Optical Properties and Conductivity of PVA–H3PO4 (Polyvinyl Alcohol–Phosphoric Acid) Film Blend Irradiated by γ-Rays
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
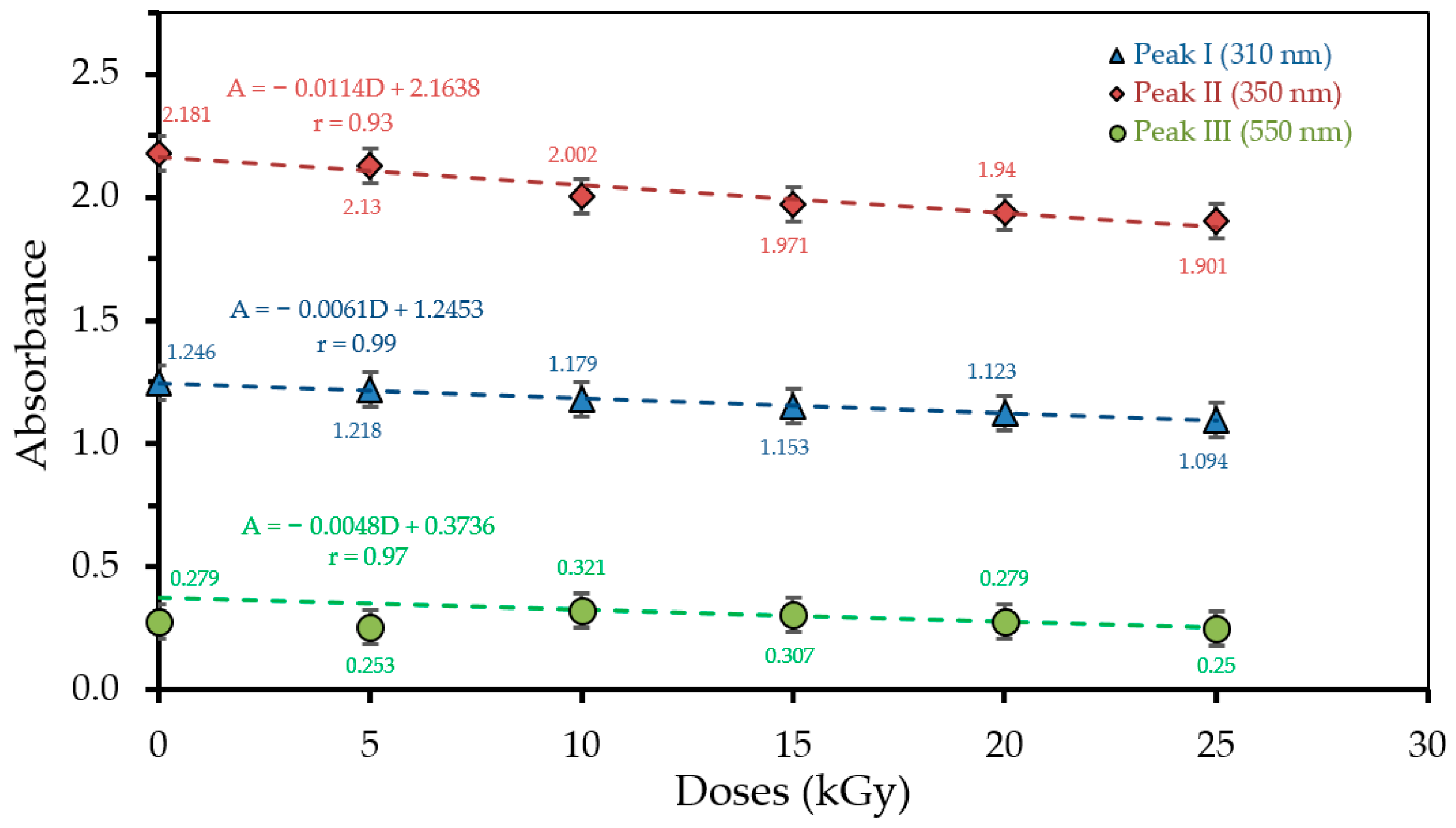
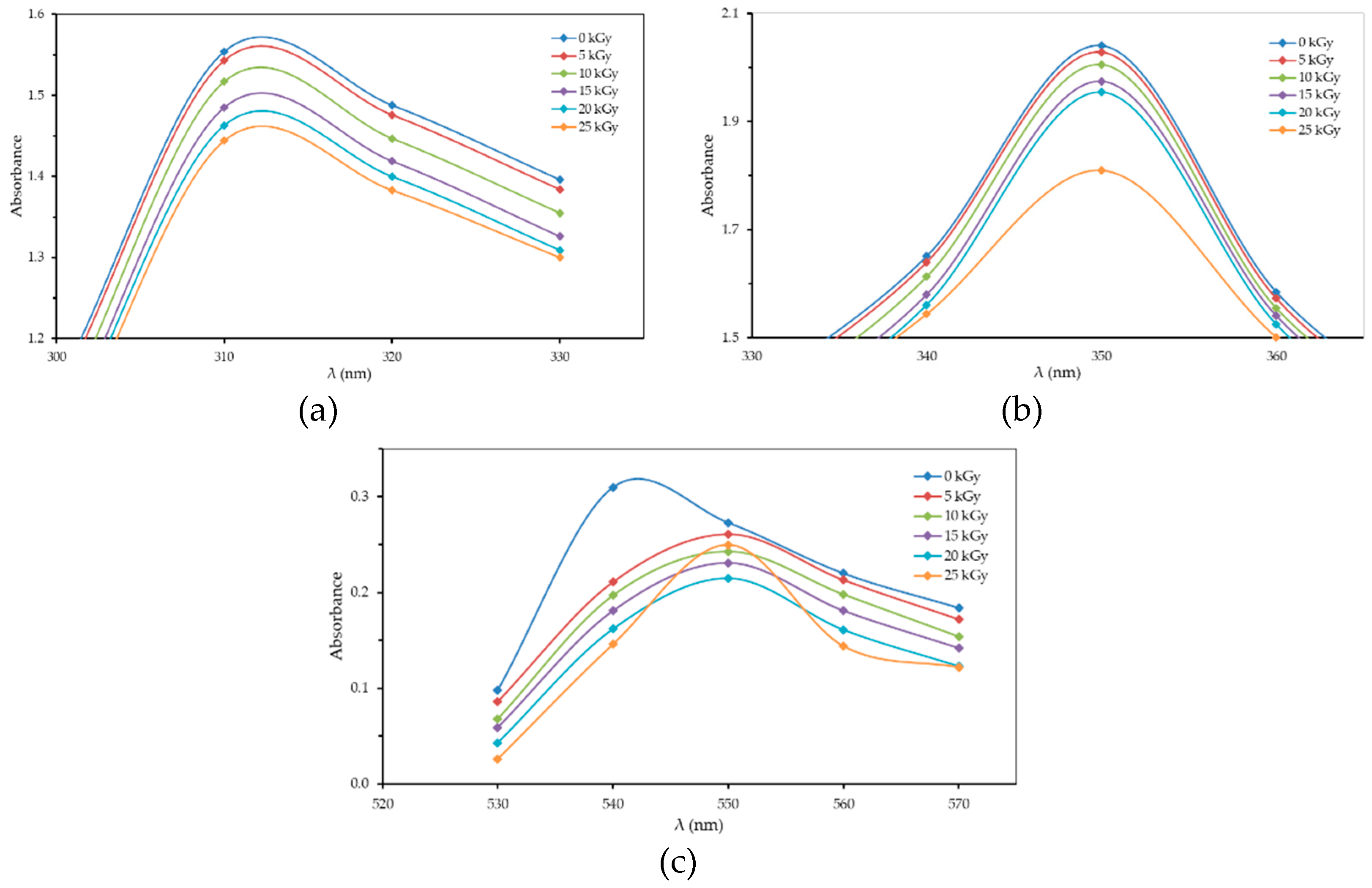
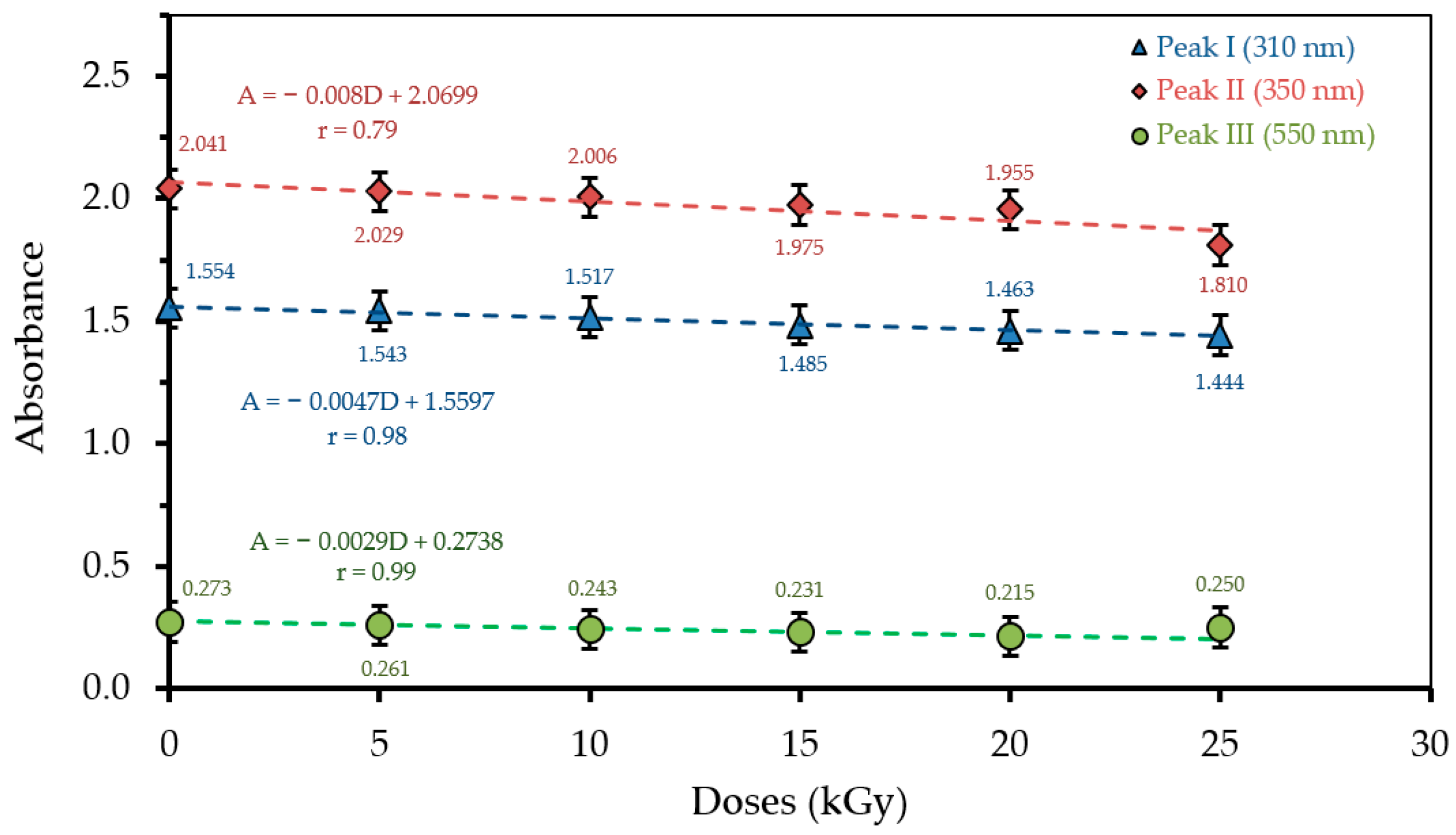
3.1. Optical Absorption
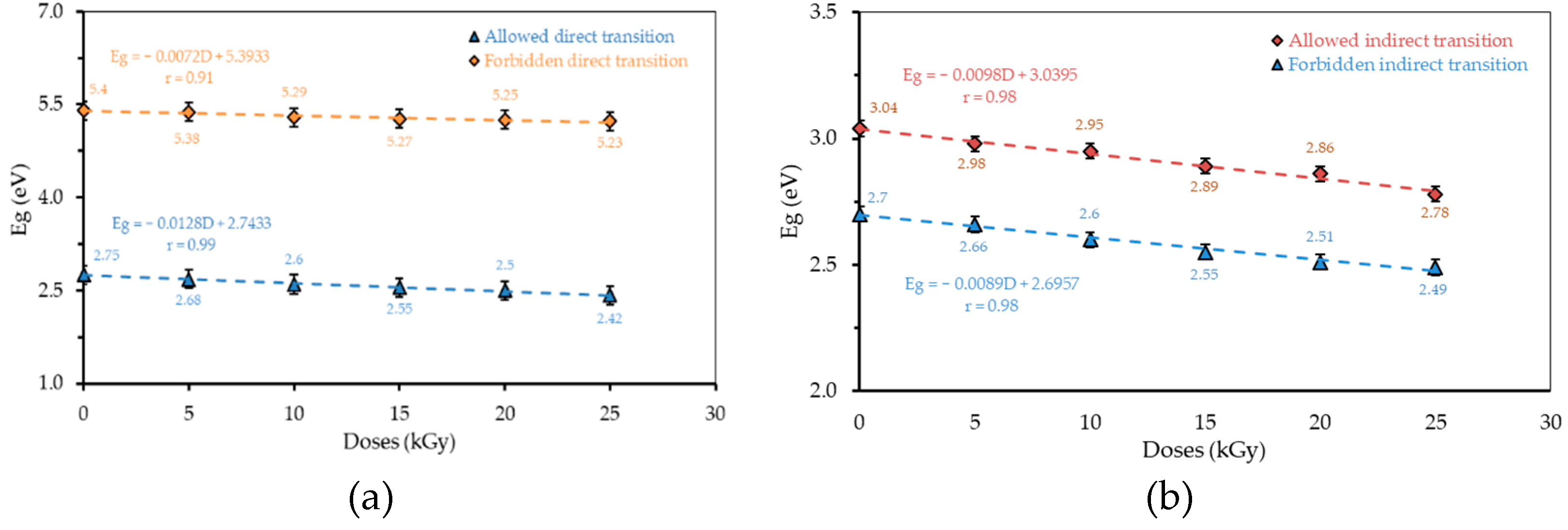
3.2. Absorption Edge
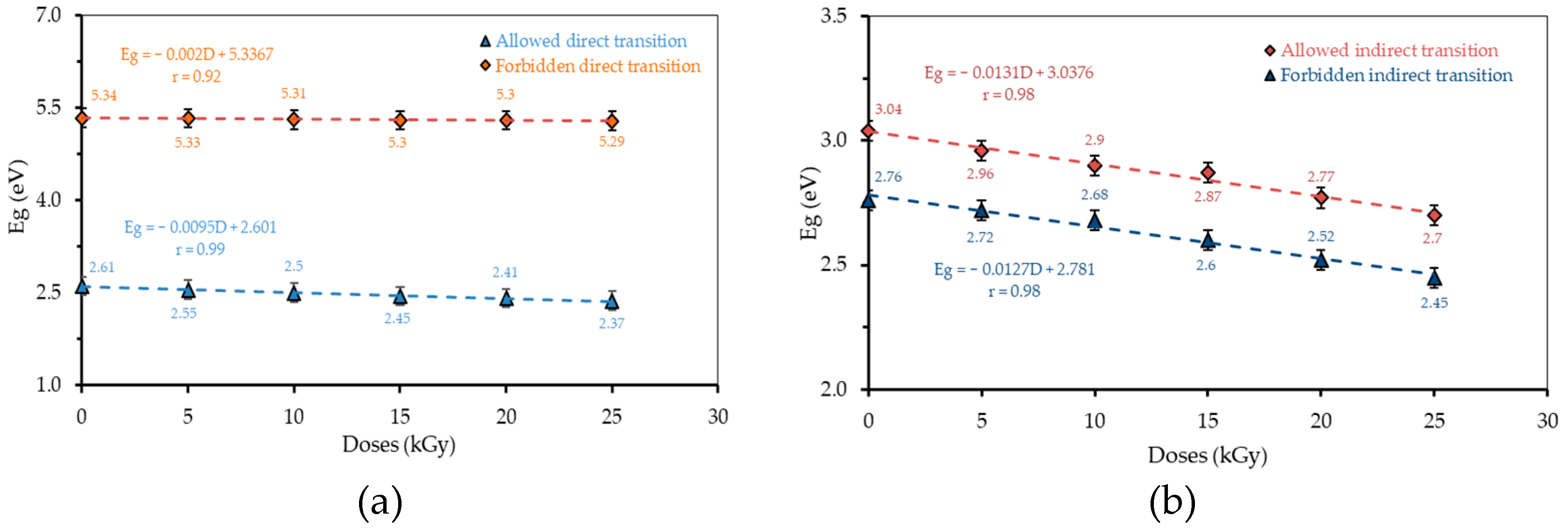
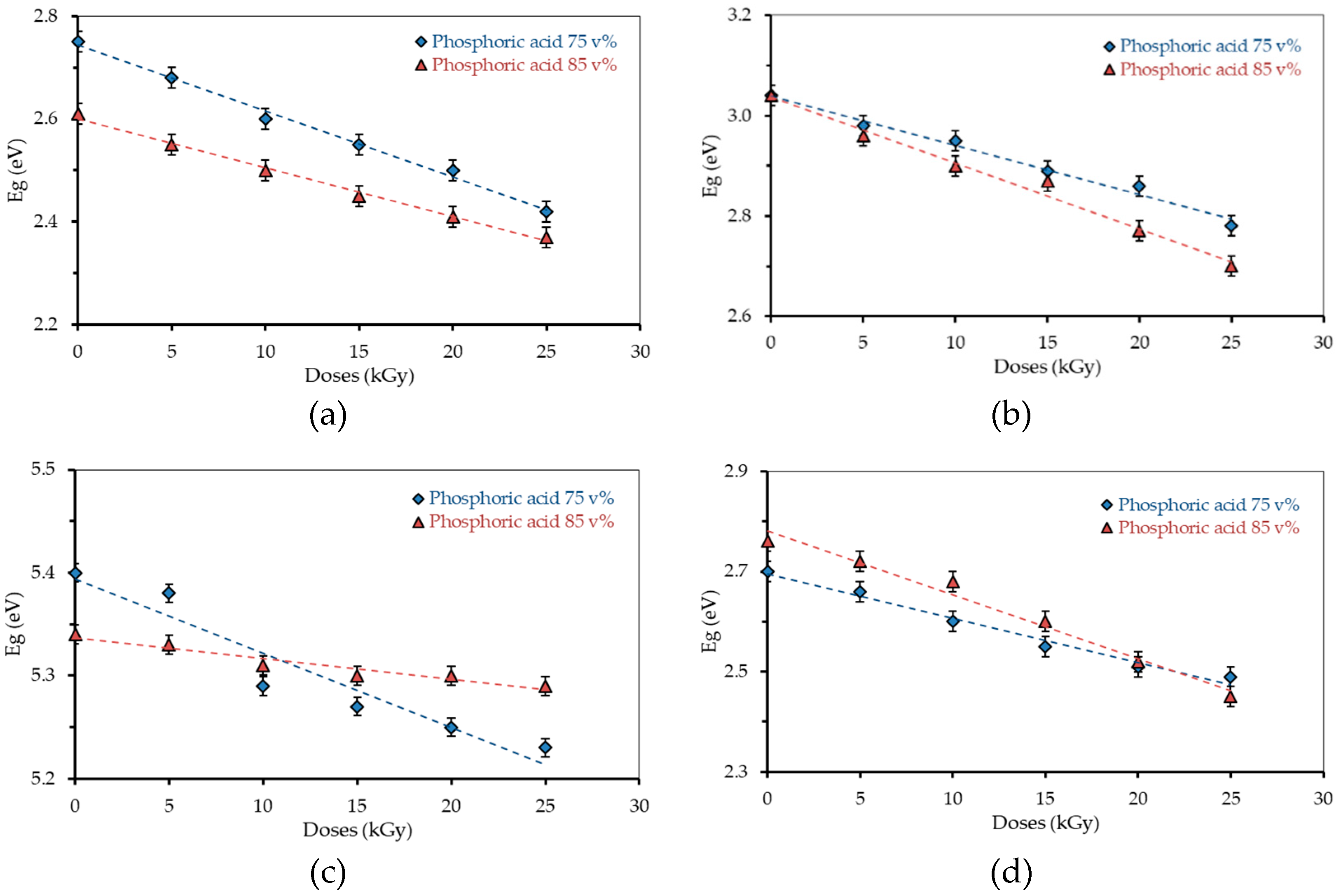
3.3. Energy Gap
3.4. Electrical Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sannasi, V.; Jose, T.P.; Jeyakumar, D. Synthesis and optical properties of poly(2,7-(9,9-dihexylfluorene)-3,3′(4,4′-dialkoxybiphenyl)). Des. Monomers Polym. 2014, 18, 51–63. [Google Scholar] [CrossRef][Green Version]
- Son, H.J.; Wang, W.; Xu, T.; Liang, Y.; Wu, Y.; Li, G.; Yu, L. Synthesis of Fluorinated Polythienothiophene-co-benzodithiophenes and Effect of Fluorination on the Photovoltaic Properties. J. Am. Chem. Soc. 2011, 133, 1885–1894. [Google Scholar] [CrossRef]
- Rehan, H. A new polymer/polymer rechargeable battery: Polyaniline/LiClO4(MeCN)/poly-1-naphthol. J. Power Sources 2003, 113, 57–61. [Google Scholar] [CrossRef]
- Grimsdale, A.C.; Chan, K.L.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of Light-Emitting Conjugated Polymers for Applications in Electroluminescent Devices. Chem. Rev. 2009, 109, 897–1091. [Google Scholar] [CrossRef] [PubMed]
- Guler, E.; Soyleyici, H.C.; Demirkol, D.O.; Ak, M.; Timur, S. A novel functional conducting polymer as an immobilization platform. Mater. Sci. Eng. C 2014, 40, 148–156. [Google Scholar] [CrossRef]
- Oyman, G.; Geyik, C.; Ayranci, R.; Ak, M.; Demirkol, D.O.; Timur, S.; Coskunol, H. Peptide-modified conducting polymer as a biofunctional surface: Monitoring of cell adhesion and proliferation. RSC Adv. 2014, 4, 53411–53418. [Google Scholar] [CrossRef]
