The Effect of Fiber Type and Yarn Diameter on Superhydrophobicity, Self-Cleaning Property, and Water Spray Resistance
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Methods
2.2.1. Hydrophobization
2.2.2. Characterization
Surface Morphology and Chemical Composition Change
Add-on Ratio and Thickness Change
Superhydrophobicity, Self-Cleaning Property, and Water Spraying Test
Water Vapor Transmission Rate (WVTR) and Air Permeability
3. Results
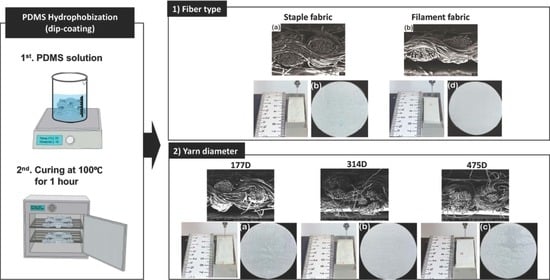
3.1. Surface Properties by the Fiber Type
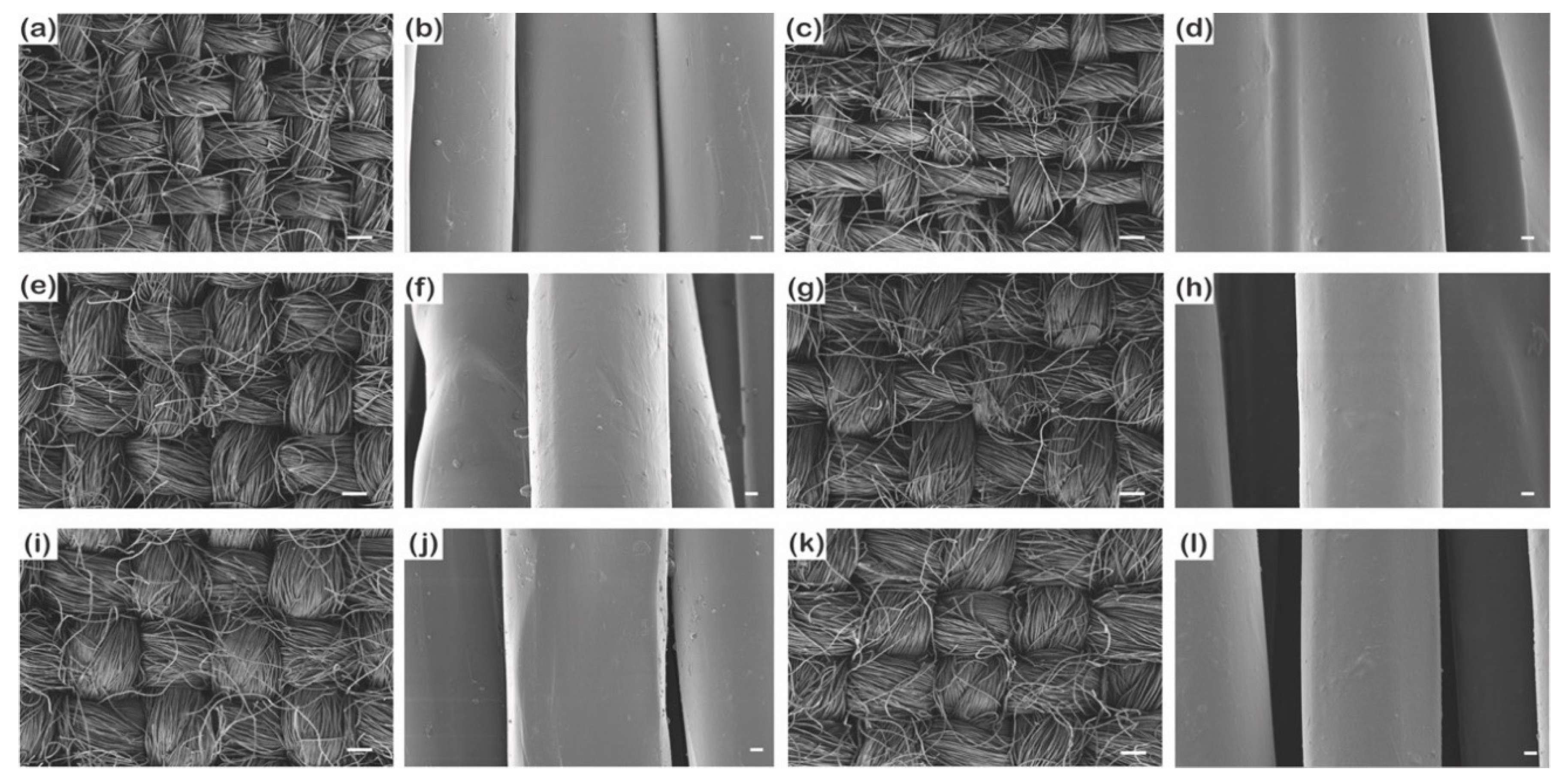
3.1.1. Changes in Surface Morphology, Add-on Ratio, and Thickness
3.1.2. Changes in Chemical Composition
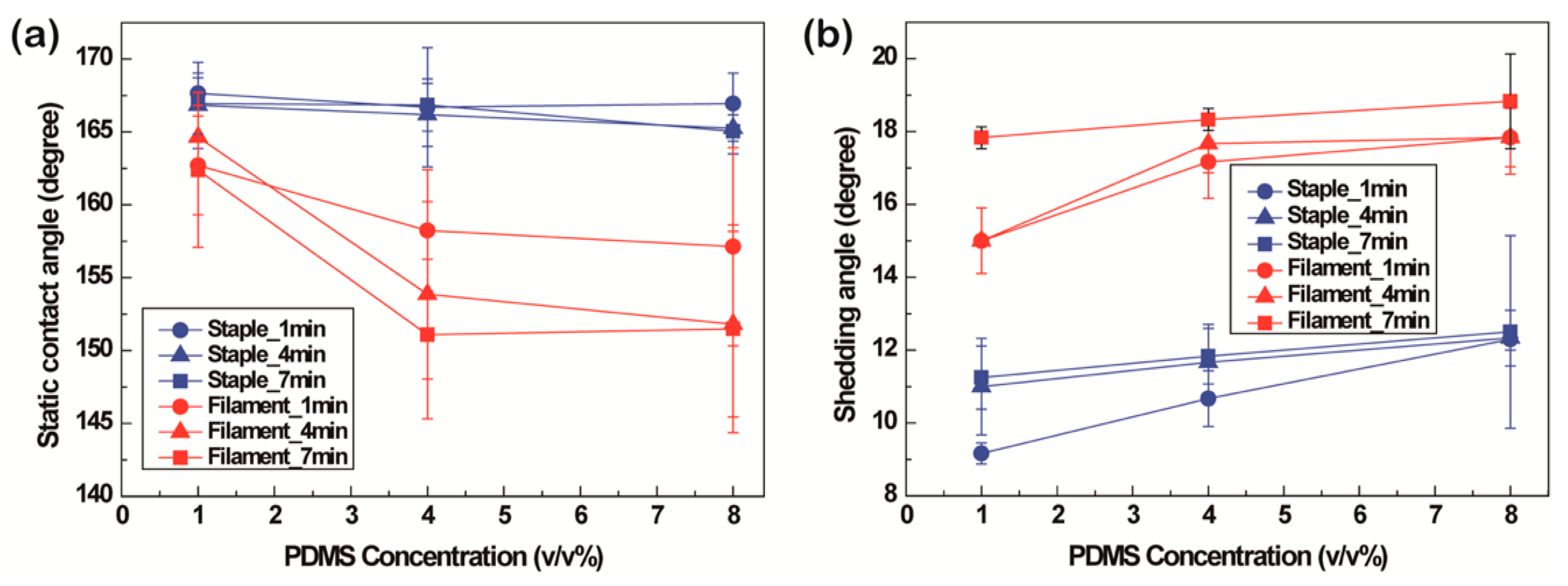
3.1.3. Superhydrophobicity
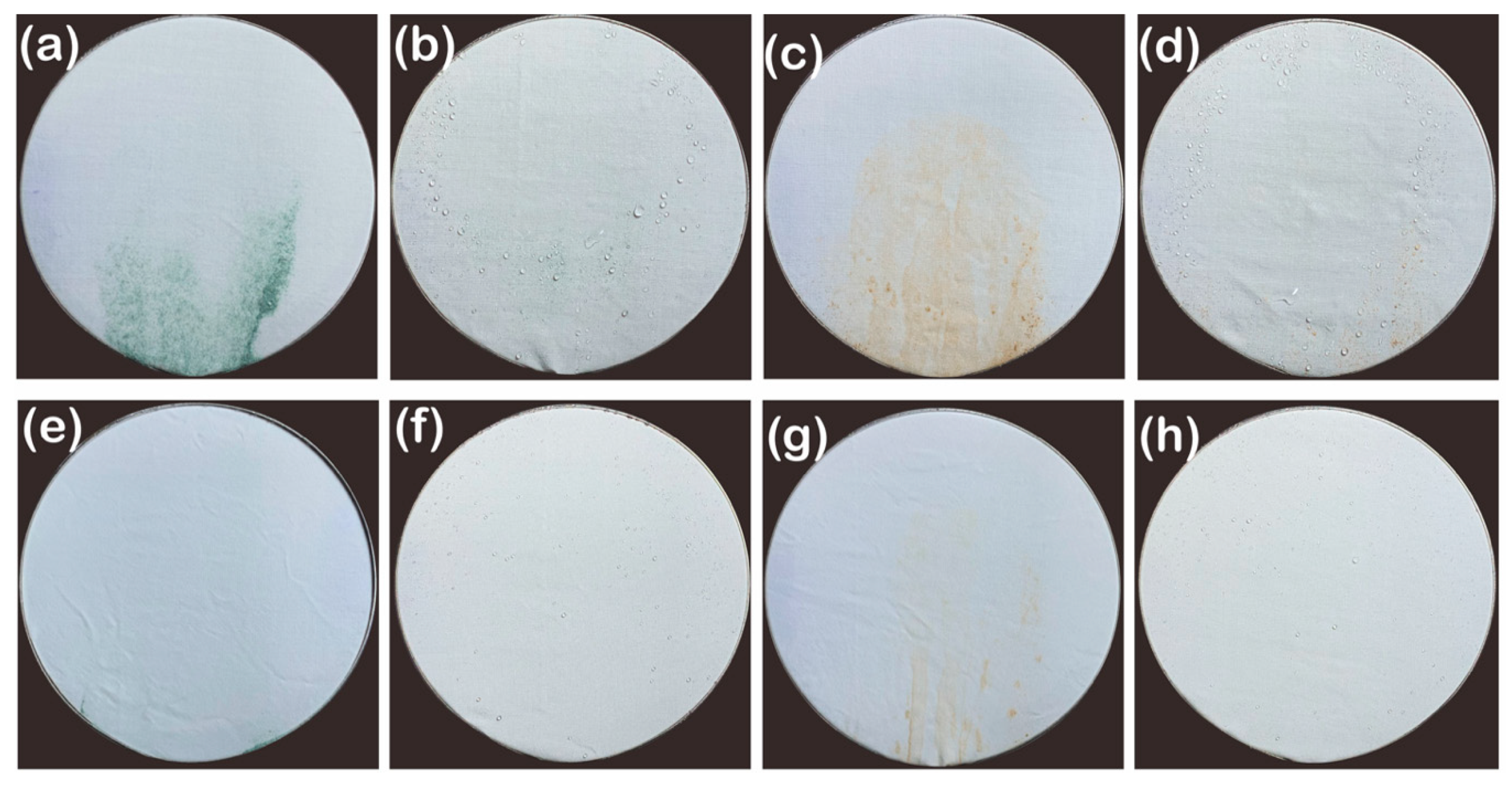
3.1.4. Self-Cleaning Property
3.1.5. Water Spray Test
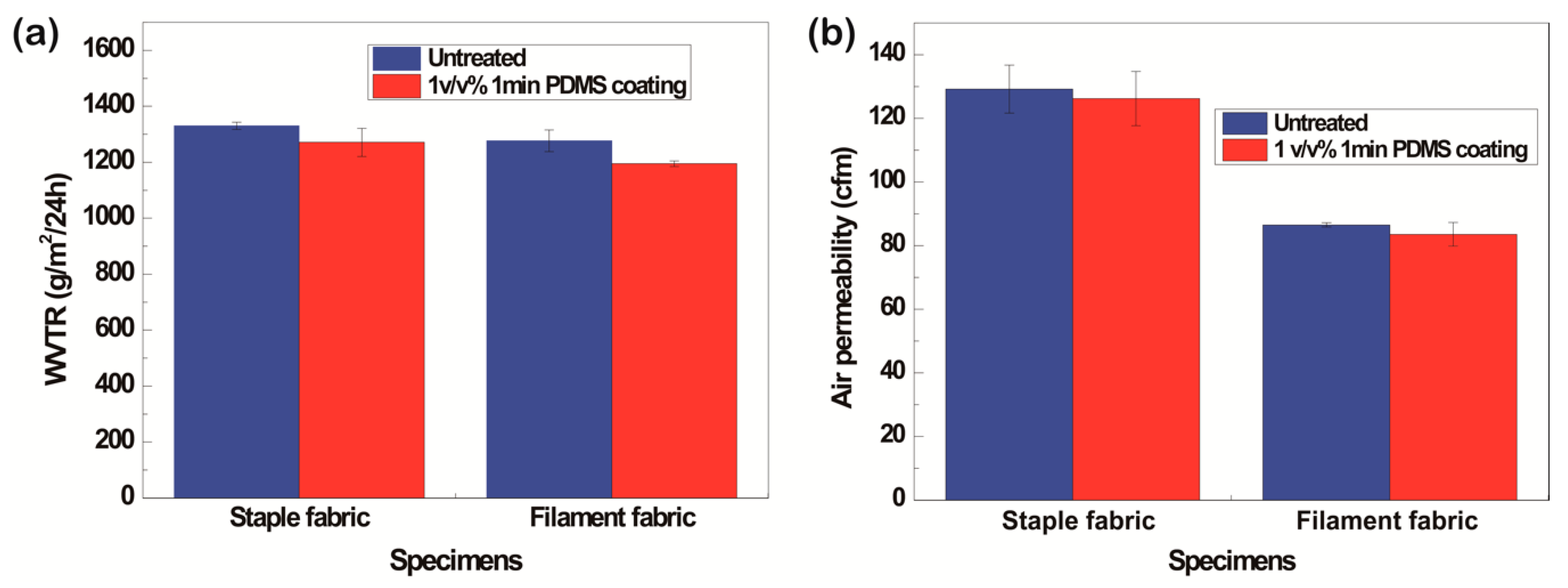
3.1.6. WVTR and Air Permeability
3.2. Surface Properties According the Yarn Diameter
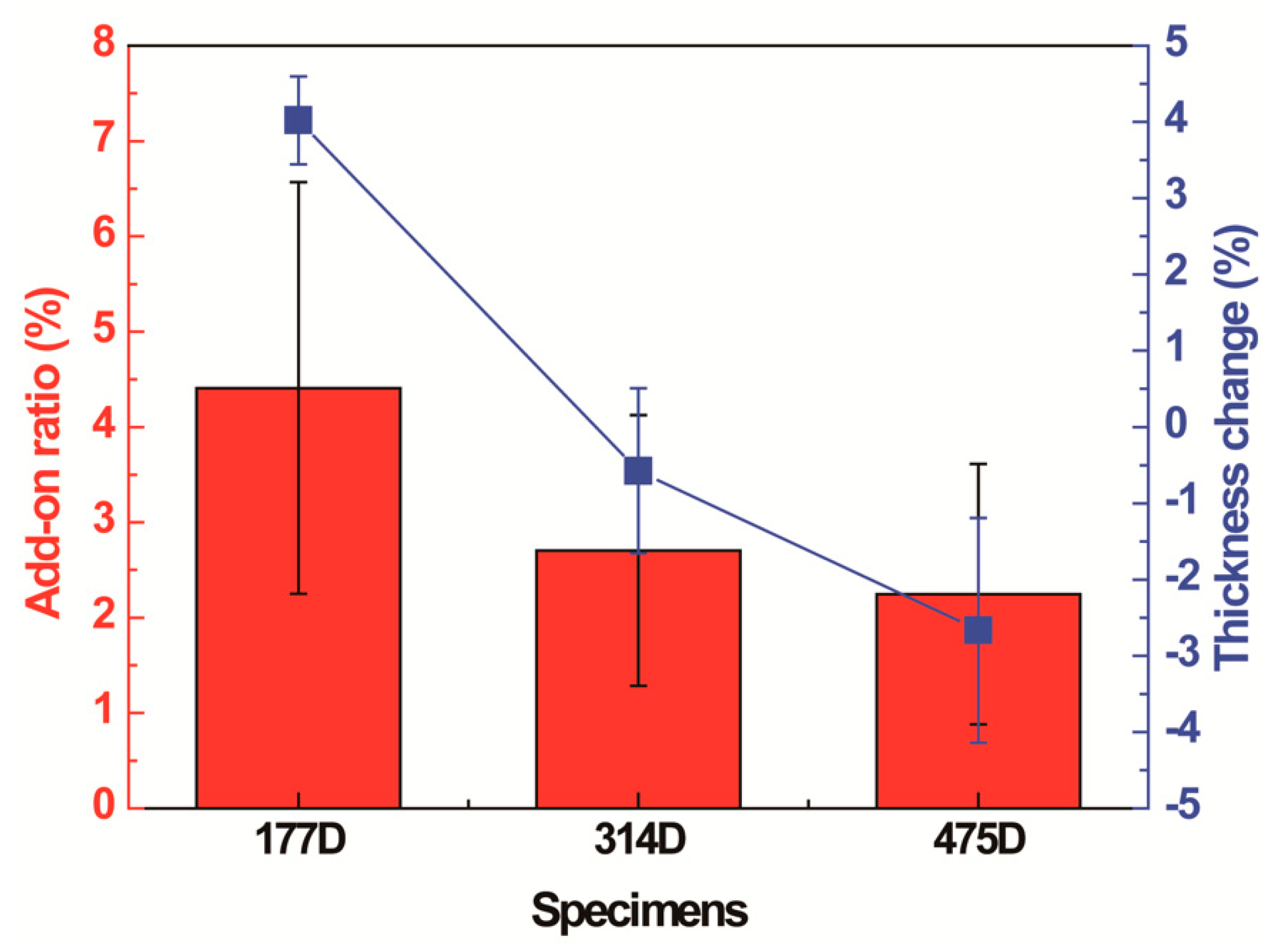
3.2.1. Changes in Surface Morphology, Add-on Ratio, and Thickness
3.2.2. Change in Chemical Composition
3.2.3. Superhydrophobicity
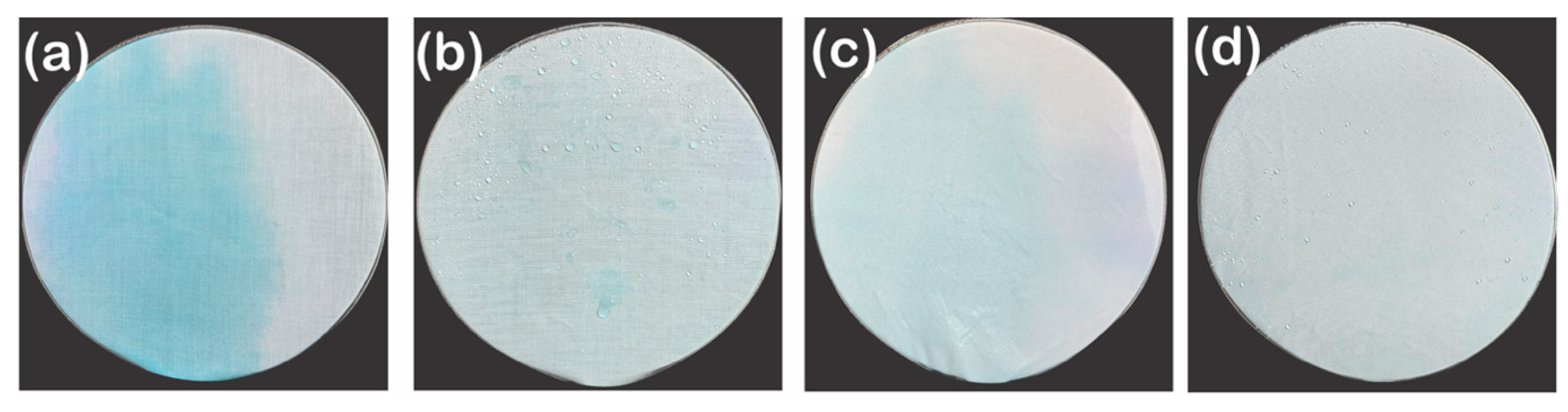
3.2.4. Self-Cleaning Property
3.2.5. Water Spraying Test
3.2.6. WVTR and Air Permeability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen-Tri, P.; Altiparmak, F.; Nguyen, N.; Tuduri, L.; Ouellet-Plamondon, C.M.; Prud’homme, R.E. Robust Superhydrophobic Cotton Fibers Prepared by Simple Dip-Coating Approach Using Chemical and Plasma-Etching Pretreatments. Acs Omega 2019, 4, 7829–7837. [Google Scholar] [CrossRef]
- Cheng, L.C.; Jonh, W.; Karim, R.; Mukarram, T.; Yi, D.; Alfredo, A. Imparting Superhydrophobicity with a Hierarchical Block Copolymer Coating. Small 2019, 1, 1905509. [Google Scholar] [CrossRef]
- Quan, Y.-Y.; Zhang, L.-Z.; Qi, R.-H.; Cai, R.-R. Self-Cleaning of Surfaces: The Role of Surface Wettability and Dust Types. Sci. Rep. 2016, 6, 38239. [Google Scholar] [CrossRef]
- Oh, J.-H.; Ko, T.-J.; Moon, M.-W.; Park, C.H. Nanostructured Fabric with Robust Superhydrophobicity Induced by a Thermal Hydrophobic Ageing Process. Rsc Adv. 2017, 7, 25597–25604. [Google Scholar] [CrossRef]
- Oh, J.-H.; Ko, T.-J.; Moon, M.-W.; Park, C.H. Nanostructured Superhydrophobic Silk Fabric Fabricated Using the Ion Beam Method. Rsc Adv. 2014, 4, 38966–38973. [Google Scholar] [CrossRef]
