Alignment Layer of Liquid Crystal Using Plant-Based Isoeugenol-Substituted Polystyrene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Isoeugenol-Modified Polystyrene
2.3. Film Preparation and LC Cell Assembly
2.4. Instrumentation
3. Results
3.1. Synthesis and Characterization of Isoeugenol-Modified Polystyrene
3.2. LC Orientation Behavior of the LC Cell Fabricated with Isoeugenol-Modified Polystyrene Film
3.3. Surface Properties of Isoeugenol-Modified Polystyrene Films
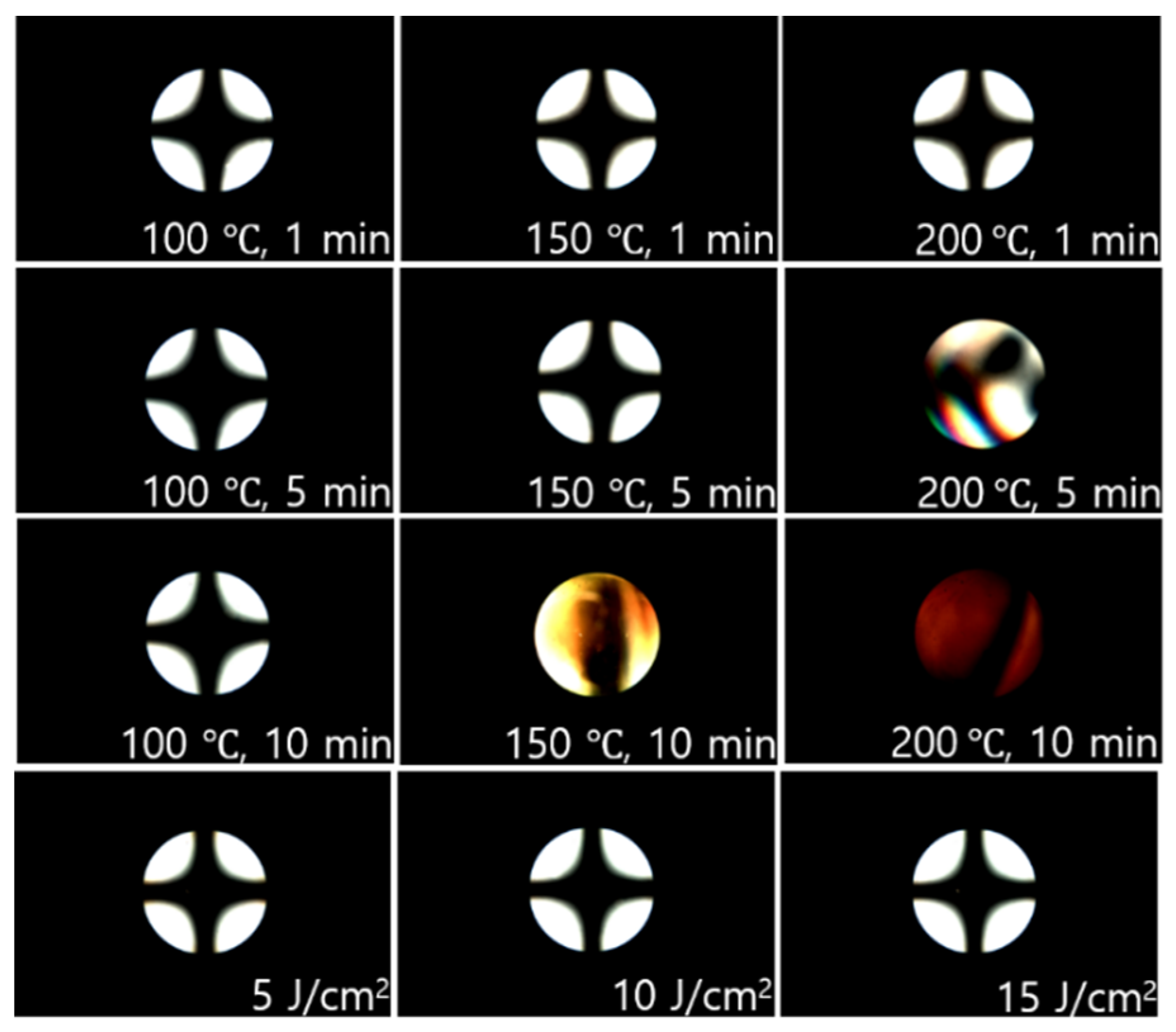
3.4. Reliability and Electro-Optical Performance of the LC Cells Fabricated with Isoeugenol-Modified Polystyrene Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enfrin, M.; Dumée, L.F.; Lee, J. Nano/microplastics in water and wastewater treatment processes–origin, impact and potential solutions. Water Res. 2019, 161, 621–638. [Google Scholar] [CrossRef]
- Our World in Data. Plastic Pollution. Available online: https://ourworldindata.org/plastic-pollution (accessed on 1 September 2018).
