False Morphology of Aerogels Caused by Gold Coating for SEM Imaging
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Aerogels
2.2. Characterization of Aerogels
2.3. Low Voltage Scanning Electron Microscopy (LVSEM)
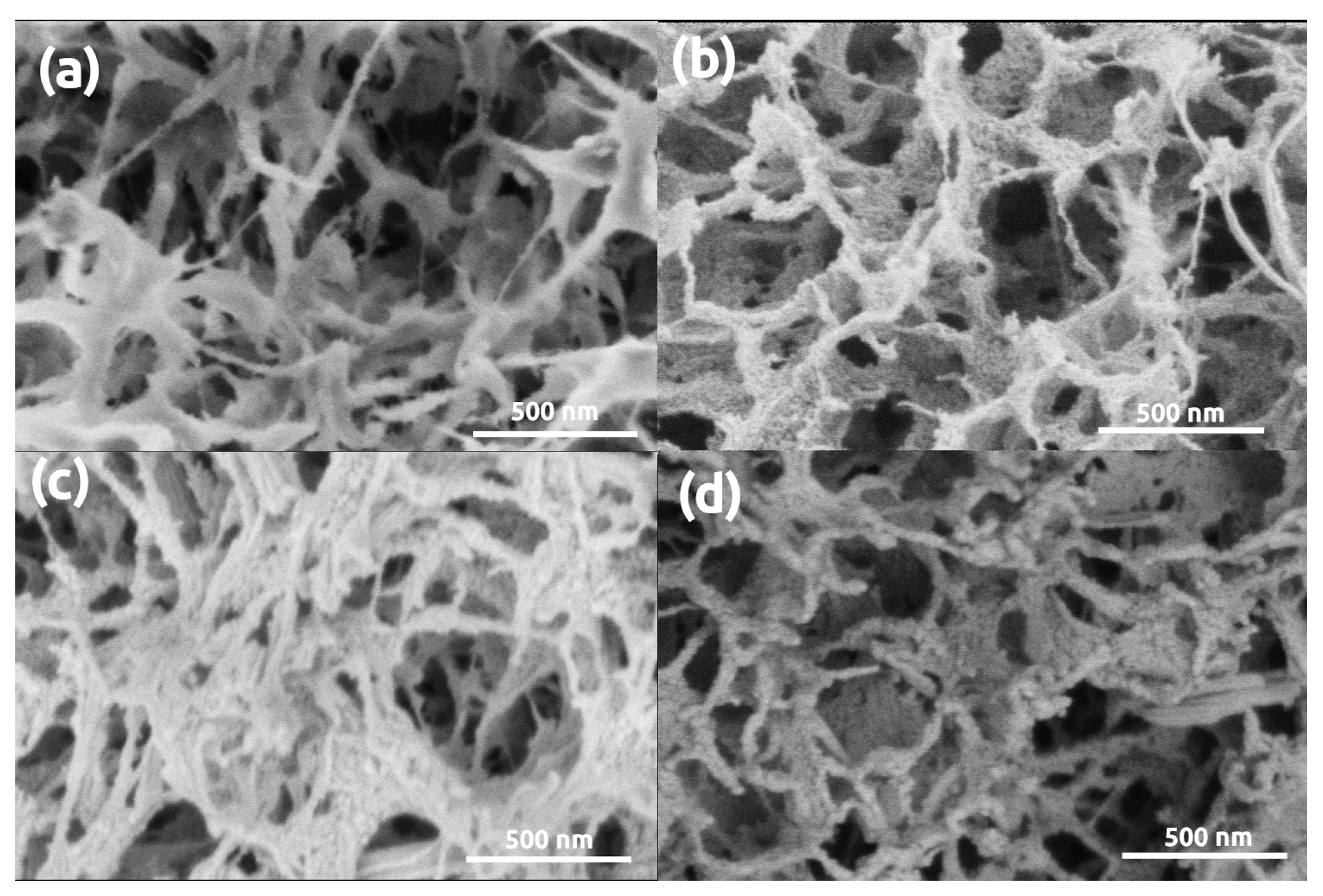
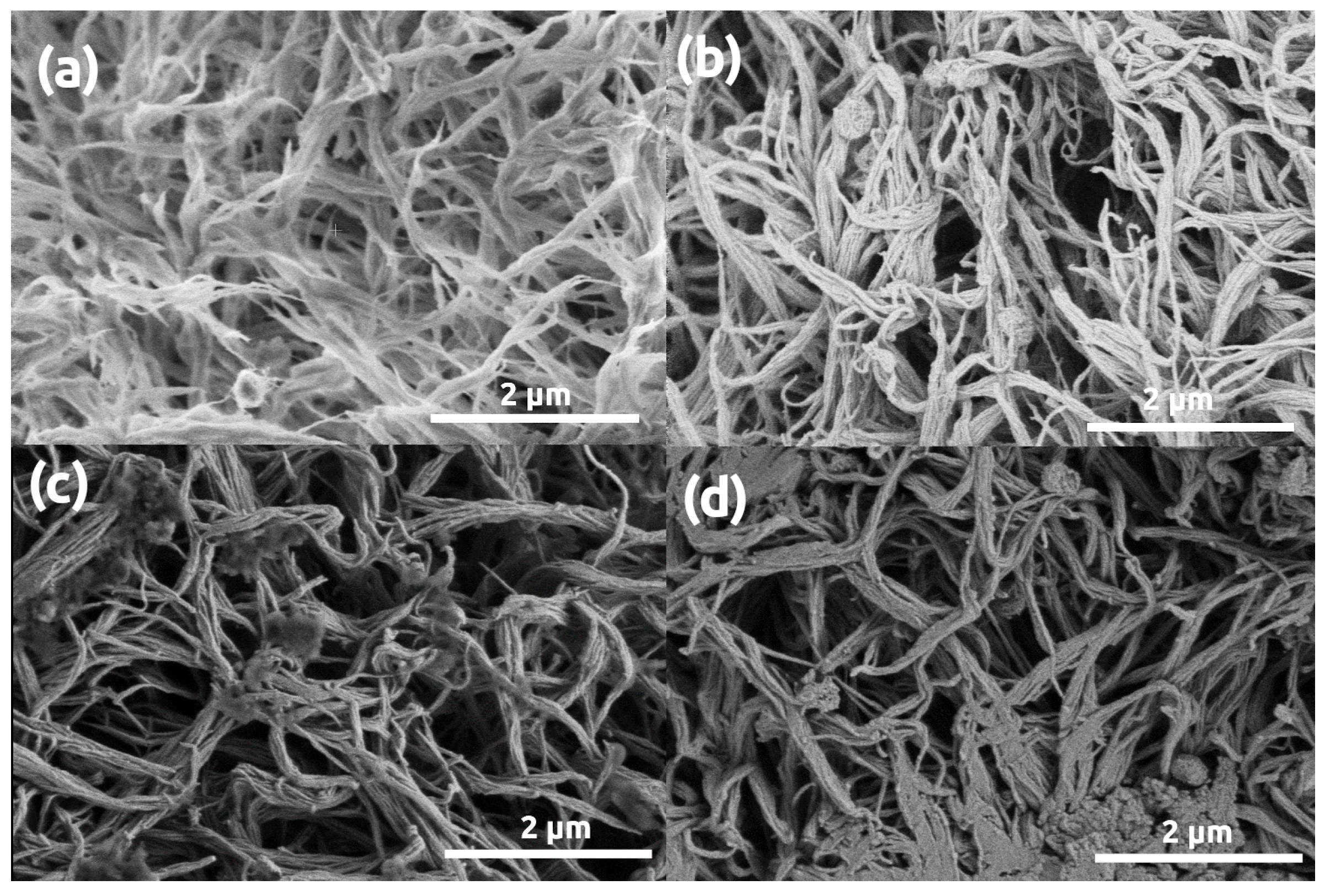
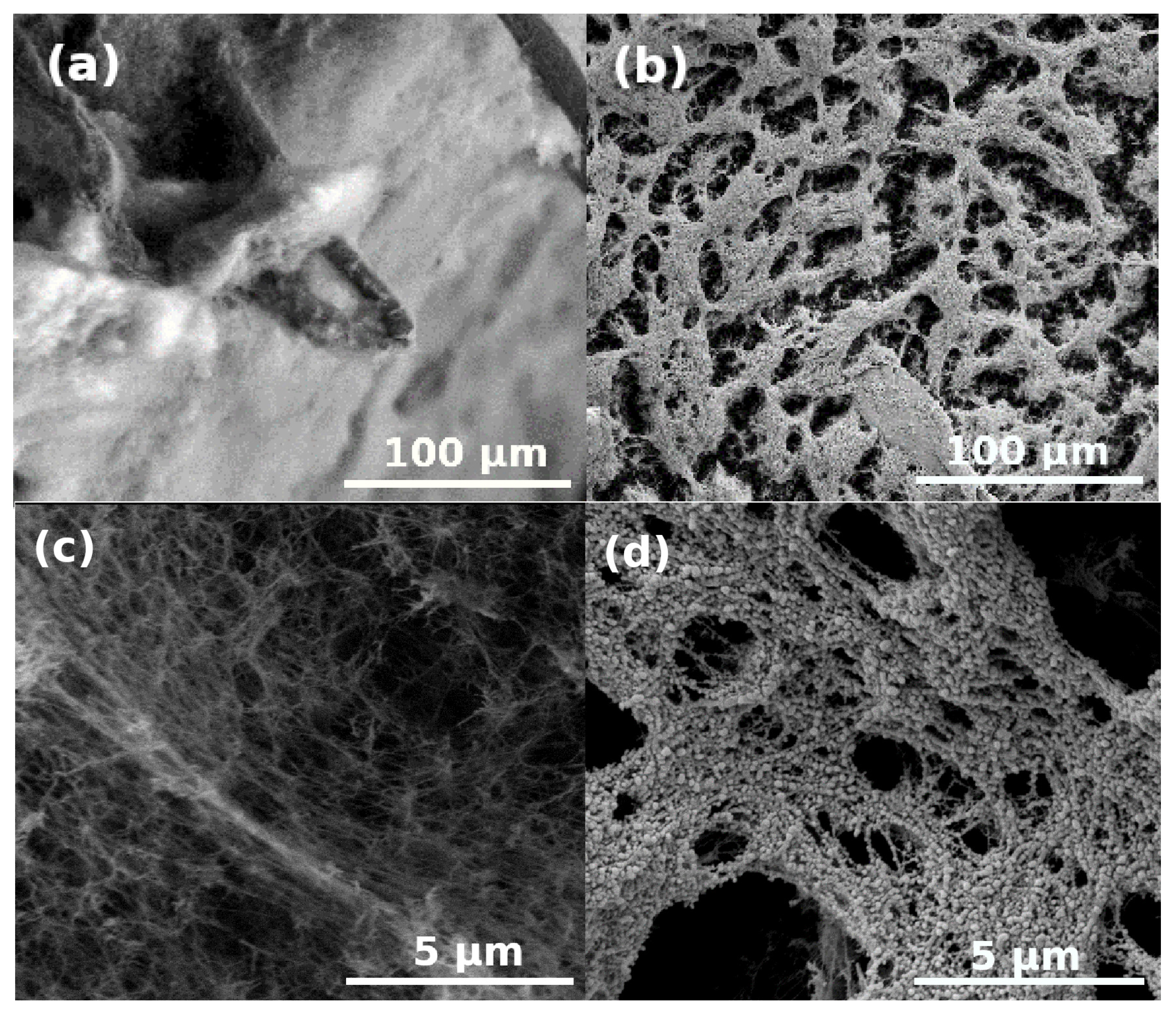
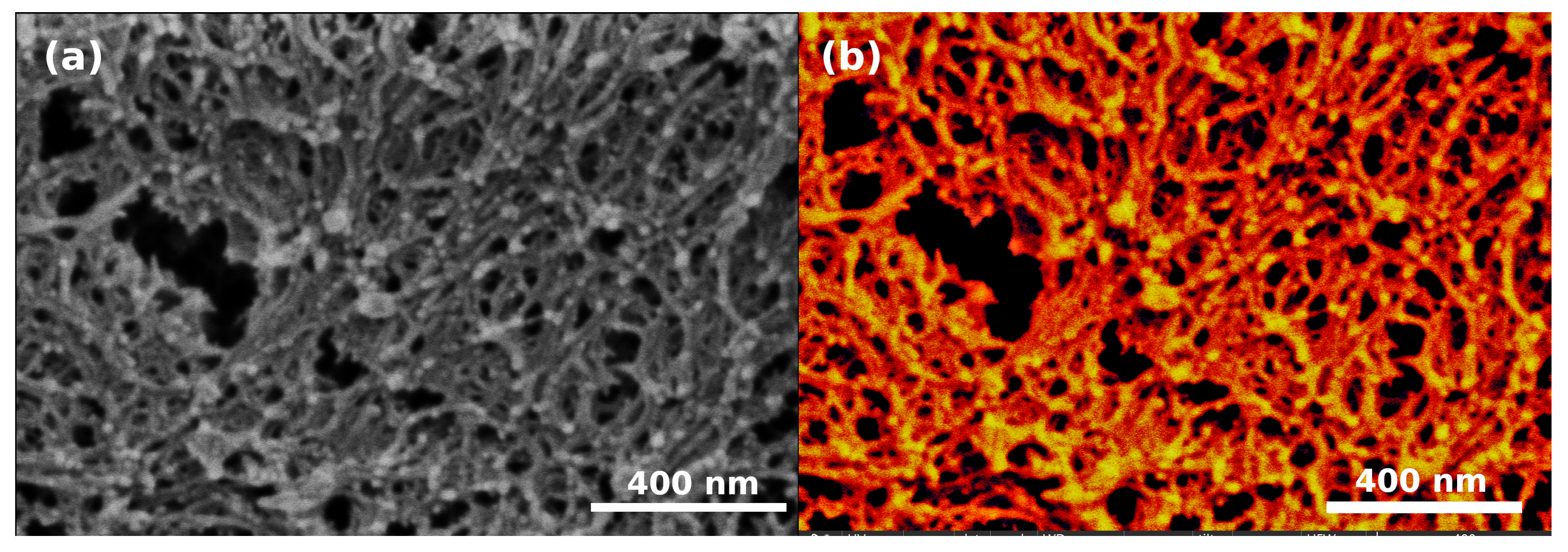
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- García-González, C.; Budtova, T.; Durães, L.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An Opinion Paper on Aerogels for Biomedical and Environmental Applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, I.; Gurikov, P. Aerogel Production: Current Status, Research Directions, and Future Opportunities. J. Supercrit. Fluids 2017, 134, 228–233. [Google Scholar] [CrossRef]
- Ülker, Z.; Erkey, C. An Emerging Platform for Drug Delivery: Aerogel Based Systems. J. Control. Release 2014, 177, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H. Recent Advances in Aerogels for Environmental Remediation Applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.; Del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and Biomedical Applications of Aerogels: Possibilities and Challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Stergar, J.; Maver, U. Review of Aerogel-based Materials in Biomedical Applications. J. Sol Gel Sci. Technol. 2016, 77, 738–752. [Google Scholar] [CrossRef]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the Production of Polysaccharide Aerogel Particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; López-Iglesias, C.; Concheiro, A.; Alvarez-Lorenzo, C. Chapter 16 Biomedical Applications of Polysaccharide and Protein Based Aerogels. In Biobased Aerogels: Polysaccharide and Protein-Based Materials; Sabu, T., Pothan, L.A., Mavelil-Sam, R., Eds.; RSC Chem.: Croydon, UK, 2018; pp. 295–323. [Google Scholar]