- Oh, J.; Park, C.H. Robust Fluorine-Free Superhydrophobic PET Fabric Using Alkaline Hydrolysis and Thermal Hydrophobic Aging Process. Macromol. Mater. Eng. 2018, 303, 1700673. [Google Scholar] [CrossRef]
- Ju, B.-J.; Oh, J.-H.; Yun, C.; Park, C.H. Development of a Superhydrophobic Electrospun Poly(Vinylidene Fluoride) Web via Plasma Etching and Water Immersion for Energy Harvesting Applications. Rsc Adv. 2018, 8, 28825–28835. [Google Scholar] [CrossRef]
- Noman, M.T.; Petru, M.; Militký, J.; Azeem, M.; Ashraf, M.A. One-Pot Sonochemical Synthesis of ZnO Nanoparticles for Photocatalytic Applications, Modelling and Optimization. Materials 2020, 13, 14. [Google Scholar] [CrossRef]
- Behera, P.; Noman, M.T.; Petrů, M. Enhanced Mechanical Properties of Eucalyptus-Basalt-Based Hybrid-Reinforced Cement Composites. Polymers 2020, 12, 2837. [Google Scholar] [CrossRef]
- Noman, M.T.; Petrů, M. Functional Properties of Sonochemically Synthesized Zinc Oxide Nanoparticles and Cotton Composites. Nanomaterials 2020, 10, 1661. [Google Scholar] [CrossRef]
- Oh, J.-H.; Moon, M.-W.; Park, C.H. Effect of Crystallinity on the Recovery Rate of Superhydrophobicity in Plasma-Nanostructured Polymers. Rsc Adv. 2020, 10, 10939–10948. [Google Scholar] [CrossRef]
- Kim, S.; Oh, J.-H.; Park, C.H. Development of Energy-Efficient Superhydrophobic Polypropylene Fabric by Oxygen Plasma Etching and Thermal Aging. Polymers 2020, 12, 2756. [Google Scholar] [CrossRef]
- Kim, H.; Oh, J.-H.; Hee Park, C. Superhydrophobic Fabric Mixed with Polyester and Cotton Yarns Modified by Alkaline Treatment and Thermal Aging. Text. Res. J. 2020, 0040517520977211. [Google Scholar] [CrossRef]
- Mazrouei-Sebdani, Z.; Khoddami, A. Alkaline Hydrolysis: A Facile Method to Manufacture Superhydrophobic Polyester Fabric by Fluorocarbon Coating. Prog. Org. Coat. 2011, 72, 638–646. [Google Scholar] [CrossRef]
- Caschera, D.; Cortese, B.; Mezzi, A.; Brucale, M.; Ingo, G.M.; Gigli, G.; Padeletti, G. Ultra Hydrophobic/Superhydrophilic Modified Cotton Textiles through Functionalized Diamond-Like Carbon Coatings for Self-Cleaning Applications. Langmuir 2013, 29, 2775–2783. [Google Scholar] [CrossRef]
- de Camargo, J.S.G.; de Menezes, A.J.; da Cruz, N.C.; Rangel, E.C.; Delgado-Silva, A.D.O. Morphological and Chemical Effects of Plasma Treatment with Oxygen (O2) and Sulfur Hexafluoride (SF6) on Cellulose Surface. Mat. Res. 2018, 20, 842–850. [Google Scholar] [CrossRef]
