Ionic Liquid-Based Surfactants: Recent Advances in Their Syntheses, Solution Properties, and Applications
Abstract
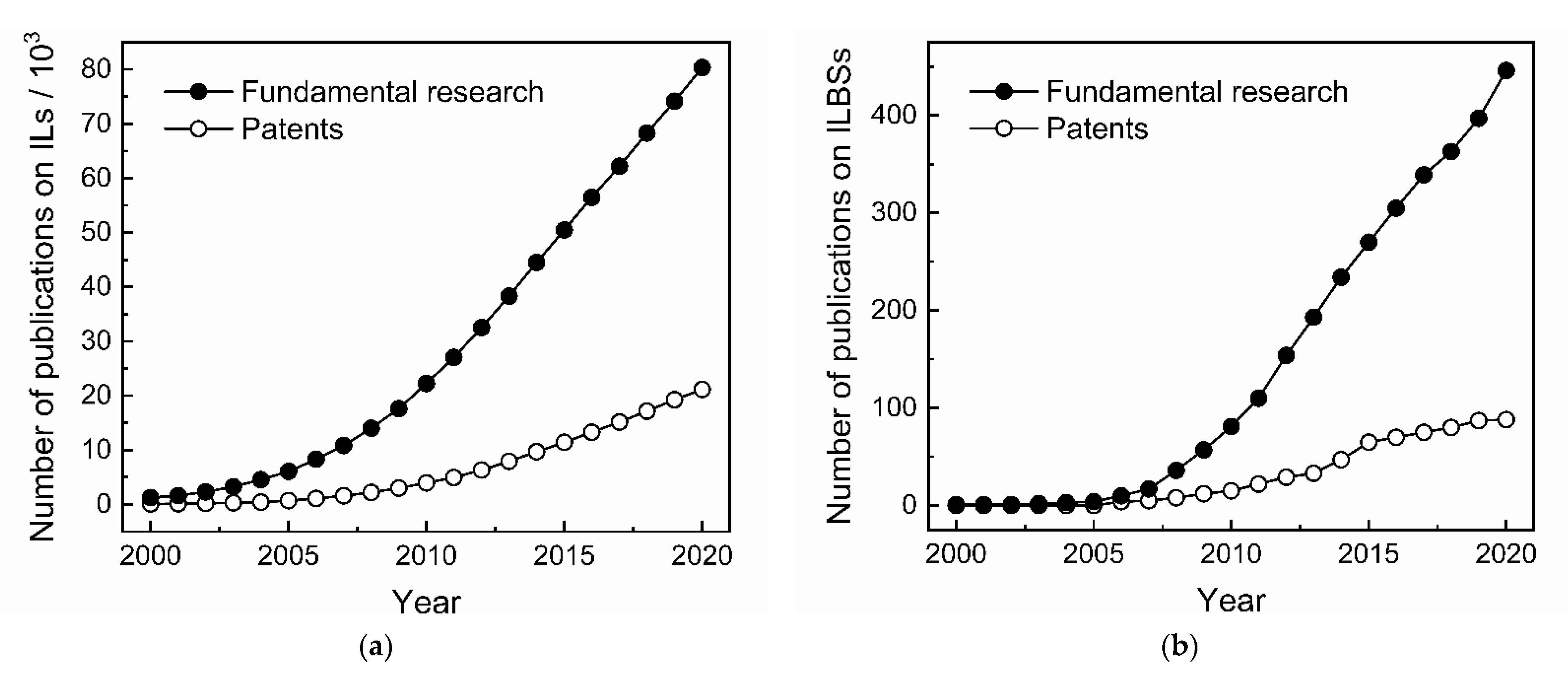
1. Introduction
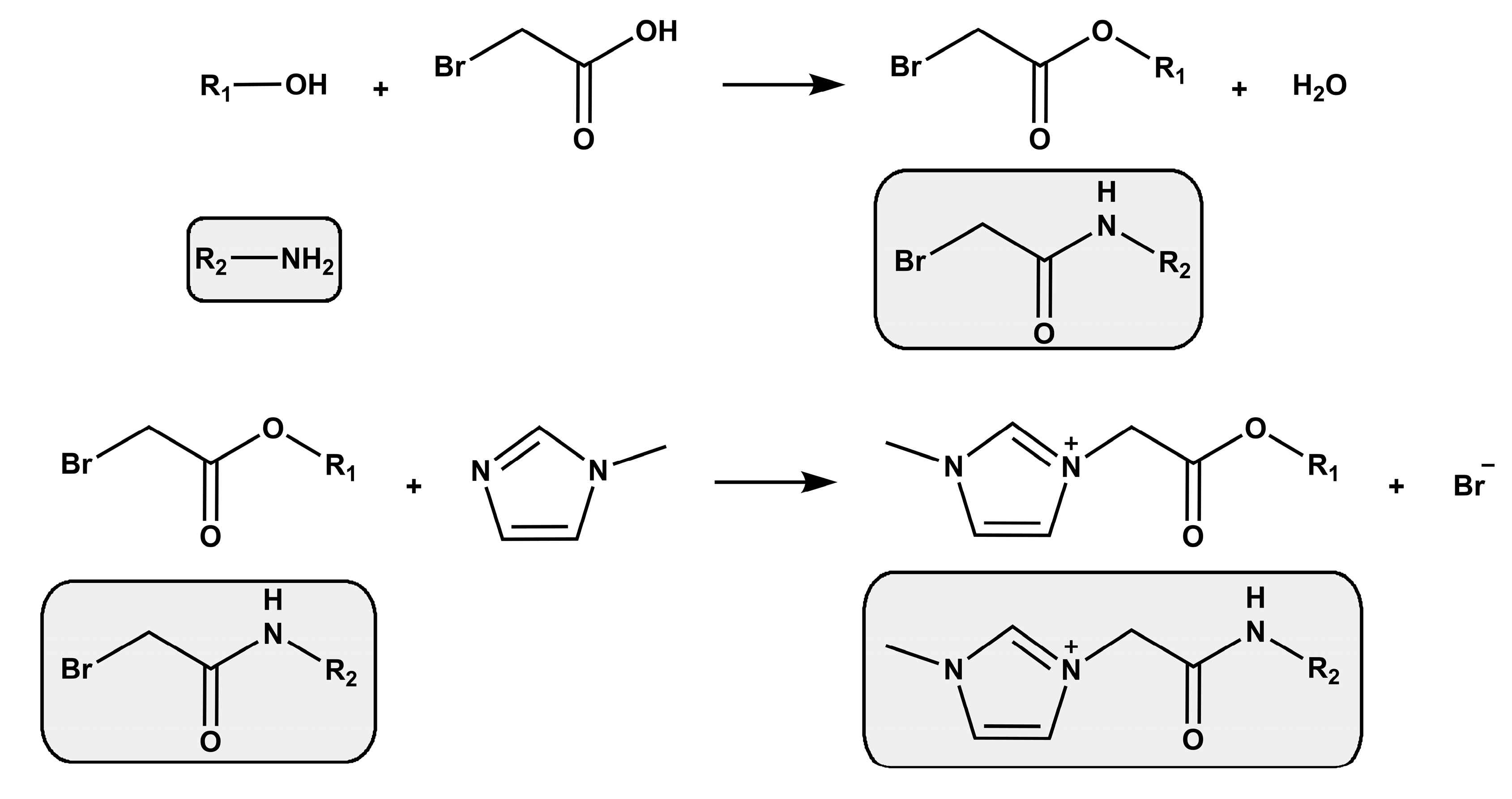
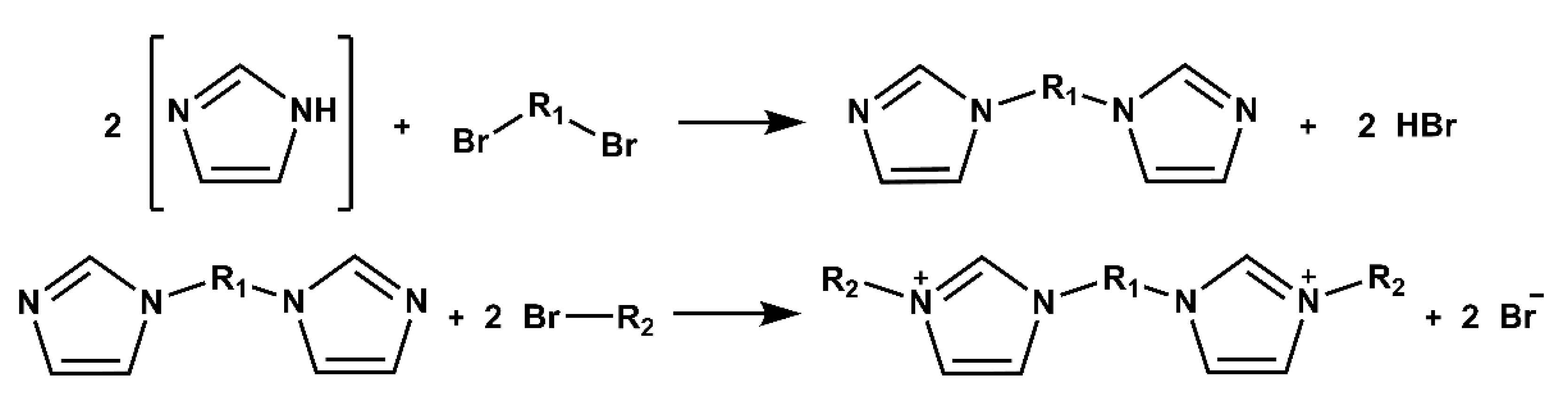
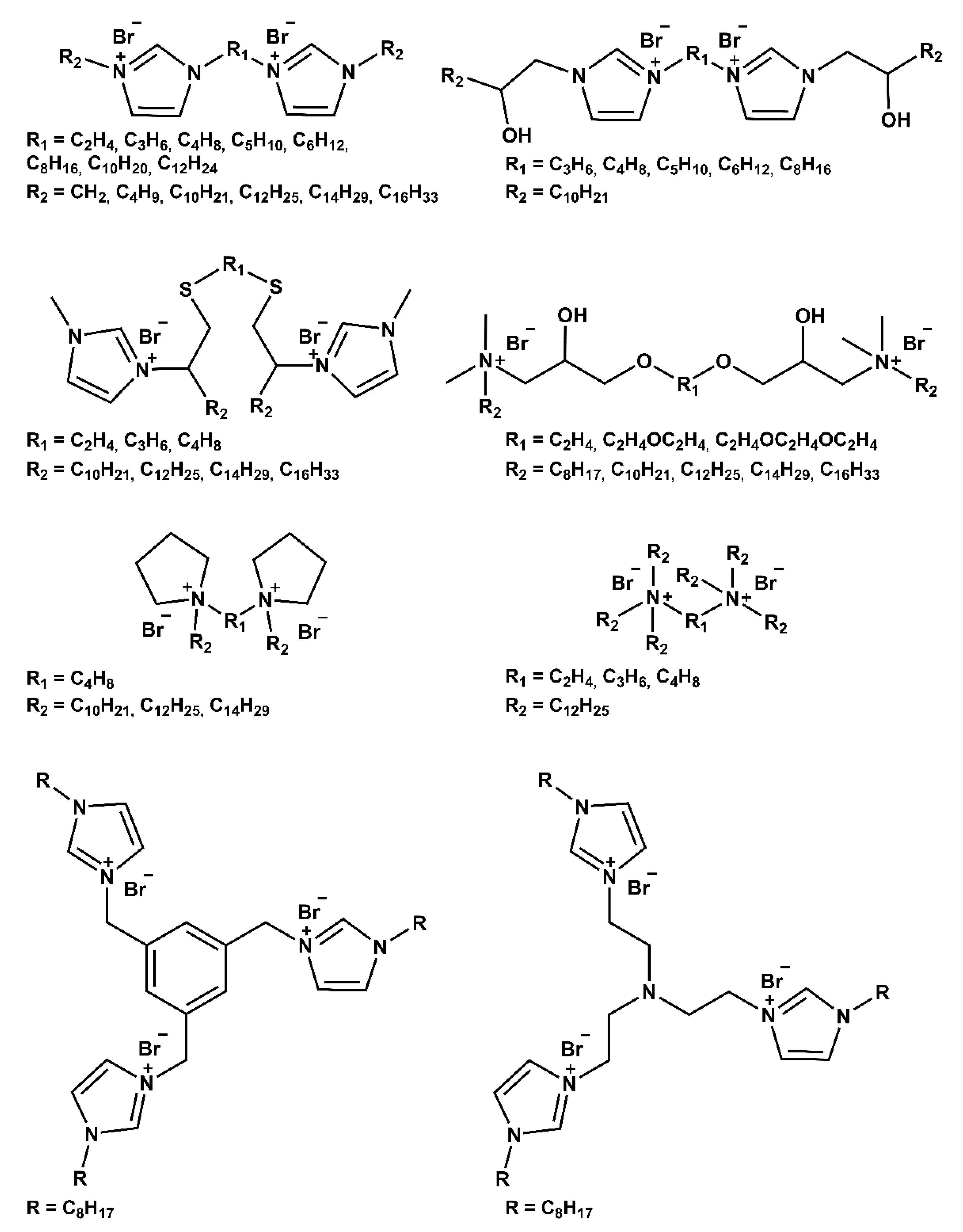
2. Strategies for Synthesizing Mono-Cationic and Gemini Ionic Liquid-Based Surfactants
3. Relevant Properties of Aqueous Solutions of ILBS
3.1. Compilation and Discussion of the Properties of Aqueous Solution of ILBSs
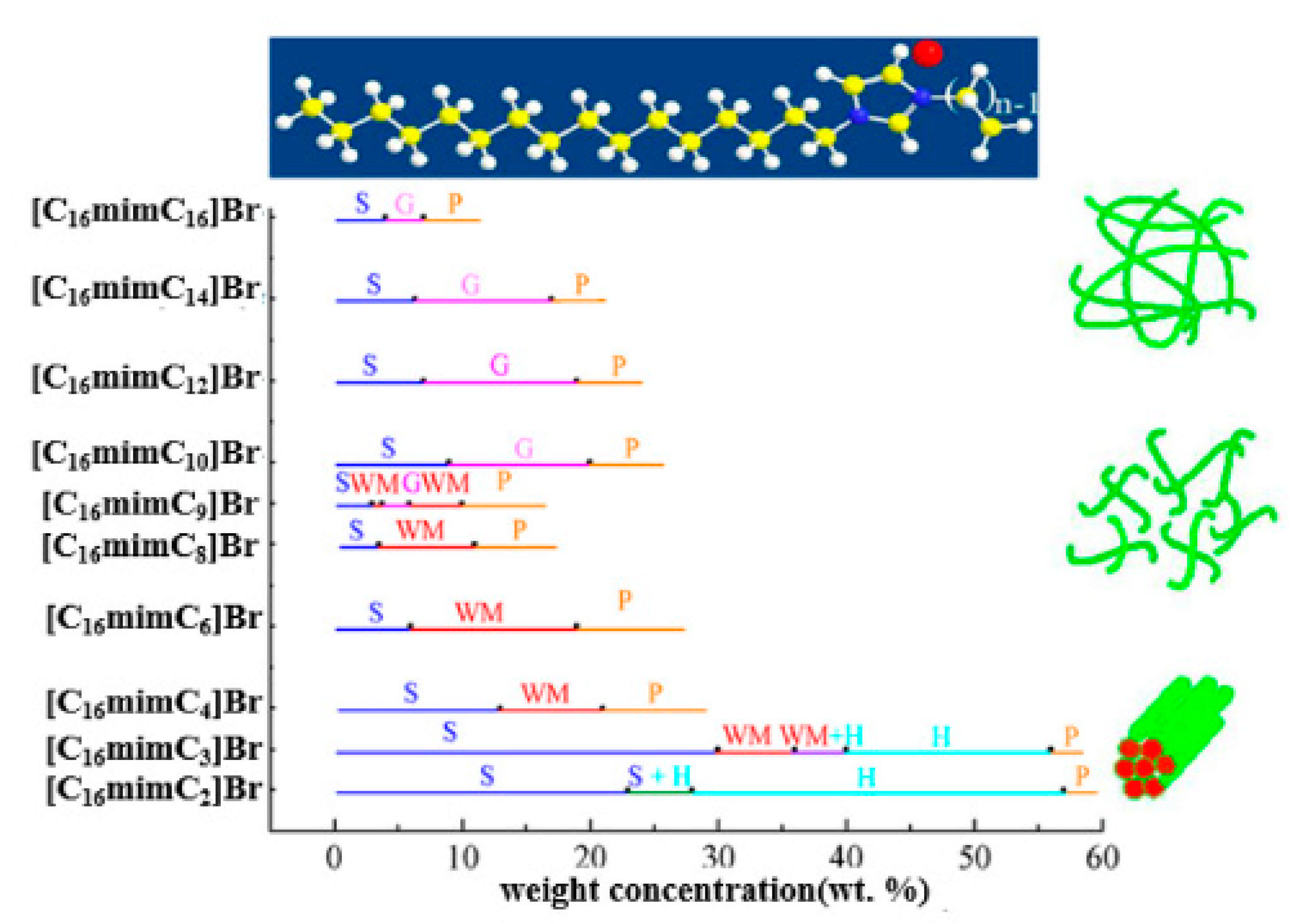
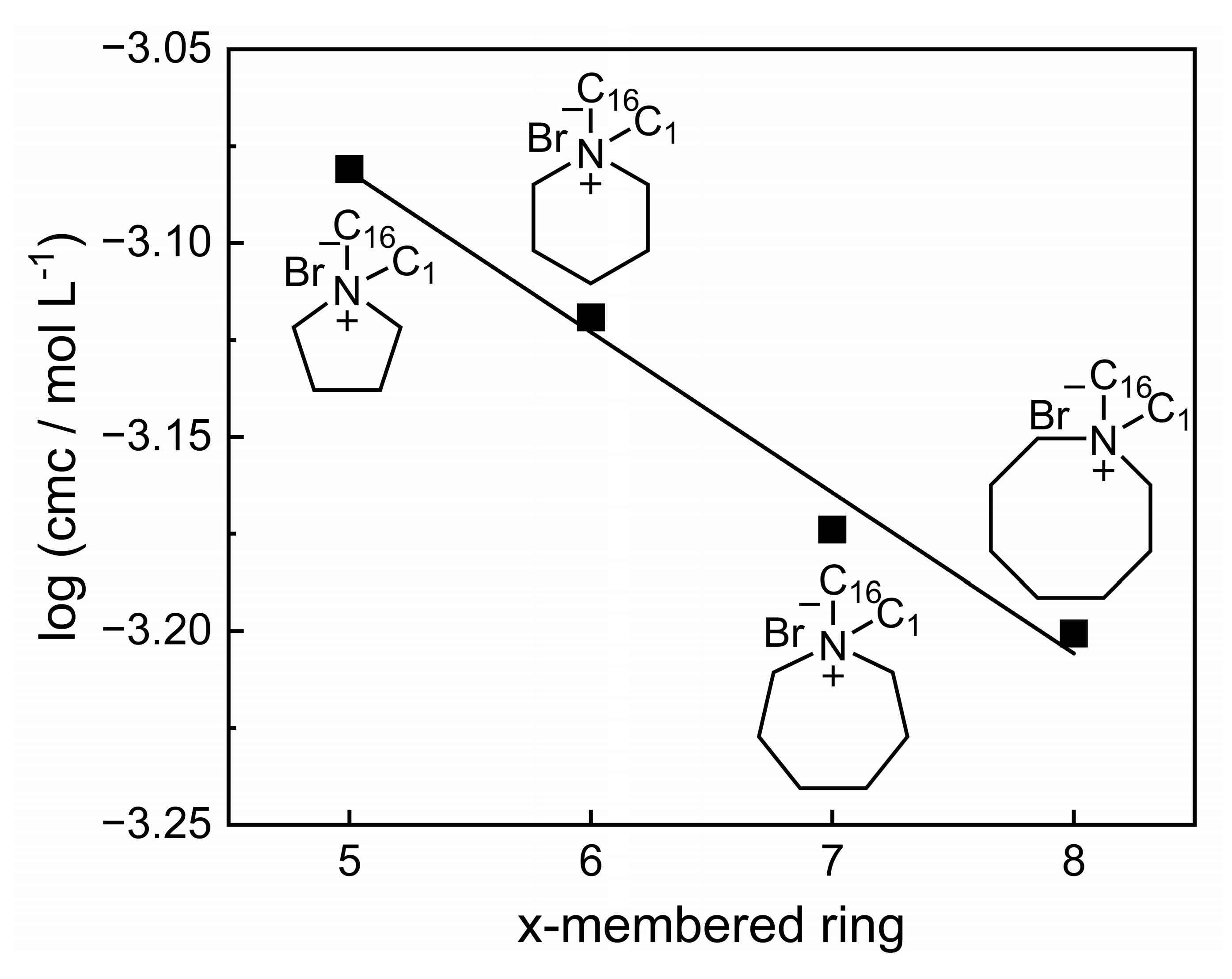
3.2. Compilation and Brief Discussion of the Properties of Aqueous Solution of GILBSs
4. Applications of IL-based Surfactants
4.1. Nanotechnology
4.2. Polymerization
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
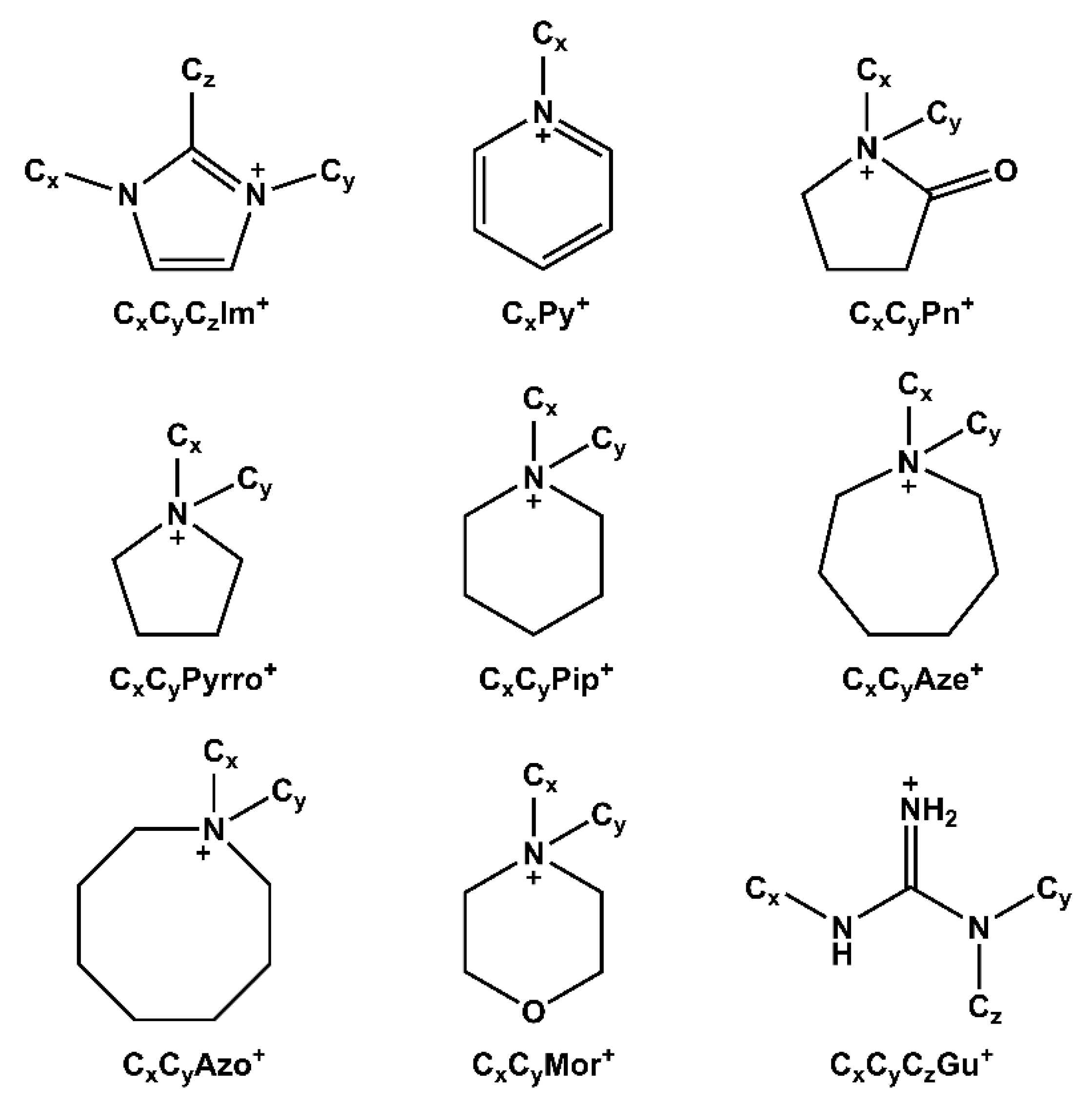
Abbreviations
| Amin | Minimum area per molecule at the water/air interface |
| AGET | Activators generated by electron transfer |
| AIBN | Azobisisobutyronitrile |
| AM | Acrylamide |
| ATRP | Atom transfer radical polymerizations |
| Aze⁺ | Azepanium |
| Azo⁺ | Azocanium |
| C₁, C₂, etc | Methyl, ethyl, etc, these alkyl groups have n-chain, unless indicated otherwise. |
| pC20 | Surface tension reduction efficiency (by 20 mN m⁻1). |
| Cmc | Critical micelle concentration |
| CTAB | Cetyltrimethylammonium bromide |
| CTACl | Cetyltrimethylammonium chloride |
| DBS | Dodecylbenzene sulphonate |
| EO | Ethylene oxide unit |
| GILBS | Gemini ionic liquid-based surfactant, where two ILBSs are joined with a tether or a spacer. |
| Gu⁺ | Guanidinium |
| HC | Surfactant hydrophobic group (or tail) |
| HG | Surfactant head-group |
| ILBS | Ionic liquid-based surfactant |
| Im+ | Imidazolium ring |
| Mn | Number average molar mass |
| Mw | Weight average molar mass |
| MM | Molar mass |
| MMA | Methyl methacrylate monomer |
| MNP | Mesoporous nanoparticles |
| MSNP | Mesoporous silica nanoparticle |
| Mor⁺ | Morpholinium |
| N⁺ | Ammonium |
| Nagg | Average aggregation number of the micellar aggregate |
| NP | Nanoparticle |
| OA | Oleic acid |
| OHIm | Hydroxyl-functionalized imidazolium ring |
| O/W | Oil-in-water emulsion or microemulsion |
| P⁺ | Phosphonium |
| Pip⁺ | Piperidinium |
| PMMA | Polymethylmethacrylate (PMMA) |
| Pn+ | 2-Pyrrolidinonium |
| PS | Polystyrene |
| Py+ | Pyridinium ring |
| Pyrro⁺ | Pyrrolidinium |
| SDS | Sodium dodecyl sulphate, SDS |
| SMeIm | Thioether-functionalized methylimidazolium ring |
| TC | An AOT analogue, based on trans-aconitic acid |
| Triton X-100 | Nonionic surfactant 2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethanol |
| Vn | Vinyl group, respectively |
| W/O | Water-in -oil emulsion or microemulsion |
| αmic | Degree of the surfactant counter-ion dissociation |
| ΔG0ads | Gibbs free energy of surfactant adsorption at the water/air interface |
| ΔG0mic | Gibbs free energy of micelle formation |
| ΔH0mic | Enthalpy of micelle formation |
| ΔS0mic | Entropy of micelle formation |
| γcmc | Surface tension at cmc |
| Γcmc | Surface pressure at cmc |
| Πcmc | Surface excess concentration at the interface |
| μE | Microemulsion |
References
- Pino, V.; Germán-Hernández, M.; Martín-Pérez, A.; Anderson, J.L. Ionic Liquid-Based Surfactants in Separation Science. Sep. Sci. Technol. 2012, 47, 264–276. [Google Scholar] [CrossRef]
- Cao, H.; Hu, Y.; Xu, W.; Wang, Y.; Guo, X. Recent Progress in the Assembly Behavior of Imidazolium-Based Ionic Liquid Surfactants. J. Mol. Liq. 2020, 319, 114354. [Google Scholar] [CrossRef]
- Hejazifar, M.; Lanaridi, O.; Bica-Schröder, K. Ionic Liquid Based Microemulsions: A Review. J. Mol. Liq. 2020, 303, 112264. [Google Scholar] [CrossRef]
- Freire, M.G.; Carvalho, P.J.; Fernandes, A.M.; Marrucho, I.M.; Queimada, A.J.; Coutinho, J.A.P. Surface Tensions of Imidazolium Based Ionic Liquids: Anion, Cation, Temperature and Water Effect. J. Colloid Interface Sci. 2007, 314, 621–630. [Google Scholar] [CrossRef]
- Lee, D.J. Enthalpy-Entropy Compensation in Ionic Micelle Formation. Colloid Polym. Sci. 1995, 273, 539–543. [Google Scholar] [CrossRef]
- Lunkenheimer, K.; Miller, R. A Criterion for Judging the Purity of Adsorbed Surfactant Layers. J. Colloid Interface Sci. 1987, 120, 176–183. [Google Scholar] [CrossRef]
- Lunkenheimer, K.; Pergande, H.J.; Krüger, H. Apparatus for Programmed High-Performance Purification of Surfactant Solutions. Rev. Sci. Instrum. 1987, 58, 2313–2316. [Google Scholar] [CrossRef]
- Freire, M.G.; Neves, C.M.S.S.; Marrucho, I.M.; Coutinho, J.A.P.; Fernandes, A.M. Hydrolysis of Tetrafluoroborate and Hexafluorophosphate Counter Ions in Imidazolium-Based Ionic Liquids. J. Phys. Chem. A 2010, 114, 3744–3749. [Google Scholar] [CrossRef]
- Martins, C.T.; Sato, B.M.; El Seoud, O.A. First Study on the Thermo-Solvatochromism in Aqueous 1-(1-Butyl)-3- Methylimidazolium Tetrafluoroborate: A Comparison between the Solvation by an Ionic Liquid and by Aqueous Alcohols. J. Phys. Chem. B 2008, 112, 8330–8339. [Google Scholar] [CrossRef]
- Xue, Z. Hydrolysis of Ionic Liquids. In Encyclopedia of Ionic Liquids; Springer Nature: Singapore, 2019; pp. 1–5. ISBN 9789811067396. [Google Scholar]
- Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Ionic Liquids Are Not Always Green: Hydrolysis of 1-Butyl-3- Methylimidazolium Hexafluorophosphate. Green Chem. 2003, 5, 361–363. [Google Scholar] [CrossRef]
- Shi, H.; Xie, Y.; Du, C.; Cong, Y.; Wang, J.; Zhao, H. Thermodynamic Study of the Solubility of 2,4′-Dihydroxydiphenyl Sulfone in Nine Organic Solvents from T = (278.15 to 313.15) K and Thermodynamic Properties of Dissolution. J. Chem. Thermodyn. 2016, 102, 79–88. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, F.; Zhang, Z.; Ren, C.; Lin, Y. Micellization and Thermodynamic Study of 1-Alkyl-3-Methylimidazolium Tetrafluoroborate Ionic Liquids in Aqueous Solution. J. Chem. Eng. Data 2014, 59, 1120–1129. [Google Scholar] [CrossRef]
- Lozano, P.; Bernal, J.M.; Vaultier, M. Towards Continuous Sustainable Processes for Enzymatic Synthesis of Biodiesel in Hydrophobic Ionic Liquids/Supercritical Carbon Dioxide Biphasic Systems. Fuel 2011, 90, 3461–3467. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, Y.; Dong, B.; Zheng, L. Interaction between the Added Long-Chain Ionic Liquid 1-Dodecyl-3-Methylimidazolium Tetrafluoroborate and Triton X-100 in Aqueous Solutions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 182–189. [Google Scholar] [CrossRef]
- Tejada-Casado, C.; Moreno-González, D.; García-Campaña, A.M.; del Olmo-Iruela, M. Use of an Ionic Liquid-Based Surfactant as Pseudostationary Phase in the Analysis of Carbamates by Micellar Electrokinetic Chromatography. Electrophoresis 2015, 36, 955–961. [Google Scholar] [CrossRef]
- Malhotra, S.V.; Kumar, V. A Profile of the in Vitro Anti-Tumor Activity of Imidazolium-Based Ionic Liquids. Bioorganic Med. Chem. Lett. 2010, 20, 581–585. [Google Scholar] [CrossRef]
- Pan, Q.; Yang, L.; Meng, X. Optimization of Enzymatic Synthesis of Tricaprylin in Ionic Liquids by Response Surface Methodology. JaocsJ. Am. Oil Chem. Soc. 2013, 90, 501–509. [Google Scholar] [CrossRef]
- Bian, M.; Zhang, Z.; Yin, H. Effects and Mechanism Characterization of Ionic Liquids as Mobile Phase Additives for the Separation of Matrine-Type Alkaloids by Liquid Chromatography. J. Pharm. Biomed. Anal. 2012, 58, 163–167. [Google Scholar] [CrossRef]
- Lozano, P.; Gomez, C.; Nieto, S.; Sanchez-Gomez, G.; García-Verdugo, E.; Luis, S.V. Highly Selective Biocatalytic Synthesis of Monoacylglycerides in Sponge-like Ionic Liquids. Green Chem. 2017, 19, 390–396. [Google Scholar] [CrossRef]
- Nozaki, Y.; Yamaguchi, K.; Tomida, K.; Taniguchi, N.; Hara, H.; Takikawa, Y.; Sadakane, K.; Nakamura, K.; Konishi, T.; Fukao, K. Phase Transition and Dynamics in Imidazolium-Based Ionic Liquid Crystals through a Metastable Highly Ordered Smectic Phase. J. Phys. Chem. B 2016, 120, 5291–5300. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, L.; Yang, C.; Xia, H.; He, Y.; Feng, M. A Complex Based on Imidazole Ionic Liquid and Copolymer of Acrylamide and Phenoxyacetamide Modification for Clay Stabilizer. J. Appl. Polym. Sci. 2015, 132, 1–11. [Google Scholar] [CrossRef]
- Zha, J.P.; Zhu, M.T.; Qin, L.; Wang, X.H. Study of Interaction between Ionic Liquids and Orange G in Aqueous Solution with UV-Vis Spectroscopy and Conductivity Meter. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 196, 178–184. [Google Scholar] [CrossRef]
- Montaño, D.F.; Casanova, H.; Cardona, W.I.; Giraldo, L.F. Functionalization of Montmorillonite with Ionic Liquids Based on 1-Alkyl-3-Methylimidazolium: Effect of Anion and Length Chain. Mater. Chem. Phys. 2017, 198, 386–392. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, C.; Chen, L.; Tan, L.; Chen, Y. Liquid-Crystalline Ionic Liquids Modified Conductive Polymers as a Transparent Electrode for Indium-Free Polymer Solar Cells. J. Mater. Chem. A 2015, 3, 22316–22324. [Google Scholar] [CrossRef]
- Kato, S.; Freitag, J.; Gostomski, P. Infinite Dilution Partial Molar Excess Entropy-Enthalpy Compensation for Thiophene, Carbon Dioxide and Water in Ionic Liquids. Fluid Phase Equilibria 2013, 339, 1–9. [Google Scholar] [CrossRef]
- Kuddushi, M.; Patel, N.K.; Rajput, S.; Shah, A.; El Seoud, O.A.; Malek, N.I. Thermo-Switchable de Novo Ionic Liquid-Based Gelators with Dye-Absorbing and Drug-Encapsulating Characteristics. ACS Omega 2018, 3, 12068–12078. [Google Scholar] [CrossRef]
- Kuddushi, M.; Kumar, A.; Ray, D.; Aswal, V.K.; El Seoud, O.A.; Malek, N.I. Concentration- And Temperature-Responsive Reversible Transition in Amide-Functionalized Surface-Active Ionic Liquids: Micelles to Vesicles to Organogel. ACS Omega 2020, 5, 24272–24284. [Google Scholar] [CrossRef]
- Wang, G.; Xu, X.; Sun, Y.; Zhuang, L.; Yao, C. Relationship between Structure and Biodegradability of Gemini Imidazolium Surface Active Ionic Liquids. J. Mol. Liq. 2019, 278, 145–155. [Google Scholar] [CrossRef]
- Ao, M.; Xu, G.; Kang, W.; Meng, L.; Gong, H.; Zhou, T. Surface Rheological Behavior of Gelatin/Ionic Liquid-Type Imidazolium Gemini Surfactant Mixed Systems. Soft Matter 2011, 7, 1199–1206. [Google Scholar] [CrossRef]
