Gum Arabic/Gelatin and Water-Soluble Soy Polysaccharides/Gelatin Blend Films as Carriers of Astaxanthin—A Comparative Study of the Kinetics of Release and Antioxidant Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. pH
2.4. Film Thickness
2.5. Film Conditioning
2.6. Microscopy
2.7. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.8. Water Affinities
2.9. Release Test
2.10. Antiradical Activity
2.11. Statistical Analysis
3. Results and Discussion
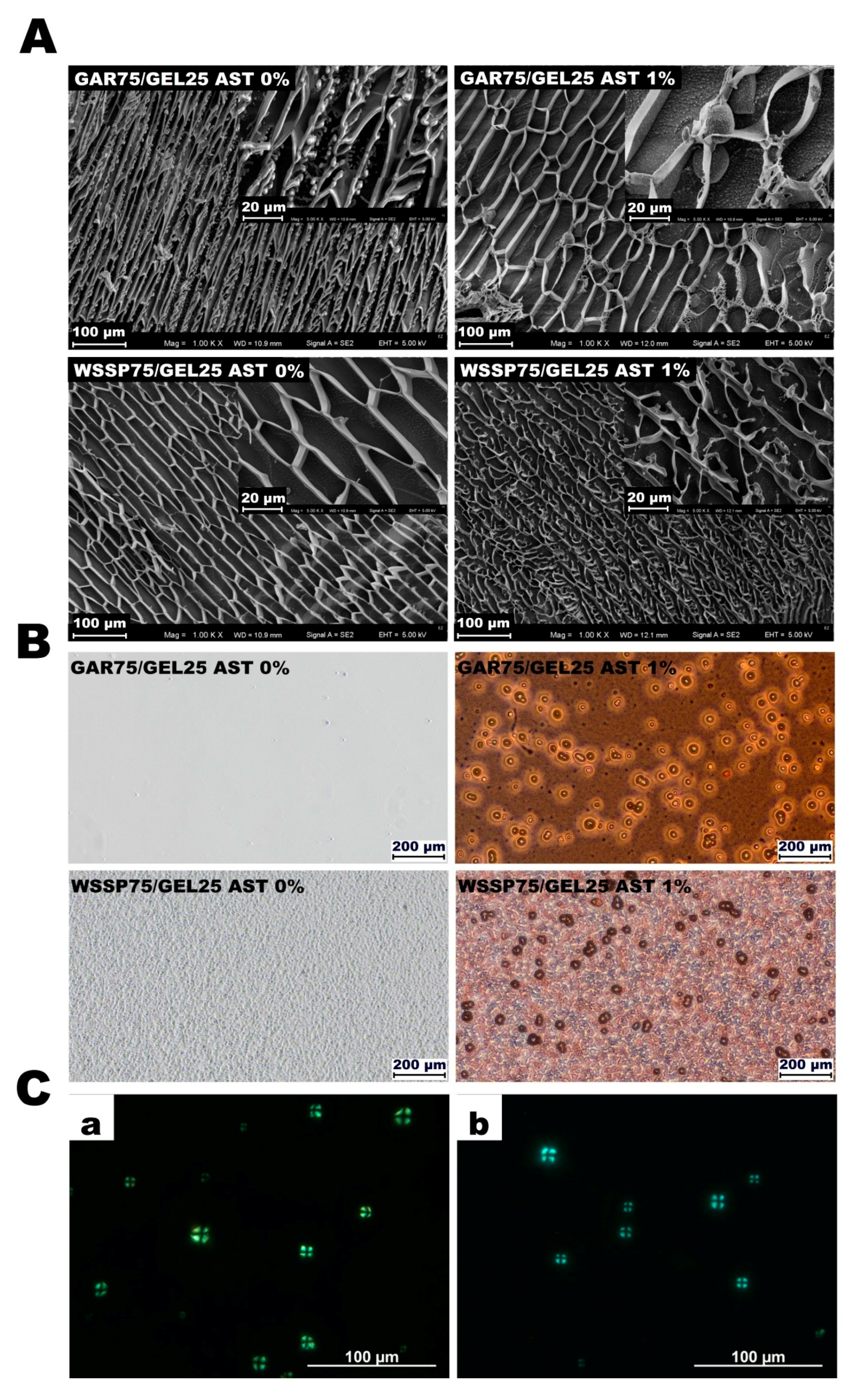
3.1. Microstructure
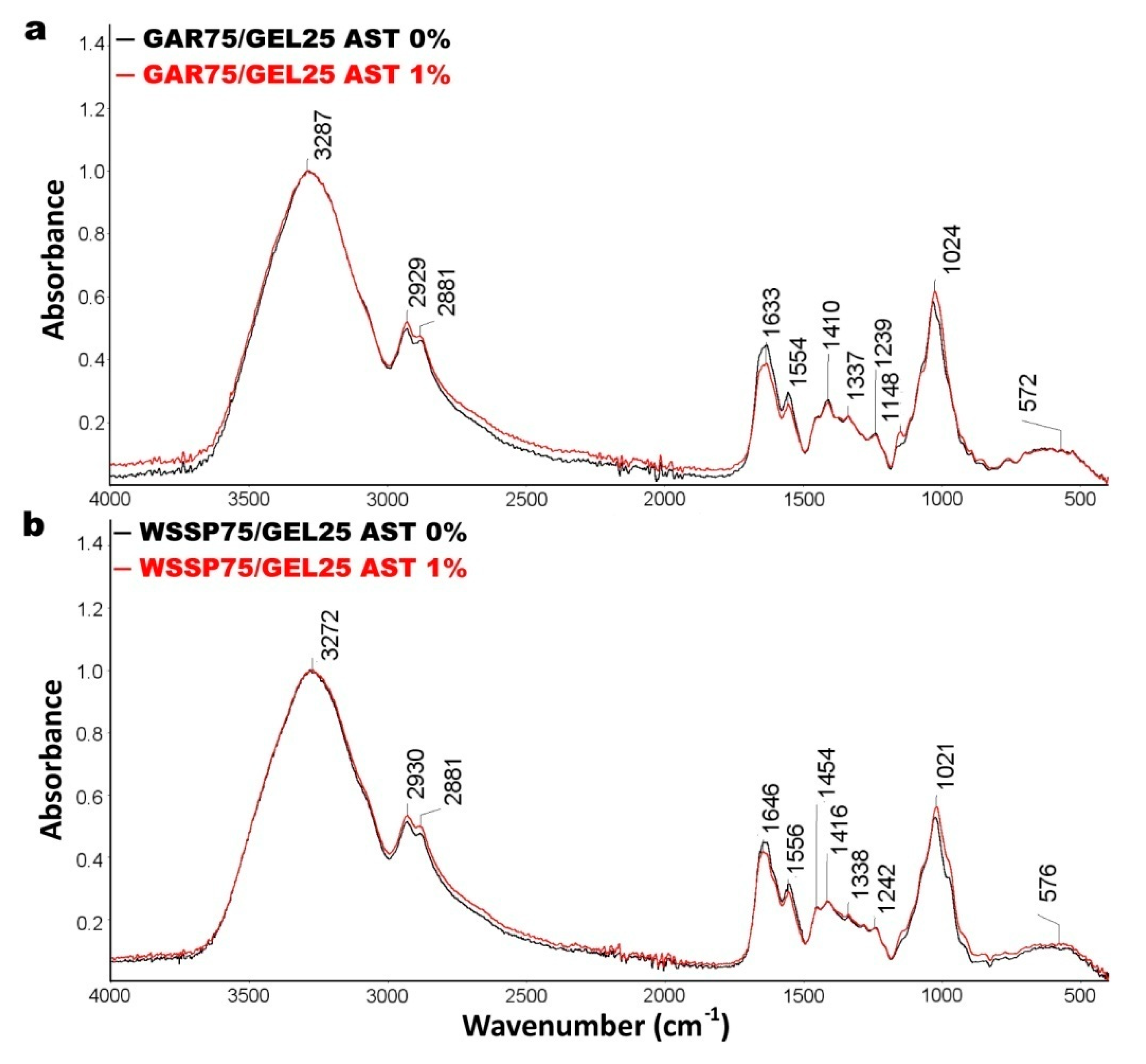
3.2. FTIR
3.3. pH, Film Thickness, and Water Affinities
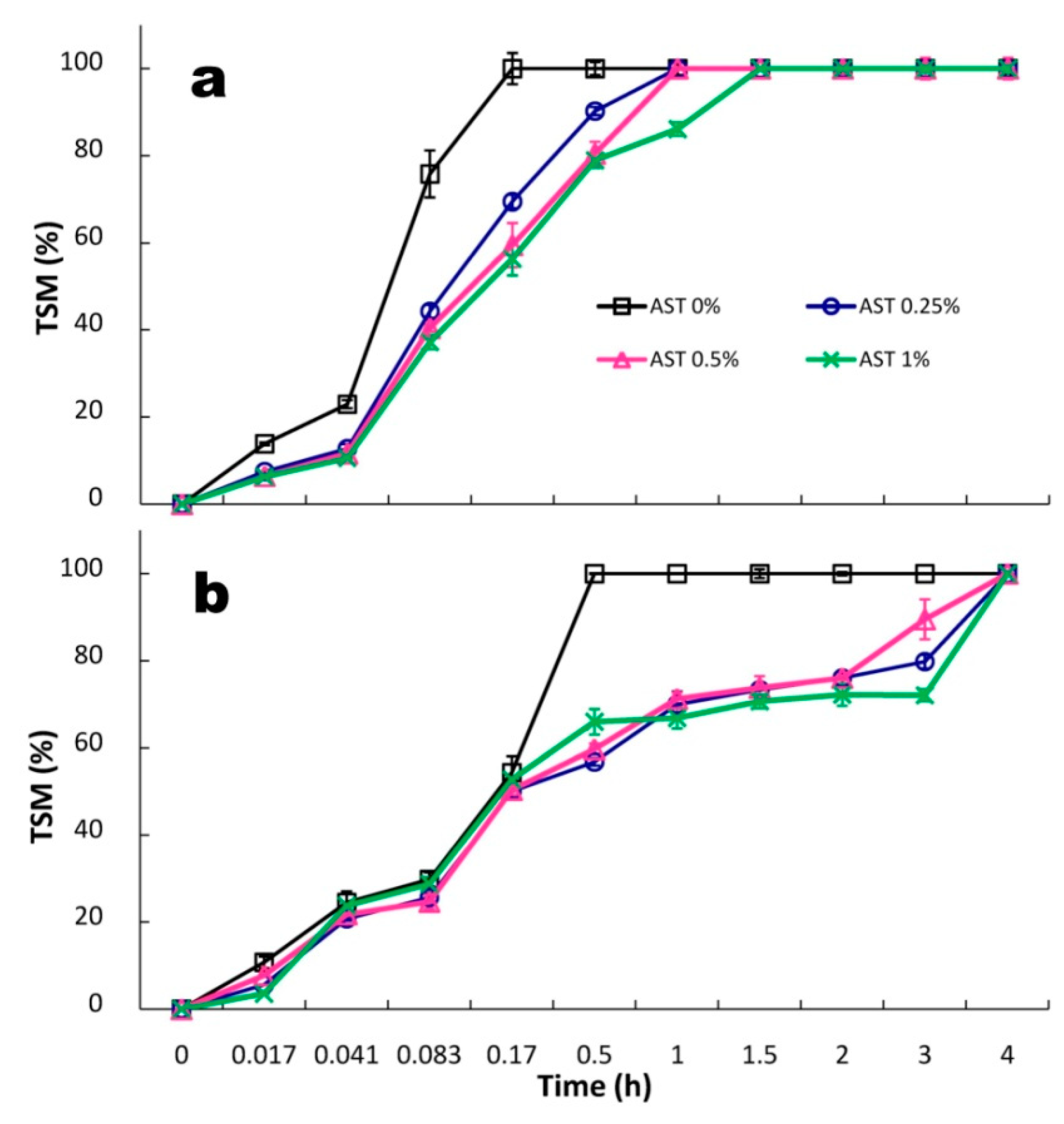
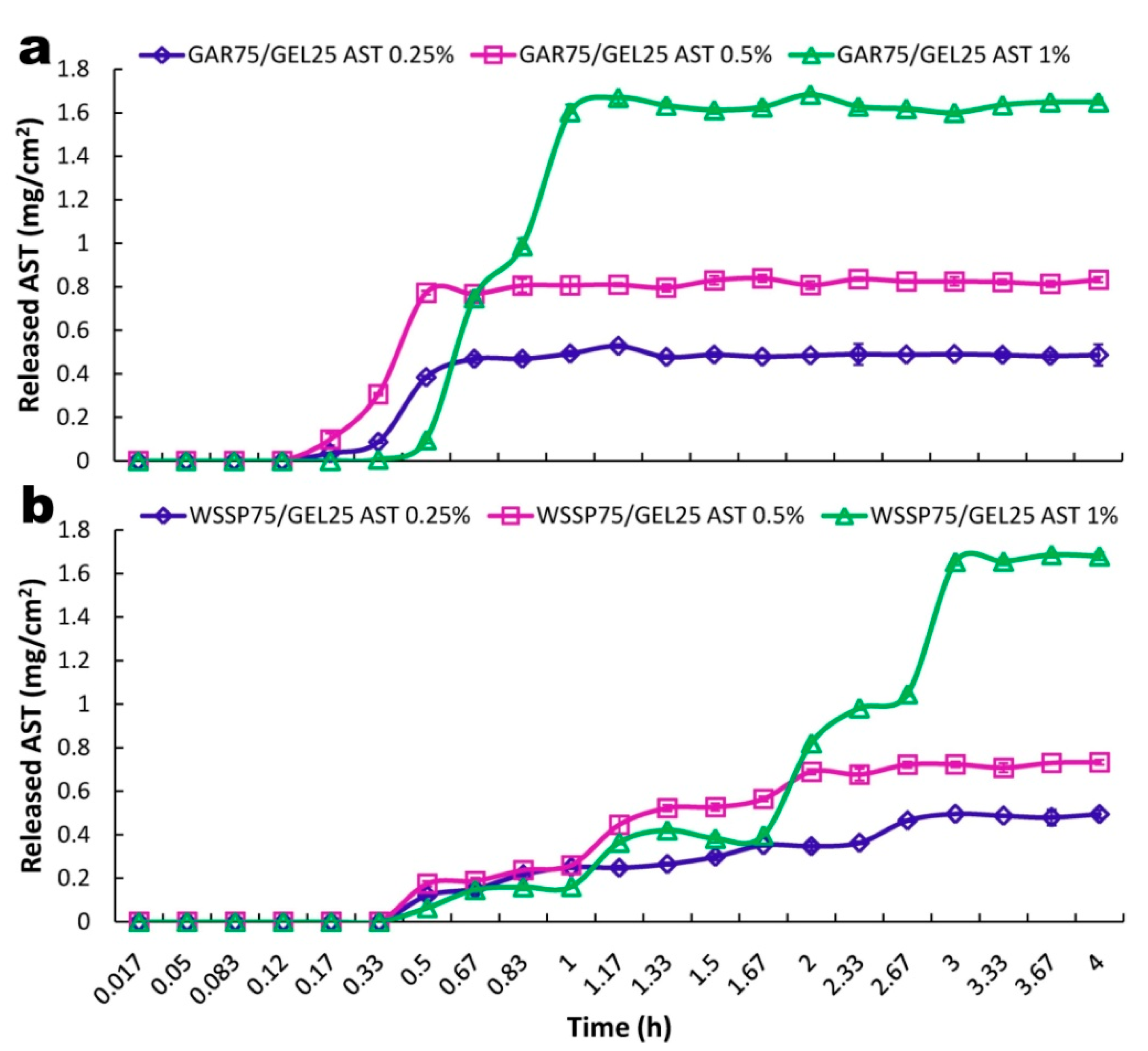
3.4. Release of ASX
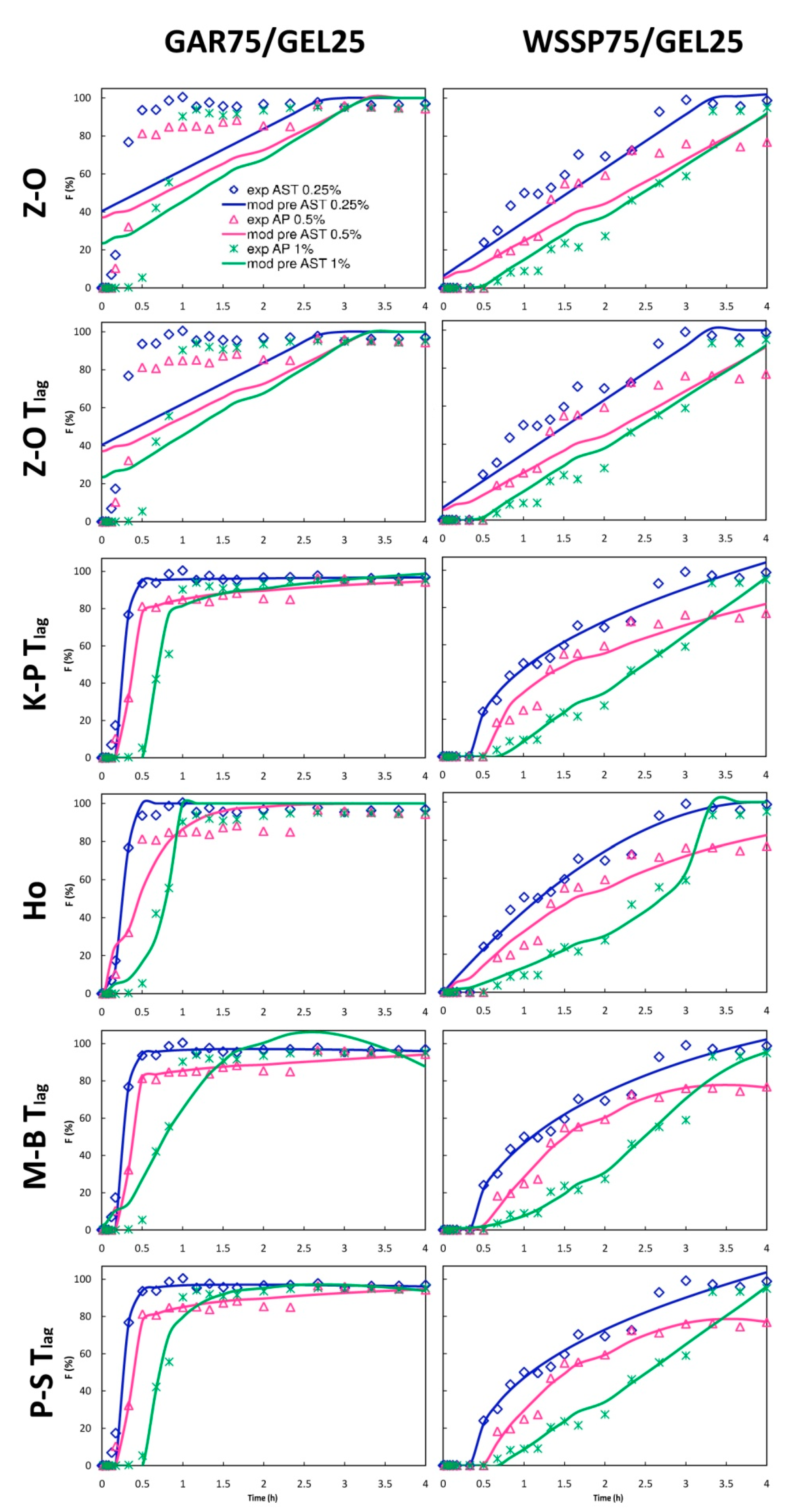
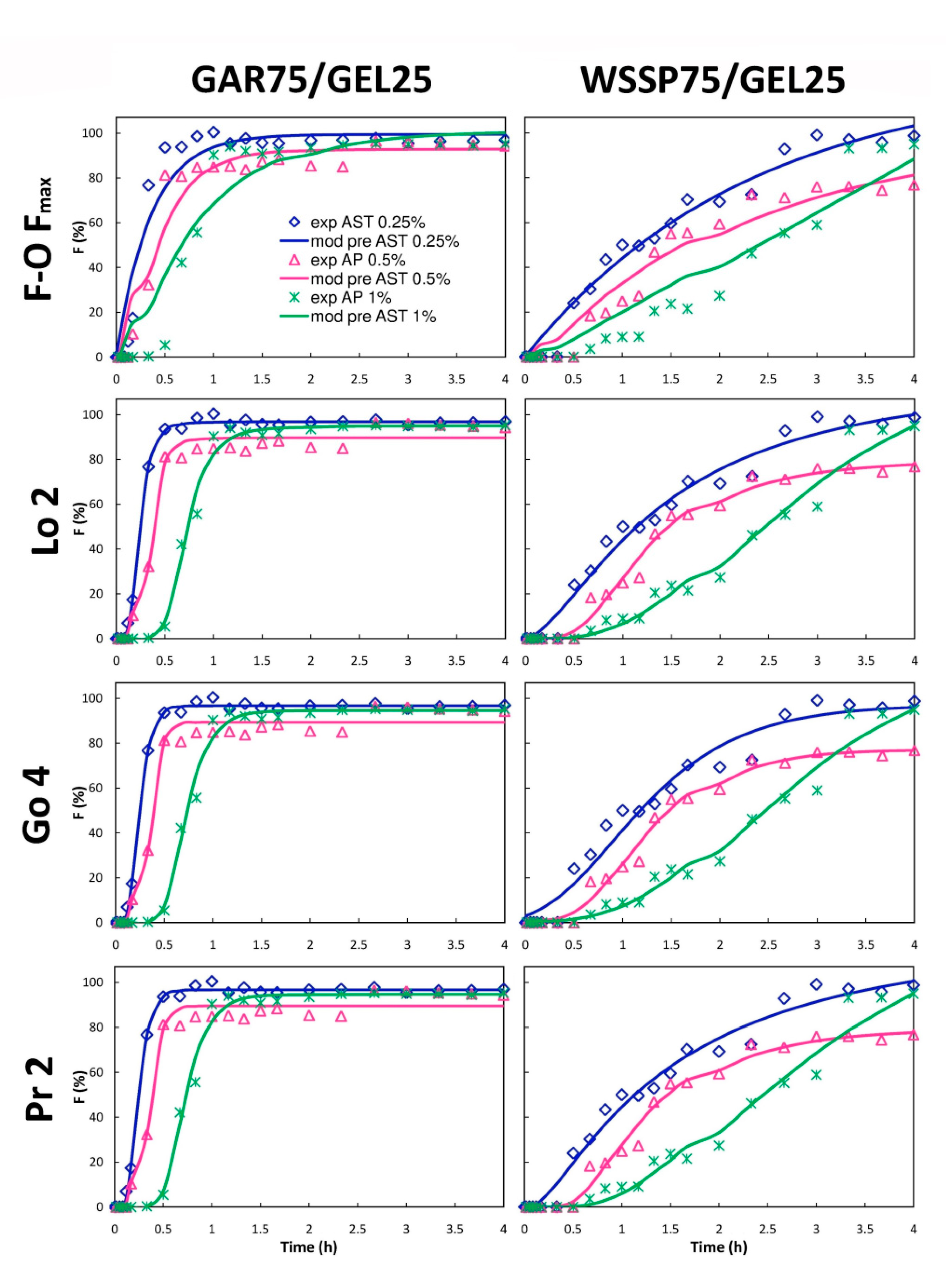
3.5. Mathematical Modeling
3.6. Antioxidant Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kontominas, M. Bioactive Food Packaging: Strategies, Quality, Safety; Kontominas, M., Ed.; DEStech Publications, Inc.: Lancaster, PA, USA, 2017. [Google Scholar]
- Cardea, S.; Baldino, L.; Reverchon, E. Comparative study of PVDF-HFP-curcumin porous structures produced by supercritical assisted processes. J. Supercrit. Fluids 2018, 133, 270–277. [Google Scholar] [CrossRef]
- Bruschi, M.L. Main mechanisms to control the drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; pp. 37–62. ISBN 9780081000922. [Google Scholar]
- Kowalczyk, D.; Baraniak, B. Effect of candelilla wax on functional properties of biopolymer emulsion films—A comparative study. Food Hydrocoll. 2014, 41, 195–209. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Kazimierczak, W.; Zięba, E.; Mężyńska, M.; Basiura-Cembala, M.; Lisiecki, S.; Karaś, M.; Baraniak, B. Ascorbic acid- and sodium ascorbate-loaded oxidized potato starch films: Comparative evaluation of physicochemical and antioxidant properties. Carbohydr. Polym. 