Application of Fluids in Supercritical Conditions in the Polymer Industry
Abstract
1. Introduction
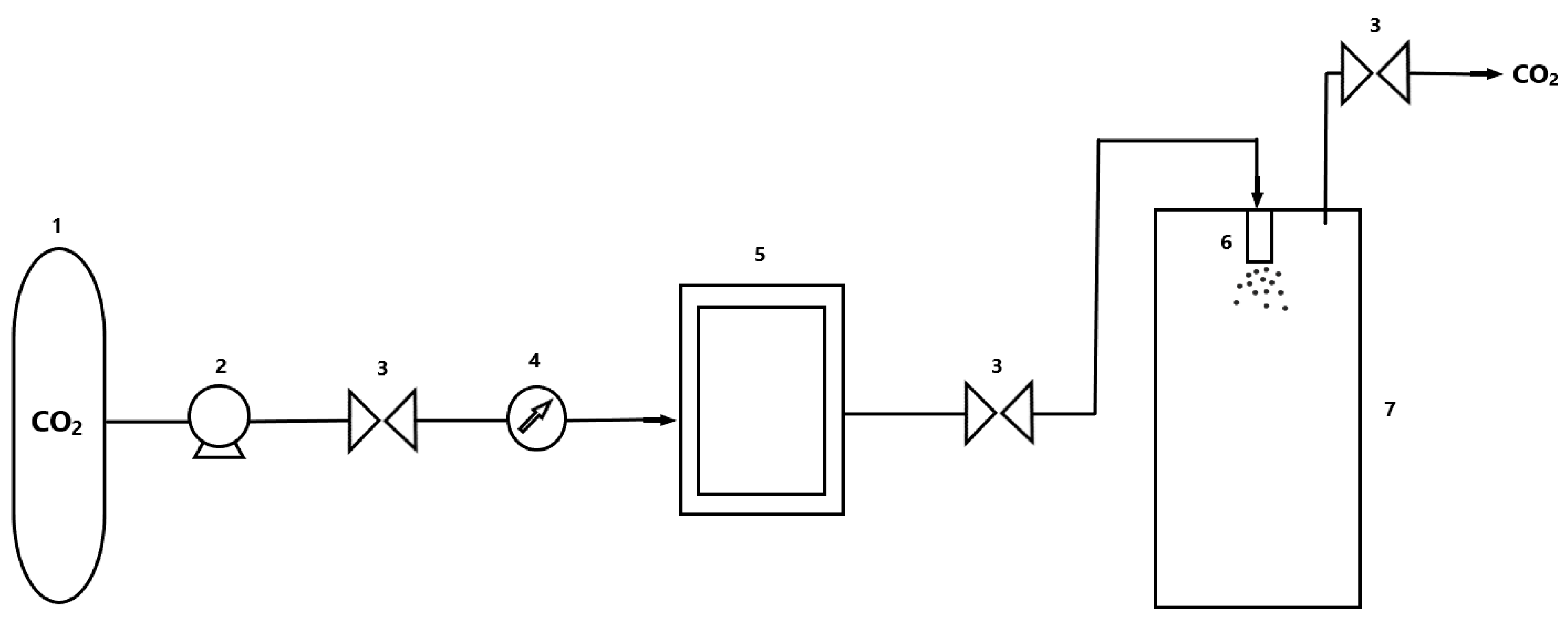
2. Supercritical Fluid Extraction (SFE)
- Operation at low temperatures, thanks to which chemical compounds that are not thermally resistant are not degraded.
- Use of nontoxic solvents, which allows for a more ecological approach to technological processes.
- The ability to selectively regulate solubility through changes in pressure and temperature increases the selectivity of a chemical reaction.
- Complete separation of the solvent from the extract reduces the contamination of products with them, as a result of which it is possible to make products intended for direct contact with humans.
- The possibility of recirculation of the solvent in the system, which reduces the costs of the process and lowers the process costs
- Fractionation of the compounds obtained by extraction during their isolation, so it is possible to obtain only the desired product.
- The extraction is carried out under anaerobic conditions, which prevents the oxidation of valuable natural substances.
- As for disadvantages, we can mention:
- A high cost of building the installation due to the need to withstand very high pressure.
- Considerable energy expenditure for solvent compression and heating.
- Incomplete knowledge of the SFE process and frequent empirical determinations, which results from previous low interest in this technology and the presence of a small number of research installations.
3. Particle Formation, Micronization and Encapsulation
- The emulsion method, consisting of obtaining an emulsion of immiscible liquids containing substances forming a shell and a core and then removing the solvent.
- Spray drying, which is based on dissolving the shell in a solvent and then dissolving, suspending or emulsifying the filling substance into this solution. The next stage is spraying the liquid through atomizers or nozzles, which causes the solvent to evaporate and the active substance to be deposited on the shell.
- Extrusion, which is used primarily for the production of microcapsules of oils in a carbohydrate matrix. Included here are three techniques: melt injection, melt extrusion and centrifugal extrusion.
- Coacervation, consisting of separating the phases in a solution of colloids or polymers and creating at least two liquid phases. The course of the coacervation process begins with phase separation in the polymer solution under the influence of temperature, pH or the addition of salt or an incompatible polymer. In this way, coacervate droplets are produced, which are adsorbed onto the surface of the active substance, thus forming the capsule shell. Adjusting the concentration of added salt, viscosity and molecular weight of the polymer allows controlling the size of the microcapsules obtained
- Polymerization in situ, which is based on the simultaneous occurrence of the shell polymerization process and the surrounding of the active substance with the produced polymer. In situ polymerization takes place without the addition of reactive agents. This process often produces capsules based on a melamine-formaldehyde film formed by the reaction of melamine with formaldehyde on the surface of an oil droplet. These casings are characterized by high strength and stability. The process of producing microcapsules by the in situ method consists of the production of melamine-formaldehyde precondensate and its prepolymerization, followed by adding oil to the solution and its emulsification. Subsequently, the temperature of the emulsion is increased, which causes the prepolymer to polymerize and form a microcapsule shell around the active ingredient.
- Lyophilization, where substances subjected to lyophilization, such as oils, are dissolved in water, and then, by reducing the pressure, the water is removed from the system, passing directly to the gaseous state. This method retains the maximum amount of volatile compounds.
