Effect of Neutralizer on Transparency of Nucleating Agent-Containing Polypropylene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
3. Measurements
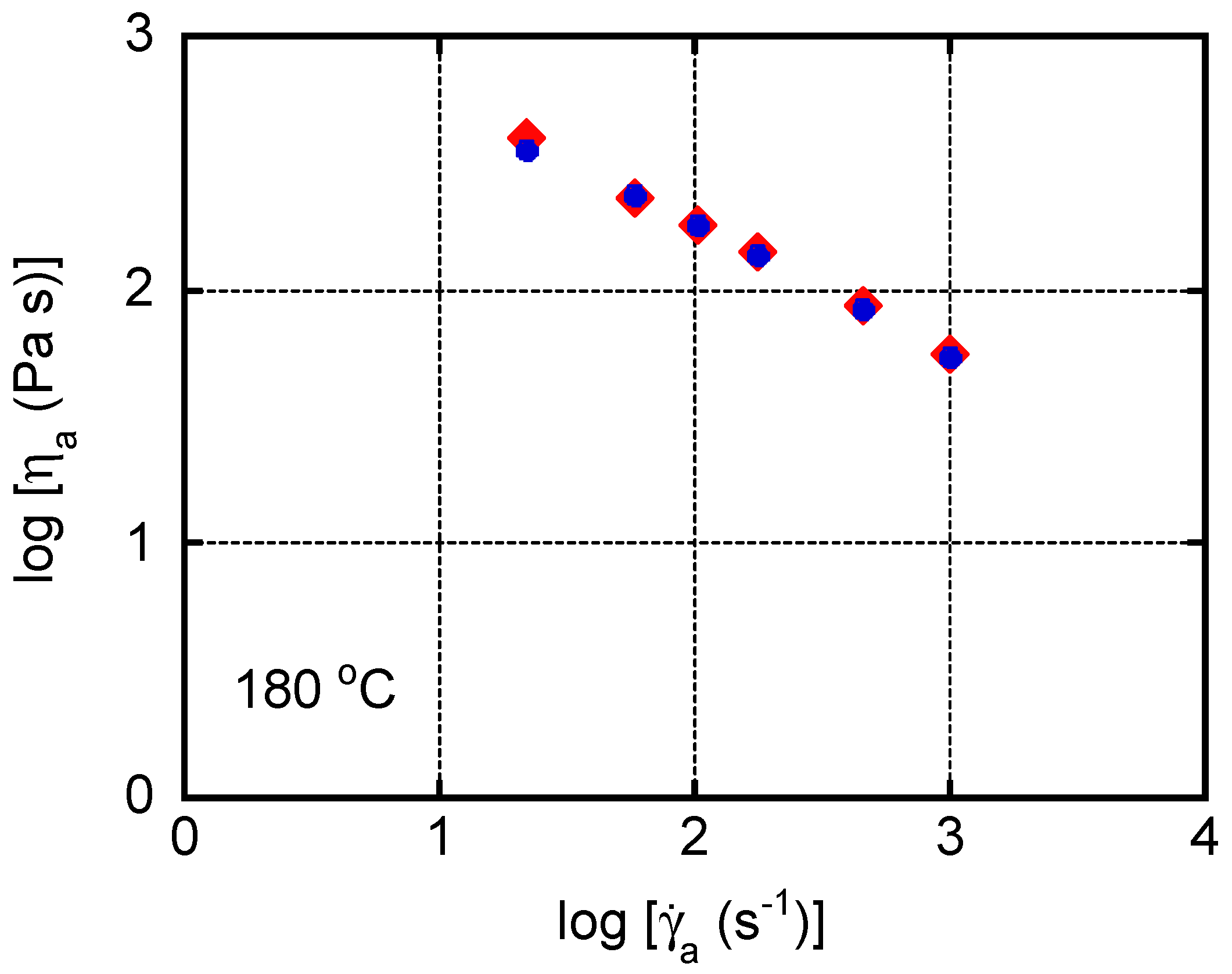
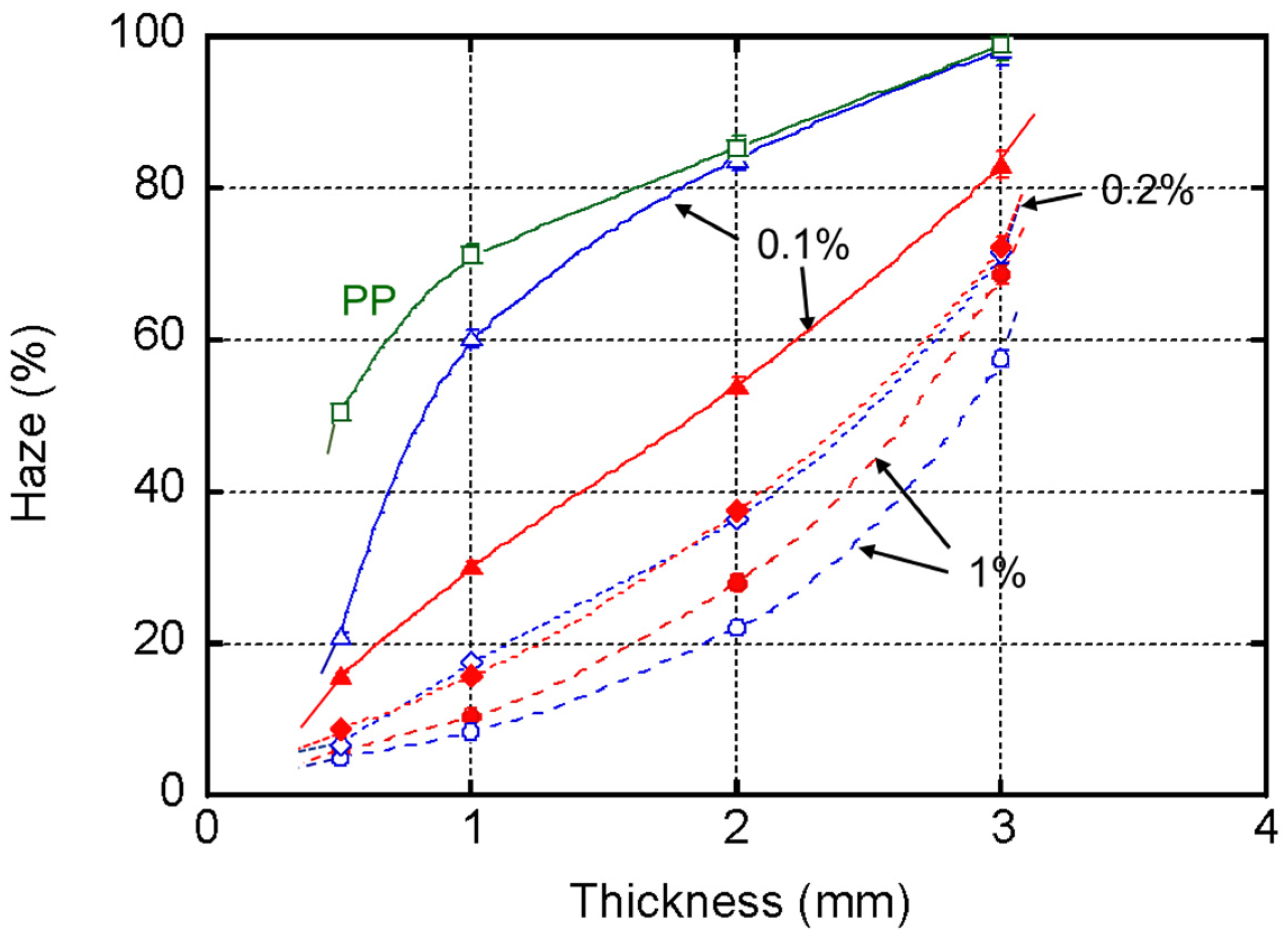
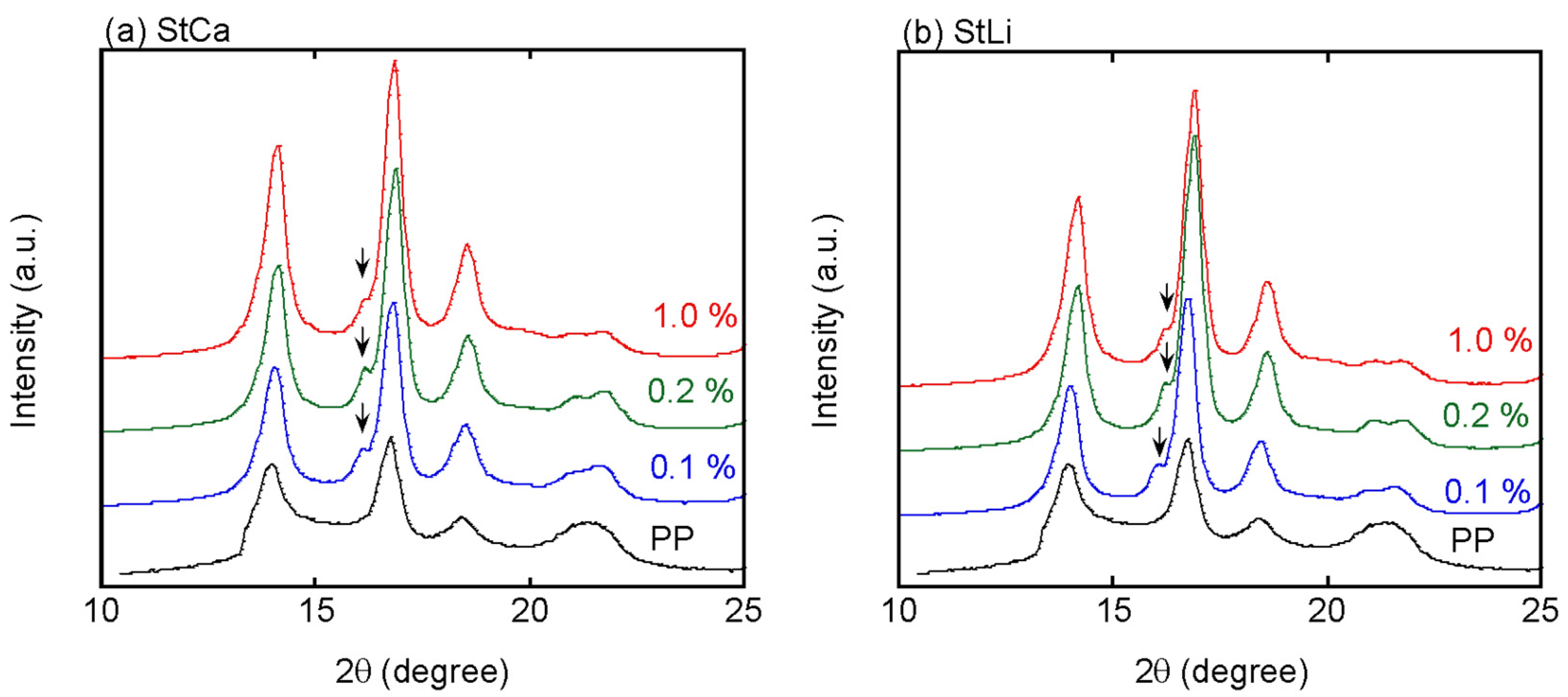
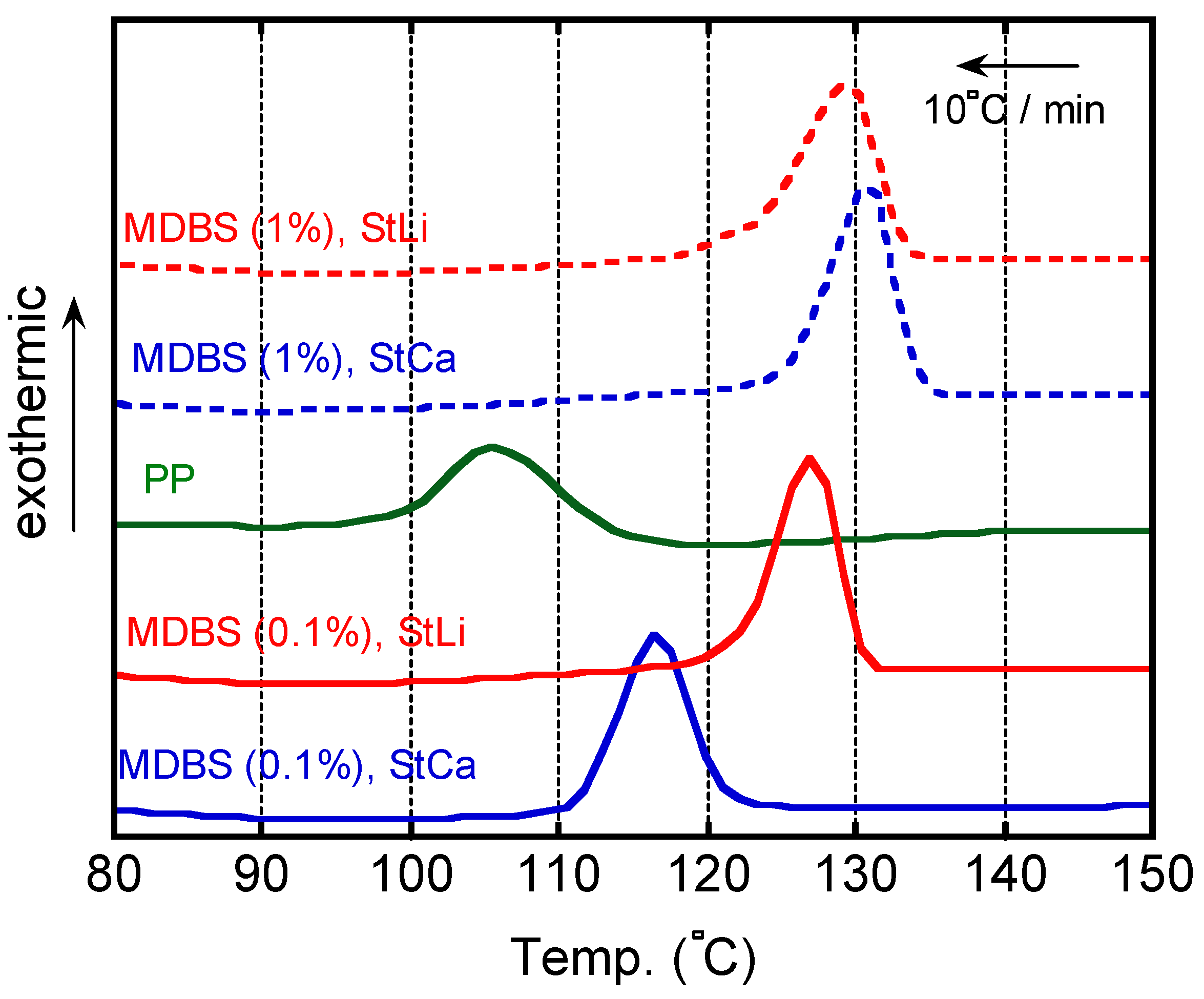
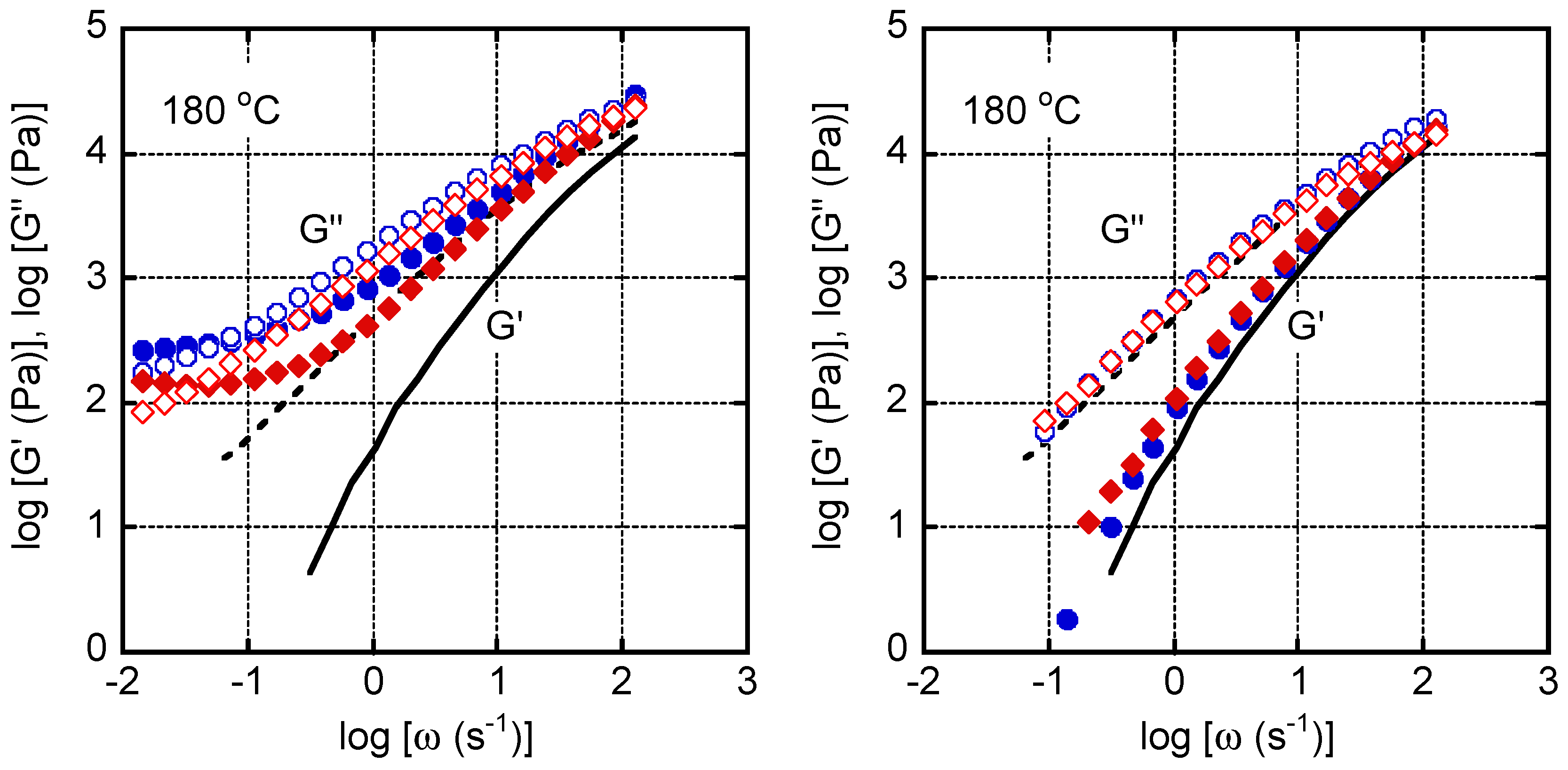
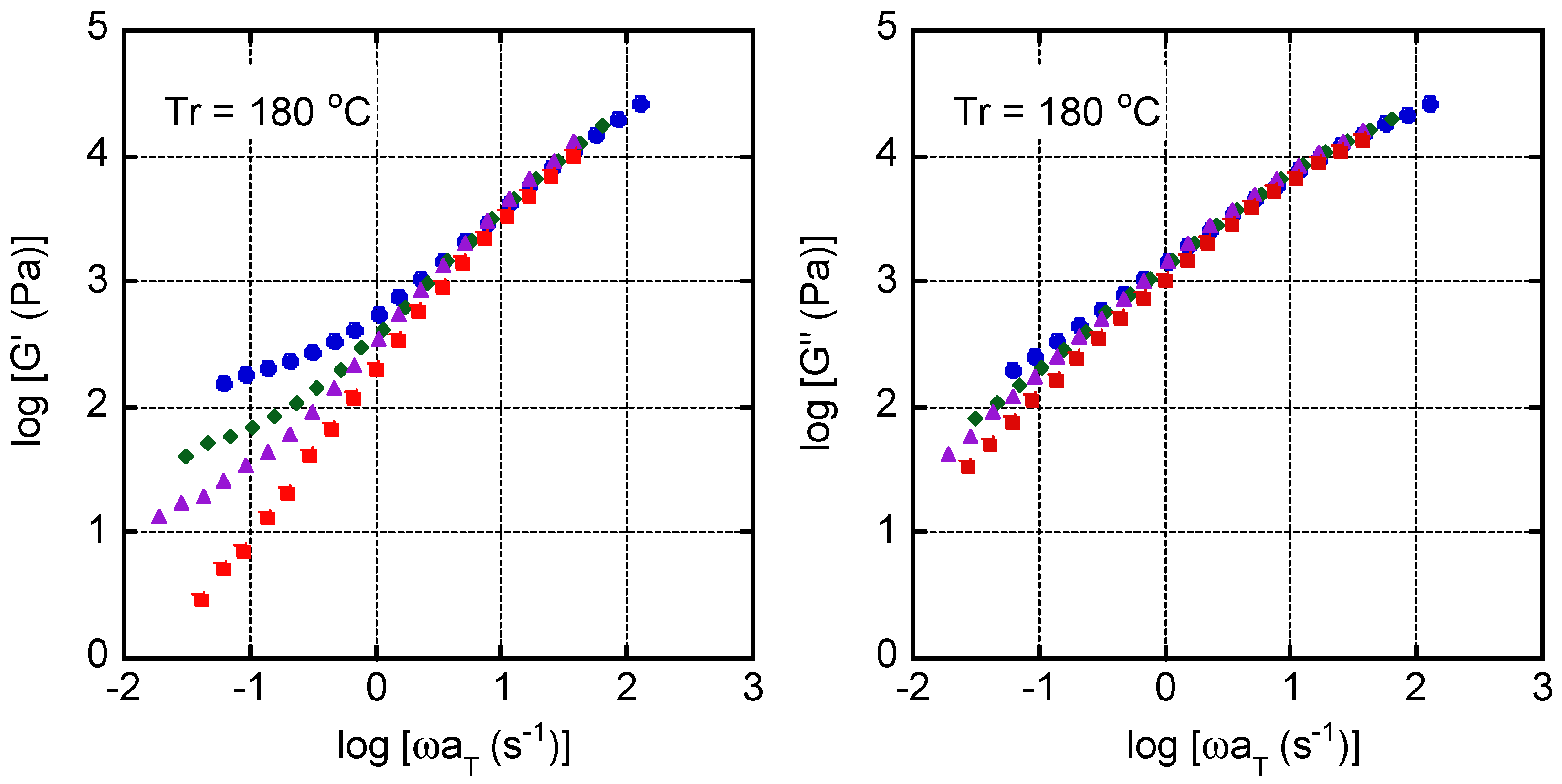
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blomenhofer, M.; Ganzleben, S.; Hanft, D.; Schmidt, H.W.; Kristiansen, M.; Smith, P.; Stoll, K.; Mäder, D.; Hoffmann, K. “Designer” nucleating agents for polypropylene. Macromolecules 2005, 38, 3688–3695. [Google Scholar] [CrossRef]
- Marco, C.; Ellis, G.; Gómez, M.A.; Arribas, J.M. Comparative study of the nucleation activity of third-generation sorbitol-based nucleating agents for isotactic polypropylene. J. Appl. Polym. Sci. 2002, 84, 2440–2450. [Google Scholar] [CrossRef]
- Libster, D.; Aserin, A.; Garti, N. Advanced nucleating agents for polypropylene. Polym. Adv. Technol. 2007, 18, 685–695. [Google Scholar] [CrossRef]
- Mileva, D.; Androsch, R.; Radusch, H.J. Effect of cooling rate on melt-crystallization of random propylene-ethylene and propylene-1-butene copolymers. Polym. Bull. 2008, 61, 643–654. [Google Scholar] [CrossRef]
- Stolte, I.; Cavallo, D.; Alfonso, G.C.; Portale, G.; Drongelen, M.V.; Androsch, R. Form I′ crystal formation in random butene-1/propylene copolymers as revealed by real-time X-ray scattering using synchrotron radiation and fast scanning chip calorimetry. Eur. Polym. J. 2014, 60, 22–32. [Google Scholar] [CrossRef]
