TEGDMA (Triethylene Glycol Dimethacrylate) Induces Both Caspase-Dependent and Caspase-Independent Apoptotic Pathways in Pulp Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Pulp Cell Culture
2.3. Monomer Exposure
2.4. Cell Counting
2.5. Fluorescence Microscopy
2.6. WST-1 (Water-Soluble Tetrazolium Salts) Colorimetric Viability Assay
2.7. Western Blotting
2.8. Plotting of Experimental Data and Statistical Analysis
3. Results
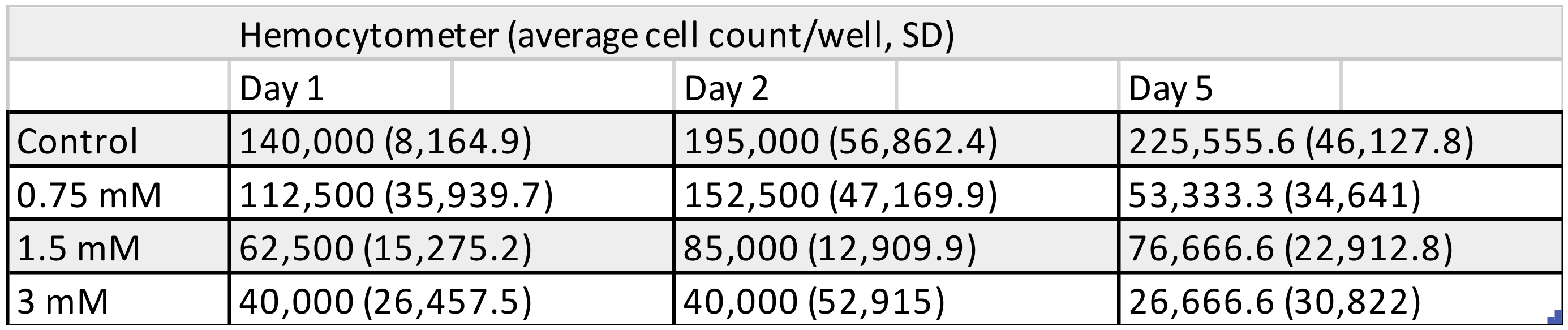
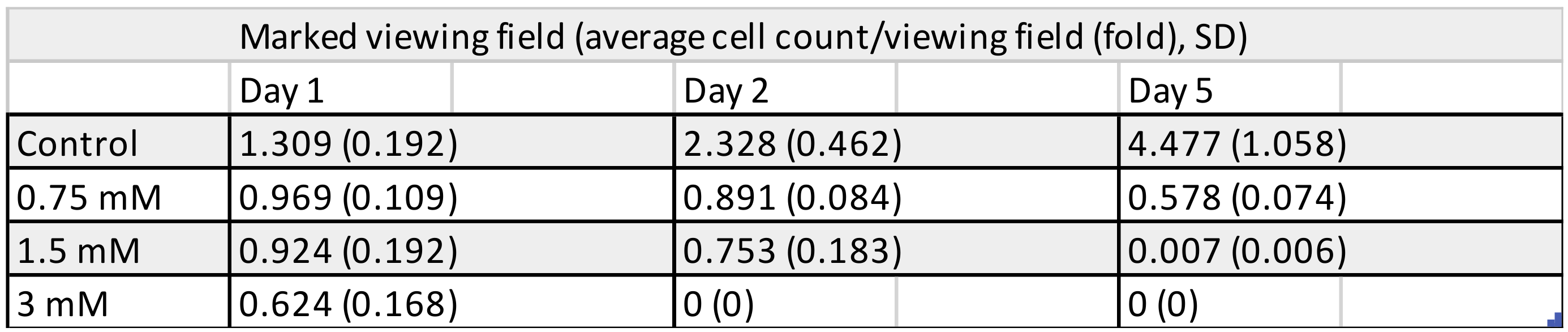
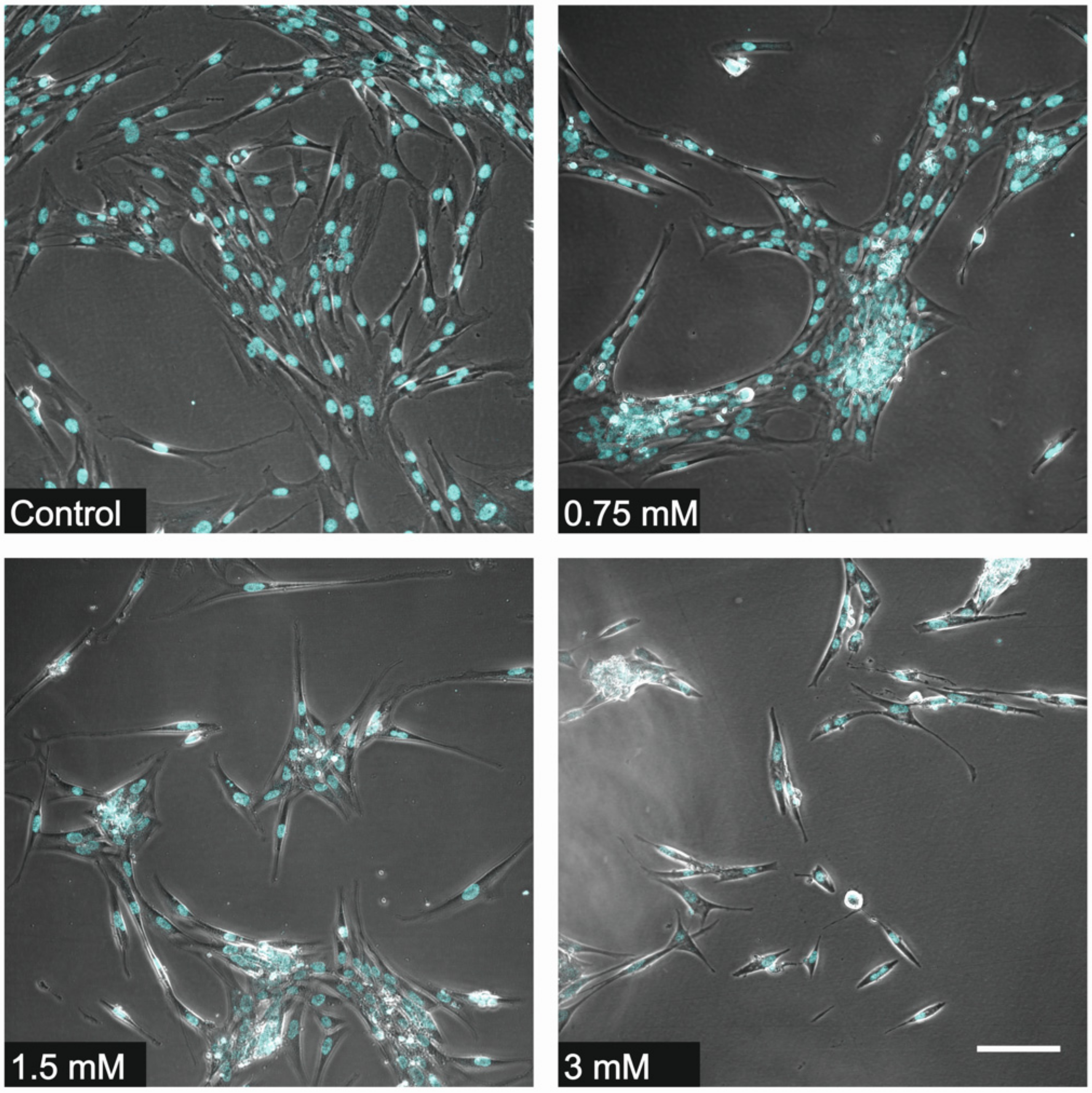
3.1. Cell Counting
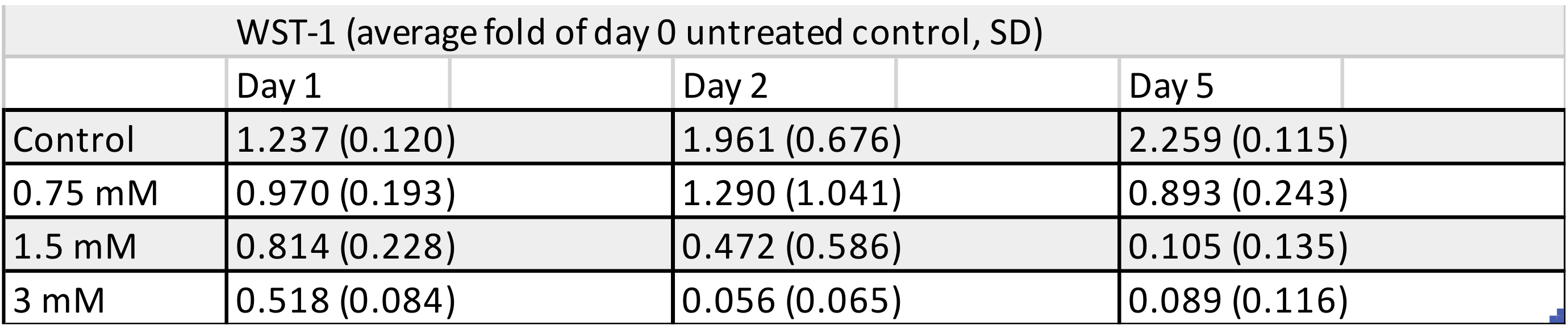
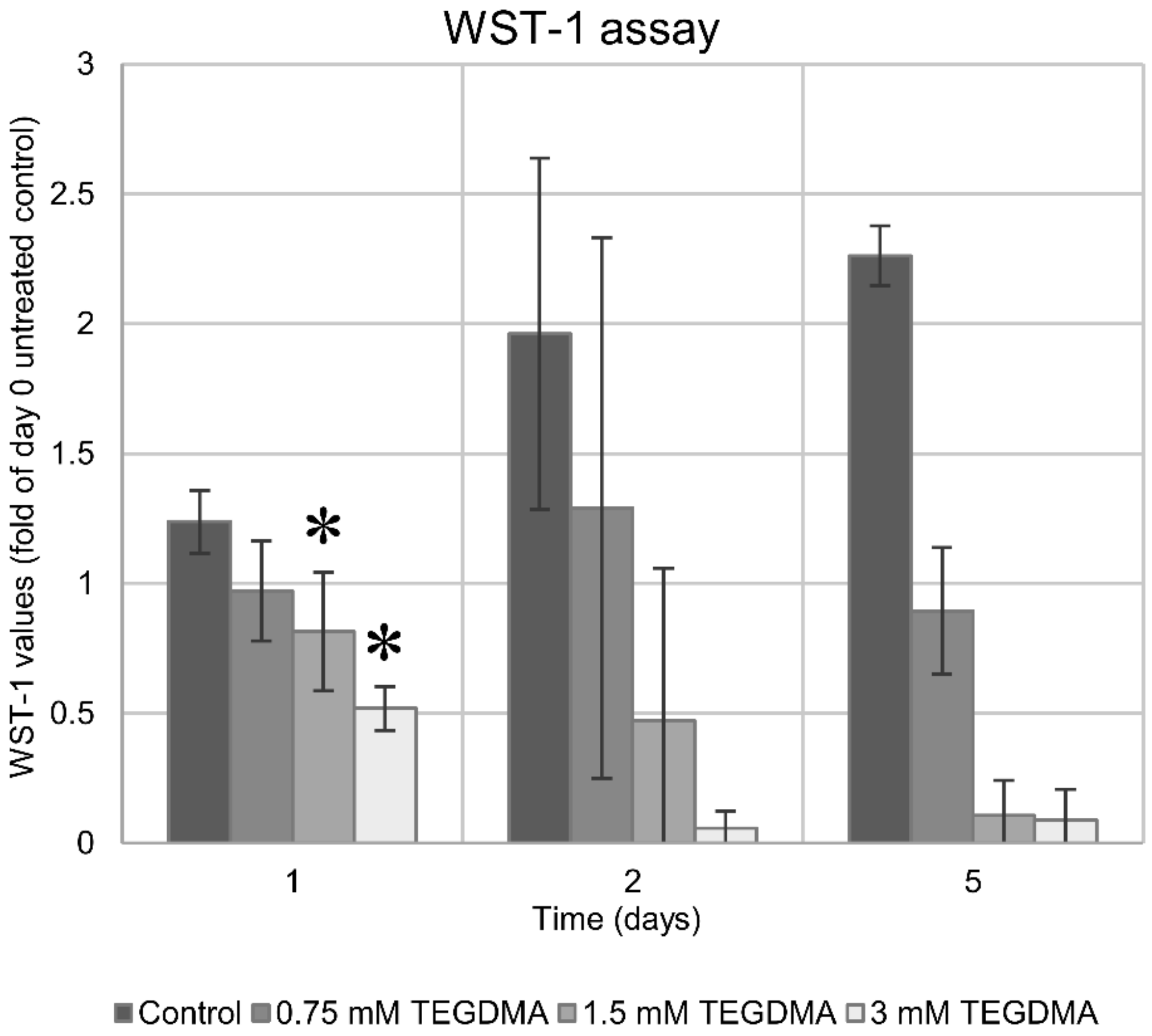
3.2. WST-1 Colorimetric Viability Assay
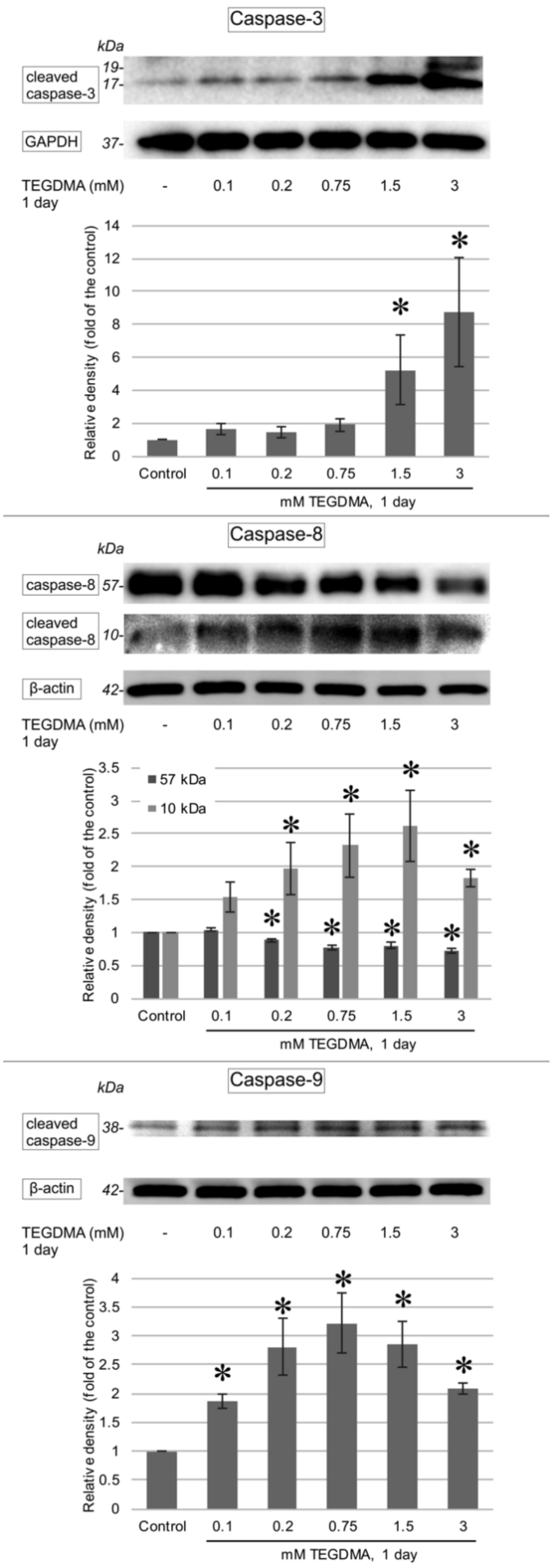
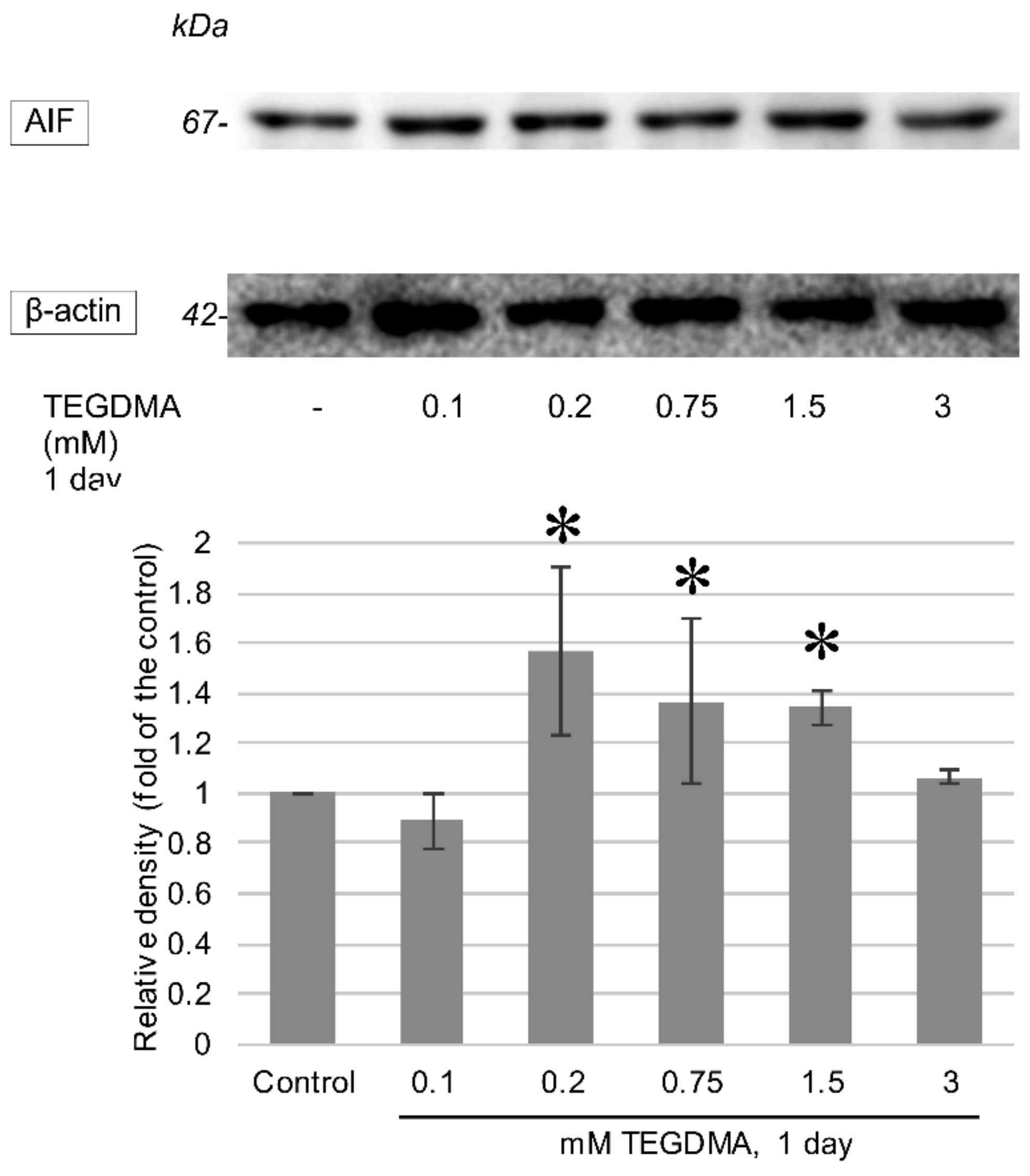
3.3. Western Blotting
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ilie, N.; Hickel, R. Resin composite restorative materials. Aust. Dent. J. 2011, 1, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Accorinte, M.L.; Loguercio, A.D.; Reis, A.; Costa, C.A. Response of human pulps capped with different self-etch adhesive systems. Clin. Oral. Investig. 