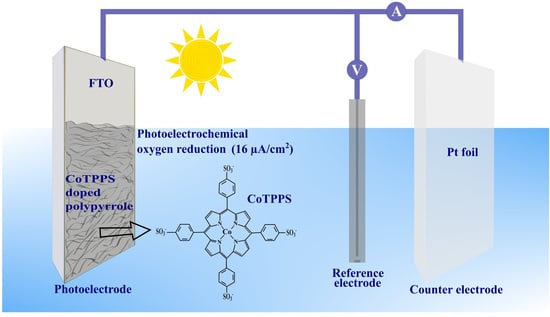
Photoelectrochemical Stability under Anodic and Cathodic Conditions of Meso-Tetra-(4-Sulfonatophenyl)-Porphyrinato Cobalt (II) Immobilized in Polypyrrole Thin Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
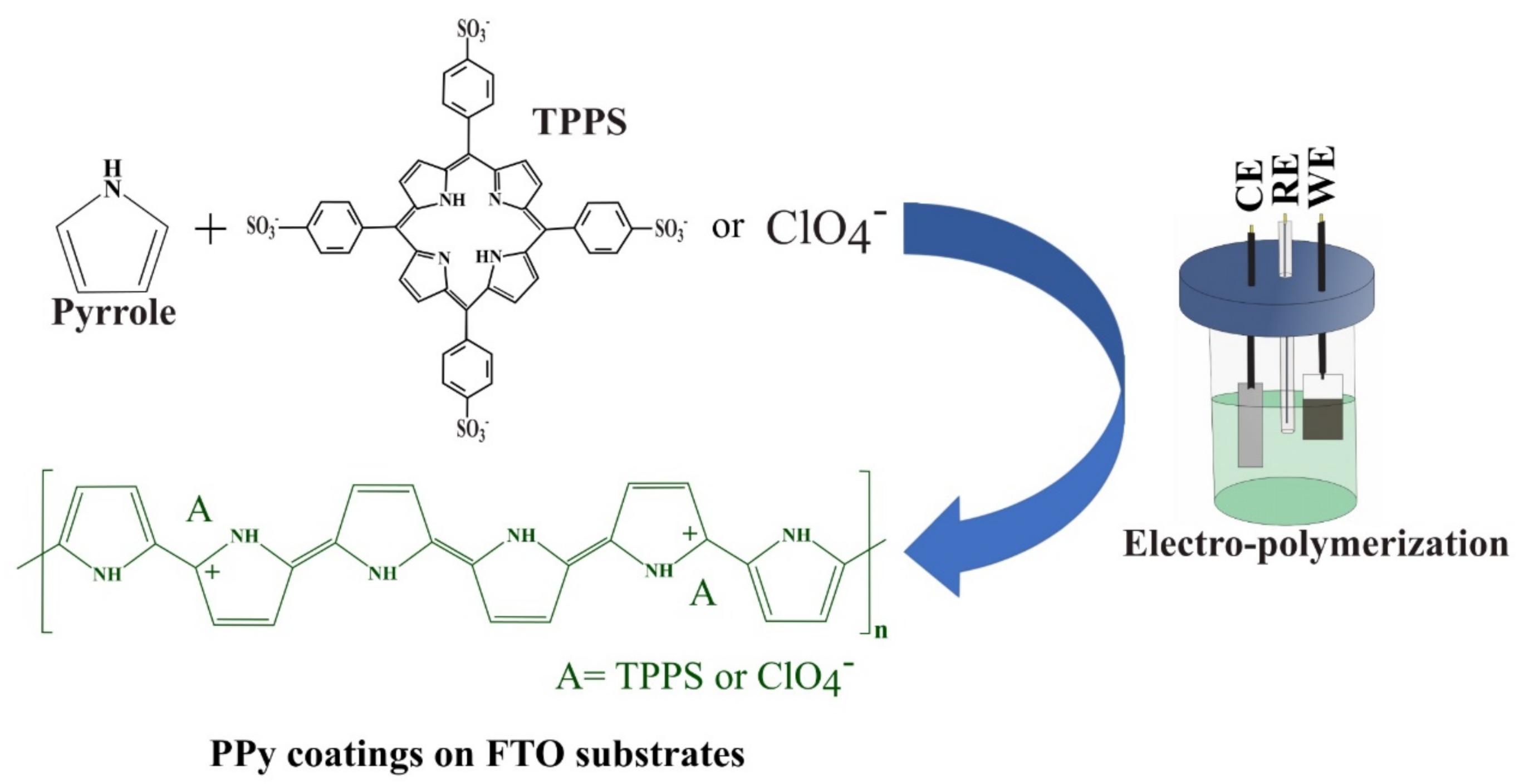
2.2. Electrochemical Synthesis of Coatings
2.3. Characterizations
3. Results and Discussion
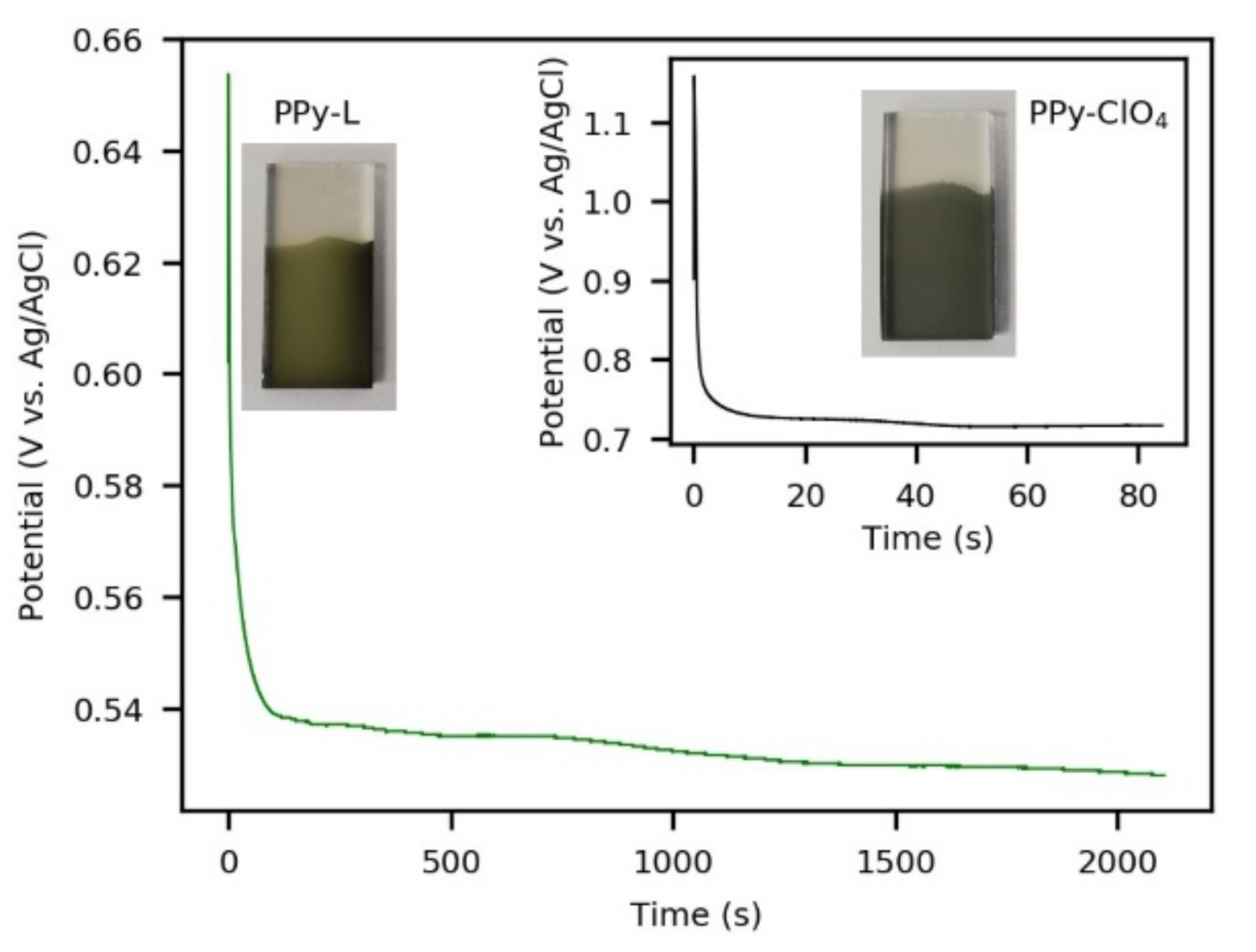
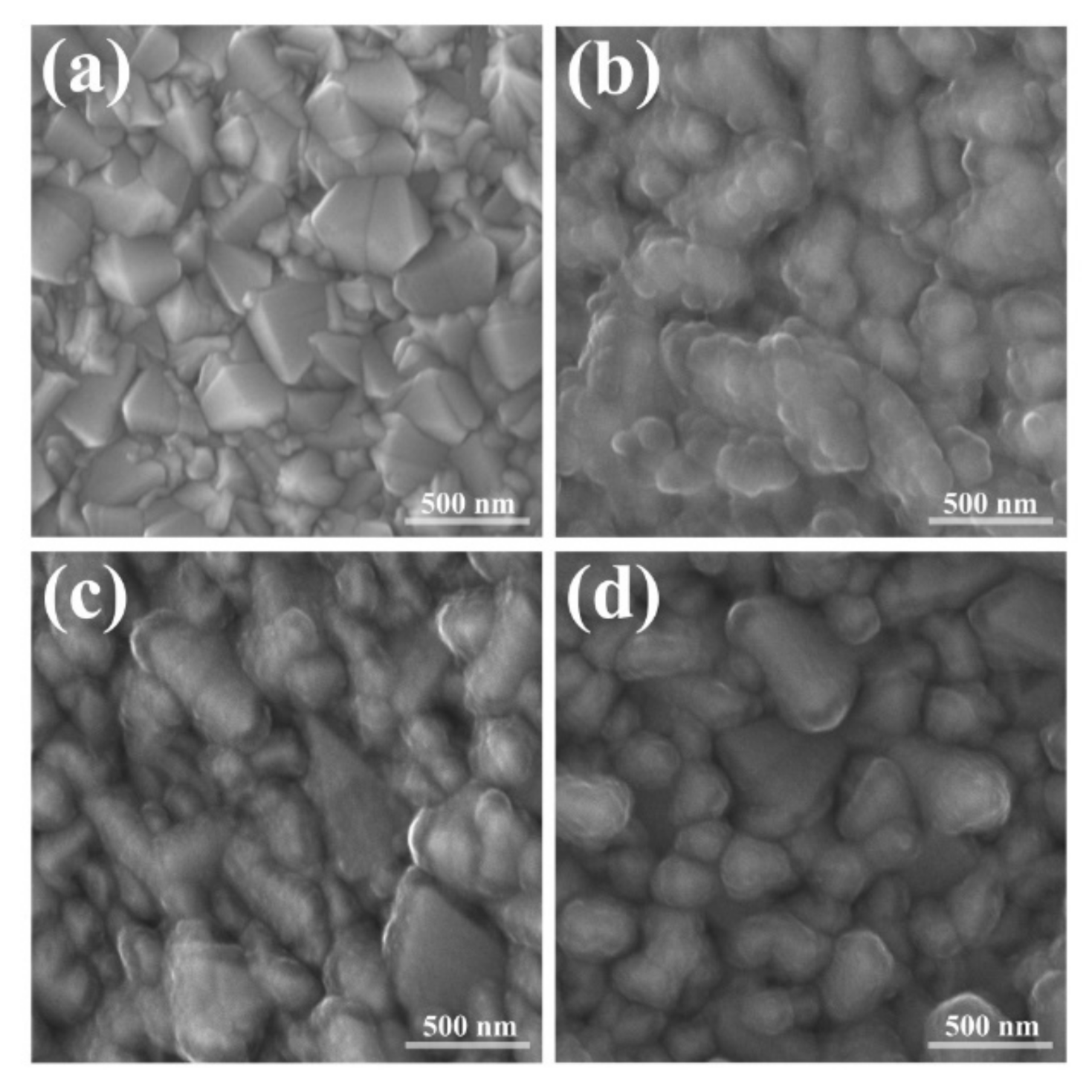
3.1. Synthesis of Coatings and Morphological Characterization
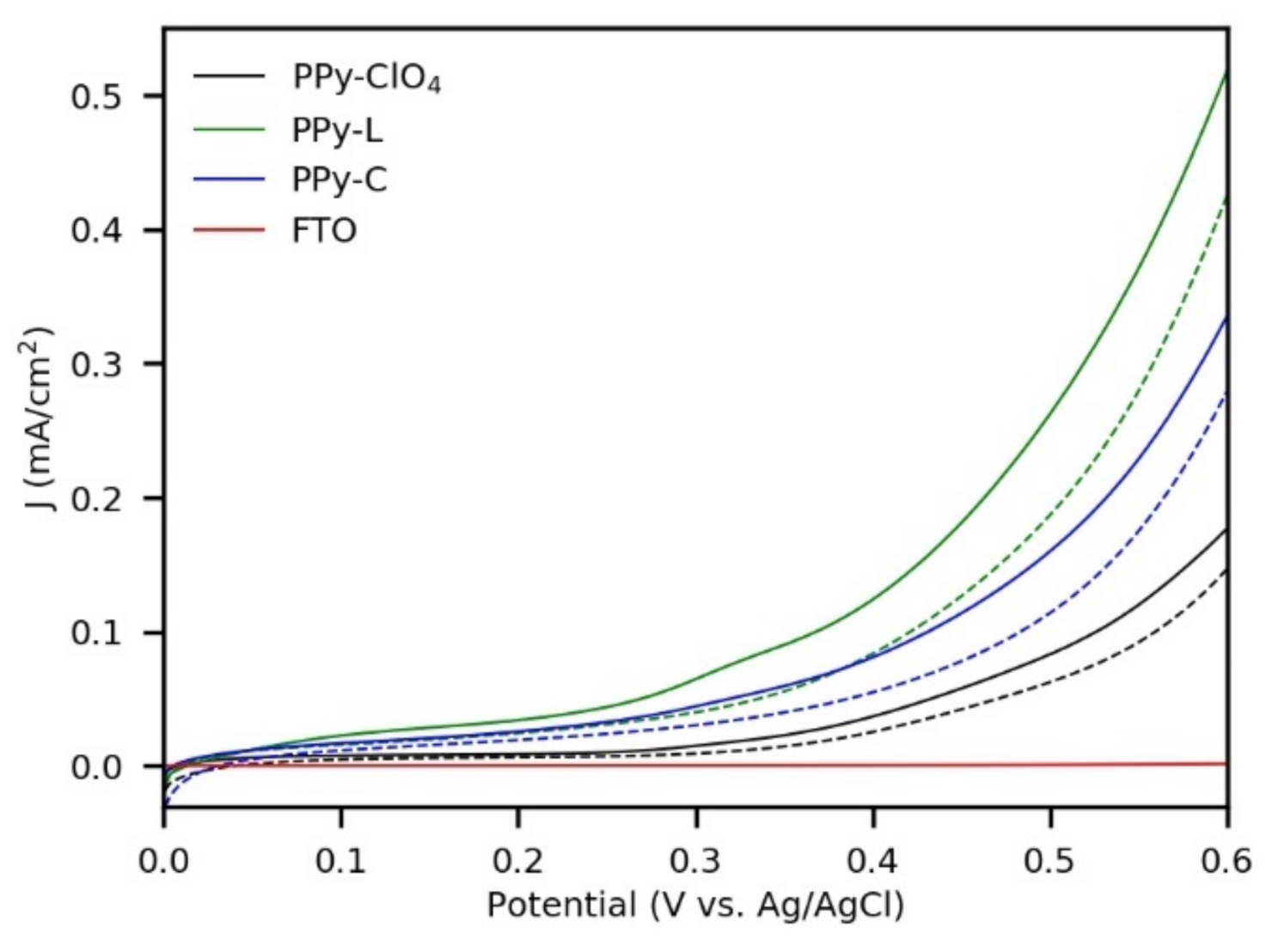
3.2. Anodic Photoresponse
3.3. Cathodic Photoresponse
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jian, J.; Jiang, G.; van de Krol, R.; Wei, B.; Wang, H. Recent advances in rational engineering of multinary semiconductors for photoelectrochemical hydrogen generation. Nano Energy 2018, 51, 457–480. [Google Scholar] [CrossRef]
- Tilley, S.D. Recent Advances and Emerging Trends in Photo-Electrochemical Solar Energy Conversion. Adv. Energy Mater. 2019, 9, 1802877. [Google Scholar] [CrossRef]
- Sun, J.; Yu, Y.; Curtze, A.E.; Liang, X.; Wu, Y. Dye-sensitized photocathodes for oxygen reduction: Efficient H2O2 production and aprotic redox reactions. Chem. Sci. 2019, 10, 5519–5527. [Google Scholar] [CrossRef]
- Saraswat, S.K.; Rodene, D.D.; Gupta, R.B. Recent advancements in semiconductor materials for photoelectrochemical water splitting for hydrogen production using visible light. Renew. Sustain. Energy Rev. 2018, 89, 228–248. [Google Scholar] [CrossRef]
- Eftekhari, A.; Babu, V.J.; Ramakrishna, S. Photoelectrode nanomaterials for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2017, 42, 11078–11109. [Google Scholar] [CrossRef]