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Landon-Lane, M. Corporate social responsibility in marine plastic debris governance. Mar. Pollut. Bull. 2018, 127, 310–319. [Google Scholar] [CrossRef]
- Kreiger, M.; Anzalone, G.C.; Mulder, M.L.; Glover, A.; Pearce, J.M. Distributed recycling of post-consumer plastic waste in rural areas. MRS Online Proc. Libr. 2012, 1492, 101–106. [Google Scholar] [CrossRef]
- The Balance. The Decomposition of Waste in Landfills. Available online: www.thebalance.com/how-long-does-it-take-garbage-to-decompose-2878033 (accessed on 22 October 2019).
- Alabi, O.A.; Ologbonjaye, K.I.; Awosolu, O.; Alalade, O.E. Public and environmental health effects of plastic wastes disposal: A review. J. Toxicol. Risk Assess. 2019, 5, 21. [Google Scholar]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- De Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future. Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution-removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kang, S.; Allen, S.; Allen, D.; Gao, T.; Sillanpää, M. Atmospheric microplastics: A review on current status and perspectives. Earth-Sci. Rev. 2020, 203, 103118. [Google Scholar] [CrossRef]
- Eriksen, M.; Thiel, M.; Prindiville, M.; Kiessling, T. Microplastic: What are the solutions. Freshw. Microplastics 2018, 58, 273–298. [Google Scholar]
- Coppock, R.L.; Cole, M.; Lindeque, P.K.; Queirós, A.M.; Galloway, T.S. A small-scale, portable method for extracting microplastics from marine sediments. Environ. Pollut. 2017, 230, 829–837. [Google Scholar] [CrossRef]
- Chen, C.; Chen, L.; Li, Y.; Fu, W.; Shi, X.; Duan, J.; Zhang, W. Impacts of microplastics on organotins’ photodegradation in aquatic environments. Environ. Pollut. 2020, 267, 115686. [Google Scholar] [CrossRef] [PubMed]
- Mitrano, D.M.; Wohlleben, W. Microplastic regulation should be more precise to incentivize both innovation and environmental safety. Nat. Commun. 2020, 11, 5324. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, F.M.; Román-Ramírez, L.A.; Wood, J. Recycling of bioplastics: Routes and benefits. J. Polym. Environ. 2020, 28, 1–21. [Google Scholar] [CrossRef]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef]
- Chen, X.; Yan, N. A brief overview of renewable plastics. Mater. Today Sustain. 2020, 7–8, 100031. [Google Scholar] [CrossRef]
