Antibacterial Activity and Protection Efficiency of Polyvinyl Butyral Nanofibrous Membrane Containing Thymol Prepared through Vertical Electrospinning
Abstract
1. Introduction
2. Experimental
2.1. Materials and Microorganisms
2.2. Preparation of PVB Antibacterial Nanofibrous Membrane Containing Thymol
2.3. Preparation of Thymol/PVB Antibacterial Nanofibrous Masks
2.4. Surface Observation of Nanofiber Membranes
2.5. Antibacterial Activity
2.5.1. Antibacterial Qualitative Tests
2.5.2. Antibacterial Quantitative Test
2.5.3. Particulate Filtration Efficiency (PFE)
Protection efficiency (%) = 100 − penetration efficiency (%)
2.5.4. Differential Pressure of Air Exchange
2.5.5. Bacterial Filtration Efficiency (BFE)
3. Results and Discussion
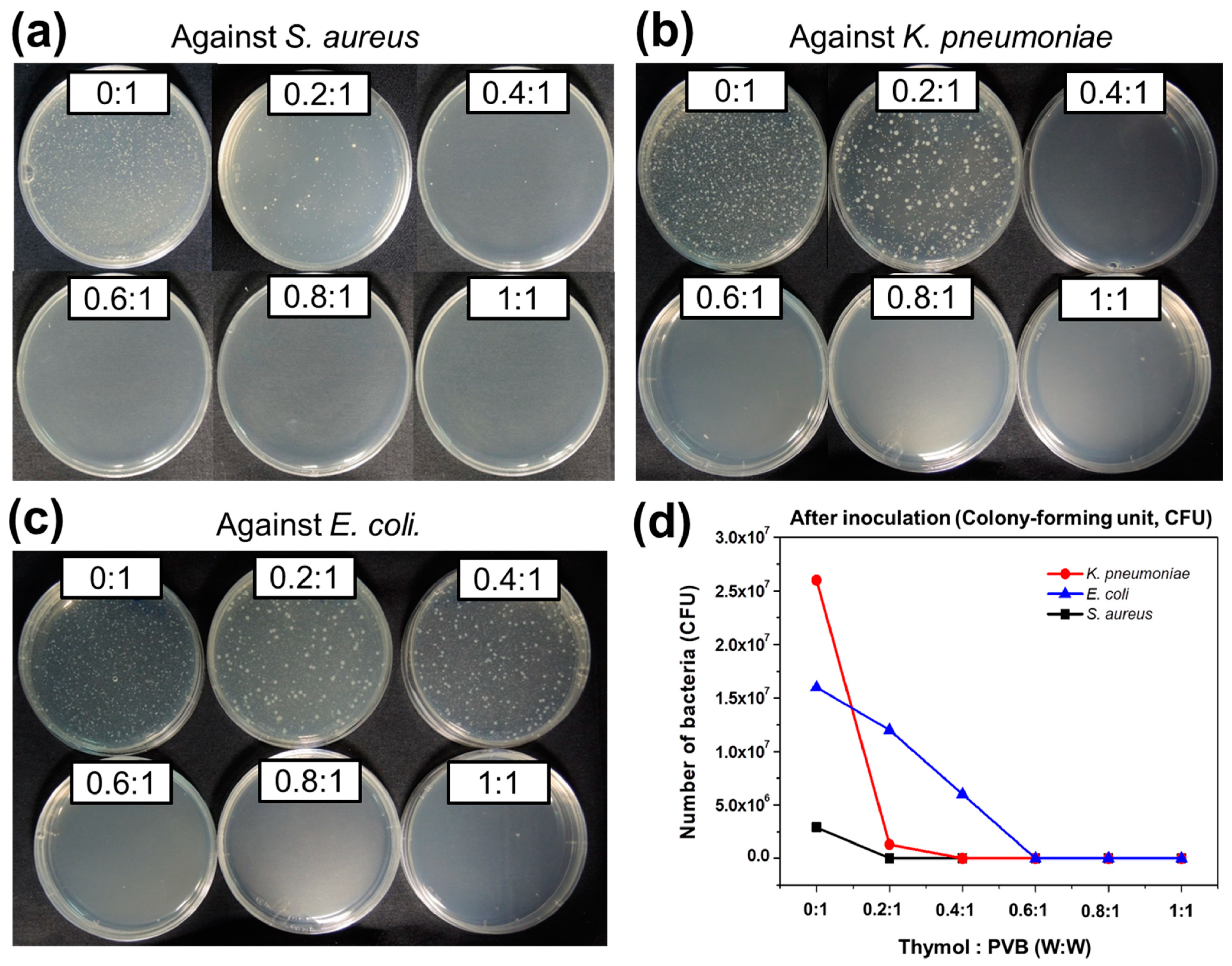
3.1. Antibacterial Qualitative Analysis for Nanofibrous Membrane of Thymol/PVB Blenders
3.2. Antibacterial Quantitative Analysis for Thymol/PVB Nanofibrous Membranes
3.2.1. Staphylococcus aureus
3.2.2. Klebsiella pneumoniae
3.2.3. Escherichia coli
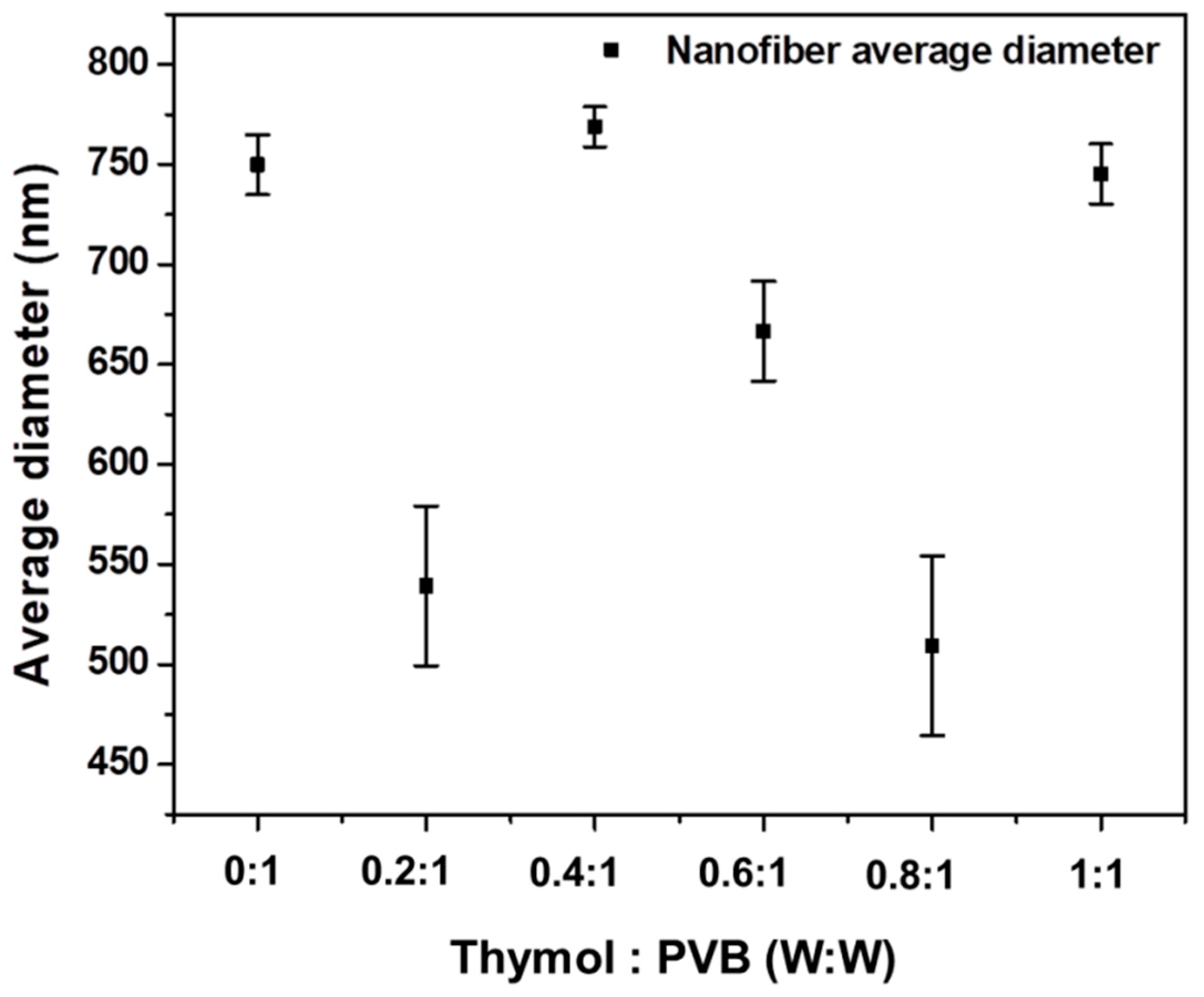
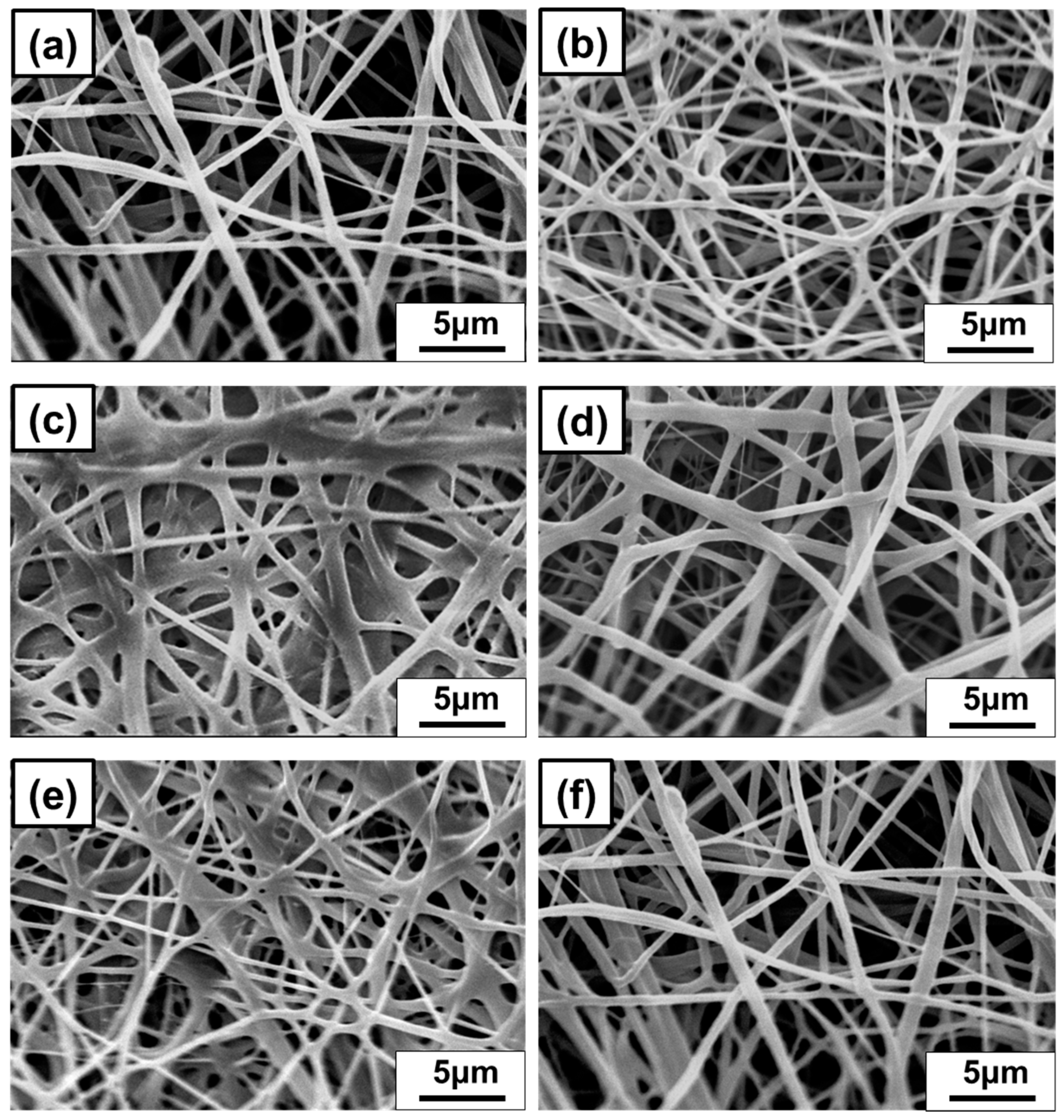
3.2.4. Diameter of Nanofibrous Membrane for Thymol/PVB Blenders
3.3. Analysis of the Protection Efficiency of Antibacterial Nanofiber Masks
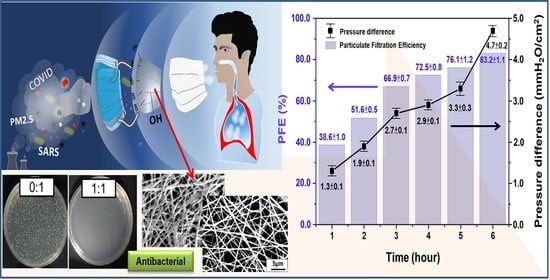
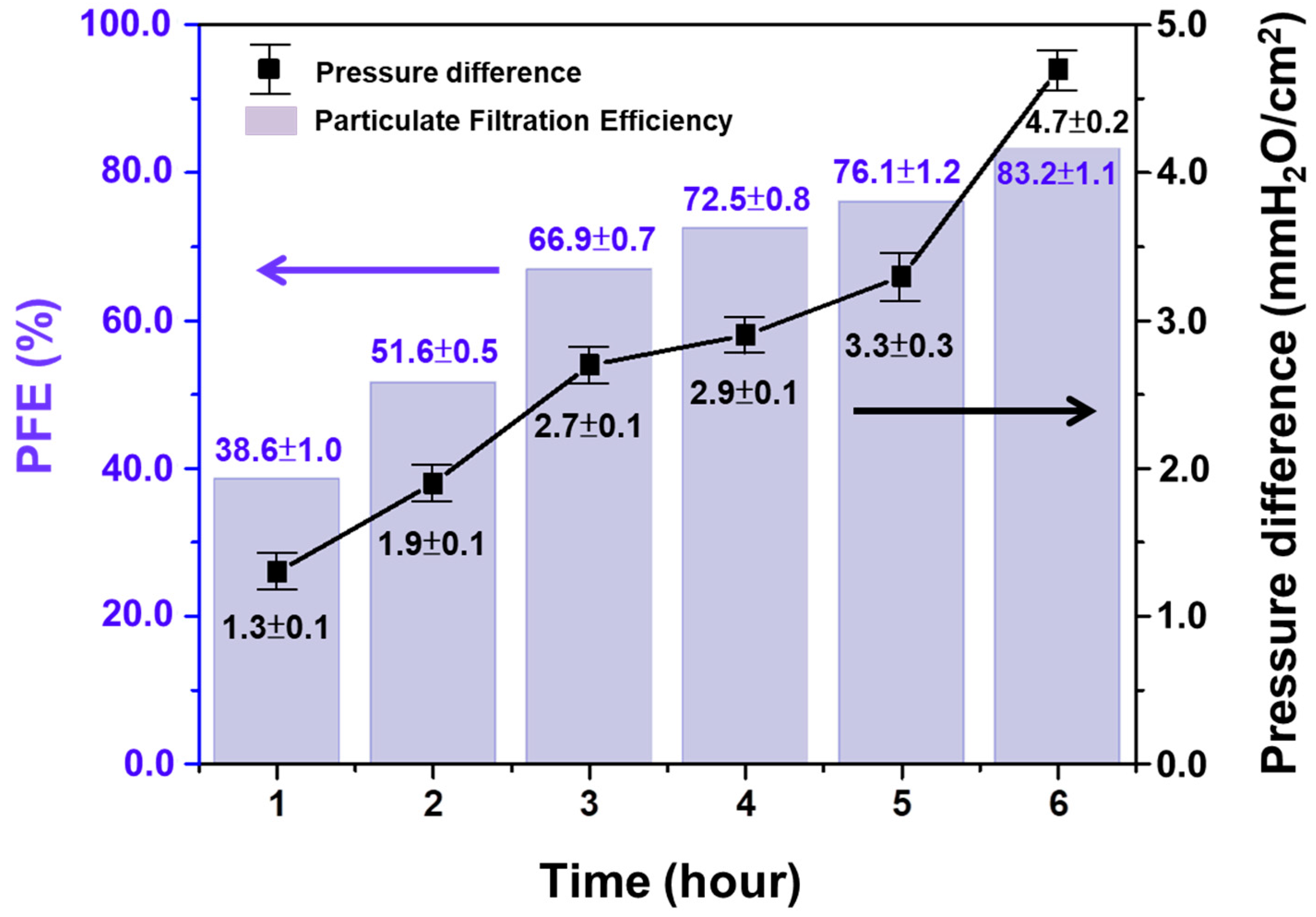
3.3.1. Submicron Particulate Filtration Efficiency (PFE) and Pressure Difference of Air Exchange
3.3.2. The Fiber Diameter and PFE
3.3.3. Spinning Parameters and PFE
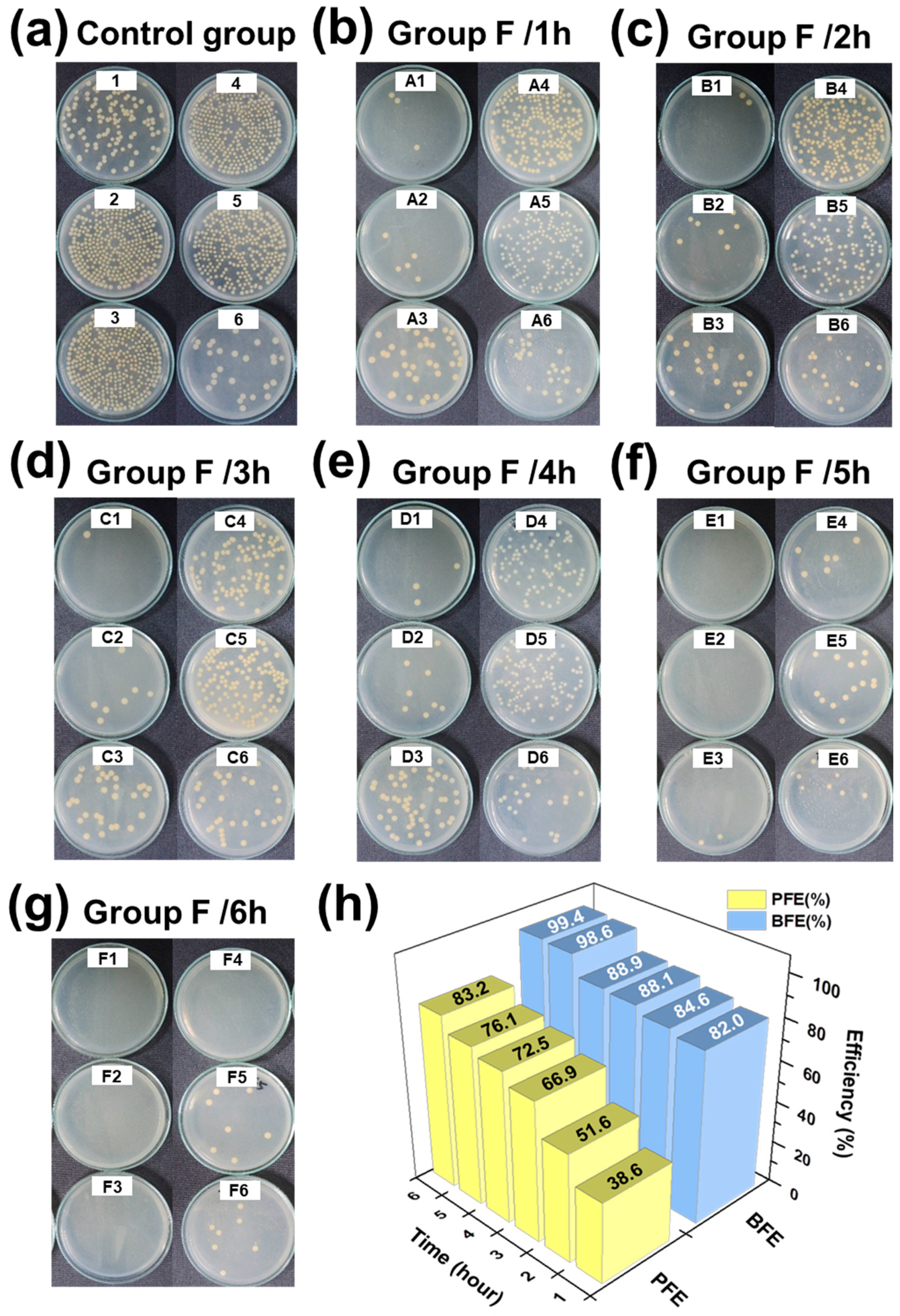
3.3.4. Bacteria Filtration Efficiency (BFE)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Bai, Y.; Xie, J.; Jiang, Q.; Qiu, Y. Synthesis and filtration properties of polyimide nanofiber membrane/carbon woven fabric sandwiched hot gas filters for removal of PM 2.5 particles. Powder Technol. 2016, 292, 54–63. [Google Scholar] [CrossRef]
- Fikenzer, S.; Uhe, T.; Lavall, D.; Rudolph, U.; Falz, R.; Busse, M.; Hepp, P.; Laufs, U. Effects of surgical and FFP2/N95 face masks on cardiopulmonary exercise capacity. Clin. Res. Cardiol. 2020, 109, 1522–1530. [Google Scholar] [CrossRef]
