Emerging Biofabrication Techniques: A Review on Natural Polymers for Biomedical Applications
Abstract
1. Introduction
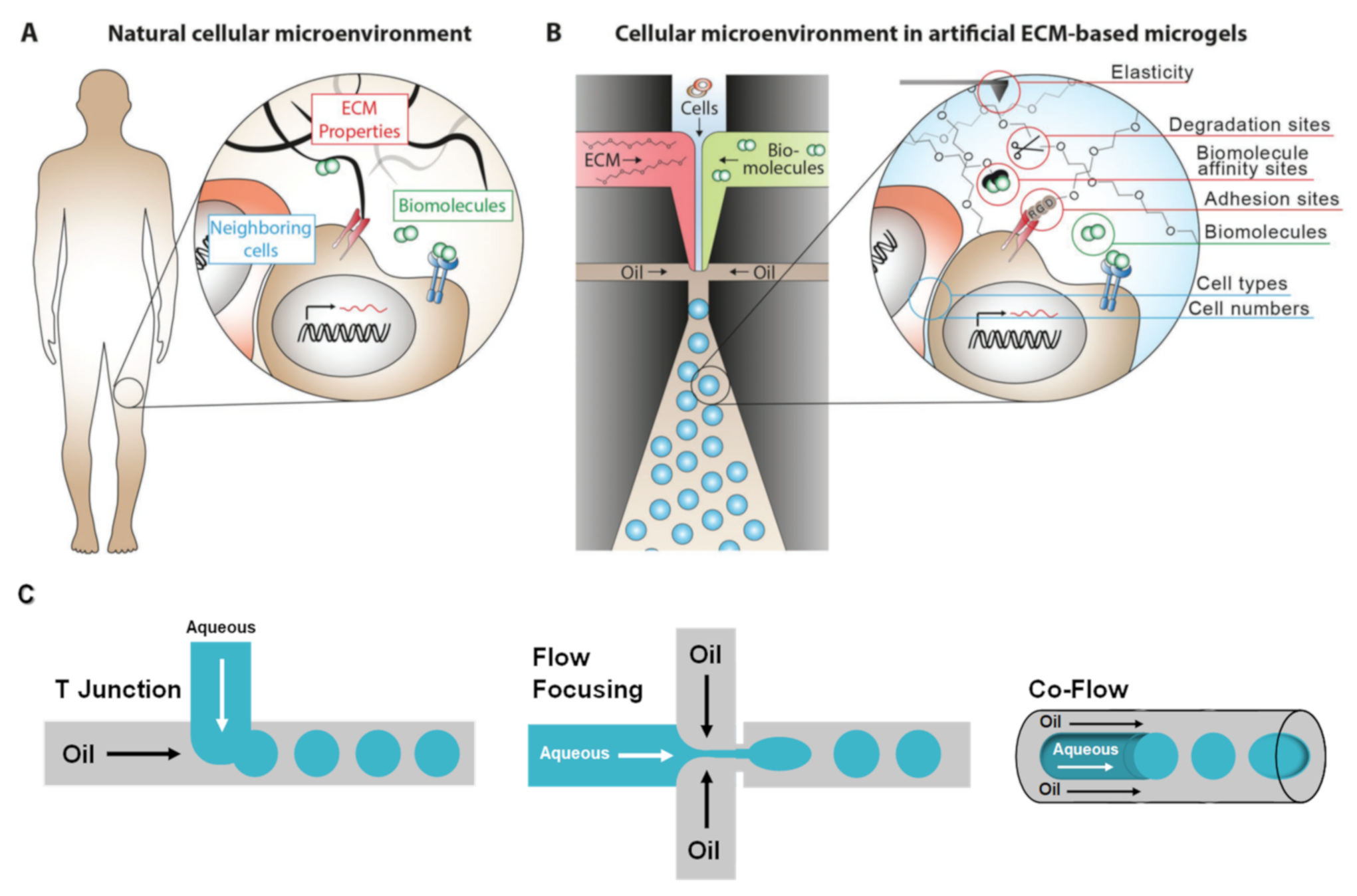
2. Microfluidics
2.1. Naturally Derived Polymer Used for the Preparation of Cell-Laden Microgels Using Microfluidics
2.1.1. Alginate
2.1.2. Hyaluronic Acid
2.1.3. Chitosan
2.1.4. Gelatin
2.1.5. Dextran
2.1.6. Heparin
2.2. Future Perspectives in Fabrication of Cell-Laden Microgels through Microfluidics
3. Cell-Electrospinning (CE) and Bio-Electrospraying (BES)
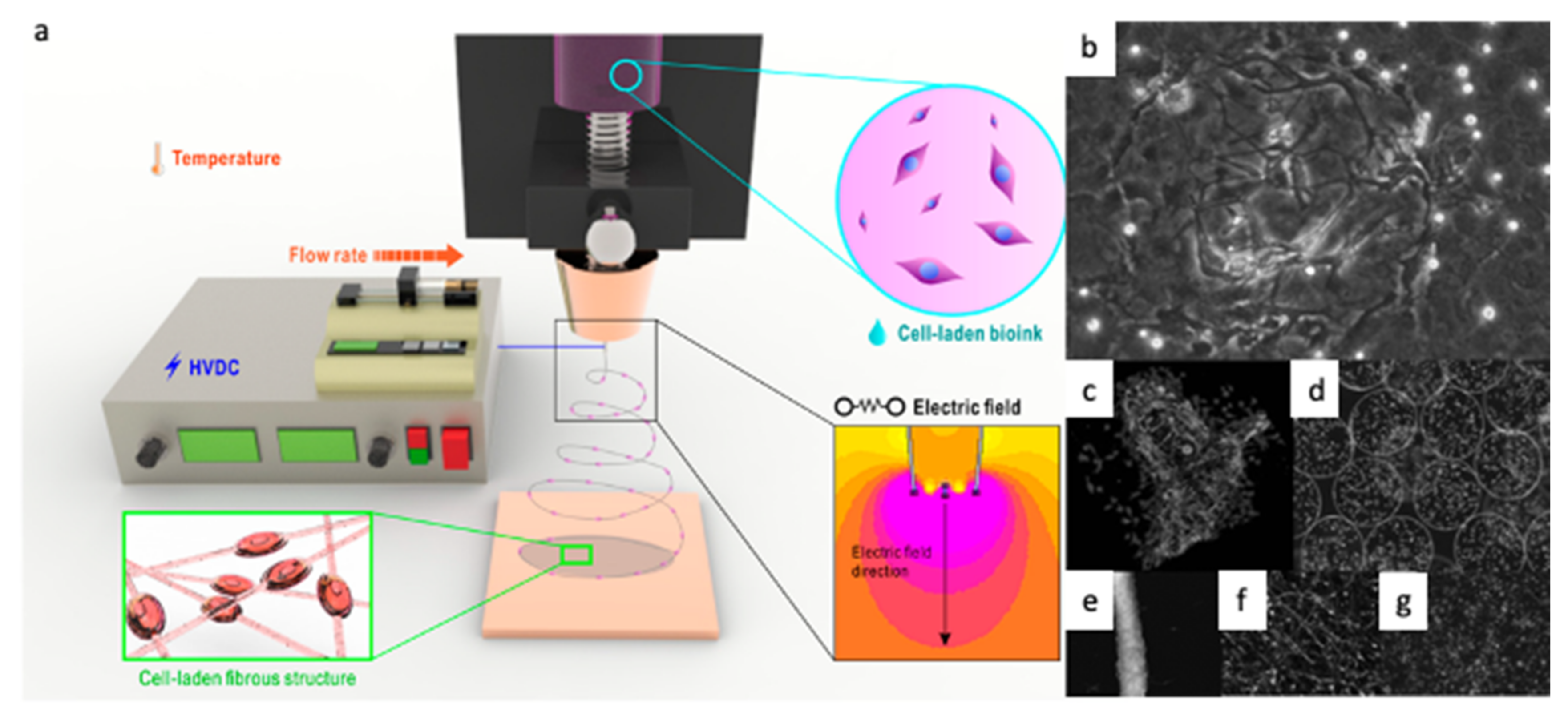
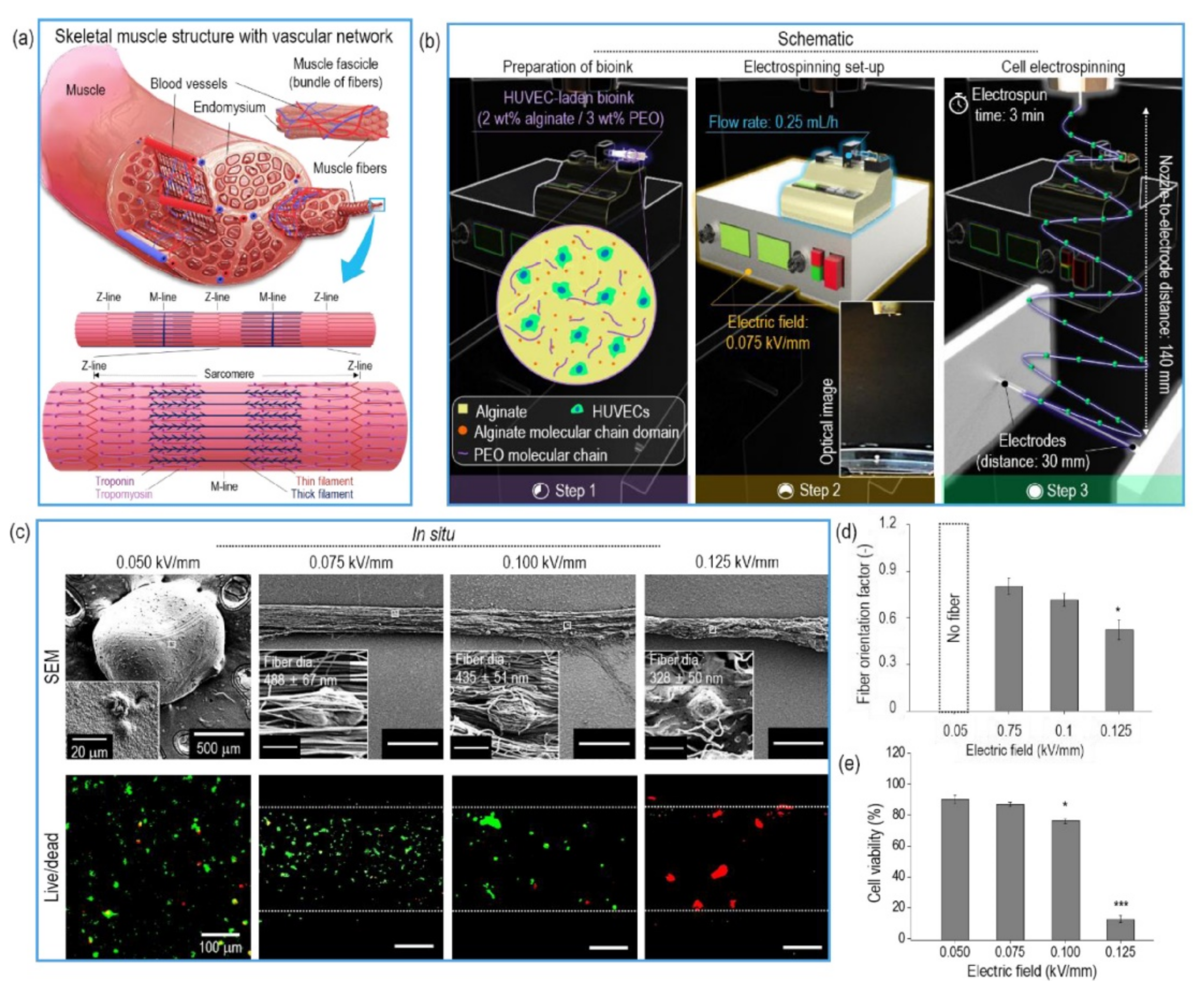
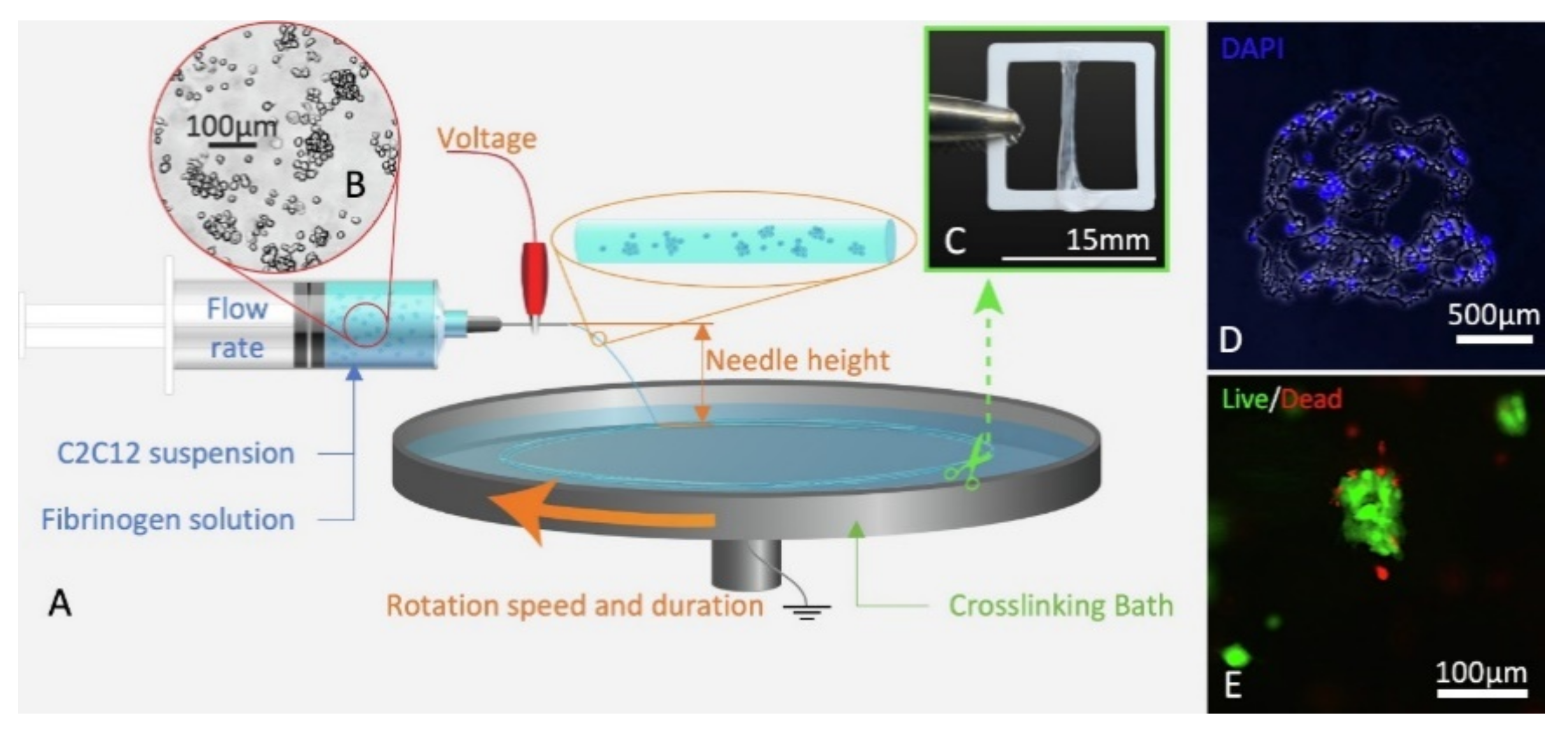
3.1. Naturally Derived Polymers for CE and BES
3.1.1. Alginate
3.1.2. Gelatin
3.1.3. Fibrin
3.1.4. Collagen
3.2. Future Trends
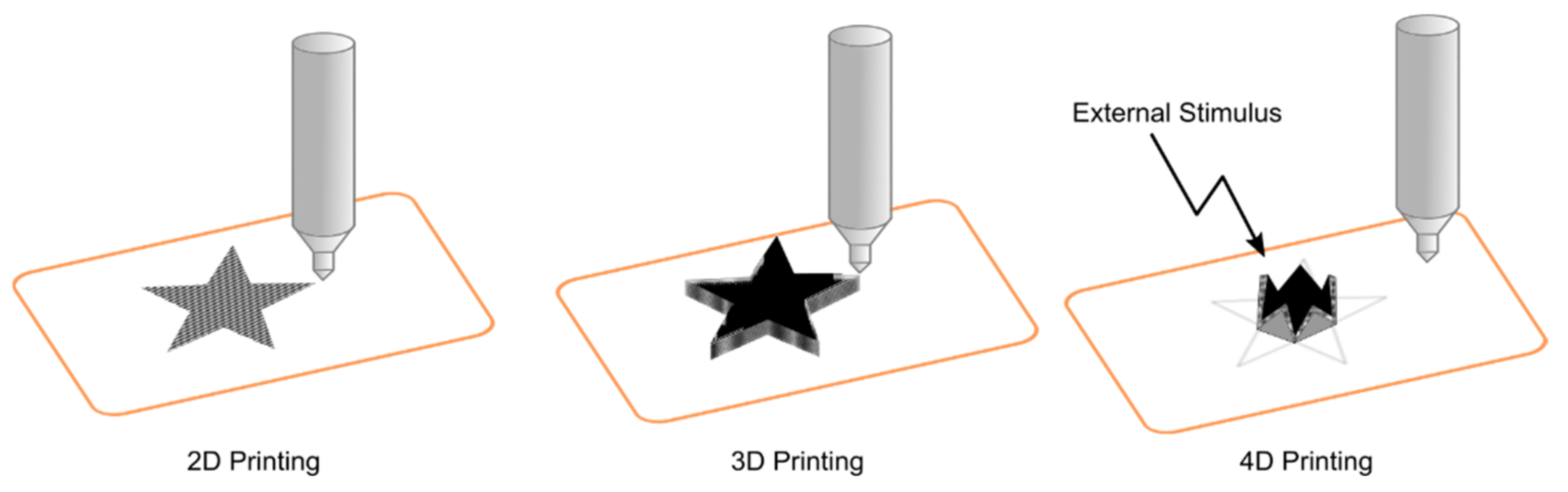
4. 3D Printing
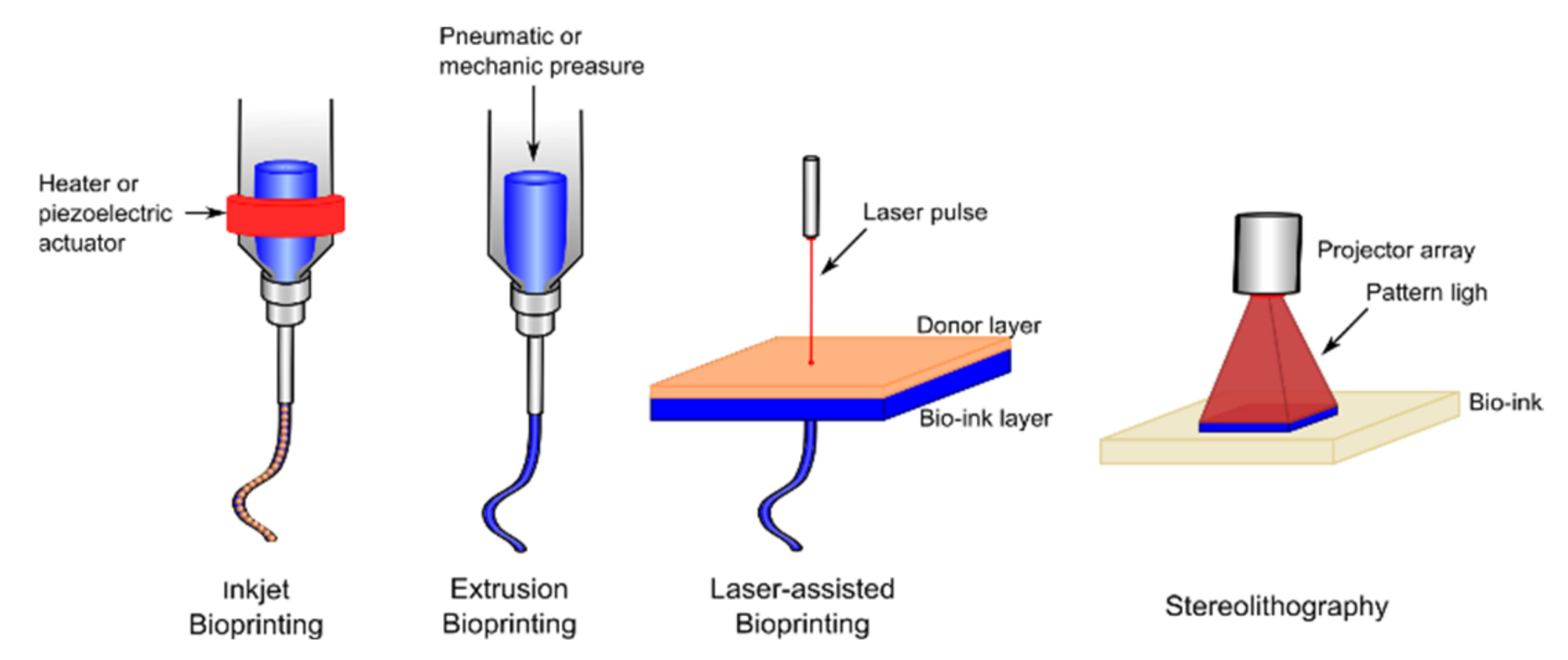
4.1. Recent Advances in Bioinks
4.1.1. Alginate Based Bioinks
4.1.2. Chitosan Based Bioinks
4.1.3. Other Natural Polymer Based Bioinks
4.1.4. Sacrificial Bioinks
4.1.5. Evolution of the Bioinks
4.2. 3D Printing for Biomedical Applications
4.2.1. Bioactive and Biodegradable Scaffolds
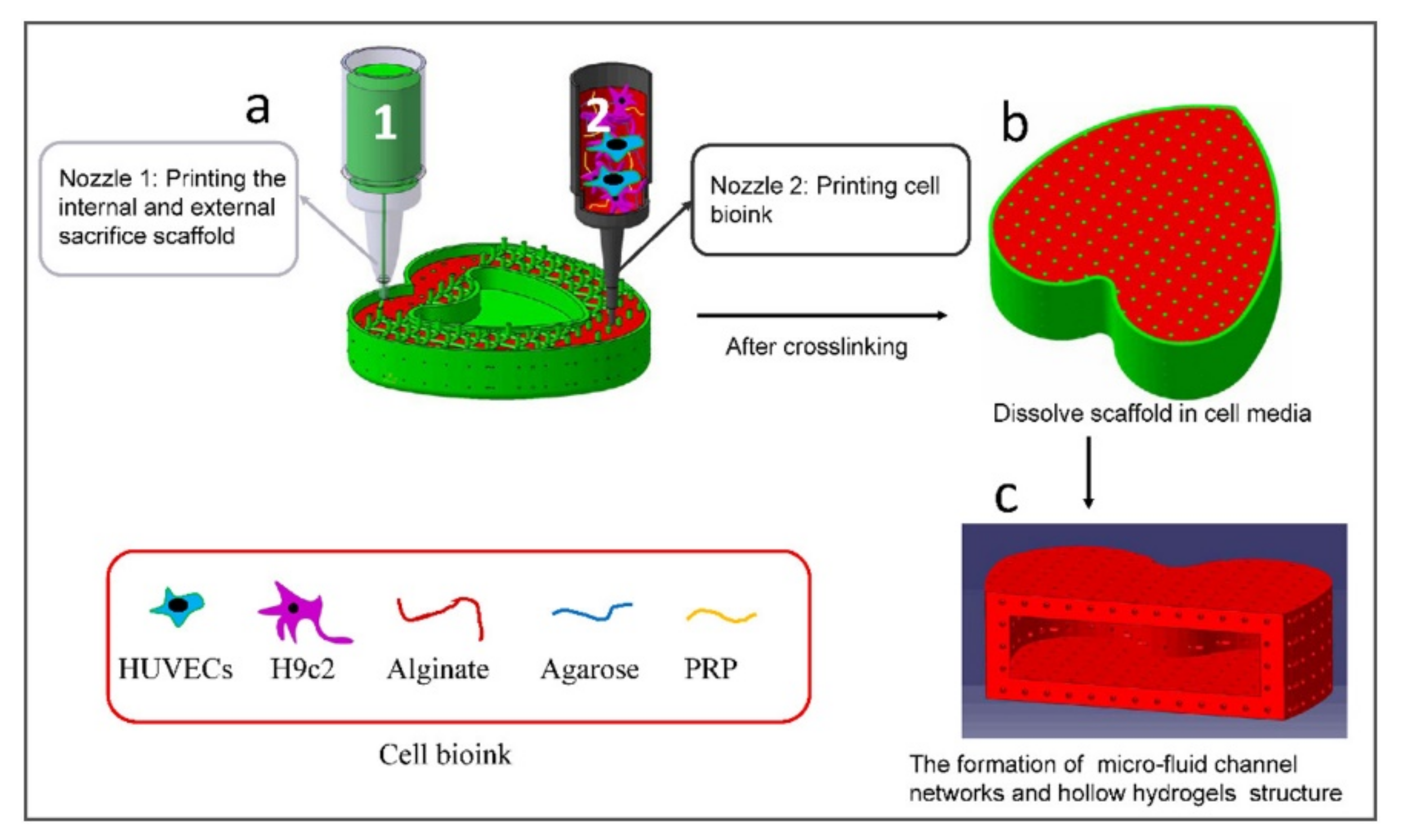
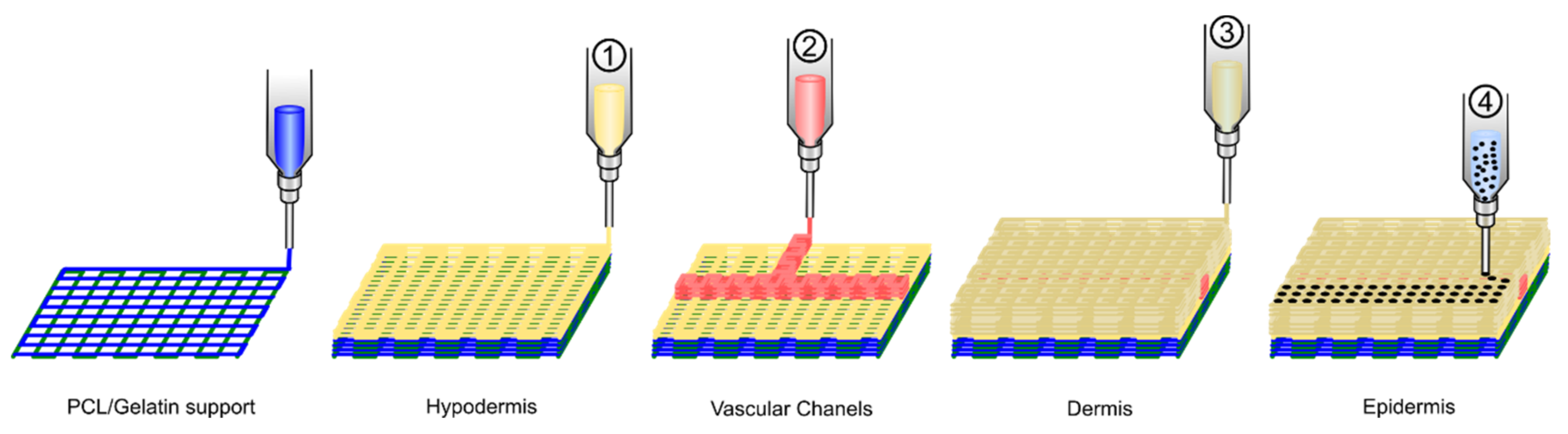
4.2.2. Directly Printing Tissue and Organs
4.3. Future Perspectives in 3D Printing
5. Summary and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tutar, R.; Motealleh, A.; Khademhosseini, A.; Kehr, N.S. Functional Nanomaterials on 2D Surfaces and in 3D Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Asadi, N.; Del Bakhshayesh, A.R.; Davaran, S.; Akbarzadeh, A. Common Biocompatible Polymeric Materials for Tissue Engineering and Regenerative Medicine. Mater. Chem. Phys. 2020, 242, 122528. [Google Scholar] [CrossRef]
- Mora-Boza, A.; Puertas-Bartolomé, M.; Vázquez-Lasa, B.; San Román, J.; Pérez-Caballer, A.; Olmeda-Lozano, M. Contribution of Bioactive Hyaluronic Acid and Gelatin to Regenerative Medicine. Methodologies of Gels Preparation and Advanced Applications. Eur. Polym. J. 2017, 95, 11–26. [Google Scholar] [CrossRef]
- Nolan, K.; Millet, Y.; Ricordi, C.; Stabler, C.L. Tissue Engineering and Biomaterials in Regenerative Medicine. Cell Transpl. 2008, 17, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Eiselt, P.; Yeh, J.; Latvala, R.K.; Shea, L.D.; Mooney, D.J. Porous Carriers for Biomedical Applications Based on Alginate Hydrogels. Biomaterials 2000, 21, 1921–1927. [Google Scholar] [CrossRef]
- Yeo, M.G.; Kim, G.H. Fabrication of Cell-Laden Electrospun Hybrid Scaffolds of Alginate-Based Bioink and PCL Microstructures for Tissue Regeneration. Chem. Eng. J. 2015, 275, 27–35. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Production and Evaluation of Dry Alginate-Chitosan Microcapsules as an Enteric Delivery Vehicle for Probiotic Bacteria. Biomacromolecules 2011, 12, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- Sideris, E.; Griffin, D.R.; Ding, Y.; Li, S.; Weaver, W.M.; Di Carlo, D.; Hsiai, T.; Segura, T. Particle Hydrogels Based on Hyaluronic Acid Building Blocks. ACS Biomater. Sci. Eng. 2016, 2, 2034–2041. [Google Scholar] [CrossRef]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan Biomaterials for Current and Potential Dental Applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef]
- Chan, H.F.; Zhang, Y.; Leong, K.W. Efficient One-Step Production of Microencapsulated Hepatocyte Spheroids with Enhanced Functions. Small 2016, 12, 2720–2730. [Google Scholar] [CrossRef]
- Zafar, M.S.; Al-Samadani, K.H. Potential Use of Natural Silk for Bio-Dental Applications. J. Taibah Univ. Med. Sci. 2014, 9, 171–177. [Google Scholar] [CrossRef]
- Zafar, M.S.; Belton, D.J.; Hanby, B.; Kaplan, D.L.; Perry, C.C. Functional Material Features of Bombyx mori Silk Light versus Heavy Chain Proteins. Biomacromolecules 2015, 16, 606–614. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chiu, C.T.; Chang, Y.P.; Wang, Y.J. Fabrication of Porous Gelatin Microfibers Using an Aqueous Wet Spinning Process. Artif. Cells Blood Substit. Biotechnol. 2009, 37, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.; Oh, J.; Kim, K.; Qiu, Y.; Joh, M.; Shin, S.R.; Wang, X.; Camci-Unal, G.; Wan, K.T.; Liao, R.; et al. Microfluidics-Assisted Fabrication of Gelatin-Silica Core-Shell Microgels for Injectable Tissue Constructs. Biomacromolecules 2014, 15, 283–290. [Google Scholar] [CrossRef]
- Feng, Q.; Li, Q.; Wen, H.; Chen, J.; Liang, M.; Huang, H.; Lan, D.; Dong, H.; Cao, X. Injection and Self-Assembly of Bioinspired Stem Cell-Laden Gelatin/Hyaluronic Acid Hybrid Microgels Promote Cartilage Repair In Vivo. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Guo, Y.; Gilbert-Honick, J.; Somers, S.M.; Mao, H.Q.; Grayson, W.L. Modified Cell-Electrospinning for 3D Myogenesis of C2C12s in Aligned Fibrin Microfiber Bundles. Biochem. Biophys. Res. Commun. 2019, 516, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Jayani, T.; Sanjeev, B.; Marimuthu, S.; Uthandi, S. Bacterial Cellulose Nano Fiber (BCNF) as Carrier Support for the Immobilization of Probiotic, Lactobacillus Acidophilus 016. Carbohydr. Polym. 2020, 250, 116965. [Google Scholar] [CrossRef]
