The Effects of Crosslinking on the Rheology and Cellular Behavior of Polymer-Based 3D-Multilayered Scaffolds for Restoring Articular Cartilage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Ca-P Materials
2.1.2. Polymeric Materials
2.2. Polymeric Solutions
2.3. Scaffold Fabrication
2.3.1. Bone Layer Suspension (B-Layer)
2.3.2. Intermediate Layer Suspension (M-Layer)
2.3.3. Cartilage Layer Suspension (T-Layer)
2.3.4. Final Step
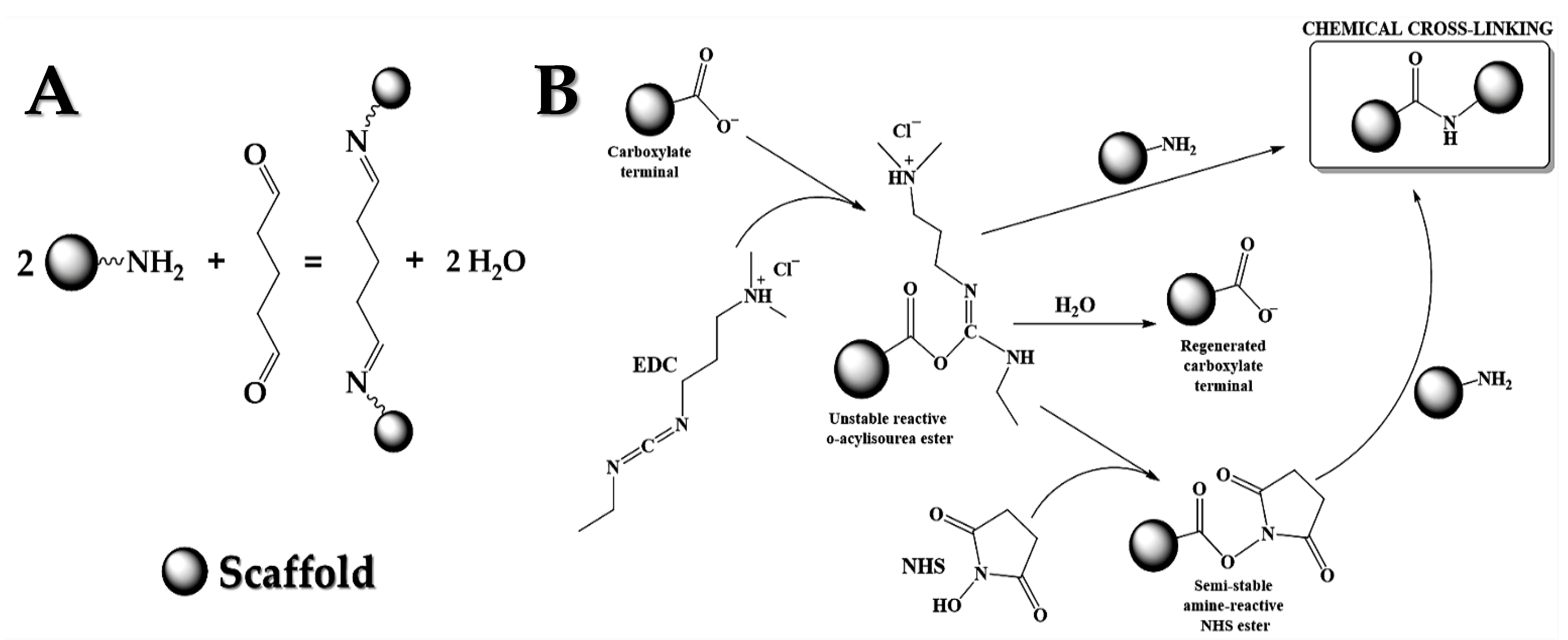
2.4. Crosslinking Process
2.4.1. Crosslinking with Glutaraldehyde
2.4.2. Crosslinking with EDC/NHS
- The NH2-terminal provided for natural polymers in the multilayer scaffold resulting, a stable amide bond;
- The NHS, which is a result of a semi-stable amine NHS ester that forms with the terminal NH2 provided by natural polymers a stable amide bond;
2.5. Morphological, Chemical, Physical, and Mechanical Characterization
2.5.1. Microstructural Morphology
2.5.2. Swelling Studies
2.5.3. Mechanical Analysis
2.6. Cell Studies
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physical-Chemical Characterization
3.1.1. Morphological Characterization
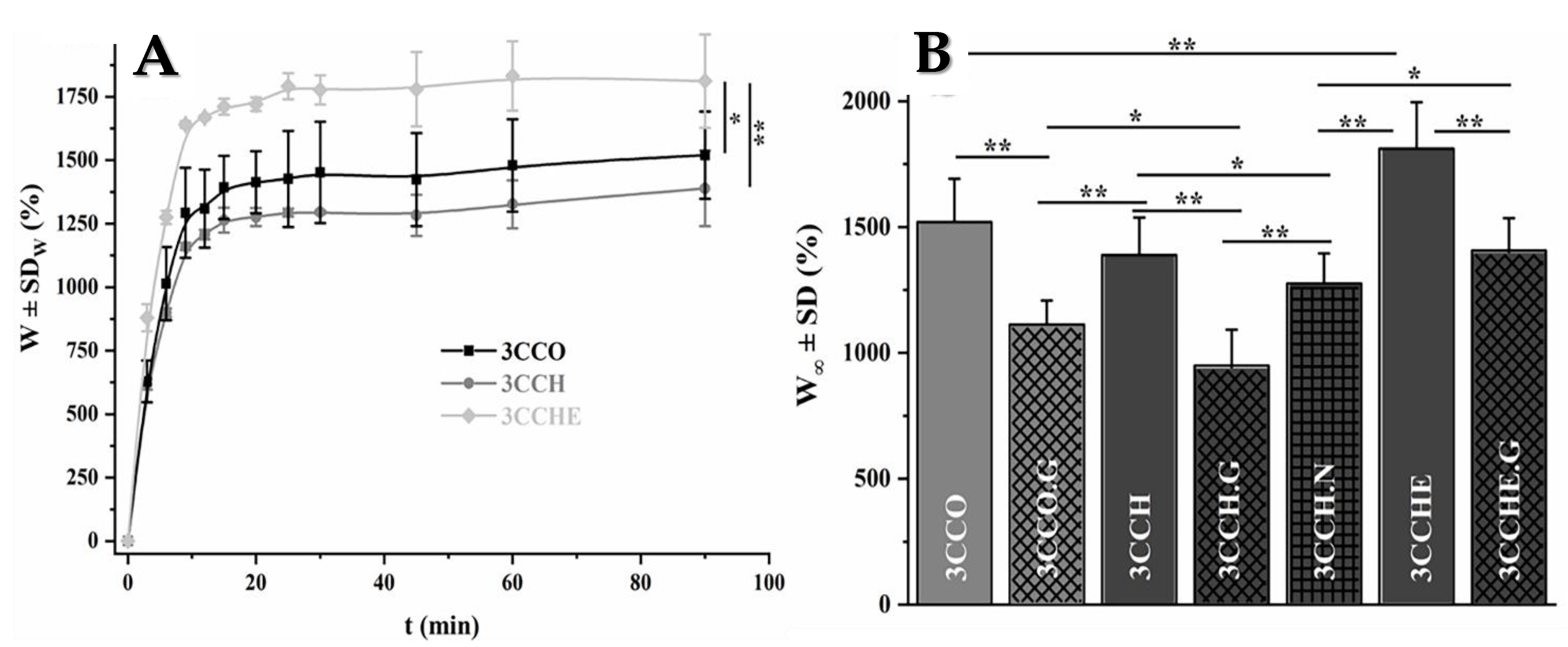
3.1.2. Swelling Studies
3.2. Rheological Properties
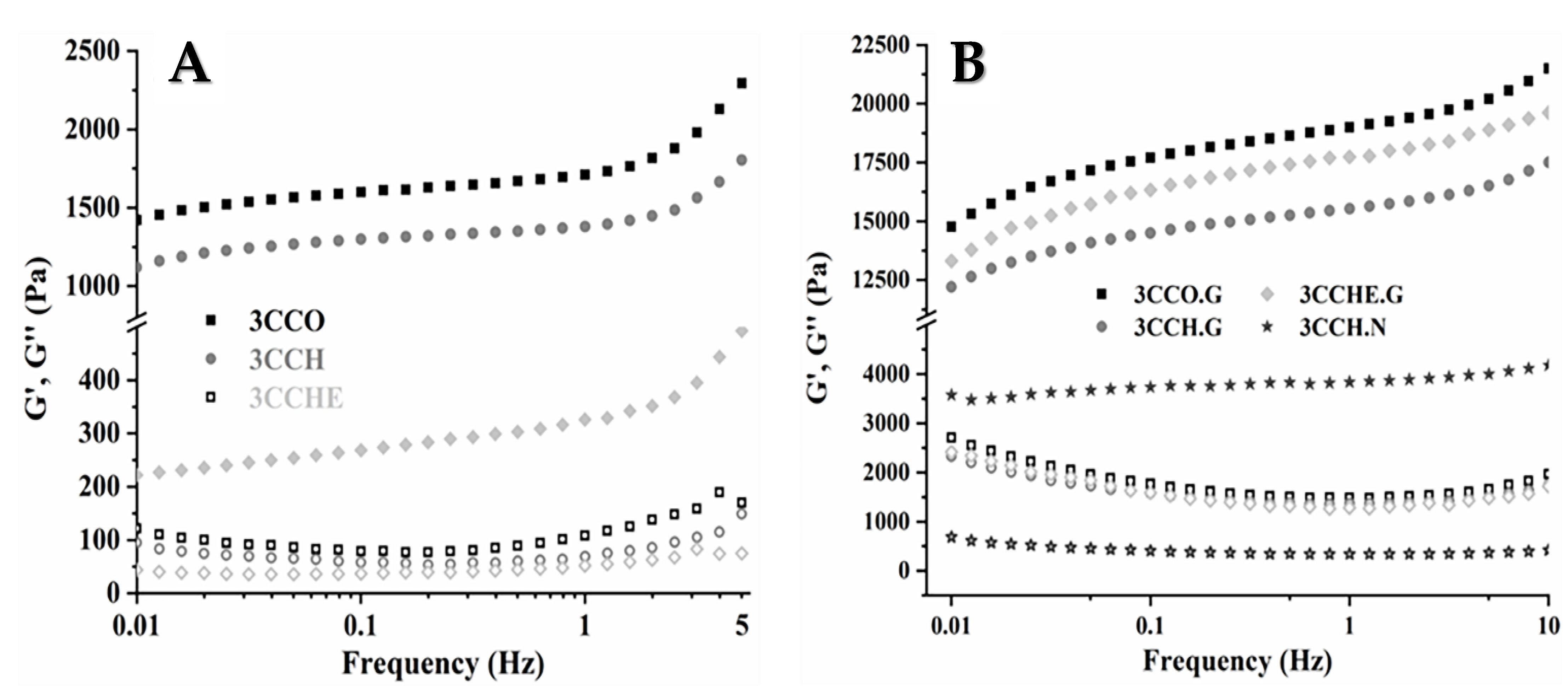
3.2.1. The Frequency Responses of Moduli
| Magnitude | 3CCO | 3CCH | 3CCHE | 3CCO.G | 3CCH.G | 3CCH.N | 3CCHE.G | Approx. Reference Values |
|---|---|---|---|---|---|---|---|---|
| G’ (kPa) | 1.71 ± 0.02 A | 1.4 ± 0.3 A | 0.33 ± 0.05 B | 19 ± 7 C | 15 ± 3 C | 3.86 ± 0.07 D | 18 ± 8 C | 1.8–7.5 (AD) [18] 1–10 (AD) [24] 0.01–3.5 (ABD) [56] 0.006–1.000 (B) [36] 0.3 (B) [66] 0.5–2.7 (AB) [79] 0.1-1 (B) [82] |
| G″ (kPa) | 0.11 ± 0.01 A | 0.07 ± 0.01 B | 0.05 ± 0.01 C | 1.5 ± 0.2 D | 1.4 ± 0.4 D | 0.3 ± 0.1 E | 1.3 ± 0.6 D | 0.02–0.75 (ABE) [18] 0.02–0.40 (ABE) [24] 0.001–0.030 [36] 0.25 [66] 0.01–0.04 [82] |
| tan | 0.064 ± 0.008 A | 0.05 ± 0.02 A | 0.16 ± 0.06 B | 0.08 ± 0.04 C | 0.09 ± 0.04 C | 0.08 ± 0.02 C | 0.07 ± 0.07 C | 0.19–0.22 [4] 0.07–0.11 (AC) [18] 0.061–0.087 (AC) [19]♦ 0.01–0.06 (A) [24] 0.096–0.19 (BC) [80]♦ 0.033-0.045 [83] |
| δ (°) | 3.7 ± 0.5 A | 3 ± 1 A | 9 ± 3 B | 5 ± 2 AC | 5 ± 2 AC | 4.6 ± 0.9A C | 4 ± 4 AC | 10.7–12.4 (B) [4]♦ 4.0–6.3 (C) [18]♦ 3.5–5.0 (A) [19] 0.5–3.5 (C) [24]♦ 5.5–11 (BC) [80] 1.8–2.6 (C) [83]♦ |
| G* (kPa) | 1.7 A | 1.4 A | 0.33 B | 19 C | 16 C | 3.9 D | 18 C | 200–250 [4] 1.8–9.5 (AD) [18] 2.0–5.5 (D) [19] 1.25–2.00 (A) [80] |
| (s) | 8 ± 4 A | 7 ± 1 A | 1.0 ± 0.5 C | 8.4 ± 0.2 A | 12.3 ± 0.4 B | 8 ± 3 A | 23 ± 1 D | 8–10 (AB) [18] |
| (s) | 50 ± 10 A | 150 ± 30 B | 18 ± 5 C | 77 ± 2 D | 85 ± 2 E | 40 ± 10 A | 180 ± 30 F | 100–110 [18] |
| (s) | 530 ± 60 A | 1400 ± 400 B | 270 ± 20 B | 860 ± 30 C | 820 ± 20 C | 460 ± 50 A | 1200 ± 300 D | 1000–1200 (BD) [18] |
3.2.2. Poroelasticity and Intrinsic Viscoelasticity
3.2.3. Time Relaxation Modulus
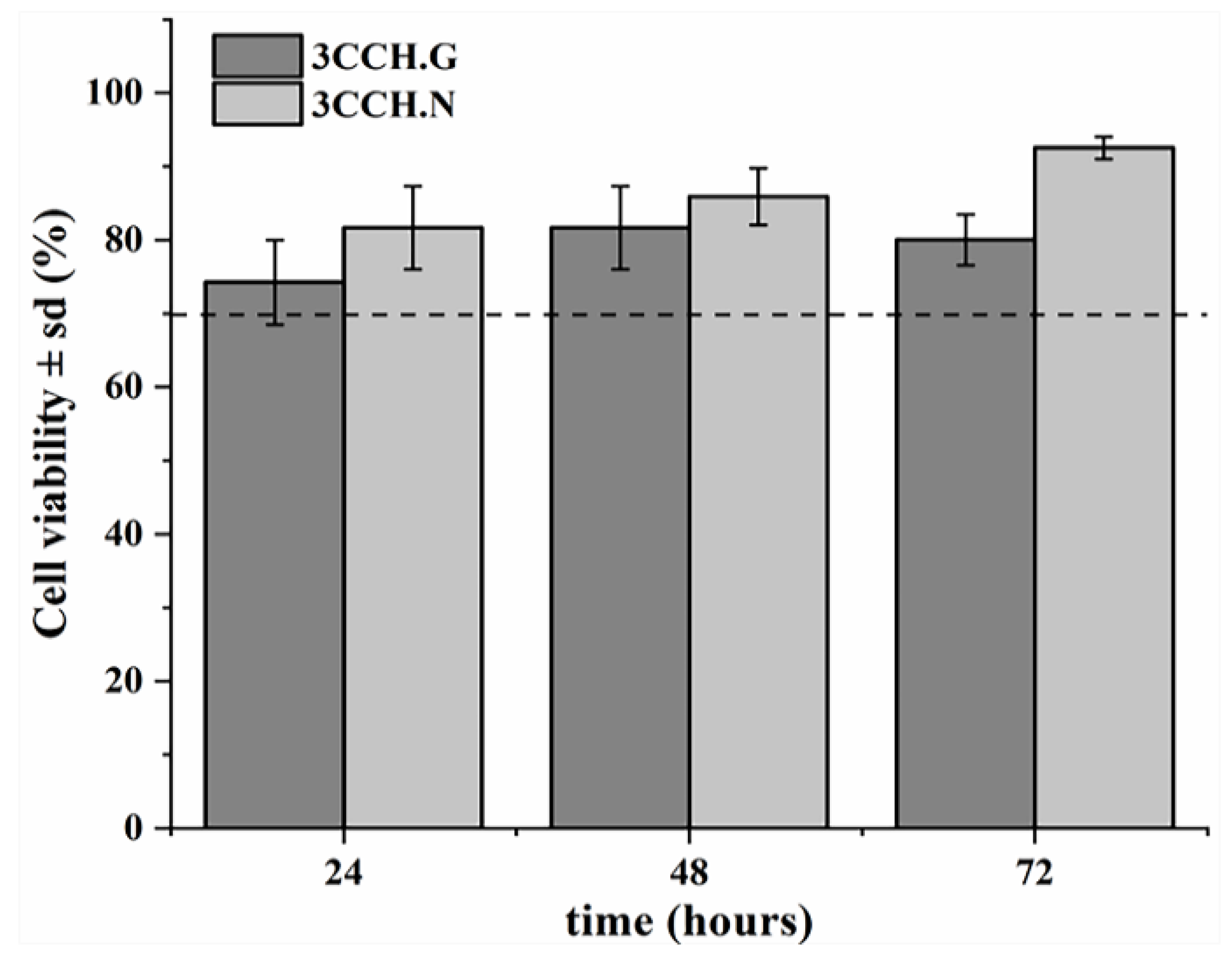
3.3. Cells Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, N.P.; Foster, R.J.; Mow, V.C. Composition and Dynamics of Articular Cartilage: Structure, Function, and Maintaining Healthy State. J. Orthop. Sports Phys. Ther. 1998, 28, 203–215. [Google Scholar] [CrossRef]
