Mesoscale Simulations of Polymer Solution Self-Assembly: Selection of Model Parameters within an Implicit Solvent Approximation
Abstract
1. Introduction
2. Model and Methods
2.1. Coarse-Grained Model with a Generalized Hamiltonian
2.2. Simulation Methods
2.3. MC-G and MC-GL Simulations
2.4. MD-GL Simulations
3. Results
3.1. Single-Chain Behavior in a Dilute Solution: Scaling Analysis
3.2. Collective Properties: Surface Tension and Chain Density
3.3. Phase Diagrams of Block Copolymer (BCP) Solutions
3.4. Evolution of Emulsified BCP Droplets
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blanazs, A.; Armes, S.P.; Ryan, A.J. Self-Assembled Block Copolymer Aggregates: From Micelles to Vesicles and their Biological Applications. Macromol. Rapid Commun. 2009, 30, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Du, J. Ultrasound and pH Dually Responsive Polymer Vesicles for Anticancer Drug Delivery. Sci. Rep. 2013, 3, srep02162. [Google Scholar] [CrossRef]
- Elsabahy, M.; Heo, G.S.; Lim, S.-M.; Sun, G.; Wooley, K.L. Polymeric Nanostructures for Imaging and Therapy. Chem. Rev. 2015, 115, 10967–11011. [Google Scholar] [CrossRef]
- Liu, C.-C.; Franke, E.; Mignot, Y.; Xie, R.; Yeung, C.W.; Zhang, J.; Chi, C.; Zhang, C.; Farrell, R.; Lai, K.; et al. Directed self-assembly of block copolymers for 7 nanometre FinFET technology and beyond. Nat. Electron. 2018, 1, 562–569. [Google Scholar] [CrossRef]
- Schacher, F.H.; Rupar, P.A.; Manners, I. Functional Block Copolymers: Nanostructured Materials with Emerging Applications. Angew. Chem. Int. Ed. 2012, 51, 7898–7921. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Walish, J.J.; Gorishnyy, T.; Thomas, E.L. Broad-wavelength-range chemically tunable block-copolymer photonic gels. Nat. Mater. 2007, 6, 957–960. [Google Scholar] [CrossRef]
- Tritschler, U.; Pearce, S.; Gwyther, J.; Whittell, G.R.; Manners, I. 50th Anniversary Perspective: Functional Nanoparticles from the Solution Self-Assembly of Block Copolymers. Macromolecules 2017, 50, 3439–3463. [Google Scholar] [CrossRef]