- Wei, R.; Gryszel, M.; Migliaccio, L.; Głowacki, E.D. Tuning photoelectrochemical performance of poly(3-hexylthiophene) electrodes via surface structuring. J. Mater. Chem. C 2020, 8, 10897–10906. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, T.; Yin, H.-J.; Gao, L.-H.; Wang, K.-Z. Electrodeposited thiophene-containing organic small molecule-modified ITO electrode with highly efficient photoelectric conversion and photoelectrochemical oxygen reduction. Electrochim. Acta 2020, 362, 137150. [Google Scholar] [CrossRef]
- Hong Trang, N.T.; Ishii, K. Photoelectrochemical Oxygen Reduction Reactions Using Phthalocyanine-Based Thin Films on an ITO Electrode. J. Phys. Chem. C 2018, 122, 3539–3547. [Google Scholar] [CrossRef]
- Zhang, W.; Lai, W.; Cao, R. Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef]
- Chen, J.; Wagner, P.; Tong, L.; Boskovic, D.; Zhang, W.; Officer, D.; Wallace, G.G.; Swiegers, G.F. A light-assisted, polymeric water oxidation catalyst that selectively oxidizes seawater with a low onset potential. Chem. Sci. 2013, 4, 2797. [Google Scholar] [CrossRef][Green Version]
- Wei, P.; Lin, K.; Meng, D.; Xie, T.; Na, Y. Photoelectrochemical Performance for Water Oxidation Improved by Molecular Nickel Porphyrin-Integrated WO3/TiO2 Photoanode. ChemSusChem 2018, 11, 1746–1750. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, J.; Wu, H.-L.; Liu, W.-Q.; Jiang, X.; Li, Z.-J.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Improved Photoelectrocatalytic Performance for Water Oxidation by Earth-Abundant Cobalt Molecular Porphyrin Complex-Integrated BiVO4 Photoanode. ACS Appl. Mater. Interfaces 2016, 8, 18577–18583. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Bhunia, K.; Patra, B.C.; Das, S.K.; Pradhan, D.; Bhaumik, A.; Pradhan, A.; Bhattacharya, S. Efficacious Electrochemical Oxygen Evolution from a Novel Co(II) Porphyrin/Pyrene-Based Conjugated Microporous Polymer. ACS Appl. Mater. Interfaces 2019, 11, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.A.; Zahran, Z.N.; Naruta, Y. Covalent bonds immobilization of cofacial Mn porphyrin dimers on an ITO electrode for efficient water oxidation in aqueous solutions. J. Catal. 2017, 352, 293–299. [Google Scholar] [CrossRef]
- Sun, Z.; Li, J.; Zheng, H.; Liu, X.; Ye, S.; Du, P. Pyrolyzed cobalt porphyrin-modified carbon nanomaterial as an active catalyst for electrocatalytic water oxidation. Int. J. Hydrogen Energy 2015, 40, 6538–6545. [Google Scholar] [CrossRef]
- Han, Y.; Wu, Y.; Lai, W.; Cao, R. Electrocatalytic Water Oxidation by a Water-Soluble Nickel Porphyrin Complex at Neutral pH with Low Overpotential. Inorg. Chem. 2015, 54, 5604–5613. [Google Scholar] [CrossRef]
- Hötger, D.; Etzkorn, M.; Morchutt, C.; Wurster, B.; Dreiser, J.; Stepanow, S.; Grumelli, D.; Gutzler, R.; Kern, K. Stability of metallo-porphyrin networks under oxygen reduction and evolution conditions in alkaline media. Phys. Chem. Chem. Phys. 2019, 21, 2587–2594. [Google Scholar] [CrossRef]
- Daniel, Q.; Ambre, R.B.; Zhang, B.; Philippe, B.; Chen, H.; Li, F.; Fan, K.; Ahmadi, S.; Rensmo, H.; Sun, L. Re-Investigation of Cobalt Porphyrin for Electrochemical Water Oxidation on FTO Surface: Formation of CoOx as Active Species. ACS Catal. 2017, 7, 1143–1149. [Google Scholar] [CrossRef]
- Nakazono, T.; Parent, A.R.; Sakai, K. Cobalt porphyrins as homogeneous catalysts for water oxidation. Chem. Commun. 2013, 49, 6325. [Google Scholar] [CrossRef]
- Apaydin, D.H.; Seelajaroen, H.; Pengsakul, O.; Thamyongkit, P.; Sariciftci, N.S.; Kunze-Liebhäuser, J.; Portenkirchner, E. Photoelectrocatalytic Synthesis of Hydrogen Peroxide by Molecular Copper-Porphyrin Supported on Titanium Dioxide Nanotubes. ChemCatChem 2018, 10, 1793–1797. [Google Scholar] [CrossRef]
