Performance Evaluation of a Novel Biosourced Co-Processed Excipient in Direct Compression and Drug Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. True Density
2.2.2. Particle Size Distribution
2.2.3. Tapped and Bulk Density
2.2.4. Angle of Repose
2.2.5. Scanning Electronic Microscopy
2.2.6. Compaction Study
Tabletability
Compressibility
Elastic Recovery
Walker and Heckel Modeling
Ejection Force
Lubricant Sensitivity
Speed and Dwell Time Effect
2.2.7. Disintegration Time
2.2.8. Melatonin Tablets Manufacturing
2.2.9. Dissolution Profile
3. Results and Discussion
3.1. Study of the Supplied Materials
3.1.1. True, Bulk and Tapped Density
3.1.2. Scanning Electronic Microscopy
3.1.3. Particle Size and Powder Flowability
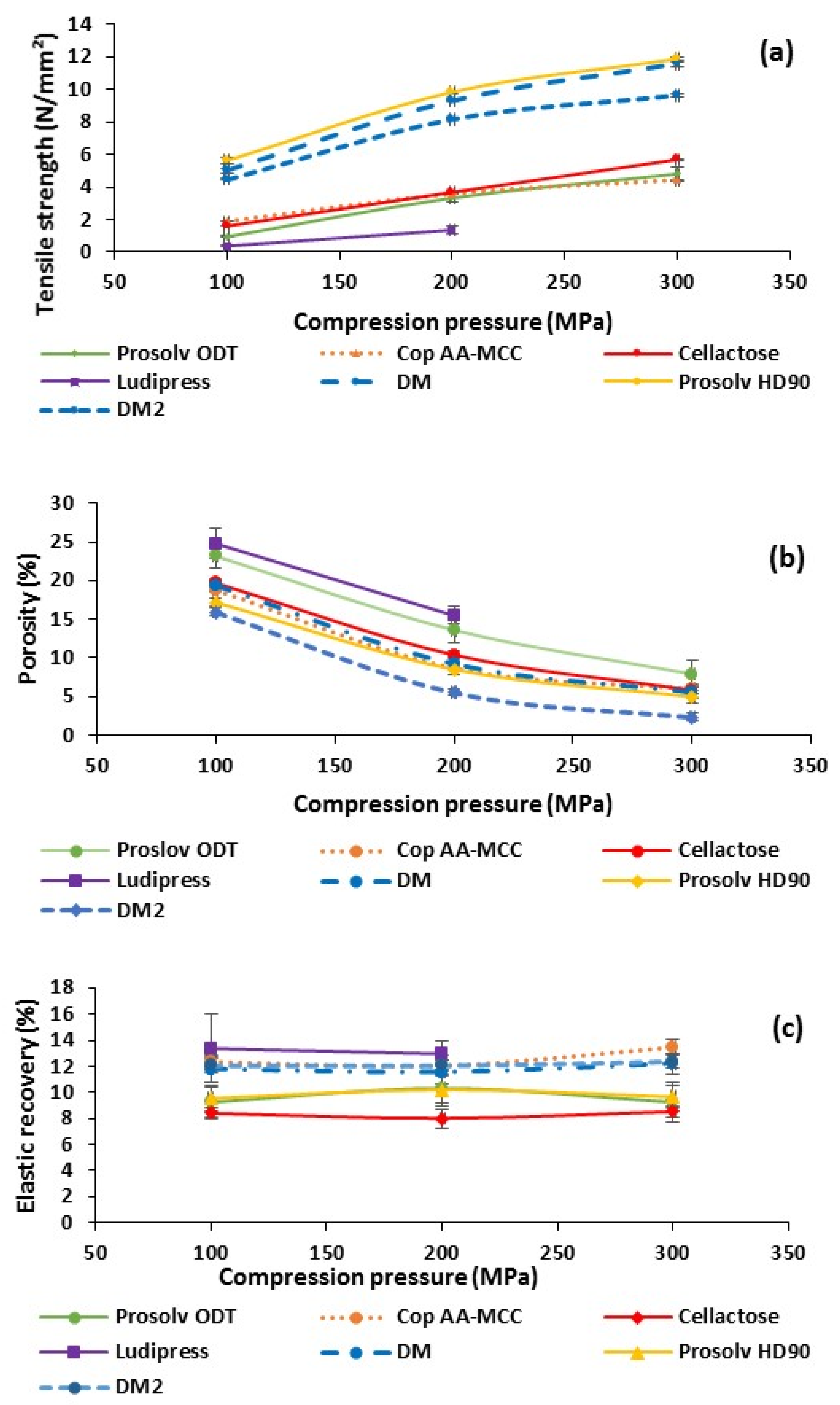
3.1.4. Powders’ Tabletability, Compressibility and Elastic Recovery of Non-Lubricated Materials
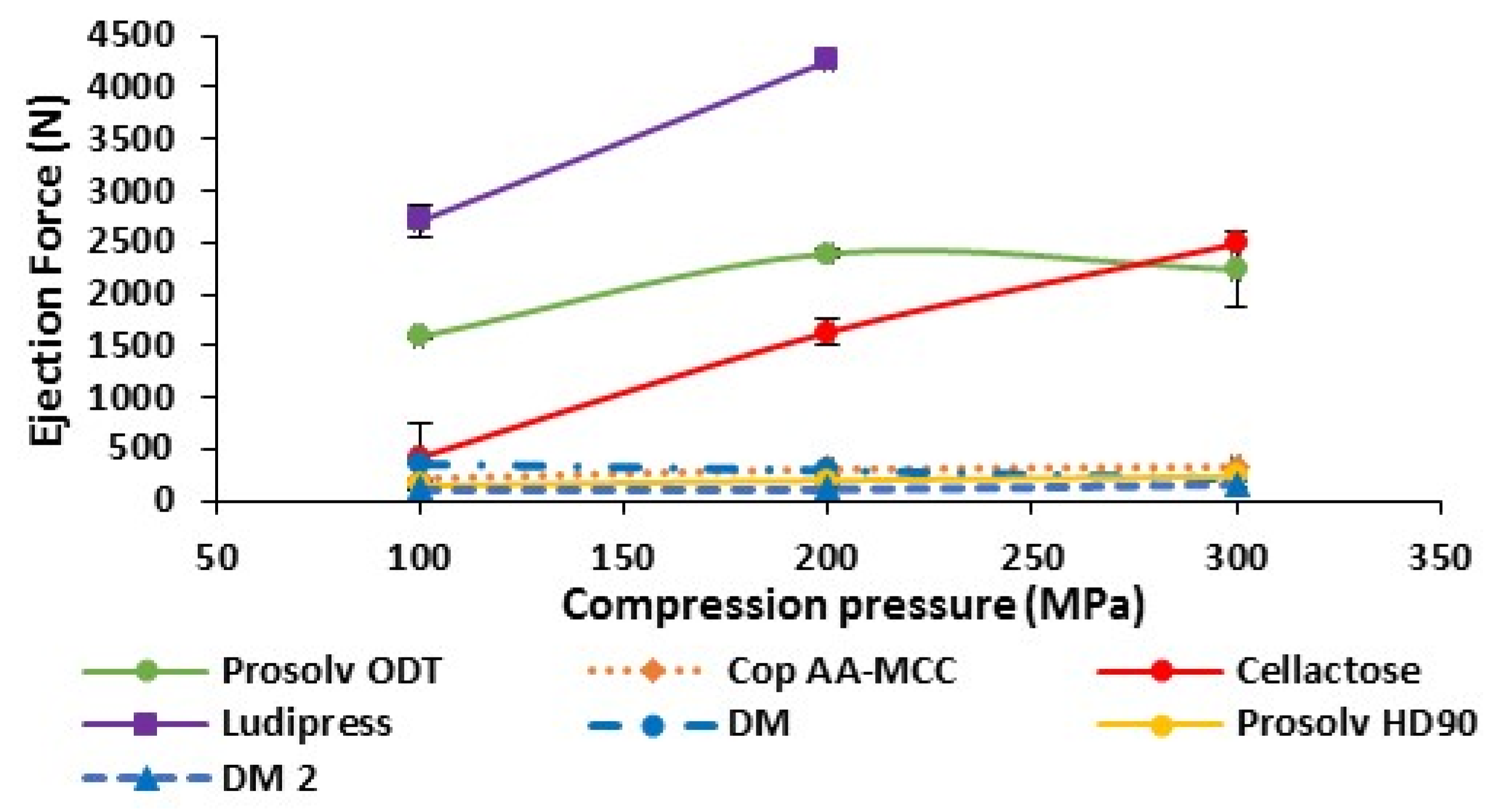