2008, 12, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Lempel, E.; Czibulya Zs Kunsági-Máté, S.; Szalma, J.; Sümegi, B.; Böddi, K. Quantification of Conversion Degree and Monomer Elution from Dental Composite Using HPLC and Micro-Raman Spectroscopy. Chromatographia 2014, 77, 1137–1144. [Google Scholar] [CrossRef]
- Lempel, E.; Czibulya, Z.; Kovács, B.; Szalma, J.; Tóth, Á.; Kunsági-Máté, S.; Varga, Z.; Böddi, K. Degree of Conversion and BisGMA, TEGDMA, UDMA Elution from Flowable Bulk Fill Composites. Int. J. Mol. Sci. 2016, 17, 732. [Google Scholar] [CrossRef]
- Geurtsen, W. Substances released from dental resin composites and glass ionomer cements. Eur. J. Oral. Sci. 1998, 106, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, M.; Ulker, M.; Ulker, E.; Sengun, A. Evaluation of cytotoxicity of six different flowable composites with the methyl tetrazolium test method. Eur. J. Gen. Dent. 2013, 2, 292–295. [Google Scholar] [CrossRef]
- Gerzina, T.; Hume, W. Diffusion of monomers from bonding resin-resin composite combinations through dentine in vitro. J. Dent. 1996, 24, 125–128. [Google Scholar] [CrossRef]
- Noda, M.; Wataha, J.C.; Kaga, M.; Lockwood, P.E.; Volkmann, K.R.; Sano, H. Components of dentinal adhesives modulate heat shock protein 72 expression in heat-stressed THP-1 human monocytes at sublethal concentrations. J. Dent. Res. 2002, 81, 265–269. [Google Scholar] [CrossRef]
