Preparation of a Novel Cellulose–Styrene Copolymer Adsorbent and Its Adsorption of Nitrobenzene from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment
2.3. Preparation of Cellulose-Styrene Copolymer
2.4. Adsorption of Nitrobenzene (NB) on Cellulose-St
2.5. Characterization
2.5.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.2. X-ray Diffraction (XRD)
2.5.3. Thermo-Gravimetric Analysis (TGA)
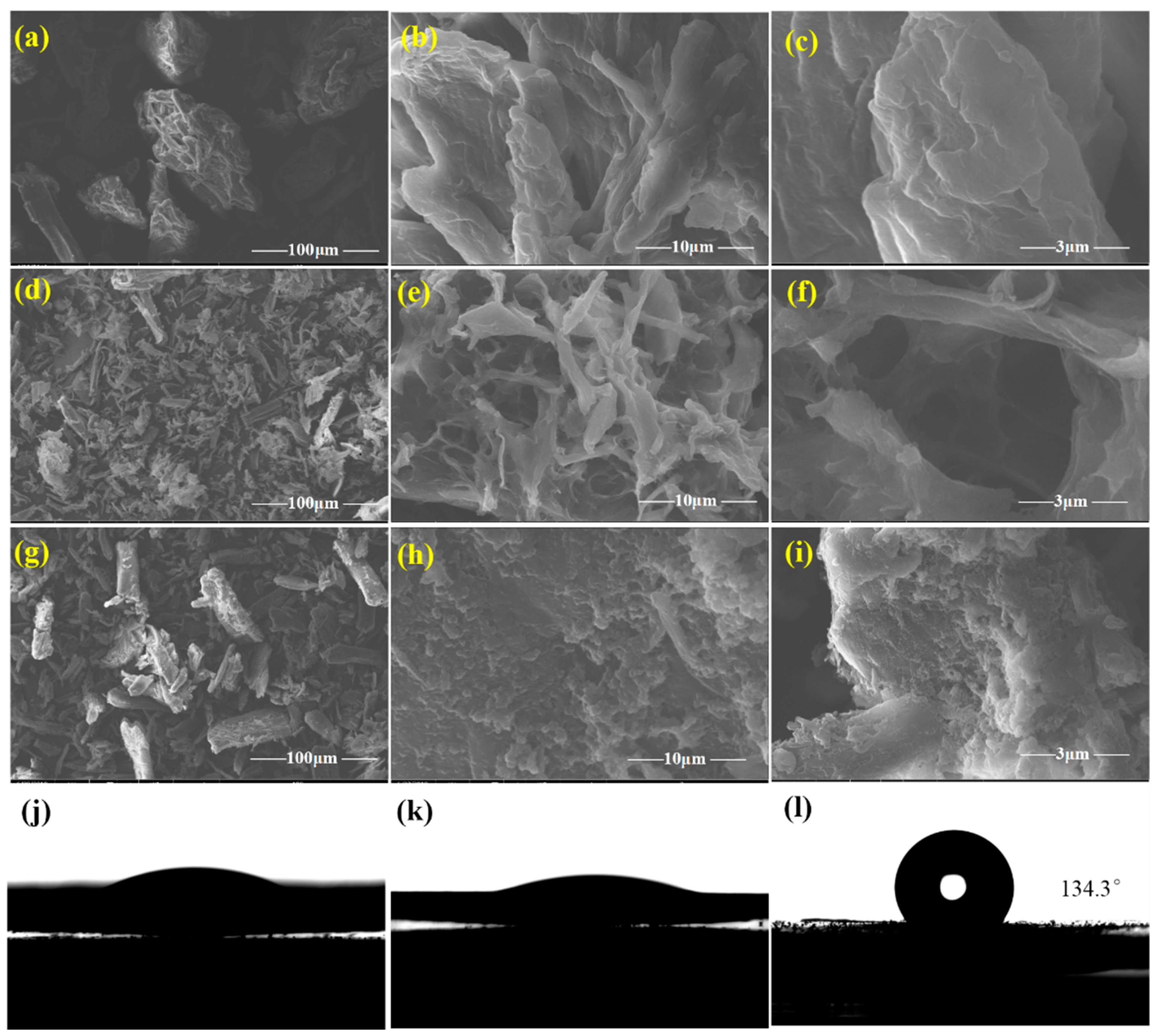
2.5.4. Scanning Electron Microscopy (SEM)
2.5.5. Carbon-13 Solid Nuclear Magnetic Resonance (13C NMR) Spectroscopy Analysis
2.5.6. Brunauer–Emmett–Teller (BET)
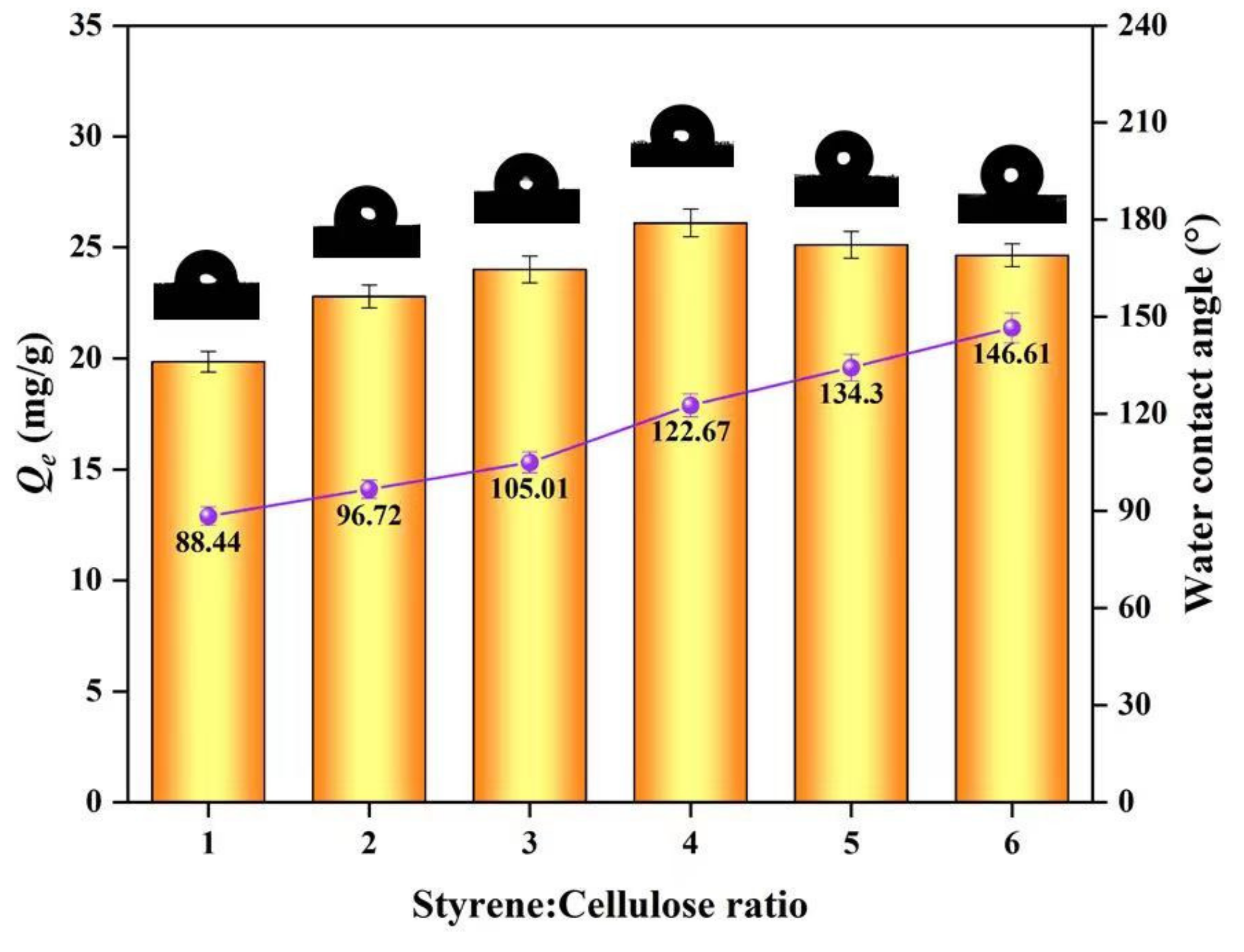
2.5.7. Contact Angle Measurements
3. Results and Discussion
3.1. Characterization
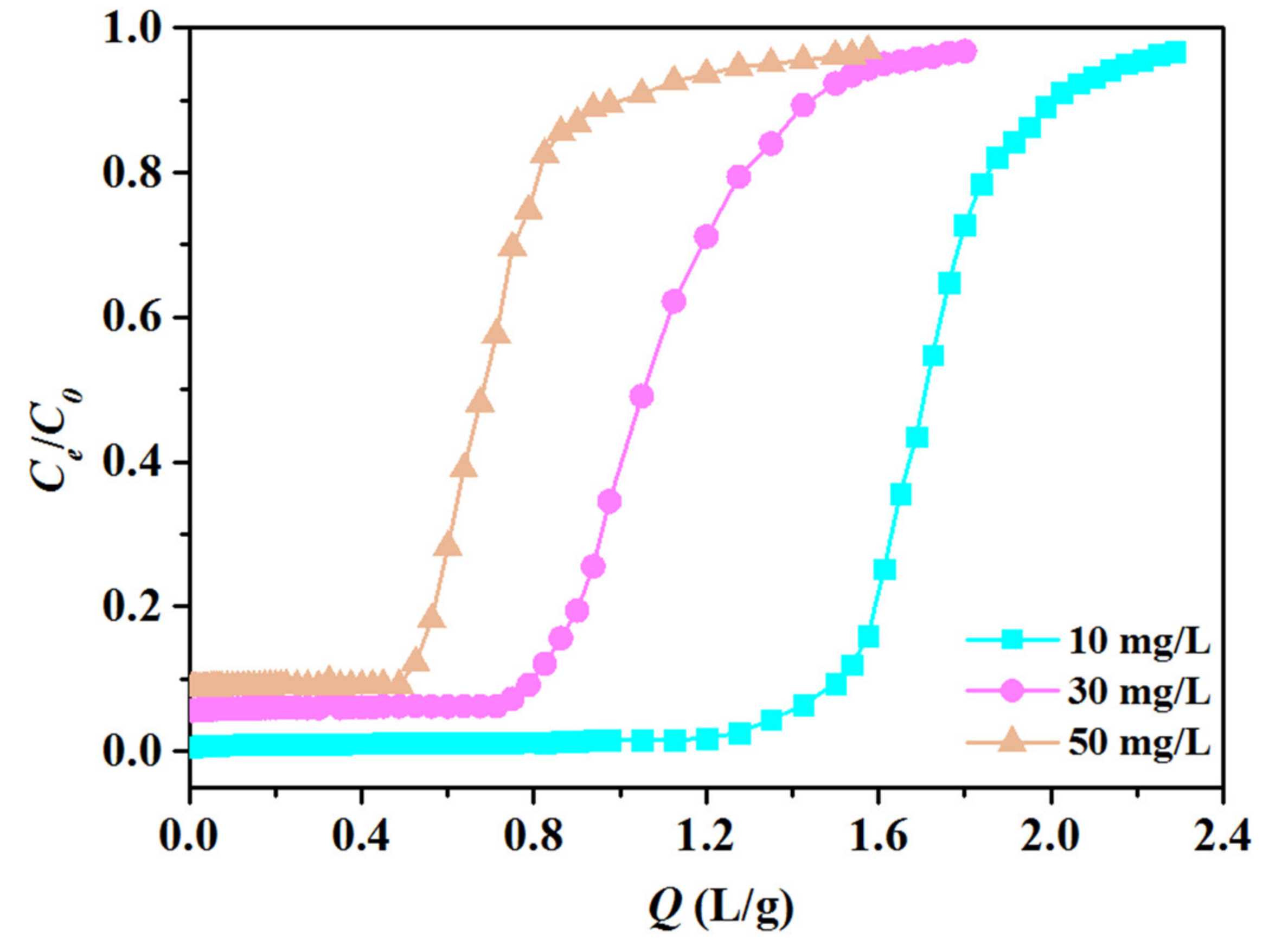
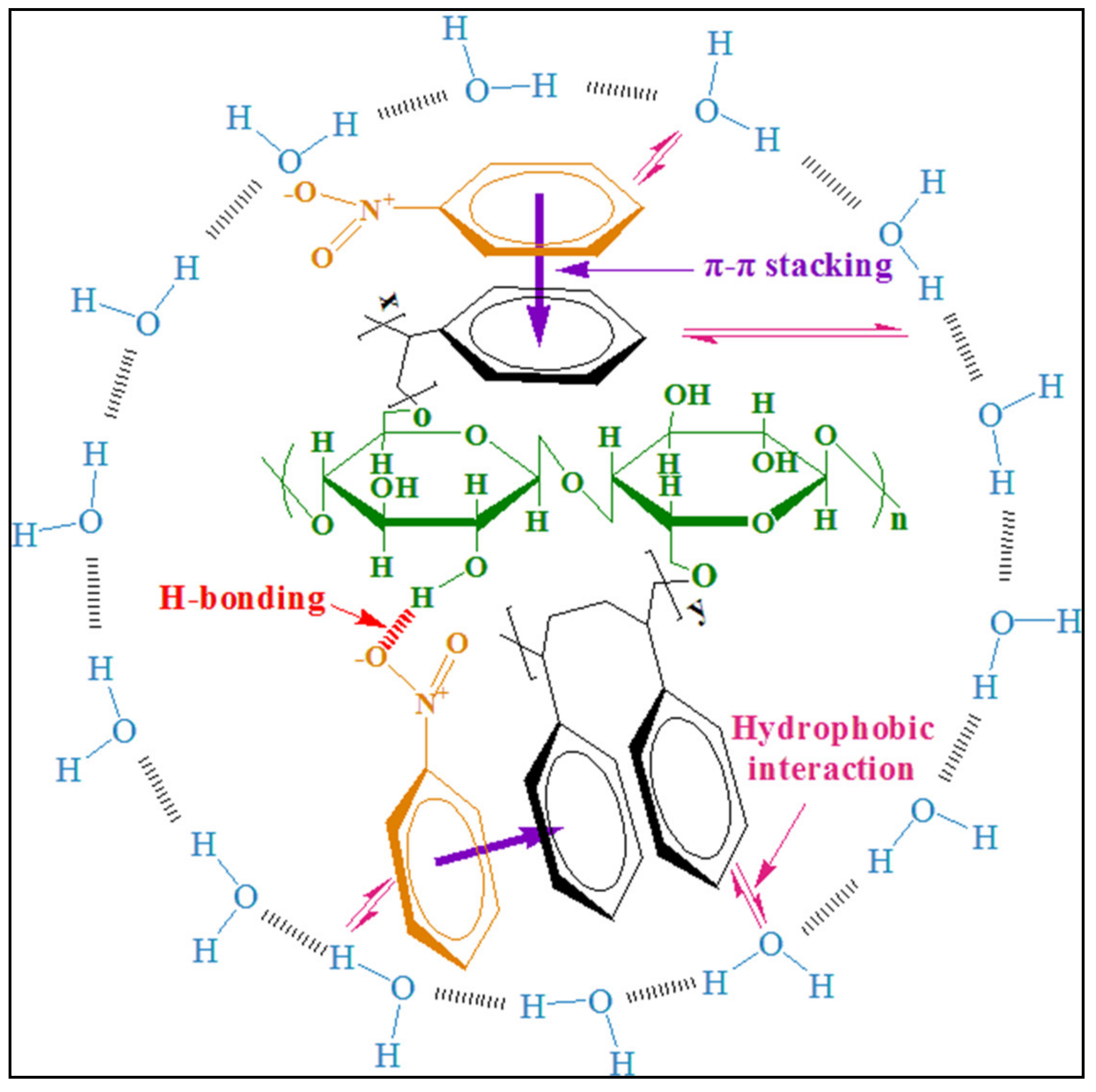
3.2. Adsorption
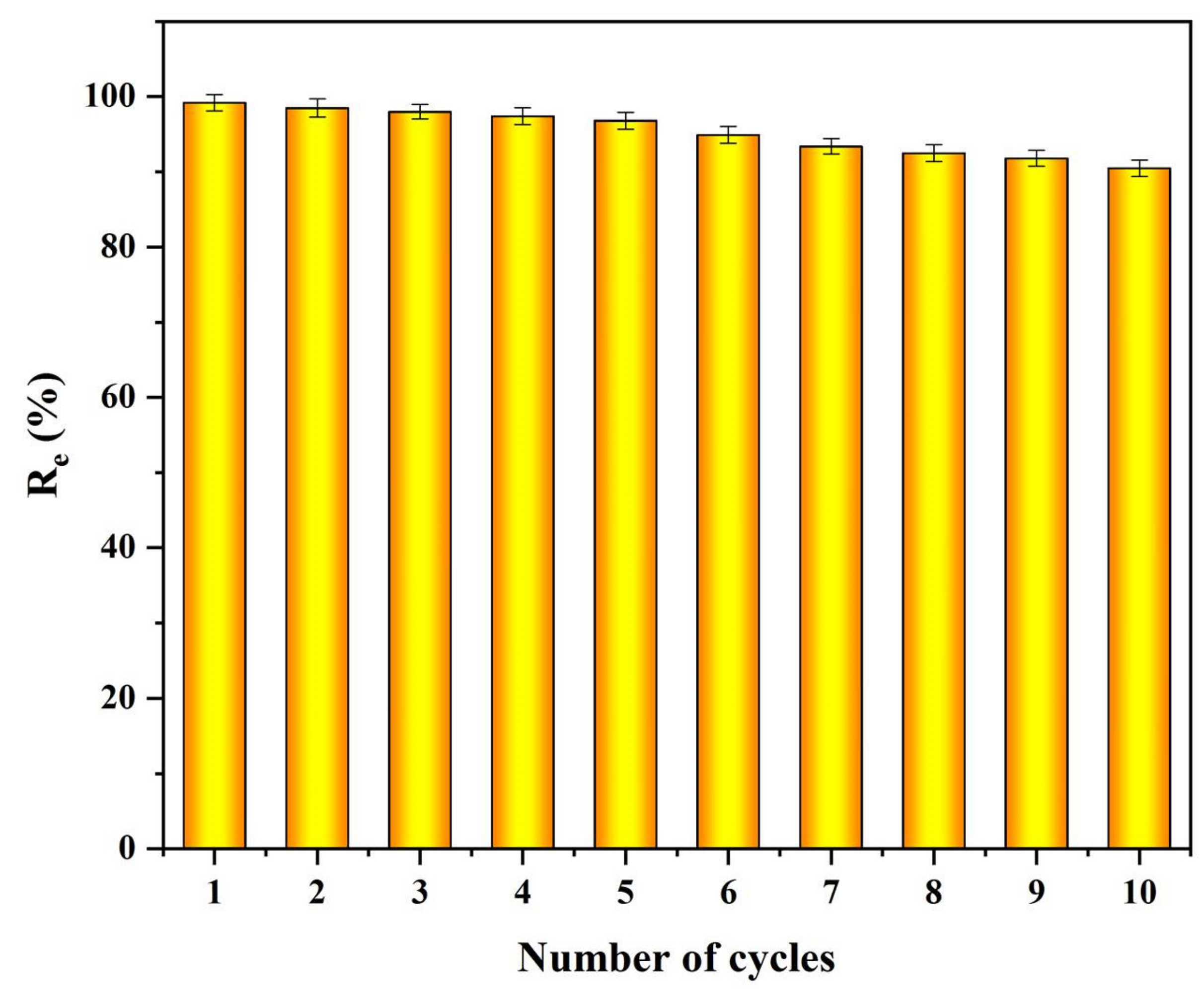
3.3. Reusability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Yang, L.; Myneni, S.C.; Deng, Y. Leaching of polycyclic aromatic hydrocarbons (PAHs) from sewage sludge-derived biochar. Chem. Eng. J. 2019, 373, 840–845. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.D.; Xu, W.; Li, Y.L.; Han, Y.F.; Sun, Q. Enhanced Nitrobenzene Adsorption in Aqueous Solution by Surface-Silanized Fly-Ash-Derived SBA-15. ACS Earth Space Chem. 2018, 2, 246–255. [Google Scholar] [CrossRef]
- World Health Organization. Nitrobenzene in drinking-water: background document for development of WHO guidelines for drinking-water quality (No. WHO/HSE/WSH/09.01/4). 2009. Available online: https://apps.who.int/iris/bitstream/handle/10665/70175/WHO_HSE_WSH_09.01_4_eng.pdf (accessed on 1 February 2021).
- Dvořák, L.; Lederer, T.; Jirků, V.; Masák, J.; Novák, L. Removal of aniline, cyanides and diphenylguanidine from industrial wastewater using a full-scale moving bed biofilm reactor. Process Biochem. 2014, 49, 102–109. [Google Scholar] [CrossRef]
