Li-Nafion Membrane Plasticised with Ethylene Carbonate/Sulfolane: Influence of Mixing Temperature on the Physicochemical Properties
Abstract
1. Introduction
2. Materials and Methods
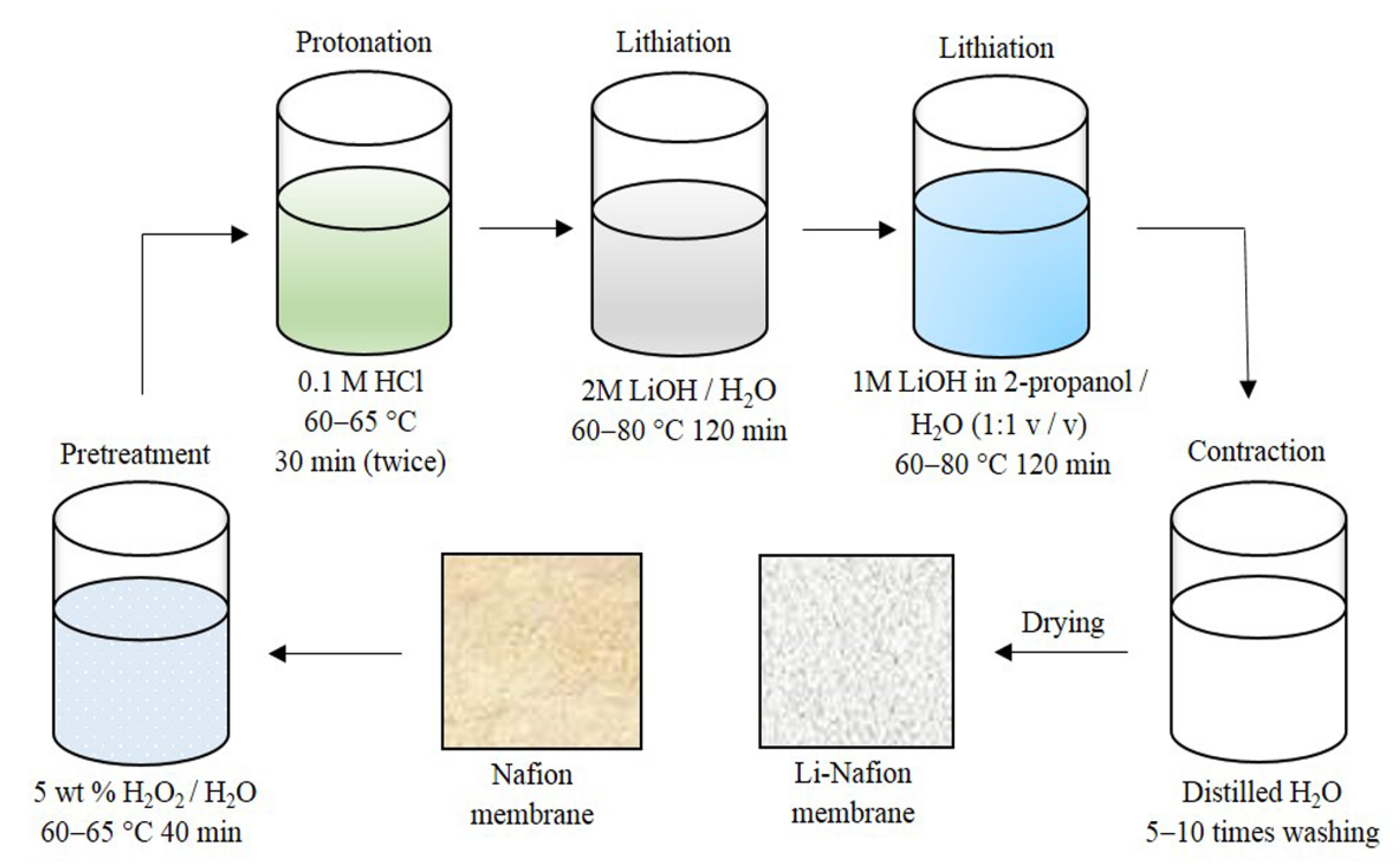
2.1. Membrane Preparation
2.2. Binary Plasticiser Preparation
2.3. Membrane Swelling
2.4. Thermal Analysis
2.5. Wide-Angle X-ray Diffraction (WAXD)
2.6. AC Conductivity Measurements
3. Results and Discussion
3.1. Characterisation of the Plasticiser
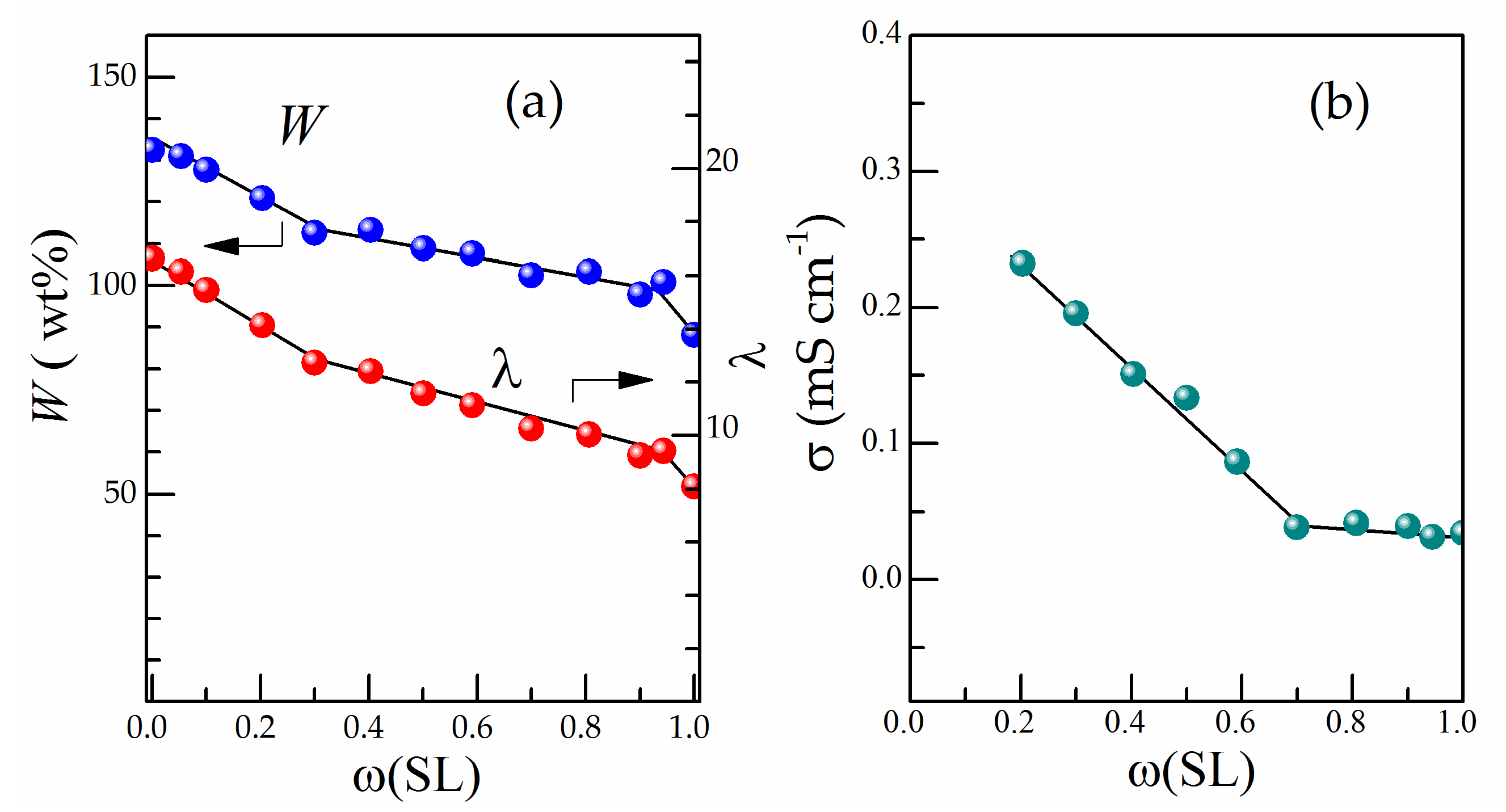
3.2. Influence of Plasticiser Composition on Solvent Uptake and Conductivity
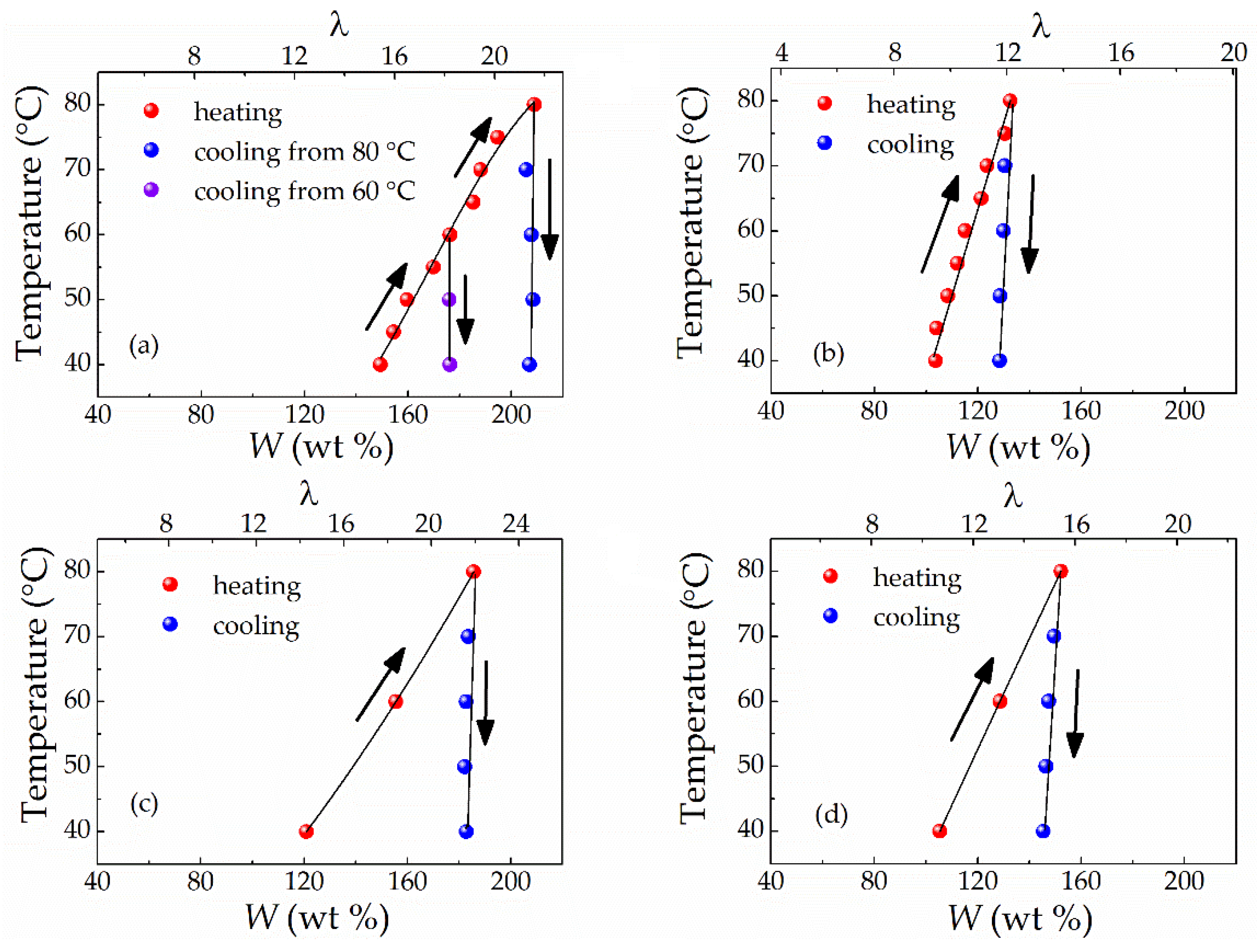
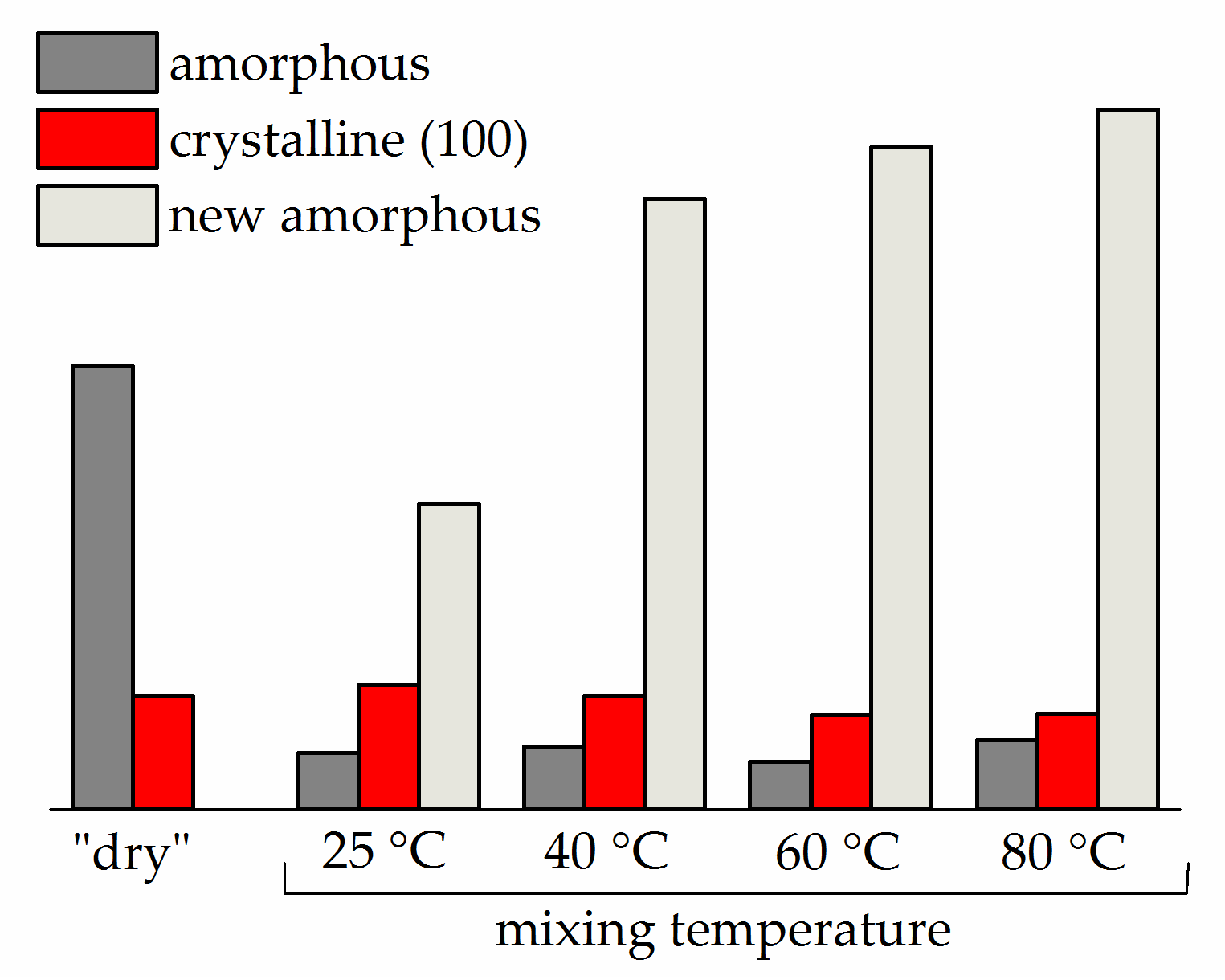
3.3. Influence of Mixing Temperature on Solvent Uptake
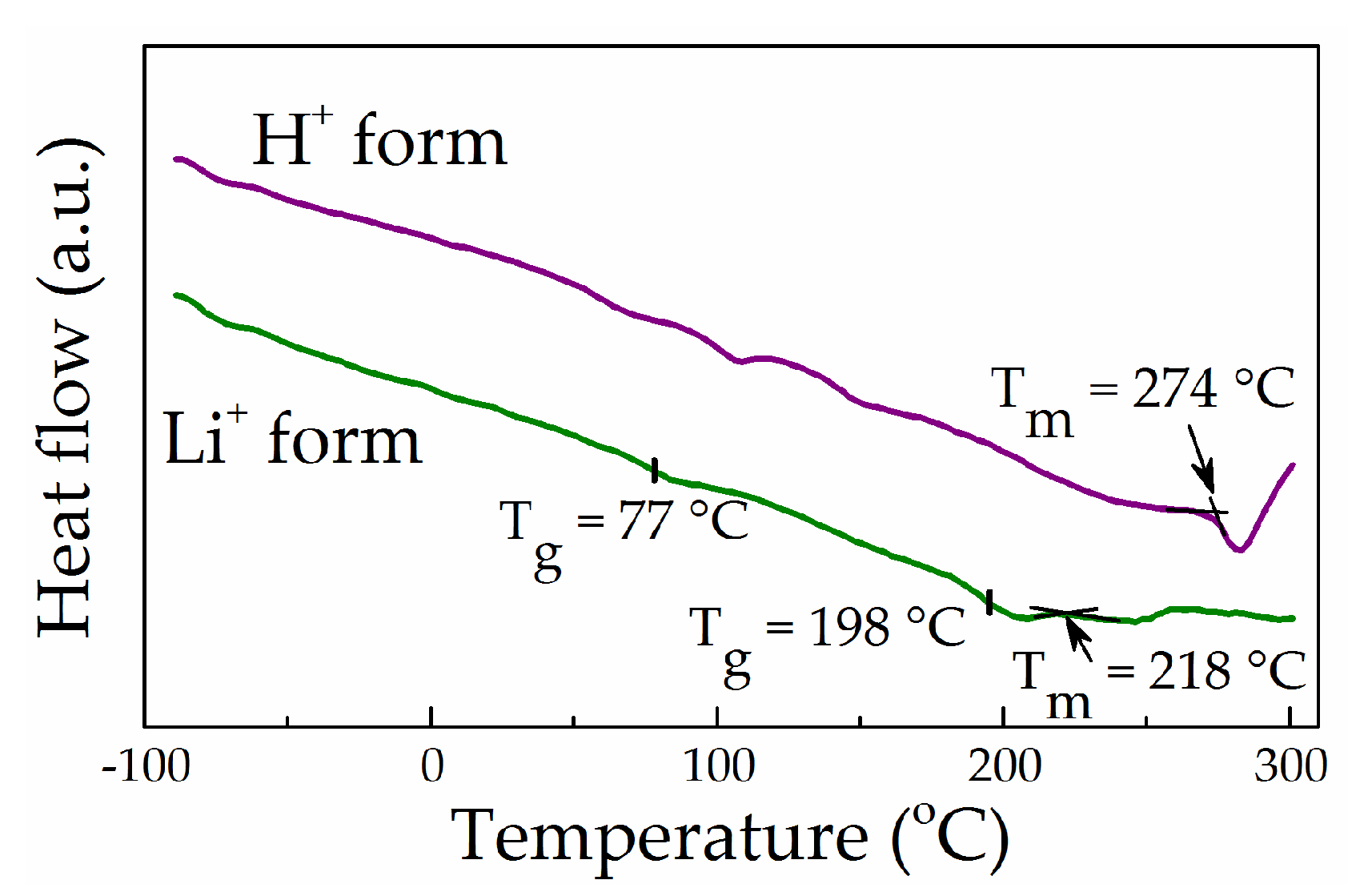
3.4. Thermal Analysis and Wide-Angle X-ray Diffraction of “Dry” Membranes
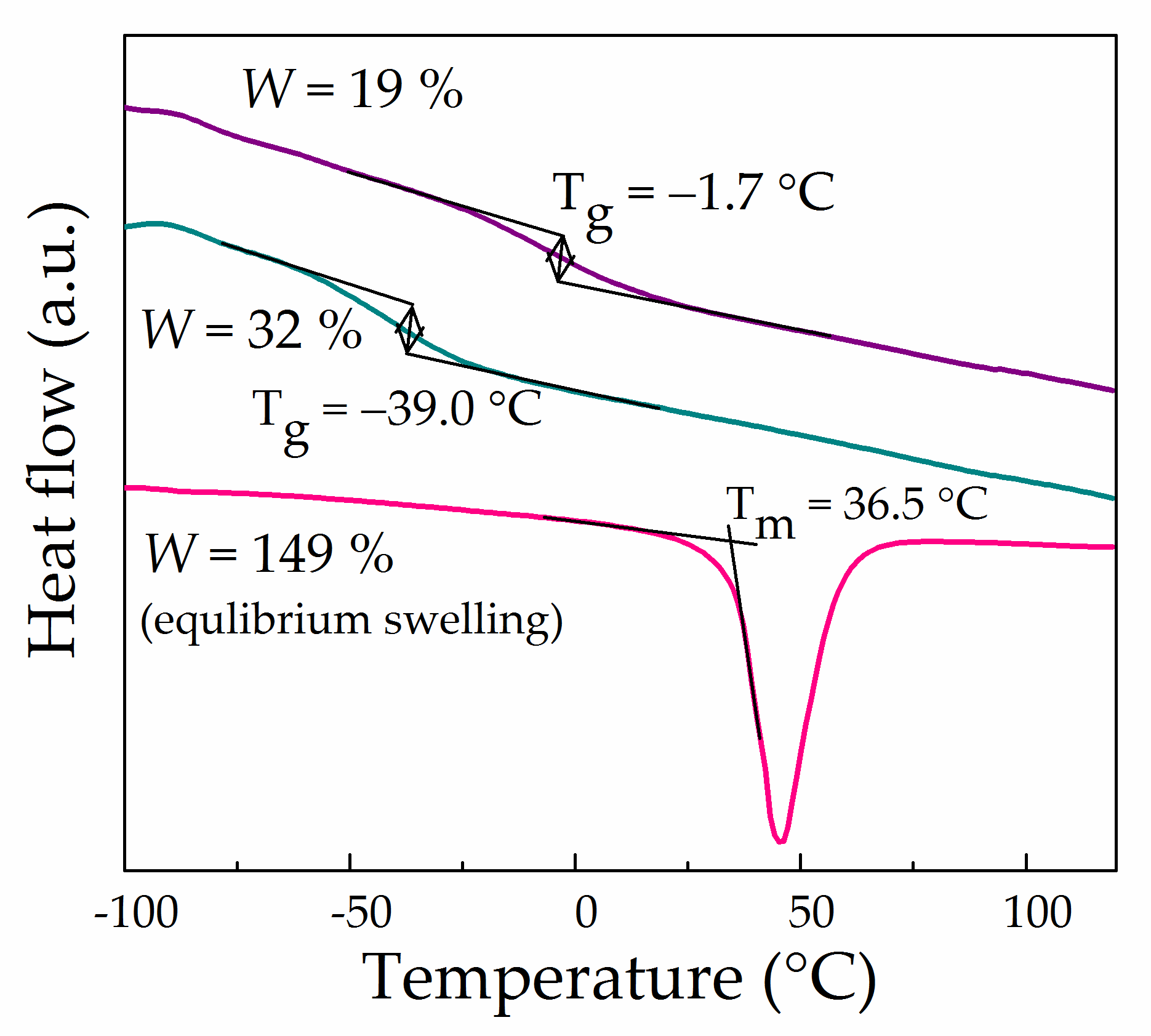
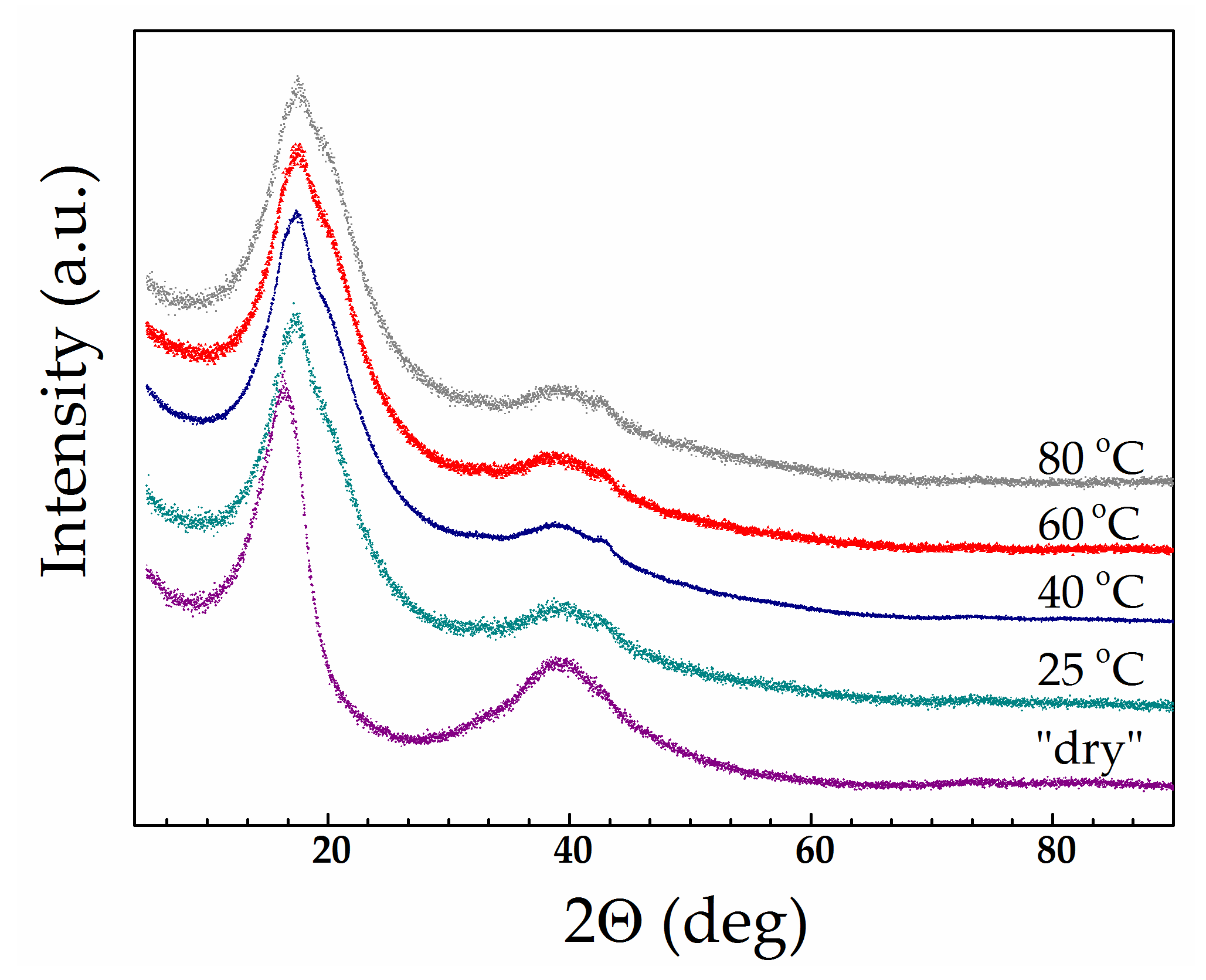
3.5. Thermal Analysis and Wide-Angle X-ray Diffraction of Swollen Li-Nafion Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kusoglu, A.; Weber, A.Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yamasaki, K.; Ishimoto, H.; Takata, Y. Ultrathin Electrolyte Membranes with PFSA-Vinylon Intermediate Layers for PEM Fuel Cells. Polymers 2020, 12, 1730. [Google Scholar] [CrossRef]
- Mazzapioda, L.; Lo Vecchio, C.; Danyliv, O.; Baglio, V.; Martinelli, A.; Navarra, M.A. Composite Nafion-CaTiO3-δ Membranes as Electrolyte Component for PEM Fuel Cells. Polymers 2020, 12, 2019. [Google Scholar] [CrossRef]
- Kayumov, R.R.; Sanginov, E.A.; Shmygleva, L.V.; Radaeva, A.P.; Karelin, A.I.; Zyubin, A.S.; Zyubina, T.S.; Anokhin, D.V.; Ivanov, D.A.; Dobrovolsky, Y.A. Ammonium form of Nafion plasticised by dimethyl sulfoxide. J. Electrochem. Soc. 2019, 166, F3216–F3226. [Google Scholar] [CrossRef]