- Groult, S.; Budtova, T. Tuning Structure and Properties of Pectin Aerogels. Eur. Polym. J. 2018, 108, 250–261. [Google Scholar] [CrossRef]
- Maleki, H.; Hüsing, N. Current Status, Opportunities and Challenges in Catalytic and Photocatalytic Applications of Aerogels: Environmental Protection Aspects. Appl. Catal. B 2018, 221, 530–555. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Fei, J.; Jia, Y.; Xue, H.; Zhao, J.; Li, J. Self-Assembled Dipeptide Aerogels with Tunable Wettability. Angew. Chem. Int. Ed. 2020, 59, 11932–11936. [Google Scholar] [CrossRef] [PubMed]
- Schwan, M.; Schettler, J.; Badaczewski, F.; Heinrich, C.; Smarsly, B.; Milow, B. The Effect of Pulverization Methods on the Microstructure of Stiff, Ductile, and Flexible Carbon Aerogels. J. Mater. Sci. 2020, 55, 5861–5879. [Google Scholar] [CrossRef]
- Rege, A.; Preibisch, I.; Schestakow, M.; Ganesan, K.; Gurikov, P.; Milow, B.; Smirnova, I.; Itskov, M. Correlating Synthesis Parameters to Morphological Entities: Predictive Modeling of Biopolymer Aerogels. Materials 2018, 11, 1670. [Google Scholar] [CrossRef] [PubMed]
- Buchtová, N.; Budtova, T. Cellulose Aero-, Cryo- and Xerogels: Towards Understanding of Morphology Control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Zu, G.; Kanamori, K.; Maeno, A.; Kaji, H.; Nakanishi, K. Superflexible Multifunctional Polyvinylpolydimethylsiloxane-Based Aerogels as Efficient Absorbents, Thermal Superinsulators, and Strain Sensors. Angew. Chem. Int. Ed. 2018, 57, 9722–9727. [Google Scholar] [CrossRef]
- Quignard, F.; Valentin, R.; Di Renzo, F. Aerogel Materials from Marine Polysaccharides. New J. Chem. 2008, 32, 1300–1310. [Google Scholar] [CrossRef]
