Bone Matrix Non-Collagenous Proteins in Tissue Engineering: Creating New Bone by Mimicking the Extracellular Matrix
Abstract
1. Introduction
2. Bone Extracellular Matrix: Characterization, Properties, and Quality
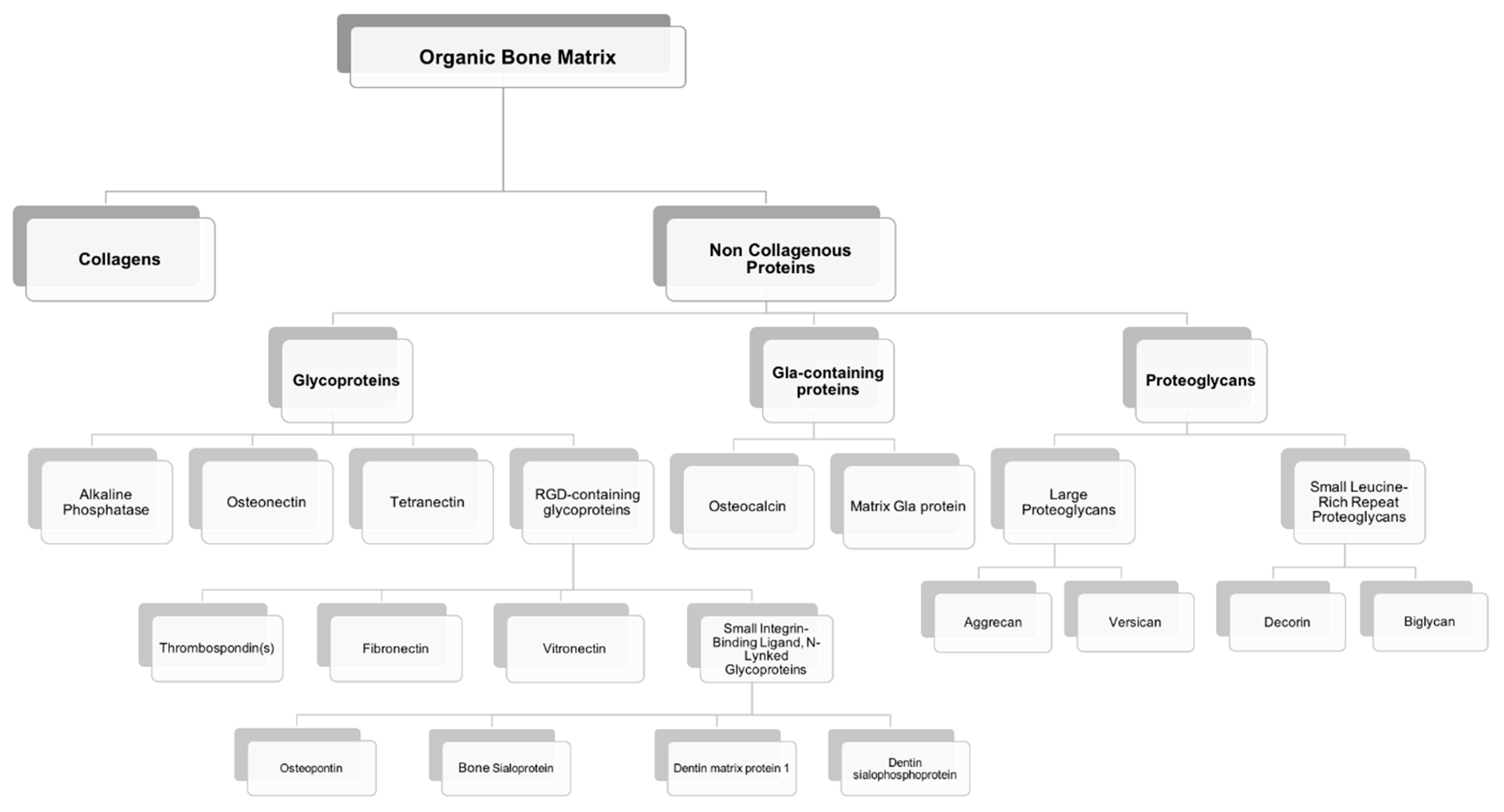
3. Non-Collagenous Bone Matrix Proteins
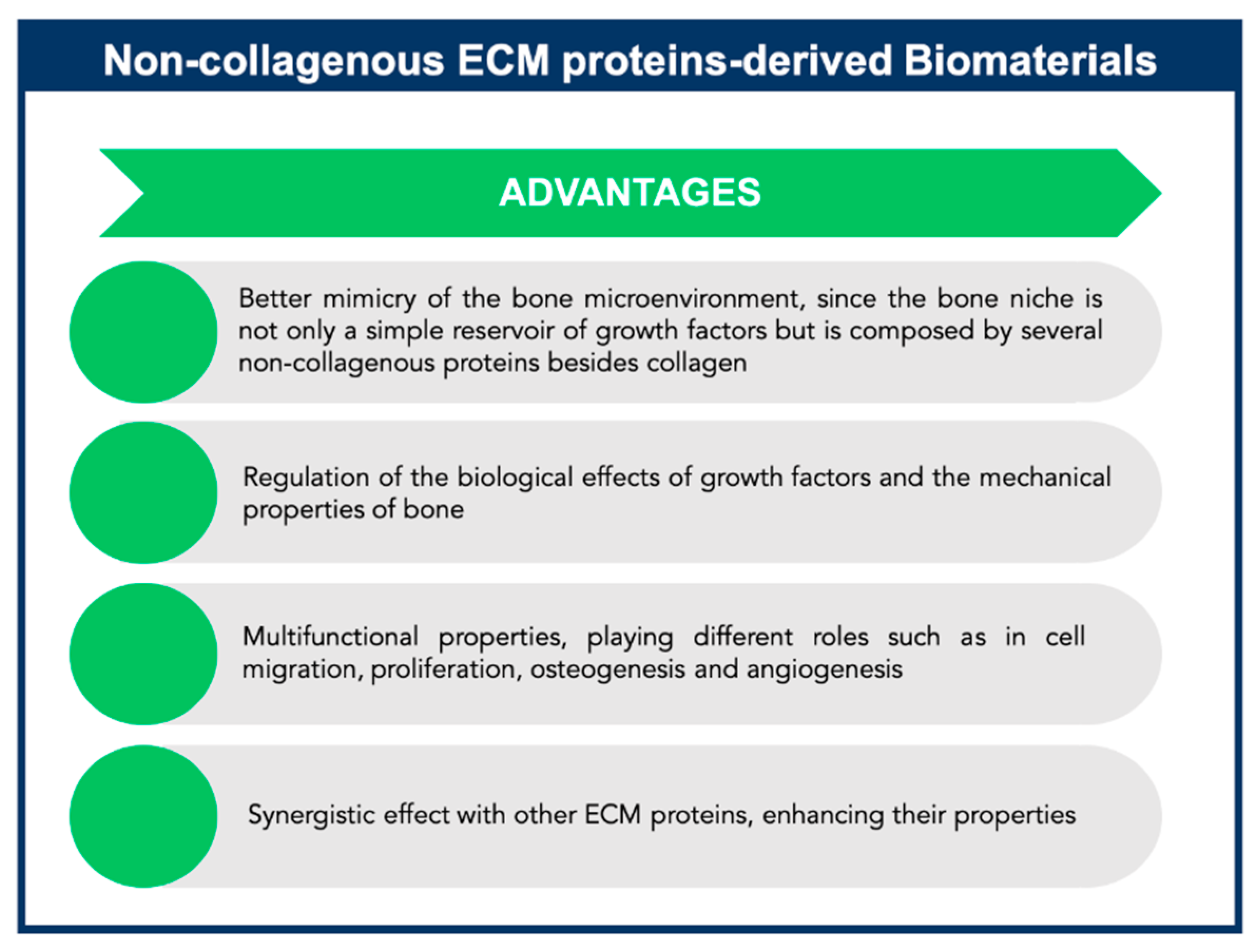
4. Exploiting Non-Collagenous Proteins in Bone Tissue Engineering Applications: An ECM Mimicking Approach
4.1. Proteoglycans
4.1.1. Large Proteoglycans
Aggrecan
Versican
4.1.2. Small Leucine-Rich Repeat Proteoglycans
Decorin
Biglycan
4.2. Glycoproteins
4.2.1. Alkaline Phosphatase
4.2.2. Osteonectin
4.2.3. Tetranectin
4.2.4. RGD-Containing Glycoproteins
Thrombospondin(s)
Fibronectin
Vitronectin
Osteopontin
Bone Sialoprotein
Dentin Matrix Proteins
Dentin Sialophosphoprotein
4.3. Gla-Containing Proteins
4.3.1. Osteocalcin
4.3.2. Matrix Gla Protein
4.4. Serum Proteins
4.5. Synergistic Biomimetic Strategies: Combination of ECM Proteins/Peptides to Elicit Bone Tissue Regeneration Responses
4.6. Native ECM as a Biomaterial Source
5. Concluding Remarks and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaszemski, M.J.; Payne, R.G.; Hayes, W.C.; Langer, R.; Mikos, A.G. Evolution of bone transplantation: Molecular, cellular and tissue strategies to engineer human bone. Biomaterials 1996, 17, 175–185. [Google Scholar] [CrossRef]
- Koond, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Stock, U.A.; Vacanti, J.P. Tissue engineering: Current state and prospects. Annu. Rev. Med. 2001, 52, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, S3–S6. [Google Scholar] [CrossRef]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Weber, F.E.; Schmoekel, H.G.; Schense, J.C.; Kohler, T.; Müller, R.; Hubbell, J.A. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat. Biotechnol. 2003, 21, 513–518. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, W.; Qiu, K.; Chen, L.; Wang, W.; Nie, W.; Mo, X.; He, C. BMP-2-derived peptide and dexamethasone incorporated mesoporous silica nanoparticles for enhanced osteogenic differentiation of bone mesenchymal stem cells. ACS Appl. Mater. Interfaces 2015, 7, 15777–15789. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Y.; Duan, Z.X.; Guo, X.D.; Li, J.F.; Lu, H.W.; Zheng, Q.X.; Quan, D.P.; Yang, S.H. Bone induction by biomimetic PLGA-(PEG-ASP)n copolymer loaded with a novel synthetic BMP-2-related peptide in vitro and in vivo. J. Control. Release 2010, 144, 190–195. [Google Scholar] [CrossRef]
- Wang, E.A.; Rosen, V.; D’Alessandro, J.S.; Bauduy, M.; Cordes, P.; Harada, T.; Israel, D.I.; Hewick, R.M.; Kerns, K.M.; LaPan, P.; et al. Recombinant human bone morphogenetic protein induces bone formation. Proc. Natl. Acad. Sci. USA 1990, 87, 2220–2224. [Google Scholar] [CrossRef]
- Friedlaender, G.E.; Perry, C.R.; Cole, J.D.; Cook, S.D.; Cierny, G.; Muschler, G.F.; Zych, G.A.; Calhoun, J.H.; LaForte, A.J.; Yin, S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J. Bone Joint Surg. Am. 2001, 83, S151–S158. [Google Scholar] [CrossRef]
- Boden, S.D.; Kang, J.; Sandhu, H.; Heller, J.G. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: A prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine 2002, 27, 2662–2673. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part. B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Woo, E.J. Adverse events after recombinant human BMP2 in nonspinal orthopaedic procedures. Clin. Orthop. Relat. Res. 2013, 471, 1707–1711. [Google Scholar] [CrossRef]
- Mi, J.; Xu, J.; Yao, H.; Li, X.; Tong, W.; Li, Y.; Dai, B.; He, X.; Chow, D.H.K.; Li, G.; et al. Calcitonin gene-related peptide enhances distraction osteogenesis by increasing angiogenesis. Tissue Eng. Part. A 2021, 27, 87–102. [Google Scholar] [CrossRef]
- Lai, M.; Yan, X.; Shen, K.; Tang, Q.; Fang, X.; Zhang, C.; Zhu, Z.; Hou, Y. The effect of calcitonin gene-related peptide functionalized TiO2 nanotubes on osteoblast and osteoclast differentiation in vitro. Colloids Surf. A 2020, 600, 124899. [Google Scholar] [CrossRef]
- Karadag, A.; Iqbai, H.; Yazici, H. Peptide-mediated bone tissue engineering. In Racing of the Surface; Li, B., Moriarty, T., Webster, T., Xing, M., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Sroga, G.E.; Karim, L.; Colon, W.; Vashishth, D. Biochemical Characterization of Major Bone-Matrix Proteins Using Nanoscale-Size Bone Samples and Proteomics Methodology. Mol. Cell. Proteomics 2011, 10, M110.006718. [Google Scholar] [CrossRef] [PubMed]
- Vashishth, D. The role of the collagen matrix in skeletal fragility. Curr. Osteoporos. Rep. 2007, 5, 62–66. [Google Scholar] [CrossRef]
- Herring, G.M.; Ashton, B.A. The isolation of soluble proteins, glycoproteins, and proteoglycans from bone. Prep. Biochem. 1974, 4, 179–200. [Google Scholar] [CrossRef]
- Roach, H.I. Why does bone matrix contain non-collagenous proteins? The possible roles of osteocalcin, osteonectin, osteopontin and bone sialoprotein in bone mineralisation and resorption. Cell Biol. Int. 1994, 18, 617–628. [Google Scholar] [CrossRef]
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The role of collagen in bone strength. Osteoporos. Int. 2006, 17, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Yerramshetty, J.S.; Akkus, O. The associations between mineral crystallinity and the mechanical properties of human cortical bone. Bone 2008, 42, 476–482. [Google Scholar] [CrossRef]
- Fratzl, P.; Paris, O.; Klaushofer, K.; Landis, W.J. Bone mineralization in an osteogenesis imperfect mouse model studied by small-angle x-ray scattering. J. Clin. Invest. 2006, 97, 396–402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dong, X.N.; Zoghi, M.; Ran, Q.; Wang, X. Collagen mutation causes changes of the microdamage morphology in bone of an OI mouse model. Bone 2002, 47, 1071–1075. [Google Scholar] [CrossRef]
- Jämsä, T.; Rho, J.Y.; Fan, Z.; MacKay, C.A.; Marks, S.C., Jr.; Tuukkanen, J. Mechanical properties in long bones of rat osteopetrotic mutations. J. Biomech. 2002, 35, 161–165. [Google Scholar] [CrossRef]
- Fantner, G.E.; Adams, J.; Turner, P.; Thurner, P.J.; Fisher, L.W.; Hansma, P.K. Nanoscale ion mediated networks in bone: Osteopontin can repeatedly dissipate large amounts of energy. Nano Letters 2007, 7, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Ritter, N.M.; Farach-Carson, M.C.; Butler, W.T. Evidence for the formation of a complex between osteopontin and osteocalcin. J. Bone Miner. Res. 1992, 7, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Poundarik, A.A.; Diab, T.; Sroga, G.E.; Ural, A.; Boskey, A.L.; Gundberg, C.M.; Vashishth, D. Dilatational band formation in bone. Proc. Natl. Acad. Sci. USA 2012, 109, 19178–19183. [Google Scholar] [CrossRef]
- Boskey, A.L. Noncollagenous matrix proteins and their role in mineralization. Bone Miner. 1989, 6, 111–123. [Google Scholar] [CrossRef]
- Ninomiya, J.T.; Tracy, R.P.; Calore, J.D.; Gendreau, M.A.; Kelm, R.J.; Mann, K.G. Heterogeneity of human bone. J. Bone Min. Res. 1990, 5, 933–938. [Google Scholar] [CrossRef]
- Marcus, R.; Dempster, D.; Cauley, J.; Feldman, D. Osteoporosis, 4th ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Bilezikian, J.P.; Raisz, L.G.; Martin, T.J. Principles of Bone Biology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Kesireddy, V.; Kasper, F.K. Approaches for building bioactive elements into synthetic scaffolds for bone tissue engineering. J. Mater. Chem. B. 2016, 4, 6773–6786. [Google Scholar] [CrossRef]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J.R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef] [PubMed]
- Maisani, M.; Pezzoli, D.; Chassande, O.; Mantovani, D. Cellularizing hydrogel-based scaffolds to repair bone tissue: How to create a physiologically relevant micro-environment? J. Tissue Eng. 2017, 8, 2041731417712073. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12. [Google Scholar] [CrossRef]