- Voropaeva, D.Y.; Novikova, S.A.; Kulova, T.L.; Yaroslavtsev, A.B. Solvation and sodium conductivity of nonaqueous polymer electrolytes based on Nafion-117 membranes and polar aprotic solvents. Solid State Ion. 2018, 324, 28–32. [Google Scholar] [CrossRef]
- Aldebert, P.; Guglielmi, M.; Pineri, M. Ionic conductivity of bulk, gels and solutions of perfluorinated ionomer membranes. Polym. J. 1991, 23, 399–406. [Google Scholar] [CrossRef][Green Version]
- Doyle, M.; Lewittes, M.E.; Roelofs, M.G.; Perusich, S.A.; Lowrey, R.E. Relationship between ionic conductivity of perfluorinated ionomeric membranes and nonaqueous solvent properties. J. Membr. Sci. 2001, 184, 257–273. [Google Scholar] [CrossRef]
- Sachan, S.; Ray, C.A.; Perusich, S.A. Lithium ion transport through nonaqueous perfluoroionomeric membranes. Polym. Eng. Sci. 2002, 42, 1469–1480. [Google Scholar] [CrossRef]
- Sanginov, E.A.; Evshchik, E.Y.; Kayumov, R.R.; Dobrovol’skii, Y.A. Lithium-ion conductivity of the Nafion membrane swollen in organic solvents. Rus. J. Electrochem. 2015, 51, 986–990. [Google Scholar] [CrossRef]
- Doyle, M.; Lewittes, M.E.; Roelofs, M.G.; Perusich, S.A. Ionic conductivity of nonaqueous solvent-swollen ionomer membranes based on fluorosulfonate, fluorocarboxylate, and sulfonate fixed ion groups. J. Phys. Chem. B 2001, 105, 9387–9394. [Google Scholar] [CrossRef]
- Su, L.; Darling, R.M.; Gallagher, K.G.; Xie, W.; Thelen, J.L.; Badel, A.F.; Barton, J.L.; Cheng, K.J.; Balsara, N.P.; Moore, J.S.; et al. An investigation of the ionic conductivity and species crossover of lithiated Nafion 117 in nonaqueous electrolytes. J. Electrochem. Soc. 2016, 163, A5253–A5262. [Google Scholar] [CrossRef]