- Zhuang, L.; Zheng, C.; Sun, J.; Yuan, A.; Wang, G. Performances of Ramie Fiber Pretreated with Dicationic Imidazolium Ionic Liquid. Fibers Polym. 2014, 15, 226–233. [Google Scholar] [CrossRef]
- Anderson, J.L.; Ding, R.; Ellern, A.; Armstrong, D.W. Structure and Properties of High Stability Geminal Dicationic Ionic Liquids. J. Am. Chem. Soc. 2005, 127, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Man, Z.; Arvina, A.; Bustam, M.A.; Nasrullah, A.; Ullah, Z.; Sarwono, A.; Muhammad, N. Dicationic Imidazolium Based Ionic Liquids: Synthesis and Properties. J. Mol. Liq. 2017, 227, 98–105. [Google Scholar] [CrossRef]
- Vélez, J.F.; Álvarez, L.V.; del Río, C.; Herradón, B.; Mann, E.; Morales, E. Imidazolium-Based Mono and Dicationic Ionic Liquid Sodium Polymer Gel Electrolytes. Electrochim. Acta 2017, 241, 517–525. [Google Scholar] [CrossRef]
- Ehsani, A.; Kowsari, E.; Boorboor Ajdari, F.; Safari, R.; Mohammad Shiri, H. Influence of Newly Synthesized Geminal Dicationic Ionic Liquid on Electrochemical and Pseudocapacitance Performance of Conductive Polymer Electroactive Film. J. Colloid Interface Sci. 2017, 505, 1158–1164. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic Liquids Toxicity—Benefits and Threats. Int. J. Mol. Sci. 2020, 21, 1–41. [Google Scholar] [CrossRef]
- Stolte, S.; Steudte, S.; Igartua, A.; Stepnowski, P. The Biodegradation of Ionic Liquids—The View from a Chemical Structure Perspective. Curr. Org. Chem. 2012, 15, 1946–1973. [Google Scholar] [CrossRef]
- Costa, S.P.F.; Azevedo, A.M.O.; Pinto, P.C.A.G.; Saraiva, M.L.M.F.S. Environmental Impact of Ionic Liquids: Recent Advances in (Eco)Toxicology and (Bio)Degradability. ChemSusChem 2017, 10, 2321–2347. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef]
- Picquet, M.; Tkatchenko, I.; Tommasi, I.; Wasserscheid, P.; Zimmermann, J. Ionic Liquids, 3. Synthesis and Utilisation of Protic Imidazolium Salts in Homogeneous Catalysis. Adv. Synth. Catal. 2003, 345, 959–962. [Google Scholar] [CrossRef]
- Thomaier, S.; Kunz, W. Aggregates in Mixtures of Ionic Liquids. J. Mol. Liq. 2007, 130, 104–107. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Pires, P.A.R.; Abdel-Moghny, T.; Bastos, E.L. Synthesis and Micellar Properties of Surface-Active Ionic Liquids: 1-Alkyl-3-Methylimidazolium Chlorides. J. Colloid Interface Sci. 2007, 313, 296–304. [Google Scholar] [CrossRef]
- Ao, M.Q.; Xu, G.Y.; Zhu, Y.Y.; Bai, Y. Synthesis and Properties of Ionic Liquid-Type Gemini Imidazolium Surfactants. J. Colloid Interface Sci. 2008, 326, 490–495. [Google Scholar] [CrossRef]
- Inoue, T.; Ebina, H.; Dong, B.; Zheng, L. Electrical Conductivity Study on Micelle Formation of Long-Chain Imidazolium Ionic Liquids in Aqueous Solution. J. Colloid Interface Sci. 2007, 314, 236–241. [Google Scholar] [CrossRef]
- Baltazar, Q.Q.; Chandawalla, J.; Sawyer, K.; Anderson, J.L. Interfacial and Micellar Properties of Imidazolium-Based Monocationic and Dicationic Ionic Liquids. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 150–156. [Google Scholar] [CrossRef]
- Kanjilal, S.; Sunitha, S.; Reddy, P.S.; Kumar, K.P.; Murty, K.P.K.; Prasad, R.B.N. Synthesis and Evaluation of Micellar Properties and Antimicrobial Activities of Imidazole-Based Surfactants. Eur. J. Lipid Sci. Technol. 2009, 111, 941–948. [Google Scholar] [CrossRef]
- Gordon, C.M.; Holbrey, J.D.; Kennedy, A.R.; Seddon, K.R. Ionic Liquid Crystals: Hexafluorophosphate Salts. J. Mater. Chem. 1998, 8, 2627–2636. [Google Scholar] [CrossRef]
- Aupoix, A.; Pégot, B.; Vo-Thanh, G. Synthesis of Imidazolium and Pyridinium-Based Ionic Liquids and Application of 1-Alkyl-3-Methylimidazolium Salts as Pre-Catalysts for the Benzoin Condensation Using Solvent-Free and Microwave Activation. Tetrahedron 2010, 66, 1352–1356. [Google Scholar] [CrossRef]
- Cravotto, G.; Gaudino, E.C.; Boffa, L.; Lévêque, J.M.; Estager, J.; Bonrath, W. Preparation of Second Generation Ionic Liquids by Efficient Solvent-Free Alkylation of N-Heterocycles with Chloroalkanes. Molecules 2008, 13, 149–156. [Google Scholar] [CrossRef]
- Deng, Y.; Morrissey, S.; Gathergood, N.; Delort, A.M.; Husson, P.; Gomes, M.F.C. The Presence of Functional Groups Key for Biodegradation in Ionic Liquids: Effect on Gas Solubility. ChemSusChem 2010, 3, 377–385. [Google Scholar] [CrossRef]
- Baker, G.A.; Pandey, S.; Pandey, S.; Baker, S.N. A New Class of Cationic Surfactants Inspired by N-Alkyl-N-Methyl Pyrrolidinium Ionic Liquids. Analyst 2004, 129, 890–892. [Google Scholar] [CrossRef]
- Lava, K.; Binnemans, K.; Cardinaels, T. Piperidinium, Piperazinium and Morpholinium Ionic Liquid Crystals. J. Phys. Chem. B 2009, 113, 9506–9511. [Google Scholar] [CrossRef]
- Song, Y.; Li, Q.; Li, Y. Self-Aggregation and Antimicrobial Activity of Alkylguanidium Salts. Colloids Surf. A: Physicochem. Eng. Asp. 2012, 393, 11–16. [Google Scholar] [CrossRef]
- Zhuang, L.H.; Yu, K.H.; Wang, G.W.; Yao, C. Synthesis and Properties of Novel Ester-Containing Gemini Imidazolium Surfactants. J. Colloid Interface Sci. 2013, 408, 94–100. [Google Scholar] [CrossRef]
- Bhadani, A.; Singh, S. Synthesis and Properties of Thioether Spacer Containing Gemini Imidazolium Surfactants. Langmuir 2011, 27, 14033–14044. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, H.; Zhao, M.; Zheng, L. Aggregation Behavior of Gemini Pyrrolidine-Based Ionic Liquids 1,1′-(Butane-1,4-Diyl)Bis(1-Alkylpyrrolidinium) Bromide ([C Npy-4-C Npy][Br 2]) in Aqueous Solution. J. Colloid Interface Sci. 2012, 372, 52–57. [Google Scholar] [CrossRef]
- Goodchild, I.; Collier, L.; Millar, S.L.; Prokeš, I.; Lord, J.C.D.; Butts, C.P.; Bowers, J.; Webster, J.R.P.; Heenan, R.K. Structural Studies of the Phase, Aggregation and Surface Behaviour of 1-Alkyl-3-Methylimidazolium Halide + Water Mixtures. J. Colloid Interface Sci. 2007, 307, 455–468. [Google Scholar] [CrossRef]
- Saien, J.; Asadabadi, S. Temperature Effect on Adsorption of Imidazolium-Based Ionic Liquids at Liquid-Liquid Interface. Colloids Surf. A Physicochem. Eng. Asp. 2013, 431, 34–41. [Google Scholar] [CrossRef]
- Vaghela, N.M.; Sastry, N.V.; Aswal, V.K. Surface Active and Aggregation Behavior of Methylimidazolium-Based Ionic Liquids of Type [Cnmim] [X], n = 4, 6, 8 and [X] = Cl-, Br-, and I- in Water. Colloid Polym. Sci. 2011, 289, 309–322. [Google Scholar] [CrossRef]
- Tourné-Péteilh, C.; Devoisselle, J.M.; Vioux, A.; Judeinstein, P.; In, M.; Viau, L. Surfactant Properties of Ionic Liquids Containing Short Alkyl Chain Imidazolium Cations and Ibuprofenate Anions. Phys. Chem. Chem. Phys. 2011, 13, 15523–15529. [Google Scholar] [CrossRef] [PubMed]
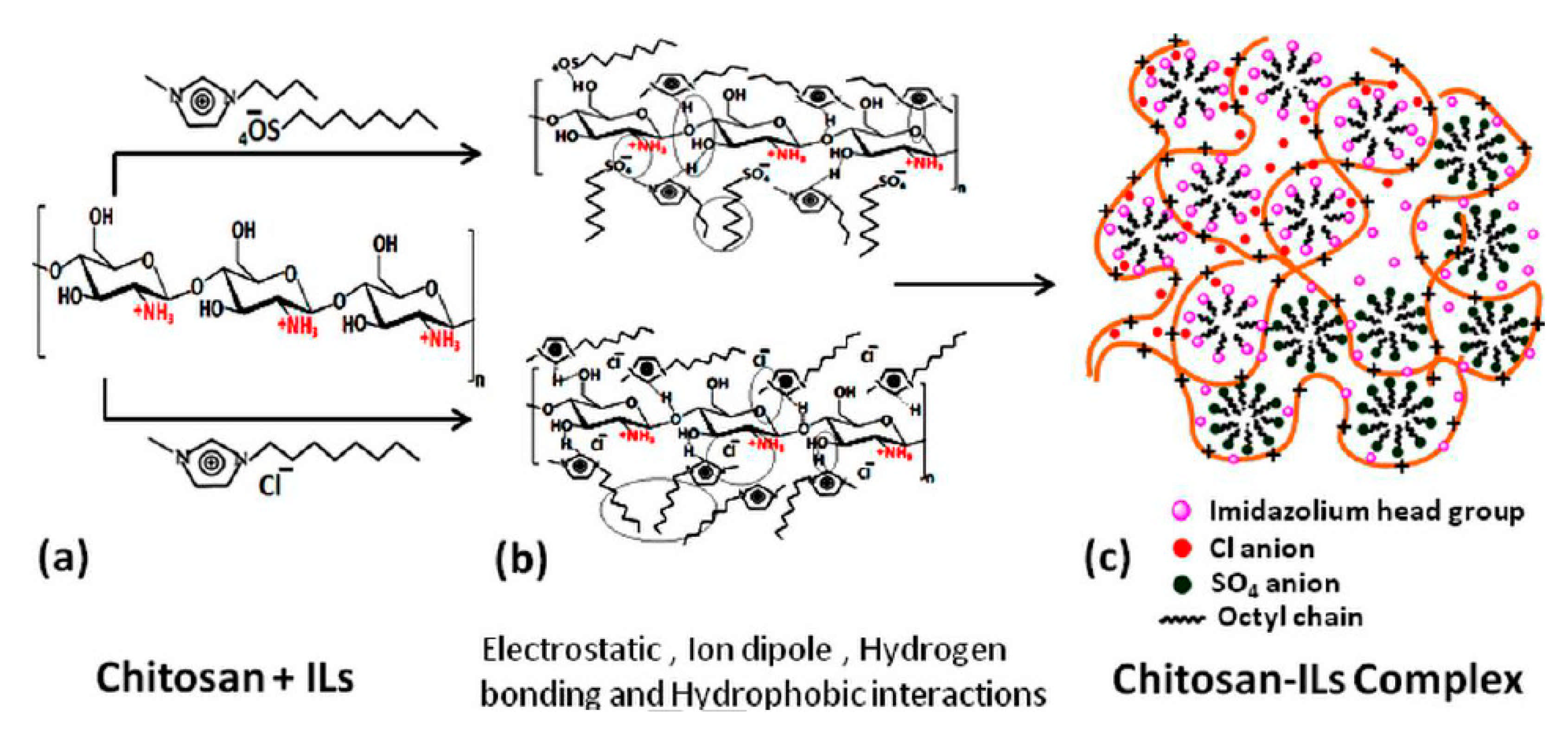
- Bharmoria, P.; Singh, T.; Kumar, A. Complexation of Chitosan with Surfactant like Ionic Liquids: Molecular Interactions and Preparation of Chitosan Nanoparticles. J. Colloid Interface Sci. 2013, 407, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Boral, S.; Bohidar, H.B.; Kumar, A. Interaction of Gelatin with Room-Temperature Ionic Liquids: A Detailed Physicochemical Study. J. Phys. Chem. B 2010, 114, 8441–8448. [Google Scholar] [CrossRef]
- Cornellas, A.; Perez, L.; Comelles, F.; Ribosa, I.; Manresa, A.; Garcia, T.T. Self-Aggregation and Antimicrobial Activity of Imidazolium and Pyridinium Based Ionic Liquids in Aqueous Solution. J. Colloid Interface Sci. 2011, 355, 164–171. [Google Scholar] [CrossRef]
- Aggarwal, R.; Singh, S. Synthesis, Characterization and Evaluation of Surface and Thermal Properties of 3-Cyclohexyloxy-2-Hydroxypropyl Pyridinium and Imidazolium Surface-Active Ionic Liquids. J. Surfactants Deterg. 2018, 21, 43–52. [Google Scholar] [CrossRef]
- Blesic, M.; Swadźba-Kwaśny, M.; Holbrey, J.D.; Canongia Lopes, J.N.; Seddon, K.R.; Rebelo, L.P.N. New Catanionic Surfactants Based on 1-Alkyl-3-Methylimidazolium Alkylsulfonates, [CnH2n+1mim][CmH 2m+1SO3]: Mesomorphism and Aggregation. Phys. Chem. Chem. Phys. 2009, 11, 4260–4268. [Google Scholar] [CrossRef]
- Sastry, N.V.; Vaghela, N.M.; Aswal, V.K. Effect of Alkyl Chain Length and Head Group on Surface Active and Aggregation Behavior of Ionic Liquids in Water. Fluid Phase Equilibria 2012, 327, 22–29. [Google Scholar] [CrossRef]
- Ali, A.; Farooq, U.; Uzair, S.; Patel, R. Conductometric and Tensiometric Studies on the Mixed Micellar Systems of Surface-Active Ionic Liquid and Cationic Surfactants in Aqueous Medium. J. Mol. Liq. 2016, 223, 589–602. [Google Scholar] [CrossRef]
- Farooq, U.; Ali, A.; Patel, R.; Malik, N.A. Self-Aggregation of Ionic Liquid-Cationic Surfactant Mixed Micelles in Water and in Diethylene Glycol–Water Mixtures: Conductometric, Tensiometric, and Spectroscopic Studies. J. Mol. Liq. 2017, 234, 452–462. [Google Scholar] [CrossRef]
- Farooq, U.; Patel, R.; Ali, A. Interaction of a Surface-Active Ionic Liquid with an Antidepressant Drug: Micellization and Spectroscopic Studies. J. Solut. Chem. 2018, 47, 568–585. [Google Scholar] [CrossRef]
- Sharma, R.; Kamal, A.; Kang, T.S.; Mahajan, R.K. Interactional Behavior of the Polyelectrolyte Poly Sodium 4-Styrene Sulphonate (NaPSS) with Imidazolium Based Surface Active Ionic Liquids in an Aqueous Medium. Phys. Chem. Chem. Phys. 2015, 17, 23582–23594. [Google Scholar] [CrossRef]
- Naderi, O.; Sadeghi, R. Effect of Temperature on the Aggregation Behaviour and Thermodynamic Properties of Surface Active Ionic Liquid 1-Decyl-3-Methylimidazolium Bromide in Aqueous Solutions: Surface Tension, Vapour Pressure Osmometery, Conductivity, Volumetric and Compressibil. J. Chem. Thermodyn. 2016, 102, 68–78. [Google Scholar] [CrossRef]
- Qin, L.; Wang, X.H. Surface Adsorption and Thermodynamic Properties of Mixed System of Ionic Liquid Surfactants with Cetyltrimethyl Ammonium Bromide. Rsc Adv. 2017, 7, 51426–51435. [Google Scholar] [CrossRef]
- Zamani, Z.; Naderi, O.; Sadeghi, R. Soluting-out Effect of Carbohydrates on the Surface Active Ionic Liquid 1-Decyl-3-Methylimidazolium Bromide in Aqueous Solutions. J. Chem. Thermodyn. 2018, 116, 289–298. [Google Scholar] [CrossRef]
- Wen, X.; Yan, Z.; Kang, Y.; Zhang, S. Apparent Molar Volume, Conductivity, and Fluorescence Studies of Ternary Systems of Dipeptides + Ionic Liquids ([Cnmim]Br, n = 10, 14) + Water at Different Temperatures. Colloid Polym. Sci. 2015, 293, 2485–2495. [Google Scholar] [CrossRef]