- Harbers, G.M.; Healy, K.E. The effect of ligand type and density on osteoblast adhesion, proliferation, and matrix mineralization. J. Biomed. Mater. Res. A 2005, 75, 855–869. [Google Scholar] [CrossRef]
- Barber, T.A.; Harbers, G.M.; Park, S.; Gilbert, M.; Healy, K.E. Ligand density characterization of peptide-modified biomaterials. Biomaterials 2005, 26, 6897–6905. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D extracellular matrix mimics: Fundamental concepts and role of materials chemistry to influence stem cell fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, Y.S.; Lee, S.J.; Chung, C.P.; Park, Y.J. Bioactive peptide-modified biomaterials for bone regeneration. Curr. Pharm. Des. 2011, 17, 2663–2676. [Google Scholar] [CrossRef]
- Koivunen, E.; Wang, B.; Dickinson, C.D.; Ruoslahti, E. Peptides in cell adhesion research. Methods Enzymol. 1994, 245, 346–369. [Google Scholar] [CrossRef]
- Shin, H.; Jo, S.; Mikos, A.G. Modulation of marrow stromal osteoblast adhesion on biomimetic oligo[poly(ethylene glycol) fumarate] hydrogels modified with Arg-Gly-Asp peptides and a poly(ethyleneglycol) spacer. J. Biomed. Mater. Res. 2002, 61, 169–179. [Google Scholar] [CrossRef]
- Schaffner, P.; Dard, M.M. Structure and function of RGD peptides involved in bone biology. Cell Mol. Life Sci. 2003, 60, 119–132. [Google Scholar] [CrossRef]
- Wang, X.; Yan, C.; Ye, K.; He, Y.; Li, Z.; Ding, J. Effect of RGD nanospacing on differentiation of stem cells. Biomaterials 2013, 34, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, K.; Li, Z.; Yan, C.; Ding, J. Adhesion, proliferation, and differentiation of mesenchymal stromal cells on RGD nanopatterns of varied nanospacings. Organogenesis 2013, 9, 280–286. [Google Scholar] [CrossRef]
- Vukicevic, S.; Luyten, F.P.; Kleinman, H.K.; Reddi, A.H. Differentiation of canalicular cell processes in bone cells by basement membrane matrix components: Regulation by discrete domains of laminin. Cell 1990, 63, 437–445. [Google Scholar] [CrossRef]
- Aota, S.; Nagai, T.; Yamada, K.M. Characterization of regions of fibronectin besides the arginine-glycine-aspartic acid sequence required for adhesive function of the cell-binding domain using site-directed mutagenesis. J. Biol. Chem. 1991, 266, 15938–15943. [Google Scholar] [CrossRef]
- Bhatnagar, R.S.; Qian, J.J.; Wedrychowska, A.; Sadeghi, M.; Wu, Y.M.; Smith, N. Design of biomimetic habitats for tissue engineering with P-15, a synthetic peptide analogue of collagen. Tissue Eng. 1999, 5, 53–65. [Google Scholar] [CrossRef]
- Reyes, C.D.; García, A.J. Alpha2beta1 integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. J. Biomed. Mater. Res. A 2004, 69, 591–600. [Google Scholar] [CrossRef]
- Reyes, C.D.; Petrie, T.A.; Burns, K.L.; Schwartz, Z.; García, A.J. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials 2007, 28, 3228–3235. [Google Scholar] [CrossRef]
- Aziz, A.H.; Wilmoth, R.L.; Ferguson, V.L.; Bryant, S.J. IDG-SW3 osteocyte differentiation and bone extracellular matrix deposition are enhanced in a 3D matrix metalloproteinase-sensitive hydrogel. ACS Appl. Biol. Mater. 2020, 16, 1666–1680. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.E.; Urist, M.R. Human bone morphogenetic protein allografting for reconstruction of femoral nonunion. Clin. Orthop. Rel. Res. 2000, 371, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, Y.; Liu, Q.; Gao, T.; Feng, J.Q.; Dechow, P.; D’Souza, R.N.; Qin, C.; Liu, X. Biomimetic engineering of nanofibrous gelatin scaffolds with noncollagenous proteins for enhanced bone regeneration. Tissue Eng. Part. A 2013, 19, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Osathanon, T.; Giachelli, C.M.; Somerman, M.J. Immobilization of alkaline phosphatase on microporous nanofibrous fibrin scaffolds for bone tissue engineering. Biomaterials 2009, 30, 4513–4521. [Google Scholar] [CrossRef] [PubMed]
- Klontzas, M.E.; Reakasame, S.; Silva, R.; Morais, J.C.F.; Vernardis, S.; MacFarlane, R.J.; Heliotis, M.; Tsiridis, E.; Panoskaltsis, N.; Boccaccini, A.R.; et al. Oxidized alginate hydrogels with the GHK peptide enhance cord blood mesenchymal stem cell osteogenesis: A paradigm for metabolomics-based evaluation of biomaterial design. Acta Biomater. 2019, 88, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Won, J.E.; Mateos-Timoneda, M.A.; Castano, O.; Planell, J.A.; Seo, S.J.; Lee, E.J.; Han, C.M.; Kim, H.W. Fibronectin immobilization on to robotic-dispensed nanobioactive glass/polycaprolactone scaffolds for bone tissue engineering. Biotechnol. Lett. 2015, 37, 935–942. [Google Scholar] [CrossRef]
- Cacchioli, A.; Ravanetti, F.; Bagno, A.; Dettin, M.; Gabbi, C. Human vitronectin-derived peptide covalently grafted onto titanium surface improves osteogenic activity: A pilot in vivo study on rabbits. Tissue Eng. Part. A 2009, 15, 2917–2926. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Zygourakis, K.; Farach-Carson, M.C.; Yaszemski, M.J.; Mikos, A.G. Attachment, proliferation, and migration of marrow stromal osteoblasts cultured on biomimetic hydrogels modified with an osteopontin-derived peptide. Biomaterials 2004, 25, 895–906. [Google Scholar] [CrossRef]
- Hamada, Y.; Egusa, H.; Kaneda, Y.; Hirata, I.; Kawaguchi, N.; Hirao, T.; Matsumoto, T.; Yao, M.; Daito, T.; Suzuki, M.; et al. Synthetic Osteopontin-derived peptide SVVYGLR can induce neovascularization in artificial bone marrow scaffold biomaterials. Dent. Mater. J. 2007, 26, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, A.; Klein, A.; Ritz, U.; Götz, H.; Mattyasovsky, S.G.; Rommens, P.M.; Hofmann, A. Evaluation of bone sialoprotein coating of three-dimensional printed calcium phosphate scaffolds in a calvarial defect model in mice. Materials 2018, 11, e2336. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Baranowski, A.; Ritz, U.; Götz, H.; Heinemann, S.; Mattyasovszky, S.; Rommens, P.M.; Hofmann, A. Effect of bone sialoprotein coated three-dimensional printed calcium phosphate scaffolds on primary human osteoblasts. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.H. Role of proteoglycan in the provisional calcification of cartilage. Clin. Orthop. Rel. Res. 1991, 262, 256–263. [Google Scholar] [CrossRef]
- Chen, C.C.; Boskey, A.L.; Rosenberg, L.C. The inhibitory effect of cartilage proteoglycans on hydroxyapatite growth. Calcif. Tissue Int. 1984, 36, 285–290. [Google Scholar] [CrossRef]
- Fedarko, N.S. Isolation and purification of proteoglycans. EXS 1994, 70, 9–35. [Google Scholar] [CrossRef]
- Weyers, A.; Linhardt, R.J. Neoproteoglycans in tissue engineering. FEBS J. 2013, 280, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Paderi, J.E.; Panitch, A. Design of a synthetic collagen-binding peptidoglycan that modulates collagen fibrillog.enesis. Biomacromolecules 2008, 9, 2562–2566. [Google Scholar] [CrossRef] [PubMed]
- Sistiabudi, R.; Paderi, J.; Panitch, A.; Ivanisevic, A. Modification of native collagen with cell-adhesive peptide to promote RPE cell attachment on Bruch’s membrane. Biotechnol. Bioeng. 2009, 102, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Stuart, K.; Paderi, J.; Snyder, P.W.; Freeman, L.; Panitch, A. Collagen-binding peptidoglycans inhibit MMP mediated collagen degradation and reduce dermal scarring. PLoS ONE 2011, 6, e22139. [Google Scholar] [CrossRef]
- Pieper, J.S.; Oosterhof, A.; Dijkstra, P.J.; Veerkamp, J.H.; van Kuppervelt, T.H. Preparation and characterization of porous crosslinked collagenous matrices containing bioavailable chondroitin sulphate. Biomaterials 1999, 20, 847–858. [Google Scholar] [CrossRef]