3.1. Rapid Expansion of Supercritical Solution (RESS)
3.2. Supercritical Antisolvent (SAS)
3.3. Aerosol Solvent Extraction System (ASES)
3.4. Particles from Gas-Saturated Solution (PGSS)
3.5. Electrospraying (ESPR)
4. Impregnation and Plasticization
5. Other Processes Using Supercritical Fluids in the Polymer Industry
5.1. Foaming
5.2. Polymerization
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakier, S.; Bajko, E. The effects of supercritical carbon dioxide treatment on bee pollen. Postępy Tech. Przetwórstwa Spoz. 2017, 1, 66–70. [Google Scholar]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Janiszewska, E.; Witrowa-Rajchert, D. Ekstrakcja nadkrytyczna w przemyśle spożywczym. Żywność Nauk. Technol. Jakość 2005, 4, 5–16. [Google Scholar]
- Singh, P.P.; Saldaña, M.D.A. Subcritical water extraction of phenolic compounds from potato peel. Food Res. Int. 2011, 44, 2452–2458. [Google Scholar] [CrossRef]
- Glišić, S.B.; Mišić, D.R.; Stamenić, M.D.; Zizovic, I.T.; Ašanin, R.M.; Skala, D.U. Supercritical carbon dioxide extraction of carrot fruit essential oil: Chemical composition and antimicrobial activity. Food Chem. 2007, 105, 346–352. [Google Scholar] [CrossRef]
- Pereira, C.G.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Pereira, C.G.; Nunes, S.B.; Padilha, F.F.; Umsza-Guez, M.A. Supercritical Fluid Extraction Using CO2: Main Applications and Future Perspectives. Sep. Sci. Technol. 2013, 48, 2741–2760. [Google Scholar] [CrossRef]
- Anekpankul, T.; Goto, M.; Sasaki, M.; Pavasant, P.; Shotipruk, A. Extraction of anti-cancer damnacanthal from roots of Morinda citrifolia by subcritical water. Sep. Purif. Technol. 2007, 55, 343–349. [Google Scholar] [CrossRef]
- McHugh, M.; Krukonis, V. Supercritical Fluid Extraction; Butterworth-Heinemann: Boston, MA, USA, 1994. [Google Scholar]
- Tabernero, A.; Martín del Valle, E.M.; Galán, M.A. Supercritical fluids for pharmaceutical particle engineering: Methods, basic fundamentals and modelling. Chem. Eng. Process. Process Intensif. 2012, 60, 9–25. [Google Scholar] [CrossRef]
- Shen, J.; Domański, K.B.; Kitao, O.; Nakanishi, K.; Carlo, M. Computer Simulation on Supercritical Carbon Dioxide Fluid. A potential model for the benzene-carbon dioxide system from ab initio calculations. Fluid Phase Equilib. 1995, 104, 375–390. [Google Scholar] [CrossRef]
- Olsen, S.A.; Tallman, D.E. Voltammetry of Ferrocene in Subcritical and Supercritical Chlorodifluoromethane. Anal. Chem. 1994, 66, 503–509. [Google Scholar] [CrossRef]
- Brunner, G.; Peter, S. On the Solubility of Glycerides and Fatty Acids in Compressed Gases in the Presence of an Entrainer. Sep. Sci. Technol. 1982, 17, 199–214. [Google Scholar] [CrossRef]
- Shende, R.V.; Lombardo, S.J. Supercritical extraction with carbon dioxide and ethylene of poly(vinyl butyral) and dioctyl phthalate from multilayer ceramic capacitors. J. Supercrit. Fluids 2002, 23, 153–162. [Google Scholar] [CrossRef]
- Hamdan, S.; Daood, H.G.; Toth-Markus, M.; Illés, V. Extraction of cardamom oil by supercritical carbon dioxide and sub-critical propane. J. Supercrit. Fluids 2008, 44, 25–30. [Google Scholar] [CrossRef]
- Cansell, F.; Rey, S.; Beslin, P. Thermodynamic aspects of supercritical fluids processing: Applications to polymers and wastes treatment. Rev. L’institute Fr. Du Pet. 1998, 53, 71–98. [Google Scholar] [CrossRef]
- Aymonier, C.; Philippot, G.; Erriguible, A.; Marre, S. Playing with chemistry in supercritical solvents and the associated technologies for advanced materials by design. J. Supercrit. Fluids 2018, 134, 184–196. [Google Scholar] [CrossRef]
- Cooper, A.I. Polymer synthesis and processing using supercritical carbon dioxide. J. Mater. Chem. 2000, 10, 207–234. [Google Scholar] [CrossRef]
- Michels, A.; Kleerekoper, L. Measurements on the dielectric constant of CO2 at 25°, 50° and 100 °C up to 1700 atmospheres. Physica 1939, 6, 586–590. [Google Scholar] [CrossRef]
- Peper, S.; Fonseca, J.M.S.; Dohrn, R. High-pressure fluid-phase equilibria: Trends, recent developments, and systems investigated (2009–2012). Fluid Phase Equilib. 2019, 484, 126–224. [Google Scholar] [CrossRef]
- Sovovà, H.; Stateva, R.P. Supercritical fluid extraction from vegetable materials. Rev. Chem. Eng. 2011, 27, 79–156. [Google Scholar] [CrossRef]
- Da Porto, C.; Voinovich, D.; Decorti, D.; Natolino, A. Response surface optimization of hemp seed (Cannabis sativa L.) oil yield and oxidation stability by supercritical carbon dioxide extraction. J. Supercrit. Fluids 2012, 68, 45–51. [Google Scholar] [CrossRef]
- Capuzzo, A.; Maffei, M.E.; Occhipinti, A. Supercritical fluid extraction of plant flavors and fragrances. Molecules 2013, 18, 7194–7238. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K. An innovative technology of algal extracts. Przem. Chem. 2014, 4, 590–592. [Google Scholar] [CrossRef]
- Ben Said, A.; Guinot, C.; Ruiz, J.C.; Grandjean, A.; Dole, P.; Joly, C.; Chalamet, Y. Supercritical CO2 extraction of contaminants from polypropylene intended for food contact: Effects of contaminant molecular structure and processing parameters. J. Supercrit. Fluids 2016, 110, 22–31. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Fathi, A.; Perego, P.; Dehghani, F. Extraction of antioxidants from winery wastes using subcritical water. J. Supercrit. Fluids 2012, 65, 18–24. [Google Scholar] [CrossRef]