- Voropaeva, D.Y.; Novikova, S.A.; Kulova, T.L.; Yaroslavtsev, A.B. Conductivity of Nafion-117 membranes intercalated by polar aprotonic solvents. Ionics 2018, 24, 1685–1692. [Google Scholar] [CrossRef]
- Voropaeva, D.Y.; Novikova, S.A.; Xu, T.; Yaroslavtsev, A.B. Polymer electrolytes for LIBs based on perfluorinated sulfocationic Nepem-117 membrane and aprotic solvents. J. Phys. Chem. B 2019, 123, 10217–10223. [Google Scholar] [CrossRef]
- Voropaeva, D.Y.; Novikova, S.A.; Yaroslavtsev, A.B. Polymer electrolytes for metal-ion batteries. Rus. Chem. Rev. 2020, 89, 1132. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Piszcz, M.; Coya, E.; Rojo, T.; Rodriguez-Martinez, L.M.; Armand, M.; Zhou, Z. Single lithium-ion conducting solid polymer electrolytes: Advances and perspectives. Chem. Soc. Rev. 2017, 46, 797–815. [Google Scholar] [CrossRef]
- Doyle, M.; Fuller, T.F.; Newman, J. The importance of the lithium ion transference number in lithium/polymer cells. Electrochim. Acta 1994, 39, 2073. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, Y.; Liu, S.; Zhang, Y. High performance of lithium-ion polymer battery based on non-aqueous lithiated perfluorinated sulfonic ion-exchange membranes. Energy Environ. Sci. 2012, 5, 5690–5693. [Google Scholar] [CrossRef]
- Yu, Q.; Nie, Y.; Cui, Y.; Zhang, J.; Jiang, F. Single-ion polyelectrolyte/mesoporous hollow-silica spheres, composite electrolyte membranes for lithium-ion batteries. Electrochim. Acta 2015, 182, 297–304. [Google Scholar] [CrossRef]
- Liang, H.Y.; Qiu, X.P.; Zhang, S.C.; Zhu, W.T.; Chen, L.Q. Study of lithiated Nafion ionomer for lithium batteries. J. Appl. Electrochem. 2004, 34, 1211–1214. [Google Scholar] [CrossRef]