- Farrell, E.; O’Brien, F.J.; Doyle, P.; Fischer, J.; Yannas, I.; Harley, B.A.; O’Connell, B.; Prendergast, P.J.; Campbell, V.A. A collagen-glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes. Tissue Eng. 2006, 12, 459–468. [Google Scholar] [CrossRef]
- Caliari, S.R.; Harley, B.A.C. Collagen-GAG scaffold biophysical properties bias MSC lineage choice in the presence of mixed soluble signals. Tissue Eng. Part. A 2014, 20, 2463–2472. [Google Scholar] [CrossRef]
- Tierney, C.M.; Jaasma, M.J.; O’Brien, F.J. Osteoblast activity on collagen-GAG scaffolds is affected by collagen and GAG concentration. J. Biomed. Mater. Res. A 2009, 91, 92–101. [Google Scholar] [CrossRef]
- Caliari, S.R.; Weisgerber, D.W.; Ramirez, M.A.; Kelkhoff, D.O.; Harley, B.A. The influence of collagen-glycosaminoglycan scaffold relative density and microstructural anisotropy on tenocyte bioactivity and transcriptomic stability. J. Mech. Behav. Biomed. Mater. 2012, 11, 27–40. [Google Scholar] [CrossRef]
- Pieper, J.S.; van Wachem, P.B.; Mja, V.L.; Brouwer, L.A.; Hafmans, T.; Veerkamp, J.H.; van Kuppevelt, T.H. Attachment of glycosaminoglycans to collagenous matrices modulates the tissue response in rats. Biomaterials 2000, 21, 1689–1699. [Google Scholar] [CrossRef]
- Harley, B.A.; Lynn, A.K.; Wissner-Gross, Z.; Bonfield, W.; Yannas, I.V.; Gibson, L.J. Design of a multiphase osteochondral scaffold. II. Fabrication of a mineralized collagen-glycosaminoglycan scaffold. J. Biomed. Mater. Res. A 2010, 92, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Pereira, C.T.; Ren, X.; Huang, W.; Bischoff, D.; Weisgerber, D.W.; Yamguchi, D.T.; Harley, B.A.; Miller, T.A. Optimizing collagen scaffolds for bone engineering: Effects of cross-linking and mineral content on structural contraction and osteogenesis. J. Craniofac. Surg. 2015, 26, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Guo, Q.; Shores, L.S.; Aly, A.; Ramakrishnan, M.; Kim, G.H.; Lu, Q.; Su, L.; Elisseeff, J.H. Use of a chondroitin sulfate bioadhesive to enhance integration of bioglass particles for repairing critical-size bone defects. J. Biomed. Mater. Res. A 2015, 103, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D.S.; Tezcaner, A.; Korkusuz, P.; Korkusuz, F.; Hasirci, V. Collagen-chondroitin sulfate-based PLLA-SAIB-coated rhBMP-2 delivery system for bone repair. Biomaterials 2005, 26, 4023–4034. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Schaller, B.; Kobayashi, E.; Hernandez, M.; Zhang, Y.; Miron, R.J. Hyaluronic acid gel-based scaffolds as potential carrier for growth factors: An in vitro bioassay on its osteogenic potential. J. Clin. Med. 2016, 5, 112. [Google Scholar] [CrossRef]
- Watanabe, H.; Yamada, Y. Chondrodysplasia of gene knockout mice for aggrecan and link protein. Glycoconjugate J. 2002, 19, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Nakamyra, M.; Sone, S.; Takahashi, I.; Mizoguchi, I.; Echigo, S.; Sasano, Y. Expression of versican and ADAMTS1, 4, and 5 during bone development in the rat mandible and hind limb. J. Histochem. Cytochem. 2005, 53, 1553–1562. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, L.; Kiani, C.; Yang, B.L.; Hu, W.; Yang, B.B. Promotion of chondrocyte proliferation by versican mediated by G1 domain and EGF-like motifs. J. Cell. Biochem. 1999, 73, 445–457. [Google Scholar] [CrossRef]
- Kjellän, L.; Lindahl, U. Proteoglycans: Structures and interactions. Annu. Rev. Biochem. 1991, 60, 443–475. [Google Scholar] [CrossRef]
- Boskey, A.L.; Spevak, L.; Doty, S.B.; Rosenberg, L. Effects of bone CS-proteoglycans, DS-decorin, and DS-biglycan on hydroxyapatite formation in a gelatin gel. Calcif. Tissue Int. 1997, 61, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Fleischmajer, R.A.; Fisher, L.W.; MacDonald, E.D.; Jacobs, L., Jr.; Perlish, J.S.; Termine, J.D. Decorin interacts with fibrillary collagen of embryonic and adult human skin. J. Struct. Biol. 1991, 106, 82–90. [Google Scholar] [CrossRef]
- Goldberg, M.; Septier, D.; Rapoport, O.; Iozzo, R.V.; Young, M.F.; Ameye, L.G. Targeted disruption of two small-leucine-rich proteoglycans, biglycan and decorin, excerpts divergent effects on enamel and dentin formation. Calcif. Tissue Int. 2005, 77, 297–310. [Google Scholar] [CrossRef]
- Ameye, L.; Young, M.F. Mice deficient in small leucine-rich proteoglycans: Novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology 2002, 12, 107R–116R. [Google Scholar] [CrossRef] [PubMed]
- Corsi, A.; Xu, T.; Chen, X.D.; Boyde, A.; Liang, J.; Mankani, M.; Sommer, B.; Iozzo, R.V.; Eichstetter, I.; Robey, P.G.; et al. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J. Bone Miner. Res. 2002, 17, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Bianco, P.; Fisher, L.W.; Longenecker, G.; Smith, E.; Goldstein, S.; Bonadio, J.; Boskey, A.L.; Heegaard, A.M.; Sommer, B.; et al. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat. Genet. 1998, 20, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.W.; Hawkins, G.R.; Tuross, N.; Termine, J.D. Purification and partial characterization of small proteoglycans I and II, bone sialoproteins I and II, and osteonectin from the mineral compartment of developing human bone. J. Biol. Chem. 1987, 262, 9702–9708. [Google Scholar] [CrossRef]
- Roach, H.I. Association of matrix acid and alkaline phosphatases with mineralization of cartilage and endochondral bone. Histochem. J. 1999, 31, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, T.; Nah, H.D.; Shapiro, I.M.; Pacifici, M. Regulated production of mineralization-competent matrix vesicles in hypertrophic chondrocytes. J. Cell. Biol. 1997, 137, 1140–1160. [Google Scholar] [CrossRef]
- Collin, P.; Nefussi, J.R.; Wetterwald, A.; Nikolas, V.; Boy-Lefever, M.L.; Fleisch, H.; Forest, N. Expression of collagen, osteocalcin and bone alkaline phosphatase in a mineralizing rat osteoblastic cell culture. Calcif. Tissue Int. 1992, 50, 175–183. [Google Scholar] [CrossRef]
- Fedde, K.N.; Blair, L.; Silverstein, J.; Coburn, S.P.; Ryan, L.M.; Weinstein, R.S.; Waymire, K.; Narisawa, S.; Millan, J.L.; MacGregor, G.R.; et al. Alkaline phosphatase knockout mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J. Bone Miner. Res. 1999, 14, 2015–2026. [Google Scholar] [CrossRef]
- Yoon, K.; Golub, E.; Rodan, G.A. Alkaline phosphatase transfected cells promote calcium and phosphate deposition. Connect. Tissue Res. 1989, 22, 17–25. [Google Scholar] [CrossRef]
- Rosset, E.M.; Bradshaw, A.D. SPARC/Osteonectin in mineralized tissue. Matrix Biol. 2016, 52, 78–87. [Google Scholar] [CrossRef]
- Stenner, D.D.; Tracy, R.G.; Riggs, B.L.; Mann, K.G. Human platelets contain and secrete Osteonectin. Proc. Natl. Acad. Sci. USA 1986, 83, 6892–6896. [Google Scholar] [CrossRef] [PubMed]
- Kelm, R.J., Jr.; Mann, K.G. The collagen binding specificity of bone and platelet osteonectin is related to differences in glycosylation. J. Biol. Chem. 1991, 266, 9632–9639. [Google Scholar] [CrossRef]
- Clezardin, P.; Malavel, L.; Ehrensperger, A.S.; Delmas, P.; Dechavanne, M.; McGregor, J.L. Complex formation of human thrombospondin with osteonectin. Eur. J. Biochem. 1988, 175, 275–284. [Google Scholar] [CrossRef]
- Termine, J.D.; Kleinman, H.K.; Whitson, S.W.; Conn, K.M.; McGarvey, M.L.; Martin, G.R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell 1981, 26, 99–105. [Google Scholar] [CrossRef]
- Delany, A.M.; Amling, M.; Priemel, M.; Howe, C.; Baron, R.; Canalis, E. Osteopenia and decreased bone formation in osteonectin-deficient mice. J. Clin. Invest. 2000, 105, 915–923. [Google Scholar] [CrossRef]
- Boskey, A.L.; Moore, D.J.; Amling, M.; Canalis, E.; Delany, A.M. Infrared analysis of the mineral and matrix in bones of osteonecin-null mice and their wild-type controls. J. Bone Miner. Res. 2003, 18, 1005–1011. [Google Scholar] [CrossRef]
- Yan, Q.; Sage, E.H. SPARC, a matricellular glycoprotein with important biological functions. J. Histochem. Cytochem. 1999, 47, 1495–1506. [Google Scholar] [CrossRef]