- Müller, M. Studying Amphiphilic Self-assembly with Soft Coarse-Grained Models. J. Stat. Phys. 2011, 145, 967–1016. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, J.; Wang, L.; Xu, Z. Theoretical modeling and simulations of self-assembly of copolymers in solution. Prog. Polym. Sci. 2017, 75, 1–30. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block Copolymers—Designer Soft Materials. Phys. Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Fredrickson, G.H.; Ganesan, V.; Drolet, F. Field-Theoretic Computer Simulation Methods for Polymers and Complex Fluids. Macromolecules 2002, 35, 16–39. [Google Scholar] [CrossRef]
- Müller, M.; de Pablo, J.J. Computational approaches for the dynamics of structure formation in self-assembling polymeric materials. Annu. Rev. Mater. Res. 2013, 43, 1–34. [Google Scholar] [CrossRef]
- Müller-Plathe, F. Coarse-graining in polymer simulation: From the atomistic to the mesoscopic scale and back. ChemPhysChem 2002, 3, 754–769. [Google Scholar] [CrossRef]
- Gartner III, T.E.; Jayaraman, A. Modeling and simulations of polymers: A Roadmap. Macromolecules 2019, 52, 755–786. [Google Scholar] [CrossRef]
- Wessels, M.G.; Jayaraman, A. Molecular dynamics simulation study of linear, bottlebrush, and star-like amphiphilic block polymer assembly in solution. Soft Matter 2019, 15, 3987–3998. [Google Scholar] [CrossRef]
- Pike, D.Q.; Detcheverry, F.A.; Müller, M.; De Pablo, J.J. Theoretically informed coarse grain simulations of polymeric systems. J. Chem. Phys. 2009, 131, 084903. [Google Scholar] [CrossRef]
- Naughton, J.R.; Matsen, M.W. Limitations of the Dilution Approximation for Concentrated Block Copolymer/Solvent Mixtures. Macromolecules 2002, 35, 5688–5696. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, J.; Lin, S. Self-Assembly Behavior of Amphiphilic Block Copolymer/Nanoparticle Mixture in Dilute Solution Studied by Self-Consistent-Field Theory/Density Functional Theory. Macromolecules 2007, 40, 5582–5592. [Google Scholar] [CrossRef]