- Ang, S.L.; Sivashankari, R.; Shaharuddin, B.; Chuah, J.-A.; Tsuge, T.; Abe, H.; Sudesh, K. Potential Applications of Polyhydroxyalkanoates as a Biomaterial for the Aging Population. Polym. Degrad. Stab. 2020, 181, 109371. [Google Scholar] [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Khalid, M.; Walvekar, R. Development of Poly(Vinyl Alcohol)-Based Polymers as Proton Exchange Membranes and Challenges in Fuel Cell Application: A Review. Polym. Rev. 2020, 60, 171–202. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Effect of vinyl silane modification on thermal and mechanical properties of starch-polyvinyl alcohol blend. Des. Monomers Polym. 2019, 22, 159–163. [Google Scholar] [CrossRef]
- Patole, S.P.; Arif, M.F.; Kumar, S. Polyvinyl alcohol incorporated buckypaper composites for improved multifunctional performance. Compos. Sci. Technol. 2018, 168, 429–436. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Bajpai, A.K.; Bajpai, J.; Sharma, A. Antimicrobial poly(vinyl alcohol) cryogel–copper nanocomposites for possible applications in biomedical fields. Des. Monomers Polym. 2015, 18, 385–400. [Google Scholar] [CrossRef][Green Version]
- Shabanpanah, S.; Omrani, A.; Lakouraj, M.M. Fabrication and characterization of PVA/NNSA/GLA/nano-silica proton conducting composite membranes for DMFC applications. Des. Monomers Polym. 2019, 22, 130–139. [Google Scholar] [CrossRef]
- Yang, C.-C. Fabrication and characterization of poly(vinyl alcohol)/montmorillonite/poly(styrene sulfonic acid) proton-conducting composite membranes for direct methanol fuel cells. Int. J. Hydrog. Energy 2011, 36, 4419–4431. [Google Scholar] [CrossRef]
- Gupta, P.; Singh, K. Characterization of H3PO4 based PVA complex system. Solid State Ion. 1996, 86–88, 319–323. [Google Scholar] [CrossRef]