- Wewer, U.M.; Ibaraki, K.; Schjorring, P.; Durkin, M.E.; Young, M.F.; Albrechtsen, R. A potential role for tetranectin in mineralization during osteogenesis. J. Cell. Biol. 1994, 127, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Iba, K.; Abe, Y.; Chikenji, T.; Kanaya, K.; Chiba, H.; Sasaki, K.; Dohke, T.; Wada, T.; Yamashita, T. Delayed fracture healing in tetranectin-deficient mice. J. Bone Miner. Metab. 2013, 31, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Iba, K.; Durkin, M.E.; Johnsen, L.; Hunziker, E.; Damgaard-Pedersen, K.; Zhang, H.; Engvall, E.; Albrechtsen, R.; Wewer, U.M. Mice with a targeted deletion of the tetranectin gene exhibit a spinal deformity. Mol. Cell. Biol. 2001, 21, 7817–7825. [Google Scholar] [CrossRef]
- Adams, J.C.; Lawler, J. The thrombospondins. Int. J. Biochem. Cell. Biol. 2004, 36, 961–968. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Bain, S.D.; Kyriakides, T.R.; Smith, E.A.; Goldstein, S.A.; Bornstein, P. Increased marrow-derived osteoprogenitor cells and endosteal bone formation in mice lacking thrombospondin 2. J. Bone Miner. Res. 2000, 15, 851–862. [Google Scholar] [CrossRef]
- Carron, J.A.; Fraser, W.C.; Gallagher, J.A. Thrombospondin promotes resorption by osteoclasts in vitro. Biochem. Biophys. Res. Commun. 1995, 213, 1017–1025. [Google Scholar] [CrossRef]
- Bornstein, P.; Kyriakides, T.R.; Yang, Z.; Armstrong, L.C.; Birk, D.E. Thrombospondin 2 modulates collagen fibrillogenesis and angiogenesis. J. Invest. Dermatol. Symp. Proc. 2000, 5, 61–66. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Zhu, Y.H.; Smith, L.T.; Bain, S.D.; Yang, Z.; Lin, M.T.; Danielson, K.G.; Iozzo, R.V.; LaMarca, M.; McKinney, C.E.; et al. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J. Cell. Biol. 1998, 140, 419–430. [Google Scholar] [CrossRef]
- Stenman, S.; Vaheri, A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J. Exp. Med. 1978, 147, 1054–1064. [Google Scholar] [CrossRef]
- Grzesik, W.J.; Robey, P.G. Bone matrix RGD glycoproteins: Immunolocalization and interaction with human primary osteoblastic bone cells in vitro. J. Bone Miner. Res. 1994, 9, 487–496. [Google Scholar] [CrossRef]
- George, E.L.; Georges-Labouesse, E.N.; Patel-King, R.S.; Rayburn, H.; Hynes, R.O. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 1993, 119, 1079–1091. [Google Scholar]
- Kumagai, T.; Lee, I.; Ono, Y.; Maeno, M.; Takagi, M. Ultrastuctural localization and biochemical characterization of vitronectin in developing rat bone. Histochem. J. 1998, 30, 111–119. [Google Scholar] [CrossRef]
- Koschnick, S.; Konstantinides, S.; Schafer, K.; Crain, K.; Loskutoff, D.J. Thrombotic phenotype of mice with a combined deficiency in plasminogen activator inhibitor 1 and vitronectin. J. Thromb. Haemost. 2005, 3, 2290–2295. [Google Scholar] [CrossRef]
- Denhardt, D.T.; Guo, X. OPN, a protein with diverse functions. FASEB J. 1993, 7, 1475–1482. [Google Scholar] [CrossRef]
- Denhardt, D.T.; Noda, M. OPN expression and function: Role in bone remodeling. J. Cell. Biochem. 1998, 72, 92–102. [Google Scholar] [CrossRef]
- Rittling, S.R.; Denhardt, D.T. OPN function in pathology: Lessons from OPN-deficient mice. Exp. Nephrol. 1999, 7, 103–113. [Google Scholar] [CrossRef]
- Denhardt, D.T.; Noda, M.; O’Regan, A.W.; Pavlliln, D.; Berman, J.S. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Invest. 2001, 107, 1055–1061. [Google Scholar] [CrossRef]
- Barry, S.T.; Ludbrook, S.B.; Murrison, E.; Horgan, C.M. Analysis of the alpha4beta1 integrin-OPN interaction. Exp. Cell Res. 2000, 258, 342–351. [Google Scholar] [CrossRef]
- Miyauchi, A.; Alvarez, J.; Greenfield, E.M.; Teti, A.; Grano, M.; Colucci, S.; Zambonin-Zallone, A.; Ross, F.P.; Teitelbaum, S.L.; Cheresh, D. Recognition of OPN and related peptides by an v3 integrin stimulates immediate cell signals in osteoclasts. J. Biol. Chem. 1991, 266, 20369–20374. [Google Scholar] [CrossRef]
- Rodriguez, D.E.; Thula-Mata, T.; Toro, E.J.; Gower, L.B. Multifunctional role of osteopontin in directing intrafibrillar mineralization of collagen and activation of osteoclasts. Acta Biomater. 2014, 10, 494–507. [Google Scholar] [CrossRef]
- Kaartinen, M.T.; Pirhonen, A.; Linnala-Kankkunen, A.; Maenpaa, P.H. Cross-linking of OPN by tissue transglutaminase increases its collagen binding properties. J. Biol. Chem. 1999, 274, 1729–1735. [Google Scholar] [CrossRef]
- Wang, L.; Guan, X.; Tang, R.; Hoyer, J.R.; Wierzbicki, A.; de Yoreo, J.J.; Nancollas, G.H. Phosphorylation of osteopontin is required for inhibition of calcium oxalate crystallization. J. Phys. Chem. B 2008, 112, 9151–9157. [Google Scholar] [CrossRef]
- Boskey, A.L. Osteopontin and related phosphorylated sialoproteins, effects on mineralization. Ann. N. Y. Acad. Sci. 1995, 760, 249–256. [Google Scholar] [CrossRef]
- Rittling, S.R.; Matsumoto, H.N.; McKee, M.D.; Nanci, A.; An, X.R.; Novick, K.E.; Kowalski, A.J.; Noda, M.; Denhardt, D.T. Mice lacking OPN show normal development and bone structure but display altered osteoclast formation in vitro. J. Bone Miner. Res. 1998, 13, 1101–1111. [Google Scholar] [CrossRef]
- Boskey, A.L.; Spevak, L.; Paschalis, E.; Doty, S.B.; McKee, M.D. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif. Tissue Inter. 2002, 71, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Christensen, B.; Taleb, H.; Sørensen, E.S. Post-translational modification of osteopontin: Effects on in vitro hydroxyapatite formation and growth. Biochem. Biophys. Res. Commun. 2012, 419, 333–338. [Google Scholar] [CrossRef]
- Asou, Y.; Rittling, S.R.; Yoshitake, H.; Tsuji, K.; Shinomiya, K.; Nifuji, A.; Denhardt, D.T.; Noda, M. Osteopontin facilitates angiogenesis, accumulation of osteoclasts and resorption in ectopic bone. Endocrinology 2001, 142, 1325–1332. [Google Scholar] [CrossRef]
- Franzen, A.; Heinegard, D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem. J. 1985, 232, 715–724. [Google Scholar] [CrossRef]
- Bianco, P.; Fisher, L.W.; Young, M.F.; Termine, J.D.; Robey, P.G. Expression of bone sialoprotein (BSP) in developing human tissues. Calcif. Tissue Int. 1991, 49, 421–426. [Google Scholar] [CrossRef]
- Gordon, J.A.; Tye, C.E.; Sampaio, A.V.; Underhill, T.M.; Hunter, G.K.; Goldberg, H.A. Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone 2007, 41, 462–473. [Google Scholar] [CrossRef]
- Hunter, G.K.; Goldberg, H.A. Nucleation of hydroxyapatite by bone sialoproteins. Proc. Natl. Acad. Sci. USA 1993, 90, 8562–8565. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Imai, T.; Fujisawa, R.; Tani, H.; Kuboki, Y. Bone sialoprotein (BSP) is a crucial factor for the expression of osteoblastic phenotypes of bone marrow cells cultured on type I collagen matrix. Calcif. Tissue Int. 2000, 66, 388–396. [Google Scholar] [CrossRef]
- Baht, G.S.; Hunter, G.K.; Goldberg, H.A. Bone sialoprotein–collagen interaction promotes hydroxyapatite nucleation. Matrix Biol. 2008, 27, 600–608. [Google Scholar] [CrossRef]
- Malaval, L.; Wade-Gueye, N.M.; Boudiffa, M.; Fei, J.; Zirngibl, R.; Chen, F.; Laroche, N.; Roux, J.P.; Burt-Pichat, B.; Duboeuf, F.; et al. Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J. Exp. Med. 2008, 205, 1145–1153. [Google Scholar] [CrossRef]
- Bouleftour, W.; Boudiffa, M.; Wade-Gueye, N.M.; Bouët, G.; Cardelli, M.; Laroche, N.; vanden-Bossche, A.; Thomas, M.; Bonnelye, E.; Aubin, J.E.; et al. Skeletal development of mice lacking bone sialoprotein (BSP)-impairment of long bone growth and progressive establishment of high trabecular bone mass. PLoS ONE 2014, 9, e95144. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Ma, S.; Zhou, J.; Zhang, H.; Feng, J.Q.; Qin, C. Roles of DMP1 processing in osteogenesis, dentinogenesis and chondrogenesis. Cells Tissues Organs 2011, 194, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.Q.; Huang, H.; Lu, Y.; Ye, L.; Xie, Y.; Tsutsui, T.W.; Kunieda, T.; Castranio, T.; Scott, G.; Bonewald, L.B.; et al. The dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J. Dent. Res. 2003, 82, 776–780. [Google Scholar] [CrossRef]