- Goncalves, V.; Gurikov, P.; Poejo, J.; Matias, A.; Heinrich, S.; Duarte, C.; Smirnova, I. Alginate-based Hybrid Aerogel Microparticles for Mucosal Drug Delivery. Eur. J. Pharm. Biopharm. 2016, 107, 160. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, P.; Chriti, D.; Raptopoulos, G.; Anyfantis, G.C. Synthetic Polymer Aerogels in Particulate Form. Materials 2019, 12, 1543. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Alemán, C.R.; Hanson, K.; Ramirez, N.; Vivod, S.L.; Wilmoth, N.; McCorkle, L. Polyimide Aerogels with Amide Cross-Links: A Low Cost Alternative for Mechanically Strong Polymer Aerogels. ACS Appl. Mater. Interfaces 2015, 7, 1240–1249. [Google Scholar] [CrossRef]
- Williams, J.C.; Nguyen, B.N.; McCorkle, L.; Scheiman, D.; Griffin, J.S.; Steiner, S.A.; Meador, M.A.B. Highly Porous, Rigid-Rod Polyamide Aerogels with Superior Mechanical Properties and Unusually High Thermal Conductivity. ACS Appl. Mater. Interfaces 2017, 9, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C.; Meador, M.A.B.; McCorkle, L.; Mueller, C.; Wilmoth, N. Synthesis and Properties of Step-Growth Polyamide Aerogels Cross-linked with Triacid Chlorides. Chem. Mater. 2014, 26, 4163–4171. [Google Scholar] [CrossRef]
- Winkler, R.; Ré, E.; Arrachart, G.; Pellet-Rostaing, S. Impact of the Solvent Structuring in Water/tert-butanol Mixtures on the Assembly of Silica Nanoparticles to Aerogels. Langmuir 2019, 35, 7905–7915. [Google Scholar] [CrossRef]
- Talley, S.; Vivod, S.; Nguyen, B.; Meador, M.; Radulescu, A.; Moore, R. Hierarchical Morphology of Poly(ether ether ketone) Aerogels. ACS Appl. Mater. Interfaces 2019, 11, 31508–31519. [Google Scholar] [CrossRef] [PubMed]
- Osorio, D.A.; Lee, B.E.; Kwiecien, J.M.; Wang, X.; Shahid, I.; Hurley, A.L.; Cranston, E.D.; Grandfield, K. Cross-linked Cellulose Nanocrystal Aerogels as Viable Bone Tissue Scaffolds. Acta Biomater. 2019, 87, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Rege, A.; Schwan, M.; Chernova, L.; Hillgärtner, M.; Itskov, M.; Milow, B. Microstructural and Mechanical Characterization of Carbon Aerogels: An In-situ and Digital Image Correlation-based Study. J. Non Cryst. Solids 2020, 529, 119568. [Google Scholar] [CrossRef]