- Ben Said, A.; Guinot, C.; Ruiz, J.C.; Grandjean, A.; Dole, P.; Joly, C.; Chalamet, Y. Modeling of supercritical CO2 extraction of contaminants from post-consumer polypropylene: Solubilities and diffusion coefficients in swollen polymer at varying pressure and temperature conditions. Chem. Eng. Res. Des. 2017, 117, 95–109. [Google Scholar] [CrossRef]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. Trac Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Hedrick, J.L.; Mulcahey, L.J.; Taylor, L.T. Mikrochimica Acta Supercritical Fluid Extraction. Mikrochim. Acta 1992, 108, 115–132. [Google Scholar] [CrossRef]
- 30. Małgorzata DJAS.; Marek HENCZKA Ekstrakcja kwasów karboksylowych z zastosowaniem ditlenku wegla w stanie nadkrytycznym. Inżynieria I Apar. Chem. 2015, 54, 314–315. [CrossRef]
- Dias, A.M.A.; Rey-Rico, A.; Oliveira, R.A.; Marceneiro, S.; Alvarez-Lorenzo, C.; Concheiro, A.; Júnior, R.N.C.; Braga, M.E.M.; De Sousa, H.C. Wound dressings loaded with an anti-inflammatory jucá (Libidibia ferrea) extract using supercritical carbon dioxide technology. J. Supercrit. Fluids 2013, 74, 34–45. [Google Scholar] [CrossRef]
- Majewska, E.; Bialecka-Florjańczyk, E. Zielona chemia w przemy ś le spo ż ywczym. Chem. Didact. Ecol. Metrol. 2010, 15, 21–27. [Google Scholar]
- Ribeiro, M.A.; Bernardo-Gil, M.G.; Esquíel, M.M. Melissa officinalis, L.: Study of antioxidant activity in supercritical residues. J. Supercrit. Fluids 2001, 21, 51–60. [Google Scholar] [CrossRef]
- Ixtaina, V.Y.; Vega, A.; Nolasco, S.M.; Tomás, M.C.; Gimeno, M.; Bárzana, E.; Tecante, A. Supercritical carbon dioxide extraction of oil from Mexican chia seed (Salvia hispanica L.): Characterization and process optimization. J. Supercrit. Fluids 2010, 55, 192–199. [Google Scholar] [CrossRef]
- Moura, L.S.; Carvalho, R.N.; Stefanini, M.B.; Ming, L.C.; Meireles, M.A.A. Supercritical fluid extraction from fennel (Foeniculum vulgare): Global yield, composition and kinetic data. J. Supercrit. Fluids 2005, 35, 212–219. [Google Scholar] [CrossRef]
- Povh, N.P.; Marques, M.O.M.; Meireles, M.A.A. Supercritical CO2 extraction of essential oil and oleoresin from chamomile (Chamomilla recutita [L.] Rauschert). J. Supercrit. Fluids 2001, 21, 245–256. [Google Scholar] [CrossRef]
- Ruetsch, L.; Daghero, J.; Mattea, M. Supercritical Extraction of Solid Matrices. Model Formulation and Experiments; Latin American Applied Research: Bahía Blanca, Argentina, 2003; Volume 33. [Google Scholar]
- Dąbek, L. Regenracja zużytych węgli aktywnych; Wydawnictwo Politechniki Śląskiej: Kielce, Poland, 2007; pp. 45–49. [Google Scholar]
- Kröber, H.; Teipel, U. Microencapsulation of particles using supercritical carbon dioxide. Chem. Eng. Process. Process Intensif. 2005, 44, 215–219. [Google Scholar] [CrossRef]
- Della Porta, G.; Castaldo, F.; Scognamiglio, M.; Paciello, L.; Parascandola, P.; Reverchon, E. Bacteria microencapsulation in PLGA microdevices by supercritical emulsion extraction. J. Supercrit. Fluids 2012, 63, 1–7. [Google Scholar] [CrossRef]
- Tsai, W.C.; Rizvi, S.S.H. Liposomal microencapsulation using the conventional methods and novel supercritical fluid processes. Trends Food Sci. Technol. 2016, 55, 61–71. [Google Scholar] [CrossRef]
- Han, F.Y.; Whittaker, A.; Howdle, S.M.; Naylor, A.; Shabir-Ahmed, A.; Smith, M.T. Sustained-Release Hydromorphone Microparticles Produced by Supercritical Fluid Polymer Encapsulation. J. Pharm. Sci. 2019, 108, 811–814. [Google Scholar] [CrossRef]
- Lasoń, E.; Ogonowski, J. Kapsułkowanie–metoda immobilizacji materiałów bioaktywnych. Lab. Lab. Apar. Bad. 2010, 15, 29–35. [Google Scholar]
- Lasoń, E.; Ogonowski, J. Kapsułkowanie w przemyśle spożywczym. Lab Lab. Apar. Bad. 2010, 15, 34–40. [Google Scholar]
- Ribeiro Dos Santos, I.; Richard, J.; Pech, B.; Thies, C.; Benoit, J.P. Microencapsulation of protein particles within lipids using a novel supercritical fluid process. Int. J. Pharm. 2002, 242, 69–78. [Google Scholar] [CrossRef]
- Prosapio, V.; Reverchon, E.; De Marco, I. Antisolvent micronization of BSA using supercritical mixtures carbon dioxide + organic solvent. J. Supercrit. Fluids 2014, 94, 189–197. [Google Scholar] [CrossRef]
- Kalogiannis, C.G.; Michailof, C.M.; Panayiotou, C.G. Microencapsulation of amoxicillin in poly(L-lactic acid) by supercritical antisolvent precipitation. Ind. Eng. Chem. Res. 2006, 45, 8738–8743. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E. Carotenoids microencapsulation by spray drying method and supercritical micronization. Food Res. Int. 2017, 99, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Karim, F.T.; Ghafoor, K.; Ferdosh, S.; Al-Juhaimi, F.; Ali, E.; Yunus, K.B.; Hamed, M.H.; Islam, A.; Asif, M.; Zaidul, I.S.M. Microencapsulation of fish oil using supercritical antisolvent process. J. Food Drug Anal. 2017, 25, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Soh, S.H.; Lee, L.Y. Microencapsulation and nanoencapsulation using supercritical fluid (SCF) techniques. Pharmaceutics 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.; Khoshnoudi-Nia, S.; Jafari, S.M. Nano/microencapsulation of anthocyanins; a systematic review and meta-analysis. Food Res. Int. 2020, 132, 109077. [Google Scholar] [CrossRef] [PubMed]
- Petermann, M. Supercritical fluid-assisted sprays for particle generation. J. Supercrit. Fluids 2018, 134, 234–243. [Google Scholar] [CrossRef]
- Knez, Z.; Škerget, M.; Knez Hrnčič, M.; Čuček, D. Particle Formation Using Sub- and Supercritical Fluids; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780444626967. [Google Scholar]
- Wang, Y.; Liu, B.; Wen, X.; Li, M.; Wang, K.; Ni, Y. Quality analysis and microencapsulation of chili seed oil by spray drying with starch sodium octenylsuccinate and maltodextrin. Powder Technol. 2017, 312, 294–298. [Google Scholar] [CrossRef]