- Mirzadeh, H.; Katbab, A.A. Eco-Friendly and Smart Polymer Systems; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-45084-7. [Google Scholar]
- Zhang, X.; Liu, S.; Salim, A.; Seeger, S. Hierarchical Structured Multifunctional Self-Cleaning Material with Durable Superhydrophobicity and Photocatalytic Functionalities. Small 2019, 15, 1901822. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Park, C.H. Influence of Micro and Nano-Scale Roughness on Hydrophobicity of a Plasma-Treated Woven Fabric. Text. Res. J. 2017, 87, 193–207. [Google Scholar] [CrossRef]
- Shim, M.H.; Kim, J.; Park, C.H. The Effects of Surface Energy and Roughness on the Hydrophobicity of Woven Fabrics. Text. Res. J. 2014, 84, 1268–1278. [Google Scholar] [CrossRef]
- Zhu, L.; Xiu, Y.; Xu, J.; Tamirisa, P.A.; Hess, D.W.; Wong, C.-P. Superhydrophobicity on Two-Tier Rough Surfaces Fabricated by Controlled Growth of Aligned Carbon Nanotube Arrays Coated with Fluorocarbon. Langmuir 2005, 24, 11208–11212. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Rodak, D.; Wong, C.; Hayden, C. Effects of Micro-and Nano-Structures on the Self-Cleaning Behaviour of Lotus Leaves. Nanotechnology 2006, 17, 1359. [Google Scholar] [CrossRef]
- Guo, X.-J.; Xue, C.-H.; Sathasivam, S.; Page, K.; He, G.; Guo, J.; Promdet, P.; Heale, F.L.; Carmalt, C.J.; Parkin, I.P. Fabrication of Robust Superhydrophobic Surfaces via Aerosol-Assisted CVD and Thermo-Triggered Healing of Superhydrophobicity by Recovery of Roughness Structures. J. Mater. Chem. A. 2019, 7, 17604–17612. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Q.; Lyons, A.M. Durable, Optically Transparent, Superhydrophobic Polymer Films. Appl. Surf. Sci. 2019, 470, 187–195. [Google Scholar] [CrossRef]
- Chauhan, P.; Kumar, A.; Bhushan, B. Self-Cleaning, Stain-Resistant and Anti-Bacterial Superhydrophobic Cotton Fabric Prepared by Simple Immersion Technique. J. Colloid Interface Sci. 2019, 535, 66–74. [Google Scholar] [CrossRef]
- Gao, L.; McCarthy, T.J. Teflon Is Hydrophilic. Comments on Definitions of Hydrophobic, Shear versus Tensile Hydrophobicity, and Wettability Characterization. Langmuir 2008, 24, 9183–9188. [Google Scholar] [CrossRef] [PubMed]
- Huovinen, E.; Hirvi, J.; Suvanto, M.; Pakkanen, T.A. Micro–Micro Hierarchy Replacing Micro–Nano Hierarchy: A Precisely Controlled Way To Produce Wear-Resistant Superhydrophobic Polymer Surfaces. Langmuir 2012, 28, 14747–14755. [Google Scholar] [CrossRef]