- Aldana, A.A.; Valente, F.; Dilley, R.; Doyle, B. Development of 3D Bioprinted GelMA-Alginate Hydrogels with Tunable Mechanical Properties. Bioprinting 2021, 21, e00105. [Google Scholar] [CrossRef]
- Lee, D.; Lee, K.; Cha, C. Microfluidics-Assisted Fabrication of Microtissues with Tunable Physical Properties for Developing an In Vitro Multiplex Tissue Model. Adv. Biosyst. 2018, 2. [Google Scholar] [CrossRef]
- Seeto, W.J.; Tian, Y.; Pradhan, S.; Kerscher, P.; Lipke, E.A. Rapid Production of Cell-Laden Microspheres Using a Flexible Microfluidic Encapsulation Platform. Small 2019, 15, e1902058. [Google Scholar] [CrossRef]
- Li, F.; Truong, V.X.; Thissen, H.; Frith, J.E.; Forsythe, J.S. Microfluidic Encapsulation of Human Mesenchymal Stem Cells for Articular Cartilage Tissue Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 8589–8601. [Google Scholar] [CrossRef]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-Dimensional Cell Culture Matrices: State of the Art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef]
- Fabbri, M.; García-Fernández, L.; Vázquez-Lasa, B.; Soccio, M.; Lotti, N.; Gamberini, R.; Rimini, B.; Munari, A.; San Román, J. Micro-Structured 3D-Electrospun Scaffolds of Biodegradable Block Copolymers for Soft Tissue Regeneration. Eur. Polym. J. 2017, 94, 33–42. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Pina, S.; Costa, J.B.; Cengiz, I.F.; García-Fernández, L.; Fernández-Gutiérrez, M.D.M.; Paiva, O.C.; Oliveira, A.L.; San-Román, J.; Oliveira, J.M.; et al. Enzymatically Cross-Linked Silk Fibroin-Based Hierarchical Scaffolds for Osteochondral Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 3781–3799. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Benito-Garzón, L.; Fung, S.; Kohn, J.; Vázquez-Lasa, B.; San Román, J. Bioadhesive Functional Hydrogels: Controlled Release of Catechol Species with Antioxidant and Antiinflammatory Behavior. Mater. Sci. Eng. C 2019, 105, 110040. [Google Scholar] [CrossRef] [PubMed]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Fu, J.; Li, X.B.; Wang, L.X.; Lv, X.H.; Lu, Z.; Wang, F.; Xia, Q.; Yu, L.; Li, C.M. One-Step Dip-Coating-Fabricated Core-Shell Silk Fibroin Rice Paper Fibrous Scaffolds for 3D Tumor Spheroid Formation. ACS Appl. Bio Mater. 2020, 3, 7462–7471. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Akagi, T.; Akashi, M. Vascularized Cardiac Tissue Construction with Orientation by Layer-by-Layer Method and 3D Printer. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, A.; Kennedy, A.R. Compression Moulding and Injection over Moulding of Porous PEEK Components. J. Mech. Behav. Biomed. Mater. 2020, 111. [Google Scholar] [CrossRef] [PubMed]
- Van Bochove, B.; Grijpma, D.W. Mechanical Properties of Porous Photo-Crosslinked Poly(Trimethylene Carbonate) Network Films. Eur. Polym. J. 2021, 143. [Google Scholar] [CrossRef]
- Peck, M.; Dusserre, N.; McAllister, T.N.; L’Heureux, N. Tissue Engineering by Self-Assembly. Mater. Today 2011, 14, 218–224. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, D.; Wu, J.; Lin, J.M. Flexible Control of Cellular Encapsulation, Permeability, and Release in a Droplet-Templated Bifunctional Copolymer Scaffold. Biomicrofluidics 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Rossow, T.; Lienemann, P.S.; Mooney, D.J. Cell Microencapsulation by Droplet Microfluidic Templating. Macromol. Chem. Phys. 2017, 218. [Google Scholar] [CrossRef]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of Electrospun Nanofibers for Biomedical and Dental Applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef]
- Zimmerling, A.; Yazdanpanah, Z.; Cooper, D.M.L.; Johnston, J.D.; Chen, X. 3D Printing PCL/NHA Bone Scaffolds: Exploring the Influence of Material Synthesis Techniques. Biomater. Res. 2021, 25, 1–12. [Google Scholar] [CrossRef]
- Vyas, C.; Zhang, J.; Øvrebø, Ø.; Huang, B.; Roberts, I.; Setty, M.; Allardyce, B.; Haugen, H.; Rajkhowa, R.; Bartolo, P.; et al. 3D Printing of Silk Microparticle Reinforced Polycaprolactone Scaffolds for Tissue Engineering Applications. Mater. Sci. Eng. C 2021, 118, 111433. [Google Scholar] [CrossRef]
- Jiang, W.; Li, M.; Chen, Z.; Leong, K.W. Cell-Laden Microfluidic Microgels for Tissue Regeneration. Lab Chip 2016, 16, 4482–4506. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.; Agrawal, R. Functional Microgels: Recent Advances in Their Biomedical Applications. Small 2018, 14, e1801724. [Google Scholar] [CrossRef]
- Newsom, J.P.; Payne, K.A.; Krebs, M.D. Microgels: Modular, Tunable Constructs for Tissue Regeneration. Acta Biomater. 2019, 88, 32–41. [Google Scholar] [CrossRef]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A. Fabrication Methods of Biopolymeric Microgels and Microgel-Based Hydrogels. Food Hydrocoll. 2017, 62, 262–272. [Google Scholar] [CrossRef]
- Tumarkin, E.; Kumacheva, E. Microfluidic Generation of Microgels from Synthetic and Natural Polymers. Chem. Soc. Rev. 2009, 38, 2161–2168. [Google Scholar] [CrossRef]
- Huang, D.; Gibeley, S.B.; Xu, C.; Xiao, Y.; Celik, O.; Ginsberg, H.N.; Leong, K.W. Engineering Liver Microtissues for Disease Modeling and Regenerative Medicine. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef] [PubMed]