- Bhatia, A.; Albazzaz, M.; Espinoza Orías, A.A.; Inoue, N.; Miller, L.M.; Acerbo, A.; George, A.; Sumner, D.R. Overexpression of DMP1 accelerates mineralization and alters cortical bone biomechanical properties in vivo. J. Mech. Behav. Biomed. Mater. 2012, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.; Srinivas, R.; Ramachandran, A.; Hao, J.; Quinn, B.; George, A. Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Proc. Natl. Acad. Sci. USA 2001, 98, 4516–4521. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.R.; Kaewpungsup, P.; Khorattanakulchai, N.; Rattanapisit, K.; Pavasant, P.; Phoolcharoen, W. Recombinant human dentin matrix protein 1 (DMP1) induces the osteogenic differentiation of human periodontal ligament cells. Biotechnol. Rep. (Amst). 2019, 23, e00348. [Google Scholar] [CrossRef] [PubMed]
- Gericke, A.; Qin, C.; Sun, Y.; Redfern, R.; Redfern, D.; Fujimoto, Y.; Taleb, H.; Butler, W.T.; Boskey, A.L. Different forms of DMP1 play distinct roles in mineralization. J. Dent. Res. 2010, 89, 355–359. [Google Scholar] [CrossRef]
- He, G.; George, A. Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J. Biol. Chem. 2004, 279, 11649–11656. [Google Scholar] [CrossRef]
- Pirotte, S.; Lamour, V.; Lambert, V.; Alvarez Gonzalez, M.L.; Ormenese, S.; Noel, A.; Mottet, D.; Castronovo, V.; Bellahcene, A. Dentin matrix protein 1induces membrane expression of VE-cadherin on endothelial cells and inhibits VEGF-induced angiogenesis by blocking VEGFR-2 phosphorylation. Blood 2011, 117, 2515–2526. [Google Scholar] [CrossRef]
- Ling, Y.; Rios, H.F.; Myers, E.R.; Lu, Y.; Feng, J.Q.; Boskey, A.L. DMP1 depletion decreases bone mineralization in vivo: An FTIR imaging analysis. J. Bone Miner. Res. 2005, 20, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.Q.; Ward, L.M.; Liu, S.; Lu, Y.; Xie, Y.; Yuan, B.; Yu, X.; Rauch, F.; Davis, S.I.; Zhang, S.; et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 2006, 38, 1310–1315. [Google Scholar] [CrossRef]
- Qin, C.; Brunn, J.C.; Cadena, E.; Ridall, A.; Tsujigiwa, H.; Nagatsuka, H.; Nagai, N.; Butler, W.T. The expression of dentin sialophosphoprotein gene in bone. J. Dent. Res. 2002, 81, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Traub, W.; Jodaikin, A.; Arad, T.; Veis, A.; Sabsay, B. Dentin phosphophoryn binding to collagen fibrils. Matrix 1992, 12, 197–201. [Google Scholar] [CrossRef]
- Boskey, A.L.; Maresca, M.; Doty, S.; Sabsay, B.; Veis, A. Concentration-dependent effects of dentin phosphophoryn in the regulation of in vitro hydroxyapatite formation and growth. Bone Miner. 1990, 11, 55–65. [Google Scholar] [CrossRef]
- Zurick, K.M.; Qin, C.; Bernards, M.T. Mineralization induction effects of osteopontin, bone sialoprotein, and dentin phosphoprotein on a biomimetic collagen substrate. J. Biomed. Mater. Res. A 2013, 101, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Sengottuvelan, A.; Balasubramanian, P.; Will, J.; Boccaccini, A.R. Bioactivation of titanium dioxide scaffolds by ALP-functionalization. Bioact. Mater. 2017, 2, 108–115. [Google Scholar] [CrossRef]
- Jafaary, F.; Hanachi, P.; Gorjipour, K. Osteoblast differentiation on collagen scaffold with immobilized alkaline phosphatase. Int. J. Organ. Transplant. Med. 2017, 8, 195–202. [Google Scholar]
- Detsch, R.; Sarker, B.; Zehnder, T.; Boccaccini, A.R.; Douglas, T.E. Additive manufacturing of cell-loaded alginate enriched with alkaline phosphatase for bone tissue engineering application. BioNanoMaterials 2014, 15, 79–87. [Google Scholar] [CrossRef]
- Pietraszek, A.; Karewicz, A.; Widnic, M.; Lachowicz, D.; Gajewska, M.; Bernasik, A.; Nowakowska, M. Halloysite-alkaline phosphatase system—A potential bioactive component of scaffold for bone tissue engineering. Colloids Surf. B Biointerfaces 2019, 173, 1–8. [Google Scholar] [CrossRef]
- Pataquiva-Mateus, A.Y.; Wu, H.C.; Lucchesi, C.; Ferraz, M.P.; Monteiro, F.J.; Spector, M. Supplementation of collagen scaffolds with SPARC to facilitate mineralization. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 862–870. [Google Scholar] [CrossRef]
- Liao, S.; Ngiam, M.; Chan, C.K.; Ramakrishna, S. Fabrication of nano-hydroxyapatite/collagen/osteonectin composites for bone graft applications. Biomed. Mater. 2009, 4, 025019. [Google Scholar] [CrossRef]
- Sarvestani, A.S.; He, X.; Jabbari, E. Osteonectin-derived peptide increases the modulus of a bone-mimetic nanocomposite. Eur. Biophys. J. 2008, 37, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Hughbanks, M.L.; Binder, B.Y.; Ingavle, G.C.; Leach, J.K. Enhanced trophic factor secretion by mesenchymal stem/stromal cells with Glycine-Histidine-Lysine (GHK)-modified alginate hydrogels. Acta Biomater. 2014, 10, 1955–1964. [Google Scholar] [CrossRef]
- Mogues, T.; Etzerodt, M.; Hall, C.; Engelich, G.; Graversen, J.H.; Hartshorn, K.L. Tetranectin binds to the kringle 1-4 form of angiostatin and modifies its functional activity. J. Biomed. Biotechnol. 2004, 2004, 73–78. [Google Scholar] [CrossRef]
- Hynes, R.O. The emergence of integrins: A personal and historical perspective. Matrix Biol. 2004, 23, 333–340. [Google Scholar] [CrossRef]
- Fisher, L.W.; Fedarko, N.S. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect. Tissue Res. 2003, 44, 33–40. [Google Scholar] [CrossRef]
- Eckrich, J.; Maas, A.; Jurk, K.; Strieth, S.; Kumm, E.; Brieger, J. The role of thrombospondin-1 in biomaterial integration of porous polyethylene implants in vivo. Laryngorhinootologie 2019, 98, S199–S200. [Google Scholar]
- Lee, S.; Lee, D.S.; Choi, I.; Pham, I.B.H.; Jang, J.H. Design of an osteoinductive extracellular fibronectin matrix protein for bone tissue engineering. Int. J. Mol. Sci. 2015, 16, 7672–7681. [Google Scholar] [CrossRef]
- Mohamadyar-Toupkanlou, F.; Vasheghani-Farahani, E.; Hanaee-Ahvaz, H.; Soheimani, M.; Dodel, M.; Havasi, P.; Ardeshirylakimi, A.; Taherzadeh, E.S. Osteogenic differentiation of MSCs on fibronectin-coated and nHA-modified scaffolds. ASAIO J. 2017, 63, 684–691. [Google Scholar] [CrossRef]
- Trujillo, S.; Gonzalez-Garcia, C.; Rico, P.; Reid, A.; Windmill, J.; Dalby, M.J.; Salmeron-Sanchez, M. Engineered 3D hydrogels with full-length fibronectin that sequester and present growth factors. Biomaterials 2020, 252, 120104. [Google Scholar] [CrossRef]
- Sangkert, S.; Kamonmattayakul, S.; Chai, W.L.; Jirut, M. A biofunctional-modified silk fibroin scaffold with mimic reconstructed extracellular matrix of decellularized pulp/collagen/fibronectin for bone tissue engineering in alveolar bone resorption. Matt. Lett. 2016, 166, 30–34. [Google Scholar] [CrossRef]
- Staines, K.A.; MacRae, V.E.; Farquharson, C. The importance of the SIBLING family of proteins on skeletal mineralisation and bone remodelling. J. Endocrinol. 2012, 214, 241–255. [Google Scholar] [CrossRef]
- Boskey, A.L.; Chiang, P.; Fermanis, A.; Brown, J.; Taleb, H.; David, V.; Rowe, P.S. MEPE’s diverse effects on mineralization. Calcif. Tissue Int. 2009, 86, 42–46. [Google Scholar] [CrossRef]
- McKee, M.D.; Nanci, A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: Ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc. Res. Tech. 1996, 33, 141–164. [Google Scholar] [CrossRef]