- Kirui, W.K.; Wu, S.; Lei, M.; Dong, R. Nitrobenzene degradation pathways and their interaction with sulfur and nitrogen transformations in horizontal subsurface flow constructed wetlands. Ecol. Eng. 2015, 84, 77–83. [Google Scholar] [CrossRef]
- Jiang, T.; Li, J.; Gao, Y.; Li, L.; Lu, T.; Pan, L. BiOBr/BiOF composites for efficient degradation of rhodamine B and nitrobenzene under visible light irradiation. J. Colloid Interface Sci. 2017, 490, 812–818. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L.; Liu, Y.; Zhang, Q. TiO2 –SA–Arg nanoparticles stabilized Pickering emulsion for photocatalytic degradation of nitrobenzene in a rotating annular reactor. Chin. J. Chem. Eng. 2017, 25, 223–231. [Google Scholar] [CrossRef]
- Ayati, A.; Tanhaei, B.; Bamoharram, F.F.; Ahmadpour, A.; Maydannik, P.; Sillanpaa, M. Photocatalytic degradation of nitrobenzene by gold nanoparticles decorated polyoxometalate immobilized TiO2 nanotubes. Sep. Purif. Technol. 2016, 171, 62–68. [Google Scholar] [CrossRef]
- Duan, H.; Liu, Y.; Yin, X.; Bai, J.; Qi, J. Degradation of nitrobenzene by Fenton-like reaction in a H2O2/schwertmannite system. Chem. Eng. J. 2016, 283, 873–879. [Google Scholar] [CrossRef]
- Wang, W.-K.; Chen, J.-J.; Li, W.-W.; Pei, D.-N.; Zhang, X.; Yu, H.-Q. Synthesis of Pt-Loaded Self-Interspersed Anatase TiO2 with a Large Fraction of (001) Facets for Efficient Photocatalytic Nitrobenzene Degradation. ACS Appl. Mater. Interfaces 2015, 7, 20349–20359. [Google Scholar] [CrossRef]
- Nitoi, I.; Oancea, P.; Raileanu, M.; Crisan, M.; Constantin, L.; Cristea, I. UV–VIS photocatalytic degradation of nitrobenzene from water using heavy metal doped titania. J. Ind. Eng. Chem. 2015, 21, 677–682. [Google Scholar] [CrossRef]
- Mohamed, R.; Ibrahim, F. Visible light photocatalytic reduction of nitrobenzene using Ag/Bi2MoO6 nanocomposite. J. Ind. Eng. Chem. 2015, 22, 28–33. [Google Scholar] [CrossRef]