- Abdul-Latif, N.; Ong, M.Y.; Nomanbhay, S.; Salman, B.; Show, P.L. Estimation of carbon dioxide (CO2) reduction by utilization of algal biomass bioplastic in malaysia using carbon emission pinch analysis (CEPA). Bioengineered 2020, 11, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Philp, J.C.; Ritchie, R.J.; Guy, K. Biobased plastics in a bioeconomy. Trends Biotechnol. 2013, 31, 65–67. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Meeker, J.D.; Sathyanarayana, S.; Swan, S.H. Phthalates and other additives in plastics: Human exposure and associated health outcomes. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2097–2113. [Google Scholar] [CrossRef]
- Talsness, C.E.; Andrade, A.J.; Kuriyama, S.N.; Taylor, J.A.; Vom Saal, F.S. Components of plastic: Experimental studies in animals and relevance for human health. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2079–2096. [Google Scholar] [CrossRef]
- Perotto, G.; Ceseracciu, L.; Simonutti, R.; Paul, U.C.; Guzman-Puyol, S.; Tran, T.; Bayer, I.S.; Athanassiou, A. Bioplastics from vegetable waste via an eco-friendly water-based process. Green Chem. 2018, 20, 894–902. [Google Scholar] [CrossRef]
- Jain, R.; Tiwari, A. Biosynthesis of planet friendly bioplastics using renewable carbon source. J. Environ. Health Sci. Eng. 2015, 13, 11. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef]
- Schoukens, G.; Breen, C.; Baschetti, M.G.; Elegir, G.; Vähä-Nissi, M.; Liu, Q.; Tiekstra, S.; Simon, P. Complex packaging structures based on wood derived products: Actual and future possibilities for 1-way food packages. J. Mater. Sci. Res. 2014, 3, 58. [Google Scholar] [CrossRef]
- Ruiz, Q.; Pourchet, S.; Placet, V.; Plasseraud, L.; Boni, G. New eco-friendly synthesized thermosets from isoeugenol-based epoxy resins. Polymers 2020, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Koeduka, T.; Fridman, E.; Gang, D.R.; Vassao, D.G.; Jackson, B.L.; Kish, C.M.; Orlova, I.; Spassova, S.M.; Lewis, N.G.; Noel, J.P. Eugenol and isoeugenol, caracteristic aromatic constituents of spices, are biosynthesized via reduction of a coniferyl alcohol ester. Proc. Natl. Acad. Sci. USA 2006, 103, 10128–10133. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, P.; Han, S.; Yan, H.; Ma, C. Metabolism of isoeugenol via isoeugenol-diol by a newly isolated strain of bacillus subtilis HS8. Appl. Microbiol. Biotechnol. 2006, 73, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.C.; Johansen, J.D. Significant exposures to isoeugenol derivatives in perfumes. Contact Derm. 2008, 58, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Topal, F. Anticholinergic and antidiabetic effects of isoeugenol from clove (Eugenia caryophylata) oil. Int. J. Food Prop. 2019, 22, 583–592. [Google Scholar] [CrossRef]
