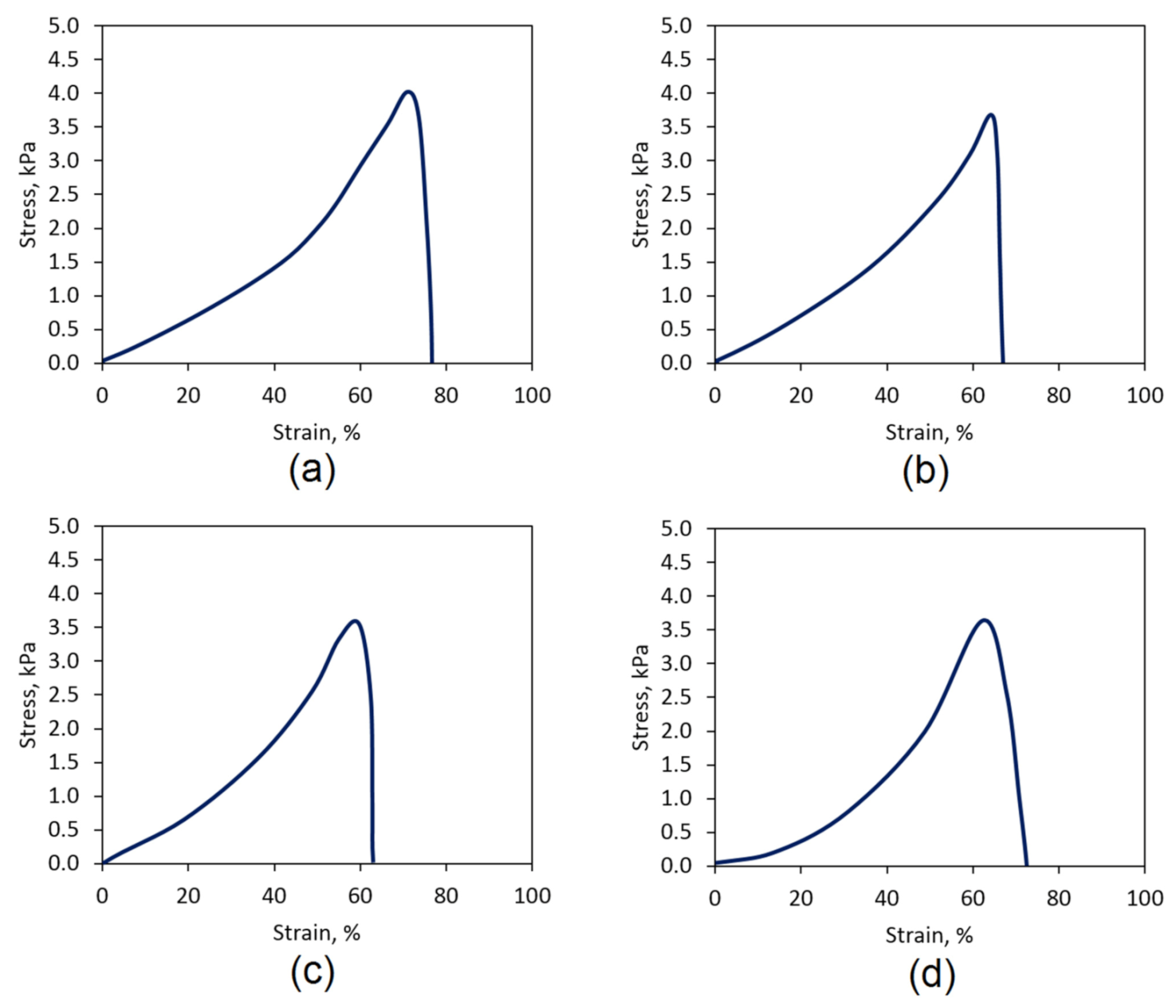
3.7. Mechanical Properties Study
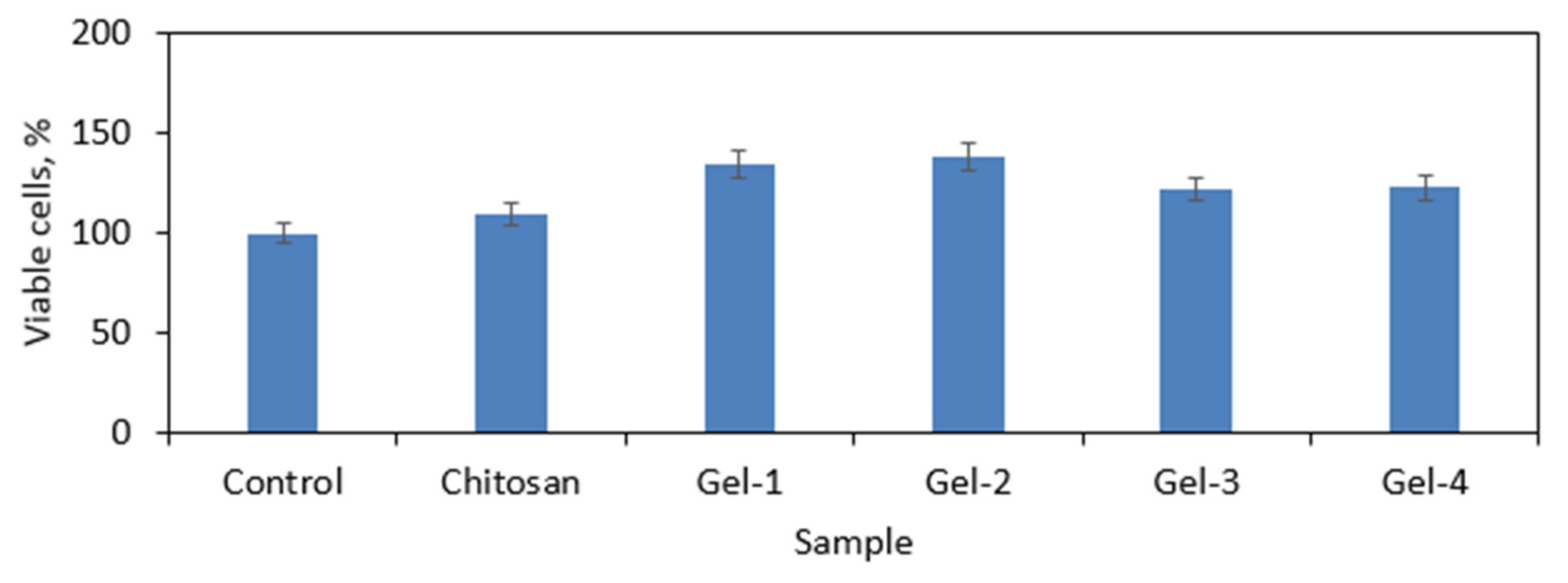
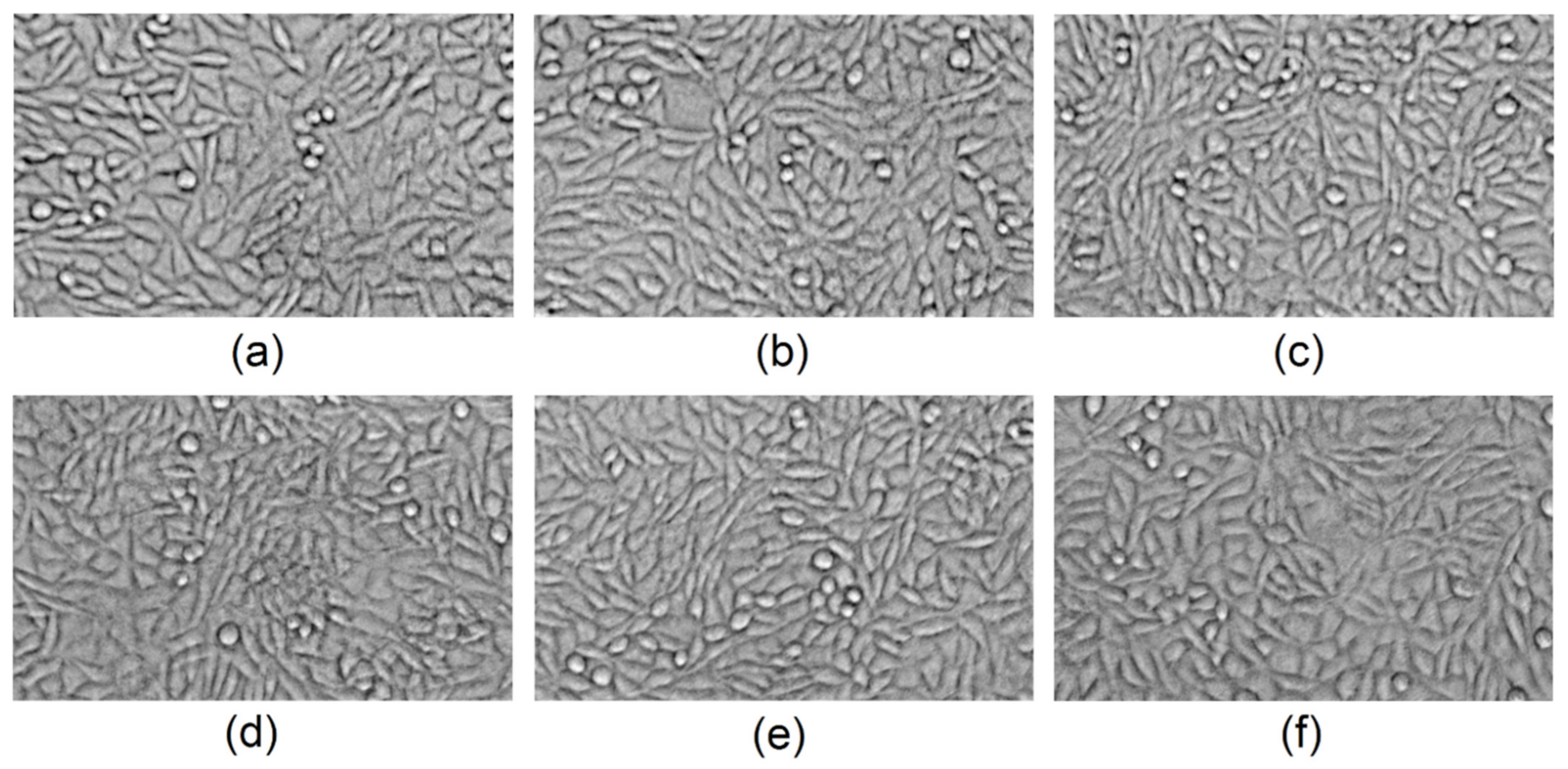
3.8. Cytotoxicity Study
3.9. Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allotey, J.K.; King, A.H.; Kumins, N.H.; Wong, V.L.; Harth, K.C.; Cho, J.S.; Kashyap, V.S. Systematic Review of Hemostatic Agents used in Vascular Surgery. J. Vasc. Surg. 2020, in press. [Google Scholar] [CrossRef]
- Bracey, A.; Shander, A.; Aronson, S.; Boucher, B.A.; Calcaterra, D.; Chu, M.W.A.; Culbertson, R.; Jabr, K.; Kehlet, H.; Lattouf, O.; et al. The Use of Topical Hemostatic Agents in Cardiothoracic Surgery. Ann. Thorac. Surg. 2017, 104, 353–360. [Google Scholar] [CrossRef]
- Galanakis, I.; Vasdev, N.; Soomro, N.N. A Review of Current Hemostatic Agents and Tissue Sealants Used in Laparoscopic Partial Nephrectomy. Rev. Urol. 2011, 13, 131–138. [Google Scholar] [PubMed]
- Nakielski, P.; Pierini, F. Blood interactions with nano- and microfibers: Recent advances, challenges and applications in nano- and microfibrous hemostatic agents. Acta Biomater. 2019, 84, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Qiao, L.; Zou, F.; Xie, Y.; Zheng, Y.; Chao, Y.; Yang, Y.; He, W.; Yang, S. Cellulose fibers-reinforced self-expanding porous composite with multiple hemostatic efficacy and shape adaptability for uncontrollable massive hemorrhage treatment. Bioact. Mater. 2021, 6, 2089–2104. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, S.; Figueroa, T.; Borges, J.; Aguayo, C.; Fernández, K. Graphene oxide-gelatin aerogels as wound dressings with improved hemostatic properties. Mater. Today Chem. 2021, 20, 100418. [Google Scholar] [CrossRef]