- Chu, D.; Nemoto, A.; Ito, H. Enhancement of Dynamic Wetting Properties by Direct Fabrication on Robust Micro–Micro Hierarchical Polymer Surfaces. Appl. Surf. Sci. 2014, 300, 117–123. [Google Scholar] [CrossRef]
- Backert, S. The Relationship Between the Structural Geometry of a Textile Fabric and Its Physical Properties: I: Literature Review. Text. Res. J. 1948, 11, 650–658. [Google Scholar] [CrossRef]
- Backer, S. The Relationship Between the Structural Geometry of a Textile Fabric and Its Physical Properties: Part IV: Interstice Geometry and Air Permeability. Text. Res. J. 1951, 10, 703–714. [Google Scholar] [CrossRef]
- Adams, D.P.; Schwarz, E.R.; Backer, S. The Relationship between the Structural Geometry of a Textile Fabric and Its Physical Properties: Part VI: Nomographic Solution of the Geometric Relationships in Cloth Geometry. Text. Res. J. 1956, 9, 653–665. [Google Scholar] [CrossRef]
- Park, J.K.; Cho, D.; Kang, T.J. A Comparison of the Interfacial, Thermal, and Ablative Properties between Spun and Filament Yarn Type Carbon Fabric/Phenolic Composites. Carbon 2004, 42, 795–804. [Google Scholar] [CrossRef]
- Hamburger, W.J. Mechanics of Abrasion of Textile Materials. Text. Res. 1945, 15, 169–177. [Google Scholar] [CrossRef]
- Hicks, E.M.; Scroggie, A.G. Taber Yarn-Sheet Abrasion Test. Text. Res. J. 1948, 18, 416–423. [Google Scholar] [CrossRef]
- Hoefnagels, H.F.; Wu, D.; de With, G.; Ming, W. Biomimetic Superhydrophobic and Highly Oleophobic Cotton Textiles. Langmuir 2007, 23, 13158–13163. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Sun, C. Robust Water-Repellent Treatment of Cotton Fabrics with Polysiloxane Modified via Thiol-Ene Click Reaction. Fibers Polym. 2018, 19, 580–586. [Google Scholar] [CrossRef]
- Davis, A.; Yeong, Y.H.; Steele, A.; Loth, E.; Bayer, I.S. Nanocomposite Coating Superhydrophobicity Recovery after Prolonged High-Impact Simulated Rain. Rsc Adv. 2014, 4, 47222–47226. [Google Scholar] [CrossRef]
- Zimmermann, J.; Seeger, S.; Reifler, F.A. Water Shedding Angle: A New Technique to Evaluate the Water-Repellent Properties of Superhydrophobic Surfaces. Text. Res. J. 2009, 79, 1565–1570. [Google Scholar] [CrossRef]
- Oh, J.; Park, C.H. Colorful Fluorine-Free Superhydrophobic Polyester Fabric Prepared via Disperse Dyeing Process. Adv. Mater. Interfaces 2020, 7, 2000127. [Google Scholar] [CrossRef]