- Jung, O.; Pegis, M.L.; Wang, Z.; Banerjee, G.; Nemes, C.T.; Hoffeditz, W.L.; Hupp, J.T.; Schmuttenmaer, C.A.; Brudvig, G.W.; Mayer, J.M. Highly Active NiO Photocathodes for H2O2 Production Enabled via Outer-Sphere Electron Transfer. J. Am. Chem. Soc. 2018, 140, 4079–4084. [Google Scholar] [CrossRef]
- Bozzini, B.; Bocchetta, P.; Kourousias, G.; Gianoncelli, A. Electrodeposition of Mn-Co/Polypyrrole Nanocomposites: An Electrochemical and In Situ Soft-X-ray Microspectroscopic Investigation. Polymers 2017, 9, 17. [Google Scholar] [CrossRef]
- Lee, D.-G.; Kim, S.H.; Joo, S.H.; Ji, H.-I.; Tavassol, H.; Jeon, Y.; Choi, S.; Lee, M.-H.; Kim, C.; Kwak, S.K.; et al. Polypyrrole-assisted oxygen electrocatalysis on perovskite oxides. Energy Environ. Sci. 2017, 10, 523–527. [Google Scholar] [CrossRef]
- Khalafallah, D.; Alothman, O.Y.; Fouad, H.; Abdelrazek Khalil, K. Hierarchical Co3O4 decorated PPy nanocasting core-shell nanospheres as a high performance electrocatalysts for methanol oxidation. Int. J. Hydrogen Energy 2018, 43, 2742–2753. [Google Scholar] [CrossRef]
- Chakraborty, R.; Sahoo, S.; Halder, N.; Rath, H.; Chattopadhyay, K. Conformational-Switch Based Strategy Triggered by [18] π Heteroannulenes toward Reduction of Alpha Synuclein Oligomer Toxicity. ACS Chem. Neurosci. 2019, 10, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Johanson, U.; Marandi, M.; Sammelselg, V.; Tamm, J. Electrochemical properties of porphyrin-doped polypyrrole films. J. Electroanal. Chem. 2005, 575, 267–273. [Google Scholar] [CrossRef]
- Patois, T.; Lakard, B.; Monney, S.; Roizard, X.; Fievet, P. Characterization of the surface properties of polypyrrole films: Influence of electrodeposition parameters. Synth. Met. 2011, 161, 2498–2505. [Google Scholar] [CrossRef]