- Shimoni, E.; Ravid, U.; Shoham, Y. Isolation of a Bacillus sp. capable of transforming isoeugenol to vanillin. J. Biotechnol. 2000, 78, 1–9. [Google Scholar] [CrossRef]
- Atsumi, T.; Fujisawa, S.; Tonosaki, K. A comparative study of the antioxidant/prooxidant activities of eugenol and isoeugenol with various concentrations and oxidation conditions. Toxicol. Vitr. 2005, 19, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Fındık, E.; Ceylan, M.; Elmastaş, M. Isoeugenol-based novel potent antioxidants: Synthesis and reactivity. Eur. J. Med. Chem. 2011, 46, 4618–4624. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.C.; Zon, M.A.; Fernández, H.; Granero, A.M.; Robledo, S.N. Determination of kinetic parameters of the enzymatic reaction between soybean peroxidase and natural antioxidants using chemometric tools. Food Chem. 2019, 275, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.F.M.; Monte, F.J.Q.; de Lemos, T.L.G.; Do Nascimento, P.G.G.; de Medeiros Costa, A.K.; De Paiva, L.M.M. Eugenol derivatives: Synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J. 2018, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Bortolomeazzi, R.; Verardo, G.; Liessi, A.; Callea, A. Formation of dehydrodiisoeugenol and dehydrodieugenol from the reaction of isoeugenol and eugenol with DPPH radical and their role in the radical scavenging activity. Food Chem. 2010, 118, 256–265. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: An updated review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of eugenol: A structure-activity relationship study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Biswas, S.; Das, R.; Banerjee, E.R. Role of free radicals in human inflammatory diseases. AIMS Biophys. 2017, 4, 596–614. [Google Scholar] [CrossRef]
- Sainz, R.M.; Lombo, F.; Mayo, J.C. Radical decisions in cancer: Redox control of cell growth and death. Cancers 2012, 4, 442–474. [Google Scholar] [CrossRef]
- Liu, Z. Chemical methods to evaluate antioxidant ability. Chem. Rev. 2010, 110, 5675–5691. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef] [PubMed]