- Lv, C.; Li, L.; Jiao, Z.; Yan, H.; Wang, Z.; Wu, Z.; Guo, M.; Wang, Y.; Zhang, P. Improved hemostatic effects by Fe3+ modified biomimetic PLLA cotton-like mat via sodium alginate grafted with dopamine. Bioact. Mater. 2021, 6, 2346–2359. [Google Scholar] [CrossRef]
- Gan, C.; Hu, H.; Meng, Z.; Zhu, X.; Gu, R.; Wu, Z.; Wang, H.; Wang, D.; Gan, H.; Wang, J.; et al. Characterization and Hemostatic Potential of Two Kaolins from Southern China. Molecules 2019, 24, 3160. [Google Scholar] [CrossRef]
- Pogorielov, V.M.; Sikora, V.Z. Chitosan as a Hemostatic Agent: Current State. Eur. J. Med. Ser. B 2015, 2, 24–33. [Google Scholar] [CrossRef]
- Whang, H.S.; Kirsch, W.; Zhu, Y.H.; Yang, C.Z.; Hudson, S.M. Hemostatic Agents Derived from Chitin and Chitosan. J. Macromol. Sci. Polym. Rev. 2005, 45, 309–323. [Google Scholar] [CrossRef]
- Lestari, W.; Yusry, W.N.A.W.; Haris, M.S.; Jaswir, I.; Idrus, E. A glimpse on the function of chitosan as a dental hemostatic agent. Jpn. Dent. Sci. Rev. 2020, 56, 147–154. [Google Scholar] [CrossRef]
- Kadam, A.A.; Shinde, S.K.; Ghodake, G.S.; Saratale, G.D.; Saratale, R.G.; Sharma, B.; Hyun, S.; Sung, J.-S. Chitosan-Grafted Halloysite Nanotubes-Fe3O4 Composite for Laccase-Immobilization and Sulfamethoxazole-Degradation. Polymers 2020, 12, 2221. [Google Scholar] [CrossRef]
- Koumentakou, I.; Terzopoulou, Z.; Michopoulou, A.; Kalafatakis, I.; Theodorakis, K.; Tzetzis, D.; Bikiaris, D. Chitosan dressings containing inorganic additives and levofloxacin as potential wound care products with enhanced hemostatic properties. Int. J. Biol. Macromol. 2020, 162, 693–703. [Google Scholar] [CrossRef]