- Iordanskii, A.; Merkulov, Y.; Moiseyev, Y.; Zaikov, G. Diffusion of phosphoric acid into polyvinyl alcohol (PVALC) films swollen in water. Polym. Sci. USSR 1974, 16, 2609–2617. [Google Scholar] [CrossRef]
- Saat, A.M.; Johan, M.R. Effect of Phosphoric Acid Concentration on the Optical Properties of Partially Phosphorylated PVA Complexes. Int. J. Polym. Sci. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Chen, F.; Liu, P. High electrically conductive polyaniline/partially phosphorylated poly(vinyl alcohol) composite films via aqueous dispersions. Macromol. Res. 2011, 19, 883–890. [Google Scholar] [CrossRef]
- Sa’Adu, L.; Hashim, M.A.; Bin-Baharuddin, M. Conductivity Studies and Characterizations of PVA-Orthophosphoric Electrolytes. J. Mater. Sci. Res. 2014, 3, 48. [Google Scholar] [CrossRef]
- Quintana, D.A.; Baca, E.; Mosquera, E.; Vargas, R.A.; Diosa, J.E. Improving the ionic conductivity in nanostructured membranes based on poly(vinyl alcohol) (PVA), chitosan (CS), phosphoric acid (H3PO4), and niobium oxide (Nb2O5). Ionics 2018, 25, 1131–1136. [Google Scholar] [CrossRef]
- Pu, H.; Huang, P. Studies on transparent and solid proton conductors based on NH4H2PO4 doped poly(vinyl alcohol). Mater. Lett. 2006, 60, 1724–1727. [Google Scholar] [CrossRef]
- Verawati, N.N.S.P. The Effect of Gamma Ray (γ) Irradiation on Optical Absorption of Polymer Film Blend. Lensa J. Kependidikan Fis. 2020, 8, 48–54. [Google Scholar] [CrossRef]
- Lazareva, T.G.; Yakovleva, O.V.; Shinkareva, E.V. Rheological and Electrical Characteristics of Polyvinyl Alcohol Composites with Phosphoric Acid. Russ. J. Appl. Chem. 2002, 75, 965–968. [Google Scholar] [CrossRef]
- Xu, T.; Chen, J.-Z.; Han, W.; Li, Z.; Yu, G. Geopolymer-Organic Polymer Composite Synthesized by the Interactions of H3PO4 with Metakaolinite Powders and Polyvinyl Alcohol. Part. Sci. Technol. 2010, 28, 539–546. [Google Scholar] [CrossRef]