- Bartczaka, Z.; Chionob, V.; Pracellab, M. Blends of propylene-ethylene and propylene-1-butene random copolymers: I. Morphology and structure. Polymer 2004, 45, 7549–7561. [Google Scholar] [CrossRef]
- Pawlak, A.; Piorkowska, E. Crystallization of isotactic polypropylene in a temperature gradient. Colloid Polym. Sci. 2001, 279, 939–946. [Google Scholar] [CrossRef]
- Lamberti, G. Isotactic polypropylene crystallization: Analysis and modeling. Eur. Polym. J. 2011, 47, 1097–1112. [Google Scholar] [CrossRef]
- Díez, F.J.; Alvarińo, C.; López, J.; Ramírez, C.; Abad, M.J.; Cano, J.; Garabal, G.S.; Barral, L. Influence of the stretching in the crystallinity of biaxially oriented polypropylene (BOPP) films. J. Therm. Anal. Calorim. 2005, 81, 21–25. [Google Scholar] [CrossRef]
- Sadeghi, F.; Carreau, P.J. Properties of uniaxially stretched polypropylene films. J. Chem. Eng. 2008, 86, 1103–1110. [Google Scholar] [CrossRef]
- Smith, T.L.; Masilamani, D.; Bui, L.K.; Khanna, Y.P.; Bray, R.G.; Hammond, W.B.; Curran, S.; Belles, J.J., Jr.; Binder-Castelli, S. The mechanism of action of sugar acetals as nucleating agents for polypropylene. Macromolecules 1994, 27, 3147–3155. [Google Scholar] [CrossRef]
- Nagasawa, S.; Fujimori, A.; Masuko, T.; Iguchi, M. Crystallisation of polypropylene containing nucleators. Polymer 2005, 46, 5241–5250. [Google Scholar] [CrossRef]
- Kristiansen, M.; Tervoort, T.; Smith, P.; Goossens, H. Polypropylene: Influence of additive concentration on polymer structure and yield behavior. Macromolecules 2005, 38, 10461–10465. [Google Scholar] [CrossRef]
- Iwasaki, S.; Inoue, M.; Takei, Y.; Nishikawa, R.; Yamaguchi, M. Modulus enhancement of polypropylene by sorbitol nucleating agent in flow field. Polym. Cryst. 2020. [Google Scholar] [CrossRef]