- Boettcher, K.; Kienle, S.; Nachtsheim, J.; Burgkart, R.; Hugel, T.; Lieleg, O. The structure and mechanical properties of articular cartilage are highly resilient towards transient dehydration. Acta Biomater. 2016, 29, 180–187. [Google Scholar] [CrossRef]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Bostan, L.; Trunfio-Sfarghiu, A.-M.; Verestiuc, L.; Popa, M.; Munteanu, F.; Rieu, J.-P.; Berthier, Y. Mechanical and tribological properties of poly(hydroxyethyl methacrylate) hydrogels as articular cartilage substitutes. Tribol. Int. 2012, 46, 215–224. [Google Scholar] [CrossRef]
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354, S32–S34. [Google Scholar] [CrossRef]
- Bougault, C.; Cueru, L.; Bariller, J.; Malbouyres, M.; Paumier, A.; Aszodi, A.; Berthier, Y.; Mallein-Gerin, F.; Trunfio-Sfarghiu, A.-M. Alteration of cartilage mechanical properties in absence of β1 integrins revealed by rheometry and FRAP analyses. J. Biomech. 2013, 46, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Heng, B.C.; Lo, E.C.M.; Zhang, C. Current Advance and Future Prospects of Tissue Engineering Approach to Dentin/Pulp Regenerative Therapy. Stem Cells Int. 2016, 2016, 9204574. [Google Scholar] [CrossRef]
- Sequeira, D.B.; Oliveira, A.R.; Seabra, C.M.; Palma, P.J.; Ramos, C.; Figueiredo, M.H.; Santos, A.C.; Cardoso, A.L.; Peça, J.; Santos, J.M. Regeneration of pulp-dentin complex using human stem cells of the apical papilla: In vivo interaction with two bioactive materials. Clin. Oral Investig. 2021, 1–13. [Google Scholar] [CrossRef]
- Xu, J.; Gou, L.; Zhang, P.; Li, H.; Qiu, S. Platelet-rich plasma and regenerative dentistry. Aust. Dent. J. 2020, 65, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Mow, V.C.; Holmes, M.H.; Lai, W.M. Fluid transport and mechanical properties of articular cartilage: A review. J. Biomech. 1984, 17, 377–394. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Kudva, A.K.; Saxena, N.S.; Roy, K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials 2011, 32, 6946–6952. [Google Scholar] [CrossRef]
- Fessler, J.H. A structural function of mucopolysaccharide in connective tissue. Biochem. J. 1960, 76, 124–132. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Hwang, Y.; Chen, A.C.; Varghese, S.; Sah, R.L. Cartilage-like mechanical properties of poly (ethylene glycol)-diacrylate hydrogels. Biomaterials 2012, 33, 6682–6690. [Google Scholar] [CrossRef]
- Suh, J.-K.F.; Matthew, H.W. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef]
- Lee, S.-H.; Shin, H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- De Torre, I.G.; Santos, M.; Quintanilla, L.; Testera, A.; Alonso, M.; Cabello, J.C.R. Elastin-like recombinamer catalyst-free click gels: Characterization of poroelastic and intrinsic viscoelastic properties. Acta Biomater. 2014, 10, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- de Torre, I.G.; Wolf, F.; Santos, M.; Rongen, L.; Alonso, M.; Jockenhoevel, S.; Rodríguez-Cabello, J.C.; Mela, P. Elastin-like recombinamer-covered stents: Towards a fully biocompatible and non-thrombogenic device for cardiovascular diseases. Acta Biomater. 2015, 12, 146–155. [Google Scholar] [CrossRef] [PubMed]