2018, 181, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Kordowska-Wiater, M.; Karaś, M.; Zięba, E.; Mężyńska, M.; Wiącek, A.E. Release kinetics and antimicrobial properties of the potassium sorbate-loaded edible films made from pullulan, gelatin and their blends. Food Hydrocoll. 2020, 101, 105539. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Pytka, M.; Szymanowska, U.; Skrzypek, T.; Łupina, K.; Biendl, M. Release kinetics and antibacterial activity of potassium salts of iso-α-acids loaded into the films based on gelatin, carboxymethyl cellulose and their blends. Food Hydrocoll. 2020, 109, 106104. [Google Scholar] [CrossRef]
- Chen, X.; Chen, M.; Xu, C.; Yam, K.L. Critical review of controlled release packaging to improve food safety and quality. Crit. Rev. Food Sci. Nutr. 2019, 59, 2386–2399. [Google Scholar] [CrossRef]
- Cook, A.B.; Perrier, S. Branched and dendritic polymer architectures: Functional nanomaterials for therapeutic delivery. Adv. Funct. Mater. 2020, 30, 1–24. [Google Scholar] [CrossRef]
- Lutz, P.J.; Peruch, F. Graft copolymers and comb-shaped homopolymers. In Polymer Science: A Comprehensive Reference, 10 Volume Set; Moeller, M., Matyjaszewski, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 6, pp. 511–542. ISBN 9780080878621. [Google Scholar]
- Sorieul, M.; Dickson, A.; Hill, S.J.; Pearson, H. Plant fibre: Molecular structure and biomechanical properties, of a complex living material, influencing its deconstruction towards a biobased composite. Materials 2016, 9, 618. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Drozłowska, E. Polysaccharide/gelatin blend films as carriers of ascorbyl palmitate—A comparative study. Food Chem. 2020, 333. [Google Scholar] [CrossRef]