- Kim, M.C.; Bae, S.; Kim, J.Y.; Park, S.Y.; Lim, J.S.; Sung, M.; Kim, S.H. Effectiveness of surgical, KF94, and N95 respirator masks in blocking SARS-CoV-2: A controlled comparison in 7 patients. Infect. Dis. 2020, 52, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, S.; Eimer, B.C.; Shaffer, R.E. Comparison of nanoparticle filtration performance of NIOSH-approved and CE-marked particulate filtering facepiece respirators. Ann. Occup. Hyg. 2009, 53, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Chalikonda, S.; Waltenbaugh, H.; Angelilli, S.; Dumont, T.; Kvasager, C.; Sauber, T.; Servello, N.; Singh, A.; Diaz-Garcia, R. Implementation of an Elastomeric Mask Program as a Strategy to Eliminate Disposable N95 Mask Use and Resterilization: Results from a Large Academic Medical Center. J. Am. Coll. Surg. 2020, 231, 333–338. [Google Scholar] [CrossRef]
- Czubryt, M.P.; Stecy, T.; Popke, E.; Aitken, R.; Jabusch, K.; Pound, R.; Lawes, P.; Ramjiawan, B.; Pierce, G.N. N95 mask reuse in a major urban hospital: COVID-19 response process and procedure. J. Hosp. Infect. 2020, 106, 277–282. [Google Scholar] [CrossRef]
- Bai, Y.; Han, C.B.; He, C.; Gu, G.Q.; Nie, J.H.; Shao, J.J.; Xiao, T.X.; Deng, C.R.; Wang, Z.L. Washable Multilayer Triboelectric Air Filter for Efficient Particulate Matter PM2.5Removal. Adv. Funct. Mater. 2018, 28, 1706680. [Google Scholar] [CrossRef]
- Cho, C.-J.; Chen, S.-Y.; Kuo, C.-C.; Veeramuthu, L.; Au-Duong, A.-N.; Chiu, Y.-C.; Chang, S.-H. Morphology and optoelectronic characteristics of organic field-effect transistors based on blends of polylactic acid and poly(3-hexylthiophene). Polym. J. 2018, 50, 975–987. [Google Scholar] [CrossRef]
- Jiang, D.H.; Chiu, P.C.; Cho, C.J.; Veeramuthu, L.; Tung, S.H.; Satoh, T.; Chiang, W.H.; Cai, X.; Kuo, C.C. Facile 3D Boron Nitride Integrated Electrospun Nanofibrous Membranes for Purging Organic Pollutants. Nanomaterials 2019, 9, 1383. [Google Scholar] [CrossRef]
- Liang, F.-C.; Ku, H.-J.; Cho, C.-J.; Chen, W.-C.; Lee, W.-Y.; Chen, W.-C.; Rwei, S.-P.; Borsali, R.; Kuo, C.-C. An intrinsically stretchable and ultrasensitive nanofiber-based resistive pressure sensor for wearable electronics. J. Mater. Chem. C 2020, 8, 5361–5369. [Google Scholar] [CrossRef]
- Lu, W.C.; Chuang, F.S.; Venkatesan, M.; Cho, C.J.; Chen, P.Y.; Tzeng, Y.R.; Yu, Y.Y.; Rwei, S.P.; Kuo, C.C. Synthesis of Water Resistance and Moisture-Permeable Nanofiber Using Sodium Alginate-Functionalized Waterborne Polyurethane. Polymers 2020, 12, 2882. [Google Scholar] [CrossRef] [PubMed]