- García-Arévalo, C.; Pierna, M.; Girotti, A.; Arias, F.J.; Rodríguez-Cabello, J.C. A comparative study of cell behavior on different energetic and bioactive polymeric surfaces made from elastin-like recombinamers. Soft Matter 2012, 8, 3239–3249. [Google Scholar] [CrossRef]
- Alvarez-Rodriguez, R.; Alonso, M.; Girotti, A.; Reboto, V.; Rodriguez-Cabello, J.C. One-pot synthesis of pH and temperature sensitive gold clusters mediated by a recombinant elastin-like polymer. Eur. Polym. J. 2010, 46, 643–650. [Google Scholar] [CrossRef]
- Girotti, A.; Reguera, J.; Arias, F.J.; Alonso, M.; Testera, A.M.; Rodríguez-Cabello, J.C. Influence of the Molecular Weight on the Inverse Temperature Transition of a Model Genetically Engineered Elastin-like pH-Responsive Polymer. Macromolecules 2004, 37, 3396–3400. [Google Scholar] [CrossRef]
- Girotti, A.; Reguera, J.; Rodríguez-Cabello, J.C.; Arias, F.J.; Alonso, M.; Testera, A.M. Design and bioproduction of a recombinant multi(bio)functional elastin-like protein polymer containing cell adhesion sequences for tissue engineering purposes. J. Mater. Sci. Mater. Med. 2004, 15, 479–484. [Google Scholar] [CrossRef]
- Testera, A.M.; Girotti, A.; De Torre, I.G.; Quintanilla, L.; Santos, M.; Alonso, M.; Rodríguez-Cabello, J.C. Biocompatible elastin-like click gels: Design, synthesis and characterization. J. Mater. Sci. Mater. Med. 2015, 26, 1–13. [Google Scholar] [CrossRef]
- Rastin, H.; Zhang, B.; Bi, J.; Hassan, K.; Tung, T.T.; Losic, D. 3D printing of cell-laden electroconductive bioinks for tissue engineering applications. J. Mater. Chem. B 2020, 8, 5862–5876. [Google Scholar] [CrossRef]
- Rastin, H.; Zhang, B.; Mazinani, A.; Hassan, K.; Bi, J.; Tung, T.T.; Losic, D. 3D bioprinting of cell-laden electroconductive MXene nanocomposite bioinks. Nanoscale 2020, 12, 16069–16080. [Google Scholar] [CrossRef]
- Soenen, S.J.; Manshian, B.; Montenegro, J.M.; Amin, F.; Meermann, B.; Thiron, T.; Cornelissen, M.; Vanhaecke, F.; Doak, S.; Parak, W.J.; et al. Cytotoxic Effects of Gold Nanoparticles: A Multiparametric Study. ACS Nano 2012, 6, 5767–5783. [Google Scholar] [CrossRef]
- Patil, R.D.; Adimurthy, S. Catalytic Methods for Imine Synthesis. Asian J. Org. Chem. 2013, 2, 726–744. [Google Scholar] [CrossRef]
- Rad, E.R.; Vahabi, H.; Formela, K.; Saeb, M.R.; Thomas, S. Injectable poloxamer/graphene oxide hydrogels with well-controlled mechanical and rheological properties. Polym. Adv. Technol. 2019, 30, 2250–2260. [Google Scholar] [CrossRef]