- Lovskaya, D.; Menshutina, N.; Mochalova, M.; Nosov, A.; Grebenyuk, A. Chitosan-Based Aerogel Particles as Highly Effective Local Hemostatic Agents. Production Process and In Vivo Evaluations. Polymers 2020, 12, 2055. [Google Scholar] [CrossRef]
- Li, T.-T.; Lou, C.-W.; Chen, A.-P.; Lee, M.-C.; Ho, T.-F.; Chen, Y.-S.; Lin, J.-H. Highly Absorbent Antibacterial Hemostatic Dressing for Healing Severe Hemorrhagic Wounds. Materials 2016, 9, 793. [Google Scholar] [CrossRef]
- Zhong, Q.-K.; Wu, Z.-Y.; Qin, Y.-Q.; Hu, Z.; Li, S.-D.; Yang, Z.-M.; Li, P.-W. Preparation and Properties of Carboxymethyl Chitosan/Alginate/Tranexamic Acid Composite Films. Membranes 2019, 9, 11. [Google Scholar] [CrossRef]
- Hu, Z.; Lu, S.; Cheng, Y.; Kong, S.; Li, S.; Li, C.; Yang, L. Investigation of the Effects of Molecular Parameters on the Hemostatic Properties of Chitosan. Molecules 2018, 23, 3147. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, M.; Zheng, X.; Li, W.; Ren, X. Chitosan/mesoporous silica hybrid aerogel with bactericidal properties as hemostatic material. Eur. Polym. J. 2021, 142, 110132. [Google Scholar] [CrossRef]
- Majaz, Q.A.; Tatiya, A.U.; Khurshid, M.; Nazim, S.; Siraj, S. The miracle plant (Kalanchoe pinnata): A phytochemical and pharmacological review. Int. J. Res. Ayurveda Pharm. 2016, 14, 1–10. [Google Scholar]
- Faboro, E.O.; Wei, L.; Liang, S.; McDonald, A.G.; Obafemi, C.A. Hepatoprotective activity of leaves of Kalanchoe pinnata Pers. J. Ethnopharmacol. 2003, 86, 197–202. [Google Scholar]