- Mora-Boza, A.; Mancipe Castro, L.M.; Schneider, R.S.; Han, W.M.; García, A.J.; Vázquez-Lasa, B.; San Román, J. Microfluidics Generation of Chitosan Microgels Containing Glycerylphytate Crosslinker for in Situ Human Mesenchymal Stem Cells Encapsulation. Mater. Sci. Eng. C 2021, 120, 111716. [Google Scholar] [CrossRef]
- Zhang, H.; Tumarkin, E.; Sullan, R.M.A.; Walker, G.C.; Kumacheva, E. Exploring Microfluidic Routes to Microgels of Biological Polymers. Macromol. Rapid Commun. 2007, 28, 527–538. [Google Scholar] [CrossRef]
- Velasco, D.; Tumarkin, E.; Kumacheva, E. Microfluidic Encapsulation of Cells in Polymer Microgels. Small 2012, 8, 1633–1642. [Google Scholar] [CrossRef]
- Utech, S.; Prodanovic, R.; Mao, A.S.; Ostafe, R.; Mooney, D.J.; Weitz, D.A. Microfluidic Generation of Monodisperse, Structurally Homogeneous Alginate Microgels for Cell Encapsulation and 3D Cell Culture. Adv. Healthc. Mater. 2015, 4, 1628–1633. [Google Scholar] [CrossRef]
- Mao, A.S.; Shin, J.W.; Utech, S.; Wang, H.; Uzun, O.; Li, W.; Cooper, M.; Hu, Y.; Zhang, L.; Weitz, D.A.; et al. Deterministic Encapsulation of Single Cells in Thin Tunable Microgels for Niche Modelling and Therapeutic Delivery. Nat. Mater. 2017, 16, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, X.; Cao, Y.; Tian, C.; Li, Y.; Wang, M.; Zhao, Y.; Zhao, G. Centrifugal Microfluidics for Ultra-Rapid Fabrication of Versatile Hydrogel Microcarriers. Appl. Mater. Today 2018, 13, 116–125. [Google Scholar] [CrossRef]
- Ma, T.; Gao, X.; Dong, H.; He, H.; Cao, X. High-Throughput Generation of Hyaluronic Acid Microgels via Microfluidics-Assisted Enzymatic Crosslinking and/or Diels-Alder Click Chemistry for Cell Encapsulation and Delivery. Appl. Mater. Today 2017, 9, 49–59. [Google Scholar] [CrossRef]
- Jang, Y.; Cha, C.; Jung, J.; Oh, J. Interfacial Compression-Dependent Merging of Two Miscible Microdroplets in an Asymmetric Cross-Junction for In Situ Microgel Formation. Macromol. Res. 2018, 26, 1143–1149. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Yildirimer, L.; Zhao, H.; Ding, R.; Wang, H.; Cui, W.; Weitz, D. Injectable Stem Cell-Laden Photocrosslinkable Microspheres Fabricated Using Microfluidics for Rapid Generation of Osteogenic Tissue Constructs. Adv. Funct. Mater. 2016, 26, 2809–2819. [Google Scholar] [CrossRef]
- Henke, S.; Leijten, J.; Kemna, E.; Neubauer, M.; Fery, A.; van den Berg, A.; van Apeldoorn, A.; Karperien, M. Enzymatic Crosslinking of Polymer Conjugates Is Superior over Ionic or UV Crosslinking for the On-Chip Production of Cell-Laden Microgels. Macromol. Biosci. 2016, 16, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Wang, H.; Wei, W.B.; Liu, H.; Jiang, L.; Qin, J.H. A Microfluidic Strategy for Controllable Generation of Water-in-Water Droplets as Biocompatible Microcarriers. Small 2018, 14, e1801095. [Google Scholar] [CrossRef]
- Siltanen, C.; Yaghoobi, M.; Haque, A.; You, J.; Lowen, J.; Soleimani, M.; Revzin, A. Microfluidic Fabrication of Bioactive Microgels for Rapid Formation and Enhanced Differentiation of Stem Cell Spheroids. Acta Biomater. 2016, 34, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tumarkin, E.; Peerani, R.; Nie, Z.; Sullan, R.M.A.; Walker, G.C.; Kumacheva, E. Microfluidic Production of Biopolymer Microcapsules with Controlled Morphology. J. Am. Chem. Soc. 2006, 128, 12205–12210. [Google Scholar] [CrossRef]
- Raz, N.; Li, J.K.; Fiddes, L.K.; Tumarkin, E.; Walker, G.C.; Kumacheva, E. Microgels with an Interpenetrating Network Structure as a Model System for Cell Studies. Macromolecules 2010, 43, 7277–7281. [Google Scholar] [CrossRef]
- Tan, W.H.; Takeuchi, S. Monodisperse Alginate Hydrogel Microbeads for Cell Encapsulation. Adv. Mater. 2007, 19, 2696–2701. [Google Scholar] [CrossRef]
- Kim, C.; Chung, S.; Kim, Y.E.; Lee, K.S.; Lee, S.H.; Oh, K.W.; Kang, J.Y. Generation of Core-Shell Microcapsules with Three-Dimensional Focusing Device for Efficient Formation of Cell Spheroid. Lab Chip 2011, 11, 246–252. [Google Scholar] [CrossRef]
- Mao, A.S.; Özkale, B.; Shah, N.J.; Vining, K.H.; Descombes, T.; Zhang, L.; Tringides, C.M.; Wong, S.W.; Shin, J.W.; Scadden, D.T.; et al. Programmable Microencapsulation for Enhanced Mesenchymal Stem Cell Persistence and Immunomodulation. Proc. Natl. Acad. Sci. USA 2019, 116, 15392–15397. [Google Scholar] [CrossRef]
- Singh, A. Biomaterials Innovation for next Generation Ex Vivo Immune Tissue Engineering. Biomaterials 2017, 130, 104–110. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, S.; Abbaspourrad, A.; Ardekani, A.M. Fabrication of Shape Controllable Janus Alginate/PNIPAAm Microgels via Microfluidics Technique and off-Chip Ionic Cross-Linking. Langmuir 2015, 31, 1885–1891. [Google Scholar] [CrossRef]
- Karakasyan, C.; Mathos, J.; Lack, S.; Davy, J.; Marquis, M.; Renard, D. Microfluidics-Assisted Generation of Stimuli-Responsive Hydrogels Based on Alginates Incorporated with Thermo-Responsive and Amphiphilic Polymers as Novel Biomaterials. Colloids Surf. B Biointerfaces 2015, 135, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Mora-Boza, A.; López-Donaire, M.L.; Saldaña, L.; Vilaboa, N.; Vázquez-Lasa, B.; San Román, J. Glycerylphytate Compounds with Tunable Ion Affinity and Osteogenic Properties. Sci. Rep. 2019, 9, 11491. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, L.; Ge, X.; Xu, B.; Zhang, W.; Qu, L.; Choi, C.H.; Xu, J.; Zhang, A.; Lee, H.; et al. Microfluidic Fabrication of Microparticles for Biomedical Applications. Chem. Soc. Rev. 2018, 47, 5646–5683. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, H.; Li, G.; Wang, Y.; Liu, L. High-Throughput Fabrication and Modular Assembly of 3D Heterogeneous Microscale Tissues. Small 2017, 13. [Google Scholar] [CrossRef]