- Zuo, A.; Dong, H.; Yu, Y.; Shu, Q.; Zheng, L.; Yu, X.; Cao, S. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin. Med. 2018, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Sohilait, H.J.; Kainama, H. Free radical scavenging activity of essential oil of eugenia caryophylata from amboina island and derivatives of eugenol. Open Chem. 2019, 17, 422–428. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Obayes, H.R.; Mohamad, A.B. Synthesis and antioxidant activities of novel 5-chlorocurcumin, complemented by semiempirical calculations. Bioinorg. Chem. Appl. 2013, 2013, 354982. [Google Scholar] [CrossRef] [PubMed]
- Aadesariya, M.K.; Ram, V.R.; Dave, P.N. Evaluation of antioxidant activities by use of various extracts from abutilon pannosum and grewia tenax leaves in the kachchh region. MOJ Food Process. Technol. 2017, 17, 359. [Google Scholar]
- Nielsen, C.K.; Kjems, J.; Mygind, T.; Snabe, T.; Schwarz, K.; Serfert, Y.; Meyer, R.L. Enhancing the antibacterial efficacy of isoeugenol by emulsion encapsulation. Int. J. Food Microbiol. 2016, 229, 7–14. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Chafer, M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Nielsen, C.K.; Subbiahdoss, G.; Zeng, G.; Salmi, Z.; Kjems, J.; Mygind, T.; Snabe, T.; Meyer, R.L. Antibacterial isoeugenol coating on stainless steel and polyethylene surfaces prevents biofilm growth. J. Appl. Microbiol. 2018, 124, 179–187. [Google Scholar] [CrossRef]
- Kather, M.; Skischus, M.; Kandt, P.; Pich, A.; Conrads, G.; Neuss, S. Functional isoeugenol-modified nanogel coatings for the design of biointerfaces. Angew. Chem. Int. 2017, 56, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, X.; Pan, J.; Zhang, G.; Gong, D. Interaction of isoeugenol with calf thymus DNA and its protective effect on DNA oxidative damage. J. Mol. Liq. 2019, 282, 356–365. [Google Scholar] [CrossRef]
- Medeiros, D.; Oliveira-Júnior, J.; Nóbrega, J.; Cordeiro, L.; Jardim, J.; Souza, H.; Silva, G.; Athayde-Filho, P.; Barbosa-Filho, J.; Scotti, L. Isoeugenol and hybrid acetamides against candida albicans isolated from the oral cavity. Pharmaceuticals 2020, 13, 291. [Google Scholar] [CrossRef]
- Ju, C.; Kim, T.; Kang, H. Renewable, eugenol-modified polystyrene layer for liquid crystal orientation. Polymers 2018, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Kim, T.; Kang, H. Liquid crystal alignment behaviors on capsaicin substituted polystyrene films. RSC Adv. 2017, 7, 41376–41383. [Google Scholar] [CrossRef]
- Ju, C.; Kang, S.; Kim, T.; Park, C.; Kang, H. Vertical liquid crystal alignment of comb-like alkyl hydroxybenzoate-substituted polystyrene. Crystals 2019, 9, 281. [Google Scholar] [CrossRef]
- Shen, Y.; Dierking, I. Perspectives in liquid-crystal-aided nanotechnology and nanoscience. Appl. Sci. 2019, 9, 2512. [Google Scholar] [CrossRef]