- Ao, M.; Kim, D. Aggregation Behavior of Aqueous Solutions of 1-Dodecyl-3-Methylimidazolium Salts with Different Halide Anions. J. Chem. Eng. Data 2013, 58, 1529–1534. [Google Scholar] [CrossRef]
- Pal, A.; Yadav, S. Thermodynamic and Surface Properties of Aqueous 1-Dodecyl-3-Methylimidazolium Chloride [C12mim][Cl] Solution in the Presence of a Series of Inorganic Salts. J. Surfactants Deterg. 2020, 23, 53–65. [Google Scholar] [CrossRef]
- Cognigni, A.; Gaertner, P.; Zirbs, R.; Peterlik, H.; Prochazka, K.; Schröder, C.; Bica, K. Surface-Active Ionic Liquids in Micellar Catalysis: Impact of Anion Selection on Reaction Rates in Nucleophilic Substitutions. Phys. Chem. Chem. Phys. 2016, 18, 13375–13384. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, M.; Kang, T.S.; Aswal, V.K. Aqueous Colloidal Systems of Bovine Serum Albumin and Functionalized Surface Active Ionic Liquids for Material Transport. RSC Adv. 2020, 10, 7073–7082. [Google Scholar] [CrossRef]
- De Freitas, D.V.; Kuhn, B.L.; Bender, C.R.; Furuyama Lima, A.M.; de Freitas Lima, M.; Tiera, M.J.; Kloster, C.L.; Frizzo, C.P.; Villetti, M.A. Thermodynamics of the Aggregation of Imidazolium Ionic Liquids with Sodium Alginate or Hydroxamic Alginate in Aqueous Solution. J. Mol. Liq. 2020, 297, 111734. [Google Scholar] [CrossRef]
- Liu, X.; Hu, J.; Huang, Y.; Fang, Y. Aggregation Behavior of Surface Active Dialkylimidazolium Ionic Liquids [C12C n Im]Br (n = 1-4) in Aqueous Solutions. J. Surfactants Deterg. 2013, 16, 539–546. [Google Scholar] [CrossRef]
- Gu, Y.; Shi, L.; Cheng, X.; Lu, F.; Zheng, L. Aggregation Behavior of 1-Dodecyl-3-Methylimidazolium Bromide in Aqueous Solution: Effect of Ionic Liquids with Aromatic Anions. Langmuir 2013, 29, 6213–6220. [Google Scholar] [CrossRef]
- Nazemi, T.; Sadeghi, R. Effect of Polar Organic Solvents on the Surface Adsorption and Micelle Formation of Surface Active Ionic Liquid 1-Dodecyl-3-Methylimidazolium Bromide in Aqueous Solutions and Comparison with the Traditional Cationic Surfactant Dodecyltrimethylammonium Bro. Colloids Surf. A Physicochem. Eng. Asp. 2014, 462, 271–279. [Google Scholar] [CrossRef]
- Geng, F.; Liu, J.; Zheng, L.; Yu, L.; Li, Z.; Li, G.; Tung, C. Micelle Formation of Long-Chain Imidazolium Ionic Liquids in Aqueous Solution Measured by Isothermal Titration Microcalorimetry. J. Chem. Eng. Data 2010, 55, 147–151. [Google Scholar] [CrossRef]
- Samarkina, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Khamatgalimov, A.R.; Zakharova, L.Y. Aggregation Capacity and Complexation Properties of a System Based on an Imidazole-Containing Amphiphile and Bovine Serum Albumin. Russ. J. Gen. Chem. 2017, 87, 2826–2831. [Google Scholar] [CrossRef]
- Blesic, M.; Lopes, A.; Melo, E.; Petrovski, Z.; Plechkova, N.V.; Canongia Lopes, J.N.; Seddon, K.R.; Rebelo, L.P.N. On the Self-Aggregation and Fluorescence Quenching Aptitude of Surfactant Ionic Liquids. J. Phys. Chem. B 2008, 112, 8645–8650. [Google Scholar] [CrossRef]
- Pal, A.; Yadav, A. Interactions Between Surface Active Ionic Liquid and Procaine Hydrochloride Drug in Aqueous Solution. J. Solut. Chem. 2018, 47, 1096–1111. [Google Scholar] [CrossRef]
- Sharma, R.; Mahajan, S.; Mahajan, R.K. Surface Adsorption and Mixed Micelle Formation of Surface Active Ionic Liquid in Cationic Surfactants: Conductivity, Surface Tension, Fluorescence and NMR Studies. Colloids Surf. A Physicochem. Eng. Asp. 2013, 427, 62–75. [Google Scholar] [CrossRef]
- Sintra, T.E.; Vilas, M.; Martins, M.; Ventura, S.P.M.; Lobo Ferreira, A.I.M.C.; Santos, L.M.N.B.F.; Gonçalves, F.J.M.; Tojo, E.; Coutinho, J.A.P. Synthesis and Characterization of Surface-Active Ionic Liquids Used in the Disruption of Escherichia Coli Cells. ChemPhysChem 2019, 20, 727–735. [Google Scholar] [CrossRef]
- Dong, B.; Zhao, X.; Zheng, L.; Zhang, J.; Li, N.; Inoue, T. Aggregation Behavior of Long-Chain Imidazolium Ionic Liquids in Aqueous Solution: Micellization and Characterization of Micelle Microenvironment. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 666–672. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, S.; Das, B. Formation of Mixed Micelle in an Aqueous Mixture of a Surface Active Ionic Liquid and a Conventional Surfactant: Experiment and Modeling. J. Chem. Eng. Data 2018, 63, 3784–3800. [Google Scholar] [CrossRef]
- Keppeler, N.; Galgano, P.D.; Santos, S.d.S.; Malek, N.I.; El Seoud, O.A. On the Effects of Head Group Volume on the Adsorption and Aggregation of 1-(n-Hexadecyl)-3-Cm-Imidazolium Bromide and Chloride Surfactants in Aqueous Solutions. J. Mol. Liq. 2021, 328, 115478. [Google Scholar] [CrossRef]
- Du, M.; Dai, C.; Chen, A.; Wu, X.; Li, Y.; Liu, Y.; Li, W.; Zhao, M. Investigation on the Aggregation Behavior of Photo-Responsive System Composed of 1-Hexadecyl-3-Methylimidazolium Bromide and 2-Methoxycinnamic Acid. RSC Advances 2015, 5, 68369–68377. [Google Scholar] [CrossRef]
- Malek, N.I.; Vaid, Z.S.; More, U.U.; El Seoud, O.A. Ionic-Liquid-Based Surfactants with Unsaturated Head Group: Synthesis and Micellar Properties of 1-(n-Alkyl)-3-Vinylimidazolium Bromides. Colloid Polym. Sci. 2015, 293, 3213–3224. [Google Scholar] [CrossRef]
- Chabba, S.; Vashishat, R.; Kang, T.S.; Mahajan, R.K. Self–Aggregation Behavior of Dialkyl Imidazolium Based Ionic Liquids in Aqueous Medium: Effect of Alkyl Chain Length. ChemistrySelect 2016, 1, 2458–2470. [Google Scholar] [CrossRef]
- Ge, L.; Wang, Q.; Wei, D.; Zhang, X.; Guo, R. Aggregation of Double-Tailed Ionic Liquid 1,3-Dioctylimidazolium Bromide and the Interaction with Triblock Copolymer F127. J. Phys. Chem. B 2013, 117, 15014–15022. [Google Scholar] [CrossRef]
- Bou Malham, I.; Letellier, P.; Turmine, M. Synthesis and Micellar Properties of 1-Decyl-2,3-Dimethylimidazolium Bromide Surfactant in Water and Water-Ethanolamine Mixtures at 298.15 K. J. Colloid Interface Sci. 2008, 328, 166–171. [Google Scholar] [CrossRef]
- Pal, A.; Yadav, A. Investigations of Drug Binding Ability of a Trisubstituted Surface Active Ionic Liquid 1-Dodecyl-2,3-Dimethylimidazolium Chloride [C12bmim][Cl]. J. Mol. Liq. 2018, 251, 167–177. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, S.; Aswal, V.K.; Mahajan, R.K. Influence of Headgroup on the Aggregation and Interactional Behavior of Twin-Tailed Cationic Surfactants with Pluronics. Langmuir 2013, 29, 11821–11833. [Google Scholar] [CrossRef]
- Sastry, N.V.; Vaghela, N.M.; Macwan, P.M.; Soni, S.S.; Aswal, V.K.; Gibaud, A. Aggregation Behavior of Pyridinium Based Ionic Liquids in Water—Surface Tension, 1H NMR Chemical Shifts, SANS and SAXS Measurements. J. Colloid Interface Sci. 2012, 371, 52–61. [Google Scholar] [CrossRef]
- Korotkikh, O.P.; Kochurova, N.N. Temperature Effects on the Aggregation of Decylpyridinium Chloride in Aqueous Solution. Russ. J. Phys. Chem. A 2007, 81, 1059–1061. [Google Scholar] [CrossRef]
- Malovikova, A.; Hayakawa, K.; Kwak, J.C.T. Surfactant-Polyelectrolyte Interactions. 4. Surfactant Chain Length Dependence of the Binding of Alkylpyridinium Cations to Dextran Sulfate. J. Phys. Chem. 1984, 88, 1930–1933. [Google Scholar] [CrossRef]
- Korotkikh, O.P.; Kochurova, N.N.; Hong, P. da Aggregation in the Aqueous Solutions of Alkylpyridinium Chlorides. Mendeleev Commun. 2008, 18, 347–349. [Google Scholar] [CrossRef]
- Banjare, R.K.; Banjare, M.K.; Panda, S. Effect of Acetonitrile on the Colloidal Behavior of Conventional Cationic Surfactants: A Combined Conductivity, Surface Tension, Fluorescence and FTIR Study. J. Solut. Chem. 2020, 49, 34–51. [Google Scholar] [CrossRef]
- Fu, D.; Gao, X.; Huang, B.; Wang, J.; Sun, Y.; Zhang, W.; Kan, K.; Zhang, X.; Xie, Y.; Sui, X. Micellization, Surface Activities and Thermodynamics Study of Pyridinium-Based Ionic Liquid Surfactants in Aqueous Solution. RSC Adv. 2019, 9, 28799–28807. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, X.; Dong, J.; Zhang, G.; Wang, C. A Novel Family of Green Ionic Liquids with Surface Activities. Sci. ChinaSer. B Chem. 2007, 50, 238–242. [Google Scholar] [CrossRef]
- Singh, D.K.; Sastry, N.V.; Trivedi, P.A. Amphiphilic Copolymers and Surface Active Ionic Liquid Systems in Aqueous Media—Surface Active and Aggregation Characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2017, 524, 111–126. [Google Scholar] [CrossRef]
- Tariq, M.; Podgoršek, A.; Ferguson, J.L.; Lopes, A.; Costa Gomes, M.F.; Pádua, A.A.H.; Rebelo, L.P.N.; Canongia Lopes, J.N. Characteristics of Aggregation in Aqueous Solutions of Dialkylpyrrolidinium Bromides. J. Colloid Interface Sci. 2011, 360, 606–616. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, M.; Zheng, L. Micelle Formation by N-Alkyl-N-Methylpyrrolidinium Bromide in Ethylammonium Nitrate. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 305–312. [Google Scholar] [CrossRef]
- Lukáč, M.; Pisárčik, M.; Lacko, I.; Devínsky, F. Surface-Active Properties of Nitrogen Heterocyclic and Dialkylamino Derivates of Hexadecylphosphocholine and Cetyltrimethylammonium Bromide. J. Colloid Interface Sci. 2010, 347, 233–240. [Google Scholar] [CrossRef]
- Sastry, N.V.; Singh, D.K. Surfactant and Gelation Properties of Acetylsalicylate Based Room Temperature Ionic Liquid in Aqueous Media. Langmuir 2016, 32, 10000–10016. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, X.; Wang, X.; Huang, D.; Chen, X. Micelle Formation by N-Alkyl-N-Methylpiperidinium Bromide Ionic Liquids in Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2012, 412, 90–95. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Lukashenko, S.S.; Yatskevich, E.I.; Kulik, N.V.; Voloshina, A.D.; Kudryavtsev, D.B.; Panteleeva, A.R.; Zobov, V.V.; Zakharova, L.Y.; Konovalov, A.I. Aggregation Behavior, Anticorrosion Effect, and Antimicrobial Activity of Alkylmethylmorpholinium Bromides. Prot. Met. Phys. Chem. Surf. 2014, 50, 538–542. [Google Scholar] [CrossRef]
- Sharma, R.; Mahajan, S.; Mahajan, R.K. Physicochemical Studies of Morpholinium Based Ionic Liquid Crystals and Their Interaction with Cyclodextrins. Fluid Phase Equilibria 2014, 361, 104–115. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Valeeva, F.G.; Zakharov, S.V.; Kuryashov, D.A.; Bashkirtseva, N.Y.; Zakharova, L.Y. Aggregation Behavior of Morpholinium Surfactants in the Presence of Organic Electrolytes. Russ. Chem. Bull. 2018, 67, 291–296. [Google Scholar] [CrossRef]