- Saion, E. Changes in the Optical Band Gap and Absorption Edge of Gamma-Irradiated Polymer Blends. J. Appl. Sci. 2005, 5, 1825–1829. [Google Scholar] [CrossRef]
- Skuja, L.; Kajihara, K.; Ikuta, Y.; Hirano, M.; Hosono, H. Urbach absorption edge of silica: Reduction of glassy disorder by fluorine doping. J. Non-Cryst. Solids 2004, 345–346, 328–331. [Google Scholar] [CrossRef]
- Aziz, S.B.; Ahmed, H.M.; Hussein, A.M.; Fathulla, A.B.; Wsw, R.M.; Hussein, R.T. Tuning the absorption of ultraviolet spectra and optical parameters of aluminum doped PVA based solid polymer composites. J. Mater. Sci. Mater. Electron. 2015, 26, 8022–8028. [Google Scholar] [CrossRef]
- Mott, N.F.; Davis, E.A. Electronic Processes in Non-Crystalline Materials, 2nd ed.; International Series of Monographs on Physics; Clarendon Press: Oxford, UK, 2012; ISBN 978-0-19-964533-6. [Google Scholar]
- Somani, P.R.; Marimuthu, R.; Viswanath, A.; Radhakrishnan, S. Thermal degradation properties of solid polymer electrolyte (poly(vinyl alcohol)+phosphoric acid)/methylene blue composites. Polym. Degrad. Stab. 2003, 79, 77–83. [Google Scholar] [CrossRef]
- Fernandes, D.; Hechenleitner, A.W.; Lima, S.; Andrade, L.; Caires, A.; Pineda, E.G. Preparation, characterization, and photoluminescence study of PVA/ZnO nanocomposite films. Mater. Chem. Phys. 2011, 128, 371–376. [Google Scholar] [CrossRef]
- Antar, E. Effect of γ-ray on optical characteristics of dyed PVA films. J. Radiat. Res. Appl. Sci. 2014, 7, 129–134. [Google Scholar] [CrossRef]
- Nakhaei, O.; Shahtahmassebi, N.; Rezaeeroknabadi, M.; Mohagheghi, M.B. Synthesis, characterization and study of optical properties of polyvinyl alcohol/ CaF2 nanocomposite films. Sci. Iran. 2012, 19, 1979–1983. [Google Scholar] [CrossRef]