- Boucher, L.J.; Katz, J.J. The Infared Spectra of Metalloporphyrins (4000-160 Cm−1). J. Am. Chem. Soc. 1967, 89, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Konev, D.V.; Istakova, O.I.; Dembinska, B.; Skunik-Nuckowska, M.; Devillers, C.H.; Heintz, O.; Kulesza, P.J.; Vorotyntsev, M.A. Electrocatalytic properties of manganese and cobalt polyporphine films toward oxygen reduction reaction. J. Electroanal. Chem. 2018, 816, 83–91. [Google Scholar] [CrossRef]
- Hatano, T.; Takeuchi, M.; Ikeda, A.; Shinkai, S. Nano-Rod Structure of Poly(ethylenedioxythiophene) and Poly(pyrrole) As Created by Electrochemical Polymerization Using Anionic Porphyrin Aggregates as Template. Org. Lett. 2003, 5, 1395–1398. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; He, T.; Liu, F. Raman and infrared spectral study of meso-sulfonatophenyl substituted porphyrins (TPPSn, n=1, 2A, 2O, 3, 4). Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2003, 59, 87–101. [Google Scholar] [CrossRef]
- Fujii, Y.; Tsukahara, Y.; Wada, Y. pH-Dependent Reversible Switching of Fluorescence of Water-Soluble Porphyrin Adsorbed on Mesoporous TiO2 Film. Bull. Chem. Soc. Jpn. 2006, 79, 561–568. [Google Scholar] [CrossRef]
- Hatano, K.; Usui, K.; Ishida, Y. Redox Reaction of the Central Metal Ions Coordinated to Tetra(p-sulfophenyl)porphine(TPPS). I. Photoreduction of Co(III)TPPS by Methanol and 2-Propanol. Bull. Chem. Soc. Jpn. 1981, 54, 413–419. [Google Scholar] [CrossRef]
- Nakazono, T.; Parent, A.R.; Sakai, K. Improving Singlet Oxygen Resistance during Photochemical Water Oxidation by Cobalt Porphyrin Catalysts. Chem. A Eur. J. 2015, 21, 6723–6726. [Google Scholar] [CrossRef] [PubMed]