- Ganesan, K.; Barowski, A.; Ratke, L.; Milow, B. Influence of Hierarchical Porous Structures on the Mechanical Properties of Cellulose Aerogels. J. Sol Gel Sci. Technol. 2019, 89, 156–165. [Google Scholar] [CrossRef]
- Ganesan, K.; Barowski, A.; Ratke, L. Gas Permeability of Cellulose Aerogels with a Designed Dual Pore Space System. Molecules 2019, 24, 2688. [Google Scholar] [CrossRef] [PubMed]
- Baldino, L.; Cardea, S.; Scognamiglio, M.; Reverchon, E. A New Tool to Produce Alginate-based Aerogels for Medical Applications, by Supercritical Gel Drying. J. Supercrit. Fluids 2019, 146, 152–158. [Google Scholar] [CrossRef]
- Maleki, H.; Shahbazi, M.A.; Montes, G.S.K.; Hosseini, S.H.; Eskandari, M.R.; Zaunschirm, S.; Verwanger, T.; Mathur, D.S.; Milow, B.; Krammer, B.; et al. Mechanically Strong Silica-Silk Fibroin Bioaerogel: A Hybrid Scaffold with Ordered Honeycomb Micromorphology and Multiscale Porosity for Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 17256–17269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Snellings, G.; Koebel, M.; Malfait, W. Superinsulating Polyisocyanate Based Aerogels: A Targeted Search for the Optimum Solvent System. ACS Appl. Mater. Interfaces 2017, 9, 18222–18230. [Google Scholar] [CrossRef]
- Rege, A.; Ratke, L.; Külcü, İ.D.; Gurikov, P. Stiffening of Biopolymer Aerogel Networks Upon Wetting: A Model-based Study. J. Non Cryst. Solids 2020, 531, 119859. [Google Scholar] [CrossRef]
- Dosta, M.; Jarolin, K.; Gurikov, P. Modelling of Mechanical Behavior of Biopolymer Alginate Aerogels Using the Bonded-Particle Model. Molecules 2019, 24, 2543. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Marco, I.D. Supercritical CO2 Adsorption of Non-steroidal Anti-inflammatory Drugs into Biopolymer Aerogels. J. CO2 Util. 2020, 36, 40–53. [Google Scholar] [CrossRef]