- Rezaie, A.; Rezaei, M.; Alboofetileh, M. Preparation of biodegradable carboxymethyl cellulose-Arabic gum composite film and evaluation of the physical, mechanical and thermal properties (in persian). Food Sci. Technol. Res. 2021, 17, 287–297. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Zięba, E.; Kazimierczak, W.; Mężyńska, M.; Basiura-Cembala, M.; Wiącek, A.E. Edible films made from blends of gelatin and polysaccharide-based emulsifiers—A comparative study. Food Hydrocoll. 2019, 96, 555–567. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Klein, K. Fruits, Vegetables, and nuts good sources of antioxidant. In Antioxidants in Food, Vitamins and Supplements; Elsevier: Amsterdam, The Netherlands, 2014; pp. 209–235. ISBN 9780124058729. [Google Scholar]
- Bogacz-Radomska, L.; Harasym, J. β-Carotene-properties and production methods. Food Qual. Saf. 2018, 2, 69–74. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Hu, I.-C. Production of potential coproducts from microalgae. In Biomass, Biofuels, Biochemicals; Pandey, A., Chang, J.-S., Soccol, C.R., Lee, D.-J., Chisti, Y.B.T.-B., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 345–358. ISBN 9780444641922. [Google Scholar]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Santocono, M.; Zurria, M.; Berrettini, M.; Fedeli, D.; Falcioni, G. Lutein, zeaxanthin and astaxanthin protect against DNA damage in SK-N-SH human neuroblastoma cells induced by reactive nitrogen species. J. Photochem. Photobiol. B Biol. 2007, 88, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Camera, E.; Mastrofrancesco, A.; Fabbri, C.; Daubrawa, F.; Picardo, M.; Sies, H.; Stahl, W. Astaxanthin, canthaxanthin and β-carotene differently affect UVA-induced oxidative damage and expression of oxidative stress-responsive enzymes. Exp. Dermatol. 