- Jassim, T.A.; Saeed, A.A. Effect of Gamma Irradiation on the Physical Properties of PVA Polymer. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 072137. [Google Scholar] [CrossRef]
- Rasheed, H.S.; Abbas, I.A.; Kadhum, A.J.; Maged, H.C. The Effect of Gamma Irradiation on the Optical Prop-Erties of (PVA-PAA-Al2O3) Films; AIP Publishing LLC: Athens, Greece, 2019; p. 020013. [Google Scholar]
- Meftah, A.M.; Gharibshahi, E.; Soltani, N.; Yunus, W.M.M.; Saion, E. Structural, Optical and Electrical Properties of PVA/PANI/Nickel Nanocomposites Synthesized by Gamma Radiolytic Method. Polymers 2014, 6, 2435–2450. [Google Scholar] [CrossRef]
- Doyan, A.; Susilawati, S. Dose Response and Optical Properties of Dyed Poly Vinyl Alcohol-Trichloroacetic Acid Polymeric Blends Irradiated with Gamma-Rays. Am. J. Appl. Sci. 2009, 6, 2071–2077. [Google Scholar] [CrossRef]
- Sato, Y.; Hishinuma, T.; Sato, S. Crystallinity of GaN Thin Films Directly Grown on Metal Foils by the Reactive Evaporation Method. Jpn. J. Appl. Phys. 2001, 40, 4516–4517. [Google Scholar] [CrossRef]
- Ali, H.E.; Abdel-Aziz, M.M.; Algarni, H.; Yahia, I.S. The structure analysis and optical performance of PVA films doped with Fe3+-metal for UV- limiter, and optoelectronics. Mater. Res. Express 2019, 6, 085334. [Google Scholar] [CrossRef]
- Elkomy, G.; Mousa, S.; Mostafa, H.A. Structural and optical properties of pure PVA/PPY and cobalt chloride doped PVA/PPY films. Arab. J. Chem. 2016, 9, S1786–S1792. [Google Scholar] [CrossRef]
- Singh, K.; Gupta, P. Polymer based solid state electrochromic display device using PVA complex electrolytes. Solid State Ion. 1995, 78, 223–229. [Google Scholar] [CrossRef]
- Devi, C.; Sharma, A.; Rao, V. Electrical and optical properties of pure and silver nitrate-doped polyvinyl alcohol films. Mater. Lett. 2002, 56, 167–174. [Google Scholar] [CrossRef]