- Menge, H.; Hotopf, S.; Pönitzsch, S.; Richter, S.; Arndt, K.-F.; Schneider, H.; Heuert, U. Investigation on the swelling behaviour in poly(dimethylsiloxane) rubber networks using nmr and compression measurements. Polymer 1999, 40, 5303–5313. [Google Scholar] [CrossRef]
- Lu, H.; Du, S. A phenomenological thermodynamic model for the chemo-responsive shape memory effect in polymers based on Flory–Huggins solution theory. Polym. Chem. 2014, 5, 1155–1162. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Leng, J.; Du, S. Qualitative separation of the physical swelling effect on the recovery behavior of shape memory polymer. Eur. Polym. J. 2010, 46, 1908–1914. [Google Scholar] [CrossRef]
- Klinger, D.; Wang, C.X.; Connal, L.A.; Audus, D.J.; Jang, S.G.; Kraemer, S.; Killops, K.L.; Fredrickson, G.H.; Kramer, E.J.; Hawker, C.J. A Facile Synthesis of Dynamic, Shape-Changing Polymer Particles. Angew. Chem. Int. Ed. 2014, 53, 7018–7022. [Google Scholar] [CrossRef]
- Jang, S.G.; Audus, D.J.; Klinger, D.; Krogstad, D.V.; Kim, B.J.; Cameron, A.; Kim, S.-W.; Delaney, K.T.; Hur, S.-M.; Killops, K.L.; et al. Striped, Ellipsoidal Particles by Controlled Assembly of Diblock Copolymers. J. Am. Chem. Soc. 2013, 135, 6649–6657. [Google Scholar] [CrossRef]
- Jeon, S.-J.; Yi, G.-R.; Yang, S.-M. Cooperative Assembly of Block Copolymers with Deformable Interfaces: Toward Nanostructured Particles. Adv. Mater. 2008, 20, 4103–4108. [Google Scholar] [CrossRef]
- Shin, J.J.; Kim, E.J.; Ku, K.H.; Lee, Y.J.; Hawker, C.J.; Kim, B.J. 100th Anniversary of Macromolecular Science Viewpoint: Block Copolymer Particles: Tuning Shape, Interfaces, and Morphology. ACS Macro Lett. 2020, 9, 306–317. [Google Scholar] [CrossRef]
- Yan, N.; Zhu, Y.; Jiang, W. Recent progress in the self-assembly of block copolymers confined in emulsion droplets. Chem. Commun. 2018, 54, 13183–13195. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.; Yin, Y.; Jiang, R.; Li, B.; Shi, A.-C. A Simulation Study of Phase Behavior of Double-Hydrophilic Block Copolymers in Aqueous Solutions. Macromolecules 2015, 48, 8897–8906. [Google Scholar] [CrossRef]