2009, 18, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.J.; Lockwood, S.F. Acute and chronic administration of disodium disuccinate astaxanthin (CardaxTM) produces marked cardioprotection in dog hearts. Mol. Cell. Biochem. 2005, 272, 221–227. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Chengli, Y.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, X.; Nakamura, A.; Burchard, W.; Hallett, F.R. Molecular characterisation of soybean polysaccharides: An approach by size exclusion chromatography, dynamic and static light scattering methods. Carbohydr. Res. 2005, 340, 2637–2644. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Idil, N.; Perçin, I.; Denizli, A. Biomedical applications of polymeric cryogels. Appl. Sci. 2019, 9, 553. [Google Scholar] [CrossRef]
- Mazurek, S.; Mucciolo, A.; Humbel, B.M.; Nawrath, C. Transmission Fourier transform infrared microspectroscopy allows simultaneous assessment of cutin and cell-wall polysaccharides of Arabidopsis petals. Plant. J. 2013, 74, 880–891. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 1–23. ISBN 9780470027318. [Google Scholar]
- Xu, J.; Wei, R.; Jia, Z.; Song, R. Characteristics and bioactive functions of chitosan/gelatin-based film incorporated with ε-polylysine and astaxanthin extracts derived from by-products of shrimp (Litopenaeus vannamei). Food Hydrocoll. 2020, 100, 105436. [Google Scholar] [CrossRef]
- Li, X.; Yan, Y. Comparative Study of the Interactions between Ovalbumin and five Antioxidants by Spectroscopic Methods. J. Fluoresc. 2017, 27, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, L.; Li, D.; Wang, Y.; Chen, Z.; Zou, C.; Liu, W.; Ma, Y.; Cao, M.J.; Liu, G.M. Re-assembled oleic acid-protein complexes as nano-vehicles for astaxanthin: Multispectral analysis and molecular docking. Food Hydrocoll. 2020, 103. [Google Scholar] [CrossRef]