- Putzeys, E.; Nys, S.; Cokic, S.M.; Duca, R.C.; Vanoirbeek, J.; Godderis, L.; Meerbeek, B.V.; Van Landuyt, K.L. Long-term elution of monomers from resin-based dental composites. Dent. Mater. 2019, 35, 477–485. [Google Scholar] [CrossRef]
- Heil, T.L.; Volkmann, K.R.; Wataha, J.C.; Lockwood, P.E. Human peripheral blood monocytes versus THP-1 monocytes for in vitro biocompatibility testing of dental material components. J. Oral. Rehabil. 2002, 29, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, J.; Leyhausen, G.; Leibfritz, D.; Geurtsen, W. Effect of TEGDMA on the intracellular glutathione concentration of human gingival fibroblasts. J. Biomed. Mater. Res. 2002, 63, 746–751. [Google Scholar] [CrossRef]
- Galler, K.M.; Schweikl, H.; Hiller, K.A.; Cavender, A.C.; Bolay, C.; D’Souza, R.N.; Schmalz, G. TEGDMA reduces mineralization in dental pulp cells. J. Dent. Res. 2011, 90, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, A.; Gerstmayr, N.; Hiller, K.A.; Bolay, C.; Waha, C.; Spagnuolo, G.; Camargo, C.; Schmalz, G.; Schweikl, H. TEGDMA-induced oxidative DNA damage and activation of ATM and MAP kinases. Biomaterials 2009, 30, 2006–2014. [Google Scholar] [CrossRef]