- Kujur, A.; Kiran, S.; Dubey, N.K.; Prakash, B. Microencapsulation of Gaultheria procumbens essential oil using chitosan-cinnamic acid microgel: Improvement of antimicrobial activity, stability and mode of action. Lwt Food Sci. Technol. 2017, 86, 132–138. [Google Scholar] [CrossRef]
- Yeo, S.D.; Kiran, E. Formation of polymer particles with supercritical fluids: A review. J. Supercrit. Fluids 2005, 34, 287–308. [Google Scholar] [CrossRef]
- Fernandes, R.V.D.B.; Borges, S.V.; Botrel, D.A. Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydr. Polym. 2014, 101, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N. Production of micro and nano particles of pharmaceutical by supercritical carbon dioxide. J. Supercrit. Fluids 2015, 100, 129–141. [Google Scholar] [CrossRef]
- Almeida, A.P.; Rodríguez-Rojo, S.; Serra, A.T.; Vila-Real, H.; Simplicio, A.L.; Delgadilho, I.; Beirão Da Costa, S.; Beirão Da Costa, L.; Nogueira, I.D.; Duarte, C.M.M. Microencapsulation of oregano essential oil in starch-based materials using supercritical fluid technology. Innov. Food Sci. Emerg. Technol. 2013, 20, 140–145. [Google Scholar] [CrossRef]
- Thote, A.J.; Gupta, R.B. Formation of nanoparticles of a hydrophilic drug using supercritical carbon dioxide and microencapsulation for sustained release. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Chiou, A.H.J.; Cheng, H.C.; Wang, D.P. Micronization and microencapsulation of felodipine by supercritical carbon dioxide. J. Microencapsul. 2006, 23, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Hee, Y.Y.; Tan, C.P.; Rahman, R.A.; Noranizan, M.; Smith, R.L.; Chong, G.H. Production of virgin coconut oil microcapsules from oil-in-water emulsion with supercritical carbon dioxide spray drying. J. Supercrit. Fluids 2017, 130, 118–124. [Google Scholar] [CrossRef]
- Bahrami, M.; Ranjbarian, S. Production of micro- and nano-composite particles by supercritical carbon dioxide. J. Supercrit. Fluids 2007, 40, 263–283. [Google Scholar] [CrossRef]
- Sihvonen, M.; Järvenpää, E.; Hietaniemi, V.; Huopalahti, R. Advances in supercritical carbon dioxide technologies. Trends Food Sci. Technol. 1999, 10, 217–222. [Google Scholar] [CrossRef]
- Mishima, K.; Matsuyama, K.; Tanabe, D.; Yamauchi, S.; Young, T.J.; Johnston, K.P. Microencapsulation of proteins by rapid expansion of supercritical solution with a nonsolvent. Mater. Interfaces Electrochem. Phenom. 2000, 46, 857–865. [Google Scholar] [CrossRef]
- Kim, J.H.; Paxton, T.E.; Tomasko, D.L. Microencapsulation of naproxen using rapid expansion of supercritical solutions. Biotechnol. Prog. 1996, 12, 650–661. [Google Scholar] [CrossRef]
- Vergara-Mendoza, M.D.S.; Ortiz-Estrada, C.H.; González-Martínez, J.; Quezada-Gallo, J.A. Microencapsulation of coenzyme Q 10 in poly(ethylene glycol) and poly(lactic acid) with supercritical carbon dioxide. Ind. Eng. Chem. Res. 2012, 51, 5840–5846. [Google Scholar] [CrossRef]
- Matsuyama, K.; Mishima, K.; Hayashi, K.I.; Matsuyama, H. Microencapsulation of TiO2 nanoparticles with polymer by rapid expansion of supercritical solution. J. Nanoparticle Res. 2003, 5, 87–95. [Google Scholar] [CrossRef]
- Meziani, M.J.; Pathak, P.; Hurezeanu, R.; Thies, M.C.; Enick, R.M.; Sun, Y.P. Supercritical-Fluid Processing Technique for Nanoscale Polymer Particles. Angew. Chem. Int. Ed. 2004, 43, 704–707. [Google Scholar] [CrossRef]
- Sun, Y.; Matsumoto, M.; Kitashima, K.; Haruki, M.; Kihara, S.I.; Takishima, S. Solubility and diffusion coefficient of supercritical-CO2 in polycarbonate and CO2 induced crystallization of polycarbonate. J. Supercrit. Fluids 2014, 95, 35–43. [Google Scholar] [CrossRef]
- Martín, Á.; Pham, H.M.; Kilzer, A.; Kareth, S.; Weidner, E. Micronization of polyethylene glycol by PGSS (Particles from Gas Saturated Solutions)-drying of aqueous solutions. Chem. Eng. Process. Process Intensif. 2010, 49, 1259–1266. [Google Scholar] [CrossRef]
- Pestieau, A.; Krier, F.; Lebrun, P.; Brouwers, A.; Streel, B.; Evrard, B. Optimization of a PGSS (particles from gas saturated solutions) process for a fenofibrate lipid-based solid dispersion formulation. Int. J. Pharm. 2015, 485, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Suttiruengwong, S.; Rolker, J.; Smirnova, I.; Arlt, W.; Seiler, M.; Lüderitz, L.; Pérez De Diego, Y.; Jansens, P.J. Hyperbranched polymers as drug carriers: Microencapsulation and release kinetics. Pharm. Dev. Technol. 2006, 11, 55–70. [Google Scholar] [CrossRef]
- De Paz, E.; Martín, Á.; Cocero, M.J. Formulation of β-carotene with soybean lecithin by PGSS (Particles from Gas Saturated Solutions)-drying. J. Supercrit. Fluids 2012, 72, 125–133. [Google Scholar] [CrossRef]
- Haq, M.; Chun, B.S. Microencapsulation of omega-3 polyunsaturated fatty acids and astaxanthin-rich salmon oil using particles from gas saturated solutions (PGSS) process. Lwt 2018, 92, 523–530. [Google Scholar] [CrossRef]
- Vijayaraghavan, M.; Stolnik, S.; Howdle, S.M.; Illum, L. Suitability of polymer materials for production of pulmonary microparticles using a PGSS supercritical fluid technique: Preparation of microparticles using PEG, fatty acids and physical or chemicals blends of PEG and fatty acids. Int. J. Pharm. 2013, 441, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Baldino, L.; Cardea, S.; Reverchon, E. A supercritical CO2 assisted electrohydrodynamic process used to produce microparticles and microfibers of a model polymer. J. Co2 Util. 2019, 33, 532–540. [Google Scholar] [CrossRef]