- Boxi, S.S.; Paria, S. Visible light induced enhanced photocatalytic degradation of organic pollutants in aqueous media using Ag doped hollow TiO2 nanospheres. RSC Adv. 2015, 5, 37657–37668. [Google Scholar] [CrossRef]
- Abdedayem, A.; Guiza, M.; Toledo, F.J.R.; Ouederni, A. Nitrobenzene degradation in aqueous solution using ozone/cobalt supported activated carbon coupling process: A kinetic approach. Sep. Purif. Technol. 2017, 184, 308–318. [Google Scholar] [CrossRef]
- Abdedayem, A.; Guiza, M.; Ouederni, A. Copper supported on porous activated carbon obtained by wetness impregnation: Effect of preparation conditions on the ozonation catalyst’s characteristics. Comptes Rendus Chim. 2015, 18, 100–109. [Google Scholar] [CrossRef]
- Chen, C.; Yan, X.; Yoza, B.A.; Zhou, T.; Li, Y.; Zhan, Y.; Wang, Q.; Li, Q.X. Efficiencies and mechanisms of ZSM5 zeolites loaded with cerium, iron, or manganese oxides for catalytic ozonation of nitrobenzene in water. Sci. Total. Environ. 2018, 612, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sui, M.; Zhang, T.; Guan, C. Effect of pH on MnOx/GAC catalyzed ozonation for degradation of nitrobenzene. Water Res. 2005, 39, 779–786. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Zhao, C.; Sun, Z.; Zhang, Z.; Mirza, Z.A.; Saylor, G.; Zhai, J.; Zheng, H. Electric field induced activated carbon fiber (ACF) cathode transition from an initiator/a promoter into an electrocatalyst in ozonation process. Chem. Eng. J. 2016, 304, 129–133. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Cho, K.H.; Khan, N.A.; Hong, D.-Y.; Jhung, S.H. Liquid-Phase Adsorption of Aromatics over a Metal–Organic Framework and Activated Carbon: Effects of Hydrophobicity/Hydrophilicity of Adsorbents and Solvent Polarity. J. Phys. Chem. C 2015, 119, 26620–26627. [Google Scholar] [CrossRef]
- Qin, Q.; Xu, Y. Enhanced nitrobenzene adsorption in aqueous solution by surface silylated MCM-41. Microporous Mesoporous Mater. 2016, 232, 143–150. [Google Scholar] [CrossRef]