- Somerman, M.J.; Prince, C.W.; Butler, W.T.; Foster, R.A.; Moehring, J.M.; Sauk, J.J. Cell attachment activity of the 44 kilodalton bone phosphoprotein is not restricted to bone cells. Matrix 1989, 9, 49–54. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Cabral, J.M.S.; da Silva, C.L.; Vashishth, D. Synergistic effect of extracellularly supplemented osteopontin and osteocalcin on stem cell proliferation, osteogenic differentiation, and angiogenic properties. J. Cell. Biochem. 2019, 120, 6555–6569. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Poundarik, A.A.; Cabral, J.M.S.; da Silva, C.L.; Vashishth, D. Biomimetic matrices for rapidly forming mineralized bone tissue based on stem cell-mediated osteogenesis. Sci. Rep. 2018, 8, 14388. [Google Scholar] [CrossRef]
- He, X.; Yang, X.; Jabbar, E. Combined effect of osteopontin and BMP-2 derived peptides grafted to an adhesive hydrogel on osteogenic and vasculogenic differentiation of marrow stromal cells. Langmuir 2012, 28, 5387–5397. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, H. Matrices and scaffold for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug. Deliv. Rev. 2007, 59, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Egusa, H.; Kaneda, Y.; Akashi, Y.; Hamada, Y.; Matsumoto, T.; Saeki, M.; Thakor, D.K.; Tabata, Y.; Matsuura, N.; Yatani, H. Enhanced bone regeneration via multimodal actions of synthetic peptide SVVYGLR on osteoprogenitors and osteoclasts. Biomaterials 2009, 30, 4676–4686. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Nokihara, K.; Okazaki, M.; Fujitani, W.; Matsumoto, T.; Matsuo, M.; Umakoshi, Y.; Takahashi, J.; Matsuura, N. Angiogenic activity of osteopontin-derived peptide SVVYGLR. Biochem. Biophy. Res. Commun. 2003, 310, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; He, H.; Meng, Q.; Zhu, Y.; Ye, X.; Xu, N.; Yu, J. Osteopontin sequence modified mesoporous calcium silicate scaffolds to promote angiogenesis in bone tissue regeneration. J. Mater. Chem. B 2020, 15, 5849–5861. [Google Scholar] [CrossRef] [PubMed]
- Damsongsang, P.; Chaikiawkeaw, D.; Phoolcharoen, W.; Rattanapisit, K.; Kaewpungsup, P.; Pavasant, P.; Hoven, V.P. Surface-immobilized plant-derived osteopontin as an effective platform to promote osteoblast adhesion and differentiation. Colloids Surf. B Biointerfaces 2019, 173, 816–824. [Google Scholar] [CrossRef]
- Klinthoopthamrong, N.; Chaikiawkeaw, D.; Phoolcharoen, W.; Rattanapisit, K.; Kaewpungsup, P.; Pavasant, P.; Hoven, V.P. Bacterial cellulose membrane conjugated with plant-derived osteopontin: Preparation and its potential for bone tissue regeneration. Int. J. Biol. Macromol. 2020, 149, 50–59. [Google Scholar] [CrossRef]
- Kruger, T.E.; Miller, A.H.; Wang, J. Collagen scaffolds in bone sialoprotein-mediated bone regeneration. Sci. World J. 2013, 2013, 812718. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, J.Y.; Chung, C.P.; Park, Y.J. Enhanced osteogenesis by collagen-binding peptide from bone sialoprotein in vitro and in vivo. J. Biomed. Mater. Res. A 2013, 101, 547–554. [Google Scholar] [CrossRef]
- Xu, L.; Anderson, A.L.; Lu, Q.; Wang, J. Role of fibrillary structure of collagenous carrier in bone sialoprotein-mediated matrix mineralization and osteoblast differentiation. Biomaterials 2007, 28, 750–761. [Google Scholar] [CrossRef]
- Gomes, S.; Leonor, I.B.; Mano, J.F.; Reis, R.L.; Kaplan, D.L. Spider silk-bone sialoprotein fusion proteins for bone tissue engineering. Soft Matter. 2011, 7, 4964–4973. [Google Scholar] [CrossRef]
- Chan, W.D.; Goldberg, H.A.; Hunter, G.K.; Dixon, S.J.; Rizkalla, A.S. Modification of polymer networks with bone sialoprotein promotes cell attachment and spreading. J. Biomed. Mater. Res. A 2010, 94, 945–952. [Google Scholar] [CrossRef]
- Rezania, A.; Healy, K.E. The effect of peptide surface density on mineralization of a matrix deposited by osteogenic cells. J. Biomed. Mater. Res. 2000, 52, 595–600. [Google Scholar] [CrossRef]
- Drevelle, O.; Bergeron, E.; Senta, H.; Lauzon, M.A.; Roux, S.; Grenier, G.; Faucheux, N. Effect of functionalized polycaprolactone on the behaviour of murine preosteoblasts. Biomaterials 2010, 31, 6468–6476. [Google Scholar] [CrossRef]
- Jha, A.K.; Jackson, W.M.; Healy, K.E. Controlling osteogenic stem cell differentiation via soft bioinspired hydrogels. PLoS ONE 2014, 9, e98640. [Google Scholar] [CrossRef]
- Rapuano, B.E.; Wu, C.; MacDonald, D.E. Osteoblast-like cell adhesion to bone sialoprotein peptides. J. Orthop. Res. 2004, 22, 353–361. [Google Scholar] [CrossRef]
- Ravindran, S.; Georg, A. Dentin matrix proteins in bone tissue engineering. Adv. Exp. Med. Biol. 2015, 881, 129–142. [Google Scholar] [CrossRef]
- Yang, B.; Chen, G.; Li, J.; Zou, Q.; Xie, D.; Chen, Y.; Wang, H.; Zheng, X.; Long, J.; Tang, W.; et al. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix-based scaffold. Biomaterials 2012, 33, 2449–2461. [Google Scholar] [CrossRef]
- Li, R.; Guo, W.; Yang, B.; Guo, L.; Sheng, L.; Chen, G.; Li, Y.; Zou, Q.; Xie, D.; An, X.; et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials 2011, 32, 4525–4538. [Google Scholar] [CrossRef]
- Chun, S.Y.; Lee, H.J.; Choi, Y.A.; Kim, K.M.; Baek, S.H.; Park, H.S.; Kim, J.Y.; Ahn, J.M.; Cho, J.Y.; Cho, D.W.; et al. Analysis of the soluble human tooth proteome and its ability to induce dentin/tooth regeneration. Tissue Eng. Part. A 2011, 17, 181–191. [Google Scholar] [CrossRef]
- Guo, W.; Gong, K.; Shi, H.; Zhu, G.; He, Y.; Ding, B.; Wen, L.; Jin, Y. Dental follicle cells and treated dentin matrix scaffold for tissue engineering the tooth root. Biomaterials 2012, 33, 1291–1302. [Google Scholar] [CrossRef]
- Alsanea, R.; Ravindran, S.; Fayad, M.I.; Johnson, B.R.; Wenckus, C.S.; Hao, J.; George, A. Biomimetic approach to perforation repair using dental pulp stem cells and dentin matrix protein 1. J. Endod. 2011, 37, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cao, B.; George, A.; Mao, C. Self-assembly and mineralization of genetically modifiable biological nanofibers driven by beta- structure formation. Biomacromolecules 2011, 12, 2193–2199. [Google Scholar] [CrossRef]
- Sreenath, T.; Thyagarajan, T.; Hall, B.; Longenecker, G.; D’Souza, R.; Hong, S.; Wright, J.T.; MacDougall, M.; Sauk, J.; Kulkarni, A.B. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J. Biol. Chem. 2003, 278, 24874–24880. [Google Scholar] [CrossRef]
- Verdelis, K.; Ling, Y.; Sreenath, T.; Haruyama, N.; MacDougall, M.; van der Meulen, M.C.; Lukashova, L.; Spevak, L.; Kulkarni, A.B.; Boskey, A.L. DSPP effects on in vivo bone mineralization. Bone 2008, 43, 983–990. [Google Scholar] [CrossRef]
- Kim, J.W.; Hu, J.C.; Lee, J.I.; Moon, S.K.; Kim, Y.J.; Jang, K.T.; Lee, S.H.; Kim, C.C.; Hahn, S.H.; Simmer, J.P. Mutational hot spot in the DSPP gene causing dentinogenesis imperfecta type II. Hum. Genet. 2005, 116, 186–191. [Google Scholar] [CrossRef]
- Boskey, A.L.; Spevak, L.; Tan, M.; Doty, S.B.; Butler, W.T. Dentin sialoprotein (DSP) has limited effects on in vitro apatite formation and growth. Calcif. Tissue Int. 2000, 67, 472–478. [Google Scholar] [CrossRef]
- Hauschka, P.V.; Lian, J.B.; Cole, D.E.C.; Gundberg, C.M. Osteocalcin and matrix Gla protein: Vitamin K-dependent proteins in bone. Physiol. Rev. 1989, 69, 990–1047. [Google Scholar] [CrossRef]
- Poser, J.W.; Esch, F.S.; Ling, N.C.; Price, P.A. Isolation and sequence of the vitamin K-dependent protein from human bone. Undercarboxylation of the first glutamic acid residue. J. Biol. Chem. 1980, 255, 8685–8691. [Google Scholar] [CrossRef]
- Calvo, M.S.; Eyre, D.R.; Caren, M.G. Molecular basis and clinical application of biological markers of bone turnover. Endocr. Rev. 1996, 17, 333–368. [Google Scholar] [CrossRef] [PubMed]
- Gundberg, C.M. Biochemical markers of bone formation. Clin. Lab. Med. 2000, 20, 489–501. [Google Scholar] [CrossRef]
- Hauschka, P.V.; Reid, M.L. Timed appearance of a calcium-binding protein containing g-carboxyglutamic acid in developing chick bone. Dev. Biol. 1978, 65, 431–436. [Google Scholar] [CrossRef]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased bone formation in osteocalcin-deficient mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef]