- Zaki, M.F. Gamma-induced modification on optical band gap of CR-39 SSNTD. Braz. J. Phys. 2008, 38, 558–562. [Google Scholar] [CrossRef]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A. Hydrogels: Experimental characterization and mathematical modelling of their mechanical and diffusive behaviour. Chem. Soc. Rev. 2018, 47, 2357–2373. [Google Scholar] [CrossRef]
- Demeter, M.; Virgolici, M.; Vancea, C.; Scarisoreanu, A.; Kaya, M.G.A.; Meltzer, V. Network structure studies on γ–irradiated collagen–PVP superabsorbent hydrogels. Radiat. Phys. Chem. 2017, 131, 51–59. [Google Scholar] [CrossRef]
- Tuleushev, A.; Harrison, F.; Kozlovskiy, A.; Zdorovets, M. Assessment of the Irradiation Exposure of PET Film with Swift Heavy Ions Using the Interference-Free Transmission UV-Vis Transmission Spectra. Polymers 2021, 13, 358. [Google Scholar] [CrossRef]
- Severin, D.; Ensinger, W.; Neumann, R.; Trautmann, C.; Walter, G.; Alig, I.; Dudkin, S. Degradation of polyimide under irradiation with swift heavy ions. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2005, 236, 456–460. [Google Scholar] [CrossRef]
- Wang, M.; Shen, W.; Ding, S.; Wang, X.; Wang, Z.; Wang, Y.; Liu, F. A coupled effect of dehydration and electrostatic interactions on selective ion transport through charged nanochannels. Nanoscale 2018, 10, 18821–18828. [Google Scholar] [CrossRef]
- Apel, P.Y.; Blonskaya, I.V.; Ivanov, O.M.; Kristavchuk, O.V.; Lizunov, N.E.; Nechaev, A.N.; Orelovich, O.L.; Polezhaeva, O.A.; Dmitriev, S.N. Creation of Ion-Selective Membranes from Polyethylene Terephthalate Films Irradiated with Heavy Ions: Critical Parameters of the Process. Membr. Membr. Technol. 2020, 2, 98–108. [Google Scholar] [CrossRef]
- Kumar, R.; Ali, S.; Singh, P.; De, U.; Virk, H.S.; Prasad, R. Physical and chemical response of 145MeV Ne6+ ion irradiated polymethylmethacrylate (PMMA) polymer. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2011, 269, 1755–1759. [Google Scholar] [CrossRef]