- Miyake, M.; Oyama, N. Effect of Amidoalkyl Group as Spacer on Aggregation Properties of Guanidine-Type Surfactants. J. Colloid Interface Sci. 2009, 330, 180–185. [Google Scholar] [CrossRef]
- Gainanova, G.A.; Vagapova, G.I.; Syakaev, V.V.; Ibragimova, A.R.; Valeeva, F.G.; Tudriy, E.V.; Galkina, I.V.; Kataeva, O.N.; Zakharova, L.Y.; Latypov, S.K.; et al. Self-Assembling Systems Based on Amphiphilic Alkyltriphenylphosphonium Bromides: Elucidation of the Role of Head Group. J. Colloid Interface Sci. 2012, 367, 327–336. [Google Scholar] [CrossRef]
- Ibragimova, A.R.; Arkhipova, D.M.; Vagapova, G.I.; Ermolaev, V.V.; Galkina, I.V.; Nigmatullina, L.S.; Rizvanov, I.K.; Zakharova, L.Y.; Milyukov, V.A.; Konovalov, A.I.; et al. Influence of the Medium Selforganization on the Catalytic Activity of Palladium Nanoparticles Stabilized by Amphiphilic Phosphonium Salts in the Suzuki Reaction. Russ. Chem. Bull. 2014, 63, 1297–1300. [Google Scholar] [CrossRef]
- Thakkar, K.; Bharatiya, B.; Shah, D.O.; Ray, D.; Aswal, V.K.; Bahadur, P. Interaction of Ionic Liquid Type Cationic Surfactants with Triton X-100 Nonionic Micelles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 547–557. [Google Scholar] [CrossRef]
- Basu Ray, G.; Ghosh, S.; Moulik, S.P. Ternary Mixtures of Alkyltriphenylphosphonium Bromides (C12TPB, C14TPB and C16TPB) in Aqueous Medium: Their Interfacial, Bulk and Fluorescence Quenching Behaviour. J. Chem. Sci. 2010, 122, 109–117. [Google Scholar] [CrossRef]
- Lu, F.; Shi, L.; Yan, H.; Yang, X.; Zheng, L. Aggregation Behavior of Dodecyltriphenylphosphonium Bromide in Aqueous Solution: Effect of Aromatic Ionic Liquids. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 203–211. [Google Scholar] [CrossRef]
- Verma, S.K.; Ghosh, K.K. Micellar and Surface Properties of Some Monomeric Surfactants and a Gemini Cationic Surfactant. J. Surfactants Deterg. 2011, 14, 347–352. [Google Scholar] [CrossRef]
- Mata, J.; Varade, D.; Bahadur, P. Aggregation Behavior of Quaternary Salt Based Cationic Surfactants. Thermochim. Acta 2005, 428, 147–155. [Google Scholar] [CrossRef]
- Sehgal, P.; Kosaka, O.; Doe, H. Interfacial and Aggregation Properties of the Binary Mixture of Decanoyl-N-Methyl-Glucamide and Hexadecyltriphenylphosphonium Bromide. Colloid Polym. Sci. 2008, 286, 275–282. [Google Scholar] [CrossRef]
- Verma, S.K.; Ghosh, K.K.; Verma, R.; Xiang, W.; Li, N.; Zhao, X. Surface, Conformational and Catalytic Activity Approach of α-Chymotrypsin and Trypsin in Micellar Media. Colloids Surf. A Physicochem. Eng. Asp. 2015, 470, 188–193. [Google Scholar] [CrossRef]
- Gaynanova, G.A.; Valeeva, F.G.; Kushnazarova, R.A.; Bekmukhametova, A.M.; Zakharov, S.V.; Mirgorodskaya, A.B.; Zakharova, L.Y. Effect of Hydrotropic Compounds on the Self-Organization and Solubilization Properties of Cationic Surfactants. Russ. J. Phys. Chem. A 2018, 92, 1400–1405. [Google Scholar] [CrossRef]
- Chakraborty, A.; Saha, S.K.; Chakraborty, S. Effect of Size of Tetraalkylammonium Counterions on the Temperature Dependent Micellization of AOT in Aqueous Medium. Colloid Polym. Sci. 2008, 286, 927–934. [Google Scholar] [CrossRef]
- Brown, P.; Butts, C.; Dyer, R.; Eastoe, J.; Grillo, I.; Guittard, F.; Rogers, S.; Heenan, R. Anionic Surfactants and Surfactant Ionic Liquids with Quaternary Ammonium Counterions. Langmuir 2011, 27, 4563–4571. [Google Scholar] [CrossRef]
- Yu, Z.J.; Zhang, X.; Xu, G.; Zhao, G.X. Physicochemical Properties of Aqueous Mixtures of Tetrabutylammonium Bromide and Anionic Surfactants. 3. Effects of Surfactant Chain Length and Salinity. J. Phys. Chem. 1990, 94, 3675–3681. [Google Scholar] [CrossRef]
- Yu, Z.J.; Xu, G. Physicochemical Properties of Aqueous Mixtures of Tetrabutylammonium Bromide and Anionic Surfactants. 1. Temperature-Induced Micellar Growth and Cloud Point Phenomenon. J. Phys. Chem. 1989, 93, 7441–7445. [Google Scholar] [CrossRef]
- Rao, K.S.; Gehlot, P.S.; Gupta, H.; Drechsler, M.; Kumar, A. Sodium Bromide Induced Micelle to Vesicle Transitions of Newly Synthesized Anionic Surface Active Ionic Liquids Based on Dodecylbenzenesulfonate. J. Phys. Chem. B 2015, 119, 4263–4274. [Google Scholar] [CrossRef]
- Jiao, J.; Dong, B.; Zhang, H.; Zhao, Y.; Wang, X.; Wang, R.; Yu, L. Aggregation Behaviors of Dodecyl Sulfate-Based Anionic Surface Active Ionic Liquids in Water. J. Phys. Chem. B 2012, 116, 958–965. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, L.; Wang, T.; Chen, P.; Bi, Y.; Yu, L. Aggregation Behavior of Dodecylsulfonate-Based Surface Active Ionic Liquids in Water. J. Mol. Liq. 2015, 212, 23–29. [Google Scholar] [CrossRef]
- Rao, K.S.; Trivedi, T.J.; Kumar, A. Aqueous-Biamphiphilic Ionic Liquid Systems: Self-Assembly and Synthesis of Gold Nanocrystals/Microplates. J. Phys. Chem. B 2012, 116, 14363–14374. [Google Scholar] [CrossRef]
- Singh, T.; Rao, K.S.; Kumar, A. Effect of Ethylene Glycol and Its Derivatives on the Aggregation Behavior of an Ionic Liquid 1-Butyl-3-Methyl Imidazolium Octylsulfate in Aqueous Medium. J. Phys. Chem. B 2012, 116, 1612–1622. [Google Scholar] [CrossRef]
- Comelles, F.; Ribosa, I.; González, J.J.; Garcia, M.T. Micellization of Sodium Laurylethoxysulfate (SLES) and Short Chain Imidazolium Ionic Liquids in Aqueous Solution. J. Colloid Interface Sci. 2014, 425, 44–51. [Google Scholar] [CrossRef]
- Pal, A.; Yadav, A. Modulations in the Aggregation Behavior of Ionic Liquid 1-Butyl-3-Methylimidazolium Octylsulfate in Aqueous Alcohol Solutions. J. Mol. Liq. 2015, 212, 569–575. [Google Scholar] [CrossRef]
- Thakkar, K.; Bharatiya, B.; Aswal, V.K.; Bahadur, P. Aggregation of 1-Alkyl-3-Methylimidazolium Octylsulphate Ionic Liquids and Their Interaction with Triton X-100 Micelles. RSC Adv. 2016, 6, 80585–80594. [Google Scholar] [CrossRef]
- Banjare, M.K.; Behera, K.; Satnami, M.L.; Pandey, S.; Ghosh, K.K. Supra-Molecular Inclusion Complexation of Ionic Liquid 1-Butyl-3-Methylimidazolium Octylsulphate with Α- and Β-Cyclodextrins. Chem. Phys. Lett. 2017, 689, 30–40. [Google Scholar] [CrossRef]
- Jiao, J.; Han, B.; Lin, M.; Cheng, N.; Yu, L.; Liu, M. Salt-Free Catanionic Surface Active Ionic Liquids 1-Alkyl-3-Methylimidazolium Alkylsulfate: Aggregation Behavior in Aqueous Solution. J. Colloid Interface Sci. 2013, 412, 24–30. [Google Scholar] [CrossRef]
- Brown, P.; Butts, C.P.; Eastoe, J.; Fermin, D.; Grillo, I.; Lee, H.C.; Parker, D.; Plana, D.; Richardson, R.M. Anionic Surfactant Ionic Liquids with 1-Butyl-3-Methyl-Imidazolium Cations: Characterization and Application. Langmuir 2012, 28, 2502–2509. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, W.; Sun, L.; Yu, L.; Jiao, J.; Wang, R. Facile Synthesis of Calcium Carbonate with an Absolutely Pure Crystal Form Using 1-Butyl-3-Methylimidazolium Dodecyl Sulfate as the Modifier. Colloid Polym. Sci. 2013, 291, 2191–2202. [Google Scholar] [CrossRef]
- Pal, A.; Yadav, S. Effect of Cationic Polyelectrolyte Poly(Diallyldimethylammonium Chloride) on Micellization Behavior of Anionic Surface Active Ionic Liquid 1-Butyl-3-Methylimidazolium Dodecylsulfate [C4mim][C12SO4] in Aqueous Solutions. Colloid Polym. Sci. 2018, 296, 1635–1650. [Google Scholar] [CrossRef]
- Pal, A.; Punia, R. Self-Aggregation Behaviour of Cationic Surfactant Tetradecyltrimethylammonium Bromide and Bi-Amphiphilic Surface Active Ionic Liquid 3-Methyl-1-Pentylimidazolium Dodecylsulfate in Aqueous Solution. J. Mol. Liq. 2020, 304, 112803. [Google Scholar] [CrossRef]
- Pal, A.; Saini, M. Effect of Alkyl Chain on Micellization Properties of Dodecylbenzenesulfonate Based Surface Active Ionic Liquids Using Conductance, Surface Tension, and Spectroscopic Techniques. J. Dispers. Sci. Technol. 2020, 41, 547–556. [Google Scholar] [CrossRef]
- Silvas, B.F.B.; Marques, E.F.; Olsson, U.; Pons, R. Headgroup Effects on the Unusual Lamellar-Lamellar Coexistence and Vesicle-to-Micelle Transition of Salt-Free Catanionic Amphiphiles. Langmuir 2010, 26, 3058–3066. [Google Scholar] [CrossRef]
- Galgano, P.D.; El Seoud, O.A. Ionic liquid-based surfactants: Synthesis, molecular structure, micellar properties and applications. In Ionic Liquid-Based Surfactant Science: Formulation, Characterization, and Applications; Paul, B.K., Moulik, S.P., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; pp. 63–99. ISBN 9781118854501. [Google Scholar]
- Evans, H.C. Alkyl Sulphates. Part I. Critical Micelle Concentration of the Sodium Salts. J. Chem. Soc. 1956, 117, 579–586. [Google Scholar] [CrossRef]
- Shimizu, S.; Pires, P.A.R.; El Seoud, O.A. Thermodynamics of Micellization of Benzyl (2-Acylaminoethyl) Dimethylammonium Chloride Surfactants in Aqueous Solutions: A Conductivity and Titration Calorimetry Study. Langmuir 2004, 20, 9551–9559. [Google Scholar] [CrossRef]
- Chatterjee, A.; Moulik, S.P.; Sanyal, S.K.; Mishra, B.K.; Puri, P.M. Thermodynamics of Micelle Formation of Ionic Surfactants: A Critical Assessment for Sodium Dodecyl Sulfate, Cetyl Pyridinium Chloride and Dioctyl Sulfosuccinate (Na Salt) by Microcalorimetric, Conductometric, and Tensiometric Measurements. J. Phys. Chem. B 2001, 105, 12823–12831. [Google Scholar] [CrossRef]
- Schnee, V.P.; Palmer, C.P. Cationic Surfactants for Micellar Electrokinetic Chromatography: 1. Characterization of Selectivity Using the Linear Solvation Energy Relationships Model. Electrophoresis 2008, 29, 767–776. [Google Scholar] [CrossRef]
- Engberts, J.B.F.N.; Nusselder, J.J.H. The Effect of Chain Packing on Surfactant Aggregation in Aqueous Solution. Pure Appl. Chem. 1990, 62, 47–55. [Google Scholar] [CrossRef][Green Version]
- Benrraou, M.; Bales, B.L.; Zana, R. Effect of the Nature of the Counterion on the Properties of Anionic Surfactants. 1. Cmc, Ionization Degree at the Cmc and Aggregation Number of Micelles of Sodium, Cesium, Tetramethylammonium, Tetraethylammonium, Tetrapropylammonium, and Tetrabutylammoniu. J. Phys. Chem. B 2003, 107, 13432–13440. [Google Scholar] [CrossRef]
- Guàrdia, E.; Skarmoutsos, I.; Masia, M. On Ion and Molecular Polarization of Halides in Water. J. Chem. Theory Comput. 2009, 5, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. Ionic Radii in Aqueous Solutions. Chem. Rev. 1988, 88, 1475–1498. [Google Scholar] [CrossRef]