- Gorshkova, N.; Brovko, O.; Palamarchuk, I.; Bogolitsyn, K.; Bogdanovich, N.; Ivakhnov, A.; Chukhchin, D.; Arkhilin, M. Formation of Supramolecular Structure in Alginate/chitosan Aerogel Materials during Sol-gel Synthesis. J. Sol Gel Sci. Technol. 2020, 95, 101–108. [Google Scholar] [CrossRef]
- Rennhofer, H.; Plappert, S.; Lichtenegger, H.; Bernstorff, S.; Fitzka, M.; Nedelec, J.M.; Liebner, F. Insight into the Nanostructure of Anisotropic Cellulose Aerogels upon compression. Soft Matter 2019, 15, 101–108. [Google Scholar] [CrossRef]
- Stachewicz, U.; Szewczyk, P.K.; Kruk, A.; Barber, A.H.; Czyrska-Filemonowicz, A. Pore Shape and Size Dependence on Cell Growth into Electrospun Fiber Scaffolds for Tissue Engineering: 2D and 3D Analyses using SEM and FIB-SEM Tomography. Mater. Sci. Eng. C 2019, 95, 397–408. [Google Scholar] [CrossRef]
- Price, C.W.; McCarthy, P.L. Low-voltage scanning electron microscopy of low-density materials. Scanning 1988, 10, 29–36. [Google Scholar] [CrossRef]
- Lázár, I.; Fábián, I. A Continuous Extraction and Pumpless Supercritical CO2 Drying System for Laboratory-Scale Aerogel Production. Gels 2016, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Kalmár, J.; Kéri, M.; Erdei, Z.; Bányai, I.; Lázár, I.; Lente, G.; Fábián, I. The Pore Network and the Adsorption Characteristics of Mesoporous Silica Aerogel: Adsorption, Kinetics on a Timescale of Seconds. RSC Adv. 2015, 5, 107237–107246. [Google Scholar] [CrossRef]
- Veres, P.; Kéri, M.; Bányai, I.; Lázár, I.; Fábián, I.; Domingo, C.; Kalmár, J. Mechanism of Drug Release from Silica-gelatin Aerogel-Relationship between Matrix Structure and Release Kinetics. Colloids Surf. B 2017, 152, 229–237. [Google Scholar] [CrossRef]