- Wei, W.; Sun, R.; Cui, J.; Wei, Z. Removal of nitrobenzene from aqueous solution by adsorption on nanocrystalline hydroxyapatite. Desalination 2010, 263, 89–96. [Google Scholar] [CrossRef]
- Hu, S.; Yao, H.; Wang, K.; Lu, C.; Wu, Y. Intensify Removal of Nitrobenzene from Aqueous Solution Using Nano-Zero Valent Iron/Granular Activated Carbon Composite as Fenton-Like Catalyst. Water Air Soil Pollut. 2015, 226, 1–13. [Google Scholar] [CrossRef]
- Dasgupta, A.; Matos, J.; Muramatsu, H.; Ono, Y.; Gonzalez, V.; Liu, H.; Rotella, C.; Fujisawa, K.; Cruz-Silva, R.; Hashimoto, Y.; et al. Nanostructured carbon materials for enhanced nitrobenzene adsorption: Physical vs. chemical surface properties. Carbon 2018, 139, 833–844. [Google Scholar] [CrossRef]
- Nezampour, F.; Ghiaci, M.; Masoomi, K. Activated Carbon and Graphitic Carbon Nitride Immobilized on Mesoporous Silica for Adsorption of Nitrobenzene. J. Chem. Eng. Data 2018, 63, 1977–1986. [Google Scholar] [CrossRef]
- Wang, F.; Ma, Y. MPC-973: A low-cost and effective adsorbent for the removal of nitrobenzene from aqueous solutions. Mater. Chem. Phys. 2018, 208, 157–162. [Google Scholar] [CrossRef]
- Zhao, C.; Si, B.; Mirza, Z.A.; Liu, Y.; He, X.; Li, J.; Wang, Z.; Zheng, H. Activated carbon fiber (ACF) enhances the UV/EF system to remove nitrobenzene in water. Sep. Purif. Technol. 2017, 187, 397–406. [Google Scholar] [CrossRef]
- Hu, S.; Wu, Y.; Yao, H.; Lu, C.; Zhang, C. Enhanced Fenton-like removal of nitrobenzene via internal microelectrolysis in nanozerovalent iron/activated carbon composite. Water Sci Technol. 2016, 73, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shen, X.; Zhang, H.; Cai, F.; Chen, W.; Gao, Q.; Julio Ortega-Calvo, J.; Tao, S.; Wang, X. Bioavailability of phenanthrene and nitrobenzene sorbed on carbonaceous materials. Carbon 2016, 110, 404–413. [Google Scholar] [CrossRef]