- Veeramuthu, L.; Li, W.-L.; Liang, F.-C.; Cho, C.-J.; Kuo, C.-C.; Chen, W.-C.; Lin, J.-H.; Lee, W.-Y.; Wang, C.-T.; Lin, W.-Y.; et al. Smart garment energy generators fabricated using stretchable electrospun nanofibers. React. Funct. Polym. 2019, 142, 96–103. [Google Scholar] [CrossRef]
- Veeramuthu, L.; Venkatesan, M.; Liang, F.C.; Benas, J.S.; Cho, C.J.; Chen, C.W.; Zhou, Y.; Lee, R.H.; Kuo, C.C. Conjugated Copolymers through Electrospinning Synthetic Strategies and Their Versatile Applications in Sensing Environmental Toxicants, pH, Temperature, and Humidity. Polymers 2020, 12, 587. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-Y.; Kuo, C.-C.; Cho, C.-J.; Liang, F.-C.; Jeng, R.-J. Novel fluorescent chemosensory filter membranes composed of electrospun nanofibers with ultra-selective and reversible pH and Hg 2+ sensing characteristics. Dyes Pigment. 2017, 143, 129–142. [Google Scholar] [CrossRef]
- Cho, C.-J.; Lu, S.-T.; Kuo, C.-C.; Liang, F.-C.; Chen, B.-Y.; Chu, C.-C. Pyrene or rhodamine derivative–modified surfaces of electrospun nanofibrous chemosensors for colorimetric and fluorescent determination of Cu 2+, Hg 2+, and pH. React. Funct. Polym. 2016, 108, 137–147. [Google Scholar] [CrossRef]
- Liang, F.C.; Kuo, C.C.; Chen, B.Y.; Cho, C.J.; Hung, C.C.; Chen, W.C.; Borsali, R. RGB-Switchable Porous Electrospun Nanofiber Chemoprobe-Filter Prepared from Multifunctional Copolymers for Versatile Sensing of pH and Heavy Metals. ACS Appl. Mater. Interfaces 2017, 9, 16381–16396. [Google Scholar] [CrossRef]
- Liang, F.C.; Luo, Y.L.; Kuo, C.C.; Chen, B.Y.; Cho, C.J.; Lin, F.J.; Yu, Y.Y.; Borsali, R. Novel Magnet and Thermoresponsive Chemosensory Electrospinning Fluorescent Nanofibers and Their Sensing Capability for Metal Ions. Polymers 2017, 9, 136. [Google Scholar] [CrossRef]
- Lin, C.C.; Jiang, D.H.; Kuo, C.C.; Cho, C.J.; Tsai, Y.H.; Satoh, T.; Su, C. Water-Resistant Efficient Stretchable Perovskite-Embedded Fiber Membranes for Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, G.-S.; He, H.-W.; Zhou, C.-F.; Yan, X.; Zhang, J.-C. Physical Structure Induced Hydrophobicity Analyzed from Electrospinning and Coating Polyvinyl Butyral Films. Adv. Condens. Matter Phys. 2019, 2019, 1–5. [Google Scholar] [CrossRef]