- Li, X.; Li, P. Study on the interaction of β-carotene and astaxanthin with trypsin and pepsin by spectroscopic techniques. Luminescence 2016, 31, 782–792. [Google Scholar] [CrossRef]
- Karamalla, K.A.; Siddig, N.E.; Osman, M.E. Analytical data for Acacia senegal var. senegal gum samples collected between 1993 and 1995 from Sudan. Food Hydrocoll. 1998, 12, 373–378. [Google Scholar] [CrossRef]
- Daoub, R.M.A.; Elmubarak, A.H.; Misran, M.; Hassan, E.A.; Osman, M.E. Characterization and functional properties of some natural Acacia gums. J. Saudi Soc. Agric. Sci. 2018, 17, 241–249. [Google Scholar] [CrossRef]
- Eren, B.; Tuncay Tanrıverdi, S.; Aydın Köse, F.; Özer, Ö. Antioxidant properties evaluation of topical astaxanthin formulations as anti-aging products. J. Cosmet. Dermatol. 2019, 18, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, A. Soluble soybean polysaccharide. In Handbook of Hydrocolloids: Second Edition; Williams, P.A., Phillips, G.O., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 693–709. ISBN 9781845694142. [Google Scholar]
- Ninan, G.; Joseph, J.; Aliyamveettil, Z.A. A comparative study on the physical, chemical and functional properties of carp skin and mammalian gelatins. J. Food Sci. Technol. 2014, 51, 2085–2091. [Google Scholar] [CrossRef]
- Martínez-Álvarez, Ó.; Calvo, M.M.; Gómez-Estaca, J. Recent advances in astaxanthin micro/nanoencapsulation to improve its stability and functionality as a food ingredient. Mar. Drugs 2020, 18, 406. [Google Scholar] [CrossRef] [PubMed]