- Jing, Z.; Peng, Y.; He, R.; Xu, Y.; Yu, T.; Hu, J. Poplar leaves reclamation for porous granules and their application in nitrobenzene removal from aqueous solution. Desalin. Water Treat. 2016, 57, 449–458. [Google Scholar] [CrossRef]
- Ilona, V.; Quik, J.T.K.; Dik, V.D.M.; Koelmans, A.A. Rapid settling of nanoparticles due to heteroaggregation with suspended sediment. Environ. Toxicol. Chem. 2014, 33, 1766–1773. [Google Scholar]
- Engler, R.E. The Complex Interaction between Marine Debris and Toxic Chemicals in the Ocean. Environ. Sci. Technol. 2012, 46, 12302–12315. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Lu, B.; Zhu, F.; Cheng, J.; Sun, Z. Nitrobenzene reduction using nanoscale zero-valent iron supported by polystyrene microspheres with different surface functional groups. Environ. Sci. Pollut. Res. 2018, 25, 7916–7923. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Liu, G. Sorption behaviors of phenanthrene, nitrobenzene, and naphthalene on mesoplastics and microplastics. Environ. Sci. Pollut. R 2019, 26, 12563–12573. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Liu, G.; Zhang, Z.; Wu, H.; Cui, B.; Bai, J.; Zhang, W. Size effect of polystyrene microplastics on sorption of phenanthrene and nitrobenzene. Ecotoxicol. Environ. Saf. 2019, 173, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Suhas Gupta, V.; Carrott, P.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Lee, S.-Y.; Wei, T.; Li, J.; Fan, Z. Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev. 2018, 47, 2837–2872. [Google Scholar] [CrossRef] [PubMed]
- Komal, K.; Gupta, K.; Kaur, S.; Kaur, J.; Kaushik, A.; Singhal, S. A comparative analysis of source based distinctly functionalized nanostructured cellulose for the adsorptive removal of toxic colorants. Cellulose 2018, 26, 1703–1724. [Google Scholar] [CrossRef]
- Zhiming, L.; Peng, W. Preparation of Hydrophobic Cellulose Aerogel Beads and Its Adsorption Performance. Chem. Ind. Forest Prod. 2018, 38, 9–17. [Google Scholar]