- Shepard, T.A.; Delsorbo, C.R.; Louth, R.M.; Walborn, J.L.; Norman, D.A.; Harvey, N.G.; Spontakj, R.J. Self-organization and polyolefin nucleation efficacy of 1,3:2,4-di-p-methylbenzylidene sorbitol. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 2617–2628. [Google Scholar] [CrossRef]
- Kobayashi, T.; Takenaka, M.; Saijo, K.; Hashimoto, T. Self-assembly and morphology of gel networks in l,3:2,4-bis-o-(p-methylbenzylidene)-D-sorbitol/n-dibutylphthalate. J. Colloid Interface Sci. 2003, 262, 456–465. [Google Scholar] [CrossRef]
- Kristiansen, M.; Werner, M.; Tervoort, T.; Smith, P.; Blomenhofer, M.; Schmidt, H. The binary system isotactic polypropylene/bis(3,4-dimethylbenzylidene)sorbitol: Phase behavior, nucleation, and optical properties. Macromolecules 2003, 36, 5150–5156. [Google Scholar] [CrossRef]
- Kobayashi, T.; Hashimoto, T. Development of self-assembling nucleators for highly transparent semi-crystalline polypropylene. Bull. Chem. Soc. Jpn. 2005, 78, 218–235. [Google Scholar] [CrossRef]
- Nogales, A.; Mitchell, G.R.; Vaughan, A.S. Anisotropic crystallization in polypropylene induced by deformation of a nucleating agent network. Macromolecules 2003, 36, 4898–4906. [Google Scholar] [CrossRef]
- Balzano, L.; Portale, G.; Peters, G.W.M.; Rastogi, S. Thermoreversible DMDBS phase separation in iPP: The effects of flow on the morphology. Macromolecules 2008, 41, 5350–5355. [Google Scholar] [CrossRef]
- Zhou, J.; Xin, Z. Relationship between molecular structure and nucleation of benzylidene acetals in isotactic polypropylene. Polym. Compos. 2012, 33, 371–378. [Google Scholar] [CrossRef]