- Kéri, M.; Forgács, A.; Papp, V.; Banyai, I.; Veres, P.; Len, A.; Dudás, Z.; Fábián, I.; Kalmár, J. Gelatin Content Governs Hydration Induced Structural Changes in Silica-gelatin Hybrid Aerogels—Implications in Drug Delivery. Acta Biomater. 2020, 105, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Lázár, I.; Forgács, A.; Horváth, A.; Király, G.; Nagy, G.; Len, A.; Dudás, Z.; Papp, V.; Balogh, Z.; Moldován, K.; et al. Mechanism of Hydration of Biocompatible Silica-casein Aerogels Probed by NMR and SANS Reveal Backbone Rigidity. Appl. Surf. Sci. 2020, 531, 147232. [Google Scholar] [CrossRef]
- Preibisch, I.; Niemeyer, P.; Yusufoglu, Y.; Gurikov, P.; Milow, B.; Smirnova, I. Polysaccharide-Based Aerogel Bead Production via Jet Cutting Method. Materials 2018, 11, 1287. [Google Scholar] [CrossRef]
- Schimper, C.B.; Pachschwoell, P.S.; Hettegger, H.; Neouze, M.A.; Nedelec, J.M.; Wendland, M.; Rosenau, T.; Liebner, F. Aerogels from Cellulose Phosphates of Low Degree of Substitution: A TBAF·H2O/DMSO Based Approach. Molecules 2020, 25, 1695. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J. Influence of Apparatus Geometry and Deposition Condition the Structure and Topography of Thick Sputtered Coatings. J. Vac. Sci. Technol. 1974, 11, 666–670. [Google Scholar] [CrossRef]
- Sree Harsha, K. Chapter 9—Nucleation and Growth of Films. In Principles of Vapor Deposition of Thin Films; Sree Harsha, K., Ed.; Elsevier: Oxford, UK, 2006; pp. 685–829. [Google Scholar]
- Greene, J. Chapter 12—Thin Film Nucleation, Growth, and Microstructural Evolution: An Atomic Scale View. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 554–620. [Google Scholar]
- Švorčík, V.; Kolská, Z.; Luxbacher, T.; Mistrík, J. Properties of Au Nanolayer Sputtered on Polyethyleneterephthalate. Mater. Lett. 2010, 64, 611–613. [Google Scholar] [CrossRef]
- Alfonso, E.; Olaya, J.; Cubillos, G. Thin Film Growth Through Sputtering Technique and Its Applications. In Crystallization; Andreeta, M.R.B., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 397–432. [Google Scholar]