- Evshchik, E.Y.; Sanginov, E.A.; Kayumov, R.R.; Zhuravlev, V.D.; Bushkova, O.V.; Dobrovolsky, Y.A. Li4Ti5O12/LiFePO4 solid-state lithium-ion full cell with lithiated Nafion membrane. Int. J. Electrochem. Sci. 2020, 15, 2216–2225. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Z.; Tan, L.; Li, L. Ion exchange membranes as electrolyte for high performance Li-ion batteries. Energy Environ. Sci. 2012, 5, 9007. [Google Scholar] [CrossRef]
- Jin, Z.; Xie, K.; Hong, X.; Hu, Z.; Liu, X. Application of lithiated Nafion ionomer film as functional separator for lithium sulfur cells. J. Power Sources 2012, 218, 163–167. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Zhang, Q.; Wei, F. Multi-functional separator/interlayer system for high-stable lithium-sulfur batteries: Progress and prospects. Energy Storage Mater. 2015, 1, 127–145. [Google Scholar] [CrossRef]
- Gao, J.; Sun, C.; Xu, L.; Chen, J.; Wang, C.; Cuo, D.; Chen, H. Lithiated Nafion as polymer electrolyte for solid-state lithium sulfur batteries using carbon-sulfur composite cathode. J. Power Sources 2018, 382, 179–189. [Google Scholar] [CrossRef]
- Gierke, T.D.; Munn, G.E.; Wilson, F.C. The morphology in nafion perfluorinated membrane products, as determined by wide- and small-angle x-ray studies. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1687. [Google Scholar] [CrossRef]
- Sanginov, E.A.; Borisevich, S.S.; Kayumov, R.R.; Istomina, A.S.; Evshchik, E.Y.; Reznitskikh, O.G.; Yaroslavtseva, T.V.; Melnikova, T.I.; Dobrovolsky, Y.A.; Bushkova, O.V. Lithiated Nafion plasticised by a mixture of ethylene carbonate and sulfolane. Electrochim. Acta 2021, 373, 137914. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kinoshita, S.-I.; Wada, S.; Hoshino, K.; Morimoto, H.; Tobishima, S. Electrochemical properties and lithium ion solvation behavior of sulfone–ester mixed electrolytes for high-voltage rechargeable lithium cells. J. Power Sources 2008, 179, 770–779. [Google Scholar] [CrossRef]
- Demakhin, A.G.; Ovsyannikov, V.M.; Ponomarenko, S.M. Elekrolitnye sistemy dlya litievyh HIT (Electrolyte Systems for Lithium Chemical Current Sources); Saratov University Publishers: Saratov, Russia, 1993. (In Russian) [Google Scholar]
- Johnson, P.H. The Properties of Ethylene Carbonate and Its Use in Electrochemical Applications: A Literature Review, Lawrence Berkeley Lab., CA (USA). 1985. Available online: https://escholarship.org/uc/item/8cg2t9r3 (accessed on 2 April 2021).
- Hess, S.; Wohlfahrt-Mehrens, M.; Wachtler, M. Flammability of Li-Ion battery electrolytes: Flash point and self-extinguishing time measurements. J. Electrochem. Soc. 2015, 162, A3084–A3097. [Google Scholar] [CrossRef]
- Reznitskikh, O.G.; Istomina, A.S.; Borisevich, S.S.; Evshchik, E.Y.; Sanginov, E.A.; Bushkova, O.V.; Dobrovolsky, Y.A. Phase diagram of ethylene carbonate–sulpholane system. Russ. J. Phys. Chtmistry A 2021, 95. (in press).
- Morita, M.; Goto, M.; Matsuda, Y. Ethylene carbonate-based organic electrolytes for electric double layer capacitors. J. Appl. Electrochem. 1992, 22, 901–908. [Google Scholar] [CrossRef]
- Liu, F.; Yi, B.; Xing, D.; Yu, J.; Zhang, H. Nafion/PTFE composite membranes for fuel cell applications. J. Membr. Sci. 2003, 212, 213–223. [Google Scholar] [CrossRef]