- Gregson, K.S.; Terrence, O.J.; Platt, J.A.; Windsor, J.L. In vitro induction of hydrolytic activity in human gingival and pulp fibroblasts by triethylene glycol dimethacrylate and monocyte chemotatic protein-1. Dent. Mater. 2008, 24, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Chang, M.C.; Huang, G.F.; Wang, Y.L.; Chan, C.P.; Wang, T.M.; Lin, P.S.; Jeng, J.H. Effect of triethylene glycol dimethacrylate on the cytotoxicity, cyclooxygenase-2 expression and prostanoids production in human dental pulp cells. Int. Endod. J. 2012, 45, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Janke, V.; von Neuhoff, N.; Schlegelberger, B.; Leyhausen, G.; Geurtsen, W. TEGDMA causes apoptosis in primary human gingival fibroblasts. J. Dent. Res. 2003, 82, 814–818. [Google Scholar] [CrossRef]
- Yeh, C.C.; Chang, J.Z.; Yang, W.H.; Chang, H.H.; Lai, E.H.; Kuo, M.Y. NADPH oxidase 4 is involved in the triethylene glycol dimethacrylate-induced reactive oxygen species and apoptosis in human embryonic palatal mesenchymal and dental pulp cells. Clin. Oral. Investig. 2015, 19, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, G.; Galler, K.; Schmalz, G.; Cosentino, C.; Rengo, S.; Schweikl, H. Inhibition of phosphatidylinositol 3-kinase amplifies TEGDMA-induced apoptosis in primary human pulp