- Bauer, T.; Thomann, R.; Mülhaupt, R. Two-component gelators and nucleating agents for polypropylene based upon supramolecular assembly. Macromolecules 1998, 31, 7651–7658. [Google Scholar] [CrossRef]
- Martin, P.; Vaughan, A.S.; Sutton, S.J.; Swingler, S.G. Crystallization behavior of a propylene/ethylene copolymer: Nucleation and a clarifying additive. J. Polym. Sci. B Polym. Phys. 2002, 40, 2178–2189. [Google Scholar] [CrossRef]
- Zhao, Y.; Vaughan, A.S.; Sutton, S.J.; Swingler, S.G. On the crystallization, morphology and physical properties of a clarified propylene/ethylene copolymer. Polymer 2001, 42, 6587–6597. [Google Scholar] [CrossRef]
- Tenma, M.; Yamaguchi, M. Structure and properties of injection-molded polypropylene with sorbitol-based clarifier. Polym. Eng. Sci. 2007, 47, 1441–1446. [Google Scholar] [CrossRef]
- Seemork, J.; Siriprumpoonthum, M.; Lee, Y.; Nobukawa, S.; Yamaguchi, M. Effect of die geometry on drawdown force of polypropylene at capillary Extrusion. Adv. Polym. Technol. 2015, 34, 21477. [Google Scholar] [CrossRef]
- Phulkerd, P.; Nakabayashi, T.; Iwasaki, S.; Yamaguchi, M. Effect of carbon nanotube addition on molecular orientation of polyethylene. J. Appl. Polym. Sci. 2019, 136, 47295. [Google Scholar] [CrossRef]
- Bernland, K.; Goossens, J.G.P.; Smith, P.; Tervoort, T.A. On clarification of haze in polypropylene. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 865–874. [Google Scholar] [CrossRef]
- Takayanagi, M.; Imada, K.; Kajiyama, T. Mechanical properties and fine structure of drawn polymers. J. Polym. Sci. C Polym. Symp. 1967, 15, 263–281. [Google Scholar] [CrossRef]
- Keane, J.J.; Stein, R.S. The scattering of light from thin polymer films. II. Scattering from polyethylene. J. Polym. Sci. 1956, 20, 327–350. [Google Scholar] [CrossRef]