- Hauck, N.; Seixas, N.; Centeno, S.P.; Schlüßler, R.; Cojoc, G.; Müller, P.; Guck, J.; Wöll, D.; Wessjohann, L.A.; Thiele, J. Droplet-Assisted Microfluidic Fabrication and Characterization of Multifunctional Polysaccharide Microgels Formed by Multicomponent Reactions. Polymers 2018, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, R. Bioengineering Hematopoietic Stem Cell Niche toward Regenerative Medicine. Adv. Drug Deliv. Rev. 2016, 99, 212–220. [Google Scholar] [CrossRef]
- Yanagawa, F.; Sugiura, S.; Kanamori, T. Hydrogel Microfabrication Technology toward Three Dimensional Tissue Engineering. Regen. Ther. 2016, 3, 45–57. [Google Scholar] [CrossRef]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of Hydrogel Biomaterials with Controlled Mechanical Properties for Tissue Engineering Applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Doshi, J.; Reneker, D.H. Electrospinning Process and Applications of Electrospun Fibers. J. Electrostat. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of Electrospinning Technique for Biomedical Applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Zanin, M.H.A.; Cerize, N.N.P.; de Oliveira, A.M. Production of Nanofibers by Electrospinning Technology: Overview and Application in Cosmetics. Nanocosmet. Nanomed. 2011, 311–332. [Google Scholar] [CrossRef]
- De Lima, G.G.; Lyons, S.; Devine, D.M.; Nugent, M.J.D. Electrospinning of Hydrogels for Biomedical Applications. Hydrogels 2018, 219–258. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and Their Applications in Tissue Engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Dotti, F.; Varesano, A.; Montarsolo, A.; Aluigi, A.; Tonin, C.; Mazzuchetti, G. Electrospun Porous Mats for High Efficiency Filtration. J. Ind. Text. 2007, 37, 151–162. [Google Scholar] [CrossRef]
- Tucker, N.; Hofman, K.; Stanger, J.; Staiger, M.; Hamid, N.A.; Torres, P.L. The History of the Science and Technology of Electrospinning from 1600 to 1995. J. Eng. Fibers Fabr. 2011, 7. [Google Scholar] [CrossRef]
- Mercante, L.A.; Scagion, V.P.; Migliorini, F.L.; Mattoso, L.H.C.; Correa, D.S. Electrospinning-Based (Bio)Sensors for Food and Agricultural Applications: A Review. TrAC Trends Anal. Chem. 2017, 91, 91–103. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Maleki, H.; Zavagna, L.; de la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-Based Electrospun Fibers for Wound Healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef]
- Tolba, E. Diversity of Electrospinning Approach for Vascular Implants: Multilayered Tubular Scaffolds. Regen. Eng. Transl. Med. 2020, 6, 383–397. [Google Scholar] [CrossRef]
- Singh, M.K.; Kumar, P.; Behera, B.K. Scaffolds for Tissue Engineering. Asian Text. J. 2009, 18, 58–62. [Google Scholar] [CrossRef]
- Chronakis, I.S. Novel Nanocomposites and Nanoceramics Based on Polymer Nanofibers Using Electrospinning Process—A Review. J. Mater. Process. Technol. 2005, 167, 283–293. [Google Scholar] [CrossRef]
- Christopherson, G.T.; Song, H.; Mao, H.Q. The Influence of Fiber Diameter of Electrospun Substrates on Neural Stem Cell Differentiation and Proliferation. Biomaterials 2009, 30, 556–564. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Li, B. Biomimetic Electrospun Nanofibrous Structures for Tissue Engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Braghirolli, D.I.; Steffens, D.; Pranke, P. Electrospinning for Regenerative Medicine: A Review of the Main Topics. Drug Discov. Today 2014, 19, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Ghanavi, J.; Farnia, P.; Velayati, A.A. Nano design of extracellular matrix for tissue engineering. In Nanoarchitectonics in Biomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 547–583. ISBN 9780128162002. [Google Scholar]
- Townsend-Nicholson, A.; Jayasinghe, S.N. Cell Electrospinning: A Unique Biotechnique for Encapsulating Living Organisms for Generating Active Biological Microthreads/Scaffolds. Biomacromolecules 2006, 7, 3364–3369. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Yeo, M.; Yang, G.H.; Kim, G. Cell-Electrospinning and Its Application for Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 6208. [Google Scholar] [CrossRef]
- Jayasinghe, S.N.; Qureshi, A.N.; Eagles, P.A.M. Electrohydrodynamic Jet Processing: An Advanced Electric-Field-Driven Jetting Phenomenon for Processing Living Cells. Small 2006, 2, 216–219. [Google Scholar] [CrossRef]
- Arumuganathar, S.; Irvine, S.; McEwan, J.R.; Jayasinghe, S.N. Pressure-Assisted Cell Spinning: A Direct Protocol for Spinning Biologically Viable Cell-Bearing Fibres and Scaffolds. Biomed. Mater. 2007, 2, 211–219. [Google Scholar] [CrossRef]
- Jayasinghe, S.N. Cell Electrospinning: A Novel Tool for Functionalising Fibres, Scaffolds and Membranes with Living Cells and Other Advanced Materials for Regenerative Biology and Medicine. Analyst 2013, 138, 2215–2223. [Google Scholar] [CrossRef]