- Li Xiaopeng, G.S. Comparative of Study of Four Thiolation Modification Methods of Cellulose Nanocrystals. J. Wuhan Univ. Nat. Sci. Ed. 2017, 63, 289–296. [Google Scholar]
- Ru, J.; Geng, B.; Tong, C.; Wang, H.; Wu, S.; Liu, H. Nanocellulose-Based Adsorption Materials. Prog. Chem. 2017, 10, 78–101. [Google Scholar]
- Segal, L.; Creely, J.; Martin, A.; Conrad, C. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Rodrigues, C.I.S.; Jackson, J.J.; Montross, M.D. A molar basis comparison of calcium hydroxide, sodium hydroxide, and potassium hydroxide on the pretreatment of switchgrass and miscanthus under high solids conditions. Ind. Crop. Prod. 2016, 92, 165–173. [Google Scholar] [CrossRef]
- Liu, M.; Wang, H.; Han, J.; Niu, Y. Enhanced hydrogenolysis conversion of cellulose to C2–C3 polyols via alkaline pretreatment. Carbohyd. Polym. 2012, 89, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Nawa Raj, B.; Jiangzheng, L.; Ajay Kumar, J. Perspective and prospective of pretreatment of corn straw for butanol production. Appl. Biochem. Biotechnol. 2014, 172, 840–853. [Google Scholar]
- Li, K.; Huang, J.; Xu, D.; Zhong, Y.; Zhang, L.; Cai, J. Mechanically strong polystyrene nanocomposites by peroxide-induced grafting of styrene monomers within nanoporous cellulose gels. Carbohydr. Polym. 2018, 199, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Tian, X.; Jiang, X.; Wang, H.; Gao, W. Modification of cellulose nanocrystal via SI-ATRP of styrene and the mechanism of its reinforcement of polymethylmethacrylate. Carbohydr. Polym. 2016, 142, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Wakida, K.; Mukasa, S.; Toyota, H. Catalytic effect on ultrasonic decomposition of cellulose. Jpn. J. Appl. Phys. 2018, 57, 07LE05. [Google Scholar] [CrossRef]
- Ahmed, F.; Arbab, A.A.; Jatoi, A.W.; Khatri, M.; Memon, N.; Khatri, Z.; Kim, I.S. Ultrasonic-assisted deacetylation of cellulose acetate nanofibers: A rapid method to produce cellulose nanofibers. Ultrason. Sonochem. 2017, 36, 319–325. [Google Scholar] [CrossRef]