- Perrut, M.; Perrut, V. Towards ingredients by combining Supercritical Fluids with other processes. J. Supercrit. Fluids 2018, 134, 214–219. [Google Scholar] [CrossRef]
- De Marco, I.; Riemma, S.; Iannone, R. Life cycle assessment of supercritical impregnation: Starch aerogel + Α-tocopherol tablets. J. Supercrit. Fluids 2019, 143, 305–312. [Google Scholar] [CrossRef]
- García-Casas, I.; Montes, A.; Valor, D.; Pereyra, C.; Martínez de la Ossa, E.J. Impregnation of mesoporous silica with mangiferin using supercritical CO2. J. Supercrit. Fluids 2018, 140, 129–136. [Google Scholar] [CrossRef]
- Löffler, S.; Seyock, S.; Nybom, R.; Jacobson, G.B.; Richter-Dahlfors, A. Electrochemically triggered release of acetylcholine from scCO2 impregnated conductive polymer films evokes intracellular Ca2 + signaling in neurotypic SH-SY5Y cells. J. Control. Release 2016, 243, 283–290. [Google Scholar] [CrossRef]
- Comin, L.M.; Temelli, F.; Saldaña, M.D.A. Impregnation of flax oil in pregelatinized corn starch using supercritical CO2. J. Supercrit. Fluids 2012, 61, 221–228. [Google Scholar] [CrossRef]
- Varona, S.; Rodríguez-Rojo, S.; Martín, Á.; Cocero, M.J.; Duarte, C.M.M. Supercritical impregnation of lavandin (Lavandula hybrida) essential oil in modified starch. J. Supercrit. Fluids 2011, 58, 313–319. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, J.; Qian, S.; Zhu, X.; Takahashi, J. Green-plasticized poly(lactic acid)/nanofibrillated cellulose biocomposites with high strength, good toughness and excellent heat resistance. Compos. Sci. Technol. 2021, 203, 108613. [Google Scholar] [CrossRef]
- Von Schnitzler, J.; Eggers, R. Mass transfer in polymers in a supercritical CO2-atmosphere. J. Supercrit. Fluids 1999, 16, 81–92. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Martirosyan, G.G. Spectroscopy of polymer/drug formulations processed with supercritical fluids: In situ ATR-IR and Raman study of impregnation of ibuprofen into PVP. Int. J. Pharm. 2002, 232, 81–90. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, J.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; de la Ossa, E.J.M. Impregnation of mango leaf extract into a polyester textile using supercritical carbon dioxide. J. Supercrit. Fluids 2017, 128, 208–217. [Google Scholar] [CrossRef]
- Goñi, M.L.; Gañán, N.A.; Barbosa, S.E.; Strumia, M.C.; Martini, R.E. Supercritical CO2-assisted impregnation of LDPE/sepiolite nanocomposite films with insecticidal terpene ketones: Impregnation yield, crystallinity and mechanical properties assessment. J. Supercrit. Fluids 2017, 130, 337–346. [Google Scholar] [CrossRef]
- Marizza, P.; Keller, S.S.; Müllertz, A.; Boisen, A. Polymer-filled microcontainers for oral delivery loaded using supercritical impregnation. J. Control. Release 2014, 173, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.P.; Braga, M.E.M.; Guerra, J.P.; Duarte, A.R.C.; Duarte, C.M.M.; Leite, E.O.B.; Gil, M.H.; de Sousa, H.C. Development of therapeutic contact lenses using a supercritical solvent impregnation method. J. Supercrit. Fluids 2010, 52, 306–316. [Google Scholar] [CrossRef]
- Barros, A.A.; Oliveira, C.; Reis, R.L.; Lima, E.; Duarte, A.R.C. Ketoprofen-eluting biodegradable ureteral stents by CO2 impregnation: In vitro study. Int. J. Pharm. 2015, 495, 651–659. [Google Scholar] [CrossRef]
- Torres, A.; Ilabaca, E.; Rojas, A.; Rodríguez, F.; Galotto, M.J.; Guarda, A.; Villegas, C.; Romero, J. Effect of processing conditions on the physical, chemical and transport properties of polylactic acid films containing thymol incorporated by supercritical impregnation. Eur. Polym. J. 2017, 89, 195–210. [Google Scholar] [CrossRef]
- Üzer, S.; Akman, U.; Hortaçsu, Ö. Polymer swelling and impregnation using supercritical CO2: A model-component study towards producing controlled-release drugs. J. Supercrit. Fluids 2006, 38, 119–128. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.M.; Tassaing, T.; Jérôme, C. Drug loading of polymer implants by supercritical CO2 assisted impregnation: A review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef]
- Hussain, Y.A.; Grant, C.S. Ibuprofen impregnation into submicron polymeric films in supercritical carbon dioxide. J. Supercrit. Fluids 2012, 71, 127–135. [Google Scholar] [CrossRef]
- López-Periago, A.; Argemí, A.; Andanson, J.M.; Fernández, V.; García-González, C.A.; Kazarian, S.G.; Saurina, J.; Domingo, C. Impregnation of a biocompatible polymer aided by supercritical CO2: Evaluation of drug stability and drug-matrix interactions. J. Supercrit. Fluids 2009, 48, 56–63. [Google Scholar] [CrossRef]
- Giufrida, W.M.; Voll, F.A.; Feihrmann, A.C.; Kunita, M.H.; Madureira, E.H.; Guilherme, M.R.; Vedoy, D.R.L.; Cabral, V.F.; Cardozo-Filho, L. Production of microparticles of PHBV polymer impregnated with progesterone by supercritical fluid technology. Can. J. Chem. Eng. 2016, 94, 1336–1341. [Google Scholar] [CrossRef]
- Milovanovic, S.; Stamenic, M.; Markovic, D.; Ivanovic, J.; Zizovic, I. Supercritical impregnation of cellulose acetate with thymol. J. Supercrit. Fluids 2015, 97, 107–115. [Google Scholar] [CrossRef]
- Milovanovic, S.; Stamenic, M.; Markovic, D.; Radetic, M.; Zizovic, I. Solubility of thymol in supercritical carbon dioxide and its impregnation on cotton gauze. J. Supercrit. Fluids 2013, 84, 173–181. [Google Scholar] [CrossRef]
- Rojas, A.; Cerro, D.; Torres, A.; Galotto, M.J.; Guarda, A.; Romero, J. Supercritical impregnation and kinetic release of 2-nonanone in LLDPE films used for active food packaging. J. Supercrit. Fluids 2015, 104, 76–84. [Google Scholar] [CrossRef]