- Sampson, S.L.; Saraiva, L.; Gustafsson, K.; Jayasinghe, S.N.; Robertson, B.D. Cell Electrospinning: An in Vitro and in Vivo Study. Small 2014, 10, 78–82. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Hu, Q. A Novel Bioactive Membrane by Cell Electrospinning. Exp. Cell Res. 2015, 338, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Onoe, H.; Takeuchi, S. Cell-Laden Microfibers for Bottom-up Tissue Engineering. Drug Discov. Today 2015, 20, 236–246. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Anu Bhushani, J.; Anandharamakrishnan, C. Electrospinning and Electrospraying Techniques: Potential Food Based Applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Bock, N.; Woodruff, M.A.; Hutmacher, D.W.; Dargaville, T.R. Electrospraying, a Reproducible Method for Production of Polymeric Microspheres for Biomedical Applications. Polymers 2011, 3, 131–149. [Google Scholar] [CrossRef]
- Greig, D.; Jayasinghe, S.N. Genomic, Genetic and Physiological Effects of Bio-Electrospraying on Live Cells of the Model Yeast Saccharomyces Cerevisiae. Biomed. Mater. 2008, 3, 34125. [Google Scholar] [CrossRef]
- Mongkoldhumrongkul, N.; Swain, S.C.; Jayasinghe, S.N.; Stürzenbaum, S. Bio-Electrospraying the Nematode Caenorhabditis Elegans: Studying Whole-Genome Transcriptional Responses and Key Life Cycle Parameters. J. R. Soc. Interface 2010, 7, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, D.; de Vos, P.; Suter, N.; Jayasinghe, S.N. Bio-Electrospraying and Cell Electrospinning: Progress and Opportunities for Basic Biology and Clinical Sciences. Adv. Healthc. Mater. 2012, 1, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Vatankhah, E.; Prabhakaran, M.P.; Ramakrishna, S. Biomimetic Nanostructures by Electrospinning and Electrospraying. Stem Cell Nanoeng. 2015, 123–141. [Google Scholar] [CrossRef]
- Jeong, S.B.; Chong, E.S.; Heo, K.J.; Lee, G.W.; Kim, H.J.; Lee, B.U. Electrospray Patterning of Yeast Cells for Applications in Alcoholic Fermentation. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tycova, A.; Prikryl, J.; Kotzianova, A.; Datinska, V.; Velebny, V.; Foret, F. Electrospray: More than Just an Ionization Source. Electrophoresis 2021, 42, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Meireles, A.B.; Corrêa, D.K.; da Silveira, J.V.W.; Millás, A.L.G.; Bittencourt, E.; de Brito-Melo, G.E.A.; González-Torres, L.A. Trends in Polymeric Electrospun Fibers and Their Use as Oral Biomaterials. Exp. Biol. Med. 2018, 243, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Huang, Y.; Agarwal, S.; Lannutti, J. Materials Selection and Residual Solvent Retention in Biodegradable Electrospun Fibers. J. Appl. Polym. Sci. 2008, 107, 1547–1554. [Google Scholar] [CrossRef]
- Chee, B.S.; Nugent, M. Electrospun Natural Polysaccharide for Biomedical Application. Nat. Polysaccharides Drug Deliv. Biomed. Appl. 2019, 589–615. [Google Scholar] [CrossRef]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A Review of Key Challenges of Electrospun Scaffolds for Tissue-Engineering Applications. J. Tissue Eng. Regen. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional Electrospun Nanofibrous Scaffolds for Biomedical Applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.C.; Sharifi, F.; Wrede, A.H.; Kimlinger, D.F.; Thomas, D.G.; Vander Wiel, J.B.; Chen, Y.; Montazami, R.; Hashemi, N.N. Microfibers as Physiologically Relevant Platforms for Creation of 3D Cell Cultures. Macromol. Biosci. 2017, 17, 1700279. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, C.H. Electrospray in the Dripping Mode for Cell Microencapsulation. J. Colloid Interface Sci. 2007, 312, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Kim, G.H. Anisotropically Aligned Cell-Laden Nanofibrous Bundle Fabricated via Cell Electrospinning to Regenerate Skeletal Muscle Tissue. Small 2018, 14, 1803491. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Kim, G.H. Micro/Nano-Hierarchical Scaffold Fabricated Using a Cell Electrospinning/3D Printing Process for Co-Culturing Myoblasts and HUVECs to Induce Myoblast Alignment and Differentiation. Acta Biomater. 2020, 107, 102–114. [Google Scholar] [CrossRef]
- Nosoudi, N.; Jacob, A.O.; Stultz, S.; Jordan, M.; Aldabel, S.; Hohne, C.; Mosser, J.; Archacki, B.; Turner, A.; Turner, P. Electrospinning Live Cells Using Gelatin and Pullulan. Bioengineering 2020, 7, 21. [Google Scholar] [CrossRef]
- Ehler, E.; Jayasinghe, S.N. Cell Electrospinning Cardiac Patches for Tissue Engineering the Heart. Analyst 2014, 139, 4449–4452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Gu, H.; Mi, H.; Rao, C.; Fu, J.; Turng, L.S. Fabrication of Scaffolds in Tissue Engineering: A Review. Front. Mech. Eng. 2018, 13, 107–119. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A Definition of Bioinks and Their Distinction from Biomaterial Inks. Biofabrication 2019, 11, 013001. [Google Scholar] [CrossRef]
- Moldovan, N.I.; Hibino, N.; Nakayama, K. Tissue Engineering. Tissue Eng. 2017, 23, 237. [Google Scholar] [CrossRef]
- Kuang, X.; Roach, D.J.; Wu, J.; Hamel, C.M.; Ding, Z.; Wang, T.; Dunn, M.L.; Qi, H.J. Advances in 4D Printing: Materials and Applications. Adv. Funct. Mater. 2019, 29, 1805290. [Google Scholar] [CrossRef]