- Norris, F.H.; Stein, R.S. The scattering of light from thin polymer films. IV. Scattering from oriented polymers. J. Polym. Sci. 1958, 22, 87–114. [Google Scholar] [CrossRef]
- Schawe, J.E.K.; Budde, F.; Alig, I. Nucleation activity at high supercooling: Sorbitol-type nucleating agents in polypropylene. Polymer 2018, 153, 587–596. [Google Scholar] [CrossRef]
- Schawe, J.E.K.; Budde, F.; Alig, I. Non-isothermal crystallization of polypropylene with sorbitol-type nucleating agents at cooling rates used in processing. Polym. Int. 2019, 68, 240–247. [Google Scholar] [CrossRef]
- Bernland, K.; Tervoort, T.; Smith, P. Phase behavior and optical- and mechanical properties of the binary system isotactic polypropylene and the nucleating/clarifying agent 1,2,3-trideoxy-4,6:5,7-bis-o-[(4-propylphenyl) methylene]-nonitol. Polymer 2009, 50, 2460–2464. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Ueda, T.; Yamanaka, K.; Kawaguchi, A.; Tobita, E.; Haruna, T. Epitaxial act of sodium 2,2′-methylene-bis-(4,6-di-t-butylphenylene)phosphate on isotactic polypropylene. Polymer 2001, 42, 9627–9631. [Google Scholar] [CrossRef]
- Horváth, Z.; Gyarmati, B.; Menyhárd, A.; Doshev, P.; Gahleitner, M.; Varga, J.; Pukánszky, B. The role of solubility and critical temperatures for the efficiency of sorbitol clarifiers in polypropylene. RSC Adv. 2014, 4, 19737–19745. [Google Scholar] [CrossRef]
- Katsuno, S.; Yoshinaga, M.; Kitade, S.; Sanada, Y.; Akiba, I.; Sakurai, K.; Masunaga, H. Crystallization kinetics of polypropylene containing a sorbitol nucleating agent. Polym. J. 2013, 45, 87–93. [Google Scholar] [CrossRef]