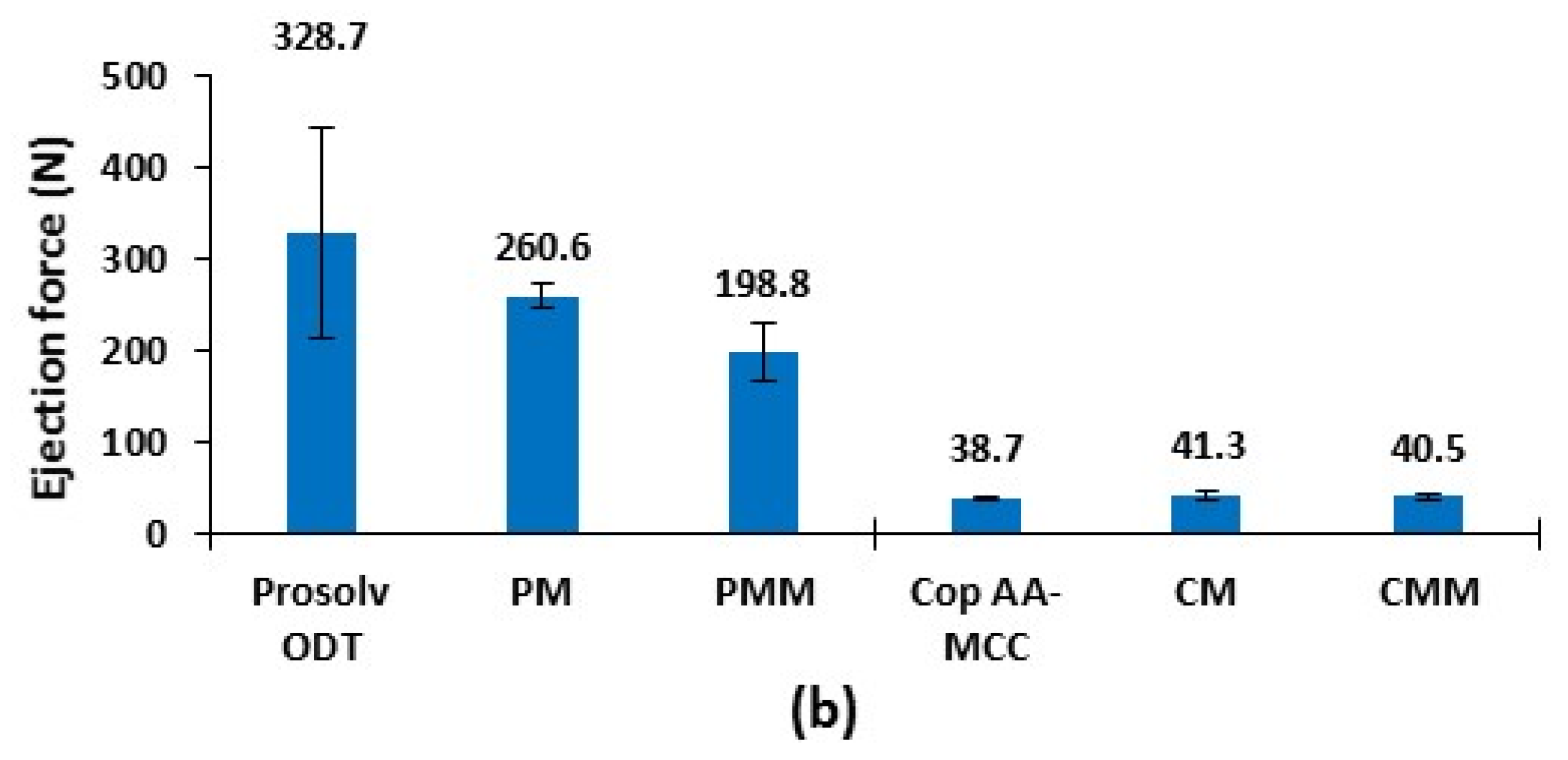
3.1.5. Ejection Force of Non-Lubricated Materials
3.1.6. Powder Deformation Behavior of Non-Lubricated Materials
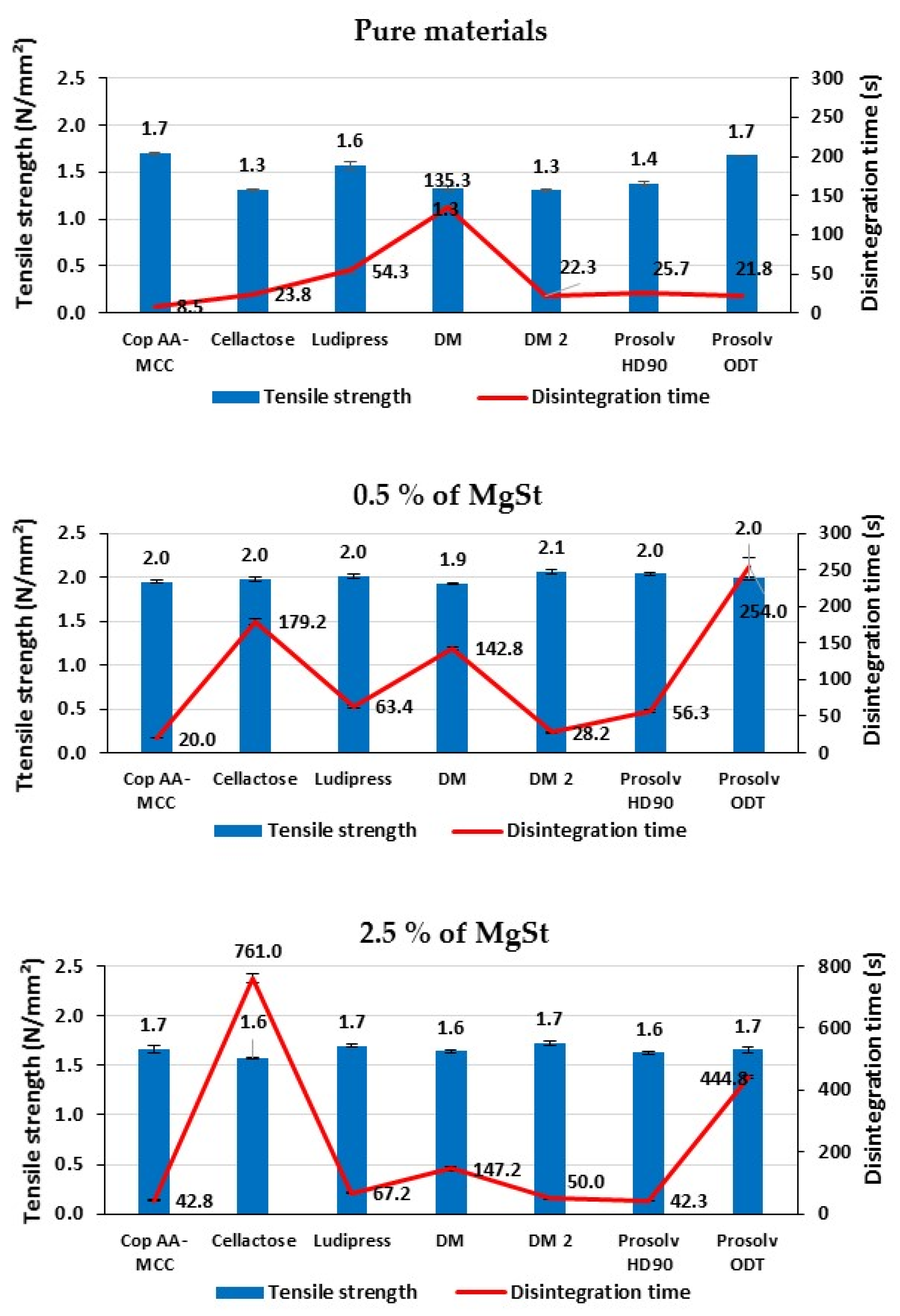
3.1.7. Disintegration Time of Non-Lubricated Materials
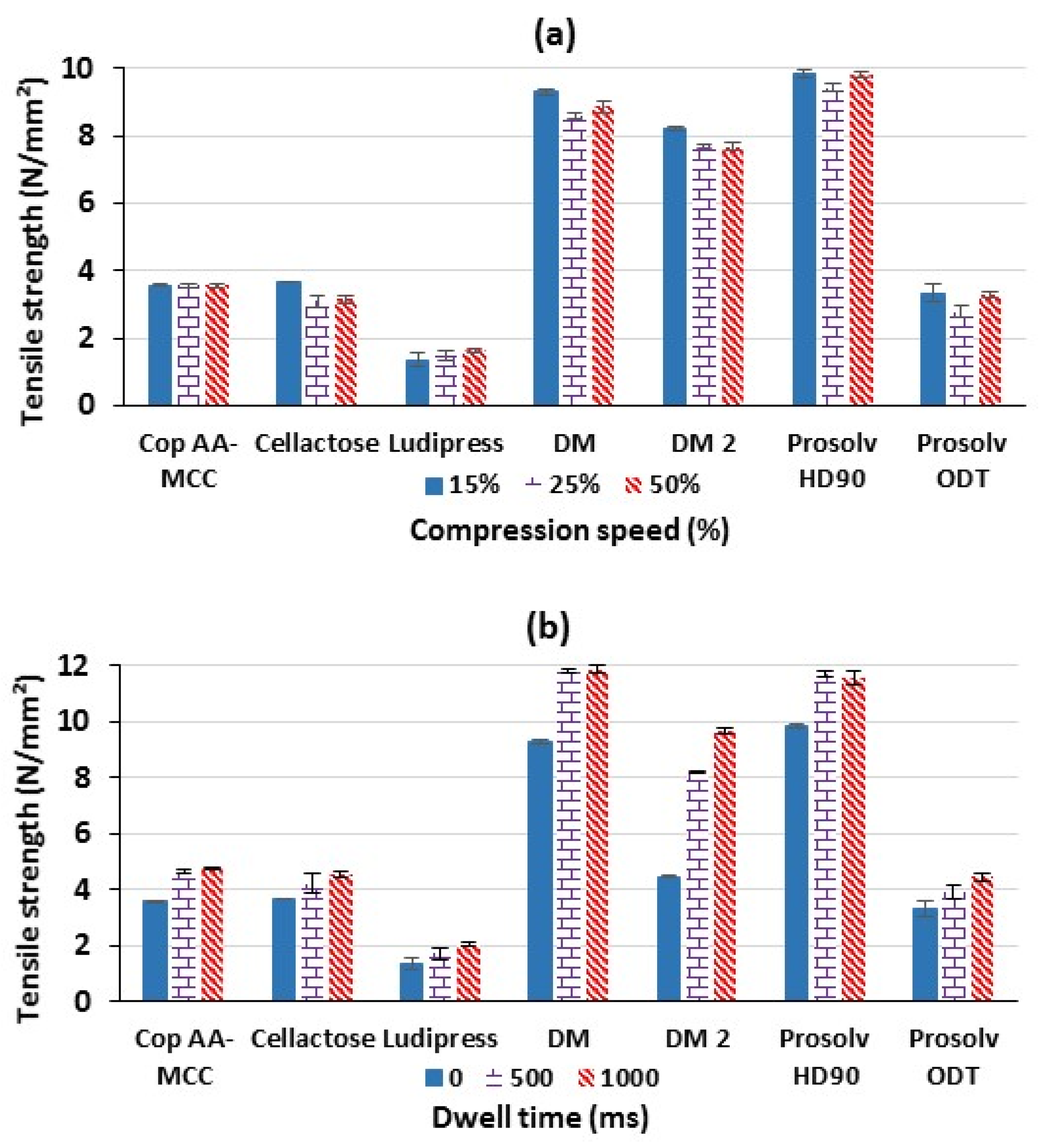
3.1.8. Effect of Compression Speed and Dwell Time on Tablet Tensile Strength
3.2. Study of Lubricated Materials
3.2.1. Effect of Lubrication on Tablet Tensile Strength
3.2.2. Effect of Lubrication on Tablet’s Disintegration
3.3. Study of a Melatonin Tablets
3.3.1. Compaction Study
3.3.2. Tablets Dissolution Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Hir, A. Formes pharmaceutiques: Comprimés. In Abrégés de Pharmacie Galénique; Masson: Paris, France, 1992. [Google Scholar]
- Augsburger, L.L.; Hoag, S.W. Pharmaceutical Dosage Forms. Tablets, 3rd ed.; Informa Healthcare: New York, NY, USA, 2008; ISBN 978-0-8493-9014-2. [Google Scholar]