- Podzimek, S. Characterization of branched polymers. In Light Scattering, Size Exclusion Chromatography and Asymmetric Flow Field Flow Fractionation: Powerful Tools for the Characterization of Polymers, Proteins and Nanoparticles; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 154–157. [Google Scholar]
- Ikeda, S.; Funami, T.; Zhang, G. Visualizing surface active hydrocolloids by atomic force microscopy. Carbohydr. Polym. 2005, 62, 192–196. [Google Scholar] [CrossRef]
- Bettini, R.; Santi, P.; Peracchia, M.T.; Catellani, P.L.; Colombo, P. Relationship between swelling and drug release in hydrogel matrices. Proc. Control. Release Soc. 1993, 19, 218–219. [Google Scholar]
- Malekjani, N.; Jafari, S.M. Modeling the release of food bioactive ingredients from carriers/nanocarriers by the empirical, semiempirical, and mechanistic models. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3–47. [Google Scholar] [CrossRef] [PubMed]
- Colín-Chávez, C.; Soto-Valdez, H.; Peralta, E.; Lizardi-Mendoza, J.; Balandrán-Quintana, R. Diffusion of natural astaxanthin from polyethylene active packaging films into a fatty food simulant. Food Res. Int. 2013, 54, 873–880. [Google Scholar] [CrossRef]
- Edelman, R.; Engelberg, S.; Fahoum, L.; Meyron-Holtz, E.G.; Livney, Y.D. Potato protein-based carriers for enhancing bioavailability of astaxanthin. Food Hydrocoll. 2019, 96, 72–80. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Lv, Y.; Yang, Y.; Ruan, Y. Protective effect of polysaccharide fractions from Radix A. sinensis against tert-butylhydroperoxide induced oxidative injury in murine peritoneal macrophages. J. Biochem. Mol. Biol. 2007, 40, 928–935. [Google Scholar] [CrossRef]