- Rastin, H.; Ormsby, R.T.; Atkins, G.J.; Losic, D. 3D Bioprinting of Methylcellulose/Gelatin-Methacryloyl (MC/GelMA) Bioink with High Shape Integrity. ACS Appl. Bio Mater. 2020, 3, 1815–1826. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Campbell, J.; Thian, E.; Watson, C.; Cameron, R. Collagen–hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010, 6, 3957–3968. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.; Bax, D.; Raynal, N.; Farndale, R.; Best, S.; Cameron, R. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Fathi, A.; Mithieux, S.M.; Martens, P.; Weiss, A.S.; Dehghani, F. The effect of elastin on chondrocyte adhesion and proliferation on poly (ɛ-caprolactone)/elastin composites. Biomaterials 2011, 32, 1517–1525. [Google Scholar] [CrossRef]
- Cigan, A.D.; Roach, B.L.; Nims, R.J.; Tan, A.R.; Albro, M.B.; Stoker, A.M.; Cook, J.L.; Vunjak-Novakovic, G.; Hung, C.T.; Ateshian, G.A. High seeding density of human chondrocytes in agarose produces tissue-engineered cartilage approaching native mechanical and biochemical properties. J. Biomech. 2016, 49, 1909–1917. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, B.; Feng, Q.; Li, S.; Chen, Y.; Luo, L. In situ-forming chitosan/nano-hydroxyapatite/collagen gel for the delivery of bone marrow mesenchymal stem cells. Carbohydr. Polym. 2011, 85, 261–267. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Chen, X.; Zimmerman, B.K.; Lu, X.L. Determine the equilibrium mechanical properties of articular cartilage from the short-term indentation response. J. Biomech. 2015, 48, 176–180. [Google Scholar] [CrossRef]
- Chung, C.-Y.; Mansour, J.M. Determination of poroelastic properties of cartilage using constrained optimization coupled with finite element analysis. J. Mech. Behav. Biomed. Mater. 2015, 42, 10–18. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Preparation of octacalcium phosphate (OCP): A direct fast method. Calcif. Tissue Int. 1985, 37, 194–197. [Google Scholar] [CrossRef]
- Fowler, B.; Moreno, E.; Brown, W. Infra-red spectra of hydroxyapatite, octacalcium phosphate and pyrolysed octacalcium phosphate. Arch. Oral Biol. 1966, 11, 477–492. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Grossin, D. Bioactive ceramics: Physical chemistry. In Comprehensive Biomaterials, 1st ed.; Ducheyne, P., Healy, K., Hutmacher, D., Grainger, D., Kirkpatrick, C., Eds.; Elsevier: Oxford, UK, 2011; pp. 187–281. [Google Scholar]
- Arellano-Jiménez, M.; García-García, R.; Reyes-Gasga, J. Synthesis and hydrolysis of octacalcium phosphate and its characterization by electron microscopy and X-ray diffraction. J. Phys. Chem. Solids 2009, 70, 390–395. [Google Scholar] [CrossRef]
- Osaka, A.; Miura, Y.; Takeuchi, K.; Asada, M.; Takahashi, K. Calcium apatite prepared from calcium hydroxide and orthophosphoric acid. J. Mater. Sci. Mater. Electron. 1991, 2, 51–55. [Google Scholar] [CrossRef]
- Fowler, B.O. Infrared studies of apatites. I. Vibrational assignments for calcium, strontium, and barium hydroxyapatites utilizing isotopic substitution. Inorg. Chem. 1974, 13, 194–207. [Google Scholar] [CrossRef]
- McConnell, D. Crystal chemistry of hydroxyapatite. Arch. Oral Biol. 1965, 10, 421–431. [Google Scholar] [CrossRef]
- Posner, A.S. Crystal chemistry of bone mineral. Physiol. Rev. 1969, 49, 760–792. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.M.; Weiss, C. Review of articular cartilage collagen research. Arthritis Rheum. 1975, 18, 553–562. [Google Scholar] [CrossRef]