- Suo, T.; Yan, D.; Yang, S.; Shi, A.-C. A Theoretical Study of Phase Behaviors for Diblock Copolymers in Selective Solvents. Macromolecules 2009, 42, 6791–6798. [Google Scholar] [CrossRef]
- Wang, J.; Guo, K.; An, L.; Muller, M.; Wang, Z.-G. Micelles of Coil−Comb Block Copolymers in Selective Solvents: Competition of Length Scales. Macromolecules 2010, 43, 2037–2041. [Google Scholar] [CrossRef]
- Rumyantsev, A.M.; Leermakers, F.A.M.; Zhulina, E.B.; Potemkin, I.I.; Borisov, O.V. Temperature-Induced Re-Entrant Morphological Transitions in Block-Copolymer Micelles. Langmuir 2018, 35, 2680–2691. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.-M.; Khaira, G.S.; Ramírez-Hernández, A.; Müller, M.; Nealey, P.F.; De Pablo, J.J. Simulation of Defect Reduction in Block Copolymer Thin Films by Solvent Annealing. ACS Macro Lett. 2015, 4, 11–15. [Google Scholar] [CrossRef]
- Warren, P.B. Hydrodynamic Bubble Coarsening in Off-Critical Vapor-Liquid Phase Separation. Phys. Rev. Lett. 2001, 87, 225702. [Google Scholar] [CrossRef] [PubMed]
- Laradji, M.; Guo, H.; Zuckermann, M.J. Off-lattice Monte Carlo simulation of polymer brushes in good solvents. Phys. Rev. E 1994, 49, 3199–3206. [Google Scholar] [CrossRef]
- Soga, K.G.; Guo, H.; Zuckermann, M.J. Polymer Brushes in a Poor Solvent. Europhys. Lett. 1995, 29, 531–536. [Google Scholar] [CrossRef]
- Soga, K.G.; Zuckermann, A.M.J.; Guo, H. Binary Polymer Brush in a Solvent. Macromolecules 1996, 29, 1998–2005. [Google Scholar] [CrossRef]
- Daoulas, K.C.; Müller, M. Comparison of Simulations of Lipid Membranes with Membranes of Block Copolymers. In Polymer Membranes/Biomembranes; Meier, W.P., Knoll, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Norizoe, Y.; Daoulas, K.C.; Müller, M. Measuring excess free energies of self-assembled membrane structures. Faraday Discuss. 2009, 144, 369–391. [Google Scholar] [CrossRef] [PubMed]
- Hömberg, M.; Müller, M. Main phase transition in lipid bilayers: Phase coexistence and line tension in a soft, solvent-free, coarse-grained model. J. Chem. Phys. 2010, 132, 155104. [Google Scholar] [CrossRef]