cells. J. Dent. Res. 2004, 83, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Krifka, S.; Petzel, C.; Bolay, C.; Hiller, K.A.; Spagnuolo, G.; Schmalz, G.; Schweikl, H. Activation of stress-regulated transcription factors by triethylene glycol dimethacrylate monomer. Biomaterials 2011, 32, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Szegezdi, E.; Fitzgerald, U.; Samali, A. Caspase-12 and ER-Stress-Mediated Apoptosis. Ann. N. Y. Acad. Sci. 2003, 1010, 186–194. [Google Scholar] [CrossRef]
- Bano, D.; Prehn, H.M.J. Apoptosis-Inducing Factor (AIF) in Physiology and Disease: The Tale of a Repented Natural Born Killer. EBioMedicine 2018, 30, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Batarseh, G.; Windsor, L.J.; Labban, N.Y.; Liu, Y.; Gregson, K. Triethylene glycol dimethacrylate induction of apoptotic proteins in pulp fibroblasts. Oper. Dent. 2014, 39, E1–E8. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, G.L.; Huang, Y.; Diwu, H.L.; Luo, Y.; Su, J.; Xiao, Y.H. The effects of 2-hydroxyethyl methacrylate on matrix metalloproteinases 2 and 9 in human pulp cells and odontoblast-like cells in vitro. Int. Endod. J. 2018, 51, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Hanks, C.T.; Wataha, J.C.; Parsell, R.R.; Strawn, S.S.; Fat, J.C. Permeability of biological and synthetic molecules through dentine. J. Oral. Rehabil. 