- Williams, C.K.; Hillmyer, M.A. Polymers from renewable resources: A perspective for a special issue of polymer reviews. Polym. Rev. 2008, 48, 1–10. [Google Scholar] [CrossRef]
- Kato, T.; Gupta, M.; Yamaguchi, D.; Gan, K.P.; Nakayama, M. Supramolecular association and nanostructure formation of liquid crystals and polymers for new functional materials. Bull. Chem. Soc. Jpn. 2021, 94, 357–376. [Google Scholar] [CrossRef]
- Manda, R.; Pagidi, S.; Heo, Y.; Lim, Y.J.; Kim, M.; Lee, S.H. Electrically tunable photonic band gap structure in monodomain blue-phase liquid crystals. NPG Asia Mater. 2020, 12, 42. [Google Scholar] [CrossRef]
- Xia, C.; Zhou, D.; Su, Y.; Zhou, G.; Yao, L.; Sun, W.; Liu, Y. A liquid-crystal-based immunosensor for the detection of cardiac troponin I. Analyst 2020, 145, 4569–4575. [Google Scholar] [CrossRef]
- Yan, Z.; Yao, J.; Hou, Y.; Zhou, J.; Sun, J.; Huang, X. Polarized optical properties in liquid crystals devices with photoaligned metal nanoparticle gratings. Appl. Phys. A 2021, 127, 1–7. [Google Scholar] [CrossRef]
- Ju, C.; Park, C.; Kim, T.; Kang, H. Vertical alignment of liquid crystals on plant-based vanillin derivative-substituted polystyrene films. RSC Adv. 2019, 9, 14188–14193. [Google Scholar] [CrossRef]
- Yoon, W.; Lee, K.M.; Evans, D.R.; McConney, M.E.; Kim, D.; Jeong, K. Giant surfactants for the construction of automatic liquid crystal alignment layers. J. Mater. Chem. C 2019, 7, 8500–8514. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, S.I.; Park, Y.H.; Reea, M.; Rim, Y.N.; Yoon, H.J.; Kim, H.C.; Kim, Y.B. Liquid-crystal alignment on the rubbed film surface of semi-flexible copolyimides containing n-alkyl side groups. Mol. Cryst. Lip. Cryst. 2000, 349, 279–282. [Google Scholar] [CrossRef]
- Kim, T.; Ju, C.; Kang, H. Vertical alignment of liquid crystal on tocopherol-substituted polystyrene films. Liq. Cryst. 2018, 45, 801–810. [Google Scholar] [CrossRef]
- Tsuda, Y.; Matsuda, Y.; Matsuda, T. Soluble polyimides bearing long-chain alkyl groups on their side chain via polymer reaction. Int. J. Polym. Sci. 2012, 2012, 972541. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Morphology evolution of functionalized styrene and methyl methacrylate copolymer latex nanoparticles by one-step emulsifier-free emulsion polymerization. Eur. Polym. J. 2020, 133, 109790. [Google Scholar] [CrossRef]