- Peer, P.; Polaskova, M.; Musilova, L. Superhydrophobic poly(vinyl butyral) nanofibrous membrane containing various silica nanoparticles. J. Text. Inst. 2019, 110, 1508–1514. [Google Scholar] [CrossRef]
- Fauzi, A.; Hapidin, D.A.; Munir, M.M.; Iskandar, F.; Khairurrijal, K. A superhydrophilic bilayer structure of a nylon 6 nanofiber/cellulose membrane and its characterization as potential water filtration media. RSC Adv. 2020, 10, 17205–17216. [Google Scholar] [CrossRef]
- Keirouz, A.; Radacsi, N.; Ren, Q.; Dommann, A.; Beldi, G.; Maniura-Weber, K.; Rossi, R.M.; Fortunato, G. Nylon-6/chitosan core/shell antimicrobial nanofibers for the prevention of mesh-associated surgical site infection. J. Nanobiotechnol. 2020, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhuang, X.; Tao, X.; Cheng, B.; Kang, W. Solution blowing nylon 6 nanofiber mats for air filtration. Fibers Polym. 2013, 14, 1485–1490. [Google Scholar] [CrossRef]
- Surgutskaia, N.S.; Martino, A.D.; Zednik, J.; Ozaltin, K.; Lovecká, L.; Bergerová, E.D.; Kimmer, D.; Svoboda, J.; Sedlarik, V. Efficient Cu2+, Pb2+ and Ni2+ ion removal from wastewater using electrospun DTPA-modified chitosan/polyethylene oxide nanofibers. Sep. Purif. Technol. 2020, 247, 116914. [Google Scholar] [CrossRef]
- Song, X.; Song, Y.; Wang, J.; Liu, Q.; Duan, Z. Insights into the pore-forming effect of polyvinyl butyral (PVB) as the polymer template to synthesize mesoporous alumina nanofibers via electrospinning. Ceram. Int. 2020, 46, 9952–9956. [Google Scholar] [CrossRef]
- Cho, C.-J.; Chang, Y.-S.; Lin, Y.-Z.; Jiang, D.-H.; Chen, W.-H.; Lin, W.-Y.; Chen, C.-W.; Rwei, S.-P.; Kuo, C.-C. Green electrospun nanofiber membranes filter prepared from novel biomass thermoplastic copolyester: Morphologies and filtration properties. J. Taiwan Inst. Chem. Eng. 2020, 106, 206–214. [Google Scholar] [CrossRef]
- Venkatesan, M.; Veeramuthu, L.; Liang, F.-C.; Chen, W.-C.; Cho, C.-J.; Chen, C.-W.; Chen, J.-Y.; Yan, Y.; Chang, S.-H.; Kuo, C.-C. Evolution of electrospun nanofibers fluorescent and colorimetric sensors for environmental toxicants, pH, temperature, and cancer cells—A review with insights on applications. Chem. Eng. J. 2020, 397, 125431. [Google Scholar] [CrossRef]