- Mandon, C.A.; Blum, L.J.; Marquette, C.A. Adding Biomolecular Recognition Capability to 3D Printed Objects: 4D Printing. Procedia Technol. 2017, 27, 1–2. [Google Scholar] [CrossRef]
- Palmara, G.; Frascella, F.; Roppolo, I.; Chiappone, A.; Chiadò, A. Functional 3D Printing: Approaches and Bioapplications. Biosens. Bioelectron. 2021, 175, 112849. [Google Scholar] [CrossRef]
- Nesaei, S.; Song, Y.; Wang, Y.; Ruan, X.; Du, D.; Gozen, A.; Lin, Y. Micro Additive Manufacturing of Glucose Biosensors: A Feasibility Study. Anal. Chim. Acta 2018, 1043, 142–149. [Google Scholar] [CrossRef]
- Kirillova, A.; Maxson, R.; Stoychev, G.; Gomillion, C.T.; Ionov, L. 4D Biofabrication Using Shape—Morphing Hydrogels. Adv. Mat. 2017, 29, 1703443. [Google Scholar] [CrossRef] [PubMed]
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and Molecular Design Criteria for 3D Printable Hydrogels. Chem. Rev. 2016, 116, 1496–1539. [Google Scholar] [CrossRef]
- Yue, Z.; Liu, X.; Coates, P.T.; Wallace, G.G. Advances in Printing Biomaterials and Living Cells: Implications for Islet Cell Transplantation. Curr. Opin. Organ Transpl. 2016, 21, 467–475. [Google Scholar] [CrossRef]
- Seol, Y.J.; Kang, H.W.; Lee, S.J.; Atala, A.; Yoo, J.J. Bioprinting Technology and Its Applications. Eur. J. Cardio Thorac. Surg. 2014, 46, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Janarthanan, G.; Tran, H.N.; Ham, H.J.; Yoon, J.H.; Noh, I. Bioink Homogeneity Control during 3D Bioprinting of Multicomponent Micro/Nanocomposite Hydrogel for Even Tissue Regeneration Using Novel Twin Screw Extrusion System. Chem. Eng. J. 2021, 415. [Google Scholar] [CrossRef]
- Muthukrishnan, L. Imminent Antimicrobial Bioink Deploying Cellulose, Alginate, EPS and Synthetic Polymers for 3D Bioprinting of Tissue Constructs. Carbohydr. Polym. 2021, 260, 117774. [Google Scholar] [CrossRef]
- Butler, H.M.; Naseri, E.; MacDonald, D.S.; Andrew Tasker, R.; Ahmadi, A. Optimization of Starch- and Chitosan-Based Bio-Inks for 3D Bioprinting of Scaffolds for Neural Cell Growth. Materialia 2020, 12. [Google Scholar] [CrossRef]
- Leucht, A.; Volz, A.C.; Rogal, J.; Borchers, K.; Kluger, P.J. Advanced Gelatin-Based Vascularization Bioinks for Extrusion-Based Bioprinting of Vascularized Bone Equivalents. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Rubio-Valle, J.F.; Perez-Puyana, V.; Jiménez-Rosado, M.; Guerrero, A.; Romero, A. Evaluation of Smart Gelatin Matrices for the Development of Scaffolds via 3D Bioprinting. J. Mech. Behav. Biomed. Mater. 2021, 115. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.Y.S.; Suntornnond, R.; Yeong, W.Y. High-Resolution Novel Indirect Bioprinting of Low-Viscosity Cell-Laden Hydrogels via Model-Support Bioink Interaction. 3D Print. Addit. Manuf. 2020, 8, 69–78. [Google Scholar] [CrossRef]
- Hauptstein, J.; Böck, T.; Bartolf-Kopp, M.; Forster, L.; Stahlhut, P.; Nadernezhad, A.; Blahetek, G.; Zernecke-Madsen, A.; Detsch, R.; Jüngst, T.; et al. Hyaluronic Acid-Based Bioink Composition Enabling 3D Bioprinting and Improving Quality of Deposited Cartilaginous Extracellular Matrix. Adv. Healthc. Mater. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, Y.; Wu, Y.; Yu, M.; Aazmi, A.; Gao, L.; Xue, Q.; Luo, Y.; Zhou, H.; Zhang, B.; et al. 3D Bioprinted Hyaluronic Acid-Based Cell-Laden Scaffold for Brain Microenvironment Simulation. BioDesign Manuf. 2020, 3, 164–174. [Google Scholar] [CrossRef]
- Wei, W.; Ma, Y.; Yao, X.; Zhou, W.; Wang, X.; Li, C.; Lin, J.; He, Q.; Leptihn, S.; Ouyang, H. Advanced Hydrogels for the Repair of Cartilage Defects and Regeneration. Bioact. Mater. 2021, 6, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Moeinzadeh, S.; Park, Y.; Lin, S.; Yang, Y.P. In-Situ Stable Injectable Collagen-Based Hydrogels for Cell and Growth Factor Delivery. Materialia 2021, 15, 100954. [Google Scholar] [CrossRef]
- Zhang, Y.; Ellison, S.T.; Duraivel, S.; Morley, C.D.; Taylor, C.R.; Angelini, T.E. 3D Printed Collagen Structures at Low Concentrations Supported by Jammed Microgels. Bioprinting 2021, 21. [Google Scholar] [CrossRef]
- Redmond, J.; McCarthy, H.; Buchanan, P.; Levingstone, T.J.; Dunne, N.J. Advances in Biofabrication Techniques for Collagen-Based 3D in Vitro Culture Models for Breast Cancer Research. Mater. Sci. Eng. C 2021, 122, 111944. [Google Scholar] [CrossRef]
- Pokusaev, B.; Vyazmin, A.; Zakharov, N.; Karlov, S.; Nekrasov, D.; Reznik, V.; Khramtsov, D. Thermokinetics and Rheology of Agarose Gel Applied to Bioprinting Technology. Therm. Sci. 2020, 24, 453. [Google Scholar] [CrossRef]
- De Melo, B.A.G.; Jodat, Y.A.; Cruz, E.M.; Benincasa, J.C.; Shin, S.R.; Porcionatto, M.A. Strategies to Use Fibrinogen as Bioink for 3D Bioprinting Fibrin-Based Soft and Hard Tissues. Acta Biomater. 2020, 117, 60–76. [Google Scholar] [CrossRef]
- Sarker, B.; Boccaccini, A.R. Alginate Utilization in Tissue Engineering and Cell Therapy. In Alginates and their Biomedical Applications; Springer: Singapore, 2018; pp. 121–155. [Google Scholar]