- Varade, V.; Honnavar, G.V.; Anjaneyulu, P.; Ramesh, K.P.; Menon, R. Probing disorder and transport properties in polypyrrole thin-film devices by impedance and Raman spectroscopy. J. Phys. D Appl. Phys. 2013, 46, 365306. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Resonance Raman Spectroscopy of Conducting Polypyrrole Nanotubes: Disordered Surface versus Ordered Body. J. Phys. Chem. A 2018, 122, 9298–9306. [Google Scholar] [CrossRef]
- Yin, P.; Yao, T.; Wu, Y.; Zheng, L.; Lin, Y.; Liu, W.; Ju, H.; Zhu, J.; Hong, X.; Deng, Z.; et al. Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chemie Int. Ed. 2016, 55, 10800–10805. [Google Scholar] [CrossRef] [PubMed]
- Konev, A.S.; Kayumov, M.Y.; Karushev, M.P.; Novoselova, Y.V.; Lukyanov, D.A.; Alekseeva, E.V.; Levin, O.V. Polymeric Metal Salen-Type Complexes as Catalysts for Photoelectrocatalytic Hydrogen Peroxide Production. ChemElectroChem 2018, 5, 3138–3142. [Google Scholar] [CrossRef]
- Jakešová, M.; Apaydin, D.H.; Sytnyk, M.; Oppelt, K.; Heiss, W.; Sariciftci, N.S.; Głowacki, E.D. Hydrogen-Bonded Organic Semiconductors as Stable Photoelectrocatalysts for Efficient Hydrogen Peroxide Photosynthesis. Adv. Funct. Mater. 2016, 26, 5248–5254. [Google Scholar] [CrossRef]
- Gryszel, M.; Markov, A.; Vagin, M.; Głowacki, E.D. Organic heterojunction photocathodes for optimized photoelectrochemical hydrogen peroxide production. J. Mater. Chem. A 2018, 6, 24709–24716. [Google Scholar] [CrossRef]
- Węcławski, M.K.; Jakešová, M.; Charyton, M.; Demitri, N.; Koszarna, B.; Oppelt, K.; Sariciftci, S.; Gryko, D.T.; Głowacki, E.D. Biscoumarin-containing acenes as stable organic semiconductors for photocatalytic oxygen reduction to hydrogen peroxide. J. Mater. Chem. A 2017, 5, 20780–20788. [Google Scholar] [CrossRef]
