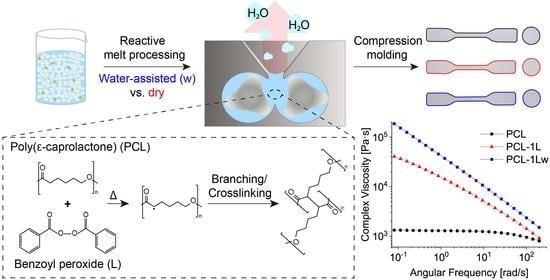
Substantial Effect of Water on Radical Melt Crosslinking and Rheological Properties of Poly(ε-Caprolactone)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
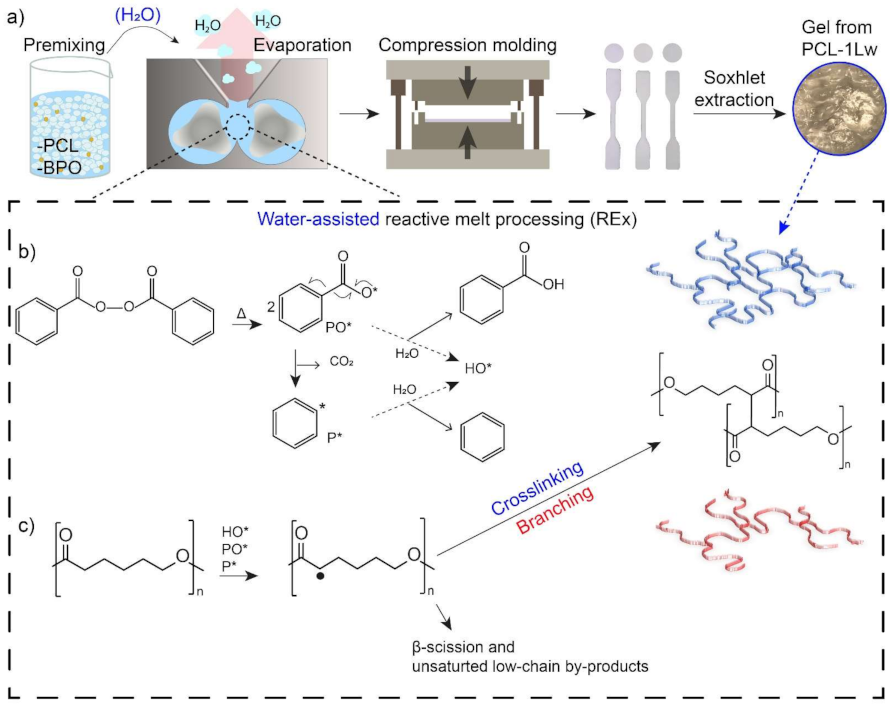
2.2. Reactive Melt Processing
2.3. Characterization Methods
3. Results and Discussion
3.1. Reactive Melt Processing
3.2. Structural Analysis
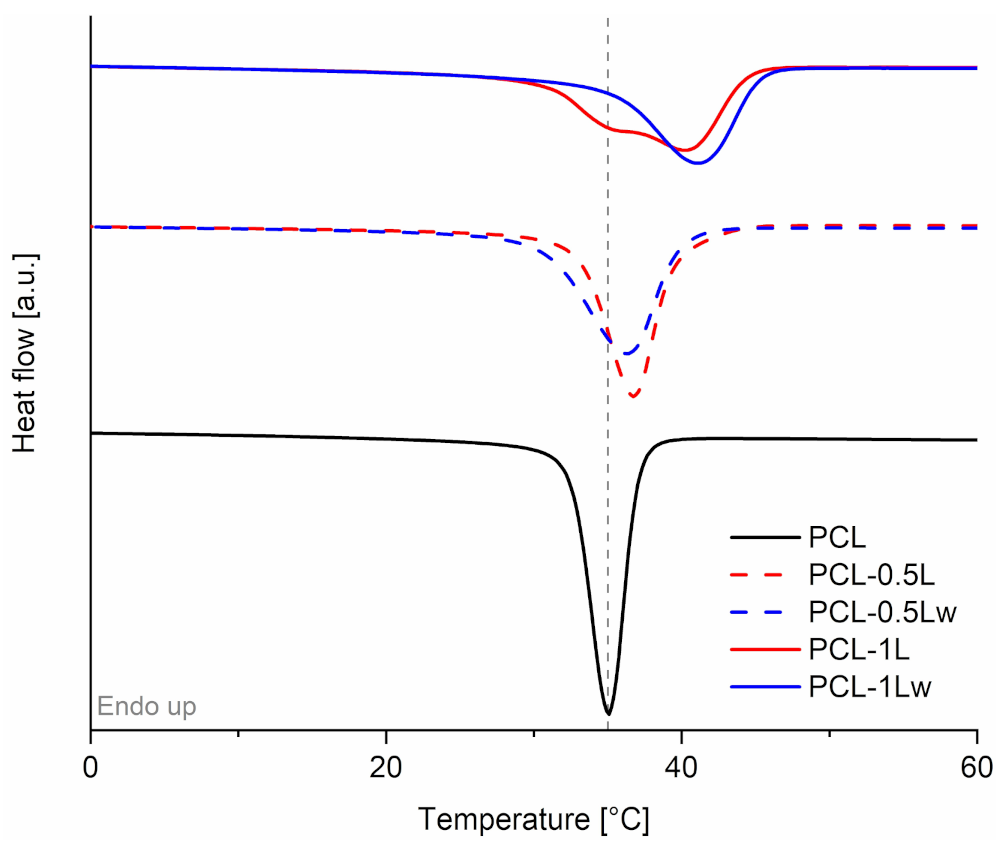
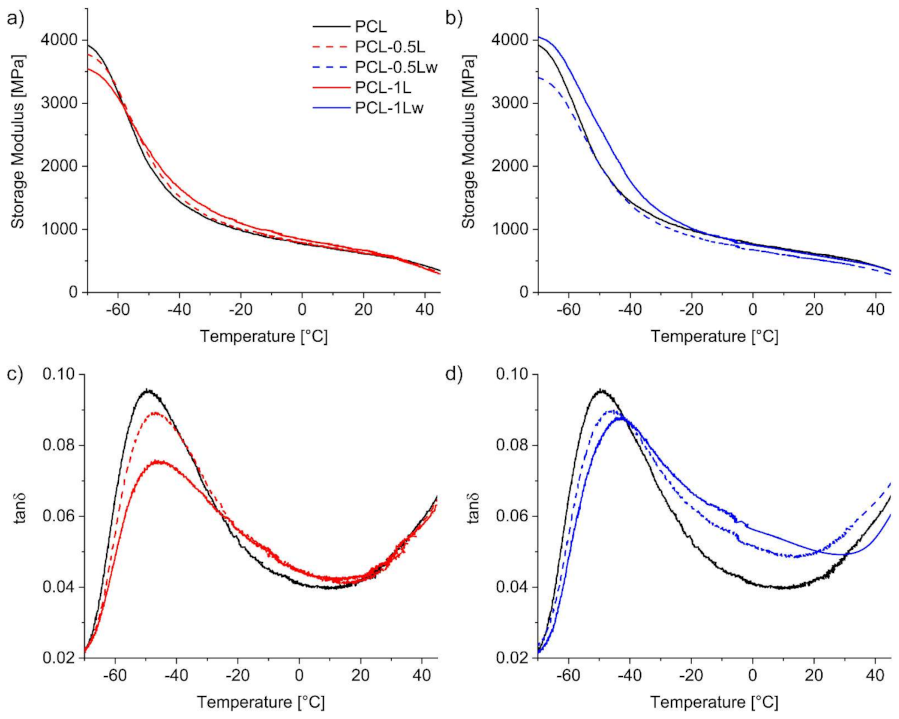
3.3. Thermal Properties
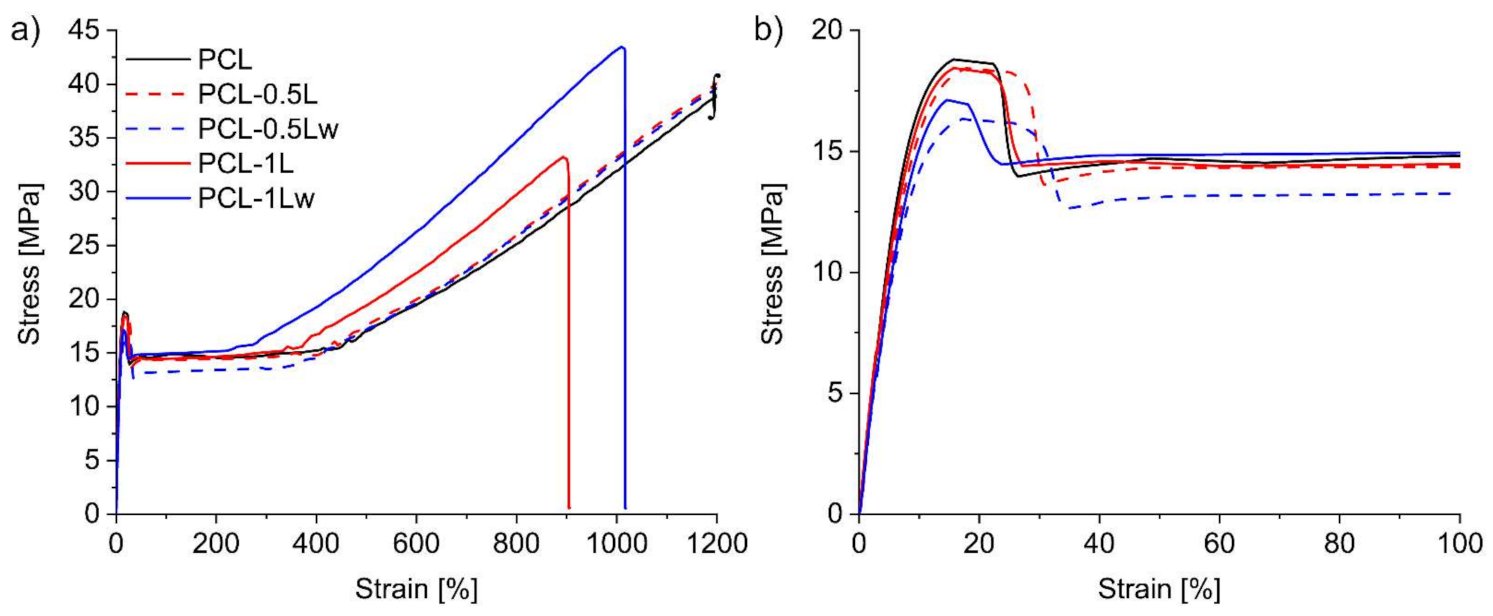
3.4. Mechanical Properties
3.5. Dynamic Rheology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Rydz, J.; Sikorska, W.; Kyulavska, M.; Christova, D. Polyester-based (bio)degradable polymers as environmentally friendly materials for sustainable development. Int. J. Mol. Sci. 2015, 16, 564–596. [Google Scholar] [CrossRef] [Green Version]
- Raquez, J.M.; Narayan, R.; Dubois, P. Recent advances in reactive extrusion processing of biodegradable polymer-based compositions. Macromol. Mater. Eng. 2008, 293, 447–470. [Google Scholar] [CrossRef]
- Kaldéus, T.; Träger, A.; Berglund, L.A.; Malmström, E.; Re, G.L. Molecular engineering of the cellulose-poly(caprolactone) bio-nanocomposite interface by reactive amphiphilic copolymer nanoparticles. ACS Nano 2019, 13, 6409–6420. [Google Scholar] [CrossRef] [PubMed]
- Lo Re, G.; Engström, J.; Wu, Q.; Malmström, E.; Gedde, U.W.; Olsson, R.T.; Berglund, L. Improved cellulose nanofibril dispersion in melt-processed polycaprolactone nanocomposites by a latex-mediated interphase and wet feeding as LDPE alternative. ACS Appl. Nano Mater. 2018, 1, 2669–2677. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Darwis, D.; Nishimura, K.; Mitomo, H.; Yoshii, F. Improvement of processability of Poly(ε-caprolactone) by radiation techniques. J. Appl. Polym. Sci. 1999, 74, 1815–1820. [Google Scholar] [CrossRef]
- Wei, L.; McDonald, A.G. Peroxide induced cross-linking by reactive melt processing of two biopolyesters: Poly(3-hydroxybutyrate) and poly(l -lactic acid) to improve their melting processability. J. Appl. Polym. Sci. 2015, 132, 1–15. [Google Scholar] [CrossRef]