- Bhor, N.J.; Bhusare, S.E.; Kare, P.T. Multifunctional Excipients: The Smart Excipients. Int. J. Pure Appl. Biosci. 2014, 2, 144–148. [Google Scholar]
- Koo, O.M.Y. Pharmaceutical Excipients: Properties, Functionality and Applications in Research and Industry, 1st ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Ren, G.; Clancy, C.; Tamer, T.M.; Schaller, B.; Walker, G.M.; Collins, M.N. Cinnamyl O-Amine Functionalized Chitosan as a New Excipient in Direct Compressed Tablets with Improved Drug Delivery. Int. J. Biol. Macromol. 2019, 141, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ballester, N.M.; Bataille, B.; Benabbas, R.; Alonso, B.; Soulairol, I. Development of Alginate Esters as Novel Multifunctional Excipients for Direct Compression. Carbohydr. Polym. 2020, 240, 116280. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, P.; Phatak, A.; Desai, U. A Review: Coprocessed Excipients-An Alternative to Novel Chemical Entities. Int. J. Pharm. Chem. Biol. Sci. 2012, 1, 1480–1498. [Google Scholar]
- Sreekanth Babu, S.; Ajay Kumar, A.; Suman, D.R. Co-Processed Excipients—A Review. Int. J. Curr. Pharm. Res. 2013, 1, 205–214. [Google Scholar]
- Patel, R.P.; Bhavsar, M. Directly Compressible Materials via Co-Processing. Int. J. Pharmtech Res. 2009, 1, 745–753. [Google Scholar]
- Vodáčková, P.; Vraníková, B.; Svačinová, P.; Franc, A.; Elbl, J.; Muselík, J.; Kubalák, R.; Solný, T. Evaluation and Comparison of Three Types of Spray Dried Coprocessed Excipient Avicel® for Direct Compression. BioMed Res. Int. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nachaegari, S.K.; Bansal, A.K. Coprocessed Excipients for Solid Dosage Forms. Pharm. Technol. 2004, 28, 52–64. [Google Scholar]
- Arida, A.I.; Al-Tabakha, M.M. Cellactose® a Co-Processed Excipient: A Comparison Study. Pharm. Dev. Technol. 2008, 13, 165–175. [Google Scholar] [CrossRef]
- Mužíková, J.; Nováková, P. A Study of the Properties of Compacts from Silicified Microcrystalline Celluloses. Drug Dev. Ind. Pharm. 2007, 33, 775–781. [Google Scholar] [CrossRef]
- Stoltenberg, I.; Breitkreutz, J. Orally Disintegrating Mini-Tablets (ODMTs)–A Novel Solid Oral Dosage Form for Paediatric Use. Eur. J. Pharm. Biopharm. 2011, 78, 462–469. [Google Scholar] [CrossRef]
- Benabbas, R.; Sanchez-Ballester, N.M.; Bataille, B.; Sharkawi, T.; Soulairol, I. Development and Pharmaceutical Performance of a Novel Co-Processed Excipient of Alginic Acid and Microcrystalline Cellulose. Powder Technol. 2021, 378, 576–584. [Google Scholar] [CrossRef]
- JRS Pharma, Prosolv® SMCC HD 90 Product Information. Available online: Https://www.Jrspharma.Com/Pharma_en/Products-Services/Excipients/Hfe/Prosolv-Smcc.Php (accessed on 5 March 2020).