- Müller, M.; Smith, G.D. Phase separation in binary mixtures containing polymers: A quantitative comparison of single-chain-in-mean-field simulations and computer simulations of the corresponding multichain systems. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 934–958. [Google Scholar] [CrossRef]
- Wang, J.; Muller, M. Microphase Separation of Mixed Polymer Brushes: Dependence of the Morphology on Grafting Density, Composition, Chain-Length Asymmetry, Solvent Quality, and Selectivity. J. Phys. Chem. B 2009, 113, 11384–11402. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Muller, M. Microphase Separation of Diblock Copolymer Brushes in Selective Solvents: Single-Chain-in-Mean-Field Simulations and Integral Geometry Analysis. Macromolecules 2009, 42, 2251–2264. [Google Scholar] [CrossRef]
- Detcheverry, F.A.; Kang, H.; Daoulas, K.C.; Müller, M.; Nealey, P.F.; De Pablo, J.J. Monte Carlo Simulations of a Coarse Grain Model for Block Copolymers and Nanocomposites. Macromolecules 2008, 41, 4989–5001. [Google Scholar] [CrossRef]
- Detcheverry, F.A.; Pike, D.Q.; Nealey, P.F.; Müller, M.; De Pablo, J.J. Monte Carlo Simulation of Coarse Grain Polymeric Systems. Phys. Rev. Lett. 2009, 102, 197801. [Google Scholar] [CrossRef]
- Weeks, J.D.; Katsov, K.; Vollmayr, K. Roles of Repulsive and Attractive Forces in Determining the Structure of Nonuniform Liquids: Generalized Mean Field Theory. Phys. Rev. Lett. 1998, 81, 4400–4403. [Google Scholar] [CrossRef]
- Katsov, K.; Weeks, J.D. On the Mean Field Treatment of Attractive Interactions in Nonuniform Simple Fluids. J. Phys. Chem. B 2001, 105, 6738–6744. [Google Scholar] [CrossRef]
- Detcheverry, F.A.; Pike, D.Q.; Nealey, P.F.; Müller, M.; De Pablo, J.J. Simulations of theoretically informed coarse grain models of polymeric systems. Faraday Discuss. 2009, 144, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Detcheverry, F.A.; Pike, D.Q.; Nagpal, U.; Nealey, P.F.; De Pablo, J.J. Theoretically informed coarse grain simulations of block copolymer melts: Method and applications. Soft Matter 2009, 5, 4858–4865. [Google Scholar] [CrossRef]