- Pan, N. Development of a Constitutive Theory for Short Fiber Yarns: Mechanics of Staple Yarn Without Slippage Effect. Text. Res. J. 1992, 62, 749–765. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, H.; Du, S.; Wang, Q.; He, J.; Cui, S. Facile Fabrication of Polyester Filament Fabric with Highly and Durable Hydrophilic Surface by Microwave-Assisted Glycolysis. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Kamath, Y.K.; Hornby, S.B. Wicking of Spin Finishes and Related Liquids into Continuous Filament Yarns. Text. Res. J. 1994, 1, 33–40. [Google Scholar] [CrossRef]
- Kawase, T.; Sekoguchi, S.; Fuj, T.; Minagawa, M. Spreading of liquids in textile assemblies: Part I: Capillary spreading of liquids. Text. Res. J. 1986, 7, 409–414. [Google Scholar] [CrossRef]
- Sethunga, G.S.M.D.P.; Karahan, H.E.; Wang, R.; Bae, T.-H. PDMS-Coated Porous PVDF Hollow Fiber Membranes for Efficient Recovery of Dissolved Biomethane from Anaerobic Effluents. J. Membr. Sci. 2019, 584, 333–342. [Google Scholar] [CrossRef]
- Xue, C.-H.; Zhang, Z.-D.; Zhang, J.; Jia, S.-T. Lasting and Self-Healing Superhydrophobic Surfaces by Coating of Polystyrene/SiO 2 Nanoparticles and Polydimethylsiloxane. J. Mater. Chem. A 2014, 2, 15001–15007. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Liu, J.-L.; Feng, X.-Q.; Wang, G.; Yu, S.-W. Mechanisms of Superhydrophobicity on Hydrophilic Substrates. J. Phys. Condens. Matter 2007, 19, 356002. [Google Scholar] [CrossRef]
- Liu, Y.; Xiu, Y.; Hess, D.W.; Wong, C.P. Silicon Surface Structure-Controlled Oleophobicity. Langmuir 2010, 26, 8908–8913. [Google Scholar] [CrossRef] [PubMed]
- Wisdom, K.M.; Watson, J.A.; Qu, X.; Liu, F.; Watson, G.S.; Chen, C.-H. Self-Cleaning of Superhydrophobic Surfaces by Self-Propelled Jumping Condensate. Proc. Natl. Acad. Sci. USA 2013, 110, 7992–7997. [Google Scholar] [CrossRef] [PubMed]
- Kale, K.H.; Palaskar, S. Atmospheric Pressure Plasma Polymerization of Hexamethyldisiloxane for Imparting Water Repellency to Cotton Fabric. Text. Res. J. 2011, 81, 608–620. [Google Scholar] [CrossRef]
- Rioboo, R.; Voué, M.; Adão, H.; Conti, J.; Vaillant, A.; Seveno, D.; De Coninck, J. Drop Impact on Soft Surfaces: Beyond the Static Contact Angles. Langmuir 2010, 26, 4873–4879. [Google Scholar] [CrossRef]