- Pondelak, A.; Rosi, F.; Maurich, C.; Miliani, C.; Škapin, S.D.; Sever Škapin, A. The Role of Relative Humidity on Crystallization of Calcium Carbonate from Calcium Acetoacetate Precursor. Appl. Surf. Sci. 2020, 506, 144768. [Google Scholar] [CrossRef]
- Sołtys-Mróz, M.; Syrek, K.; Pierzchała, J.; Wiercigroch, E.; Malek, K.; Sulka, G.D. Band Gap Engineering of Nanotubular Fe2O3-TiO2 Photoanodes by Wet Impregnation. Appl. Surf. Sci. 2020, 517, 146195. [Google Scholar] [CrossRef]
- Erdman, N.; Bell, D.C.; Reichelt, R. Scanning Electron Microscopy. In Springer Handbook of Microscopy; Hawkes, P., Spence, J.C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 229–318. [Google Scholar]
- Huber, L.; Zhao, S.; Malfait, W.J.; Vares, S.; Koebel, M.M. Fast and Minimal-Solvent Production of Superinsulating Silica Aerogel Granulate. Angew. Chem. Int. Ed. 2017, 56, 4753–4756. [Google Scholar] [CrossRef] [PubMed]
- Pircher, N.; Carbajal, L.; Schimper, C.B.; Bacher, M.; Rennhofer, H.; Nedelec, J.M.; Lichtenegger, H.; Rosenau, T.; Liebner, F. Impact of Selected Solvent Systems on the Pore and Solid Structure of Cellulose Aerogels. Cellulose 2016, 23, 1949–1966. [Google Scholar] [CrossRef] [PubMed]
- Hovington, P.; Drouin, D.; Gauvin, R. CASINO: A new monte carlo code in C language for electron beam interaction—Part I: Description of the program. Scanning 1997, 19. [Google Scholar] [CrossRef]
- Al-Alwany, R. Effect of Working Pressure on the Structural and Morphological Properties of Gold Nanoparticles Prepared by a DC Magnetron Sputtering Technique. J. Crit. Rev. 2020, 7, 1–5. [Google Scholar] [CrossRef]
- Barna, P.; Adamik, M. Fundamental Structure Forming Phenomena of Polycrystalline Films and the Structure Zone Models. Thin Solid Film. 1998, 317, 27–33. [Google Scholar] [CrossRef]