- Niknejad, A.S.; Bazgir, S.; Sadeghzadeh, A.; Shirazi, M.M.A. Styrene-acrylonitrile (SAN) nanofibrous membranes with unique properties for desalination by direct contact membrane distillation (DCMD) process. Desalination 2020, 488, 114502. [Google Scholar] [CrossRef]
- Döğüşcü, D.K.; Damlıoğlu, Y.; Alkan, C. Poly (Styrene-Co-Divinylbenzene-Co-Acrylamide)/N-octadecane microencapsulated phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2019, 198, 5–10. [Google Scholar] [CrossRef]
- Straus, S.; Madorsky, S.L. Thermal stability of polydivinylbenzene and of copolymers of styrene with divinylbenzene and with trivinylbenzene. J. Res. Natl. Bur. Stand. A Phys. Chem. 1961, 65, 243. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kwon, K.; Kang, D.; Lee, J. Enhanced, perpendicular liquid-crystal alignment on rubbed films of a coumarin-containing polystyrene. Macromol. Chem. Phys. 2007, 208, 1853–1861. [Google Scholar] [CrossRef]
- Kim, T.; Ju, C.; Kang, H. Vertical liquid crystal orientation of phytochemical-based oryzanol modified polystyrene. RSC Adv. 2018, 8, 1569–1575. [Google Scholar] [CrossRef]
- Kim, T.; Ju, C.; Park, C.; Kang, H. Enhanced, parallel liquid crystal alignment based on polystyrene substituted with phthalimidoyl groups. RSC Adv. 2019, 9, 9755–9761. [Google Scholar] [CrossRef]
- Kang, H.; Lee, J.; Nam, B.; Bae, J.W. Liquid crystal alignment behavior on transparent cellulose films. RSC Adv. 2015, 5, 38654–38659. [Google Scholar] [CrossRef]
- Li, X.; Yanagimachi, T.; Bishop, C.; Smith, C.; Dolejsi, M.; Xie, H.; Kurihara, K.; Nealey, P.F. Engineering the anchoring behavior of nematic liquid crystals on a solid surface by varying the density of liquid crystalline polymer brushes. Soft Matter 2018, 14, 7569–7577. [Google Scholar] [CrossRef]
- Cai, F.; Zheng, F.; Lu, X.; Lu, Q. Control of the alignment of liquid crystal molecules on a sequence-polymerized film by surface migration and polarized light irradiation. Polym. Chem. 2017, 8, 7316–7324. [Google Scholar] [CrossRef]
- Costa, E.; Aquilano, D. Experimental value of the specific surface energy of the cleavage {10.4} calcite rhombohedron in the presence of its saturated aqueous solution. Crystals 2018, 8, 238. [Google Scholar] [CrossRef]
- Bandzierz, K.; Reuvekamp, L.; Dryzek, J.; Dierkes, W.; Blume, A.; Bielinski, D. Influence of network structure on glass transition temperature of elastomers. Materials 2016, 9, 607. [Google Scholar] [CrossRef]
- White, R.P.; Lipson, J.E. Polymer free volume and its connection to the glass transition. Macromolecules 2016, 49, 3987–4007. [Google Scholar] [CrossRef]