- Voué, M.; Rioboo, R.; Bauthier, C.; Conti, J.; Charlot, M.; De Coninck, J. Dissipation and Moving Contact Lines on Non-Rigid Substrates. J. Eur. Ceram. Soc. 2003, 23, 2769–2775. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, P.; Xue, J.; Wang, Y.; Chen, Q.; Ding, J.; Wang, Q. Superhydrophobic Stability of Nanotube Array Surfaces under Impact and Static Forces. Acs Appl. Mater. Interfaces 2014, 6, 8073–8079. [Google Scholar] [CrossRef]
- Lyons, W.J. Influence of Fiber Ends on the Adhesion of Bare Tire Cords. Text. Res. J. 1950, 20, 654–656. [Google Scholar] [CrossRef]
- Save, N.S.; Jassal, M.; Agrawal, A.K. Polyacrylamide Based Breathable Coating for Cotton Fabric. J. Ind. Text. 2002, 32, 119–138. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Bolling, L.; Haile, M.; Grunlan, J.C. Improving Oxygen Barrier and Reducing Moisture Sensitivity of Weak Polyelectrolyte Multilayer Thin Films with Crosslinking. Rsc Adv. 2012, 2, 12355. [Google Scholar] [CrossRef]
- Nassar, M.A.; El-Sakhawy, M.; Madkour, H.M.F.; El-ziaty, A.K.; Mohamed, S.A. Novel Coating of Bagasse Paper Sheets by Gelatin and Chitosan. Nord. Pulp. Pap. Res. J. 2014, 29, 741–746. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, J.H.; Choi, C.-H. Cotton Fabrics with Single-Faced Superhydrophobicity. Langmuir 2012, 28, 17426–17434. [Google Scholar] [CrossRef]
- El-Newashy, R.F.; Mowafi, S.; Haggag, K.; Abou Taleb, M.; El-Sayed, H. Evaluation of Comfort Attributes of Polyester Knitted Fabrics Treated with Sericin. Fibers Polym. 2019, 20, 1992–2001. [Google Scholar] [CrossRef]
- Xue, C.-H.; Li, X.; Jia, S.-T.; Guo, X.-J.; Li, M. Fabrication of Robust Superhydrophobic Fabrics Based on Coating with PVDF/PDMS. Rsc Adv. 2016, 6, 84887–84892. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Zhang, D.; Li, L.; Zhu, Y. Preparation and Characterization of Superhydrophobic Surface Based on Polydimethylsiloxane (PDMS). J. Adhes. Sci. Technol. 2019, 33, 1870–1881. [Google Scholar] [CrossRef]
- Shim, M.H.; Kim, J.; Park, C.H. Development of Superhydrophobic Fabrics by Surface Fluorination and Formation of CNT-Induced Roughness. Mater. Sci. 2015, 21, 68–73. [Google Scholar] [CrossRef][Green Version]
- Tian, D.; Zhang, X.; Tian, Y.; Wu, Y.; Wang, X.; Zhai, J.; Jiang, L. Photo-Induced Water–Oil Separation Based on Switchable Superhydrophobicity–Superhydrophilicity and Underwater Superoleophobicity of the Aligned ZnO Nanorod Array-Coated Mesh Films. J. Mater. Chem. 2012, 22, 19652. [Google Scholar] [CrossRef]