- Kong, H.; Yang, J.; Zhang, Y.; Fang, Y.; Nishinari, K.; Phillips, G.O. Synthesis and antioxidant properties of gum arabic-stabilized selenium nanoparticles. Int. J. Biol. Macromol. 2014, 65, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kchaou, H.; Benbettaïeb, N.; Jridi, M.; Abdelhedi, O.; Karbowiak, T.; Brachais, C.H.; Léonard, M.L.; Debeaufort, F.; Nasri, M. Enhancement of structural, functional and antioxidant properties of fish gelatin films using Maillard reactions. Food Hydrocoll. 2018, 83, 326–339. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Szymanowska, U.; Skrzypek, T.; Basiura-Cembala, M.; Łupina, K.; Biendl, M. Edible films based on gelatin, carboxymethyl cellulose, and their blends as carriers of potassium salts of iso-α-acids: Structural, physicochemical and antioxidant properties. Food Hydrocoll. 2021, 115. [Google Scholar] [CrossRef]
- Nurilmala, M.; Hizbullah, H.H.; Karnia, E.; Kusumaningtyas, E.; Ochiai, Y. Characterization and antioxidant activity of collagen, gelatin, and the derived peptides from Yellowfin Tuna (Thunnus albacares) skin. Mar. Drugs 2020, 18, 98. [Google Scholar] [CrossRef]



| Film | AST (%) | pH of FFSs | Thickness (µm) | Sw (%) |
|---|---|---|---|---|
| GAR75/GEL25 | 0 | 5.31 ± 0.01 g | 85.27 ± 2.77 a | 499.40 ± 25.88 d |
| 0.25 | 5.28 ± 0.01 f | 86.65 ± 3.75 a | 416.05 ± 24.58 c | |
| 0.5 | 5.25 ± 0.01 e | 87.65 ± 3.12 a | 416.51 ± 20.07 c | |
| 1 | 5.15 ± 0.00 d | 86.96 ± 3.80 a | 381.66 ± 15.49 c | |
| WSSP75/GEL25 | 0 | 5.01 ± 0.01 c | 85.53 ± 3.84 a | 301.32 ± 26.52 b |
| 0.25 | 4.91 ± 0.01 a | 87.09 ± 2.93 a | 269.96 ± 18.86 ab | |
| 0.5 | 4.94 ± 0.00 b | 89.90 ± 3.77 a | 262.19 ± 28.58 a | |
| 1 | 4.94 ± 0.01 b | 89.02 ± 2.53 a | 255.86 ± 14.63 a |
| Film | AST (%) | t25% (min) | t50% (min) | R2adjusted ^ | t25%ABTS * (min) | t50%ABTS * (min) |
|---|---|---|---|---|---|---|
| GAR75/GEL25 | 0 | n.d | n.d | n.d. | 42.10 | 120.76 |
| 0.25 | 11.28 | 14.64 | 0.999 (Lo 2) | 7.51 | 101.47 | |
| 0.5 | 10.2 | 21.72 | 0.992 (K-P Tlag) | 0.64 | 1.94 | |