- Yang, F.; Li, L.; Li, Q.; Tan, W.; Liu, W.; Xian, M. Enhancement of enzymatic in situ saccharification of cellulose in aqueous-ionic liquid media by ultrasonic intensification. Carbohydr. Polym. 2010, 81, 311–316. [Google Scholar] [CrossRef]
- Wang, X.L.; Fang, G.Z.; Hu, C.P. Influence of ultrasonic wave-activating treatment on structure and oxidation reactivity of microcrystalline cellulose. Chem. J. Chin. U 2007, 28, 565–567. [Google Scholar]
- Tanaka, A.; Onoda, H.; Nitta, K.-H. Molecular Aggregation and Ultrasonic Properties of Hydroxypropyl-Cellulose Films. Polym. J. 2000, 32, 665–669. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Ning, L.; Jin, H.; Dufresne, A. Highly alkynyl-functionization of cellulose nanocrystals and advanced nanocomposites thereof via click chemistry. Polym. Chem. 2015, 6, 4385–4395. [Google Scholar] [CrossRef]
- Wang, D.; Shan, H.; Sun, X.; Zhang, H.; Wu, Y. Removal of nitrobenzene from aqueous solution by adsorption onto carbonized sugarcane bagasse. Adsorpt. Sci. Technol. 2018, 36, 1366–1385. [Google Scholar] [CrossRef]
- Wu, Y.; Qi, H.; Li, B.; Zhanhua, H.; Li, W.; Liu, S. Novel hydrophobic cotton fibers adsorbent for the removal of nitrobenzene in aqueous solution. Carbohydr. Polym. 2017, 155, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Sun, R.; Jin, Z.; Cui, J.; Wei, Z. Hydroxyapatite–gelatin nanocomposite as a novel adsorbent for nitrobenzene removal from aqueous solution. Appl. Surf. Sci. 2014, 292, 1020–1029. [Google Scholar] [CrossRef]
- Patil, D.V.; Rallapalli, P.B.S.; Dangi, G.P.; Tayade, R.J.; Somani, R.S.; Bajaj, H.C. MIL-53(AI): An Efficient Adsorbent for the Removal of Nitrobenzene from Aqueous Solutions. Ind. Eng. Chem. Res. 2011, 50, 10516–10524. [Google Scholar] [CrossRef]




| Sample | Crystallinity (%) | ||
|---|---|---|---|
| cellulose | 685 | 106 | 84.53 |