- Soltan, N.; Ning, L.; Mohabatpour, F.; Papagerakis, P.; Chen, X. Printability and Cell Viability in Bioprinting Alginate Dialdehyde-Gelatin Scaffolds. ACS Biomater. Sci. Eng. 2019, 5, 2976–2987. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Singh, R.; Sarker, B.; Papageorgiou, D.G.; Juhasz, J.A.; Roether, J.A.; Cicha, I.; Kaschta, J.; Schubert, D.W.; Chrissafis, K.; et al. Hybrid Hydrogels Based on Keratin and Alginate for Tissue Engineering. J. Mater. Chem. B 2014, 2, 5441–5451. [Google Scholar] [CrossRef] [PubMed]
- Peniche, H.; Reyes-Ortega, F.; Aguilar, M.R.; Rodríguez, G.; Abradelo, C.; García-Fernández, L.; Peniche, C.; Román, J.S. Thermosensitive Macroporous Cryogels Functionalized with Bioactive Chitosan/Bemiparin Nanoparticles. Macromol. Biosci. 2013, 13, 1556–1567. [Google Scholar] [CrossRef] [PubMed]
- De La Mata, A.; Nieto-Miguel, T.; López-Paniagua, M.; Galindo, S.; Aguilar, M.R.; García-Fernández, L.; Gonzalo, S.; Vázquez, B.; Román, J.S.; Corrales, R.M.; et al. Chitosan-Gelatin Biopolymers as Carrier Substrata for Limbal Epithelial Stem Cells. J. Mater. Sci. Mater. Med. 2013, 24, 2819–2829. [Google Scholar] [CrossRef]
- Fernández-Gutiérrez, M.; Pérez-Köhler, B.; Benito-Martínez, S.; García-Moreno, F.; Pascual, G.; García-Fernández, L.; Aguilar, M.R.; Vázquez-Lasa, B.; Bellón, J.M. Development of Biocomposite Polymeric Systems Loaded with Antibacterial Nanoparticles for the Coating of Polypropylene Biomaterials. Polymers 2020, 12, 1829. [Google Scholar] [CrossRef] [PubMed]
- Nakal-Chidiac, A.; García, O.; García-Fernández, L.; Martín-Saavedra, F.M.; Sánchez-Casanova, S.; Escudero-Duch, C.; San Román, J.; Vilaboa, N.; Aguilar, M.R. Chitosan-Stabilized Silver Nanoclusters with Luminescent, Photothermal and Antibacterial Properties. Carbohydr. Polym. 2020, 250. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Song, M.; Hourston, D.J. Novel Chitosan-Based Films Cross-Linked by Genipin with Improved Physical Properties. Biomacromolecules 2004, 5, 162–168. [Google Scholar] [CrossRef]
- Mora-Boza, A.; Włodarczyk-Biegun, M.K.; Del Campo, A.; Vázquez-Lasa, B.; Román, J.S. Glycerylphytate as an Ionic Crosslinker for 3D Printing of Multi-Layered Scaffolds with Improved Shape Fidelity and Biological Features. Biomater. Sci. 2020, 8, 506–516. [Google Scholar] [CrossRef]
- He, Y.; Wang, F.; Wang, X.; Zhang, J.; Wang, D.; Huang, X. A Photocurable Hybrid Chitosan/Acrylamide Bioink for DLP Based 3D Bioprinting. Mater. Des. 2021, 202, 109588. [Google Scholar] [CrossRef]
- Jian, Z.; Zhuang, T.; Qinyu, T.; Liqing, P.; Kun, L.; Xujiang, L.; Diaodiao, W.; Zhen, Y.; Shuangpeng, J.; Xiang, S.; et al. 3D Bioprinting of a Biomimetic Meniscal Scaffold for Application in Tissue Engineering. Bioact. Mater. 2021, 6, 1711–1726. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Włodarczyk-Biegun, M.K.; Del Campo, A.; Vázquez-Lasa, B.; Román, J.S. 3D Printing of a Reactive Hydrogel Bio-Ink Using a Static Mixing Tool. Polymers 2020, 12, 1986. [Google Scholar] [CrossRef]
- Skardal, A.; Zhang, J.; McCoard, L.; Xu, X.; Oottamasathien, S.; Prestwich, G.D. Photocrosslinkable Hyaluronan-Gelatin Hydrogels for Two-Step Bioprinting. Tissue Eng. Part A 2010, 16, 2675–2685. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.; Soker, S. Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Skin Wounds. Stem Cells Transl. Med. 2012, 1, 792–802. [Google Scholar] [CrossRef]
- Zou, Q.; Grottkau, B.E.; He, Z.; Shu, L.; Yang, L.; Ma, M.; Ye, C. Biofabrication of Valentine-Shaped Heart with a Composite Hydrogel and Sacrificial Material. Mater. Sci. Eng. C 2020, 108, 110205. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, J.; Noh, I. Recent Trends in Bioinks for 3D Printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef]
- Raphael, B.; Khalil, T.; Workman, V.L.; Smith, A.; Brown, C.P.; Streuli, C.; Saiani, A.; Domingos, M. 3D Cell Bioprinting of Self-Assembling Peptide-Based Hydrogels. Mater. Lett. 2017, 190, 103–106. [Google Scholar] [CrossRef]
- Hassan, M.; Dave, K.; Chandrawati, R.; Dehghani, F.; Gomes, V.G. 3D Printing of Biopolymer Nanocomposites for Tissue Engineering: Nanomaterials, Processing and Structure-Function Relation. Eur. Polym. J. 2019, 121, 109340. [Google Scholar] [CrossRef]
- Murata, D.; Kunitomi, Y.; Harada, K.; Tokunaga, S.; Takao, S.; Nakayama, K. Osteochondral Regeneration Using Scaffold-Free Constructs of Adipose Tissue-Derived Mesenchymal Stem Cells Made by a Bio Three-Dimensional Printer with a Needle-Array in Rabbits. Regen. Ther. 2020, 15, 77–89. [Google Scholar] [CrossRef]
- Hernández-Arriaga, A.M.; del Cerro, C.; Urbina, L.; Eceiza, A.; Corcuera, M.A.; Retegi, A.; Auxiliadora Prieto, M. Genome Sequence and Characterization of the Bcs Clusters for the Production of Nanocellulose from the Low PH Resistant Strain Komagataeibacter Medellinensis ID13488. Microb. Biotechnol. 2019, 12, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Wang, X.; Ni, Z.; Ni, Y.; Huan, W.; Lv, Y.; Bai, S. Effects of Nanocellulose on Alginate/Gelatin Bio-Inks for Extrusion-Based 3D Printing. BioResources 2020, 15, 7357–7373. [Google Scholar] [CrossRef]
- Gao, J.; Ding, X.; Yu, X.; Chen, X.; Zhang, X.; Cui, S.; Shi, J.; Chen, J.; Yu, L.; Chen, S.; et al. Cell-Free Bilayered Porous Scaffolds for Osteochondral Regeneration Fabricated by Continuous 3D-Printing Using Nascent Physical Hydrogel as Ink. Adv. Healthc. Mater. 2021, 10, 2001404. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, C.; Wang, Y.; Zhang, L.; Zhang, J.; Shi, J.; Si, J.; Yuan, Y.; Liu, C. Direct Three-Dimensional Printing of a Highly Customized Freestanding Hyperelastic Bioscaffold for Complex Craniomaxillofacial Reconstruction. Chem. Eng. J. 2021, 411, 128541. [Google Scholar] [CrossRef]