- Knott, L.; Bailey, A. Collagen cross-links in mineralizing tissues: A review of their chemistry, function, and clinical relevance. Bone 1998, 22, 181–187. [Google Scholar] [CrossRef]
- Pillai, C.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Pal, S. Mechanical Properties of Biological Materials, 1st ed.; Springer Science & Business Media: New York, NY, USA, 2013; pp. 23–40. [Google Scholar]
- Bian, L.; Hou, C.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials 2013, 34, 413–421. [Google Scholar] [CrossRef]
- Mao, Z. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- McGann, M.E.; Bonitsky, C.M.; Ovaert, T.C.; Wagner, D.R. The effect of collagen crosslinking on the biphasic poroviscoelastic cartilage properties determined from a semi-automated microindentation protocol for stress relaxation. J. Mech. Behav. Biomed. Mater. 2014, 34, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Vanderhooft, J.L.; Alcoutlabi, M.; Magda, J.J.; Prestwich, G.D. Rheological Properties of Cross-Linked Hyaluronan-Gelatin Hydrogels for Tissue Engineering. Macromol. Biosci. 2009, 9, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Birkhead, J.R.; Suen, L.F.; Yamin, R.; Mizuno, S.; Glowacki, J.; Arbiser, J.L.; Apperley, J.F. Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. J. Clin. Investig. 1994, 94, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, O.; Garric, X.; Lavigne, J.-P.; Berghe, H.V.D.; Coudane, J. Multilayer, degradable coating as a carrier for the sustained release of antibiotics: Preparation and antimicrobial efficacy in vitro. J. Control. Release 2012, 162, 492–501. [Google Scholar] [CrossRef]
- Nuernberger, S.; Cyran, N.; Albrecht, C.; Redl, H.; Vécsei, V.; Marlovits, S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials 2011, 32, 1032–1040. [Google Scholar] [CrossRef]
- Cao, B.; Peng, R.; Li, Z.; Ding, J. Effects of spreading areas and aspect ratios of single cells on dedifferentiation of chondrocytes. Biomaterials 2014, 35, 6871–6881. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Matsiko, A.; Dickson, G.R.; O’Brien, F.J.; Gleeson, J.P. A biomimetic multi-layered collagen-based scaffold for osteochondral repair. Acta Biomater. 2014, 10, 1996–2004. [Google Scholar] [CrossRef]
- Nukavarapu, S.P.; Dorcemus, D.L. Osteochondral tissue engineering: Current strategies and challenges. Biotechnol. Adv. 2013, 31, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Klika, V.; Gaffney, E.A.; Chen, Y.-C.; Brown, C.P. An overview of multiphase cartilage mechanical modelling and its role in understanding function and pathology. J. Mech. Behav. Biomed. Mater. 2016, 62, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, N.; Ravichandran, P.; Reddy, P.N.; Ramamurty, N.; Pal, S.; Rao, K.P. Collagen–chitosan polymeric scaffolds for the in vitro culture of human epidermoid carcinoma cells. Biomaterials 2001, 22, 1943–1951. [Google Scholar] [CrossRef]