- Shi, S.; Weber, A.Z.; Kusoglu, A. Structure/property relationship of Nafion XL composite membranes. J. Membr. Sci. 2016, 516, 123–134. [Google Scholar] [CrossRef]
- Starkweather, H.W., Jr. Crystallinity in perfluorosulfonic acid ionomers and related polymers. Macromolecules 1982, 15, 320–323. [Google Scholar] [CrossRef]
- Ozerin, A.N.; Rebrov, A.V.; Yakunin, A.N.; Bogovtseva, L.P.; Timashev, S.F.; Bakeev, N.F. Structural changes in perfluorinated membranes in the processes of saponification and orientation stretching. Polym. Sci. U.S.S.R. 1986, 28, 275–282. [Google Scholar] [CrossRef]
- Gebel, G.; Aldebert, P.; Pineri, M. Structure and related properties of solution-cast perfluosulfonated ionomer films. Macromolecules 1987, 20, 1425–1428. [Google Scholar] [CrossRef]
- Fujimura, M.; Hashimoto, T.; Kawai, H. Small-angle x-ray scattering study of perfluorinated ionomer membranes. 1. Origin of two scattering maxima. Macromolecules 1981, 14, 1309–1315. [Google Scholar] [CrossRef]
- Hink, S.; Henkensmeier, D.; Jang, J.H.; Kim, H.-J.; Han, J.; Nam, S.-W. Reduced in-plan swelling of Nafion by a biaxial modification process. Macromol. Chem. Phys. 2015, 216, 1235–1243. [Google Scholar] [CrossRef]
- Gao, J.; Shao, Q.; Chen, J. Lithiated Nafion-garnet ceramic composite electrolyte membrane for solid-state lithium metal battery. J. Energy Chem. 2020, 46, 237–247. [Google Scholar] [CrossRef]
- Jung, H.-Y.; Kim, J.W. Role of the glass transition temperature of Nafion 117 membrane in the preparation of the membrane electrode assembly in a direct methanol fuel cell (DMFC). Int. J. Hydrogen Energy 2012, 37, 12580–12585. [Google Scholar] [CrossRef]
- Tager, A.A. Physical Chemistry of Polymers; Mir Publishers: Moscow, Russia, 1978. [Google Scholar]