- Gedde, U. Polymer Physics; Springer: Dordrecht, The Netherlands, 1995; pp. 79–82. [Google Scholar]
- Kang, H.; Seo, J.G.; Kang, D.; Lee, J. Liquid crystal alignment properties of poly (styrenesulphonate)/alkyltrimethylammonium complexes. Liq. Cryst. 2013, 40, 492–498. [Google Scholar] [CrossRef]
- Kang, H.; Park, J.S.; Sohn, E.; Kang, D.; Rosenblatt, C.; Lee, J. Polyimide blend alignment layers for control of liquid crystal pretilt angle through baking. Polymer 2009, 50, 5220–5227. [Google Scholar] [CrossRef]
- Liu, B.; Meng, C.; Chen, L. Role of monomer alkyl chain length in pretilt angle control of polymer-stabilized liquid crystal alignment system. J. Phys. Chem. C 2017, 121, 21037–21044. [Google Scholar] [CrossRef]

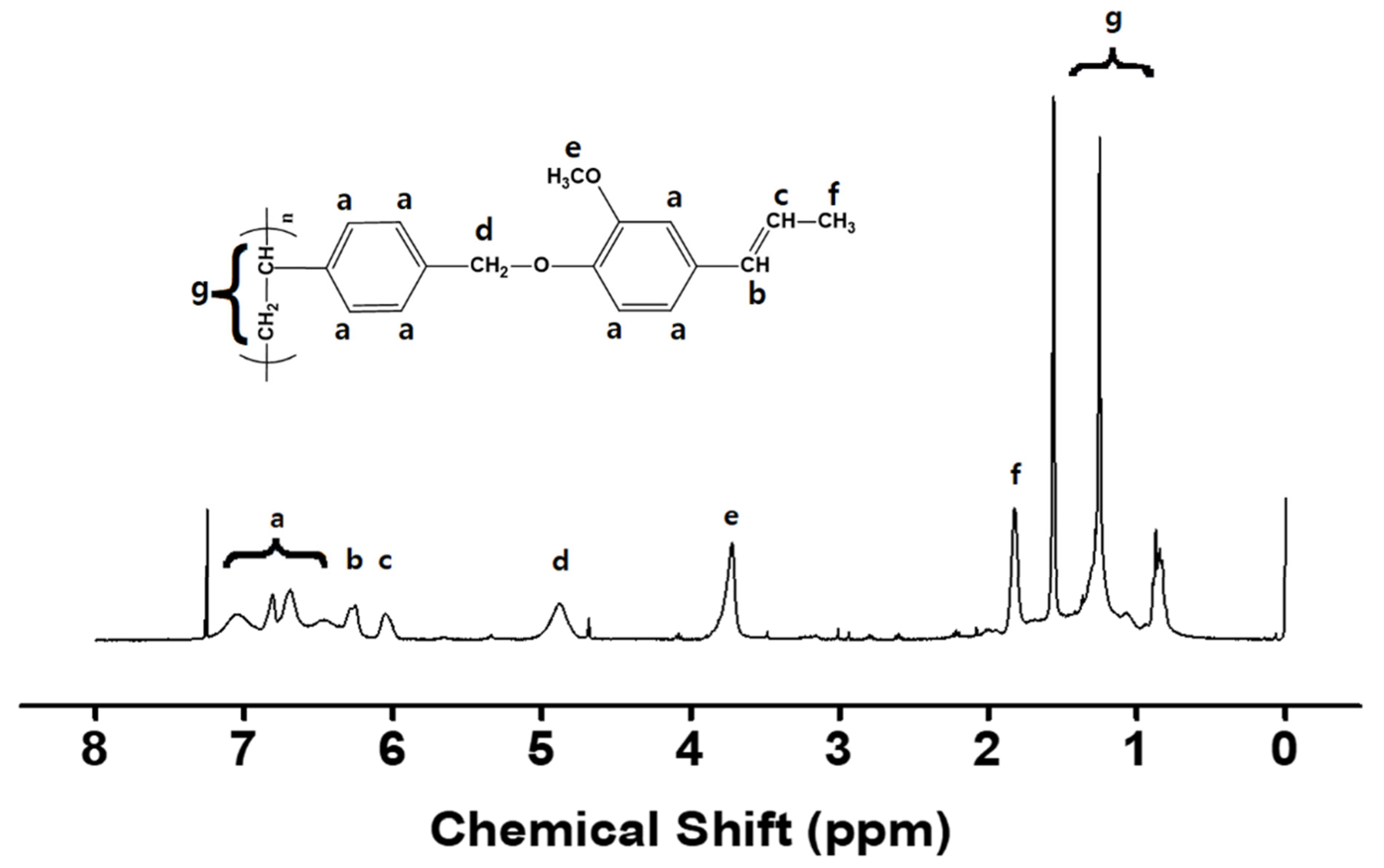
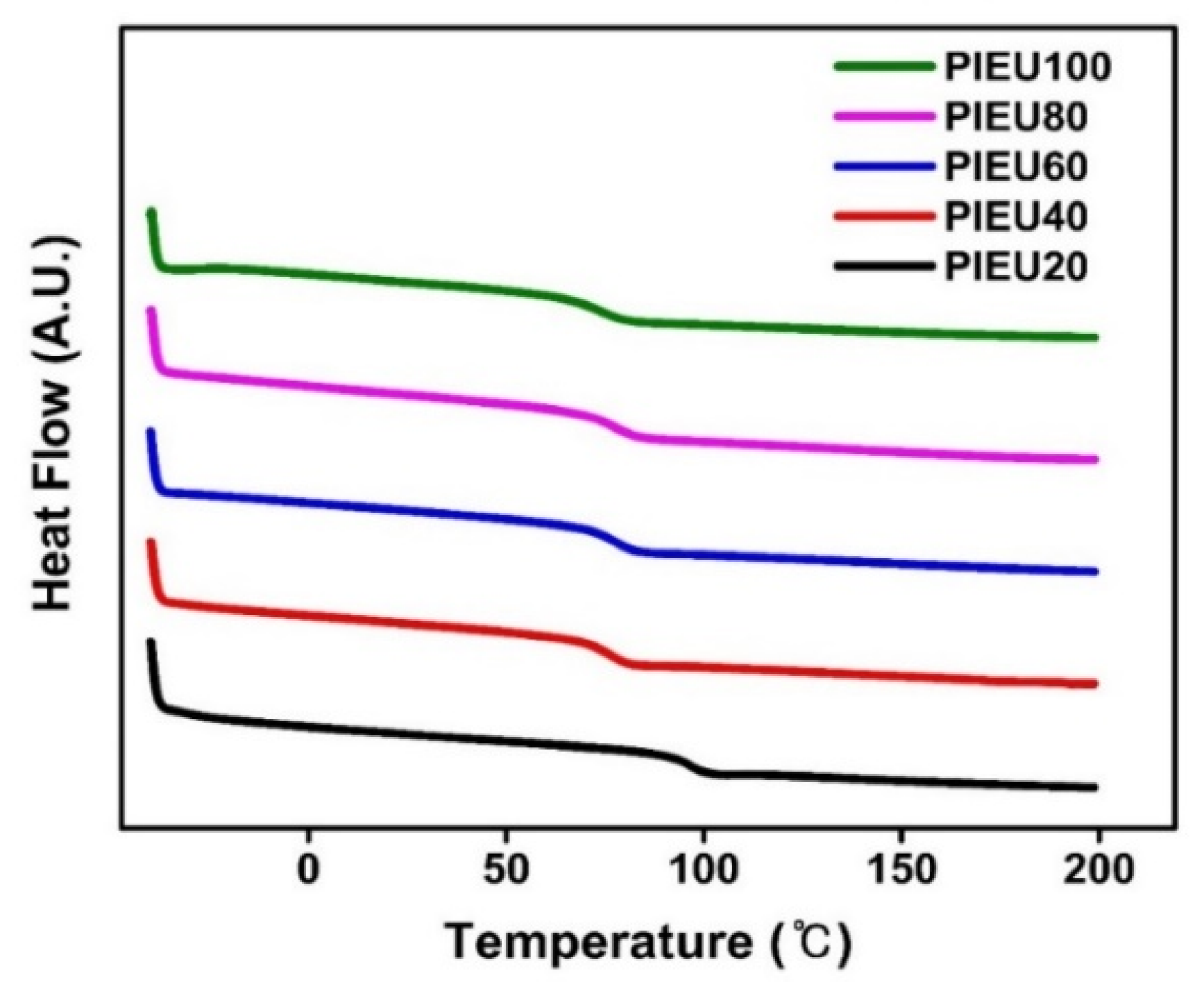

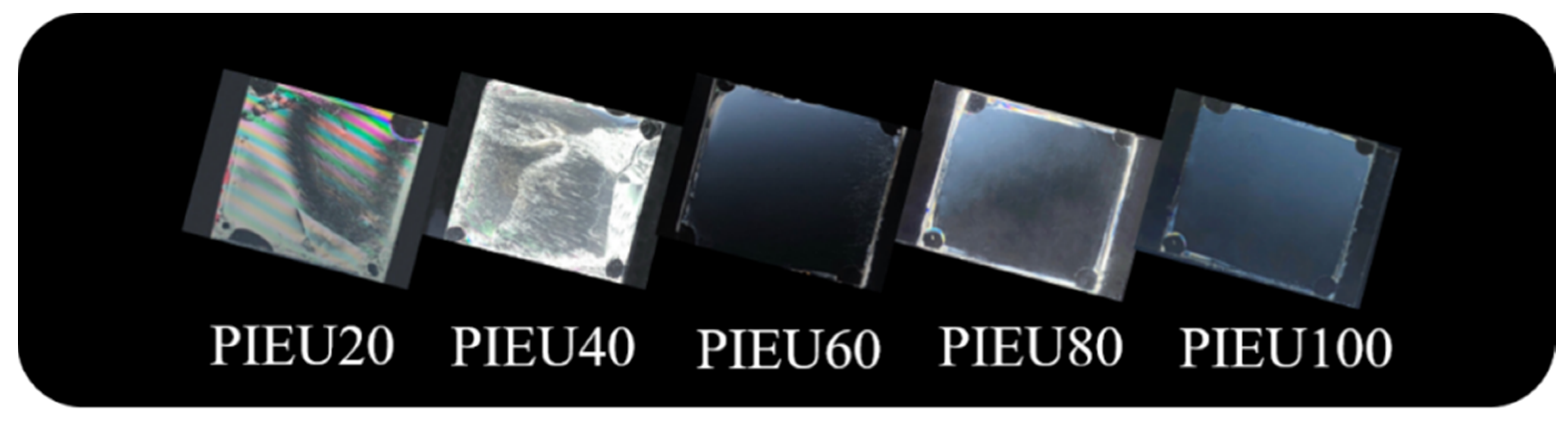

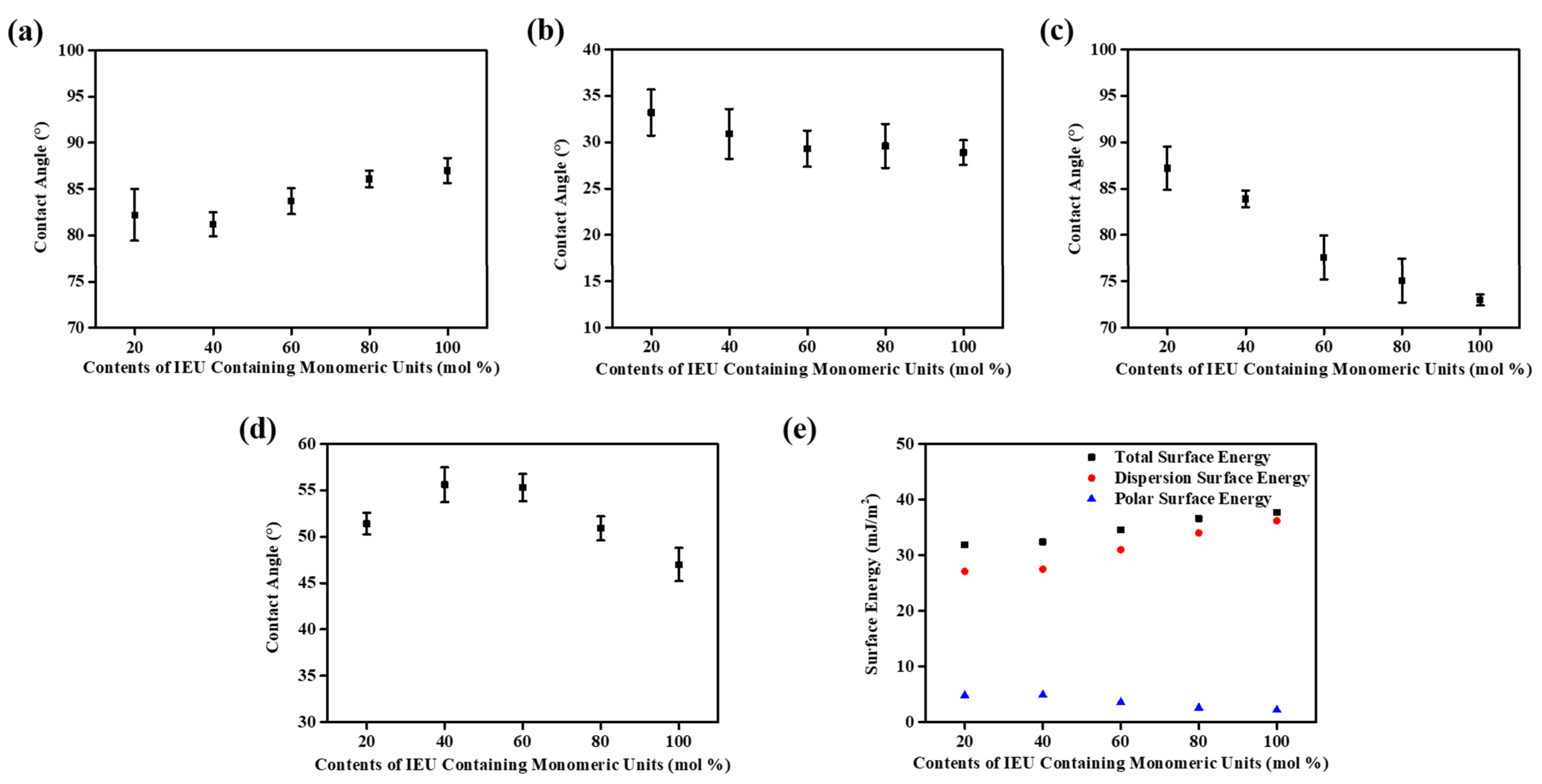
| Polymer Designation | Feed Ratio of Isoeugenol [mol%] | Degree of Substitution [%] | Tg [°C] |
|---|---|---|---|
| PIEU20 | 20 | 20 | 96 |
| PIEU40 | 40 | 40 | 76 |
| PIEU60 | 60 | 60 | 77 |
| PIEU80 | 80 | 80 | 77 |
| PIEU100 | 150 | 100 | 74 |
| Polymer Designation | Contact Angle (°) a | Surface Energy (mJ/m2) b | LC Aligning Ability c | |||||
|---|---|---|---|---|---|---|---|---|
| Water | Diiodo Methane | Formamide | Ethylene Glycol | Polar | Dispersion | Total | ||
| PIEU20 | 82.2 | 33.2 | 87.2 | 51.4 | 4.8 | 27.1 | 31.9 | X |
| PIEU40 | 81.3 | 30.9 | 83.9 | 55.6 | 4.9 | 27.5 | 32.4 | X |
| PIEU60 | 83.7 | 29.3 | 77.6 | 55.3 | 3.6 | 31.0 | 34.6 | O |
| PIEU80 | 86.1 | 29.6 | 75.1 | 50.9 | 2.6 | 34.0 | 36.6 | O |
| PIEU100 | 87.1 | 28.9 | 73.0 | 47.0 | 2.2 | 36.2 | 38.4 | O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Seo, K.; Kang, H. Alignment Layer of Liquid Crystal Using Plant-Based Isoeugenol-Substituted Polystyrene. Polymers 2021, 13, 547. https://doi.org/10.3390/polym13040547
Yang D, Seo K, Kang H. Alignment Layer of Liquid Crystal Using Plant-Based Isoeugenol-Substituted Polystyrene. Polymers. 2021; 13(4):547. https://doi.org/10.3390/polym13040547
Chicago/Turabian StyleYang, DaEun, Kyutae Seo, and Hyo Kang. 2021. "Alignment Layer of Liquid Crystal Using Plant-Based Isoeugenol-Substituted Polystyrene" Polymers 13, no. 4: 547. https://doi.org/10.3390/polym13040547
APA StyleYang, D., Seo, K., & Kang, H. (2021). Alignment Layer of Liquid Crystal Using Plant-Based Isoeugenol-Substituted Polystyrene. Polymers, 13(4), 547. https://doi.org/10.3390/polym13040547