- Chan, H.W.; Cho, C.J.; Hsu, K.H.; He, C.L.; Kuo, C.C.; Chu, C.C.; Chen, Y.H.; Chen, C.W.; Rwei, S.P. Smart Wearable Textiles with Breathable Properties and Repeatable Shaping in In Vitro Orthopedic Support from a Novel Biomass Thermoplastic Copolyester. Macromol. Mater. Eng. 2019, 304, 1900103. [Google Scholar] [CrossRef]
- Hsu, K.H.; Chen, C.W.; Wang, L.Y.; Chan, H.W.; He, C.L.; Cho, C.J.; Rwei, S.P.; Kuo, C.C. Bio-based thermoplastic poly(butylene succinate-co-propylene succinate) copolyesters: Effect of glycerol on thermal and mechanical properties. Soft Matter 2019, 15, 9710–9720. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Michalska-Sionkowska, M.; Walczak, M.; Sionkowska, A. Antimicrobial activity of collagen material with thymol addition for potential application as wound dressing. Polym. Test. 2017, 63, 360–366. [Google Scholar] [CrossRef]
- Marino, M.; Bersani, C.; Comi, G. Antimicrobial activity of the essential oils of Thymus vulgaris L. measured using a bioimpedometric method. J. Food Prot. 1999, 62, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Blanco, A.R.; Cannatelli, M.A.; Enea, V.; Flamini, G.; Morelli, I.; Sudano Roccaro, A.; Alonzo, V. Susceptibility of methicillin-resistant staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef]
- Kavoosi, G.; Dadfar, S.M.; Purfard, A.M. Mechanical, physical, antioxidant, and antimicrobial properties of gelatin films incorporated with thymol for potential use as nano wound dressing. J. Food Sci. 2013, 78, E244–E250. [Google Scholar] [CrossRef]
- Karami, Z.; Rezaeian, I.; Zahedi, P.; Abdollahi, M. Preparation and performance evaluations of electrospun poly(ε-caprolactone), poly(lactic acid), and their hybrid (50/50) nanofibrous mats containing thymol as an herbal drug for effective wound healing. J. Appl. Polym. Sci. 2013, 129, 756–766. [Google Scholar] [CrossRef]
- Koosehgol, S.; Ebrahimian-Hosseinabadi, M.; Alizadeh, M.; Zamanian, A. Preparation and characterization of in situ chitosan/polyethylene glycol fumarate/thymol hydrogel as an effective wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 66–75. [Google Scholar] [CrossRef]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun Nanofibers Membranes for Effective Air Filtration. Macromol. Mater. Eng. 2017, 302, 1600353. [Google Scholar] [CrossRef]