- Almeida, S.; Kawano, Y. Thermal behavior of Nafion membrane. J. Therm Anal. Calorim. 1999, 58, 569–577. [Google Scholar] [CrossRef]
- Corti, H.R.; Nores-Pondal, F.; Buera, M.P. Low temperature thermal properties of Nafion 117 membranes in water and methanol-water mixtures. J. Power Sources 2006, 161, 799–805. [Google Scholar] [CrossRef]
- Moore, R.B.; Martin, C.R. Chemical and morphological properties of solution-cast perfluorosulfonate ionomers. Macromolecules 1988, 21, 1334–1339. [Google Scholar] [CrossRef]
- Ozerin, A.N.; Rebrov, A.V.; Yakunin, A.N.; Bessonova, N.P.; Dreiman, N.A.; Sokolov, L.F.; Bakeyev, N.F. Effect of low molecular weight fractions on the structure of the perfluorinated sulphocationite ion-exchange membrane. Polym. Sci. U.S.S.R. 1986, 28, 2559–2565. [Google Scholar] [CrossRef]
- Sun, L.Y.; Thrasher, J.S. Studies of the thermal behavior of Nafion® membranes treated with aluminum (III). Polym. Degrad. Stab. 2005, 89, 43–49. [Google Scholar] [CrossRef]
- Stefanithis, I.D.; Mauritz, K.A. Microstructural evolution of a silicon oxide phase in a perfluorosulfonic acid ionomer by an in situ sol-gel reaction. 3. Thermal analysis studies. Macromolecules 1990, 23, 2397–2402. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, F.; Dong, S. A single ionic conductor based on Nafion and its electrochemical properties used as lithium polymer electrolyte. J. Phys. Chem. B 2004, 108, 1365–1370. [Google Scholar] [CrossRef]
- Yeo, R.S. Dual cohesive energy densities of perfluorosulphonic acid (Nafion) membrane. Polymer 1980, 21, 432. [Google Scholar] [CrossRef]
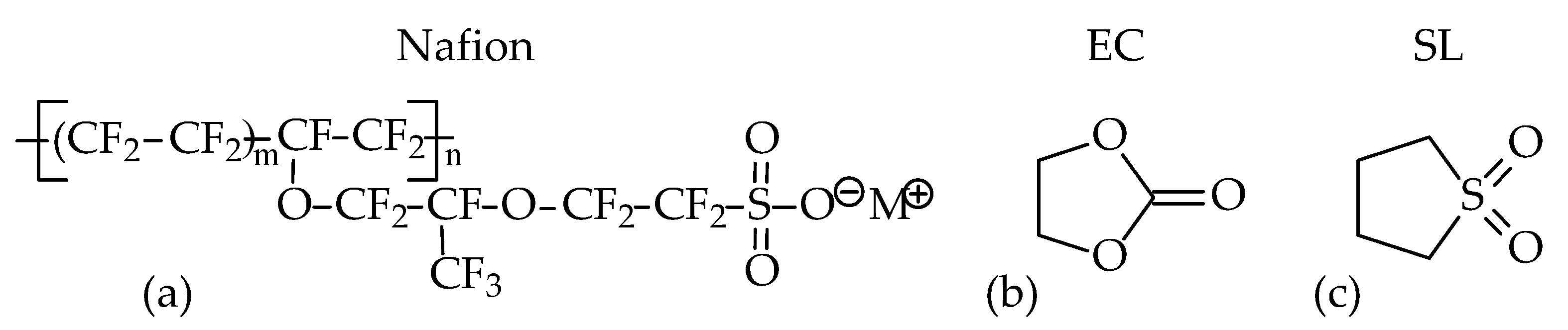
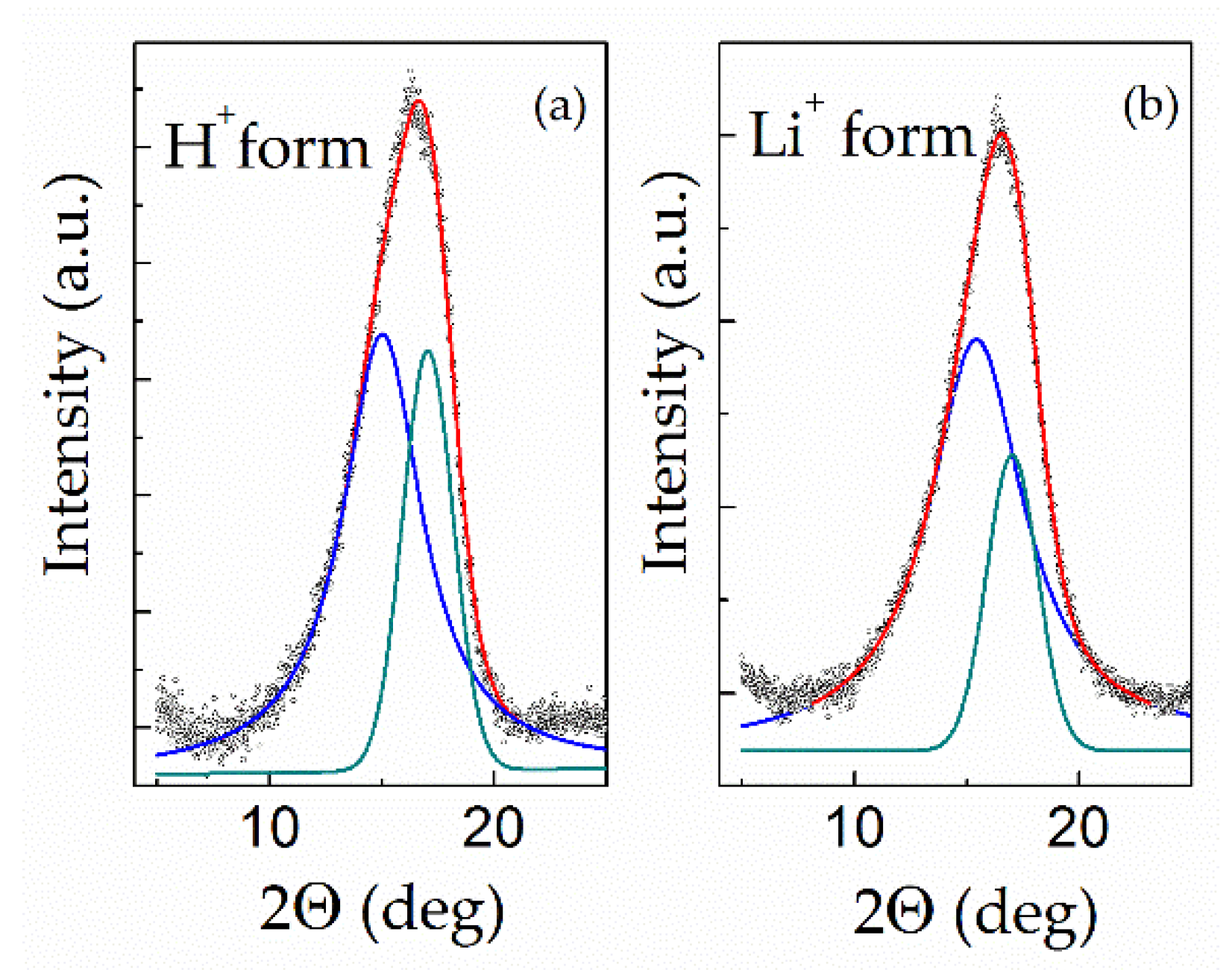
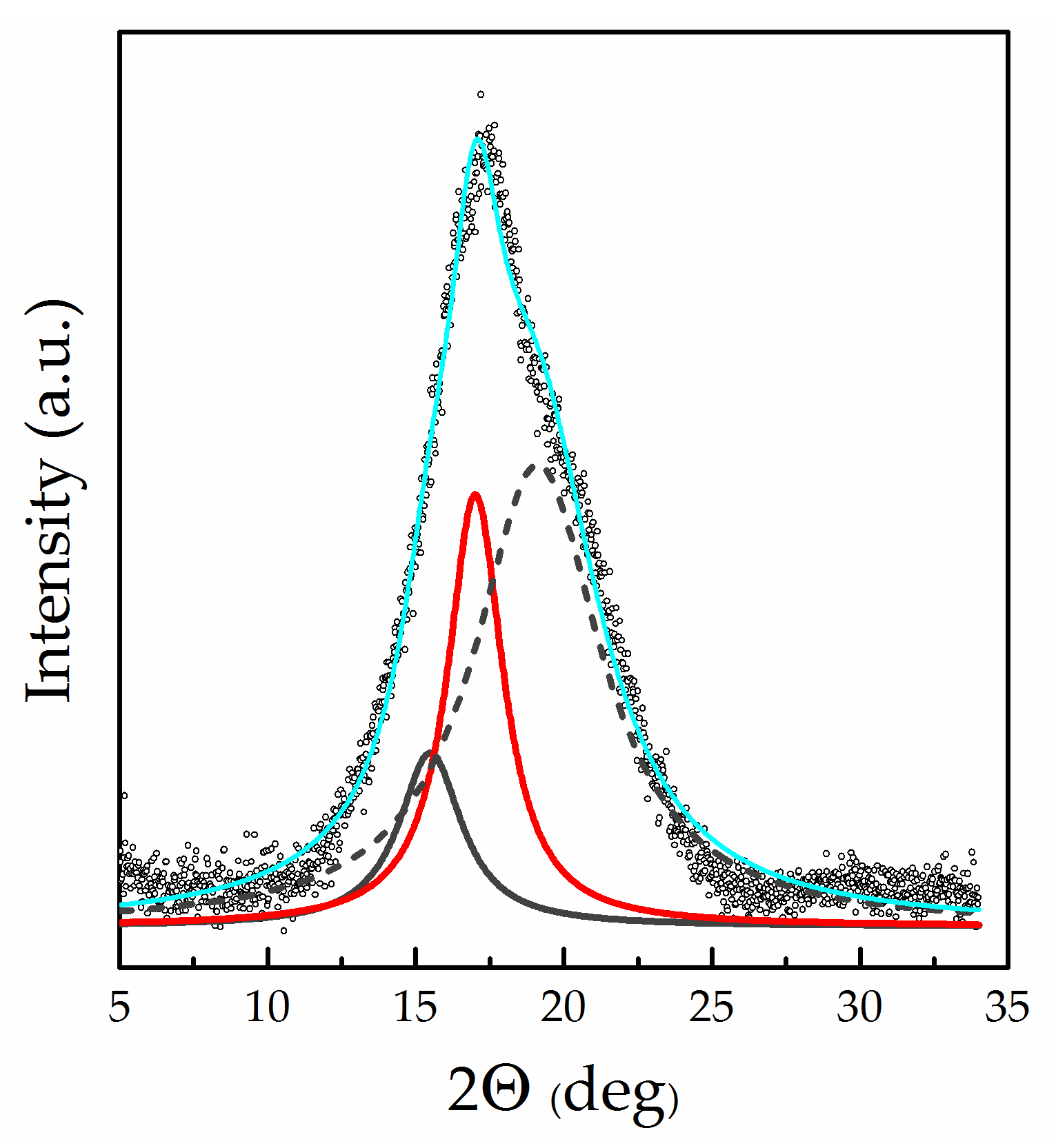