- De Souza, A.C.; Dias, A.M.A.; Sousa, H.C.; Tadini, C.C. Impregnation of cinnamaldehyde into cassava starch biocomposite films using supercritical fluid technology for the development of food active packaging. Carbohydr. Polym. 2014, 102, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.A.; Silva, J.M.; Craveiro, R.; Paiva, A.; Reis, R.L.; Duarte, A.R.C. Green solvents for enhanced impregnation processes in biomedicine. Curr. Opin. Green Sustain. Chem. 2017, 5, 82–87. [Google Scholar] [CrossRef]
- Cabezas, L.I.; Fernández, V.; Mazarro, R.; Gracia, I.; De Lucas, A.; Rodríguez, J.F. Production of biodegradable porous scaffolds impregnated with indomethacin in supercritical CO2. J. Supercrit. Fluids 2012, 63, 155–160. [Google Scholar] [CrossRef]
- Mustapa, A.N.; Martin, A.; Sanz-Moral, L.M.; Rueda, M.; Cocero, M.J. Impregnation of medicinal plant phytochemical compounds into silica and alginate aerogels. J. Supercrit. Fluids 2016, 116, 251–263. [Google Scholar] [CrossRef]
- Braga, M.E.M.; Pato, M.T.V.; Silva, H.S.R.C.; Ferreira, E.I.; Gil, M.H.; Duarte, C.M.M.; de Sousa, H.C. Supercritical solvent impregnation of ophthalmic drugs on chitosan derivatives. J. Supercrit. Fluids 2008, 44, 245–257. [Google Scholar] [CrossRef]
- Goñi, M.L.; Gañán, N.A.; Strumia, M.C.; Martini, R.E. Eugenol-loaded LLDPE films with antioxidant activity by supercritical carbon dioxide impregnation. J. Supercrit. Fluids 2016, 111, 28–35. [Google Scholar] [CrossRef]
- Ngo, T.T.; Liotta, C.L.; Eckert, C.A.; Kazarian, S.G. Supercritical fluid impregnation of different azo-dyes into polymer: In situ UV/Vis spectroscopic study. J. Supercrit. Fluids 2003, 27, 215–221. [Google Scholar] [CrossRef]
- Popov, V.K.; Bagratashvili, V.N.; Krasnov, A.P.; Said-Galiyev, E.E.; Nikitin, L.N.; Afonicheva, O.V.; Aliev, A.D. Modification of tribological properties of polyarylate by supercritical fluid impregnation of copper(II) hexafluoroacetylacetonate. Tribol. Lett. 1998, 5, 297–301. [Google Scholar] [CrossRef]
- Alessi, P.; Cortesi, A.; Kikic, I.; Vecchione, F. Plasticization of polymers with supercritical carbon dioxide: Experimental determination of glass-transition temperatures. J. Appl. Polym. Sci. 2003, 88, 2189–2193. [Google Scholar] [CrossRef]
- Hao, J.; Whitaker, M.J.; Wong, B.; Serhatkulu, G.; Shakesheff, K.M.; Howdle, S.M. Plasticization and Spraying of Poly (DL-lactic acid) Using Supercritical Carbon Dioxide: Control of Particle Size. J. Pharm. Sci. 2004, 93, 1083–1090. [Google Scholar] [CrossRef]
- Fleming, O.S.; Kazarian, S.G. Polymer Processing with Supercritical Fluids. Supercrit. Carbon Dioxide Polym. React. Eng. 2006, 42, 205–238. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Cran, M.J.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa, E.J.; Bigger, S.W. Effect of supercritical CO2 and olive leaf extract on the structural, thermal and mechanical properties of an impregnated food packaging film. J. Supercrit. Fluids 2019, 145, 181–191. [Google Scholar] [CrossRef]
- Weidner, E. Impregnation via supercritical CO2–What we know and what we need to know. J. Supercrit. Fluids 2018, 134, 220–227. [Google Scholar] [CrossRef]
- Marizza, P.; Pontoni, L.; Rindzevicius, T.; Alopaeus, J.F.; Su, K.; Zeitler, J.A.; Keller, S.S.; Kikic, I.; Moneghini, M.; De Zordi, N.; et al. Supercritical impregnation of polymer matrices spatially confined in microcontainers for oral drug delivery: Effect of temperature, pressure and time. J. Supercrit. Fluids 2016, 107, 145–152. [Google Scholar] [CrossRef]
- Partap, S.; Hebb, A.K.; Ur Rehman, I.; Darr, J.A. Formation of porous natural-synthetic polymer composites using emulsion templating and supercritical fluid assisted impregnation. Polym. Bull. 2007, 58, 849–860. [Google Scholar] [CrossRef]
- Muth, O.; Hirth, T.; Vogel, H. Polymer modification by supercritical impregnation. J. Supercrit. Fluids 2000, 17, 65–72. [Google Scholar] [CrossRef]
- Kikic, I.; Vecchione, F. Supercritical impregnation of polymers. Curr. Opin. Solid State Mater. Sci. 2003, 7, 399–405. [Google Scholar] [CrossRef]
- Ginty, P.J.; Howard, D.; Upton, C.E.; Barry, J.J.A.; Rose, F.R.A.J.; Shakesheff, K.M.; Howdle, S.M. A supercritical CO2 injection system for the production of polymer/mammalian cell composites. J. Supercrit. Fluids 2008, 43, 535–541. [Google Scholar] [CrossRef]
- Chen, C.X.; Liu, Q.Q.; Xin, X.; Guan, Y.X.; Yao, S.J. Pore formation of poly(ε-caprolactone) scaffolds with melting point reduction in supercritical CO2 foaming. J. Supercrit. Fluids 2016, 117, 279–288. [Google Scholar] [CrossRef]
- Fanovich, M.A.; Jaeger, P. Sorption and diffusion of compressed carbon dioxide in polycaprolactone for the development of porous scaffolds. Mater. Sci. Eng. C 2012, 32, 961–968. [Google Scholar] [CrossRef]
- Kravanja, G.; Hrnčič, M.K.; Škerget, M.; Knez, Ž. Interfacial tension and gas solubility of molten polymer polyethylene glycol in contact with supercritical carbon dioxide and argon. J. Supercrit. Fluids 2016, 108, 45–55. [Google Scholar] [CrossRef]
- Guo, H.; Kumar, V. Solid-state poly(methyl methacrylate) (PMMA) nanofoams. Part I: Low-temperature CO2 sorption, diffusion, and the depression in PMMA glass transition. Polymer (Guildf) 2015, 57, 157–163. [Google Scholar] [CrossRef]
- Karimi, M.; Heuchel, M.; Weigel, T.; Schossig, M.; Hofmann, D.; Lendlein, A. Formation and size distribution of pores in poly(ε-caprolactone) foams prepared by pressure quenching using supercritical CO2. J. Supercrit. Fluids 2012, 61, 175–190. [Google Scholar] [CrossRef]