- Di Maio, E.; Iannace, S.; Marrazzo, C.; Narkis, M.; Nicolais, L. Effect of molecular modification on PCL foam formation and morphology of PCL. Macromol. Symp. 2005, 228, 219–228. [Google Scholar] [CrossRef]
- Niaounakis, M. Introduction. In Biopolymers: Processing and Products; William Andrew: Kidlington, UK, 2015; pp. 1–77. ISBN 9780323266987. [Google Scholar]
- Fortelny, I.; Ujcic, A.; Fambri, L.; Slouf, M. Phase structure, compatibility, and toughness of PLA/PCL blends: A review. Front. Mater. 2019, 6, 206. [Google Scholar] [CrossRef] [Green Version]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable compatibilized polymer blends for packaging applications: A literature review. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef] [Green Version]
- Przybysz-Romatowska, M.; Haponiuk, J.; Formela, K. Reactive extrusion of biodegradable aliphatic polyesters in the presence of free-radical-initiators: A review. Polym. Degrad. Stab. 2020, 182, 109383. [Google Scholar] [CrossRef]
- Koenig, M.F.; Huang, S.J. Evaluation of crosslinked poly(caprolactone) as a biodegradable, hydrophobic coating. Polym. Degrad. Stab. 1994, 45, 139–144. [Google Scholar] [CrossRef]
- Jarrett, P.; Benedict, C.V.; Bell, J.P.; Cameron, J.A.; Huang, S.J. Mechanism of the biodegradation of polycaprolactone. In Polymers as Biomaterials; Shalaby, S.W., Hoffman, A.S., Ratner, B.D., Horbett, T.A., Eds.; Springer: Boston, MA, USA, 1984; pp. 181–192. ISBN 978-1-4613-2433-1. [Google Scholar]
- Yoshii, F.; Darwis, D.; Mitomo, H.; Makuuchi, K. Crosslinking of poly(ε-caprolactone) by radiation technique and its biodegradability. Radiat. Phys. Chem. 2000, 57, 417–420. [Google Scholar] [CrossRef]
- Kim, C.H.; Cho, K.Y.; Park, J.K. Grafting of glycidyl methacrylate onto polycaprolactone: Preparation and characterization. Polymer 2001, 42, 5135–5142. [Google Scholar] [CrossRef]