- Bijma, K.; Engberts, J.B.F.N. Effect of Counterions on Properties of Micelles Formed by Alkylpyridinium Surfactants. 1. Conductometry and 1H-NMR Chemical Shifts. Langmuir 1997, 13, 4843–4849. [Google Scholar] [CrossRef]
- Ao, M.; Huang, P.; Xu, G.; Yang, X.; Wang, Y. Aggregation and Thermodynamic Properties of Ionic Liquid-Type Gemini Imidazolium Surfactants with Different Spacer Length. Colloid Polym. Sci. 2009, 287, 395–402. [Google Scholar] [CrossRef]
- Pal, A.; Datta, S.; Aswal, V.K.; Bhattacharya, S. Small-Angle Neutron-Scattering Studies of Mixed Micellar Structures Made of Dimeric Surfactants Having Imidazolium and Ammonium Headgroups. J. Phys. Chem. B 2012, 116, 13239–13247. [Google Scholar] [CrossRef]
- Maurya, J.K.; Khan, A.B.; Dohare, N.; Ali, A.; Kumar, A.; Patel, R. Effect of Aromatic Amino Acids on the Surface Properties of 1-Dodecyl-3-(4-(3-Dodecylimidazolidin-1-Yl)Butyl)Imidazolidine Bromide Gemini Surfactant. J. Dispers. Sci. Technol. 2018, 39, 174–180. [Google Scholar] [CrossRef]
- Kamboj, R.; Singh, S.; Bhadani, A.; Kataria, H.; Kaur, G. Gemini Imidazolium Surfactants: Synthesis and Their Biophysiochemical Study. Langmuir 2012, 28, 11969–11978. [Google Scholar] [CrossRef]
- Tawfik, S.M. Simple One Step Synthesis of Gemini Cationic Surfactant-Based Ionic Liquids: Physicochemical, Surface Properties and Biological Activity. J. Mol. Liq. 2015, 209, 320–326. [Google Scholar] [CrossRef]
- Li, H.; Yu, C.; Chen, R.; Li, J.; Li, J. Novel Ionic Liquid-Type Gemini Surfactants: Synthesis, Surface Property and Antimicrobial Activity. Colloids Surf. A Physicochem. Eng. Asp. 2012, 395, 116–124. [Google Scholar] [CrossRef]
- Lu, F.; Zhang, S.; Zheng, L. Dispersion of Multi-Walled Carbon Nanotubes (MWCNTs) by Ionic Liquid-Based Phosphonium Surfactants in Aqueous Solution. J. Mol. Liq. 2012, 173, 42–46. [Google Scholar] [CrossRef]
- Nacham, O.; Martín-Pérez, A.; Steyer, D.J.; Trujillo-Rodríguez, M.J.; Anderson, J.L.; Pino, V.; Afonso, A.M. Interfacial and Aggregation Behavior of Dicationic and Tricationic Ionic Liquid-Based Surfactants in Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 224–234. [Google Scholar] [CrossRef]
- In, M.; Zana, R. Phase Behavior of Gemini Surfactants. J. Dispers. Sci. Technol. 2007, 28, 143–154. [Google Scholar] [CrossRef]
- Welton, T. Ionic Liquids in Catalysis. Coord. Chem. Rev. 2004, 248, 2459–2477. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of Ionic Liquids in the Chemical Industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Weingärtner, H. Understanding Ionic Liquids at the Molecular Level: Facts, Problems, and Controversies. Angew. Chem. Int. Ed. 2008, 47, 654–670. [Google Scholar] [CrossRef]
- Sawant, A.D.; Raut, D.G.; Darvatkar, N.B.; Salunkhe, M.M. Recent Developments of Task-Specific Ionic Liquids in Organic Synthesis. Green Chem. Lett. Rev. 2011, 4, 41–54. [Google Scholar] [CrossRef]
- Dupont, J.; Itoh, T.; Lozano, P.; Malhotra, S.V. (Eds.) Environmentally Friendly Syntheses Using Ionic Liquids; CRC Press: New York, NY, USA, 2015. [Google Scholar]
- Mohammad, A.I. (Ed.) Green Solvents II: Properties and Applications of Ionic Liquids; Springer Verlag: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Kuddushi, M.; Patel, N.K.; Gawali, S.L.; Mata, J.P.; Montes-Campos, H.; Varela, L.M.; Hassan, P.A.; Malek, N.I. Thermo-Switchable de Novo Ionogel as Metal Absorbing and Curcumin Loaded Smart Bandage Material. J. Mol. Liq. 2020, 306, 112922. [Google Scholar] [CrossRef]
- Kuddushi, M.; Patel, N.K.; Rajput, S.; El Seoud, O.A.; Mata, J.P.; Malek, N.I. Temperature-Responsive Low Molecular Weight Ionic Liquid Based Gelator: An Approach to Fabricate an Anti-Cancer Drug-Loaded Hybrid Ionogel. ChemSystemsChem 2020, 2, 1–13. [Google Scholar] [CrossRef]
- Rajput, S.M.; Mondal, K.; Kuddushi, M.; Jain, M.; Ray, D.; Aswal, V.K.; Malek, N.I. Formation of Hydrotropic Drug/Gemini Surfactant Based Catanionic Vesicles as Efficient Nano Drug Delivery Vehicles. Colloids Interface Sci. Commun. 2020, 37, 100273. [Google Scholar] [CrossRef]
- Kulshrestha, A.; Gehlot, P.S.; Kumar, A. Magnetic Proline-Based Ionic Liquid Surfactant as a Nano-Carrier for Hydrophobic Drug Delivery. J. Mater. Chem. B 2020, 8, 3050–3057. [Google Scholar] [CrossRef]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic Liquids as a Potential Tool for Drug Delivery Systems. MedChemComm 2016, 7, 1881–1897. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Barber, P.S.; Rogers, R.D. Ionic Liquids: Novel Applications in Drug Delivery. Expert Opin. Drug Deliv. 2013, 10, 1367–1381. [Google Scholar] [CrossRef]
- Kuddushi, M.; Ray, D.; Aswal, V.; Hoskins, C.; Malek, N. Poly(Vinyl Alcohol) and Functionalized Ionic Liquid-Based Smart Hydrogels for Doxorubicin Release. Acs Appl. Bio Mater. 2020, 3, 4883–4894. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic Liquid-Mediated Formation of 5-Hydroxymethylfurfural-A Promising Biomass-Derived Building Block. Chem. Rev. 2011, 111, 397–417. [Google Scholar] [CrossRef]
- Passos, H.; Freire, M.G.; Coutinho, J.A.P. Ionic Liquids Solutions as Extractive Solvents of Value-Added Compounds from Biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic Liquid Processing of Cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic Liquids and Their Interaction with Cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef]
- Amarasekara, A.S. Acidic Ionic Liquids. Chem. Rev. 2016, 116, 6133–6183. [Google Scholar] [CrossRef]
- Mai, N.L.; Kim, C.K.; Park, B.; Park, H.J.; Lee, S.H.; Koo, Y.M. Prediction of Cellulose Dissolution in Ionic Liquids Using Molecular Descriptors Based QSAR Model. J. Mol. Liq. 2016, 215, 541–548. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Kuroda, K. Rational Design of Mesoporous Metals and Related Nanomaterials by a Soft-Template Approach. Chem. Asian J. 2008, 3, 664–676. [Google Scholar] [CrossRef]
- Tian, T.; Hu, Q.; Wang, Y.; Gao, Y.; Yu, L. Effect of Imidazolium-Based Surface-Active Ionic Liquids on the Orientation of Liquid Crystals at Various Fluid/Liquid Crystal Interfaces. Langmuir 2016, 32, 11745–11753. [Google Scholar] [CrossRef] [PubMed]
- Goossens, K.; Lava, K.; Bielawski, C.W.; Binnemans, K. Ionic Liquid Crystals: Versatile Materials. Chem. Rev. 2016, 116, 4643–4807. [Google Scholar] [CrossRef] [PubMed]
- Kuddushi, M.; Mata, J.; Malek, N. Self-Sustainable, Self-Healable, Load Bearable and Moldable Stimuli Responsive Ionogel for the Selective Removal of Anionic Dyes from Aqueous Medium. J. Mol. Liq. 2020, 298, 112048. [Google Scholar] [CrossRef]
- Shah, A.; Kuddushi, M.; Ray, D.; Aswal, V.K.; Malek, N.I. Sodium Salicylate Mediated Ionic Liquid Based Catanionic Coacervates as Membrane-Free Microreactors for the Selective Sequestration of Dyes and Curcumin. ChemSystemsChem 2020, 2. [Google Scholar] [CrossRef]
- Kuddushi, M.; Rajput, S.; Shah, A.; Mata, J.; Aswal, V.K.; El Seoud, O.A.; Kumar, A.; Malek, N.I. Stimuli Responsive, Self-Sustainable, and Self-Healable Functionalized Hydrogel with Dual Gelation, Load-Bearing, and Dye-Absorbing Properties. ACS Appl. Mater. Interfaces 2019, 11, 19572–19583. [Google Scholar] [CrossRef]
- Shah, A.; Kuddushi, M.; Rajput, S.; El Seoud, O.A.; Malek, N.I. Ionic Liquid-Based Catanionic Coacervates: Novel Microreactors for Membrane-Free Sequestration of Dyes and Curcumin. ACS Omega 2018, 3, 17751–17761. [Google Scholar] [CrossRef]
- Shah, A.; Jain, M.; Lad, V.N.; Ray, D.; Aswal, V.K.; Malek, N.I. Selective Accumulation of Dyes and Curcumin in a Macroscopic Complex Coacervates Composed of Morpholinium Based Ester Functionalized Ionic Liquid and Sodium Salicylate. J. Mol. Liq. 2020, 317. [Google Scholar] [CrossRef]
- Qi, L.; Gong, Y.; Fang, M.; Jia, Z.; Cheng, N.; Yu, L. Surface-Active Ionic-Liquid-Encapsulated Polyoxometalate Nanospheres: Construction, Self-Assembly, Adsorption Behavior, and Application for Dye Removal. ACS Appl. Nano Mater. 2020, 3, 375–383. [Google Scholar] [CrossRef]
- Cheng, N.; Hu, Q.; Guo, Y.; Wang, Y.; Yu, L. Efficient and Selective Removal of Dyes Using Imidazolium-Based Supramolecular Gels. ACS Appl. Mater. Interfaces 2015, 7, 10258–10265. [Google Scholar] [CrossRef]
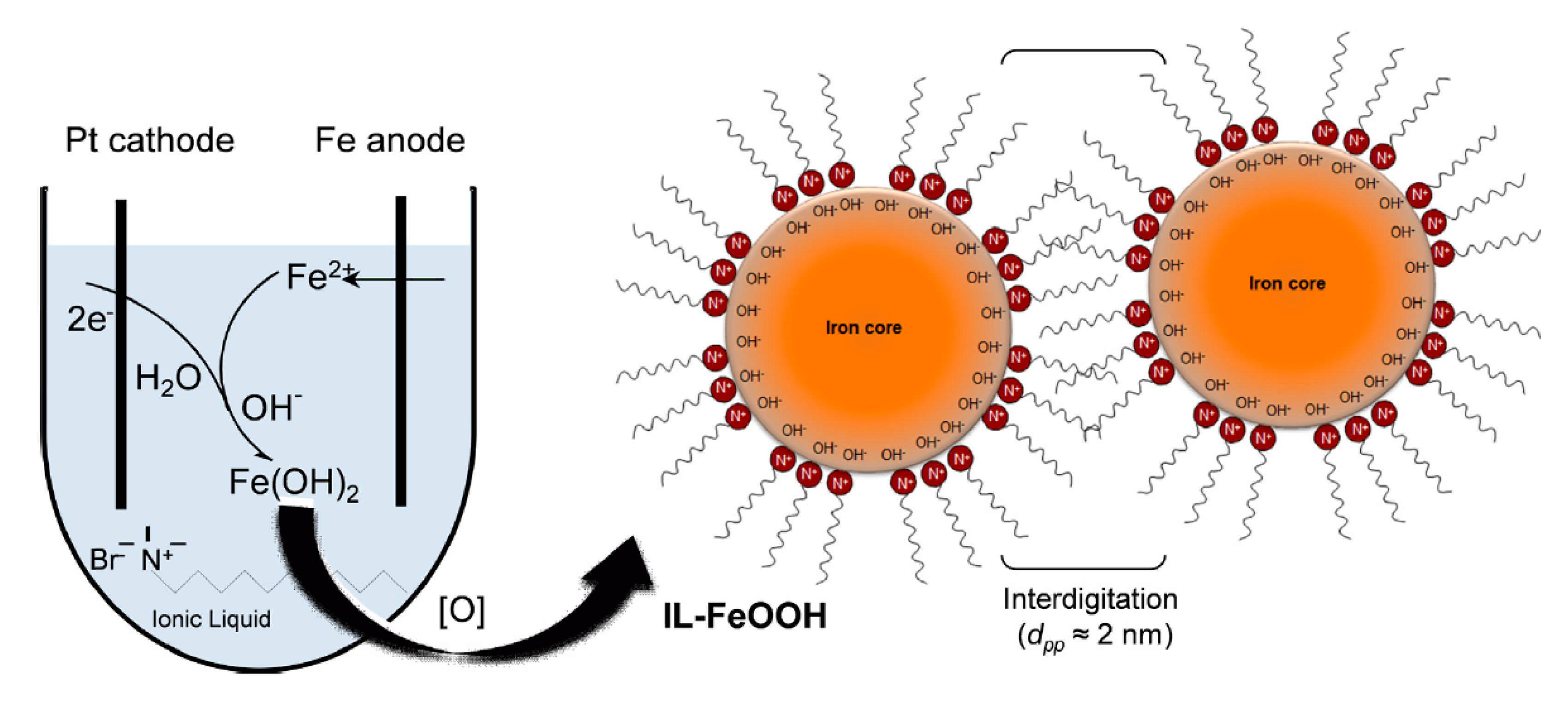
- Jusoh, R.; Jalil, A.A.; Triwahyono, S.; Idris, A.; Haron, S.; Sapawe, N.; Jaafar, N.F.; Jusoh, N.W.C. Synthesis of Reverse Micelle α-FeOOH Nanoparticles in Ionic Liquid as an Only Electrolyte: Inhibition of Electron-Hole Pair Recombination for Efficient Photoactivity. Appl. Catal. A Gen. 2014, 469, 33–44. [Google Scholar] [CrossRef]
- Han, P.; Liu, T.; Ji, X.; Tang, S. Morphology-Controlled Synthesis of Mesoporous Silica with Co-Template of Surfactant P123 and Ionic Liquid [Dmim]Cl. Chin. Chem. Lett. 2018, 29, 1305–1309. [Google Scholar] [CrossRef]
- Brevet, D.; Jouannin, C.; Tourné-Péteilh, C.; Devoisselle, J.M.; Vioux, A.; Viau, L. Self-Encapsulation of a Drug-Containing Ionic Liquid into Mesoporous Silica Monoliths or Nanoparticles by a Sol-Gel Process. RSC Adv. 2016, 6, 82916–82923. [Google Scholar] [CrossRef]
- Isa, E.D.M.; Ahmad, H.; Rahman, M.B.A. Long Chain Imidazolium Ionic Liquids as Templates in the Formation of Mesoporous Silica Nanospheres. Solid State Phenom. 2020, 301 SSP, 209–216. [Google Scholar] [CrossRef]
- Mohamed Isa, E.D.; Abdul Rahman, M.B.; Ahmad, H. Monodispersed Mesoporous Silica Nanospheres Based on Pyridinium Ionic Liquids. J. Porous Mater. 2018, 25, 1439–1446. [Google Scholar] [CrossRef]
- Isa, E.D.M.; Ahmad, H.; Rahman, M.B.A. Optimization of Synthesis Parameters of Mesoporous Silica Nanoparticles Based on Ionic Liquid by Experimental Design and Its Application as a Drug Delivery Agent. J. Nanomater. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Zaharudin, N.S.; Mohamed Isa, E.D.; Ahmad, H.; Abdul Rahman, M.B.; Jumbri, K. Functionalized Mesoporous Silica Nanoparticles Templated by Pyridinium Ionic Liquid for Hydrophilic and Hydrophobic Drug Release Application. J. Saudi Chem. Soc. 2020, 24, 289–302. [Google Scholar] [CrossRef]
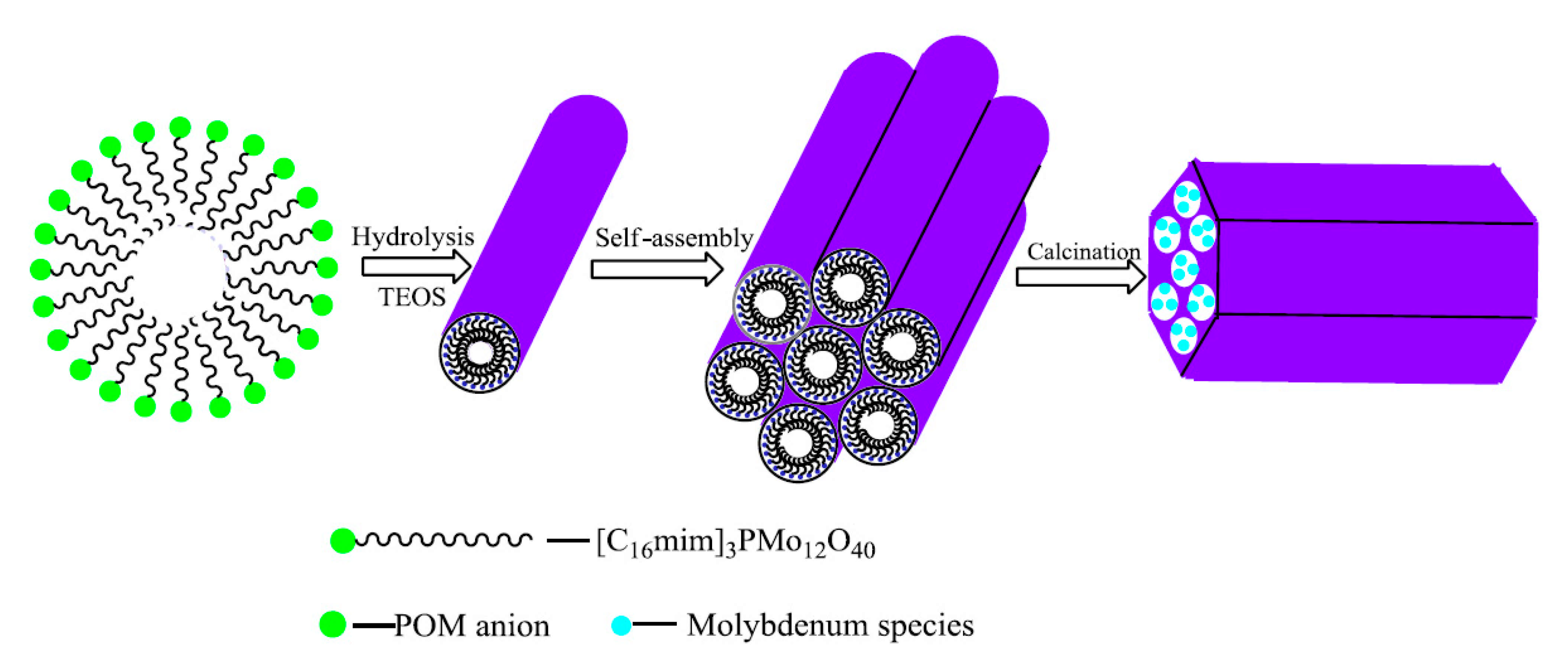
- Zhang, M.; Zhu, W.; Li, H.; Li, M.; Yin, S.; Li, Y.; Wei, Y.; Li, H. Facile Fabrication of Molybdenum-Containing Ordered Mesoporous Silica Induced Deep Desulfurization in Fuel. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 174–181. [Google Scholar] [CrossRef]