- Primožič, M.; Čolnik, M.; Knez, Ž.; Leitgeb, M. Advantages and disadvantages of using SC CO2 for enzyme release from halophilic fungi. J. Supercrit. Fluids 2019, 143, 286–293. [Google Scholar] [CrossRef]
- Frerich, S.C. Biopolymer foaming with supercritical CO2-Thermodynamics, foaming behaviour and mechanical characteristics. J. Supercrit. Fluids 2015, 96, 349–358. [Google Scholar] [CrossRef]
- Gedler, G.; Antunes, M.; Velasco, J.I. Effects of graphene nanoplatelets on the morphology of polycarbonate-graphene composite foams prepared by supercritical carbon dioxide two-step foaming. J. Supercrit. Fluids 2015, 100, 167–174. [Google Scholar] [CrossRef]
- Di Maio, E.; Kiran, E. Foaming of polymers with supercritical fluids and perspectives on the current knowledge gaps and challenges. J. Supercrit. Fluids 2018, 134, 157–166. [Google Scholar] [CrossRef]
- Watkins, J.J.; McCarthy, T.J. Polymerization in Supercritical Fluid-Swollen Polymers: A New Route to Polymer Blends. Macromolecules 1994, 27, 4845–4847. [Google Scholar] [CrossRef]
- Jiang, R.; Yao, S.; Chen, Y.; Liu, T.; Xu, Z.; Park, C.B.; Zhao, L. Effect of chain topological structure on the crystallization, rheological behavior and foamability of TPEE using supercritical CO2 as a blowing agent. J. Supercrit. Fluids 2019, 147, 48–58. [Google Scholar] [CrossRef]
- Tsai, W.C.; Wang, Y. Progress of supercritical fluid technology in polymerization and its applications in biomedical engineering. Prog. Polym. Sci. 2019, 98, 101161. [Google Scholar] [CrossRef]
- Boyère, C.; Jérôme, C.; Debuigne, A. Input of supercritical carbon dioxide to polymer synthesis: An overview. Eur. Polym. J. 2014, 61, 45–63. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Casimiro, T.; Aguiar-Ricardo, A.; Simplício, A.L.; Duarte, C.M.M. Supercritical fluid polymerisation and impregnation of molecularly imprinted polymers for drug delivery. J. Supercrit. Fluids 2006, 39, 102–106. [Google Scholar] [CrossRef]
- Nalawade, S.P.; Picchioni, F.; Janssen, L.P.B.M. Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications. Prog. Polym. Sci. 2006, 31, 19–43. [Google Scholar] [CrossRef]
- Haldorai, Y.; Shim, J.J.; Lim, K.T. Synthesis of polymer-inorganic filler nanocomposites in supercritical CO2. J. Supercrit. Fluids 2012, 71, 45–63. [Google Scholar] [CrossRef]
- Yue, B.; Yang, J.; Huang, C.Y.; Dave, R.; Pfeffer, R. Synthesis of macroporous PMMA/silica nanocomposite monoliths in supercritical carbon dioxide. Macromol. Rapid Commun. 2005, 26, 1406–1411. [Google Scholar] [CrossRef]
- Meziani, M.J.; Pathak, P.; Desai, T.; Sun, Y.P. Supercritical fluid processing of nanoscale particles from biodegradable and biocompatible polymers. Ind. Eng. Chem. Res. 2006, 45, 3420–3424. [Google Scholar] [CrossRef]
- Reverchon, E.; Antonacci, A. Polymer microparticles production by supercritical assisted atomization. J. Supercrit. Fluids 2007, 39, 444–452. [Google Scholar] [CrossRef]
- Sproule, T.L.; Lee, J.A.; Li, H.; Lannutti, J.J.; Tomasko, D.L. Bioactive polymer surfaces via supercritical fluids. J. Supercrit. Fluids 2004, 28, 241–248. [Google Scholar] [CrossRef]
- Ngo, T.T.; Blair, S.; Kuwahara, K.; Christensen, D.; Barrera, I.; Domingo, M.; Singamneni, S. Drug impregnation for laser sintered poly(methyl methacrylate) biocomposites using supercritical carbon dioxide. J. Supercrit. Fluids 2018, 136, 29–36. [Google Scholar] [CrossRef]
- Watson, M.S.; Whitaker, M.J.; Howdle, S.M.; Shakesheff, K.M. Incorporation of proteins into polymer materials by a novel supercritical fluid processing method. Adv. Mater. 2002, 14, 1802–1804. [Google Scholar] [CrossRef]
- Chen, A.Z.; Li, Y.; Chau, F.T.; Lau, T.Y.; Hu, J.Y.; Zhao, Z.; Mok, D.K. wah Microencapsulation of puerarin nanoparticles by poly(l-lactide) in a supercritical CO2 process. Acta Biomater. 2009, 5, 2913–2919. [Google Scholar] [CrossRef] [PubMed]
- Randolph, T.W.; Randolph, A.D.; Mebes, M.; Yeung, S. Sub-Micrometer-Sized Biodegradable Particles of Poly(L-Lactic Acid) via the Gas Antisolvent Spray Precipitation Process. Biotechnol. Prog. 1993, 9, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Kuska, R.; Milovanovic, S.; Frerich, S.; Ivanovic, J. Thermal analysis of polylactic acid under high CO2 pressure applied in supercritical impregnation and foaming process design. J. Supercrit. Fluids 2019, 144, 71–80. [Google Scholar] [CrossRef]
- Ivanovic, J.; Knauer, S.; Fanovich, A.; Milovanovic, S.; Stamenic, M.; Jaeger, P.; Zizovic, I.; Eggers, R. Supercritical CO2 sorption kinetics and thymol impregnation of PCL and PCL-HA. J. Supercrit. Fluids 2016, 107, 486–498. [Google Scholar] [CrossRef]
- Prieto, C.; Calvo, L. Supercritical fluid extraction of emulsions to nanoencapsulate vitamin E in polycaprolactone. J. Supercrit. Fluids 2017, 119, 274–282. [Google Scholar] [CrossRef]
- Akolade, J.O.; Balogun, M.; Swanepoel, A.; Ibrahim, R.B.; Yusuf, A.A.; Labuschagne, P. Microencapsulation of eucalyptol in polyethylene glycol and polycaprolactone using particles from gas-saturated solutions. Rsc Adv. 2019, 9, 34039–34049. [Google Scholar] [CrossRef]
- Kiran, E. Supercritical fluids and polymers-The year in review-2014. J. Supercrit. Fluids 2016, 110, 126–153. [Google Scholar] [CrossRef]
- Pham, M.; Pollak, S.; Petermann, M. Micronisation of poly(ethylene oxide) solutions and separation of water by PGSS-Drying. J. Supercrit. Fluids 2012, 64, 19–24. [Google Scholar] [CrossRef]