- John, J.; Tang, J.; Yang, Z.; Bhattacharya, M. Synthesis and characterization of anhydride-functional polycaprolactone. J. Polym. Sci. Part. A Polym. Chem. 1997, 35, 1139–1148. [Google Scholar] [CrossRef]
- Semba, T.; Kitagawa, K.; Ishiaku, U.S.; Hamada, H. The effect of crosslinking on the mechanical properties of polylactic acid/polycaprolactone blends. J. Appl. Polym. Sci. 2006, 101, 1816–1825. [Google Scholar] [CrossRef]
- Przybysz, M.; Marć, M.; Klein, M.; Saeb, M.R.; Formela, K. Structural, mechanical and thermal behavior assessments of PCL/PHB blends reactively compatibilized with organic peroxides. Polym. Test. 2018, 67, 513–521. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Rayón, E.; Carbonell-Verdu, A.; Lopez-Martinez, J.; Balart, R. Improvement of the compatibility between poly(3-hydroxybutyrate) and poly(ε-caprolactone) by reactive extrusion with dicumyl peroxide. Eur. Polym. J. 2017, 86, 41–57. [Google Scholar] [CrossRef] [Green Version]
- Sedov, I.; Magsumov, T.; Abdullin, A.; Yarko, E.; Mukhametzyanov, T.; Klimovitsky, A.; Schick, C. Influence of the cross-link density on the rate of crystallization of poly(ε-Caprolactone). Polymers 2018, 10, 902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, K.; Kriz, D.; Salovey, R.; Narkis, M.; Wallerstein, R. Crosslinking of polycaprolactone in the pre-gelation region. Polym. Eng. Sci. 1988, 28, 1484–1490. [Google Scholar] [CrossRef]
- Przybysz, M.; Hejna, A.; Haponiuk, J.; Formela, K. Structural and thermo-mechanical properties of poly(ε-caprolactone) modified by various peroxide initiators. Polymers 2019, 11, 1101. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Ran, X.; Su, X.; Zhang, K.; Liu, N. Effect of peroxide crosslinking on thermal and mechanical properties of poly (ε-caprolactone). Polym. Int. 2007, 600, 593–600. [Google Scholar] [CrossRef]
- Tolinski, M. Crosslinking. In Additives for Polyolefins; William Andrew: Kidlington, UK, 2009; pp. 215–220. [Google Scholar]
- Denisov, E.T.; Denisov, E.T. Effect of solvent on free radical reactions. In Liquid-Phase Reaction Rate Constants; Springer: Boston, MA, USA, 1974; pp. 427–441. [Google Scholar]