- Migliaccio, L.; Gryszel, M.; Đerek, V.; Pezzella, A.; Głowacki, E.D. Aqueous photo(electro)catalysis with eumelanin thin films. Mater. Horiz. 2018, 5, 984–990. [Google Scholar] [CrossRef]
- Wang, H.; Sun, H.-T.; Zhang, Y.-Y.; Zhang, C.-C.; Cheng, Q.-R.; Hou, S.-M.; Liao, J.-H.; Wang, K.-Z. Three-dimensional high-rate electropolymerized thin film with exceptionally high photocurrent based on a triphenylamine-containing ruthenium complex. Electrochim. Acta 2019, 298, 265–278. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, C.-X.; Li, Y.-J.; Fu, Y.-H.; Yin, Z.-H.; Gao, L.-H.; Wang, K.-Z. A 3D electropolymerized thin film based on a thiophene-functionalized Ru(II) complex: Electrochemical and photoelectrochemical insights. Inorg. Chem. Front. 2019, 6, 3518–3528. [Google Scholar] [CrossRef]

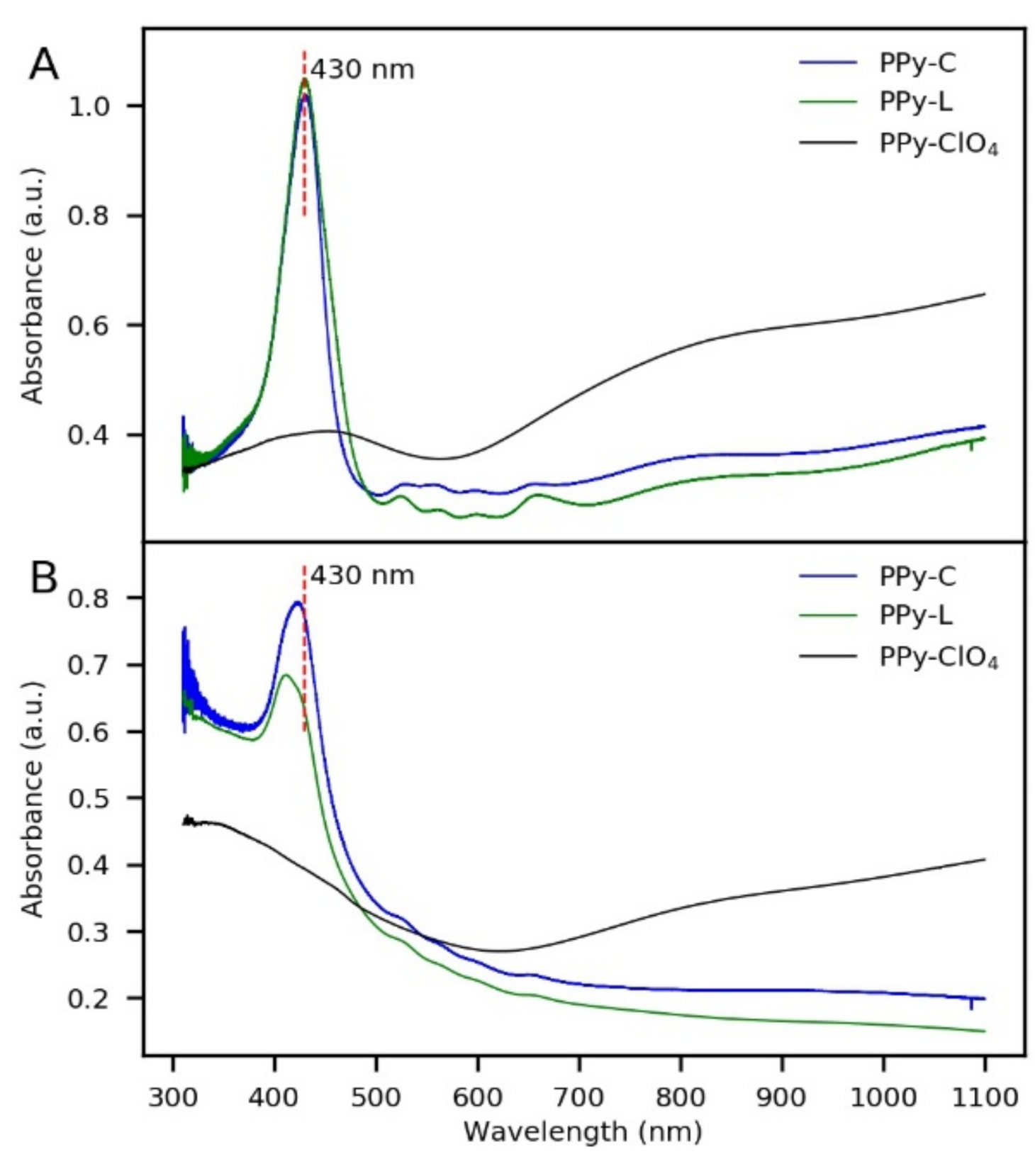
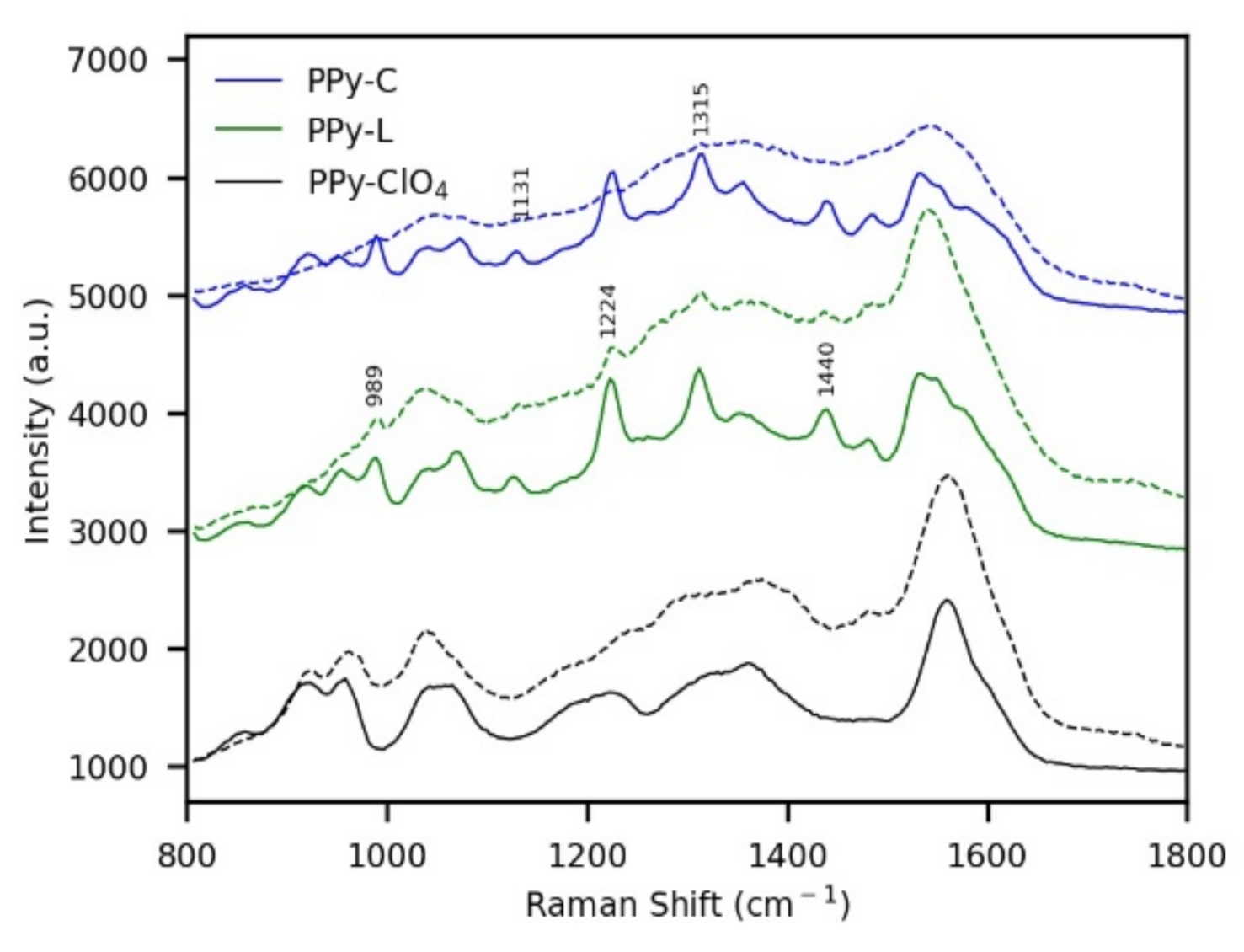

| System | Soret Band (nm) | Q Bands (nm) |
|---|---|---|
| TPPS in solution [31,32] | 413 | 515. 552, 580, 634 |
| TPPS in solution (this work) | 414 | 516, 553, 581, 635 |
| TPPS immobilized in PPy | 430 | 524, 561, 598, 660 |
| CoTPPS in solution [33,34] | 425 | 425, 538 |
| CoTPPS immobilized in PPy | 431 | (528 and 558 overlapped), 598, 658 |
| Photoelectrode | Bias | Electrolyte | JPh (μA/cm2) | Reference |
|---|---|---|---|---|
| CoTPPS-doped PPy (PPy-C) | −0.4 V vs. Ag/AgCl | 0.1 M Na2SO4 | 16 | This work |
| P3HT treated with oxygen plasma (3-7 P3HT-PS) | 0 V vs. Ag/AgCl | H2SO4 pH 1 | ~15 | [6] |
| Thin film based on functionalized thiophene (Film(dimer-L2)) | 0 V vs. SCE | 0.1 M Na2SO4 | 26.89 | [7] |
| Zn Pc-D and PVDF mixture on ITO (2Zn) | −0.12 V vs. NHE | 0.5 M KNO3 | 15.5 | [8] |
| Mg Pc-D and PVDF mixture on ITO (1Mg) | −0.12 V vs. NHE | 0.5 M KNO3 | 9.3 | [8] |