- Guggillia, P.; Chilvery, A.; Powell, R. Reducing the Bandgap Energy via Doping Process in Lead-Free Thin Film Nanocomposites. Res. Rev. J. Mater. Sci. 2017, 5, 5. [Google Scholar] [CrossRef]
- Mohan, K.R.; Achari, V.; Rao, V.; Sharma, A. Electrical and optical properties of (PEMA/PVC) polymer blend electrolyte doped with NaClO4. Polym. Test. 2011, 30, 881–886. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Rsaul, H.A.; Ahmed, H.M. In situ synthesis of CuS nanoparticle with a distinguishable SPR peak in NIR region. J. Mater. Sci. Mater. Electron. 2016, 27, 4163–4171. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Rasheed, M.A. A novel polymer composite with a small optical band gap: New approaches for photonics and optoelectronics. J. Appl. Polym. Sci. 2017, 134, 134. [Google Scholar] [CrossRef]
- Brédas, J.L.; Chance, R.R.; Silbey, R. Comparative theoretical study of the doping of conjugated polymers: Polarons in polyacetylene and polyparaphenylene. Phys. Rev. B 1982, 26, 5843–5854. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Park, J.-S.; Kim, Y.-A.; Yang, S.-J.; Jeong, S.-I.; Lee, J.-Y.; Lim, Y.-M. Gamma Ray-Induced Polymerization and Cross-Linking for Optimization of PPy/PVP Hydrogel as Biomaterial. Polymers 2020, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Goto, H. A Possibility for Construction of an Iodine Cleaning System Based on Doping for π-Conjugated Polymers. Polymers 2011, 3, 875–885. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, W.; Wang, C.; Wen, T.-C.; Wei, Y. One-dimensional conducting polymer nanocomposites: Synthesis, properties and applications. Prog. Polym. Sci. 2011, 36, 671–712. [Google Scholar] [CrossRef]


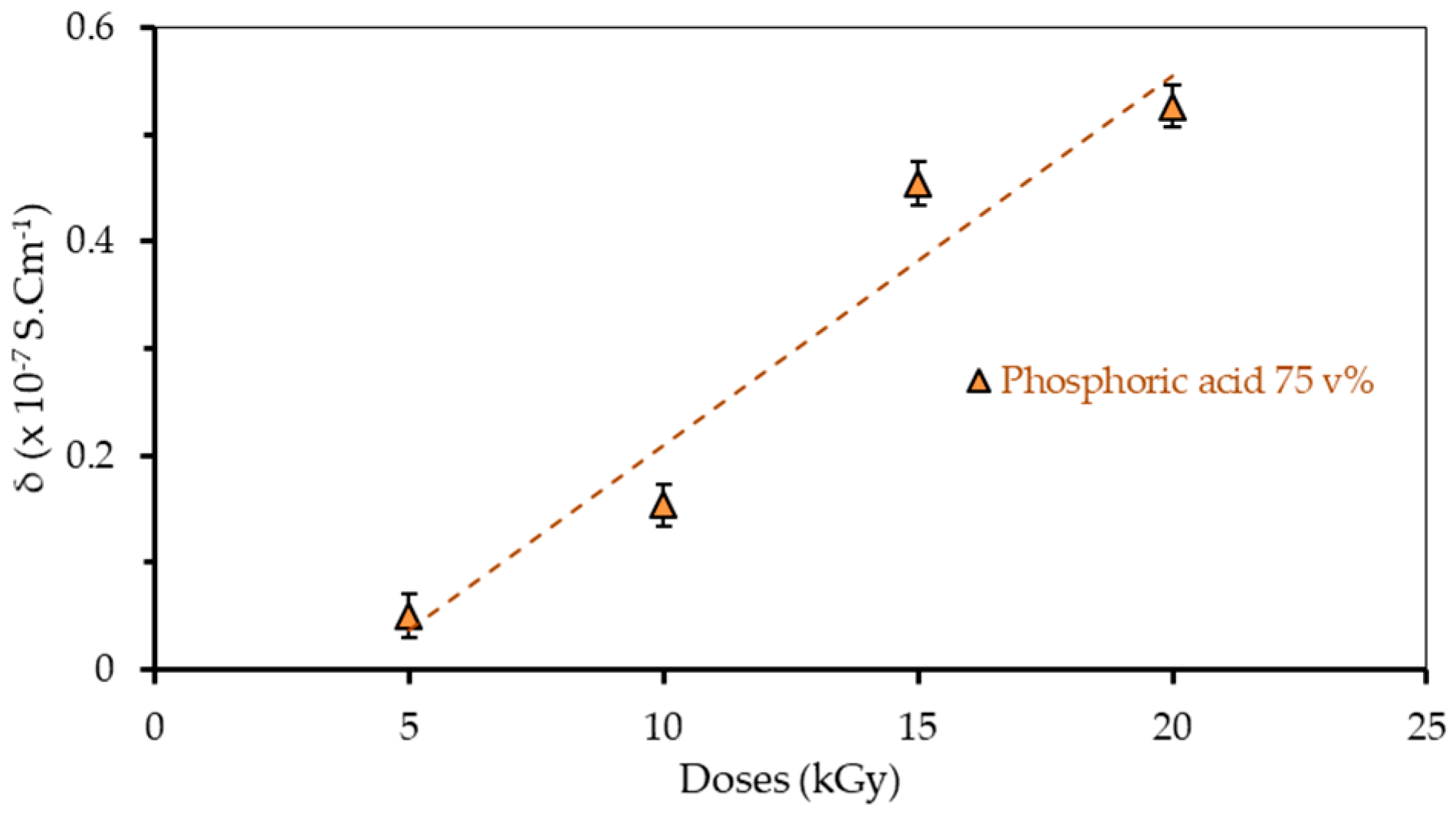