- Zare, A.; Lashanizadegan, A.; Darvishi, P.; Zerafat, M.M. Synthesis and Characterization of NaP Zeolite Nanocrystals Using [C12mim][Cl] Ionic Liquid. Chem. Pap. 2020, 74, 2163–2174. [Google Scholar] [CrossRef]
- Hu, J.; Yang, Q.; Yang, L.; Zhang, Z.; Su, B.; Bao, Z.; Ren, Q.; Xing, H.; Dai, S. Confining Noble Metal (Pd, Au, Pt) Nanoparticles in Surfactant Ionic Liquids: Active Non-Mercury Catalysts for Hydrochlorination of Acetylene. ACS Catal. 2015, 5, 6724–6731. [Google Scholar] [CrossRef]
- Huang, B.; Huang, C.; Chen, J.; Sun, X. Size-Controlled Synthesis and Morphology Evolution of Nd2O3 Nano-Powders Using Ionic Liquid Surfactant Templates. J. Alloy. Compd. 2017, 712, 164–171. [Google Scholar] [CrossRef]
- Komal; Kaur, H.; Kainth, M.; Meena, S.S.; Kang, T.S. Sustainable Preparation of Sunlight Active α-Fe2O3 Nanoparticles Using Iron Containing Ionic Liquids for Photocatalytic Applications. RSC Adv. 2019, 9, 41803–41810. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Ye, X.; Wang, Z.; Wu, T.; Li, C. Controlled Synthesis of Icosahedral Gold Nanocrystals, and Their Self-Assembly with an Ionic Liquid for Enhanced Immunosensing of Squamous Cell Carcinoma Antigen. Microchim. Acta 2017, 184, 3565–3572. [Google Scholar] [CrossRef]
- Xu, Z.B.; Lu, G.P.; Cai, C. Palladium Nanoparticles Stabilized by Aqueous Vesicles Self-Assembled from a PEGylated Surfactant Ionic Liquid for the Chemoselective Reduction of Nitroarenes. Catal. Commun. 2017, 99, 57–60. [Google Scholar] [CrossRef]
- Duan, W.; Li, A.; Chen, Y.; Zhuo, K.; Liu, J.; Wang, J. Ionic Liquid-Assisted Synthesis of Reduced Graphene Oxide–Supported Hollow Spherical PtCu Alloy and Its Enhanced Electrocatalytic Activity toward Methanol Oxidation. J. Nanoparticle Res. 2018, 20. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Nabavizadeh, M.; Gholami, A.; Aleyasin, Z.S.; Dorostkar, S.; Saliminasab, M.; Ghasemi, Y.; Hemmateenejad, B.; Sharghi, H. Positively Charged Imidazolium-Based Ionic Liquid-Protected Silver Nanoparticles: A Promising Disinfectant in Root Canal Treatment. Int. Endod. J. 2015, 48, 790–800. [Google Scholar] [CrossRef]
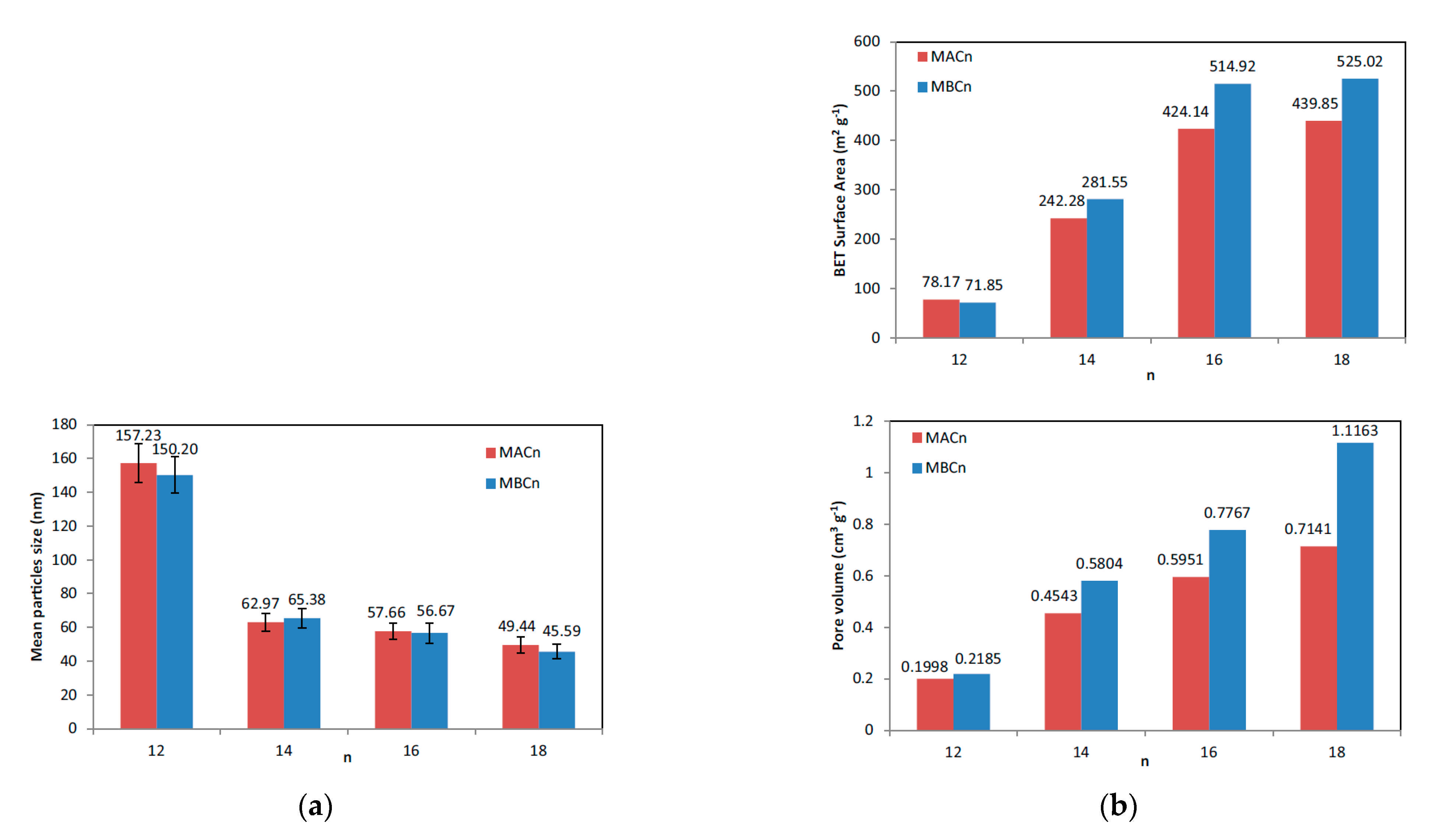
- Lv, X.; Zhang, L.; Xing, F.; Lin, H. Controlled Synthesis of Monodispersed Mesoporous Silica Nanoparticles: Particle Size Tuning and Formation Mechanism Investigation. Microporous Mesoporous Mater. 2016, 225, 238–244. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L. Ionic Liquids: Perspectives for Organic and Catalytic Reactions. J. Mol. Catal. A Chem. 2002, 182–183, 419–437. [Google Scholar] [CrossRef]
- Cole, A.C.; Jensen, J.L.; Ntai, I.; Tran, K.L.T.; Weaver, K.J.; Forbes, D.C.; Davis, J.H. Novel Brønsted Acidic Ionic Liquids and Their Use as Dual Solvent-Catalysts. J. Am. Chem. Soc. 2002, 124, 5962–5963. [Google Scholar] [CrossRef]
- Planellas, M.; Pleixats, R.; Shafir, A. Palladium Nanoparticles in Suzuki Cross-Couplings: Tapping into the Potential of Tris-Imidazolium Salts for Nanoparticle Stabilization. Adv. Synth. Catal. 2012, 354, 651–662. [Google Scholar] [CrossRef]
- MacLeod, M.J.; Johnson, J.A. PEGylated N-Heterocyclic Carbene Anchors Designed to Stabilize Gold Nanoparticles in Biologically Relevant Media. J. Am. Chem. Soc. 2015, 137, 7974–7977. [Google Scholar] [CrossRef]
- Yasukawa, T.; Miyamura, H.; Kobayashi, S. Cellulose-Supported Chiral Rhodium Nanoparticles as Sustainable Heterogeneous Catalysts for Asymmetric Carbon-Carbon Bond-Forming Reactions. Chem. Sci. 2015, 6, 6224–6229. [Google Scholar] [CrossRef] [PubMed]
- Visbal, R.; Gimeno, M.C. N-Heterocyclic Carbene Metal Complexes: Photoluminescence and Applications. Chem. Soc. Rev. 2014, 43, 3551–3574. [Google Scholar] [CrossRef] [PubMed]
- Naderi, O.; Nyman, M.; Amiri, M.; Sadeghi, R. Synthesis and Characterization of Silver Nanoparticles in Aqueous Solutions of Surface Active Imidazolium-Based Ionic Liquids and Traditional Surfactants SDS and DTAB. J. Mol. Liq. 2019, 273, 645–652. [Google Scholar] [CrossRef]
- Janiak, C. Ionic Liquids for the Synthesis and Stabilization of Metal Nanoparticles. Z. Fur Nat. Sect. B J. Chem. Sci. 2013, 68, 1059–1089. [Google Scholar] [CrossRef]
- Manojkumar, K.; Sivaramakrishna, A.; Vijayakrishna, K. A Short Review on Stable Metal Nanoparticles Using Ionic Liquids, Supported Ionic Liquids, and Poly(Ionic Liquids). J. Nanoparticle Res. 2016, 18. [Google Scholar] [CrossRef]
- Tshemese, Z.; Masikane, S.C.; Mlowe, S.; Revaprasadu, N. Progress in Green Solvents for the Stabilisation of Nanomaterials: Imidazolium Based Ionic Liquids. In Recent Advances in Ionic Liquids; Rahman, M.M., Ed.; IntechOpen: London, UK, 2018; pp. 69–89. [Google Scholar]
- Verma, M.; Singh, K.; Bakshi, M.S. Surface Active Magnetic Iron Oxide Nanoparticles for Extracting Metal Nanoparticles across an Aqueous-Organic Interface. J. Mater. Chem. C 2019, 7, 10623–10634. [Google Scholar] [CrossRef]
- Galgano, P.D.; El Seoud, O.A. Surface Active Ionic Liquids: Study of the Micellar Properties of 1-(1-Alkyl)-3-Methylimidazolium Chlorides and Comparison with Structurally Related Surfactants. J. Colloid Interface Sci. 2011, 361, 186–194. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, H.; Yu, Y.; Hua, L.; Li, H.; Feng, B.; Hou, Z. Amphiphilic Ionic Liquid Stabilizing Palladium Nanoparticles for Highly Efficient Catalytic Hydrogenation. Phys. Chem. Chem. Phys. 2011, 13, 13492–13500. [Google Scholar] [CrossRef]
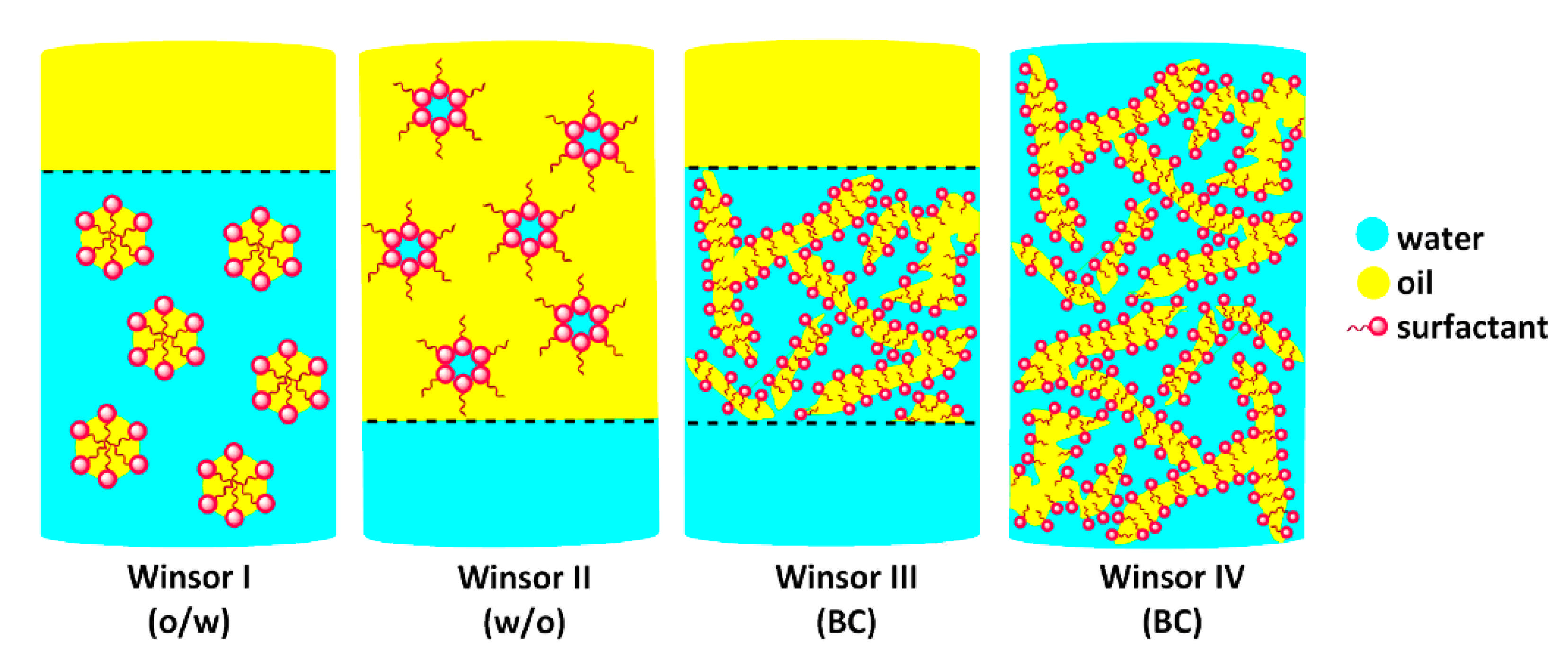
- Danielsson, I.; Lindman, B. The Definition of Microemulsion. Colloids Surf. 1981, 3, 391–392. [Google Scholar] [CrossRef]
- Moulik, S.P.; Paul, B.K. Structure, Dynamics and Transport Properties of Micro Emulsions. Adv. Colloid Interface Sci. 1998, 78, 99–195. [Google Scholar] [CrossRef]
- Winsor, P.A. Hydrotropy, Solubilisation and Related Emulsification Processes. Part I. Trans. Faraday Soc. 1948, 44, 376–398. [Google Scholar] [CrossRef]
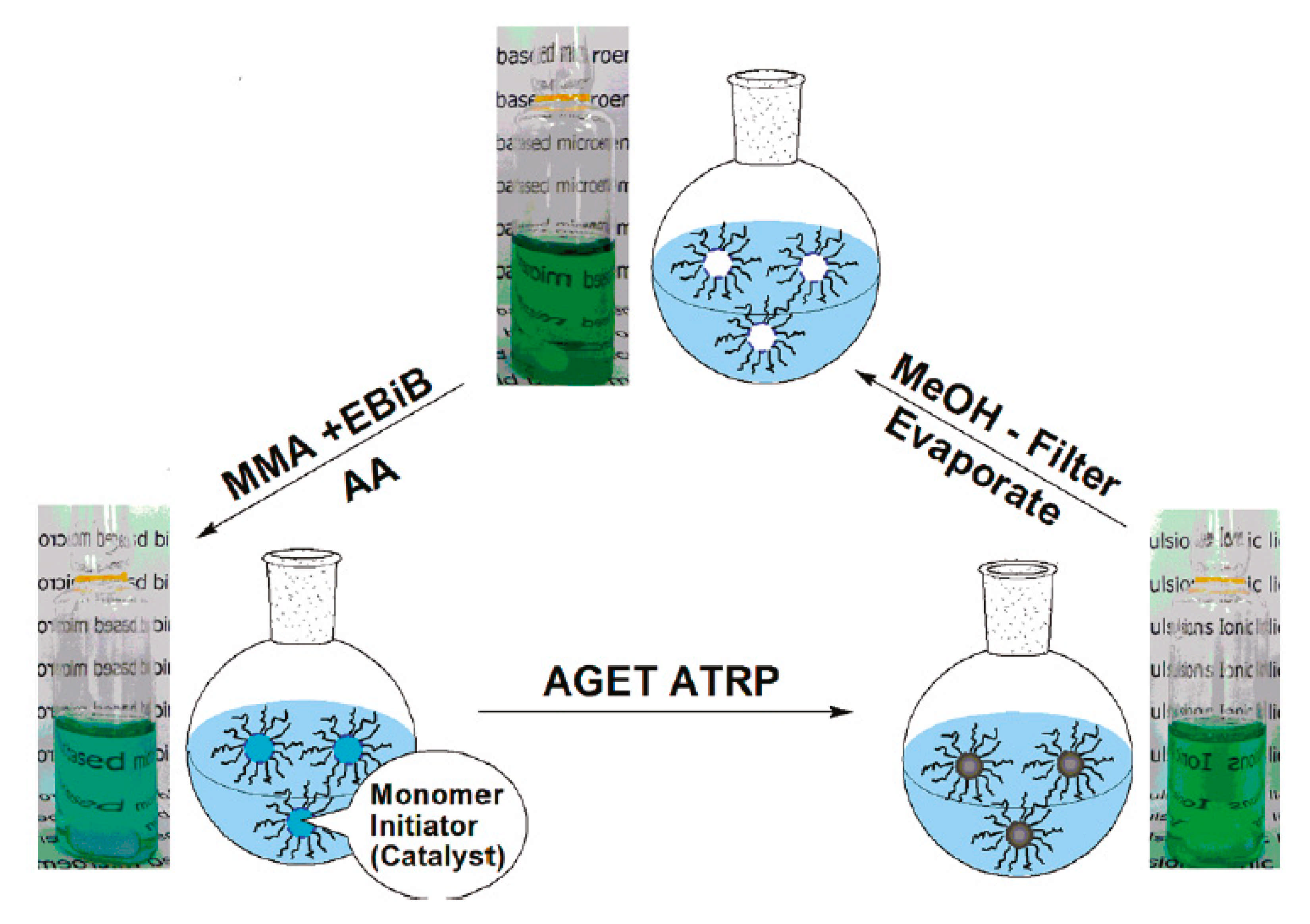
- Yan, F.; Texter, J. Surfactant Ionic Liquid-Based Microemulsions for Polymerization. Chem. Commun. 2006, 2696–2698. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, F.; Qiu, L.; Lu, J.; Zhou, Y.; Chen, J.; Tang, Y.; Texter, J. Sustainable Polymerizations in Recoverable Microemulsions. Langmuir 2010, 26, 3803–3806. [Google Scholar] [CrossRef]
- Wang, G.X.; Lu, M.; Liu, L.C.; Wu, H.; Zhong, M. Fe-Mediated ARGET Atom Transfer Radical Polymerization of Methyl Methacrylate in Ionic Liquid-Based Microemulsion. J. Appl. Polym. Sci. 2013, 128, 3077–3083. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, L.; Deng, Z.; Texter, J.; Yan, F. Low-Temperature AGET ATRP of Methyl Methacrylate in Ionic Liquid-Based Microemulsions. Macromolecules 2011, 44, 7948–7955. [Google Scholar] [CrossRef]
- Lu, J.; Ding, Y.; Yu, Y.; Wu, S.; Feng, W. Effect of Two Kinds of Imidazolium Ionic Liquids on the Microemulsion Polymerization of Methyl Methylacrylate. Macromol. Symp. 2010, 298, 167–173. [Google Scholar] [CrossRef]
- Mirhoseini, F.; Salabat, A. Ionic Liquid Based Microemulsion Method for the Fabrication of Poly(Methyl Methacrylate)-TiO2 Nanocomposite as a Highly Efficient Visible Light Photocatalyst. RSC Adv. 2015, 5, 12536–12545. [Google Scholar] [CrossRef]
- Wu, L.G.; Shen, J.N.; Du, C.H.; Wang, T.; Teng, Y.; van der Bruggen, B. Development of AgCl/Poly(MMA-Co-AM) Hybrid Pervaporation Membranes Containing AgCl Nanoparticles through Synthesis of Ionic Liquid Microemulsions. Sep. Purif. Technol. 2013, 114, 117–125. [Google Scholar] [CrossRef]
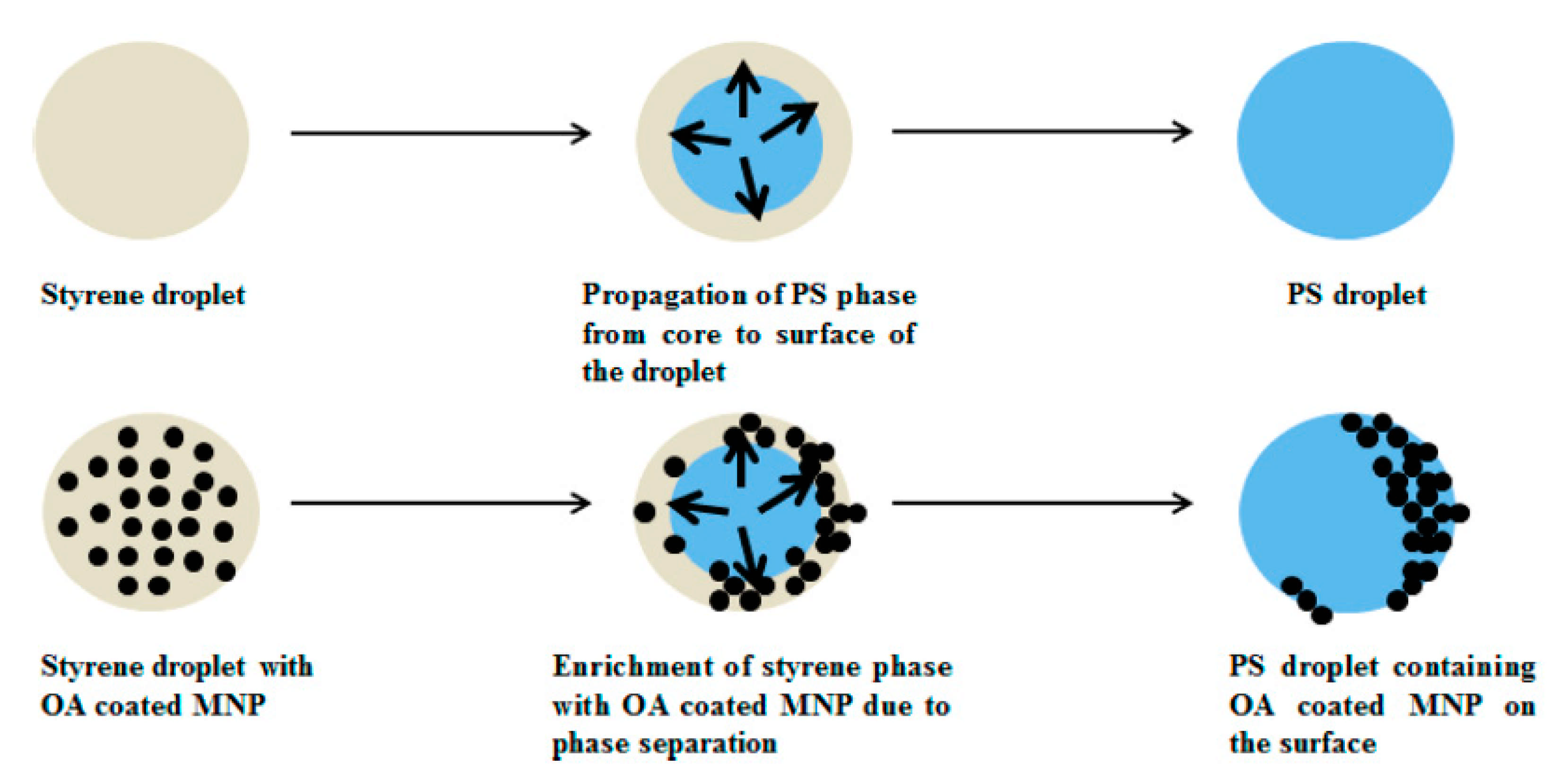
- Chakraborty, S.; Jähnichen, K.; Komber, H.; Basfar, A.A.; Voit, B. Synthesis of Magnetic Polystyrene Nanoparticles Using Amphiphilic Ionic Liquid Stabilized RAFT Mediated Miniemulsion Polymerization. Macromolecules 2014, 47, 4186–4198. [Google Scholar] [CrossRef]
- England, D.; Tambe, N.; Texter, J. Stimuli-Responsive Nanolatexes: Porating Films. ACS Macro Lett. 2012, 1, 310–314. [Google Scholar] [CrossRef]
- Bonnenfold, A.; Ibarra, M.; Mecerreyes, D.; Leiza, J.R. Adding Magnetic Ionic Liquid Monomers to the Emulsion PolymerizationTool-Box: Towards Polymer Latexes and Coatings with New Properties. J. Polym. Sci. A Polym. Chem. 2016, 54, 1145–1152. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Gracia, R.; Leal, P.G.; Paulis, M.; Mecerreyes, D. Simple route to prepare stable liquid marbles using poly(ionic liquid)s. Polymer 2014, 55, 3397–3403. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Mantione, D.; Gracia, R.; Leiza, J.R.; Paulis, M.; Mecerreyes, D. From Polymer Latexes to Multifunctional Liquid Marbles. ACS Appl. Mater. Interfaces 2015, 7, 4433–4441. [Google Scholar] [CrossRef] [PubMed]
- Hanamertani, A.S.; Pilus, R.M.; Irawan, S. A Review on the Application of Ionic Liquids for Enhanced Oil Recovery. In Icipeg 2016; Springer: Singapore, 2017; pp. 133–147. ISBN 9789811036507. [Google Scholar]
- Nandwani, S.K.; Malek, N.I.; Chakraborty, M.; Gupta, S. Insight into the Application of Surface-Active Ionic Liquids in Surfactant Based Enhanced Oil Recovery Processes-A Guide Leading to Research Advances. Energy Fuels 2020, 34, 6544–6557. [Google Scholar] [CrossRef]
- Pletnev, I.V.; Smirnova, S.V.; Shvedene, N.V. New Directions in Using Ionic Liquids in Analytical Chemistry. 1: Liquid–Liquid Extraction. J. Anal. Chem. 2019, 74, 625–658. [Google Scholar] [CrossRef]
- Pletnev, I.V.; Smirnova, S.V.; Shvedene, N.V. New Directions in Using Ionic Liquids in Analytical Chemistry. 2: Electrochemical Methods. J. Anal. Chem. 2019, 74, 1–10. [Google Scholar] [CrossRef]
- Nawała, J.; Dawidziuk, B.; Dziedzic, D.; Gordon, D.; Popiel, S. Applications of Ionic Liquids in Analytical Chemistry with a Particular Emphasis on Their Use in Solid-Phase Microextraction. Trac Trends Anal. Chem. 2018, 105, 18–36. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Nan, H.; Varona, M.; Emaus, M.N.; Souza, I.D.; Anderson, J.L. Advances of Ionic Liquids in Analytical Chemistry. Anal. Chem. 2019, 91, 505–531. [Google Scholar] [CrossRef]




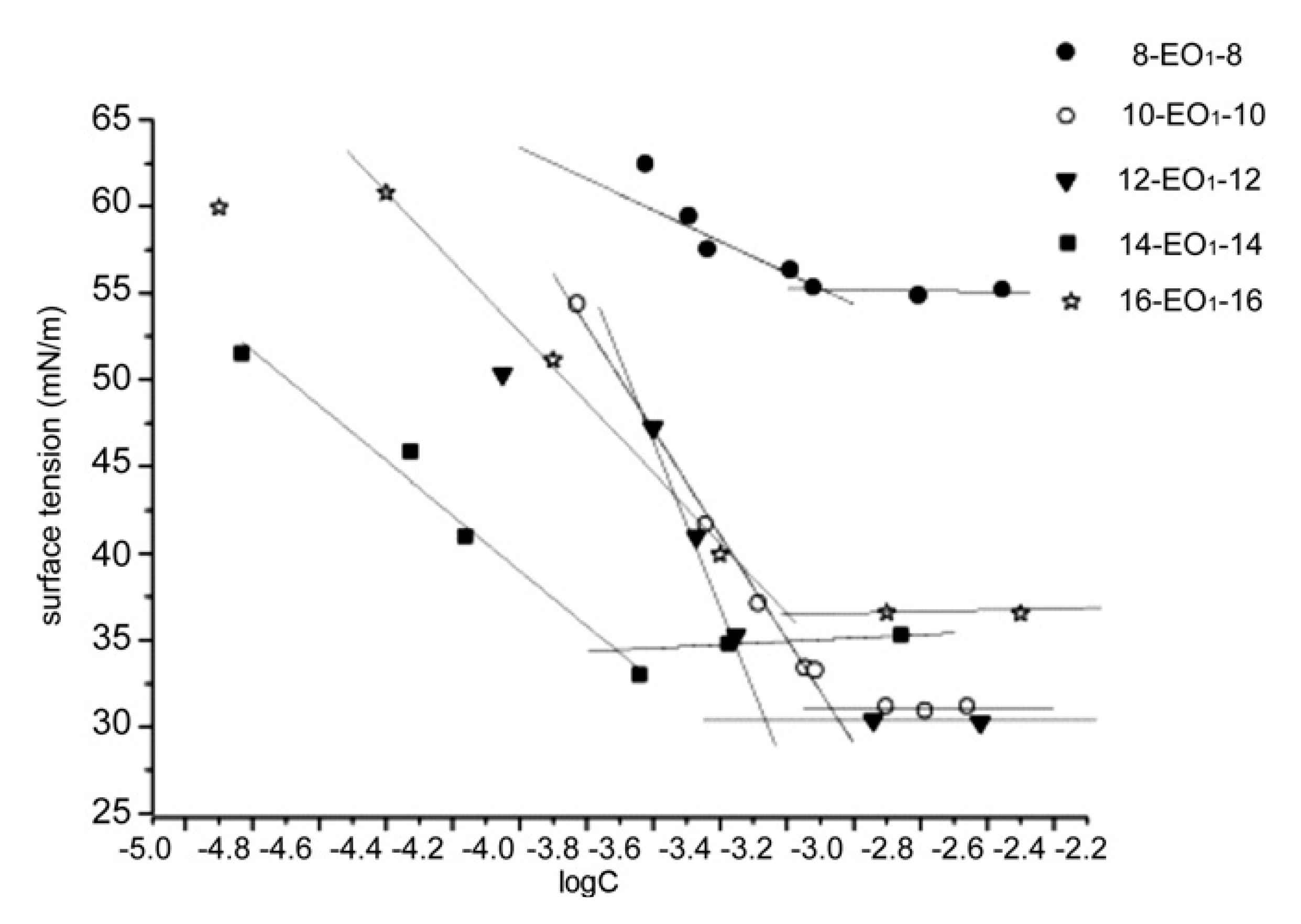
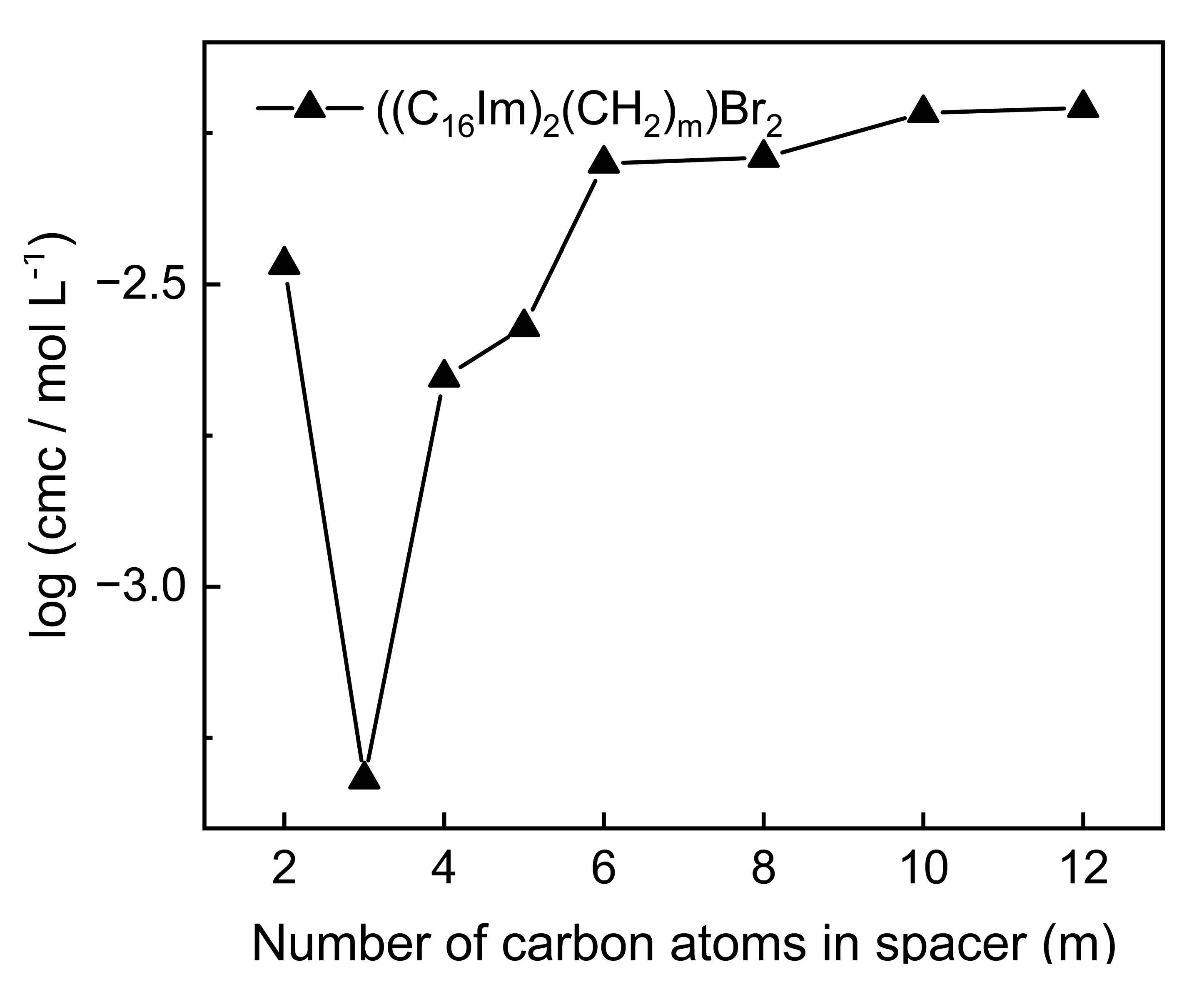
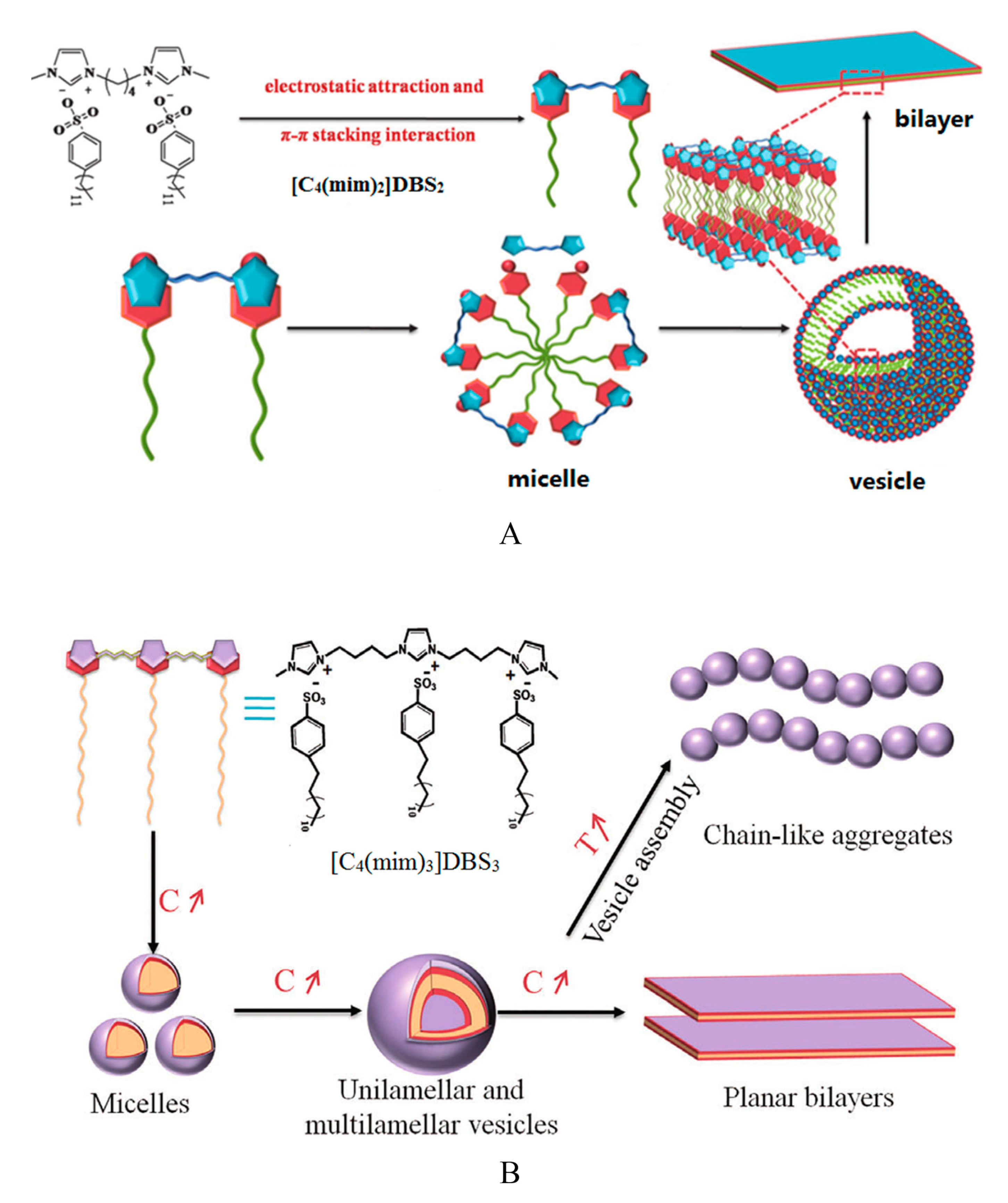
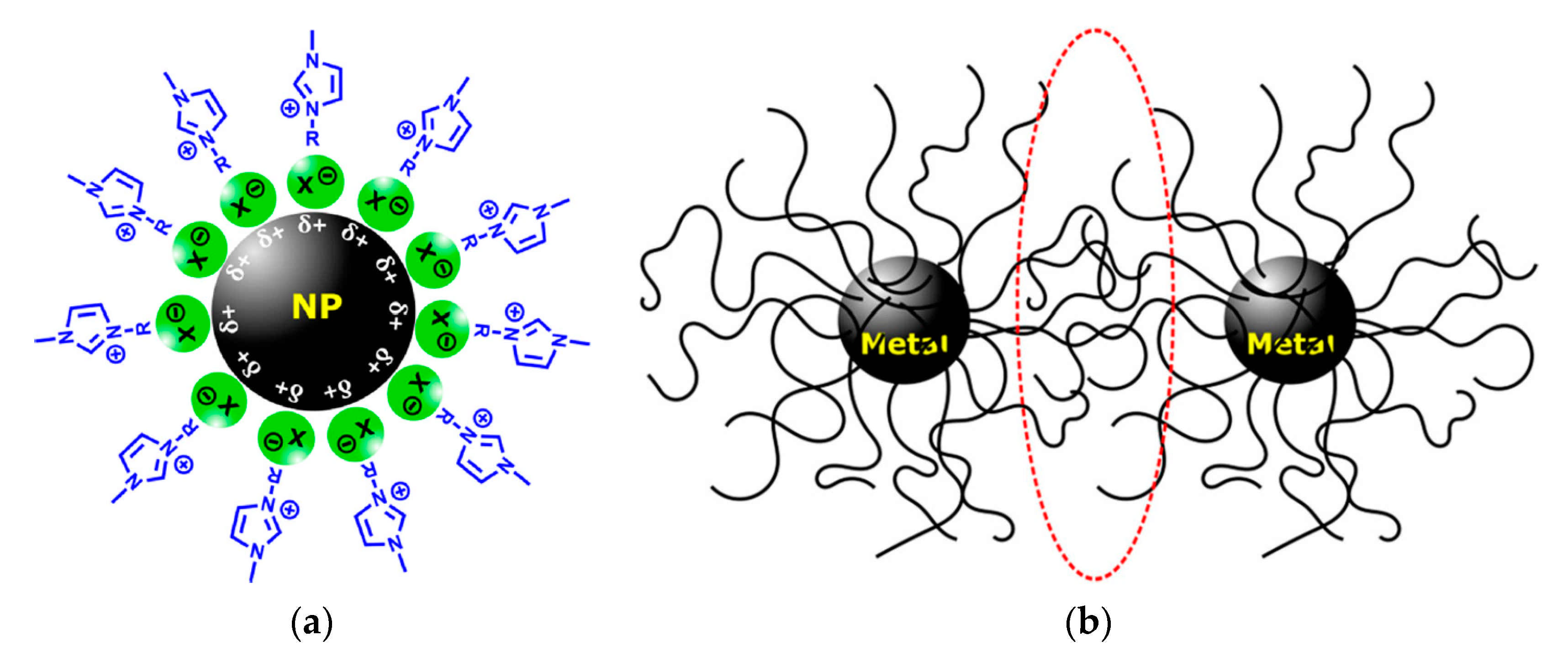





| Entry | Cation 1 | Anion 2 | cmc × 103 (mol L−1) 3 | γcmc (mN m−1) ⁴ | Πcmc (mN m−1) ⁵ | Γmax (mol m−2) ⁶ | Amin (Å2) ⁷ | pC20 ⁸ | ΔG0ads (kJ mol−1) ⁹ |