- Peng, L.; Cheng, X.R.; Wang, J.W.; Xu, D.X.; Wang, G. Preparation and Evaluation of Porous Chitosan/Collagen Scaffolds for Periodontal Tissue Engineering. J. Bioact. Compat. Polym. 2006, 21, 207–220. [Google Scholar] [CrossRef]
- Mirahmadi, F.; Tafazzoli-Shadpour, M.; Shokrgozar, M.A.; Bonakdar, S. Enhanced mechanical properties of thermosensitive chitosan hydrogel by silk fibers for cartilage tissue engineering. Mater. Sci. Eng. C 2013, 33, 4786–4794. [Google Scholar] [CrossRef]
- Sambudi, N.S.; Sathyamurthy, M.; Lee, G.M.; Bin Park, S. Electrospun chitosan/poly(vinyl alcohol) reinforced with CaCO3 nanoparticles with enhanced mechanical properties and biocompatibility for cartilage tissue engineering. Compos. Sci. Technol. 2015, 106, 76–84. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Yang, W.; Song, Y. Compressive mechanical properties and microstructure of PVA–HA hydrogels for cartilage repair. RSC Adv. 2016, 6, 20166–20172. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, H.; Sun, S.; Zhou, T.; Wu, J.; Wan, Y. Designed composites for mimicking compressive mechanical properties of articular cartilage matrix. J. Mech. Behav. Biomed. Mater. 2014, 36, 32–46. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Meyvis, T.K.; Stubbe, B.G.; Van Steenbergen, M.J.; Hennink, W.E.; De Smedt, S.C.; Demeester, J. A comparison between the use of dynamic mechanical analysis and oscillatory shear rheometry for the characterisation of hydrogels. Int. J. Pharm. 2002, 244, 163–168. [Google Scholar] [CrossRef]
- Kavanagh, G.M.; Ross-Murphy, S.B. Rheological characterisation of polymer gels. Prog. Polym. Sci. 1998, 23, 533–562. [Google Scholar] [CrossRef]
- Tschoegl, N.W. The Phenomenological Theory of Linear Viscoelastic Behavior: An Introduction, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 51–63. [Google Scholar]
- Kiss, M.Z.; Varghese, T.; Hall, T.J. Viscoelastic characterization ofin vitrocanine tissue. Phys. Med. Biol. 2004, 49, 4207–4218. [Google Scholar] [CrossRef]
- Kiss, M.Z.; Hobson, M.A.; Varghese, T.; Harter, J.; Kliewer, M.A.; Hartenbach, E.M.; Zagzebski, J.A. Frequency-dependent complex modulus of the uterus: Preliminary results. Phys. Med. Biol. 2006, 51, 3683–3695. [Google Scholar] [CrossRef]
- Yu, Q.; Zhou, J.; Fung, Y.C. Neutral axis location in bending and Young’s modulus of different layers of arterial wall. Am. J. Physiol. Circ. Physiol. 1993, 265, H52–H60. [Google Scholar] [CrossRef]
- Erkamp, R.; Wiggins, P.; Skovoroda, A.; Emelianov, S.; O’Donnell, M. Measuring the Elastic Modulus of Small Tissue Samples. Ultrason. Imaging 1998, 20, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Freeman, P.M.; Natarajan, R.N.; Kimura, J.H.; Andriacchi, T.P. Chondrocyte cells respond mechanically to compressive loads. J. Orthop. Res. 1994, 12, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Du, C.; Toh, W.S.; Wan, A.C.; Gao, S.J.; Kurisawa, M. Modulation of chondrocyte functions and stiffness-dependent cartilage repair using an injectable enzymatically crosslinked hydrogel with tunable mechanical properties. Biomaterials 2014, 35, 2207–2217. [Google Scholar] [CrossRef]