- Kaur, I.; Gautam, N.; Khanna, N.D. Modification of polypropylene through intercrosslinking graft copolymerization of poly(vinyl alcohol): Synthesis and characterization. J. Appl. Polym. Sci. 2008, 107, 2238–2245. [Google Scholar] [CrossRef]
- Vöhringer-Martinez, E.; Hansmann, B.; Hernandez, H.; Francisco, J.S.; Troe, J.; Abel, B. Water catalysis of a radical-molecule gas-phase reaction. Science 2007, 315, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Troe, J. The Polanyi lecture. The colourful world of complex-forming bimolecular reactions. J. Chem. Soc. Faraday Trans. 1994, 90, 2303–2317. [Google Scholar] [CrossRef]
- D’Haene, P.; Remsen, E.E.; Asrar, J. Preparation and characterization of a branched bacterial polyester. Macromolecules 1999, 32, 5229–5235. [Google Scholar] [CrossRef]
- Dean, K.M.; Petinakis, E.; Meure, S.; Yu, L.; Chryss, A. Melt strength and rheological properties of biodegradable poly(lactic aacid) modified via alkyl radical-based reactive extrusion processes. J. Polym. Environ. 2012, 20, 741–747. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, W.; Papadaki, M.; Mannan, M.S.; Mashuga, C.V.; Cheng, Z. Thermal decomposition of solid benzoyl peroxide using advanced reactive system screening tool: Effect of concentration, confinement and selected acids and bases. J. Loss Prev. Process. Ind. 2019, 60, 28–34. [Google Scholar] [CrossRef]
- Cicogna, F.; Coiai, S.; Rizzarelli, P.; Carroccio, S.; Gambarotti, C.; Domenichelli, I.; Yang, C.; Dintcheva, N.T.; Filippone, G.; Pinzino, C.; et al. Functionalization of aliphatic polyesters by nitroxide radical coupling. Polym. Chem. 2014, 5, 5656–5667. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Mattoso, L.H.C.; Orts, W.J.; Medeiros, E.S. Structural and morphological characterization of micro and nanofibers produced by electrospinning and solution blow spinning: A comparative study. Adv. Mater. Sci. Eng. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Castilla-Cortázar, I.; Vidaurre, A.; Marí, B.; Campillo-Fernández, A.J. Morphology, crystallinity, and molecular weight of poly(ε-caprolactone)/graphene oxide hybrids. Polymers 2019, 11, 1099. [Google Scholar] [CrossRef] [Green Version]