- Hamidinejad, M.; Zhao, B.; Zandieh, A.; Moghimian, N.; Filleter, T.; Park, C.B. Enhanced Electrical and Electromagnetic Interference Shielding Properties of Polymer-Graphene Nanoplatelet Composites Fabricated via Supercritical-Fluid Treatment and Physical Foaming. Acs Appl. Mater. Interfaces 2018, 10, 30752–30761. [Google Scholar] [CrossRef]
- Chang, S.H.; Park, S.C.; Shim, J.J. Phase equilibria of supercritical fluid-polymer systems. J. Supercrit. Fluids 1998, 13, 113–119. [Google Scholar] [CrossRef]
- Si-Moussa, C.; Belghait, A.; Khaouane, L.; Hanini, S.; Halilali, A. Novel density-based model for the correlation of solid drugs solubility in supercritical carbon dioxide. Comptes Rendus Chim. 2017, 20, 559–572. [Google Scholar] [CrossRef]
- Asgarpour Khansary, M.; Amiri, F.; Hosseini, A.; Hallaji Sani, A.; Shahbeig, H. Representing solute solubility in supercritical carbon dioxide: A novel empirical model. Chem. Eng. Res. Des. 2015, 93, 355–365. [Google Scholar] [CrossRef]
- Li, H.; Jia, D.; Zhu, Q.; Shen, B. Determination, correlation and prediction of the solubilities of niflumic acid, clofenamic acid and tolfenamic acid in supercritical CO2. Fluid Phase Equilib. 2015, 392, 95–103. [Google Scholar] [CrossRef]
- Bitencourt, R.G.; Palma, A.M.; Coutinho, J.A.P.; Cabral, F.A.; Meirelles, A.J.A. Prediction of solid solute solubility in supercritical CO2 with cosolvents using the CPA EoS. Fluid Phase Equilib. 2019, 482, 1–10. [Google Scholar] [CrossRef]
- Kikic, I. Polymer-supercritical fluid interactions. J. Supercrit. Fluids 2009, 47, 458–465. [Google Scholar] [CrossRef]
- Reddy, S.N.; Madras, G. Modeling of ternary solubilities of solids in supercritical carbon dioxide in the presence of cosolvents or cosolutes. J. Supercrit. Fluids 2012, 63, 105–114. [Google Scholar] [CrossRef]
- Hizaddin, H.F.; Hadj-Kali, M.K.; Alnashef, I.M.; Mjalli, F.S.; Hashim, M.A. Prediction of CO2 solubility in ionic liquids using the PSRK model. J. Supercrit. Fluids 2015, 100, 184–193. [Google Scholar] [CrossRef]
- Funazukuri, T. Concerning the determination and predictive correlation of diffusion coefficients in supercritical fluids and their mixtures. J. Supercrit. Fluids 2018, 134, 28–32. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A.; Razmimanesh, F. Solubility of an antiarrhythmic drug (amiodarone hydrochloride) in supercritical carbon dioxide: Experimental and modeling. Fluid Phase Equilib. 2017, 450, 149–159. [Google Scholar] [CrossRef]
- Caputo, G.; Scognamiglio, M.; De Marco, I. Nimesulide adsorbed on silica aerogel using supercritical carbon dioxide. Chem. Eng. Res. Des. 2012, 90, 1082–1089. [Google Scholar] [CrossRef]
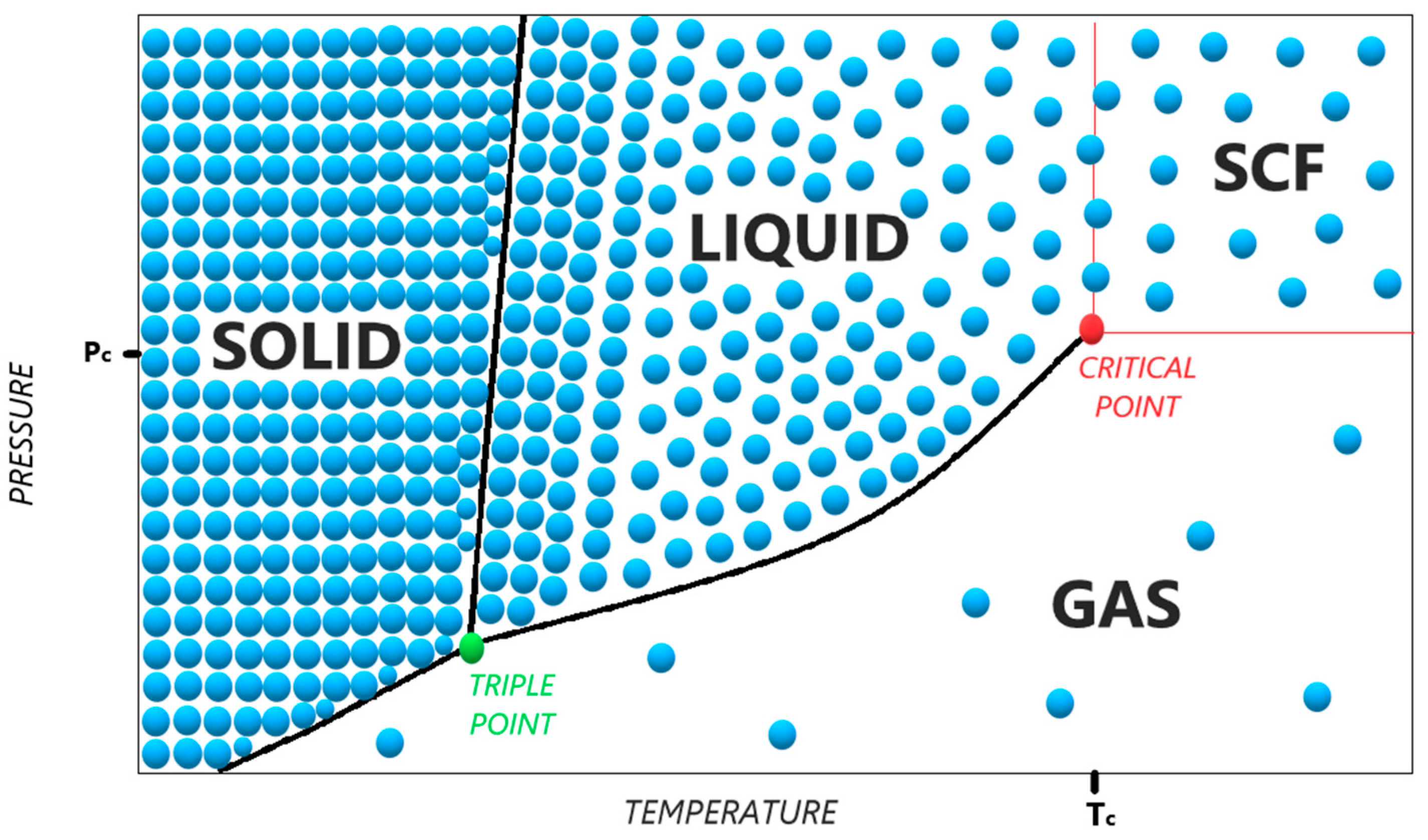




| Phase | Density (kg/m3) | Viscosity (µPa × s) | Diffusivity (mm2/s) |
|---|---|---|---|
| Gas | 1 | 10 | 1–10 |
| SCF | 100–1000 | 50–100 | 0.01–0.1 |
| Liquid | 1000 | 500–1000 | 0.001 |
| Solvent | Critical Temperature (K) | Critical Pressure (MPa) | Critical Density (g/cm3) | Ref. |
|---|---|---|---|---|
| Acetone | 508.1 | 4.7 | 0.278 | [3] |
| Ammonia | 405.6 | 11.3 | 0.235 | [4] |
| Carbon dioxide | 304.2 | 7.4 | 0.468 | [5] |
| Diethyl ether | 467.6 | 3.6 | 0.265 | [3] |
| Methanol | 512.6 | 8.1 | 0.272 | [6] |
| Toluene | 591.7 | 4.1 | 0.292 | [7] |
| Water | 647.3 | 22.0 | 0.322 | [8] |
| Benzene | 562.2 | 4.9 | 0.304 | [9,10,11] |
| Chlorodifluoromethane | 384.9 | 3.9 | 0.522 | [9,10,12] |
| Ethane | 305.6 | 4.9 | 0.212 | [9,10,13] |
| Ethylene | 282.5 | 5.1 | 0.220 | [9,10,14] |
| n-Propane | 367.0 | 4.3 | 0.225 | [9,10,15] |
| Cyclohexane | 553.3 | 4.0 | 0.270 | [16,17] |
| Nitrogen dioxide | 309.4 | 7.2 | 0.457 | [16] |
| n-Pentane | 469.6 | 3.4 | 0.232 | [16] |
| Isopropanol | 508.6 | 5.4 | 0.274 | [16] |
| Methane | 190.5 | 46.4 | 0.16 | [18] |
| C2F6 | 292.9 | 30.6 | 0.62 | [18] |
| SF6 | 318.6 | 37.2 | 0.73 | [18] |
| Propylene | 364.9 | 46.1 | 0.24 | [18] |
| Ethanol | 516.5 | 63.8 | 0.28 | [18] |
| Isobutanol | 548.1 | 43.0 | 0.27 | [18] |
| Pyridine | 647.2 | 220.5 | 0.32 | [18] |
| Name of the Natural Matrix | Functional Compound | Group of Functional Activity | Supercritical Fluids | Conditions of Extraction | Reference |
|---|---|---|---|---|---|
| Melissa | Phenol | Antioxidant | CO2 | 100 bar, 35 °C | [33] |
| Saffron | Volatile Oil | Antimicrobial | CO2 and isopropilic alcohol | 300 bar, 40 °C | [7] |
| Sage | Oil | Hipocholesterolemic | CO2 | 250 bar, 60 °C | [34] |
| Sage | Essential Oil | Antispasmodic | CO2 | 128 bar, 50 °C | [7] |
| Anis Seed | Triglycerides | Diuretic | CO2 | 250 bar, 40 °C | [35] |
| Chamomile | Oleoresin | Anti-inflammatory | CO2 | 160 bar, 40 °C | [36] |
| Clove bud | Essential Oil | Antiseptic | CO2 | 120 bar, 50 °C | [37] |
| Stevia | Glycosides | Hypoglycemic Hypotensive | CO2 | 200 bar, 30 °C | [7] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutek, K.; Masek, A.; Kosmalska, A.; Cichosz, S. Application of Fluids in Supercritical Conditions in the Polymer Industry. Polymers 2021, 13, 729. https://doi.org/10.3390/polym13050729
Tutek K, Masek A, Kosmalska A, Cichosz S. Application of Fluids in Supercritical Conditions in the Polymer Industry. Polymers. 2021; 13(5):729. https://doi.org/10.3390/polym13050729
Chicago/Turabian StyleTutek, Karol, Anna Masek, Anna Kosmalska, and Stefan Cichosz. 2021. "Application of Fluids in Supercritical Conditions in the Polymer Industry" Polymers 13, no. 5: 729. https://doi.org/10.3390/polym13050729
APA StyleTutek, K., Masek, A., Kosmalska, A., & Cichosz, S. (2021). Application of Fluids in Supercritical Conditions in the Polymer Industry. Polymers, 13(5), 729. https://doi.org/10.3390/polym13050729