- Shen, H.; Niu, L.; Fan, K.; Li, J.; Guan, X.; Song, J. Application of Solubility Parameters in 1,3:2,4-Bis(3,4-dimethylbenzylidene)sorbitol organogel in binary organic mixtures. Langmuir 2014, 30, 9176–9182. [Google Scholar] [CrossRef]
- Nasr, P.; Corradini, M.G.; Hill, J.; Read, S.T.; Rosendahl, S.M.; Weiss, R.G.; Auzanneau, F.I.; Rogers, M.A. Hansen solubility parameters clarify the role of the primary and secondary hydroxyl groups on the remarkable self-assembly of 1:3,2:4-dibenzylidene sorbitol. J. Phys. Chem. C 2020, 124, 26455–26466. [Google Scholar] [CrossRef]
- Roy, S.; Sciont, V.; Jan, S.C.; Wesdemiotis, C.; Pischera, A.M.; Espe, M.P. Sorbitol-POSS interactions on development of isotactic polypropylene composites. Macromolecules 2011, 44, 8064–8079. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Dutkiewicz, M.; Sterzyński, T.; Laura, M.; Lorenzo, D. Isotactic polypropylene modified with sorbitol-based derivative and siloxane-silsesquioxane resin. Eur. Polym. J. 2016, 85, 62–71. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Miyata, H.; Tan, V.; Gogos, C.G. Relation between molecular structure and flow instability for ethylene/α-olefin copolymers. Polymer 2002, 43, 5249–5255. [Google Scholar] [CrossRef]
- Ahn, S.; White, J.L. Die Wall Effects, Contact angles and slippage in the flow of polymer melts and polymer-particulate compounds with carboxylic acids in a slit die. Intern. Polym. Process. 2004, 19, 21–26. [Google Scholar] [CrossRef]
- Samuels, R. Morphology of deformed polypropylene. Quantitative relations by combined X-ray, optical, and sonic methods. J. Polym. Sci. 1965, A3, 1741. [Google Scholar] [CrossRef]