- Dantas de Araújo, E.R.; Félix-Silva, J.; Xavier-Santos, J.B.; Morais Fernandes, J.; Guerra, G.C.B.; de Araújo, A.A.; de Souza Araújo, D.F.; de Santis Ferreira, L.; Júnior, A.S.; Fernandes-Pedrosa, M.-F.; et al. Local anti-inflammatory activity: Topical formulation containing Kalanchoe brasiliensis and Kalanchoe pinnata leaf aqueous extract. Biomed. Pharmacother. 2019, 113, 108721. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Shanmugam, V.K. Anti-inflammatory activity screening of Kalanchoe pinnata methanol extract and its validation using a computational simulation approach. Inform. Med. Unlocked. 2019, 14, 6–14. [Google Scholar] [CrossRef]
- Mora-Pérez, A.; Hernández-Medel, M.D.R. Anticonvulsant activity of methanolic extract from Kalanchoe pinnata (Lam.) stems and roots in mice: A comparison to diazepam. Neurologia 2016, 31, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Menon, N.; Sparks, J.; Omoruyi, F. Hypoglycemic and hypocholesterolemic activities of the aqueous preparation of Kalanchoe pinnata leaves in streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2015, 5, 3–9. [Google Scholar] [CrossRef]
- De Araújo, E.R.D.; Guerra, G.C.B.; Araújo, D.F.d.S.; De Araújo, A.A.; Fernandes, J.M.; De Araújo Júnior, R.F.; Da Silva, V.C.; De Carvalho, T.G.; Ferreira, L.D.S.; Zucolotto, S.M. Gastroprotective and Antioxidant Activity of Kalanchoe brasiliensis and Kalanchoe pinnata Leaf Juices against Indomethacin and Ethanol-Induced Gastric Lesions in Rats. Int. J. Mol. Sci. 2018, 19, 1265. [Google Scholar] [CrossRef]
- Faboro, E.O.; Wei, L.; Liang, S.; McDonald, A.G.; Obafemi, C.A. Phytochemical Analyzes from the Leaves of Bryophyllum pinnatum. EJMP 2006, 14, 1–10. [Google Scholar] [CrossRef]