- Chen, P.-C.; Xu, Z.-K. Mineral-Coated Polymer Membranes with Superhydrophilicity and Underwater Superoleophobicity for Effective Oil/Water Separation. Sci. Rep. 2013, 3, 2776. [Google Scholar] [CrossRef]
- Zhang, E.; Cheng, Z.; Lv, T.; Qian, Y.; Liu, Y. Anti-Corrosive Hierarchical Structured Copper Mesh Film with Superhydrophilicity and Underwater Low Adhesive Superoleophobicity for Highly Efficient Oil–Water Separation. J. Mater. Chem. A 2015, 3, 13411–13417. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Wang, C.; Liu, J.; Zhai, H.; Liu, B.; Zhao, X.; Fang, D. Facile Fabrication of Zinc Oxide Coated Superhydrophobic and Superoleophilic Meshes for Efficient Oil/Water Separation. Rsc Adv. 2018, 8, 35150–35156. [Google Scholar] [CrossRef]
- Guan, Y.; Cheng, F.; Pan, Z. Superwetting Polymeric Three Dimensional (3D) Porous Materials for Oil/Water Separation: A Review. Polymers 2019, 11, 806. [Google Scholar] [CrossRef]
- Chen, C.-M.; Yang, S. Directed Water Shedding on High-Aspect-Ratio Shape Memory Polymer Micropillar Arrays. Adv. Mater. 2014, 26, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiao, Z.; Chan, P.C.H.; Lee, Y.-K.; Li, Z. A Comparative Study of Droplet Impact Dynamics on a Dual-Scaled Superhydrophobic Surface and Lotus Leaf. Appl. Surf. Sci. 2011, 257, 8857–8863. [Google Scholar] [CrossRef]
- Yang, C.; Tartaglino, U.; Persson, B.N.J. Influence of Surface Roughness on Superhydrophobicity. Phys. Rev. Lett. 2006, 97, 116103. [Google Scholar] [CrossRef]
- Cortese, B.; D’Amone, S.; Manca, M.; Viola, I.; Cingolani, R.; Gigli, G. Superhydrophobicity Due to the Hierarchical Scale Roughness of PDMS Surfaces. Langmuir 2008, 24, 2712–2718. [Google Scholar] [CrossRef] [PubMed]
- Souma, D. Water and Stain Repellent Textiles, Using New Plasma Technology. Master’s Thesis, Chalmers University of Technology, Göteborg, Sweden, 2013. [Google Scholar]









| Fiber | Weave | Sample Code | Fiber Type | Yarn Count | Twist (turns/m) | Weave Density (Wrap/Weft) | Cover Factor | Cloth Cover Factor | Thickness (mm) | Solid Volume Fraction | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wrap | Weft | ||||||||||
| PET | Plain | Staple fabric | Staple | 177D | 865.8 | 66/66 | 12.1 | 12.1 | 19.0 | 0.28 ± 0.01 | 0.33 |
| Filament fabric | Filament | 150D/144f | 0 | 72/72 | 12.1 | 12.1 | 19.0 | 0.21 ± 0.01 | 0.38 | ||
| 177D | Staple | 177D | 865.8 | 53/53 | 9.7 | 9.7 | 16.0 | 0.28 ± 0.01 | 0.24 | ||