- Kaźmierczak, D.; Guzińska, K.; Dymel, M. Antibacterial Activity of PLA Fibres Estimated by Quantitative Methods. Fibres Text. East. Eur. 2016, 24, 126–130. [Google Scholar] [CrossRef]
- Pinho, E.; Magalhães, L.; Henriques, M.; Oliveira, R. Antimicrobial activity assessment of textiles: Standard methods comparison. Ann. Microbiol. 2010, 61, 493–498. [Google Scholar] [CrossRef]
- Lee, S.-A.; Chen, Y.-L.; Hwang, D.-C.; Wu, C.-C.; Chen, J.-K. Performance Evaluation of Full Facepiece Respirators with Cartridges. Aerosol. Air Qual. Res. 2017, 17, 1316–1328. [Google Scholar] [CrossRef]
- Bundjaja, V.; Santoso, S.P.; Angkawijaya, A.E.; Yuliana, M.; Soetaredjo, F.E.; Ismadji, S.; Ayucitra, A.; Gunarto, C.; Ju, Y.H.; Ho, M.H. Fabrication of cellulose carbamate hydrogel-dressing with rarasaponin surfactant for enhancing adsorption of silver nanoparticles and antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111542. [Google Scholar] [CrossRef]
- Deng, L.; Kang, X.; Liu, Y.; Feng, F.; Zhang, H. Effects of surfactants on the formation of gelatin nanofibres for controlled release of curcumin. Food Chem. 2017, 231, 70–77. [Google Scholar] [CrossRef]
- Demirdogen, R.E.; Kilic, D.; Emen, F.M.; Aşkar, Ş.; Karaçolak, A.İ.; Yesilkaynak, T.; Ihsan, A. Novel antibacterial cellulose acetate fibers modified with 2-fluoropyridine complexes. J. Mol. Struct. 2020, 1204, 127537. [Google Scholar] [CrossRef]
- Guzinska, K.; Kazmierczak, D.; Dymel, M.; Pabjanczyk-Wlazlo, E.; Bogun, M. Anti-bacterial materials based on hyaluronic acid: Selection of research methodology and analysis of their anti-bacterial properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 800–808. [Google Scholar] [CrossRef] [PubMed]
- NO, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Park, S.J.; Park, Y.M. Eco-dyeing and antimicrobial properties of chlorophyllin copper complex extracted from Sasa veitchii. Fibers Polym. 2010, 11, 357–362. [Google Scholar] [CrossRef]
- Yim, W.; Cheng, D.; Patel, S.H.; Kou, R.; Meng, Y.S.; Jokerst, J.V. KN95 and N95 Respirators Retain Filtration Efficiency despite a Loss of Dipole Charge during Decontamination. ACS Appl. Mater. Interfaces 2020, 12, 54473–54480. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A.; Shuvho, M.B.A.; Shahid, M.A.; Haque, A.; Kashem, M.A.; Lam, S.S.; Ong, H.C.; Uddin, M.A.; Mofijur, M. Prospect of biobased antiviral face mask to limit the coronavirus outbreak. Environ. Res. 2021, 192, 110294. [Google Scholar] [CrossRef] [PubMed]