- Gabriel, H.; Agbor, A.; Vinson, J.A.; Donnelly, P.E. Folin-Ciocalteau Reagent for Polyphenolic Assay. Int. J. Food Sci. Nutr. Diet. 2014, 3, 147–156. [Google Scholar]
- Bezuneh, T.T.; Kebede, E.M. UV—Visible Spectrophotometric Quantification of Total Polyphenol in Selected Fruits. Int. J. Food Sci. Nutr. 2015, 4, 397–401. [Google Scholar] [CrossRef][Green Version]
- Abbas, O.; Compère, G.; Larondelle, Y.; Pompeu, D.; Rogez, H.; Baeten, V. Phenolic compound explorer: A mid-infrared spectroscopy database. Vib. Spectrosc. 2017, 92, 111–118. [Google Scholar] [CrossRef]
- Hama, T.; Kouchi, A.; Watanabe, N.; Enami, S.; Shimoaka, T.; Hasegawa, T. In Situ Nondestructive Analysis of Kalanchoe pinnata Leaf Surface Structure by Polarization-Modulation Infrared Reflection−Absorption Spectroscopy. J. Phys. Chem. B 2017, 121, 11124–11131. [Google Scholar] [CrossRef]
- Radwan-Pragłowska, J.; Piątkowski, M.; Deineka, V.; Janus, Ł.; Korniienko, V.; Husak, E.; Holubnycha, V.; Liubchak, I.; Zhurba, V.; Sierakowska, A.; et al. Chitosan-Based Bioactive Hemostatic Agents with Antibacterial Properties—Synthesis and Characterization. Molecules 2019, 24, 2629. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Wellard, M.; Woodruff, M.A.; Klein, T.J. Tailoring Hydrogel Viscoelasticity with Physical and Chemical Crosslinking. Polymers 2015, 7, 2650–2669. [Google Scholar] [CrossRef]
- Park, J.K.; Nah, J.-W.; Choi, C. Thermosensitive Chitosan-based Hydrogel with Growth Factor as Adhesion Barrier. Polym. Korea 2015, 39, 480–486. [Google Scholar] [CrossRef]