| 314D | Staple | 314D | 570.8 | 40/40 | 9.7 | 9.7 | 16.0 | 0.45 ± 0.01 | 0.33 | ||
| 475D | Staple | 475D | 491.2 | 32/32 | 9.6 | 9.6 | 15.9 | 0.55 ± 0.01 | 0.33 | ||
| PDMS Coating | ||
|---|---|---|
| Concentration (v/v%) | Duration (min) | Code |
| 1 | 1 | P1T1 |
| 4 | P1T4 | |
| 7 | P1T7 | |
| 4 | 1 | P4T1 |
| 4 | P4T4 | |
| 7 | P4T7 | |
| 8 | 1 | P8T1 |
| 4 | P8T4 | |
| 7 | P8T7 | |
| Static Contact Angle (°) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 177D | 314D | 475D | |||||||
| v/v% min | 1 | 4 | 7 | 1 | 4 | 7 | 1 | 4 | 7 |
| 1 | 168.1 ± 3.0 | 168.3 ± 2.6 | 164.1 ± 2.1 | 0.0 ± 0.0 | 168.0 ± 1.6 | 169.0 ± 2.5 | 0.0 ± 0.0 | 165.7 ± 2.6 | 167.6 ± 2.4 |
| 4 | 168.6 ± 0.4 | 166.5 ± 2.1 | 164.8 ± 1.5 | 118.7±32.6 | 167.2 ± 2.1 | 168.8 ± 2.6 | 0.0 ± 0.0 | 165.7 ± 1.4 | 168.2 ± 2.0 |
| 8 | 166.1 ± 1.6 | 166.9 ± 1.3 | 163.5 ± 2.2 | 154.6±10.4 | 167.1 ± 2.7 | 168.9 ± 3.5 | 105.3 ± 48.7 | 166.7 ± 3.2 | 168.4 ± 1.2 |
| Shedding Angle (°) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 177D | 314D | 475D | ||||||||
| min | 1 | 4 | 7 | 1 | 4 | 7 | 1 | 4 | 7 | |
| v/v% | ||||||||||
| 1 | 8.8 ± 0.8 | 8.5 ± 0.0 | 10.2 ± 1.8 | 45.0 ± 0.0 | 8.8 ± 0.3 | 9.5 ± 0.0 | 45.0 ± 0.0 | 12.5 ± 0.5 | 11.5 ± 0.3 | |
| 4 | 8.3 ± 0.6 | 10.3 ± 0.3 | 10.5 ± 0.9 | 45.0 ± 0.0 | 9.7 ± 0.8 | 9.4 ± 0.3 | 45.0 ± 0.0 | 11.2 ± 0.3 | 11.7 ± 1.0 | |
| 8 | 11.3 ± 1.2 | 12.7 ± 1.0 | 13.0 ± 1.3 | 17.3 ± 5.9 | 9.3 ± 1.5 | 9.4 ± 0.3 | 45.0 ± 0.0 | 11.8 ± 0.6 | 11.6 ± 0.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.H.; Park, C.H. The Effect of Fiber Type and Yarn Diameter on Superhydrophobicity, Self-Cleaning Property, and Water Spray Resistance. Polymers 2021, 13, 817. https://doi.org/10.3390/polym13050817
Oh JH, Park CH. The Effect of Fiber Type and Yarn Diameter on Superhydrophobicity, Self-Cleaning Property, and Water Spray Resistance. Polymers. 2021; 13(5):817. https://doi.org/10.3390/polym13050817
Chicago/Turabian StyleOh, Ji Hyun, and Chung Hee Park. 2021. "The Effect of Fiber Type and Yarn Diameter on Superhydrophobicity, Self-Cleaning Property, and Water Spray Resistance" Polymers 13, no. 5: 817. https://doi.org/10.3390/polym13050817
APA StyleOh, J. H., & Park, C. H. (2021). The Effect of Fiber Type and Yarn Diameter on Superhydrophobicity, Self-Cleaning Property, and Water Spray Resistance. Polymers, 13(5), 817. https://doi.org/10.3390/polym13050817