- Rammelt, S.; Neumann, M.; Hanisch, U.; Reinstorf, A.; Pompe, W.; Zwipp, H.; Biewener, A. Osteocalcin enhances bone remodeling around hydroxyapatite/collagen composites. J. Biomed. Mater. Res. A 2005, 73, 284–294. [Google Scholar] [CrossRef]
- Chenu, C.; Colucci, S.; Grano, M.; Zigrino, P.; Barattolo, R.; Zambonin, G.; Baldini, N.; Vergnaud, P.; Delmas, P.D.; Zallone, A.Z. Osteocalcin induces chemotaxis, secretion of matrix proteins, and calcium-mediated intracellular signaling in human osteoclast-like cells. J. Biol. Chem. 1994, 127, 1149–1158. [Google Scholar] [CrossRef]
- Bodine, P.V.; Komm, B.S. Evidence that conditionally immortalized human osteoblasts express an osteocalcin receptor. Bone 1999, 25, 535–543. [Google Scholar] [CrossRef]
- Glowacki, J.; Lian, J.B. Impaired recruitment and differentiation of osteoclast progenitors by osteocalcin-deplete bone implants. Cell. Differ. 1987, 21, 247–254. [Google Scholar] [CrossRef]
- Ferron, M.; Hinoi, E.; Karsenty, G.; Ducy, P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl. Acad. Sci. USA 2008, 105, 5266–5270. [Google Scholar] [CrossRef]
- Wei, J.; Karsenty, G. An overview of the metabolic functions of osteocalcin. Rev. Endocr. Metab. Disord. 2015, 16, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Lee, D.S.; Lee, S.; Jang, J.H. Osteocalcin/fibronectin-functionalized collagen matrices for bone tissue engineering. J. Biomed. Mater. Res. A 2015, 103, 2133–2140. [Google Scholar] [CrossRef]
- Cantatore, F.P.; Crivellato, E.; Nico, B.; Ribatti, D. Osteocalcin is angiogenic in vivo. Cell Biol. Inter. 2005, 29, 583–585. [Google Scholar] [CrossRef]
- Price, P.A.; Williamson, M.K. Primary structure of bovine matrix Gla protein, a new vitamin K-dependent bone protein. J. Biol. Chem. 1985, 260, 14791–14975. [Google Scholar] [CrossRef]
- Murshed, M.; Schinke, T.; McKee, M.D.; Karsenty, G. Extracellular matrix mineralization is regulated locally: Different roles of two Gla-containing proteins. J. Cell. Biol. 2004, 165, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, Z.; Yan, K.; Wang, Y.; Yang, Y.; Wu, X. Matrix gla protein promotes the bone formation by up-regulating Wnt/β-catenin signaling pathway. Front. Endocrinol. (Lausanne) 2019, 10, 891. [Google Scholar] [CrossRef]
- Garnett, J.; Dieppe, P. The effects of serum and human albumin on calcium hydroxyapatite crystal growth. Biochem. J. 1990, 266, 863–868. [Google Scholar]
- Carvalho, M.S.; Silva, J.C.; Hoff, C.M.; Cabral, J.M.S.; Linhardt, R.J.; da Silva, C.L.; Vashishth, D. Loss and rescue of osteocalcin and osteopontin modulate osteogenic and angiogenic features of mesenchymal stem/stromal cells. J. Cell. Physiol. 2020, 235, 7496–7515. [Google Scholar] [CrossRef]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix:usingnature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef]
- Petersen, T.H.; Calle, E.A.; Zhao, L.; Lee, E.J.; Gui, L.; Raredon, M.B.; Gavrilov, K.; Yi, T.; Zhuang, Z.W.; Breuer, C.; et al. Tissue-engineered lungs for in vivo implantation. Science 2010, 329, 538–541. [Google Scholar] [CrossRef]
- Papadimitropoulos, A.; Scotti, C.; Bourgine, P.; Scherberich, A.; Martin, I. Engineered decellularized matrices to instruct bone regeneration processes. Bone 2015, 70, 66–72. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Khan, Y. Regenerative engineering. Sci. Transl. Med. 2012, 4, 160ed9. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, L.E.; McDevitt, T.C. Cell-derived matrices for tissue engineering and regenerative medicine application. Biomater. Sci. 2015, 3, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R.; Silverman, B.F.; Buring, K.; Dubuc, F.L.; Rosenberg, J.M. The bone induction principle. Clin. Orthop. Relat. Res. 1967, 53, 243–283. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; Somers, A.; Mellonig, J.T.; Carnes, D.L., Jr.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation is dependent on donor age but not gender. J. Periodontol. 1998, 69, 470–478. [Google Scholar] [CrossRef]
- Munting, E.; Wilmart, J.F.; Wijne, A.; Hennebert, P.; Delloye, C. Effect of sterilization on osteoinduction. Comparison of five methods in demineralized rat bone. Acta Orthop. Scand. 1988, 59, 34–38. [Google Scholar] [CrossRef]
- Bourgine, P.E.; Scotti, C.; Pigeot, S.; Tchang, L.A.; Todorov, A.; Martin, I. Osteoinductivity of engineered cartilaginous templates devitalized by inducible apoptosis. Proc. Natl. Acad. Sci. USA 2014, 111, 17426–17431. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Silva, J.C.; Cabral, J.M.S.; da Silva, C.L.; Vashishth, D. Cultured cell-derived extracellular matrices to enhance the osteogenic differentiation and angiogenic properties of human mesenchymal stem/stromal cells. J. Tissue Eng. Regen. Med. 2019, 13, 1544–1558. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Carvalho, M.S.; Udangawa, R.N.; Moura, C.S.; Cabral, J.M.S.; da Silva, C.L.; Ferreira, F.C.; Vashishth, D.; Linhardt, R.J. Extracellular matrix decorated polycaprolactone scaffolds for improved mesenchymal stem/stromal cell osteogenesis towards a patient-tailored bone tissue engineering approach. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2153–2166. [Google Scholar] [CrossRef]
- Lin, H.; Yang, G.; Tan, J.; Tuan, R.S. Influence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential. Biomaterials 2012, 33, 4480–4489. [Google Scholar] [CrossRef]
- Datta, N.; Holtorf, H.L.; Sikavitsas, V.I.; Jansen, J.A.; Mikos, A.G. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials 2005, 26, 971–977. [Google Scholar] [CrossRef]
- Datta, N.; Pham, Q.P.; Sharma, U.; Sikavitsas, V.I.; Jansen, J.A.; Mikos, A.G. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc. Natl. Acad. Sci. USA 2006, 103, 2488–2493. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Silva, J.C.; Udangawa, R.N.; Cabral, J.M.S.; Ferreira, F.C.; da Silva, C.L.; Linhardt, R.J.; Vashishth, D. Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 99, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Kasper, F.K.; Mistry, A.S.; Sharma, U.; Yasko, A.W.; Jansen, J.A. Analysis of the osteoinductive capacity and angiogenicity of an in vitro generated extracellular matrix. J. Biomed. Mater. Res. A 2008, 88, 295–303. [Google Scholar] [CrossRef]
- Sadr, N.; Pippenger, B.E.; Scherberich, A.; Wendt, D.; Mantero, S.; Martin, I. Enhancing the biological performance of synthetic polymeric materials by decoration with engineered, decellularized extracellular matrix. Biomaterials 2012, 33, 5085–5093. [Google Scholar] [CrossRef] [PubMed]

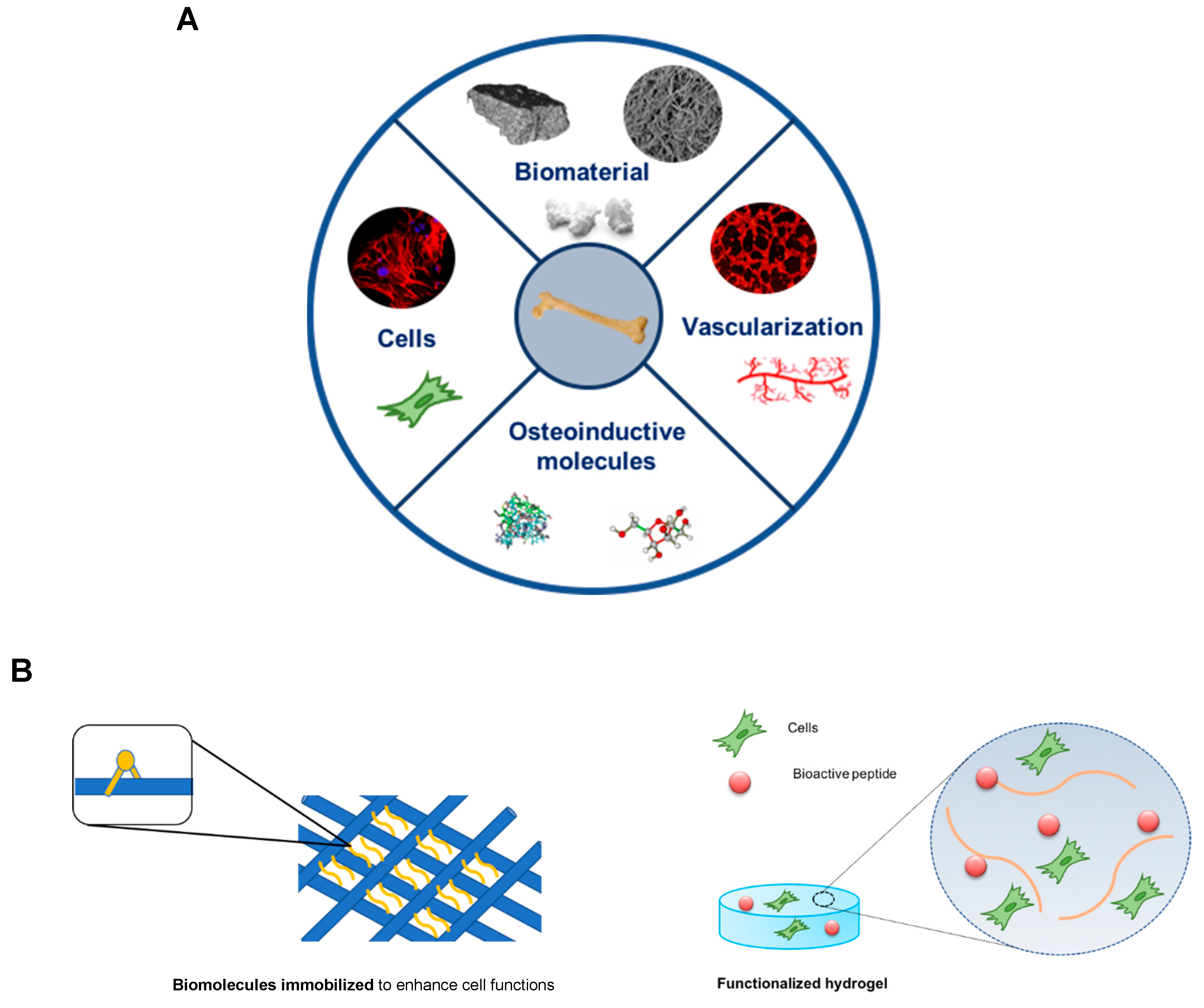

| Non-Collagenous Protein | Modified Biomaterial | Outcomes | References |
|---|---|---|---|
| Alkaline Phosphatase | ALP-immobilized on microporous nanofibrous fibrin scaffolds by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride/N-hydroxysuccinimide (EDC/NHS) method. | Supported cell proliferation and osteogenic differentiation in vitro. In vivo, these scaffolds promoted bone formation. | [54] |
| Osteonectin | Oxidized alginate hydrogels with the GHK peptide, a fragment of osteonectin. The free aldehyde groups present in the oxidized alginate can form covalent bonds with molecules that contain amino groups, such as GHK (self-crosslinking). | Improved osteogenic differentiation of MSCs, demonstrated by enhanced gene expression, alkaline phosphatase activity and bone extracellular matrix deposition. | [55] |
| Fibronectin | Fibronectin-immobilized nanobioactive glass/polycaprolactone scaffolds by EDC/NHS treatment. | Improved cellular adhesion and proliferation. | [56] |