- JRS Pharma, Prosolv® ODT Product Information. Available online: Https://www.Jrspharma.Com/Pharma_en/Products-Services/Excipients/Hfe/Prosolv-Odt-G2.Php (accessed on 5 March 2020).
- BASF, Ludipress® Product Information. Available online: Https://Pharmaceutical.Basf.Com/Global/En/Drug-Formulation/Products/Ludipress.Html (accessed on 5 March 2020).
- Bulk density and tapped density of powders (monograph 2.9.34). In European Pharmacopeia; European Directorate for the Quality of Medicines &HealthCare, Council of Europe: Strasbourg, France, 2019; pp. 384–387.
- Powder flow (monograph 2.9.36). In European Pharmacopeia; European Directorate for the Quality of Medicines &HealthCare, Council of Europe: Strasbourg, France, 2019; pp. 387–391.
- Hersay, J.A.; Rees, J.E. Deformation of Particles during Briquetting. Nat. Phys. Sci. 1971, 230, 96. [Google Scholar] [CrossRef]
- Walker, E.E. The Properties of Powders. Part VI. The Compressibility of Powders. Trans. Faraday Soc. 1923, 19, 73–82. [Google Scholar] [CrossRef]
- Disintegration of tablets and capsules (monograph 2.9.34). In European Pharmacopeia; European Directorate for the Quality of Medicines &HealthCare, Council of Europe: Strasbourg, France, 2019; pp. 323–325.
- Dissolution test for solid dosage forms. In European Pharmacopeia; European Directorate for the Quality of Medicines &HealthCare, Council of Europe: Strasbourg, France, 2016; pp. 302–309.
- Sun, C.; Grant, D. Influence of Crystal Structure on the Tableting Properties of Sulfamerazine Polymorphs. Pharm. Res. 2001, 18, 274–280. [Google Scholar] [CrossRef]
- Heckel, R.W. Density-Pressure Relationship in Powder Compaction. Trans. Metall. Soc. AIME 1961, 221, 671–675. [Google Scholar]
- Narang, A.S.; Mantri, R.V.; Raghavan, K.S. Excipient Compatibility and Functionality. In Developing Solid Oral Dosage Forms; Elsevier: Amsterdam, The Netherlands, 2017; pp. 151–179. ISBN 978-0-12-802447-8. [Google Scholar]
- Badawy, S.I.F.; Gray, D.B.; Hussain, M.A. A Study on the Effect of Wet Granulation on Microcrystalline Cellulose Particle Structure and Performance. Pharm. Res. 2006, 23, 634–640. [Google Scholar] [CrossRef]
- Hlinak, A.J.; Kuriyan, K.; Morris, K.R.; Reklaitis, G.V.; Basu, P.K. Understanding Critical Material Properties for Solid Dosage Form Design. J. Pharm. Innov. 2006, 1, 12–17. [Google Scholar] [CrossRef]
- Pifferi, G.; Santoro, P.; Pedrani, M. Quality and Functionality of Excipients. Farmaco 1999, 54, 1–14. [Google Scholar] [CrossRef]
- Tran, D.T.; Majerová, D.; Veselý, M.; Kulaviak, L.; Ruzicka, M.C.; Zámostný, P. On the Mechanism of Colloidal Silica Action to Improve Flow Properties of Pharmaceutical Excipients. Int. J. Pharm. 2019, 556, 383–394. [Google Scholar] [CrossRef]
- Schmid, W.; Picker-Freyer, K.M. Tableting and Tablet Properties of Alginates: Characterisation and Potential for Soft Tableting. Eur. J. Pharm. Biopharm. 2009, 72, 165–172. [Google Scholar] [CrossRef]
- Al-Ibraheemi, Z.A.M.; Anuar, M.S.; Taip, F.S.; Amin, M.C.I.; Tahir, S.M.; Mahdi, A.B. Deformation and Mechanical Characteristics of Compacted Binary Mixtures of Plastic (Microcrystalline Cellulose), Elastic (Sodium Starch Glycolate), and Brittle (Lactose Monohydrate) Pharmaceutical Excipients. Part. Sci. Technol. 2013, 31, 561–567. [Google Scholar] [CrossRef]