|---|---|---|---|---|---|---|---|---|---|
| Cationic ILBSs | |||||||||
| 1 | C8C1Im+ | Cl− | 116 [58], 101.7 [59], 190 [60], 108.9 [61], 170.2 [62] | 28.3 [59], 33.5 [61], 37.3 [62] | 43.3 [59], 32.2 [60], 38.5 [61] | 2.682 [58], 1.6 [59], 1.52 [61], 1.24 [62] | 104 [59], 84 [60], 109 [61], 133 [62] | 1.8 [59], 1.67 [60], 1.92 [61] | −43.33 [58], −47.6 [61], −24.6 [62] |
| 2 | Br− | 121 [45], 120.0 [59], 170 [63], 119.29 [64] | 41 [45], 28.7 [59], 41.3 [63], 29.33 [64] | 44.9 [59], 42.87 [64] | 2.7 [59], 3.065 [64] | 60 [59], 124 [63], 53 [64] | 1.8 [59] | −36.5 [64] | |
| 3 | I− | 94.9 [59] | 28.2 [59] | 44.4 [59] | 1.4 [59] | 117 [59] | 2.0 [59] | ||
| 4 | C1SO3− | 220 [65] | 29.0 [65] | 1.0 [65] | |||||
| 5 | C4SO3− | 140 [65] | 28.5 [65] | 1.6 [65] | |||||
| 8 | C10C1Im+ | Cl− | 39.90 [42], 45 [60], 40.00 [66], 40.0 [67], 40 [68], 40 [69], 27.7 [70] | 27.3 [66] | 33.7 [60], 44.5 [66] | 1.9 [66], 1.84 [67], 1.84 [68], 1.84 [69] | 85 [42], 92 [60], 85 [66], 90 [67], 90 [68], 90 [69] | 2.55 [60], 2.5 [66], 2.60 [68] | −30.16 [42], −48.55 [67], −48.00 [68], −48.55 [69] |
| 9 | Br− | 20 [45], 33 [63], 36.7 [71], 27.24 [72], 39.6 [73] | 39 [45], 39.1 [63], 37.3 [71], 48.95 [72], 36.04 [73] | 35.5 [71], 23.02 [72], 33.37 [73] | 1.82 [71], 1.75 [72], 1.71 [73] | 91 [63], 57.6 [74], 91.4 [71], 94.61 [72], 97.01 [73] | 1.61 [72] | ||
| 10 | C1SO3− | 60 [65] | 28.5 [65] | 1.5 [65] | |||||
| 12 | C12C1Im+ | Cl− | 13.17 [42], 14.80 [66], 13.8 [70], 16.8 [75], 13.86 [76], 13.25 [77], 11.62 [78] | 38.7 [66], 38.4 [75], 30.45 [76] | 33.6 [66], 41.55 [76], 28.6 [77] | 2.3 [66], 2.91 [75], 2.04 [76] | 72 [42], 72 [66], 57 [75], 81.39 [76], 72 [77] | 2.4 [66], 2.16 [75], 2.72 [76] | −32.03 [42] |
| 13 | Br− | 4.3 [45], 9.0 [63], 9.19 [72], 10.6 [75], 9.29 [77], 10.35 [79], 8.22 [80], 11.21 [81], 8.73 [82], 9.68 [83], 9.0 [84], 9 [85] | 35 [45], 37.2 [63], 46.71 [72], 36.8 [75], 38.96 [80], 42.9 [81], 38.68 [82], 37.40 [83] | 25.26 [72], 29.3 [77], 33.06 [80], 33.94 [82] | 1.80 [72], 3.03 [75], 4.48 [79], 3.36 [80], 3.095 [82] | 67 [63], 92.49 [72], 64 [74], 55 [75], 71 [77], 37.1 [79], 49.42 [80], 53.7 [82] | 2.35 [72], 2.16 [75], 3.06 [79], 2.54 [80], 2.45 [82] | ||
| 14 | I− | 4.6 [75], 4.76 [77] | 31.7 [75] | 37.7 [77] | 4.47 [75] | 37 [75], 62 [77] | 2.80 [75] | ||
| 15 | C1SO3− | 14 [65] | 28.5 [65] | 2.1 [65] | |||||
| 17 | C14C1Im+ | Cl− | 2.98 [42], 3.10 [70], 3.63 [86] | 34.15 [86] | 36.65 [86] | 2.25 [86] | 56 [42], 74 [86] | 2.89 [86] | −33.81 [42], −55.55 [86] |
| 18 | Br− | 1.9 [63], 2.69 [83], 2.76 [87], 2.6 [88], 2.8 [89] | 37.2 [63], 37.70 [83], 37 [88], 39.2 [89] | 41.4 [87], 33.8 [89] | 1.74 [87], 1.26 [88], 1.96 [89] | 67 [63], 64.8 [74], 95 [87], 132 [88], 84.7 [89] | 3.6 [87], 3.03 [88], 3.33 [89] | −47.48 [87] | |
| 19 | C16C1Im+ | Cl− | 0.87 [42], 1.14 [66], 0.89 [90], 0.87 [91] | 37.0 [66], 40.9 [91] | 34.8 [66], 28.8 [90] | 3.4 [66], 2.06 [90], 3.4 [91] | 49 [42], 49 [66], 80 [90], 49 [91] | 3.2 [66], 3.4 [90], 3.39 [91] | −35.23 [42], −35.64 [91] |
| 20 | Br− | 0.8 [45], 0.610 [72], 0.78 [79], 0.51 [83], 0.55 [89], 0.71 [91], 0.566 [92] | 41 [45], 44.53 [72], 37.41 [83], 39.1 [89], 38.7 [91], 38.98 [92] | 27.44 [72], 33.9 [89], 33.05 [92] | 2.00 [72], 4.20 [79], 2.03 [89], 3.0 [91], 2.06 [92] | 83.14 [72], 39.6 [79], 81.6 [89], 54 [91], 80.7 [92] | 3.42 [72], 3.85 [79], 3.78 [89], 3.55 [91] | −36.83 [91] | |
| 21 | C18C1Im+ | Cl− | 0.40 [66] | 42.0 [66] | 29.8 [66] | 3.7 [66] | 45 [66] | 3.6 [66] | |
| 22 | C12C2Im+ | Br− | 6.40 [80] | 38.09 [80] | 33.93 [80] | 3.09 [80] | 53.74 [80] | 2.70 [80] | |
| 23 | C16C2Im+ | Cl− | 0.88 [91] | 35.4 [91] | 2.6 [91] | 63 [91] | 3.62 [91] | −38.29 [91] | |
| 24 | Br− | 0.55 [91], 0.26 [93] | 39.8 [91], 32.2 [93] | 40.3 [93] | 2.7 [91], 2.58 [93] | 61 [91], 64.2 [93] | 3.65 [91], 4.22 [93] | −38.19 [91], −66.32 [93] | |
| 25 | C10VnIm+ | Br− | 27.20 [93] | 34.5 [93] | 37.3 [93] | 1.86 [93] | 89.3 [93] | 2.44 [93] | −50.60 [93] |
| 26 | C12VnIm+ | Br− | 7.00 [93] | 34.1 [93] | 38.4 [93] | 2.03 [93] | 81.8 [93] | 2.80 [93] | −56.31 [93] |
| 28 | C14VnIm+ | Br− | 1.85 [93] | 33.8 [93] | 38.6 [93] | 2.18 [93] | 76.2 [93] | 3.40 [93] | −61.30 [93] |
| 29 | C16VnIm+ | Br− | 0.60 [91], 0.48 [93] | 37.7 [91], 33.5 [93] | 39.1 [93] | 3.1 [91], 2.53 [93] | 53 [91], 65.7 [93] | 3.63 [91], 4.00 [93] | −37.09 [91], −65.47 [93] |
| 30 | C12C3Im+ | Br− | 5.05 [80] | 37.56 [80] | 34.46 [80] | 2.20 [80] | 75.48 [80] | 2.85 [80] | |
| 31 | C16C3Im+ | Cl− | 0.71 [91] | 35.2 [91] | 2.2 [91] | 75 [91] | 3.82 [91] | −40.78 [91] | |
| 32 | Br− | 0.44 [91] | 39.7 [91] | 2.3 [91] | 73 [91] | 3.80 [91] | −40.46 [91] | ||
| 33 | C16AlIm+ | Br− | 0.51 [91] | 38.7 [91] | 2.6 [91] | 63 [91] | 3.74 [91] | −38.90 [91] | |
| 34 | C8C4Im+ | Br− | 41 [45], 75.8 [94] | 40 [45], 33.2 [94] | 37 [94] | 1.3 [94] | 126.9 [94] | 2.3 [94] | −51.1 [94] |
| 35 | C10C4Im+ | Br− | 6.3 [45] | 36 [45] | |||||
| 36 | C12C4Im+ | Br− | 2.4 [45], 3.68 [80], 5.3 [94] | 38 [45], 34.92 [80], 33.0 [94] | 37.10 [80], 37.2 [94] | 2.02 [80], 2.1 [94] | 82.21 [80], 76.9 [94] | 3.18 [80], 2.9 [94] | −53.2 [94] |
| 37 | C16C4Im+ | Cl− | 0.50 [91] | 38.0 [91] | 2.1 [91] | 80 [91] | 3.89 [91] | −41.86 [91] | |
| 38 | Br− | 0. 1 [45], 0.40 [91] | 45 [45], 40.3 [91] | 1.9 [91] | 86 [91] | 3.92 [91] | −42.73 [91] | ||
| 39 | C16C5Im+ | Cl− | 0.35 [91] | 39.6 [91] | 1.6 [91] | 106 [91] | 4.15 [91] | −46.37 [91] | |
| 40 | C8C8Im+ | Br− | 5.6 [45], 8.0 [95] | 32 [45], 25.9 [95] | |||||
| 41 | C12C12Im+ | Br− | 0.1 [45] | 28 [45] | |||||
| 42 | C10C1C1Im+ | Br− | 43.0 [96] | 30.9 [96] | 1.7 [96] | 99.4 [96] | |||
| 43 | C12C1C1Im+ | Cl− | 12.27 [97] | 31.21 [97] | 40.79 [97] | 0.95 [97] | 1.75 [97] | 2.75 [97] | −78.78 [97] |
| 44 | C10C1C10Im+ | Cl− | 1.23 [98] | 32.7 [98] | 1.98 [98] | 83.5 [98] | −46.66 [98] | ||
| 45 | C8Py+ | Cl− | 181 [99] | 36.84 [99] | 34.6 [99] | 1.70 [99] | 98 [99] | 1.6 [99] | |
| 46 | Br− | 180 [63] | 41.9 [63] | 66 [63] | |||||
| 48 | C10Py+ | Cl− | 65.5 [100] | ||||||
| 49 | Br− | 30 [63] | 40.7 [63] | 61 [63] | |||||
| 50 | C11Py+ | Br− | 19.5 [101] | ||||||
| 51 | C12Py+ | Cl− | 14.0 [101] | ||||||
| 52 | Br− | 9.3 [63] | 39.3 [63] | 71 [63] | |||||
| 53 | C13Py+ | Br− | 4.57 [101] | ||||||
| 54 | C14Py+ | Cl− | 3.20 [102] | ||||||
| 55 | Br− | 2.2 [63], 2.65 [101] | 38.0 [63] | 86 [63] | |||||
| 56 | C16Py+ | Cl− | 0.99 [103] | 49 [103] | 23.0 [103] | 1.17 [103] | 142.0 [103] | 3.00 [103] | −25.7 [103] |
| 57 | Br− | 0.62 [101], 0.9 [103] | 49 [103] | 23.0 [103] | 0.91 [103] | 142 [103] | 3.03 [103] | −25.70 [103] | |
| 58 | C8-(o-C1)Py+ | Cl− | 166 [99] | 31.80 [99] | 40.1 [99] | 1.67 [99] | 99 [99] | 3.0 [99] | |
| 63 | C8-(m-C1)Py+ | Cl− | 170 [99] | 32.38 [99] | 39.2 [99] | 1.71 [99] | 97 [99] | 1.9 [99] | |
| 65 | C10-(m-C1)Py+ | Cl− | 45 [85] | 27.9 [85] | 121 [85] | 1.62 [85] | |||
| 66 | C12-(m-C1)Py+ | Cl− | 13 [85] | 27.9 [85] | 108 [85] | 2.22 [85] | |||
| 67 | Br− | 10 [85], 9.83 [104] | 39.2 [104] | 32.9 [104] | 1.84 [104] | 90.3 [104] | 2.8 [104] | −55.00 [104] | |
| 68 | C14-(m-C1)Py+ | Cl− | 3.1 [85] | 28.0 [85] | 92 [85] | 2.82 [85] | |||
| 69 | Br− | 2.26 [104] | 38.8 [104] | 33.3 [104] | 2.09 [104] | 79.4 [104] | 3.1 [104] | −58.39 [104] | |
| 70 | C16-(m-C1)Py+ | Cl− | 0.8 [85] | 27.9 [85] | 80 [85] | 3.39 [85] | |||
| 71 | Br− | 0.508 [104] | 37.6 [104] | 34.5 [104] | 2.38 [104] | 69.7 [104] | 3.8 [104] | −63.00 [104] | |
| 72 | C18-(m-C1)Py+ | Cl− | 0.3 [85] | 27.8 [85] | 76 [85] | 3.87 [85] | |||
| 73 | C8-(p-C1)Py+ | Cl− | 175 [99] | 31.00 [99] | 40.7 [99] | 1.65 [99] | 101 [99] | 2.2 [99] | |
| 79 | C10C1Pn+ | Br− | 31 [105] | 28.6 [105] | 3.8 [105] | 44 [105] | |||
| 80 | C12C1Pn+ | Br− | 16 [105] | 25.1 [105] | 4.4 [105] | 38 [105] | |||
| 81 | C14C1Pn+ | Br− | 7.4 [105] | 28.7 [105] | 4.5 [105] | 37 [105] | |||
| 82 | C16C1Pn+ | Br− | 3.3 [105] | 31.2 [105] | 5.2 [105] | 32 [105] | |||
| 83 | C18C1Pn+ | Br− | 1.5 [105] | 32.7 [105] | 4.4 [105] | 38 [105] | |||
| 86 | C12C1Pyrro+ | Cl− | 19.60 [106] | 34.4 [106] | 36.6 [106] | 2.4 [106] | 69 [106] | 2.3 [106] | |
| 87 | Br− | 15 [107], 13.5 [108] | 42.4 [108] | 30.3 [108] | 3.03 [108] | 54.8 [108] | |||
| 88 | C14C1Pyrro+ | Br− | 3.30 [108] | 42.7 [108] | 30.0 [108] | 3.55 [108] | 46.8 [108] | ||
| 89 | C16C1Pyrro+ | Br− | 0.860 [108], 0.72 [109] | 41.2 [108], 36.7 [109] | 31.5 [108] | 3.67 [108] | 45.2 [108], 51 [109] | ||
| 90 | C18C1Pyrro+ | Cl− | 0.42 [66] | 36.5 [66] | 35.3 [66] | 3.5 [66] | 48 [66] | 3.7 [66] | |
| 92 | C8C4Pyrro+ | Br− | 150 [107] | ||||||
| 93 | C12C4Pyrro+ | Br− | 6 [107] | ||||||
| 98 | C12C1Pip+ | Cl− | 19.79 [110] | 36.5 [110] | 35.3 [110] | 2.4 [110] | 68.5 [110] | 3.35 [110] | |
| 99 | Br− | 11 [85], 11.83 [111] | 41.43 [111] | 31.57 [111] | 2.31 [111] | 71.82 [111] | 2.33 [111] | ||
| 100 | C14C1Pip+ | Br− | 3.22 [111] | 41.23 [111] | 31.77 [111] | 2.35 [111] | 70.65 [111] | 2.90 [111] | |
| 101 | C16C1Pip+ | Br− | 0.68 [109], 0.73 [111] | 37.3 [109], 41.14 [111] | 31.86 [111] | 2.63 [111] | 61 [109], 63.22 [111] | 3.51 [111] | |
| 102 | C18C1Pip+ | Cl− | 0.45 [66] | 37.7 [66] | 34.1 [66] | 3.3 [66] | 50 [66] | 3.8 [66] | |
| 103 | C16C1Aze+ | Br− | 0.590 [109] | 37.9 [109] | 65 [109] | ||||
| 104 | C16C1Azo+ | Br− | 0.51 [109] | 38.8 [109] | 75 [109] | ||||
| 105 | C10C1Mor+ | Br− | 30 [112] | ||||||
| 106 | C12C1Mor+ | Cl− | 21.80 [106] | 34.6 [106] | 36.4 [106] | 2.5 [106] | 66 [106] | 2.3 [106] | |
| 107 | Br− | 9.6 [112] | |||||||
| 108 | C14C1Mor+ | Br− | 4.0 [112], 2.93 [113], 4.10 [114] | 41.2 [113] | 2.79 [113] | 59 [113] | 3.2 [113] | −15.4 [113] | |
| 109 | C16C1Mor+ | Br− | 0.74 [109], 1.0 [112], 1.02 [113], 1.00 [114] | 29.9 [109] | 38.5 [113] | 2.69 [113], 1.82 [114] | 71 [109], 61 [113], 91 [114] | 3.6 [113] | −9.5 [113], −42 [114] |
| 110 | C18C1Mor+ | Br− | 0.33 [112] | ||||||
| 111 | C8Gu+ | Cl− | 75 [53] | 24.5 [53] | 4.60 [53] | 36.2 [53] | |||
| 112 | C10Gu+ | Cl− | 22 [53] | 23.5 [53] | 3.78 [53] | 44.2 [53] | |||
| 113 | C12Gu+ | Cl− | 5.5 [53] | 24.1 [53], 24.5 [115] | 3.93 [53], 4.54 [115] | 42.3 [53], 36.6 [115] | |||
| 115 | C8C1C1Gu+ | Cl− | 7.2 [53] | 35.4 [53] | 3.66 [53] | 45.4 [53] | |||
| 116 | C10C1C1Gu+ | Cl− | 2.1 [53] | 33.3 [53] | 3.40 [53] | 48.9 [53] | |||