| 1 | 26.46 | 34.02 | 0.993 (Pr 2) | 0.22 | 0.53 | |
| WSSP75/GEL25 | 0 | n.d | n.d | n.d. | 31.23 | 101.01 |
| 0.25 | 31.68 | 65.16 | 0.988 (K-P Tlag) | 12.94 | 54.95 | |
| 0.5 | 48.48 | 78.60 | 0.989 (Go 4) | 2.34 | 30.21 | |
| 1 | 87.54 | 142.80 | 0.983 (Ho) | 0.91 | 1.23 |
| Film | AST (%) | R2 * | R2 ** |
|---|---|---|---|
| GAR75/GEL25 | 0.25 | 0.93 | 0.87 |
| 0.5 | 0.96 | 0.83 | |
| 1 | 0.91 | 0.34 | |
| WSSP75/GEL25 | 0.25 | 0.89 | 0.93 |
| 0.5 | 0.88 | 0.89 | |
| 1 | 0.70 | 0.72 | |
| All formulations | 0.89 | 0.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łupina, K.; Kowalczyk, D.; Kazimierczak, W. Gum Arabic/Gelatin and Water-Soluble Soy Polysaccharides/Gelatin Blend Films as Carriers of Astaxanthin—A Comparative Study of the Kinetics of Release and Antioxidant Properties. Polymers 2021, 13, 1062. https://doi.org/10.3390/polym13071062
Łupina K, Kowalczyk D, Kazimierczak W. Gum Arabic/Gelatin and Water-Soluble Soy Polysaccharides/Gelatin Blend Films as Carriers of Astaxanthin—A Comparative Study of the Kinetics of Release and Antioxidant Properties. Polymers. 2021; 13(7):1062. https://doi.org/10.3390/polym13071062
Chicago/Turabian StyleŁupina, Katarzyna, Dariusz Kowalczyk, and Waldemar Kazimierczak. 2021. "Gum Arabic/Gelatin and Water-Soluble Soy Polysaccharides/Gelatin Blend Films as Carriers of Astaxanthin—A Comparative Study of the Kinetics of Release and Antioxidant Properties" Polymers 13, no. 7: 1062. https://doi.org/10.3390/polym13071062
APA StyleŁupina, K., Kowalczyk, D., & Kazimierczak, W. (2021). Gum Arabic/Gelatin and Water-Soluble Soy Polysaccharides/Gelatin Blend Films as Carriers of Astaxanthin—A Comparative Study of the Kinetics of Release and Antioxidant Properties. Polymers, 13(7), 1062. https://doi.org/10.3390/polym13071062