- Keshavarz, L.; Pishnamazi, M.; Rao Khandavilli, U.B.; Shirazian, S.; Collins, M.N.; Walker, G.M.; Frawley, P.J. Tailoring Crystal Size Distributions for Product Performance, Compaction of Paracetamol. Arab. J. Chem. 2021, 14, 103089. [Google Scholar] [CrossRef]
- De Boer, A.H.; Vromans, H.; Lerk, C.F.; Bolhuis, G.K.; Kussendrager, K.D.; Bosch, H. Studies on Tableting Properties of Lactose. III. The Consolidation Behaiour of Sieve Fractions of Crystalline α-Lactose Monohydrate. Pharm. Weekbl. 1986, 8, 145–150. [Google Scholar]
- Tarlier, N.; Soulairol, I.; Bataille, B.; Baylac, G.; Ravel, P.; Nofrerias, I.; Lefèvre, P.; Sharkawi, T. Compaction Behavior and Deformation Mechanism of Directly Compressible Textured Mannitol in a Rotary Tablet Press Simulator. Int. J. Pharm. 2015, 495, 410–419. [Google Scholar] [CrossRef]
- Tye, C.K.; Sun, C.; Amidon, G.E. Evaluation of the Effects of Tableting Speed on the Relationships between Compaction Pressure, Tablet Tensile Strength, and Tablet Solid Fraction. J. Pharm. Sci. 2005, 94, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Benabbas, R.; Sanchez-Ballester, N.M.; Bataille, B.; Leclercq, L.; Sharkawi, T.; Soulairol, I. Structure-Properties Relationship in the Evaluation of Alginic Acid Functionality for Tableting. AAPS PharmSciTech 2020, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline Cellulose, a Direct Compression Binder in a Quality by Design Environment—A Review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef]
- Chaheen, M.; Sanchez-Ballester, N.M.; Bataille, B.; Yassine, A.; Belamie, E.; Sharkawi, T. Development of Coprocessed Chitin-Calcium Carbonate as Multifunctional Tablet Excipient for Direct Compression. J. Pharm. Sci. 2018, 107, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.; Clarke, F.C. A Modern Approach to the Heckel Equation: The Effect of Compaction Pressure on the Yield Pressure of Ibuprofen and Its Sodium Salt. J. Nanomed. Nanotechnol. 2016, 7, 381. [Google Scholar] [CrossRef]
- Osei-Yeboah, F.; Feng, Y.; Sun, C.C. Evolution of Structure and Properties of Granules Containing Microcrystalline Cellulose and Polyvinylpyrrolidone During High-Shear Wet Granulation. J. Pharm. Sci. 2014, 103, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Smallenbroek, A.; Bolhuis, G.; Lerk, C. The Effect of Particle Size of Disintegrants on the Disintegration of Tablets. Pharm. Weekbl. 1981, 3, 1048–1051. [Google Scholar] [CrossRef]
- Roberts, R.J.; Rowe, R.C. The Effect of Punch Velocity on the Compaction of a Variety of Materials. J. Pharm. Pharmacol. 1985, 37, 377–384. [Google Scholar] [CrossRef]
- Akande, O.F.; Ford, J.L.; Rowe, P.H.; Rubinstein, M.H. Pharmaceutics: The Effects of Lag-Time and Dwell-Time on the Compaction Properties of 1:1 Paracetamol/Microcrystalline Cellulose Tablets Prepared by Pre-Compression and Main Compression. J. Pharm. Pharmacol. 1998, 50, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, P.J.; Parrott, E.L. Effect of Lubricants on Tensile Strengths of Tablets. Drug Dev. Ind. Pharm. 1984, 10, 259–273. [Google Scholar] [CrossRef]
- Takeuchi, H.; Nagira, S.; Aikawa, M.; Yamamoto, H.; Kawashima, Y. Effect of Lubrication on the Compaction Properties of Pharmaceutical Excipients as Measured by Die Wall Pressure. J. Drug Deliv. Sci. Technol. 2005, 15, 177–182. [Google Scholar] [CrossRef]