| 117 | C12C1C1Gu+ | Cl− | 0.67 [53] | 32.5 [53] | 3.05 [53] | 54.5 [53] | |||
| 118 | C8Ph3P+ | Br− | 32 [116] | ||||||
| 120 | C10Ph3P+ | Br− | 6.1 [116], 9.0 [117], 6.60 [118] | 40.00 [118] | |||||
| 122 | C12Ph3P+ | Br− | 2.0 [116], 1.51 [118], 2.35 [119], 2.00 [120], 2.00 [121] | 40.80 [118], 46.0 [120] | 26.5 [120], 32.4 [121] | 2.24 [119], 1.36 [121] | 74 [119], 122 [121] | 3.01 [120] | −60.2 [119], −64.2 [121] |
| 123 | C14Ph3P+ | Br− | 0.33 [116], 0.61 [118], 0.61 [119], 0.60 [122] | 40.60 [118], 41.3 [122] | 1.88 [119] | 88 [119], 82.2 [122] | −56.0 [119] | ||
| 124 | C16Ph3P+ | Br− | 0.10 [116], 0.15 [118], 0.24 [119], 0.14 [121], 0.15 [123], 0.10 [124], 0.10 [125] | 40.25 [118] | 25.8 [121], 27.2 [123], 31.4 [124] | 1.02 [119], 1.38 [121], 1.39 [123], 1.40 [124] | 163 [119], 120 [121], 119 [123], 118.0 [124] | −75.3 [119], −71.1 [121], −51.3 [123], −58.9 [124] | |
| 125 | C18Ph3P+ | Br− | 0.018 [116] | ||||||
| Anionic ILBSs | |||||||||
| 127 | (CH3)4N+ | AOT− | 2.90 [126], 2.48 [127] | 29.4 [127] | 1.60 [126] | 104 [126], 96 [127] | |||
| 129 | (C2H5)4N+ | AOT− | 2.45 [126], 2.07 [127] | 28.7 [127] | 1.43 [126] | 116 [126], 101 [127] | |||
| 130 | (C3H7)4N+ | C12SO4− | 1.46 [127] | 31.8 [127] | 67 [127] | ||||
| 131 | AOT− | 0.97 [126], 1.27 [127] | 26.1 [127] | 1.71 [126] | 97 [126], 96 [127] | ||||
| 132 | (C4H9)4N+ | C8SO4− | 24.5 [128] | 32.0 [128] | 3.75 [128] | ||||
| 133 | C10SO4− | 4.17 [128] | 31.6 [128] | 4.03 [128] | |||||
| 134 | C12SO4− | 0.525 [128] | 31.2 [128] | 4.47 [128] | |||||
| 135 | C14SO4− | 0.26 [129] | 5.43 [129] | 31 [129] | |||||
| 136 | AOT− | 0.77 [126] | 1.63 [126] | 102 [126] | |||||
| 137 | C4Py+ | DBS− | 0.92 [130] | 29.69 [130] | 42.31 [130] | 2.31 [130] | 72.08 [130] | 3.92 [130] | |
| 138 | C4C1Pyrro+ | C12SO4− | 2.7 [131] | 34.3 [131] | 37.9 [131] | 2.27 [131] | 74 [131] | 3.5 [131] | |
| 139 | C4C1Im+ | C8SO3− | 135 [65] | 40.0 [65] | 1.6 [65] | ||||
| 140 | C12SO3− | 4.4 [132] | 36.9 [132] | 35.9 [132] | 1.14 [132] | 145 [132] | 3.05 [132] | ||
| 141 | C8SO4− | 33.4 [61], 23.0 [62], 34.9 [133], 30.5 [134], 30 [135], 34.5 [136], 31.9 [137], 30.0 [138] | 26.1 [61], 30.5 [62], 26.1 [133], 29.6 [134], 31.5 [135], 30.3 [136], 32.8 [137], 34.5 [138] | 45.9 [61], 45.9 [133], 42.4 [134], 41.0 [136], 37.5 [138] | 2.44 [61], 1.63 [62], 1.9 [133], 2.18 [134], 1.87 [135], 1.39 [136], 2.079 [138] | 68 [61], 102 [62], 87.1 [133], 76 [134], 89 [135], 12 [136], 69 [137], 131 [138] | 2.60 [61], 2.5 [133], 2.56 [135], 1.52 [138] | −49.7 [61], −38.7 [62], −49.5 [134] | |
| 142 | C10SO4− | 8.8 [139] | 34.7 [139] | 37.9 [139] | 2.81 [139] | 59 [139] | 2.7 [139] | ||
| 143 | C12SO4− | 1.8 [131], 2.4 [133], 2.30 [140], 1.9 [141], 1.84 [142] | 31.9 [131], 34.4 [133], 32.9 [140], 31.90 [142] | 40.3 [131], 37.6 [133], 39.90 [142] | 2.53 [131], 2.4 [133], 2.48 [142] | 66 [131], 67.8 [133], 56 [140], 67 [142] | 3.4 [131], 3.3 [133], 3.82 [142] | ||
| 144 | C14SO4− | 0.5 [139] | 30.5 [139] | 42.1 [139] | 1.66 [139] | 10 [139] | 4.2 [139] | ||
| 145 | DBS− | 1.08 [130] | 29.18 [130] | 42.82 [130] | 2.09 [130] | 79.86 [130] | 3.94 [130] | ||
| 146 | AOT− | 1.78 [140] | 25.7 [140] | 86 [140] | |||||
| 147 | TC− | 0.55 [140] | 24.8 [140] | 111 [140] | |||||
| 148 | C5C1Im+ | C12SO4− | 1.6 [143] | 57.86 [143] | 13.64 [143] | 0.96 [143] | 173.4 [143] | −59.23 [143] | |
| 149 | DBS− | 0.32 [144] | 30.92 [144] | 42.38 [144] | 1.91 [144] | 86.76 [144] | −1.03 [144] | ||
| 150 | C6C1Im+ | C8SO4− | 14.2 [133], 18.6 [137] | 25.6 [133], 30.3 [137] | 45.2 [133] | 1.9 [133] | 84.7 [133], 66 [137] | 2.9 [133] | |
| 151 | C12SO4− | 1.1 [133], 0.8 [139] | 27.1 [133], 30.0 [139] | 44.9 [133], 42.6 [139] | 2.4 [133], 2.08 [139] | 68.5 [133], 80 [139] | 4.1 [133], 4.0 [139] | ||
| 152 | C7C1Im+ | DBS− | 0.12 [144] | 34.21 [144] | 39.09 [144] | 1.35 [144] | 122.64 [144] | −1.15 [144] | |
| Biamphiphilic ILBSs | |||||||||
| 153 | C8C1Im+ | C8SO3− | 12 [65] | 43.7 [65] | 2.9 [65] | ||||
| 154 | C8SO4− | 4.1 [133] | 24.4 [133] | 47.6 [133] | 2.5 [133] | 66.0 [133] | 3.3 [133] | ||
| 155 | C12SO4− | 0.4 [133], 0.3 [139] | 26.0 [133], 26.9 [139] | 46.0 [133], 45.7 [139] | 2.4 [133], 2.33 [139] | 68.5 [133], 71 [139] | 4.3 [133], 4.5 [139] | ||
| 156 | C10C1Im+ | C12SO4− | 0.1 [139] | 25.4 [139] | 47.2 [139] | 2.36 [139] | 70 [139] | 5.0 [139] | |
| 157 | C16Py+ | C8SO3− | 54 [145] | ||||||
| 158 | C8SO4− | 24 [145] | |||||||
| 162 | C16(CH3)3N+ | C8SO3− | 88 [145] | ||||||
| 163 | C8SO4− | 26 [145] | |||||||
| Entry | Cation 1 | cmc × 103 (mol L−1) | γcmc (mN m−1) | Πcmc (mN m−1) | Γmax (mol m−2) | Amin (Å2) | pC20 | ΔG0ads (kJ mol−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | (C12Im)2C2)2+ | 0.55 [156] | 33.6 [156] | 1.26 [156] | 135 [156] | 4.54 [156] | ||
| 2 | (C16Im)2C2)2+ | 0.0341 [157] | ||||||
| 3 | (C16Im)2C3)2+ | 0.0048 [157] | ||||||
| 4 | (C10Im)2C4)2+ | 4.50 [43], 2 [45] | 35.2 [43], 35 [45] | 1.25 [43] | 133 [43] | 3.14 [43] | ||
| 5 | (C12Im)2C4)2+ | 0.72 [43], 0.76 [158] | 35.7 [43], 35.0 [158] | 37.8 [43] | 1.19 [43], 16.30 [158] | 140 [43], 101.43 [158] | 3.94 [43] | −50.93 [158] |
| 6 | (C14Im)2C4)2+ | 0.10 [43] | 37.2 [43] | 0.88 [43] | 188 [43] | 5.04 [43] | ||
| 7 | (C16Im)2C4)2+ | 0.0222 [157] | ||||||
| 8 | (C16Im)2C5)2+ | 0.0269 [157] | ||||||
| 9 | (C12Im)2C6)2+ | 0.78 [156] | 39.5 [156] | 1.16 [156] | 143 [156] | 3.73 [156] | ||
| 10 | (C16Im)2C6)2+ | 0.0501 [157] | ||||||
| 11 | (C4Im)2C8)2+ | 32.3 [45] | 47 [45] | |||||
| 12 | (C16Im)2C8)2+ | 0.0512 [157] | ||||||
| 13 | (C1Im)2C10)2+ | 14.9 [45] | 38 [45] | |||||
| 14 | (C4Im)2C10)2+ | 11.7 [45] | 38 [45] | |||||
| 15 | (C16Im)2C10)2+ | 0.0607 [157] | ||||||
| 16 | (C4Im)2C12)2+ | 7.2 [45] | 46 [45] | |||||
| 17 | (C10Im)2C12)2+ | 0.6 [45] | 48 [45] | |||||
| 18 | (C16Im)2C12)2+ | 0.0619 [157] | ||||||
| 19 | ((C12SMeIm)2C2)2+ | 0.32 [55] | 39.7 [55] | 2.60 [55] | 63 [55] | 3.93 [55] | −45.57 [55] | |
| 20 | ((C14SMeIm)2C2)2+ | 0.072 [55] | 42.9 [55] | 2.12 [55] | 78 [55] | 4.53 [55] | −47.80 [55] | |
| 22 | ((C12SMeIm)2C3)2+ | 0.26 [55] | 40.7 [55] | 2.13 [55] | 77 [55] | 4.06 [55] | −48.21 [55] | |
| 23 | ((C14SMeIm)2C3)2+ | 0.063 [55] | 45.8 [55] | 3.09 [55] | 53 [55] | 4.42 [55] | −42.31 [55] | |
| 25 | ((C12SMeIm)2C4)2+ | 0.22 [55] | 40.8 [55] | 2.06 [55] | 80 [55] | 4.12 [55] | −47.51 [55] | |
| 26 | ((C14SMeIm)2C4)2+ | 0.058 [55] | 46.6 [55] | 3.10 [55] | 53 [55] | 4.39 [55] | −42.82 [55] | |
| 28 | ((C12OHIm)2C3)2+ | 0.72 [159] | 30.0 [159] | 2.53 [159] | 65 [159] | 3.91 [159] | −49.67 [159] | |
| 29 | ((C12OHIm)2C4)2+ | 0.76 [159] | 28.1 [159] | 2.33 [159] | 71 [159] | 4.11 [159] | −50.63 [159] | |
| 30 | ((C12OHIm)2C5)2+ | 1.02 [159] | 32.9 [159] | 2.29 [159] | 72 [159] | 3.72 [159] | −52.46 [159] | |
| 31 | ((C12OHIm)2C6)2+ | 1.07 [159] | 35.2 [159] | 2.98 [159] | 55 [159] | 3.44 [159] | −44.78 [159] | |
| 32 | ((C12OHIm)2C8)2+ | 1.14 [159] | 37.6 [159] | 1.90 [159] | 87 [159] | 3.58 [159] | −49.45 [159] | |
| 33 | ((C12)3N)2C2)2+ | 1.995 [160] 2 | 39 [160] | 0.769 [160] | 251.7 [160] | 5.1 [160] | −20.19 [160] | |
| 34 | ((C12)3N)2C3)2+ | 1.412 [160] 2 | 40 [160] | 1.012 [160] | 147.3 [160] | 5.1 [160] | −19.30 [160] | |
| 35 | ((C12)3N)2C6)2+ | 1.445 [160] 2 | 42 [160] | 1.131 [160] | 146.8 [160] | 6.9 [160] | −19.63 [160] | |
| 36 | ((C8C1C1N)2(OE)3Gly)2+ | 1.02 [161] 2 | 55.2 [161] | 0.802 [161] | −47.5 [161] | |||
| 37 | ((C10C1C1N)2(OE)3Gly)2+ | 0.859 [161] | 32.55 [161] | 1.466 [161] | 3.863 [161] | −53.9 [161] | ||
| 38 | ((C12C1C1N)2(OE)3Gly)2+ | 0.711 [161] | 30.57 [161] | 3 [161] | 3.64 [161] | −37.1 [161] | ||
| 39 | ((C14C1C1N)2(OE)3Gly)2+ | 0.243 [161] | 34.5 [161] | 0.947 [161] | 4.727 [161] | −69.6 [161] | ||
| 40 | ((C16C1C1N)2(OE)3Gly)2+ | 0.631 [161] | 37 [161] | 1.314 [161] | 4.14 [161] | −55 [161] | ||
| 41 | ((C8C1C1N)2(OE)4Gly)2+ | 1.822 [161] | 49.08 [161] | 0.519 [161] | 3.417 [161] | −80.7 [161] | ||
| 42 | ((C10C1C1N)2(OE)4Gly)2+ | 1.7 [161] | 46.96 [161] | 0.417 [161] | 3.222 [161] | −85.3 [161] | ||
| 43 | ((C12C1C1N)2(OE)4Gly)2+ | 1.239 [161] | 28.54 [161] | 1.06 [161] | 4.124 [161] | −67.1 [161] | ||
| 44 | ((C14C1C1N)2(OE)4Gly)2+ | 0.333 [161] | 34.81 [161] | 0.813 [161] | 4.63 [161] | −75 [161] | ||
| 45 | ((C16C1C1N)2(OE)4Gly)2+ | 0.389 [161] | 36.51 [161] | 0.785 [161] | 4.71 [161] | −75.1 [161] | ||
| 46 | ((C8C1C1N)2(OE)5Gly)2+ | 0.701 [161] | 52.22 [161] | 0.519 [161] | −83 [161] | |||
| 47 | ((C10C1C1N)2(OE)5Gly)2+ | 0.649 [161] | 48.28 [161] | 0.827 [161] | 3.539 [161] | −62.5 [161] | ||
| 48 | ((C12C1C1N)2(OE)5Gly)2+ | 0.607 [161] | 33.78 [161] | 1.4 [161] | 4 [161] | −55.1 [161] | ||
| 49 | ((C14C1C1N)2(OE)5Gly)2+ | 0.502 [161] | 33.61 [161] | 1.04 [161] | 4.389 [161] | −65.2 [161] | ||
| 50 | ((C16C1C1N)2(OE)5Gly)2+ | 0.398 [161] | 43.92 [161] | 0.925 [161] | 5.91 [161] | −60.0 [161] | ||
| 51 | (C10Pyrro)2C4)2+ | 3.3 [56] | 43.2 [56] | 1.37 [56] | 121.2 [56] | 2.96 [56] | ||
| 52 | (C12Pyrro)2C4)2+ | 0.5 [162], 0.53 [56] | 41.7 [56] | 1.46 [56] | 113.3 [56] | 3.80 [56] | ||
| 53 | (C14Pyrro)2C4)2+ | 0.1 [162], 0.108 [56] | 40.4 [56] | 1.59 [56] | 104.4 [56] | 4.41 [56] | ||
| 54 | ((C8Im)3Am)3+ | 4.3 [163] | 33 [163] | 1.11 [163] | 1.50 [163] | 3.13 [163] | ||
| 55 | ((C8Im)3Bn)3+ | 2.2 [163] | 40 [163] | 1.37 [163] | 1.21 [163] | 2.81 [163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Seoud, O.A.; Keppeler, N.; Malek, N.I.; Galgano, P.D. Ionic Liquid-Based Surfactants: Recent Advances in Their Syntheses, Solution Properties, and Applications. Polymers 2021, 13, 1100. https://doi.org/10.3390/polym13071100
El Seoud OA, Keppeler N, Malek NI, Galgano PD. Ionic Liquid-Based Surfactants: Recent Advances in Their Syntheses, Solution Properties, and Applications. Polymers. 2021; 13(7):1100. https://doi.org/10.3390/polym13071100
Chicago/Turabian StyleEl Seoud, Omar A., Nicolas Keppeler, Naved I. Malek, and Paula D. Galgano. 2021. "Ionic Liquid-Based Surfactants: Recent Advances in Their Syntheses, Solution Properties, and Applications" Polymers 13, no. 7: 1100. https://doi.org/10.3390/polym13071100
APA StyleEl Seoud, O. A., Keppeler, N., Malek, N. I., & Galgano, P. D. (2021). Ionic Liquid-Based Surfactants: Recent Advances in Their Syntheses, Solution Properties, and Applications. Polymers, 13(7), 1100. https://doi.org/10.3390/polym13071100