- Franzén, H.M.; Draget, K.I.; Langebäck, J.; Nilsen-Nygaard, J. Characterization and Properties of Hydrogels Made from Neutral Soluble Chitosans. Polymers 2015, 7, 373–389. [Google Scholar] [CrossRef]
- Bashir, S.; Teo, Y.Y.; Ramesh, S.; Ramesh, K.; Rizwan, M.; Rizwan, M. Synthesis and characterization of pH-sensitive N-succinyl chitosan hydrogel and properties for biomedical applications. J. Chil. Chem. Soc. 2019, 64, 0717–9707. [Google Scholar] [CrossRef]
- Costa, C.N.; Teixeira, V.G.; Delpech, M.C.; Souza, J.V.; Costa, M.A. Viscometric study of chitosan solutions in acetic acid/sodium acetate and acetic acid/sodium chloride. Carbohydr. Polym. 2015, 133, 245–250. [Google Scholar] [CrossRef]
- Rohindra, T.R.; Nand, A.V.; Khurma, J.R. Swelling properties of chitosan hydrogels. SPJNAS 2004, 22, 32–35. [Google Scholar] [CrossRef]
- Wahba, M.I. Enhancement of the mechanical properties of chitosan. J. Biomater. Sci. Polym. Ed. 2019, 31, 350–375. [Google Scholar] [CrossRef]
- Le, H.R.; Qu, S.; Mackay, R.E.; Rothwell, R. Fabrication and mechanical properties of chitosan composite membrane containing hydroxyapatite particles. J. Adv. Ceram. 2012, 1, 66–71. [Google Scholar] [CrossRef]
- Grząbka-Zasadzińska, A.; Amietszajew, T.; Borysiak, S. Thermal and mechanical properties of chitosan nanocomposites with cellulose modified in ionic liquids. J. Therm. Anal. Calorim. 2017, 130, 143–154. [Google Scholar] [CrossRef]
- Notin, L.; Viton, C.; David, L.; Alcouffe, P.; Rochas, C.; Domard, A. Morphology and mechanical properties of chitosan fibers obtained by gel-spinning: Influence of the dry-jet-stretching step and ageing. Acta Biomater. 2006, 2, 387–402. [Google Scholar] [CrossRef]