- Yamamura, T.; Ohta, T.; Taira, T.; Ogawa, Y.; Sakai, Y.; Moribe, K.; Yamamoto, K. Effects of Automated External Lubrication on Tablet Properties and the Stability of Eprazinone Hydrochloride. Int. J. Pharm. 2009, 370, 1–7. [Google Scholar] [CrossRef]
- Kamiya, T.; Kondo, H.; Hiroma, H.; Yamashita, K.; Hakomori, T.; Sako, K.; Iwao, Y.; Noguchi, S.; Itai, S. Impact of Process Parameters on Mg–St Content and Tablet Surface Wettability in the External Lubrication Method for a Rotary Tablet Press. Adv. Powder Technol. 2016, 27, 193–198. [Google Scholar] [CrossRef]
- Priyanka, S.; Vandana, S. A Review Article on: Superdisintegrants. Int. J. Drug Dev. Res. 2013, 3, 76–87. [Google Scholar]
- Quodbach, J.; Kleinebudde, P. A Critical Review on Tablet Disintegration. Pharm. Dev. Technol. 2015, 21, 1–12. [Google Scholar] [CrossRef]
- Rojas, J.; Aristizabal, J.; Henao, M. Screening of Several Excipients for Direct Compression of Tablets: A New Perspective Based on Functional Properties. J. Appl. Pharm. Sci. 2013, 34, 17–23. [Google Scholar]
- Moriton, R.C. Disintegrants in tableting. In Pharmaceutical Dosage Forms: Tablets; Informa Healthcare: New York, NY, USA, 2008; Volume 2, pp. 217–249. [Google Scholar]
- Soulairol, I.; Sanchez-Ballester, N.M.; Aubert, A.; Tarlier, N.; Bataille, B.; Quignard, F.; Sharkawi, T. Evaluation of the Super Disintegrant Functionnalities of Alginic Acid and Calcium Alginate for the Design of Orodispersible Mini Tablets. Carb. Polym. 2018, 197, 576–585. [Google Scholar] [CrossRef] [PubMed]







| Excipients | Cop AA-MCC | Cellactose | Ludipress | DM | DM2 | Prosolv HD90 | Prosolv ODT |
|---|---|---|---|---|---|---|---|
| TRD * (g/mL) | 1.49 | 1.47 | 1.46 | 1.51 | 1.45 | 1.53 | 1.51 |
| BD * (g/mL) | 0.36 | 0.41 | 0.55 | 0.33 | 0.35 | 0.47 | 0.60 |
| TPD * (g/mL) | 0.43 | 0.49 | 0.64 | 0.44 | 0.46 | 0.56 | 0.74 |
| Cop AA-MCC | Cellactose | Ludipress | DM | DM2 | Prosolv HD90 | Prosolv ODT | |
|---|---|---|---|---|---|---|---|
| PS (µm) | 209 ± 1 | 197 ± 4 | 220 ± 13 | 86 ± 7 | 280 ± 5 | 151 ± 1 | 160 ± 5 |
| AOR (°) * | 37.9 | 38.2 | 30.1 | 47.1 | 42.4 | 35.8 | 37.9 |
| CI * | 15.5 | 18.0 | 14.3 | 24.9 | 22.8 | 15.9 | 19.7 |
| HR ** | 1.18 | 1.22 | 1.17 | 1.33 | 1.29 | 1.19 | 1.24 |
| Flow property | Good/fair | Fair | Good | Poor | Passable | Good/fair | Fair |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benabbas, R.; Sanchez-Ballester, N.M.; Aubert, A.; Sharkawi, T.; Bataille, B.; Soulairol, I. Performance Evaluation of a Novel Biosourced Co-Processed Excipient in Direct Compression and Drug Release. Polymers 2021, 13, 988. https://doi.org/10.3390/polym13060988
Benabbas R, Sanchez-Ballester NM, Aubert A, Sharkawi T, Bataille B, Soulairol I. Performance Evaluation of a Novel Biosourced Co-Processed Excipient in Direct Compression and Drug Release. Polymers. 2021; 13(6):988. https://doi.org/10.3390/polym13060988
Chicago/Turabian StyleBenabbas, Rihab, Noelia M. Sanchez-Ballester, Adrien Aubert, Tahmer Sharkawi, Bernard Bataille, and Ian Soulairol. 2021. "Performance Evaluation of a Novel Biosourced Co-Processed Excipient in Direct Compression and Drug Release" Polymers 13, no. 6: 988. https://doi.org/10.3390/polym13060988
APA StyleBenabbas, R., Sanchez-Ballester, N. M., Aubert, A., Sharkawi, T., Bataille, B., & Soulairol, I. (2021). Performance Evaluation of a Novel Biosourced Co-Processed Excipient in Direct Compression and Drug Release. Polymers, 13(6), 988. https://doi.org/10.3390/polym13060988