- Das, O.; Neisiany, R.E.; Capezza, A.J.; Hedenqvist, M.S.; Forsth, M.; Xu, Q.; Jiang, L.; Ji, D.; Ramakrishna, S. The need for fully bio-based facemasks to counter coronavirus outbreaks: A perspective. Sci. Total Environ. 2020, 736, 139611. [Google Scholar] [CrossRef] [PubMed]
- El-Atab, N.; Qaiser, N.; Badghaish, H.; Shaikh, S.F.; Hussain, M.M. Flexible Nanoporous Template for the Design and Development of Reusable Anti-COVID-19 Hydrophobic Face Masks. ACS Nano 2020, 14, 7659–7665. [Google Scholar] [CrossRef]
- Fadare, O.O.; Okoffo, E.D. Covid-19 face masks: A potential source of microplastic fibers in the environment. Sci. Total Environ. 2020, 737, 140279. [Google Scholar] [CrossRef]
- Xiao, B.; Huang, Q.; Chen, H.; Chen, X.; Long, G. A Fractal Model for Capillary Flow through a Single Tortuous Capillary with Roughened Surfaces in Fibrous Porous Media. Fractals 2021, 29, 2150017. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, W.; Zhang, X.; Long, G.; Fan, J.; Chen, H.; Deng, L. A novel fractal solution for permeability and Kozeny-Carman constant of fibrous porous media made up of solid particles and porous fibers. Powder Technol. 2019, 349, 92–98. [Google Scholar] [CrossRef]
| Thymol:PVB (w:w) | Thymol (g) | PVB (g) | 5 wt% PVB (g) |
|---|---|---|---|
| 0:1 | 0 | 2.5 | 50 |
| 0.2:1 | 0.5 | 2.5 | 50 |
| 0.4:1 | 1 | 2.5 | 50 |
| 0.6:1 | 1.5 | 2.5 | 50 |
| 0.8:1 | 2 | 2.5 | 50 |
| 1:1 | 2.5 | 2.5 | 50 |
| Method | JIS L1902 (Halo Method) | AATCC147 (Parallel Streak Method) | |||
|---|---|---|---|---|---|
| Carrier | Aluminum Foil | Net | Aluminum Foil | Net | |
| Pressing with glass | none | yes | yes | yes | |
| group | A | B | C | D | |
| Thymol:PVB (w:w) | |||||
| 0:1 | c NI | c NI | c NI | c NI | |
| 0.2:1 | c NI | c NI | c NI | c NI | |
| 0.4:1 | c NI | c NI | c NI | c NI | |
| 0.6:1 | c NI | c NI | c NI | c NI | |
| 0.8:1 | a CZ 2.0 mm | c NI | a CZ 2.1 mm | c NI | |
| 1:1 | a CZ 5.1 mm | c NI | a CZ 6.4 mm | c NI | |
| Group | Spinning Time (min) | CNS 14755 | CNS 14777 | |
|---|---|---|---|---|
| PFE (%) | Inspiratory Impedance (mmH2O) | Pressure Difference (mmH2O/cm2) | ||