- Huang, M.; Khor, E.; Lim, L.-Y. Uptake and cytotoxicity of chitosan molecules and nanoparticles: Effects of molecular weight and degree of deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef]
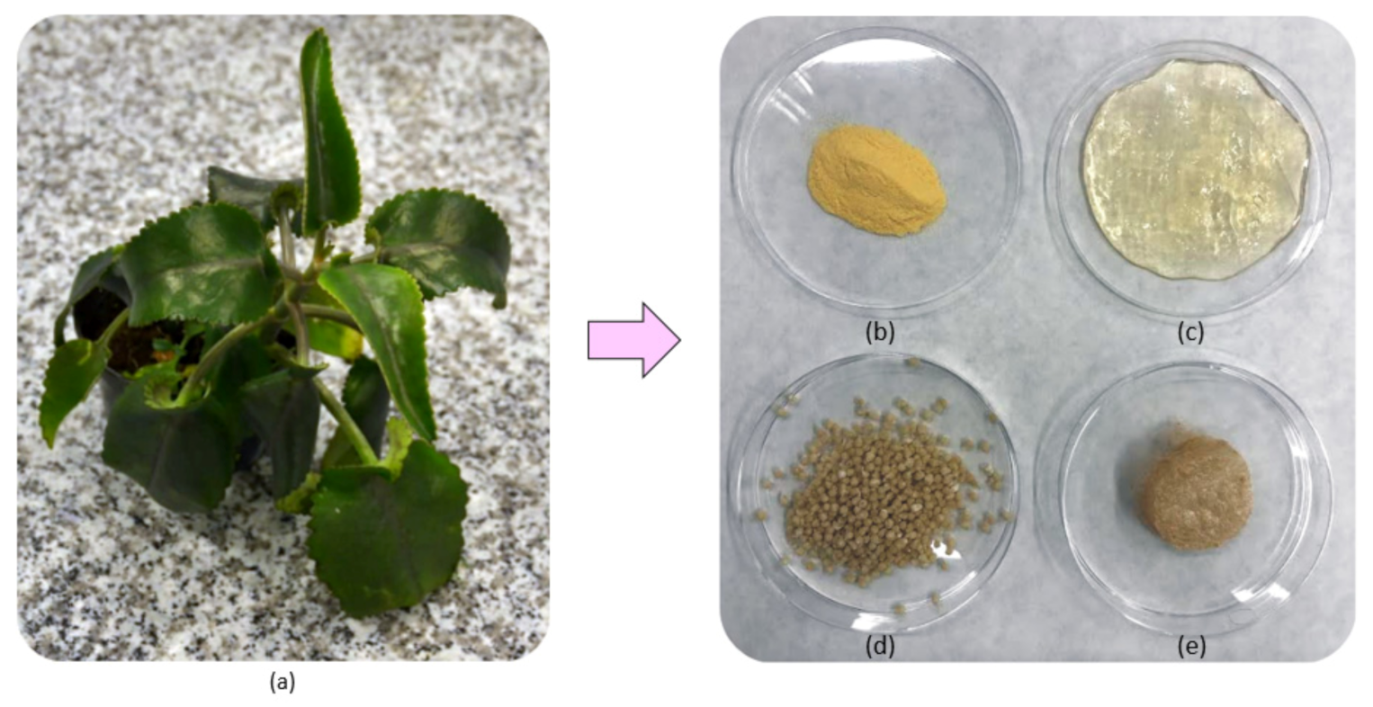
| Sample | Glu:Asp, g:g | Lyophilization | Form |
|---|---|---|---|
| Gel1-0 | 0.90:0.10 | No | Patch |
| Gel2-0 | 0.75:0.25 | No | Patch |
| Gel3-0 | 0.25:0.75 | No | Patch |
| Gel4-0 | 0.10:0.90 | No | Patch |
| Gel-1 | 0.9:0.10 | Yes | Patch |
| Gel-2 | 0.75:0.25 | Yes | Patch |
| Gel-3 | 0.25:0.75 | Yes | Patch |
| Gel-4 | 0.10:0.90 | Yes | Patch |
| Gel-1-B | 0.9:0.10 | Yes | Beads |
| Gel-2-B | 0.75:0.25 | Yes | Beads |
| Gel-3-B | 0.25:0.75 | Yes | Beads |
| Gel-4-B | 0.10:0.90 | Yes | Beads |
| Sample | O–H | C–H | C=C–H | C=O | C=C | C–O |
|---|---|---|---|---|---|---|
| cm−1 | cm−1 | cm−1 | cm−1 | cm−1 | cm−1 | |
| Kalanchoe pinnata leaf | 3324 | 2923 | - | 1722 | 1429 | 1006 |
| 2878 | ||||||
| Commercially available extract | 3187 | 2943 | - | 1713 | 1591 | 1118 |
| 2875 | 1394 | |||||
| 1089 | ||||||
| Plant extract prepared using 50% ethanol solution | 3351 | 2924 | 3009 | 1720 | 1599 | 1176 |
| 2856 | 1396 | |||||
| 1092 | ||||||
| Plant extract prepared using 95% ethanol solution | 3304 | 2923 | 3009 | 1721 | 1601 | 1173 |
| 2858 | 1386 | |||||
| 1074 | ||||||
| Plant extract prepared using 95% ethanol solution and ultrasounds | 3308 | 2975 | - | 1714 | 1589 | 1236 |
| 2925 | 1394 | |||||
| 1076 |
| Sample | O–H | C–H | C=O | –NH2 | C–O | –COO– |
|---|---|---|---|---|---|---|
| cm−1 | cm−1 | cm−1 | cm−1 | cm−1 | cm−1 | |
| Raw fungal chitosan | 3359 | 2925 | 1653 | 1593 | 1029 | - |