| Sample | Amorphous Galo | (100) | Crystalline Degree, % | ||
|---|---|---|---|---|---|
| 2θ, Deg | Fwhm | 2θ, Deg | Fwhm | ||
| Nafion 115 (H+) | 15.00 ± 0.10 | 4.29 | 17.03 ± 0.04 | 2.25 | 29 |
| Nafion 115 (Li+) | 15.41 ± 0.06 | 5.14 | 17.01 ± 0.03 | 2.27 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Istomina, A.S.; Yaroslavtseva, T.V.; Reznitskikh, O.G.; Kayumov, R.R.; Shmygleva, L.V.; Sanginov, E.A.; Dobrovolsky, Y.A.; Bushkova, O.V. Li-Nafion Membrane Plasticised with Ethylene Carbonate/Sulfolane: Influence of Mixing Temperature on the Physicochemical Properties. Polymers 2021, 13, 1150. https://doi.org/10.3390/polym13071150
Istomina AS, Yaroslavtseva TV, Reznitskikh OG, Kayumov RR, Shmygleva LV, Sanginov EA, Dobrovolsky YA, Bushkova OV. Li-Nafion Membrane Plasticised with Ethylene Carbonate/Sulfolane: Influence of Mixing Temperature on the Physicochemical Properties. Polymers. 2021; 13(7):1150. https://doi.org/10.3390/polym13071150
Chicago/Turabian StyleIstomina, Aigul S., Tatyana V. Yaroslavtseva, Olga G. Reznitskikh, Ruslan R. Kayumov, Lyubov V. Shmygleva, Evgeny A. Sanginov, Yury A. Dobrovolsky, and Olga V. Bushkova. 2021. "Li-Nafion Membrane Plasticised with Ethylene Carbonate/Sulfolane: Influence of Mixing Temperature on the Physicochemical Properties" Polymers 13, no. 7: 1150. https://doi.org/10.3390/polym13071150
APA StyleIstomina, A. S., Yaroslavtseva, T. V., Reznitskikh, O. G., Kayumov, R. R., Shmygleva, L. V., Sanginov, E. A., Dobrovolsky, Y. A., & Bushkova, O. V. (2021). Li-Nafion Membrane Plasticised with Ethylene Carbonate/Sulfolane: Influence of Mixing Temperature on the Physicochemical Properties. Polymers, 13(7), 1150. https://doi.org/10.3390/polym13071150