- Masuko, T.; Tanaka, H.; Okajima, S. Studies on biaxial stretching of polypropylene film. IV. Overall orientation during one-step biaxial stretching and its application to the estimation of the principal refractive indices of isotactic polypropylene. J. Polym. Sci. A-2 1970, 8, 1565–1574. [Google Scholar] [CrossRef]
- Russo, R.; Vittoria, V. Determination of intrinsic birefringence of smectic phase in isotactic polypropylene. J. Appl. Polym. Sci. 1996, 60, 955–961. [Google Scholar] [CrossRef]
- Uchiyama, Y.; Iwasaki, S.; Ueoka, C.; Fukui, T.; Okamoto, K.; Yamaguchi, M. Molecular orientation and mechanical anisotropy of polypropylene sheet containing N,N′-dicyclohexyl-2,6-naphthalenedicarboxamide. J. Polym. Sci. B Polym. Phys. 2009, 47, 424–434. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Suzuki, K.; Miyata, H. Structure and mechanical properties for binary blends of i-PP with ethylene-1-hexene copolymers. J. Polym. Sci. B Polym. Phys. 1999, 37, 701–713. [Google Scholar] [CrossRef]
- Tenma, M.; Mieda, N.; Takamatsu, S.; Yamaguchi, M. Structure and properties for transparent polypropylene containing sorbitol-based clarifier. J. Polym. Sci. B Polym. Phys. 2008, 46, 41–47. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Miyata, H. Influence of stereoregularity of polypropylene on miscibility with ethylene-1-hexene copolymer. Macromolecules 1999, 32, 5911–5916. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Wagner, M.H. Impact of processing history on rheological properties for branched polypropylene. Polymer 2006, 47, 3629–3635. [Google Scholar] [CrossRef]
- Scheirs, J. Modern Polyesters; Scheirs, J., Long, T.E., Eds.; Wiley: New York, NY, USA, 2005; pp. 495–540. [Google Scholar]