- Ron, R.; Gachet, D.; Rechav, K.; Salomon, A. Direct Fabrication of 3D Metallic Networks and Their Performance. Adv. Mater. 2017, 29, 1604018. [Google Scholar] [CrossRef]
- Kim, H.R.; Park, H.H. The Effect of Ar+Ion Bombardment on SiO2 Aerogel Film. JJAP 1998, 37, 6955–6958. [Google Scholar] [CrossRef]
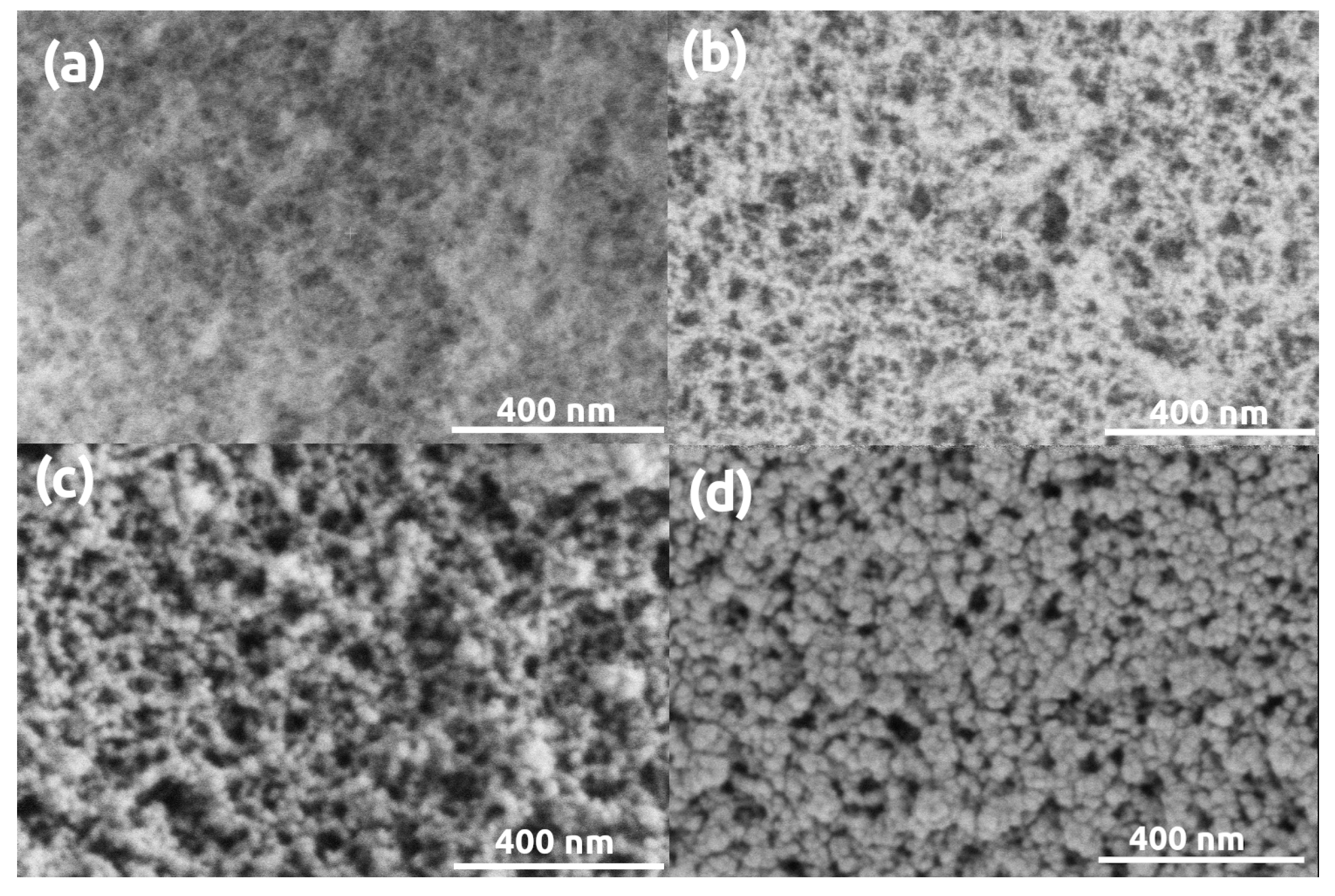
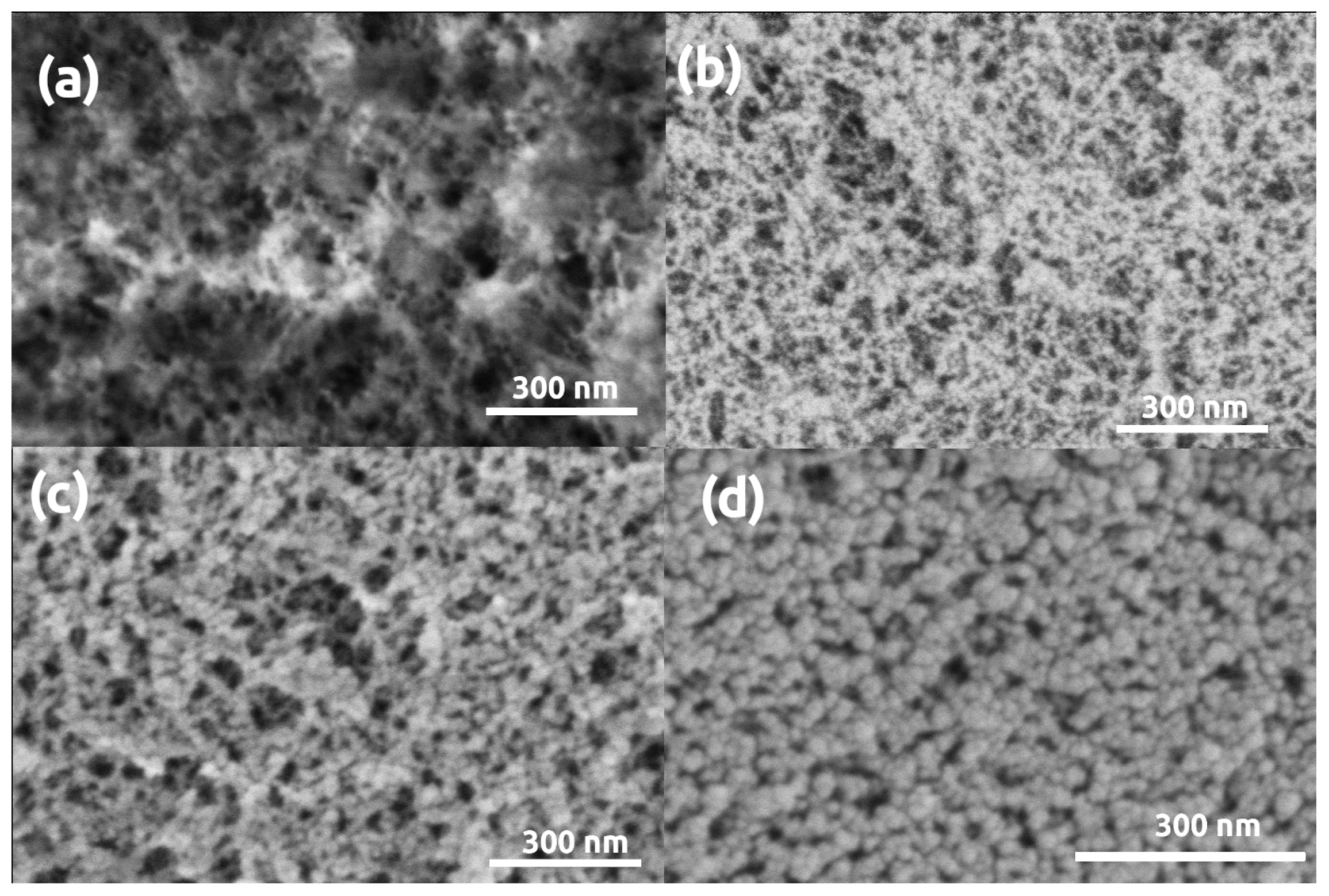
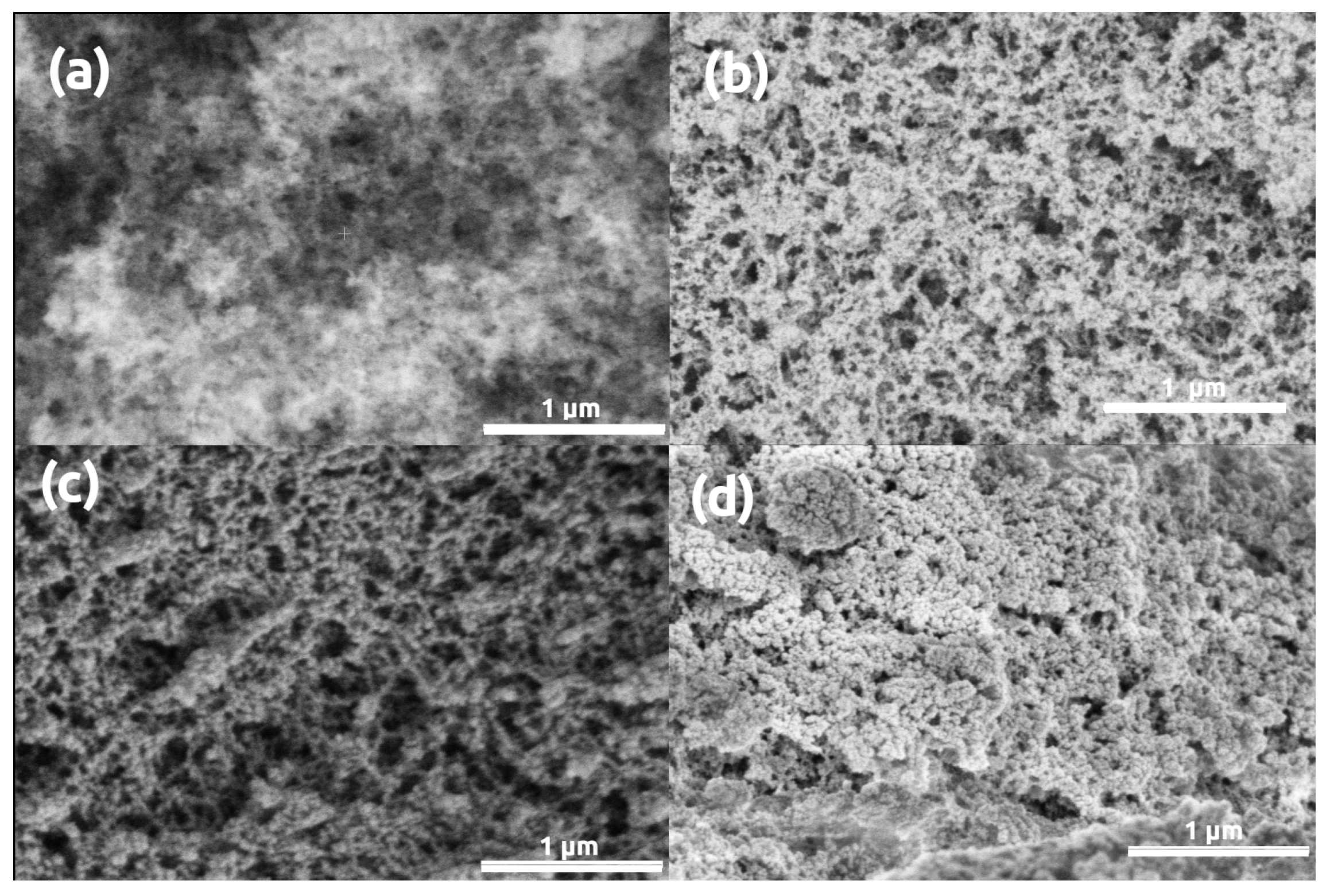
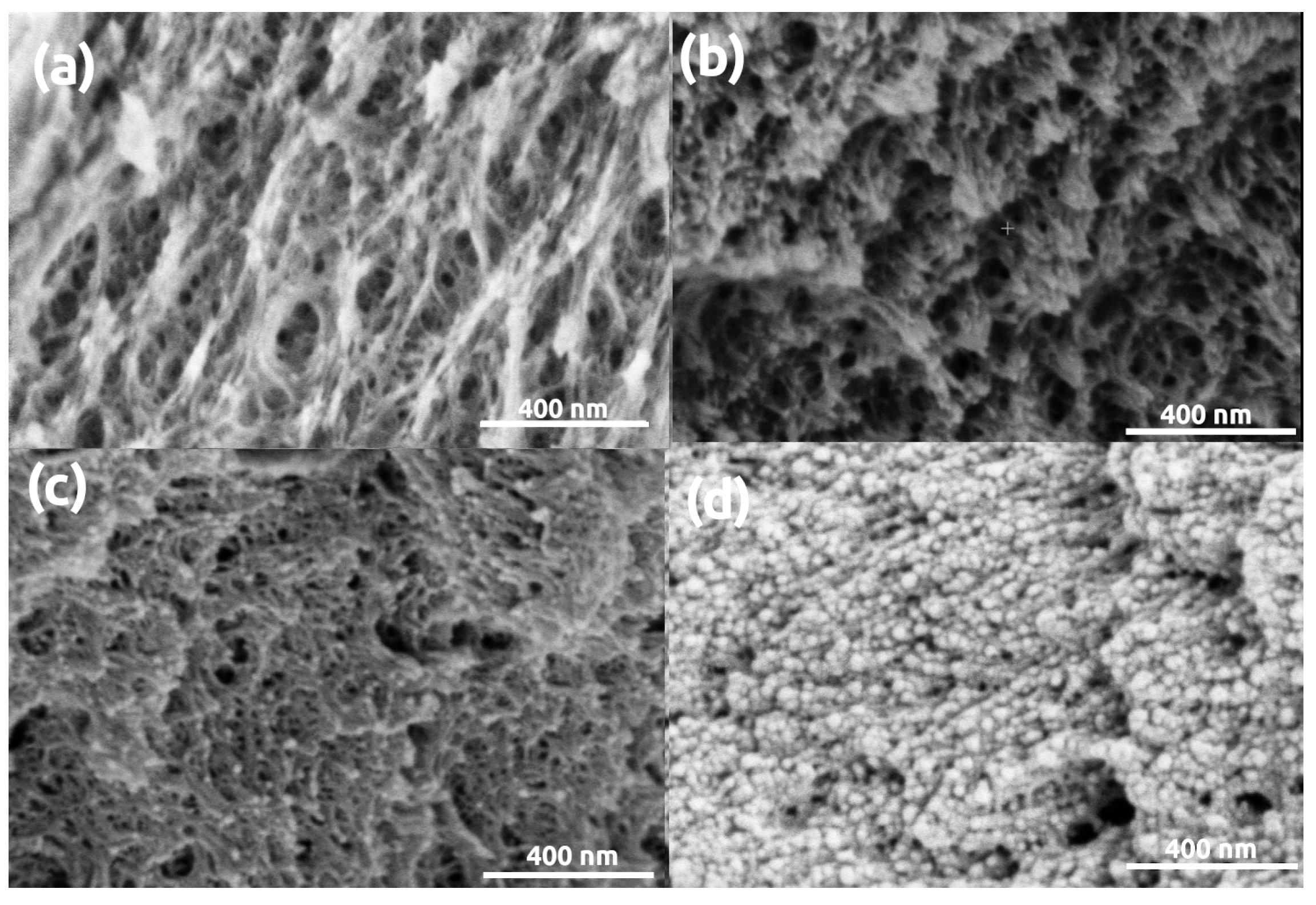

| Parameter | Silica | Silica- Gelatin | Silica- Casein | Ca- Alginate | Poly- Imide | Poly- Amide | Poly- Amide (Ca(II)) | Method |
|---|---|---|---|---|---|---|---|---|
| Specific | ||||||||
| surface area (m g) | 898 | 742 | 750 | 544 | 297 | 245 | 251 | BET |
| Mean pore | ||||||||
| size (nm) | 15 | 14 | 17 | 42 | 3.8 | 3.4 | 4.3 | BJH |
| Total pore | ||||||||
| volume (cm g) | 7.5 | 3.2 | 3.2 | 7.8 | 0.8 | 0.8 | 0.7 | BJH |
| C-constant | 109 | 62 | 71 | - | 55 | 45 | 59 | BET |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhász, L.; Moldován, K.; Gurikov, P.; Liebner, F.; Fábián, I.; Kalmár, J.; Cserháti, C. False Morphology of Aerogels Caused by Gold Coating for SEM Imaging. Polymers 2021, 13, 588. https://doi.org/10.3390/polym13040588
Juhász L, Moldován K, Gurikov P, Liebner F, Fábián I, Kalmár J, Cserháti C. False Morphology of Aerogels Caused by Gold Coating for SEM Imaging. Polymers. 2021; 13(4):588. https://doi.org/10.3390/polym13040588
Chicago/Turabian StyleJuhász, Laura, Krisztián Moldován, Pavel Gurikov, Falk Liebner, István Fábián, József Kalmár, and Csaba Cserháti. 2021. "False Morphology of Aerogels Caused by Gold Coating for SEM Imaging" Polymers 13, no. 4: 588. https://doi.org/10.3390/polym13040588
APA StyleJuhász, L., Moldován, K., Gurikov, P., Liebner, F., Fábián, I., Kalmár, J., & Cserháti, C. (2021). False Morphology of Aerogels Caused by Gold Coating for SEM Imaging. Polymers, 13(4), 588. https://doi.org/10.3390/polym13040588