| cellulose-OH | 343 | 152 | 55.69 |
| cellulose-St | 248 | 103 | / |
| Model | C | R2 | ||
|---|---|---|---|---|
| intraparticle diffusion | 1.82801 | 1.49433 | 0.99129 | |
| 0.71501 | 10.50543 | 0.96981 | ||
| 0.05756 | 21.49101 | 0.99727 |
| Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|
| (min−1) | (mg/g) | R2 | (g·mg−1·min−1) | (mg/g) | R2 |
| 0.01238 | 22.7503 | 0.9843 | 0.04182 | 22.7503 | 0.9975 |
| Adsorbent | Desorption Method | Reuse (times) | Re (%) | References |
|---|---|---|---|---|
| cellulose-St | ethanol | ten | 90.1 | this work |
| MPCs | ethanol | six | 82.2 | [25] |
| SCB | ethanol | three | 8.27 | [56] |
| CH3–MCM-41 | toluene and acetone | five | 19 | [20] |
| HCF | ethanol | four | 93 | [57] |
| HAP-GEL | 50% methanol | three | 16.9 | [58] |
| MIL-53(Al) | methanol | three | 87.4 | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Lin, N.; Li, Y.; Ye, X.; Liu, Y.; Lv, Y.; Lin, C.; Liu, M. Preparation of a Novel Cellulose–Styrene Copolymer Adsorbent and Its Adsorption of Nitrobenzene from Aqueous Solutions. Polymers 2021, 13, 609. https://doi.org/10.3390/polym13040609
Yang G, Lin N, Li Y, Ye X, Liu Y, Lv Y, Lin C, Liu M. Preparation of a Novel Cellulose–Styrene Copolymer Adsorbent and Its Adsorption of Nitrobenzene from Aqueous Solutions. Polymers. 2021; 13(4):609. https://doi.org/10.3390/polym13040609
Chicago/Turabian StyleYang, Guifang, Na Lin, Yuan Li, Xiaoxia Ye, Yifan Liu, Yuancai Lv, Chunxiang Lin, and Minghua Liu. 2021. "Preparation of a Novel Cellulose–Styrene Copolymer Adsorbent and Its Adsorption of Nitrobenzene from Aqueous Solutions" Polymers 13, no. 4: 609. https://doi.org/10.3390/polym13040609
APA StyleYang, G., Lin, N., Li, Y., Ye, X., Liu, Y., Lv, Y., Lin, C., & Liu, M. (2021). Preparation of a Novel Cellulose–Styrene Copolymer Adsorbent and Its Adsorption of Nitrobenzene from Aqueous Solutions. Polymers, 13(4), 609. https://doi.org/10.3390/polym13040609