- Tsugawa, N.; Yamaguchi, M. Effect of lithium salt addition on the structure and optical properties of PMMA/PVB blends. Polymer 2018, 146, 242–248. [Google Scholar] [CrossRef]
- Saari, R.A.; Maeno, R.; Tsuyuguchi, R.; Marujiwat, W.; Phukerd, P.; Yamaguchi, M. Impact of lithium halides on rheological properties of aqueous solution of poly(vinyl alcohol). J. Polym. Res. 2020, 27, 218. [Google Scholar] [CrossRef]
- Saari, R.A.; Maeno, R.; Marujiwat, W.; Nasri, M.S.; Matsumura, K.; Yamaguchi, M. Modification of poly(vinyl alcohol) fibers with lithium bromide. Polymer 2021, 213, 123193. [Google Scholar] [CrossRef]
- Saari, R.A.; Nasri, M.S.; Marujiwat, W.; Maeno, R.; Yamaguchi, M. Application of Hofmeister series to the structure and properties of poly(vinyl alcohol) films containing metal salt. Polym. J. 2021. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, S.; Uchiyama, Y.; Tenma, M.; Yamaguchi, M. Effect of Neutralizer on Transparency of Nucleating Agent-Containing Polypropylene. Polymers 2021, 13, 680. https://doi.org/10.3390/polym13050680
Iwasaki S, Uchiyama Y, Tenma M, Yamaguchi M. Effect of Neutralizer on Transparency of Nucleating Agent-Containing Polypropylene. Polymers. 2021; 13(5):680. https://doi.org/10.3390/polym13050680
Chicago/Turabian StyleIwasaki, Shohei, Yohei Uchiyama, Miwa Tenma, and Masayuki Yamaguchi. 2021. "Effect of Neutralizer on Transparency of Nucleating Agent-Containing Polypropylene" Polymers 13, no. 5: 680. https://doi.org/10.3390/polym13050680
APA StyleIwasaki, S., Uchiyama, Y., Tenma, M., & Yamaguchi, M. (2021). Effect of Neutralizer on Transparency of Nucleating Agent-Containing Polypropylene. Polymers, 13(5), 680. https://doi.org/10.3390/polym13050680