| Polymeric metal salen-type complex ([Ni(MeOSalen)]n) | 0 V vs. Ag/AgCl | 1 M Na2SO4 + HCl pH 1 | 23 | [38] |
| Thin film of hydrogen-bonded organic pigment (EPI) | 0 V vs. Ag/AgCl | 0.1 M HCl | 15 | [39] |
| Thin film of biscoumarin-containing acene | 0 V vs. Ag/AgCl | Na2SO4 + HCl pH 2 | 3 | [41] |
| Thin film of eumelanin | 0 V vs. Ag/AgCl | pH 1 | 8 | [42] |
| Ru complex polymerized on ITO (Poly(RuL3)1) | 0 V vs. SCE | 0.1 M Na2SO4 | 10.7 | [43] |
| Ru complex polymerized on ITO (Poly(RuL3)2) | 0 V vs. SCE | 0.1 M Na2SO4 | 1.36 | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puerres, J.; Díaz, M.; Hurtado, J.; Ortiz, P.; Cortés, M.T. Photoelectrochemical Stability under Anodic and Cathodic Conditions of Meso-Tetra-(4-Sulfonatophenyl)-Porphyrinato Cobalt (II) Immobilized in Polypyrrole Thin Films. Polymers 2021, 13, 657. https://doi.org/10.3390/polym13040657
Puerres J, Díaz M, Hurtado J, Ortiz P, Cortés MT. Photoelectrochemical Stability under Anodic and Cathodic Conditions of Meso-Tetra-(4-Sulfonatophenyl)-Porphyrinato Cobalt (II) Immobilized in Polypyrrole Thin Films. Polymers. 2021; 13(4):657. https://doi.org/10.3390/polym13040657
Chicago/Turabian StylePuerres, Jhon, Mauro Díaz, John Hurtado, Pablo Ortiz, and María T. Cortés. 2021. "Photoelectrochemical Stability under Anodic and Cathodic Conditions of Meso-Tetra-(4-Sulfonatophenyl)-Porphyrinato Cobalt (II) Immobilized in Polypyrrole Thin Films" Polymers 13, no. 4: 657. https://doi.org/10.3390/polym13040657
APA StylePuerres, J., Díaz, M., Hurtado, J., Ortiz, P., & Cortés, M. T. (2021). Photoelectrochemical Stability under Anodic and Cathodic Conditions of Meso-Tetra-(4-Sulfonatophenyl)-Porphyrinato Cobalt (II) Immobilized in Polypyrrole Thin Films. Polymers, 13(4), 657. https://doi.org/10.3390/polym13040657