1994, 21, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Paschalidis, T.; Bakopoulou, A.; Papa, P.; Leyhausen, G.; Geurtsen, W.; Koidis, P. Dental pulp stem cells’ secretome enhances pulp repair processes and compensates TEGDMA-induced cytotoxicity. Dent. Mater. 2014, 30, e405–e418. [Google Scholar] [CrossRef]
- Ginzkey, C.; Zinnitsch, S.; Steussloff, G.; Koehler, C.; Hackenberg, S.; Hagen, R.; Kleinsasser, N.H.; Froelich, K. Assessment of HEMA and TEGDMA induced DNA damage by multiple genotoxicological endpoints in human lymphocytes. J. Dent. Mater. 2015, 31, 865–876. [Google Scholar] [CrossRef]
- Huang, F.M.; Kuan, Y.H.; Lee, S.S.; Chang, Y.C. Cytotoxicity and genotoxicity of triethyleneglycol-dimethacrylate in macrophages involved in DNA damage and caspases activation. Environ. Toxicol. 2015, 30, 581–588. [Google Scholar] [CrossRef]
- Schneider, T.R.; Hakami-Tafreshi, R.; Tomasino-Perez, A.; Tayebi, L.; Lobner, D. Effects of dental composite resin monomers on dental pulp cells. Dent. Mater. J. 2019, 31, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Zhou, L.; Lei, Y.; Zhang, Y.; Huang, C. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox. Biol. 2019, 25, 101047. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lovász, B.V.; Berta, G.; Lempel, E.; Sétáló, G., Jr.; Vecsernyés, M.; Szalma, J. TEGDMA (Triethylene Glycol Dimethacrylate) Induces Both Caspase-Dependent and Caspase-Independent Apoptotic Pathways in Pulp Cells. Polymers 2021, 13, 699. https://doi.org/10.3390/polym13050699
Lovász BV, Berta G, Lempel E, Sétáló G Jr., Vecsernyés M, Szalma J. TEGDMA (Triethylene Glycol Dimethacrylate) Induces Both Caspase-Dependent and Caspase-Independent Apoptotic Pathways in Pulp Cells. Polymers. 2021; 13(5):699. https://doi.org/10.3390/polym13050699
Chicago/Turabian StyleLovász, Bálint Viktor, Gergely Berta, Edina Lempel, György Sétáló, Jr., Mónika Vecsernyés, and József Szalma. 2021. "TEGDMA (Triethylene Glycol Dimethacrylate) Induces Both Caspase-Dependent and Caspase-Independent Apoptotic Pathways in Pulp Cells" Polymers 13, no. 5: 699. https://doi.org/10.3390/polym13050699
APA StyleLovász, B. V., Berta, G., Lempel, E., Sétáló, G., Jr., Vecsernyés, M., & Szalma, J. (2021). TEGDMA (Triethylene Glycol Dimethacrylate) Induces Both Caspase-Dependent and Caspase-Independent Apoptotic Pathways in Pulp Cells. Polymers, 13(5), 699. https://doi.org/10.3390/polym13050699