- Magnani, C.; Idström, A.; Nordstierna, L.; Müller, A.J.; Dubois, P.; Raquez, J.M.; Lo Re, G. Interphase design of cellulose nanocrystals/poly(hydroxybutyrate- ran-valerate) bionanocomposites for mechanical and thermal properties tuning. Biomacromolecules 2020, 21, 1892–1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chellquist, E.M.; Gorman, W.G. Benzoyl peroxide solubility and stability in hydric solvents. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1992, 9, 1341–1346. [Google Scholar] [CrossRef]
- Mardyukov, A.; Sanchez-Garcia, E.; Crespo-Otero, R.; Sander, W. Interaction and reaction of the phenyl radical with water: A source of OH radicals. Angew. Chem. Int. Ed. 2009, 48, 4804–4807. [Google Scholar] [CrossRef]
- Navarro, R.; Burillo, G.; Adem, E.; Marcos-Fernández, A. Effect of ionizing radiation on the chemical structure and the physical properties of polycaprolactones of different molecular weight. Polymers 2018, 10, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Re, G.; Spinella, S.; Boujemaoui, A.; Vilaseca, F.; Larsson, P.T.; Adås, F.; Berglund, L.A.; Larsson, P.T.; Ada, F.; Berglund, L.A. Poly(ε-caprolactone) biocomposites based on acetylated cellulose fibers and wet compounding for improved mechanical performance. ACS Sustain. Chem. Eng. 2018, 6, 6753–6760. [Google Scholar] [CrossRef] [Green Version]
- Carlson, D.; Dubois, P.; Nie, L.; Narayan, R. Free radical branching of polylactide by reactive extrusion. Polym. Eng. Sci. 1998, 38, 311–321. [Google Scholar] [CrossRef]
- Skoglund, P.; Fransson, A. Continuous cooling and isothermal crystallization of polycaprolactone. J. Appl. Polym. Sci. 1996, 61, 2455–2465. [Google Scholar] [CrossRef]
- Kim, D.J.; Kim, W.S.; Lee, D.H.; Min, K.E.; Park, L.S.; Kang, I.K.; Jeon, I.R.; Seo, K.H. Modification of poly(butylene succinate) with peroxide: Crosslinking, physical and thermal properties, and biodegradation. J. Appl. Polym. Sci. 2001, 81, 1115–1124. [Google Scholar] [CrossRef]