- Szarko, M.; Muldrew, K.; Bertram, J.E. Freeze-thaw treatment effects on the dynamic mechanical properties of articular cartilage. BMC Musculoskelet. Disord. 2010, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A. Molecular interactions in collagen and chitosan blends. Biomaterials 2004, 25, 795–801. [Google Scholar] [CrossRef]
- Cho, J.; Heuzey, M.-C.; Bégin, A.; Carreau, P.J. Physical Gelation of Chitosan in the Presence of β-Glycerophosphate: The Effect of Temperature. Biomacromolecules 2005, 6, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xiong, D.; Gao, F. Viscoelastic behavior of nano-hydroxyapatite reinforced poly(vinyl alcohol) gel biocomposites as an articular cartilage. J. Mater. Sci. Mater. Electron. 2008, 19, 1963–1969. [Google Scholar] [CrossRef]
- Hamann, N.; Zaucke, F.; Heilig, J.; Oberländer, K.D.; Brüggemann, G.-P.; Niehoff, A. Effect of different running modes on the morphological, biochemical, and mechanical properties of articular cartilage. Scand. J. Med. Sci. Sports 2012, 24, 179–188. [Google Scholar] [CrossRef]
- Kalyanam, S.; Yapp, R.D.; Insana, M.F. Poro-Viscoelastic Behavior of Gelatin Hydrogels Under Compression-Implications for Bioelasticity Imaging. J. Biomech. Eng. 2009, 131, 081005. [Google Scholar] [CrossRef] [PubMed]
- Strange, D.G.T.; Fletcher, T.L.; Tonsomboon, K.; Brawn, H.; Zhao, X.; Oyen, M.L. Separating poroviscoelastic deformation mechanisms in hydrogels. Appl. Phys. Lett. 2013, 102, 31913. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, H.; Kawazoe, N.; Chen, G. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater. 2014, 10, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Sefat, F.; Raja, T.I.; Zafar, M.S.; Sultan, Z.K. Chapter 3—Nanoengineered biomaterials for cartilage repair. In Nanoengineered Biomaterials for Regenerative Medicine, 1st ed.; Mozafari, M., Rajadas, J., Kaplan, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 39–71. [Google Scholar]
- Habeeb, A.; Hiramoto, R. Reaction of proteins with glutaraldehyde. Arch. Biochem. Biophys. 1968, 126, 16–26. [Google Scholar] [CrossRef]
- Qian, Y.; Engel, M.H.; Macko, S.A.; Carpenter, S.; Deming, J.W. Kinetics of peptide hydrolysis and amino acid decomposition at high temperature. Geochim. et Cosmochim. Acta 1993, 57, 3281–3293. [Google Scholar] [CrossRef]
- Sewald, N.; Jakubke, H.-D. Peptides: Chemistry & Biology; Wiley-VCH Verlag: New York, NY, USA, 2002; Print ISBN:9783527304059, Online ISBN:9783527600687. [Google Scholar] [CrossRef]
- Kusuhara, H.; Isogai, N.; Enjo, M.; Otani, H.; Ikada, Y.; Jacquet, R.; Lowder, E.; Landis, W.J. Tissue engineering a model for the human ear: Assessment of size, shape, morphology, and gene expression following seeding of different chondrocytes. Wound Repair Regen. 2009, 17, 136–146. [Google Scholar] [CrossRef]