- Groot, R.D.; Warren, P.B. Dissipative particle dynamics: Bridging the gap between atomistic and mesoscopic simulation. J. Chem. Phys. 1997, 107, 4423–4435. [Google Scholar] [CrossRef]
- Espanol, P.; Warren, P.B. Statistical Mechanics of Dissipative Particle Dynamics. Europhys. Lett. 1995, 30, 191–196. [Google Scholar] [CrossRef]
- Anderson, J.A.; Glaser, J.; Glotzer, S.C. HOOMD-blue: A Python package for high-performance molecular dynamics and hard particle Monte Carlo simulations. Comput. Mater. Sci. 2020, 173, 109363. [Google Scholar] [CrossRef]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: New York, NY, USA, 2003; Volume 23. [Google Scholar]
- Wang, R.; Wang, Z.-G. Theory of Polymer Chains in Poor Solvent: Single-Chain Structure, Solution Thermodynamics, and Θ Point. Macromolecules 2014, 47, 4094–4102. [Google Scholar] [CrossRef]
- Zhou, Z.; Daivis, P.J. Molecular dynamics study of polymer conformation as a function of concentration and solvent quality. J. Chem. Phys. 2009, 130, 224904. [Google Scholar] [CrossRef]
- Steinhauser, M.O. A molecular dynamics study on universal properties of polymer chains in different solvent qualities. Part I. A review of linear chain properties. J. Chem. Phys. 2005, 122, 094901. [Google Scholar] [CrossRef] [PubMed]
- Kholodenko, A.; Freed, K.F. Theta point (“tricritical”) region behavior for a polymer chain: Transition to collapse. J. Chem. Phys. 1984, 80, 900–924. [Google Scholar] [CrossRef]
- Jacobberger, R.M.; Thapar, V.; Wu, G.-P.; Chang, T.-H.; Saraswat, V.; Way, A.J.; Jinkins, K.R.; Ma, Z.; Nealey, P.F.; Hur, S.-M.; et al. Boundary-directed epitaxy of block copolymers. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Evangelio, L.; Fernández-Regúlez, M.; Fraxedas, J.; Mueller, M.; Pérez-Murano, F. Role of Penetrability into a Brush-Coated Surface in Directed Self-Assembly of Block Copolymers. ACS Appl. Mater. Interfaces 2019, 11, 3571–3581. [Google Scholar] [CrossRef]