| Doses (kGy) | Resistivity ρ (Ω·cm) | Conductivity σ (S cm−1) |
|---|---|---|
| 5 | (20.0 ± 0.6) × 107 | (0.050 ± 0.6) × 10−7 |
| 10 | (6.5 ± 0.8) × 107 | (0.154 ± 0.8) × 10−7 |
| 15 | (2.2 ± 0.4) × 107 | (0.454 ± 0.4) × 10−7 |
| 20 | (1.9 ± 0.5) × 107 | (0.526 ± 0.5) × 10−7 |
| Polymer Film | Electrical Conducitivity |
|---|---|
| PVA | Dry PVA, σ = 10−10–10−14 S cm−1 PVA in water, σ ≈ 10−9 S cm−1 [14] |
| PVA + H3PO4 | Increased in conductivity of six-fold of the pure PVA (σ ≈ 10−9 S·cm−1) [14] |
| (PVA + H3PO4) + Gamma irradiation | H3PO4 85 v%, irradiation 5 kGy, σ = (0.21 ± 0,4) × 10−5 S cm−1 H3PO4 75 v%, irradiation 5 kGy, σ = (0.050 ± 0,6) × 10−7 S cm−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susilawati, S.; Prayogi, S.; Arif, M.F.; Ismail, N.M.; Bilad, M.R.; Asy’ari, M. Optical Properties and Conductivity of PVA–H3PO4 (Polyvinyl Alcohol–Phosphoric Acid) Film Blend Irradiated by γ-Rays. Polymers 2021, 13, 1065. https://doi.org/10.3390/polym13071065
Susilawati S, Prayogi S, Arif MF, Ismail NM, Bilad MR, Asy’ari M. Optical Properties and Conductivity of PVA–H3PO4 (Polyvinyl Alcohol–Phosphoric Acid) Film Blend Irradiated by γ-Rays. Polymers. 2021; 13(7):1065. https://doi.org/10.3390/polym13071065
Chicago/Turabian StyleSusilawati, Susilawati, Saiful Prayogi, Muhamad F. Arif, Noor Maizura Ismail, Muhammad Roil Bilad, and Muhammad Asy’ari. 2021. "Optical Properties and Conductivity of PVA–H3PO4 (Polyvinyl Alcohol–Phosphoric Acid) Film Blend Irradiated by γ-Rays" Polymers 13, no. 7: 1065. https://doi.org/10.3390/polym13071065
APA StyleSusilawati, S., Prayogi, S., Arif, M. F., Ismail, N. M., Bilad, M. R., & Asy’ari, M. (2021). Optical Properties and Conductivity of PVA–H3PO4 (Polyvinyl Alcohol–Phosphoric Acid) Film Blend Irradiated by γ-Rays. Polymers, 13(7), 1065. https://doi.org/10.3390/polym13071065