| C | 0 | 32.8 ± 2.5 | 3.9 ± 0.3 | 1.4 ± 0.2 |
| C | 15 | 65.0 ± 2.5 | 9.1 ± 0.9 | 3.8 ± 0.1 |
| C | 30 | 67.1 ± 2.0 | 11.4 ± 0.6 | 4.4 ± 0.1 |
| C | 45 | 89.5 ± 1.0 | 38.7 ± 0.8 | 13.7 ± 1.8 |
| C | 60 | 94.2 ± 0.3 | 54.2 ± 8.6 | 14.1 ± 4.5 |
| C | 120 | 89.5 ± 1.6 | 42.7 ± 4.9 | 12.6 ± 2.0 |
| D | 0 | 7.5 ± 0.1 | 1.8 ± 0.1 | 0.8 ± 0.1 |
| D | 45 | 85.1 ± 0.7 | 29.9 ± 0.4 | 9.7 ± 0.1 |
| E | 60 | 62.9 ± 1.2 | 7.3 ± 0.2 | 2.6 ± 0.2 |
| E | 120 | 82.7 ± 1.0 | 12.3 ± 0.4 | 4.5 ± 0.3 |
| Group/Spinning Time | D/45 min | E/2 h |
|---|---|---|
| Voltage | 25 kV | 15 kV |
| Flow rate | 5 mL/h | 2 mL/h |
| PFE(%) | 85.1 | 82.7 |
| Inspiratory impedance (mmH2O) | 29.9 | 12.3 |
| Pressure difference (mmH2O/cm2) | 9.7 | 4.5 |
| Average diameter (nm) | 836 | 375 |
| Standard deviation | 329 | 69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, W.-C.; Chen, C.-Y.; Cho, C.-J.; Venkatesan, M.; Chiang, W.-H.; Yu, Y.-Y.; Lee, C.-H.; Lee, R.-H.; Rwei, S.-P.; Kuo, C.-C. Antibacterial Activity and Protection Efficiency of Polyvinyl Butyral Nanofibrous Membrane Containing Thymol Prepared through Vertical Electrospinning. Polymers 2021, 13, 1122. https://doi.org/10.3390/polym13071122
Lu W-C, Chen C-Y, Cho C-J, Venkatesan M, Chiang W-H, Yu Y-Y, Lee C-H, Lee R-H, Rwei S-P, Kuo C-C. Antibacterial Activity and Protection Efficiency of Polyvinyl Butyral Nanofibrous Membrane Containing Thymol Prepared through Vertical Electrospinning. Polymers. 2021; 13(7):1122. https://doi.org/10.3390/polym13071122
Chicago/Turabian StyleLu, Wen-Chi, Ching-Yi Chen, Chia-Jung Cho, Manikandan Venkatesan, Wei-Hung Chiang, Yang-Yen Yu, Chen-Hung Lee, Rong-Ho Lee, Syang-Peng Rwei, and Chi-Ching Kuo. 2021. "Antibacterial Activity and Protection Efficiency of Polyvinyl Butyral Nanofibrous Membrane Containing Thymol Prepared through Vertical Electrospinning" Polymers 13, no. 7: 1122. https://doi.org/10.3390/polym13071122
APA StyleLu, W.-C., Chen, C.-Y., Cho, C.-J., Venkatesan, M., Chiang, W.-H., Yu, Y.-Y., Lee, C.-H., Lee, R.-H., Rwei, S.-P., & Kuo, C.-C. (2021). Antibacterial Activity and Protection Efficiency of Polyvinyl Butyral Nanofibrous Membrane Containing Thymol Prepared through Vertical Electrospinning. Polymers, 13(7), 1122. https://doi.org/10.3390/polym13071122