- Collins, J.S.; Goldsmith, T.H. Spectral properties of fluorescence induced by glutaraldehyde fixation. J. Histochem. Cytochem. 1981, 29, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, D. Theoretical and practical aspects of glutaraldehyde fixation. In Fixation in Histochemistry; Stoward, P.J., Ed.; Springer: Boston, MA, USA, 1973; pp. 47–83. [Google Scholar] [CrossRef]




| Sample Code | B-Layer | M-Layer | T-Layer | Cross-Linked |
|---|---|---|---|---|
| 3CCO | COL:CHI (1:1) + 2% Ca-P | COL:CHI (1:1) | COL:CHI (3:1) | No |
| 3CCH | COL:CHI (1:1) | No | ||
| 3CCHE | COL:ELR(1:1) | No | ||
| 3CCO.G | COL:CHI (1:1) | Yes (G) | ||
| 3CCH.G | COL:CHI (1:1) | Yes (G) | ||
| 3CCH.N | COL:CHI (1:1) | Yes (N) | ||
| 3CCHE.G | COL:ELR(1:1) | Yes (G) |
| Sample | Slope (Pa/Hz½) |
|---|---|
| 3CCO | 166 ± 3 |
| 3CCH | 115 ± 3 |
| 3CCHE | 71 ± 2 |
| 3CCO.G | 1010 ± 20 |
| 3CCH.G | 850 ± 20 |
| 3CCH.N | 139 ± 7 |
| 3CCHE.G | 920 ± 20 |
| Parameter | 3CCO | 3CCH | 3CCHE | 3CCO.G | 3CCH.G | 3CCH.N | 3CCHE.G |
|---|---|---|---|---|---|---|---|
| Geq | 0.576 ± 0.006 | 0.29 ± 0.06 | 0.208 ± 0.004 | 0.387 ± 0.003 | 0.267 ± 0.003 | 0.27 ± 0.01 | 0.31 ± 0.03 |
| G1 | 0.06 ± 0.02 | 0.17 ± 0.02 | 3 ± 1 | 0.247 ± 0.003 | 0.155 ± 0.002 | 0.12 ± 0.03 | 0.212 ± 0.007 |
| (s) | 8 ± 4 | 7 ± 1 | 1.9 ± 0.3 | 8.4 ± 0.2 | 12.3 ± 0.4 | 8 ± 3 | 23 ± 1 |
| G2 | 0.10 ± 0.01 | 0.14 ± 0.02 | 0.334 ± 0.005 | 0.192 ± 0.002 | 0.218 ± 0.002 | 0.20 ± 0.03 | 0.18 ± 0.02 |
| (s) | 50 ± 10 | 150 ± 30 | 210 ± 10 | 77 ± 2 | 85 ± 2 | 40 ± 10 | 180 ± 30 |
| G3 | 0.207 ± 007 | 0.41 ± 0.03 | --- | 0.272 ± 0.002 | 0.406 ± 0.002 | 0.39 ± 0.01 | 0.316 ± 0.009 |
| (s) | 530 ± 60 | 1400 ± 400 | --- | 860 ± 30 | 820 ± 20 | 460 ± 50 | 1200 ± 300 |
| G% | 58% | 39% | 19% | 42% | 31% | 31% | 37% |
| (%) | 98.23 | 99.07 | 95.89 | 99.97 | 99.98 | 98.79 | 99.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, Y.; Sola, F.J.; Fuentes, G.; Quintanilla, L.; Almirall, A.; Cruz, L.J.; Rodríguez-Cabello, J.C.; Tabata, Y. The Effects of Crosslinking on the Rheology and Cellular Behavior of Polymer-Based 3D-Multilayered Scaffolds for Restoring Articular Cartilage. Polymers 2021, 13, 907. https://doi.org/10.3390/polym13060907
Campos Y, Sola FJ, Fuentes G, Quintanilla L, Almirall A, Cruz LJ, Rodríguez-Cabello JC, Tabata Y. The Effects of Crosslinking on the Rheology and Cellular Behavior of Polymer-Based 3D-Multilayered Scaffolds for Restoring Articular Cartilage. Polymers. 2021; 13(6):907. https://doi.org/10.3390/polym13060907
Chicago/Turabian StyleCampos, Yaima, Francisco J. Sola, Gastón Fuentes, Luis Quintanilla, Amisel Almirall, Luis J. Cruz, José C. Rodríguez-Cabello, and Yasuhiko Tabata. 2021. "The Effects of Crosslinking on the Rheology and Cellular Behavior of Polymer-Based 3D-Multilayered Scaffolds for Restoring Articular Cartilage" Polymers 13, no. 6: 907. https://doi.org/10.3390/polym13060907
APA StyleCampos, Y., Sola, F. J., Fuentes, G., Quintanilla, L., Almirall, A., Cruz, L. J., Rodríguez-Cabello, J. C., & Tabata, Y. (2021). The Effects of Crosslinking on the Rheology and Cellular Behavior of Polymer-Based 3D-Multilayered Scaffolds for Restoring Articular Cartilage. Polymers, 13(6), 907. https://doi.org/10.3390/polym13060907