- Cubo, N.; Garcia, M.; Del Cañizo, J.F.; Velasco, D.; Jorcano, J.L. 3D Bioprinting of Functional Human Skin: Production and in Vivo Analysis. Biofabrication 2017, 9, 015006. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, B.J.; Hunter-Smith, D.J.; Rozen, W.M. Vascularized versus Nonvascularized Bone Grafts: What Is the Evidence? Clin. Orthop. Relat. Res. 2016, 474, 1319–1327. [Google Scholar] [CrossRef]
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D.W. 3D Cell Printing of Perfusable Vascularized Human Skin Equivalent Composed of Epidermis, Dermis, and Hypodermis for Better Structural Recapitulation of Native Skin. Adv. Healthc. Mater. 2019, 8, 1801019. [Google Scholar] [CrossRef]
| Polymer | Microfluidics Approach | Crosslinking Strategy | Microgel Size Range | Additives | Cell Type | Ref. |
|---|---|---|---|---|---|---|
| Alginate | Flow-focusing | Ionic crosslinking (Calcium-EDTA) | 10–50 µm | No | MSCs | [48] |
| Alginate | Flow-focusing | Ionic crosslinking (calcium) | 20–50 µm | poly-D-lysine | bMSCs | [49] |
| Alginate | Flow-focusing | Ionic crosslinking (Calcium-EDTA) | ≈140 µm | PNiPAM | HepG2 | [33] |
| Alginate | Centrifugal microfluidics | Ionic crosslinking (calcium) | Tunable (also fibers) | No | HepG2 | [50] |
| Alginate | Double emulsion (w/o/w) flow focusing | Ionic crosslinking (calcium) | ≤200 µm | Collagen | Hepatocytes and endothelial cells | [11] |
| Acrylamide hyaluronic acid | Flow-focusing | Enzymatic reaction and photopolymerization | ≈80 µm | No | Human dermal fibroblasts | [8] |
| Furylamine and tyramine hyaluronic acid | T-junction | Enzymatic crosslinking, Diels-Alder click chemistry, or a combination | ≈250 µm | MAL-PEG-MAL | ATDC-5 cells | [51] |
| N-carboxylic chitosan | Asymmetric cross-section | Schiff base reaction | ≈200 µm | Oxidized dextran | NIH-3T3 fibroblasts | [52] |
| Chitosan Lactate | Flow-focusing | Ionic crosslinking (G1Phy and TPP) | 100–130 µm | No | hMSCs | [45] |
| GelMA | Double flow-focusing | Photopolymerization | 100–200 µm | No | macrophages | [20] |
| GelMA | Capillary | Photopolymerization | ≈165 µm | no | bMSCs | [53] |
| GelMA | T-junction | Photopolymerization | 300–1100 µm | PEGDA Poly(ethylene glycol)-fibrinogen | ECFCs breast cancer cells hiPSCs | [21] |
| GelNB | Capillary | Photopolymerization | 300–600 µm | PEG-SH | bMSCs | [22] |
| Thiolated gelatin | T-junction | Thiol-Michael addition reaction | 100–250 µm | Vinyl sulfonated hyaluronic acid | bMSCs | [16] |
| Dextran-tyramine | Flow-focusing | enzymatic crosslinking | 120–200 µm | No | hMSCs | [54] |
| Dextran | Flow-focusing | Ionic crosslinking(calcium) | ≈90 µm | PEG and Alginate | rat pancreatic islet | [55] |
| Methacrylated heparin | Flow-focusing | Michael addition | 60–120 µm | PEG diacrylate monomers with 8-arm PEG-thiol | mESCs | [56] |
| Compound | Advantages | Disadvantages | Bioprinting Technique | Ref. | |
|---|---|---|---|---|---|
| Natural Polymers | Alginate | Low cytotoxicity, biodegradable, allow cell adhesion | Low mechanical properties | Extrusion | [19,130,131] |
| Chitosan | Low cytotoxicity, biodegradable, antibacterial activity, allow cell adhesion | Low mechanical properties and depends on the origin and MW | Extrusion | [131,132] | |
| Gelatin | Lox cytotoxicity, improved cell adhesion, biodegradable | Poor mechanical properties and depends on the temperature. Low viscosity | Extrusion, Inkjet, Laser-assisted | [133,134,135] | |
| Hyaluronic acid | Similar to the ECM, biocompatible and biodegradable | Low mechanical strength and rapid degradation | Extrusion, Inkjet | [136,137,138] | |
| Collagen | Improved cell adhesion, good biocompatibility | Low mechanical strength and low viscosity | Extrusion, Inkjet, Laser-assisted | [139,140,141] | |
| Agarose | Good mechanical properties, biodegradable | Low cell adhesion | Extrusion | [142] | |
| Fibrin | Biocompatible, improved cell adhesion, non-cytotoxic | Low mechanical properties, rapid degradation | Extrusion, Inkjet | [143] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puertas-Bartolomé, M.; Mora-Boza, A.; García-Fernández, L. Emerging Biofabrication Techniques: A Review on Natural Polymers for Biomedical Applications. Polymers 2021, 13, 1209. https://doi.org/10.3390/polym13081209
Puertas-Bartolomé M, Mora-Boza A, García-Fernández L. Emerging Biofabrication Techniques: A Review on Natural Polymers for Biomedical Applications. Polymers. 2021; 13(8):1209. https://doi.org/10.3390/polym13081209
Chicago/Turabian StylePuertas-Bartolomé, María, Ana Mora-Boza, and Luis García-Fernández. 2021. "Emerging Biofabrication Techniques: A Review on Natural Polymers for Biomedical Applications" Polymers 13, no. 8: 1209. https://doi.org/10.3390/polym13081209
APA StylePuertas-Bartolomé, M., Mora-Boza, A., & García-Fernández, L. (2021). Emerging Biofabrication Techniques: A Review on Natural Polymers for Biomedical Applications. Polymers, 13(8), 1209. https://doi.org/10.3390/polym13081209