| Vitronectin | Vitronectin-derived peptide covalently grafted onto titanium scaffolds. Pretreated (oxidized and silanized) constructs were peptide-grafted by immersion overnight into a 1 mg/mL peptide solution. | The presence of the vitronectin-peptide bound to the titanium constructs improved the osteogenic activity immediately after implantation, accelerating bone ongrowth. | [57] |
| Osteopontin | Oligo(poly(ethylene glycol)) fumarate hydrogels modified with OPN-derived peptide. Peptides were coupled to acrylated-PEG by NHS treatment. | Improved osteoblast proliferation and migration. | [58] |
| Osteopontin | CO3 apatite-collagen sponges containing the SVVYGLR motif (amino acids residues 12–18 of OPN). CO3 apatite-collagen sponges were immersed in 10 ng/mL of SVVYGLR peptide solution. | In vivo studies presented improved angiogenesis. | [59] |
| Bone Sialoprotein | Bone sialoprotein coated 3D printed calcium phosphate scaffolds. 3D printed calcium phosphate scaffolds were coated with BSP via physisorption. Incubation was performed with different concentrations of BSP solution (50 and 200 µg/mL) under mechanical stirring at 8 °C. | Improved osteoblast viability and in vivo studies showed that BSP coated 3D printed calcium phosphate scaffolds promoted increased bone formation in comparison to uncoated scaffolds. | [60,61] |
| Proteoglycans | In Vivo Studies | Functions | References |
|---|---|---|---|
| Aggrecan | Aggrecan deficient mice presented cartilage matrix deficiency and were characterized by perinatal lethal dwarfism and craniofacial abnormalities. | Can have an important role in preventing cartilage calcification. | [80] |
| Versican | Versican deficient mice have presented an early lethality. | Can have an important role in preventing cartilage calcification. | [81,82,83] |
| Decorin | Decorin-knockout mice showed skin laxity and fragility and their bones did not demonstrate any visible bone phenotype. However, their teeth sowed alteration in matrix properties, presenting a hypomineralized dentin. | Binds to collagen and can regulate fibril diameter and orientation. Can prevent premature osteoid calcification and regulate the collagen-matrix interactions. | [84,85,86,87,88] |
| Biglycan | The biglycan-knockout mice presented reduced skeletal growth, having shorter femora and decreased bone mass. | Binds to collagen and can regulate fibril diameter and orientation. Can prevent premature osteoid calcification and regulate the collagen-matrix interactions. | [84,86,87,88,89] |
| Glycoproteins | In Vivo Studies | Functions | References |
|---|---|---|---|
| Alkaline Phosphatase | Mice with null mutations for the tissue –nonspecific alkaline phosphatase showed increased osteoid and defective growth plate development. | Possible role in mineralization. ALP can act as a potential Ca2+ carrier and hydrolyzes inhibitors of mineralization such as pyrophosphates. | [91,92,93,94,95] |
| Osteonectin | Osteonectin deficient mice have presented a poor bone status, developing osteopenia. | Can promote mineral deposition and regulate growth and proliferation of mineral crystals, supporting bone remodeling. May influence cell functions, binding to growth factors and through cell-matrix interactions. | [96,97,98,99,100,101,102,103] |
| Tetranectin | Tetranectin deficient mice have presented a delayed fracture healing. | Can regulate matrix mineralization, playing a role in tissue formation and remodeling. | [104,105,106] |
| Thrombospondin | Thrombospondin deficient mice presented disordered collagen in their soft tissues, increased cortical bone thickness and density and altered fibroblast attachment. | Role in cell attachment. It binds to several ECM proteins. Role in bone development and remodeling, collagen fibrillogenesis and ECM organization. | [107,108,109,110,111] |
| Fibronectin | Elimination of fibronectin gene in transgenic animals is lethal in utero, since connective tissues do not form. | Role in cell attachment. It binds to several matrix proteins and cell surface proteins, like collagen. | [112,113,114] |
| Vitronectin | Vitronectin deficient mice have been shown to have a thrombolytic phenotype, but skeletal defects were not apparent in these mice. | Role in cell attachment. It can bind to collagen. | [113,115,116] |
| Osteopontin | Osteopontin deficient mice presented larger crystal size and an increased mineral content. | Role in cell attachment. It binds with other molecules present in bone matrix. Can regulate mineralization by regulating the nucleation of mineral crystals. Can regulate bone resorption through osteoclasts attachment and migration. Play a specific role in angiogenesis. | [117,118,119,120,121,122,123,124,125,126,127,128,129,130] |
| Bone Sialoprotein | Bone sialoprotein deficient mice presented shorter, hypomineralized bones with higher trabecular bone mass and with lower bone formation rate. | Role in cell attachment and matrix mineralization induction. It acts as a hydroxyapatite nucleator since it has high affinity for calcium. Can have an important role in osteoclasts formation and bone resorption. | [131,132,133,134,135,136,137,138] |
| Dentin matrix protein-1 | Dentin matrix protein-1 deficient mice have significantly lower mineral content when compared with their controls. | Role in cell attachment. It binds to collagen. If phosphorylated, may inhibit the formation and growth of hydroxyapatite, if dephosphorylated it facilitates nucleation of hydroxyapatite crystals, inducing mineralization. Can play a role in angiogenesis. | [139,140,141,142,143,144,145,146,147,148] |
| Dentin sialophosphoprotein | Dentin sialophosphoprotein deficient mice have shown decreased mineral content. | Can regulate type I collagen fibrillogenesis and acts as nucleator of hydroxyapatite formation at lower concentrations and inhibitor at higher concentrations. | [149,150,151,152] |
| Gla-proteins. | In Vivo Studies | Functions | References |
|---|---|---|---|
| Osteocalcin | Osteocalcin deficient mice presented increased bone formation without impairing bone resorption. | Can influence bone mineralization. It has high affinity to calcium, accelerating nucleation of hydroxyapatite and playing an active role in the early stages of bone healing. Can regulate activity of osteoclasts and bone resorption. Acts as a hormone regulating insulin secretion and glucose homeostasis. | [202,203,204,205,206,207,208,209,210,211,212,213] |
| Matrix Gla Protein | Matrix Gla Protein deficient mice died prematurely due to massive calcification of their tracheal cartilage and blood vessels, indicating an important role in preventing mineralization. | Can function in cartilage metabolism inhibiting mineralization. | [214,215,216,217,218,219,220,221,222,223,224,225,226,227] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, M.S.; Cabral, J.M.S.; da Silva, C.L.; Vashishth, D. Bone Matrix Non-Collagenous Proteins in Tissue Engineering: Creating New Bone by Mimicking the Extracellular Matrix. Polymers 2021, 13, 1095. https://doi.org/10.3390/polym13071095
Carvalho MS, Cabral JMS, da Silva CL, Vashishth D. Bone Matrix Non-Collagenous Proteins in Tissue Engineering: Creating New Bone by Mimicking the Extracellular Matrix. Polymers. 2021; 13(7):1095. https://doi.org/10.3390/polym13071095
Chicago/Turabian StyleCarvalho, Marta S., Joaquim M. S. Cabral, Cláudia L. da Silva, and Deepak Vashishth. 2021. "Bone Matrix Non-Collagenous Proteins in Tissue Engineering: Creating New Bone by Mimicking the Extracellular Matrix" Polymers 13, no. 7: 1095. https://doi.org/10.3390/polym13071095
APA StyleCarvalho, M. S., Cabral, J. M. S., da Silva, C. L., & Vashishth, D. (2021). Bone Matrix Non-Collagenous Proteins in Tissue Engineering: Creating New Bone by Mimicking the Extracellular Matrix. Polymers, 13(7), 1095. https://doi.org/10.3390/polym13071095