- Silmore, K.S.; Howard, M.P.; Panagiotopoulos, A.Z. Vapour—Liquid phase equilibrium and surface tension of fully flexible Lennard–Jones chains. Mol. Phys. 2017, 115, 320–327. [Google Scholar] [CrossRef]
- Hur, S.-M.; García-Cervera, C.J.; Fredrickson, G.H. Chebyshev Collocation in Polymer Field Theory: Application to Wetting Phenomena. Macromolecules 2012, 45, 2905–2919. [Google Scholar] [CrossRef]
- Lodge, T.P.; Pudil, B.; Hanley, K.J. The Full Phase Behavior for Block Copolymers in Solvents of Varying Selectivity. Macromolecules 2002, 35, 4707–4717. [Google Scholar] [CrossRef]
- Hanley, K.J.; Lodge, T.P.; Huang, C.-I. Phase Behavior of a Block Copolymer in Solvents of Varying Selectivity. Macromolecules 2000, 33, 5918–5931. [Google Scholar] [CrossRef][Green Version]
- Hanley, K.J.; Lodge, T.P. Effect of dilution on a block copolymer in the complex phase window. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 3101–3113. [Google Scholar] [CrossRef]
- McConnell, G.A.; Gast, A.P. Melting of Ordered Arrays and Shape Transitions in Highly Concentrated Diblock Copolymer Solutions. Macromolecules 1997, 30, 435–444. [Google Scholar] [CrossRef]
- Lai, C.; Russel, W.B.; Register, R.A. Phase Behavior of Styrene—Isoprene Diblock Copolymers in Strongly Selective Solvents. Macromolecules 2002, 35, 841–849. [Google Scholar] [CrossRef]
- Shin, J.M.; Kim, Y.; Yun, H.; Yi, G.-R.; Kim, B.J. Morphological Evolution of Block Copolymer Particles: Effect of Solvent Evaporation Rate on Particle Shape and Morphology. ACS Nano 2017, 11, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Song, D.-P.; Zhao, T.H.; Guidetti, G.; Vignolini, S.; Parker, R.M. Hierarchical Photonic Pigments via the Confined Self-Assembly of Bottlebrush Block Copolymers. ACS Nano 2019, 13, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, J.; Yang, Y.; Wang, K.; Xu, N.; Li, J.; Liang, R.; Shen, L.; Xie, X.; Tao, J.; et al. Block Copolymer Capsules with Structure-Dependent Release Behavior. Angew. Chem. Int. Ed. 2016, 55, 14633–14637. [Google Scholar] [CrossRef]