| 2871 | 1151 | 885 | ||||
| Gel-1 | 3229 | 2924 | 1658 | 1576 | 1070 | - |
| 2855 | 1154 | 897 | ||||
| Gel-2 | 3229 | 2924 | 1658 | 1575 | 1066 | - |
| 2284 | 1148 | 891 | ||||
| Gel-3 | 3250 | 2924 | 1645 | 1581 | 1063 | 1732 |
| 2849 | 1148 | 903 | ||||
| Gel-4 | 3208 | 2923 | 1651 | 1573 | 1066 | 1738 |
| 2860 | 1154 | 897 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Sierakowska, A.; Galek, T.; Szajna, E.; Bogdał, D.; Tupaj, M. Fungal Chitosan-Derived Biomaterials Modified with Kalanchoe pinnata as Potential Hemostatic Agents—Development and Characterization. Polymers 2021, 13, 1300. https://doi.org/10.3390/polym13081300
Radwan-Pragłowska J, Janus Ł, Piątkowski M, Sierakowska A, Galek T, Szajna E, Bogdał D, Tupaj M. Fungal Chitosan-Derived Biomaterials Modified with Kalanchoe pinnata as Potential Hemostatic Agents—Development and Characterization. Polymers. 2021; 13(8):1300. https://doi.org/10.3390/polym13081300
Chicago/Turabian StyleRadwan-Pragłowska, Julia, Łukasz Janus, Marek Piątkowski, Aleksandra Sierakowska, Tomasz Galek, Ernest Szajna, Dariusz Bogdał, and Mirosław Tupaj. 2021. "Fungal Chitosan-Derived Biomaterials Modified with Kalanchoe pinnata as Potential Hemostatic Agents—Development and Characterization" Polymers 13, no. 8: 1300. https://doi.org/10.3390/polym13081300
APA StyleRadwan-Pragłowska, J., Janus, Ł., Piątkowski, M., Sierakowska, A., Galek, T., Szajna, E., Bogdał, D., & Tupaj, M. (2021). Fungal Chitosan-Derived Biomaterials Modified with Kalanchoe pinnata as Potential Hemostatic Agents—Development and Characterization. Polymers, 13(8), 1300. https://doi.org/10.3390/polym13081300