- Yan, N.; Zhu, Y.; Jiang, W. Self-Assembly of AB Diblock Copolymer Confined in a Soft Nano-Droplet: A Combination Study by Monte Carlo Simulation and Experiment. J. Phys. Chem. B 2016, 120, 12023–12029. [Google Scholar] [CrossRef]
- Chi, P.; Wang, Z.; Li, B.; Shi, A.-C. Soft Confinement-Induced Morphologies of Diblock Copolymers. Langmuir 2011, 27, 11683–11689. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Liu, H.; Zhu, Y.; Jiang, W.; Dong, Z. Entropy-Driven Hierarchical Nanostructures from Cooperative Self-Assembly of Gold Nanoparticles/Block Copolymers under Three-Dimensional Confinement. Macromolecules 2015, 48, 5980–5987. [Google Scholar] [CrossRef]

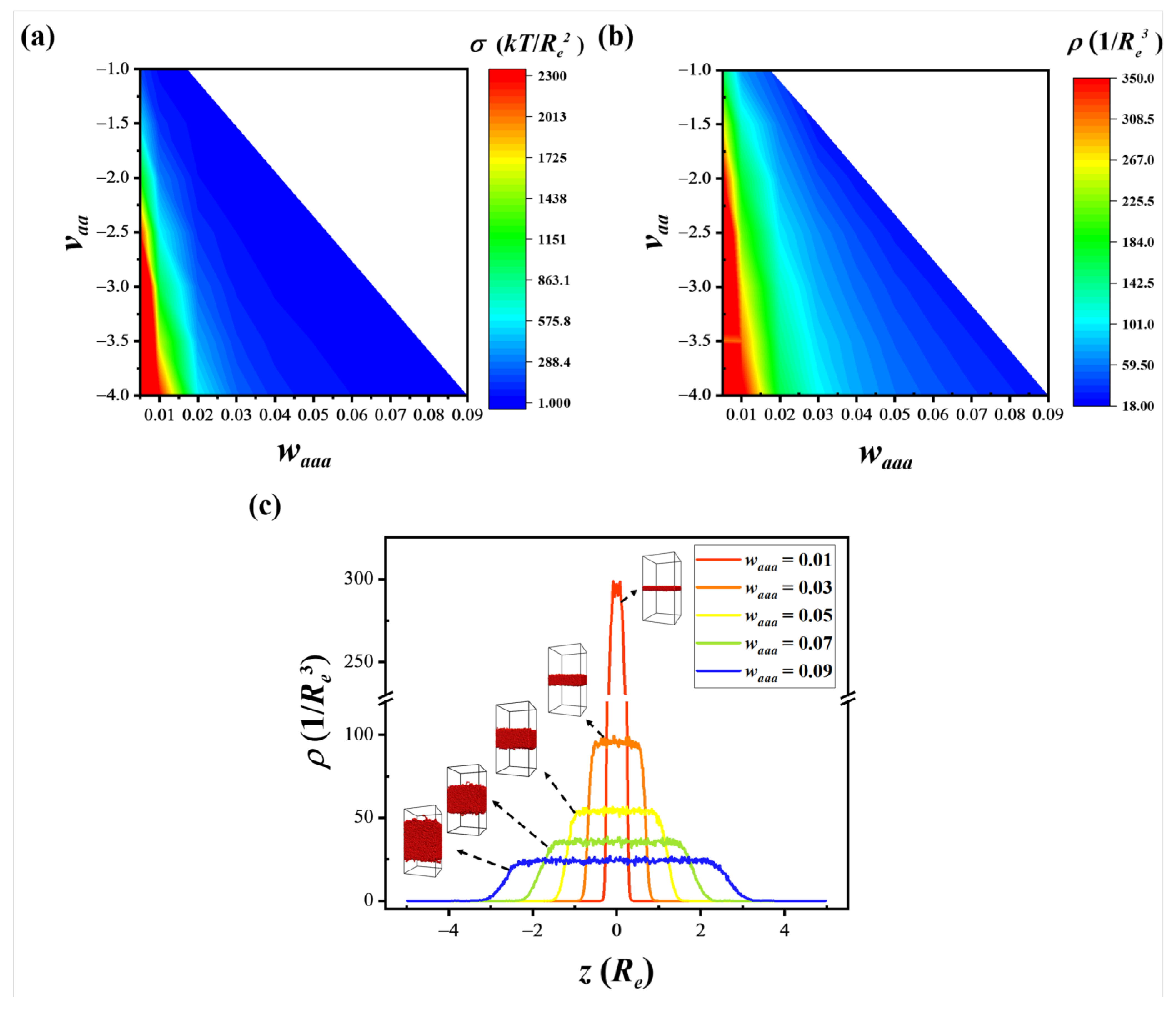
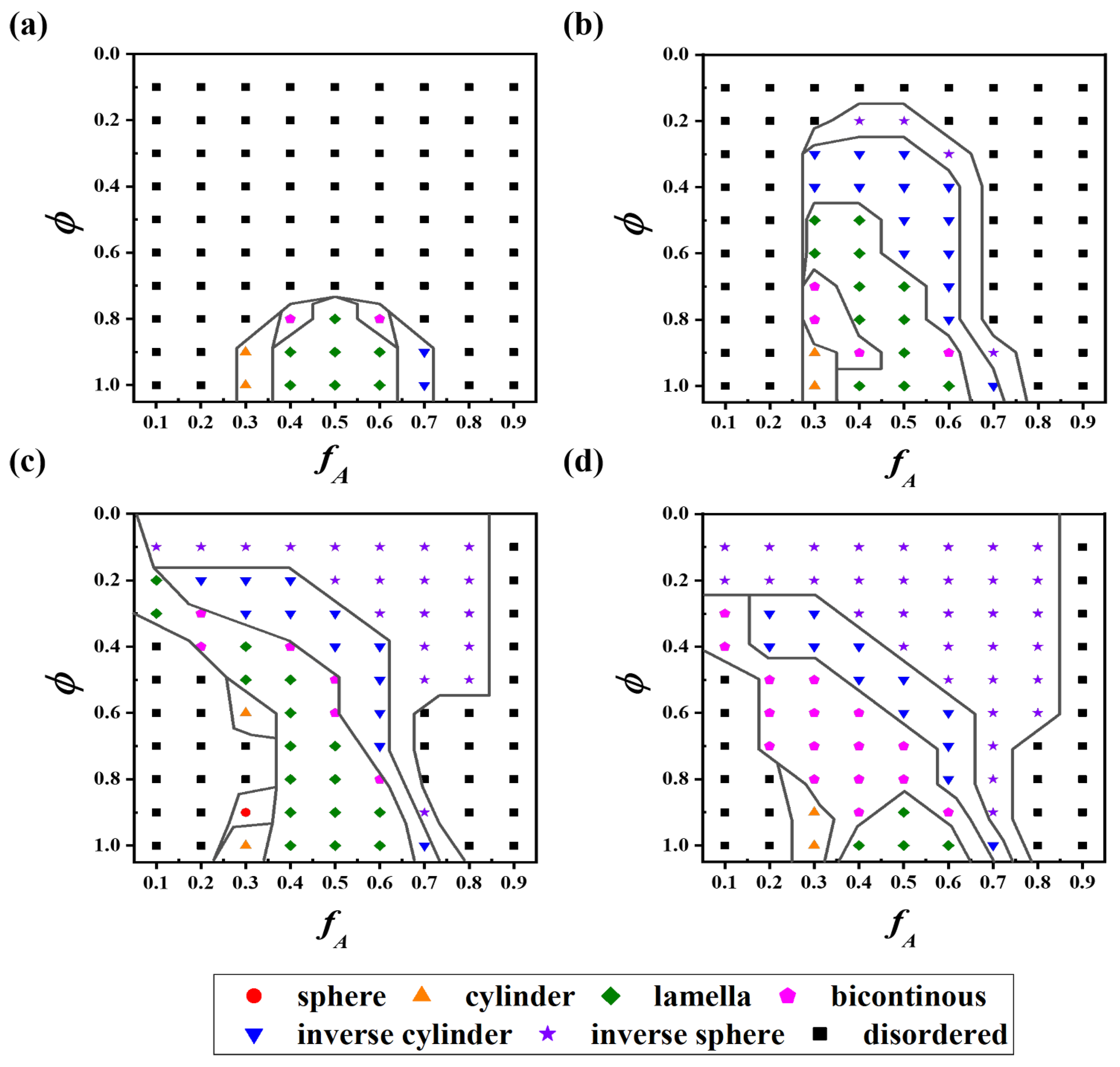

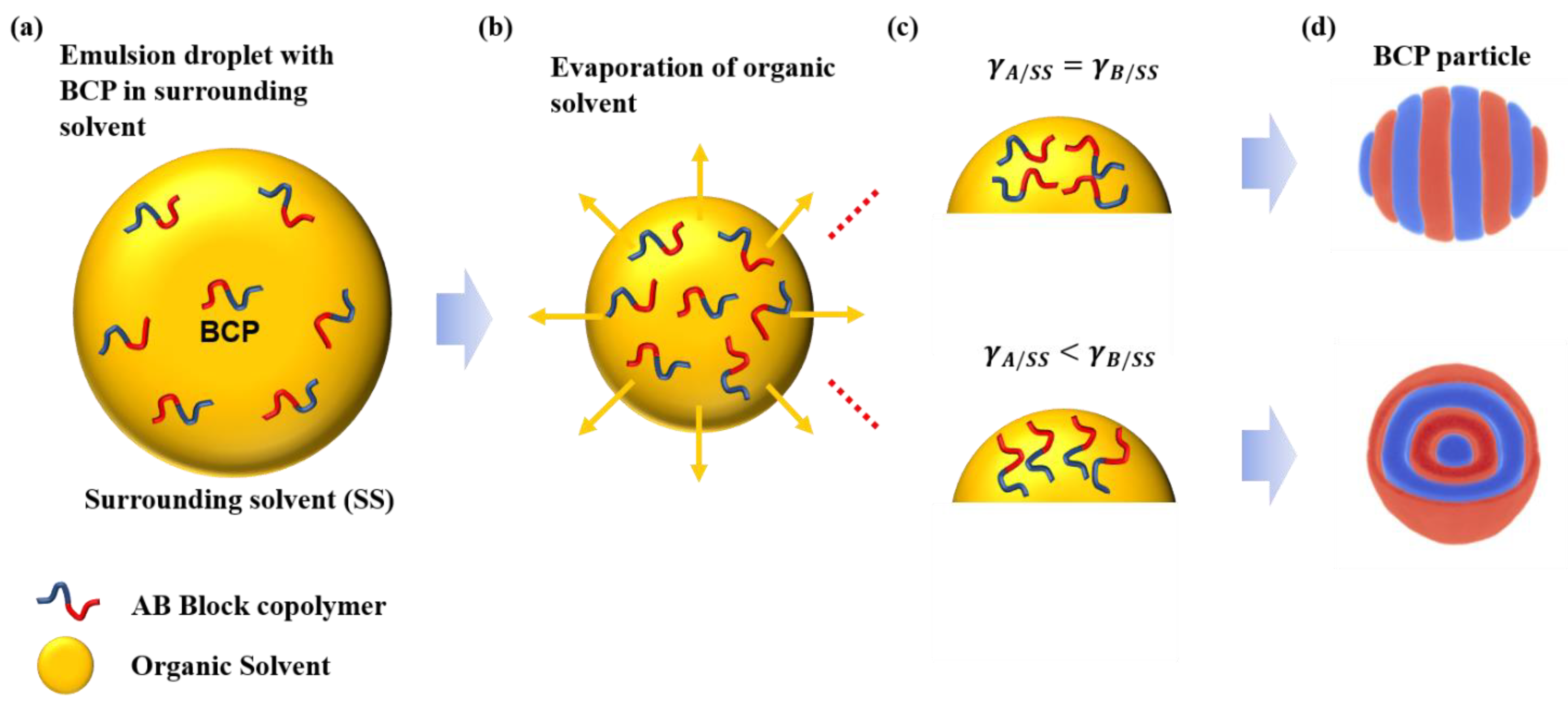

| vaa | waaa | vbb | wbbb | |
|---|---|---|---|---|
| Figure 3a–dilute | 2.0 | 0.0 | 2.0 | 0.0 |
| Figure 3b–dilute | 2.0 | 0.0 | −0.1656 | 0.00564 |
| Figure 3c–dilute | 2.0 | 0.0 | −4.0 | 0.04 |
| Figure 3d–dilute | 2.0 | 0.0 | −1.6094 | 0.00934 |
| Melt | −1.6094 | 0.00934 | −1.6094 | 0.00934 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Ramírez-Hernández, A.; Thapar, V.; Hur, S.-M. Mesoscale Simulations of Polymer Solution Self-Assembly: Selection of Model Parameters within an Implicit Solvent Approximation. Polymers 2021, 13, 953. https://doi.org/10.3390/polym13060953
Park J, Ramírez-Hernández A, Thapar V, Hur S-M. Mesoscale Simulations of Polymer Solution Self-Assembly: Selection of Model Parameters within an Implicit Solvent Approximation. Polymers. 2021; 13(6):953. https://doi.org/10.3390/polym13060953
Chicago/Turabian StylePark, Juhae, Abelardo Ramírez-Hernández, Vikram Thapar, and Su-Mi Hur. 2021. "Mesoscale Simulations of Polymer Solution Self-Assembly: Selection of Model Parameters within an Implicit Solvent Approximation" Polymers 13, no. 6: 953. https://doi.org/10.3390/polym13060953
APA StylePark, J., Ramírez-Hernández, A., Thapar, V., & Hur, S.-M. (2021). Mesoscale Simulations of Polymer Solution Self-Assembly: Selection of Model Parameters within an Implicit Solvent Approximation. Polymers, 13(6), 953. https://doi.org/10.3390/polym13060953