- Mishra, J.K.; Chang, Y.W.; Kim, W. The effect of peroxide crosslinking on thermal, mechanical, and rheological properties of polycaprolactone/epoxidized natural rubber blends. Polym. Bull. 2011, 66, 673–681. [Google Scholar] [CrossRef]
- Takamura, M.; Nakamura, T.; Kawaguchi, S.; Takahashi, T.; Koyama, K. Molecular characterization and crystallization behavior of peroxide-induced slightly crosslinked poly(L-lactide) during extrusion. Polym. J. 2010, 42, 600–608. [Google Scholar] [CrossRef]
- Kolahchi, A.R.; Kontopoulou, M. Chain extended poly(3-hydroxybutyrate) with improved rheological properties and thermal stability, through reactive modification in the melt state. Polym. Degrad. Stab. 2015, 121, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Signori, F.; Boggioni, A.; Righetti, M.C.; Rondán, C.E.; Bronco, S.; Ciardelli, F. Evidences of transesterification, chain branching and cross-linking in a biopolyester commercial blend upon reaction with dicumyl peroxide in the melt. Macromol. Mater. Eng. 2015, 300, 153–160. [Google Scholar] [CrossRef]
- Ai, X.; Wang, D.; Li, X.; Pan, H.; Kong, J.; Yang, H.; Zhang, H.; Dong, L. The properties of chemical cross-linked poly(lactic acid) by bis(tert-butyl dioxy isopropyl) benzene. Polym. Bull. 2019, 76, 575–594. [Google Scholar] [CrossRef]
- Mishra, J.K.; Chang, Y.W.; Kim, D.K. Green thermoplastic elastomer based on polycaprolactone/epoxidized natural rubber blend as a heat shrinkable material. Mater. Lett. 2007, 61, 3551–3554. [Google Scholar] [CrossRef]
- Suhartini, M.; Mitomo, H.; Nagasawa, N.; Yoshii, F.; Kume, T. Radiation crosslinking of poly(butylene succinate) in the presence of low concentrations of trimethallyl isocyanurate and its properties. J. Appl. Polym. Sci. 2003, 88, 2238–2246. [Google Scholar] [CrossRef]
- Trinkle, S.; Freidrich, C. Van Gurp-Palmen-plot: A way to characterize polydispersity of linear polymers. Rheol. Acta 2001, 40, 322–328. [Google Scholar] [CrossRef]
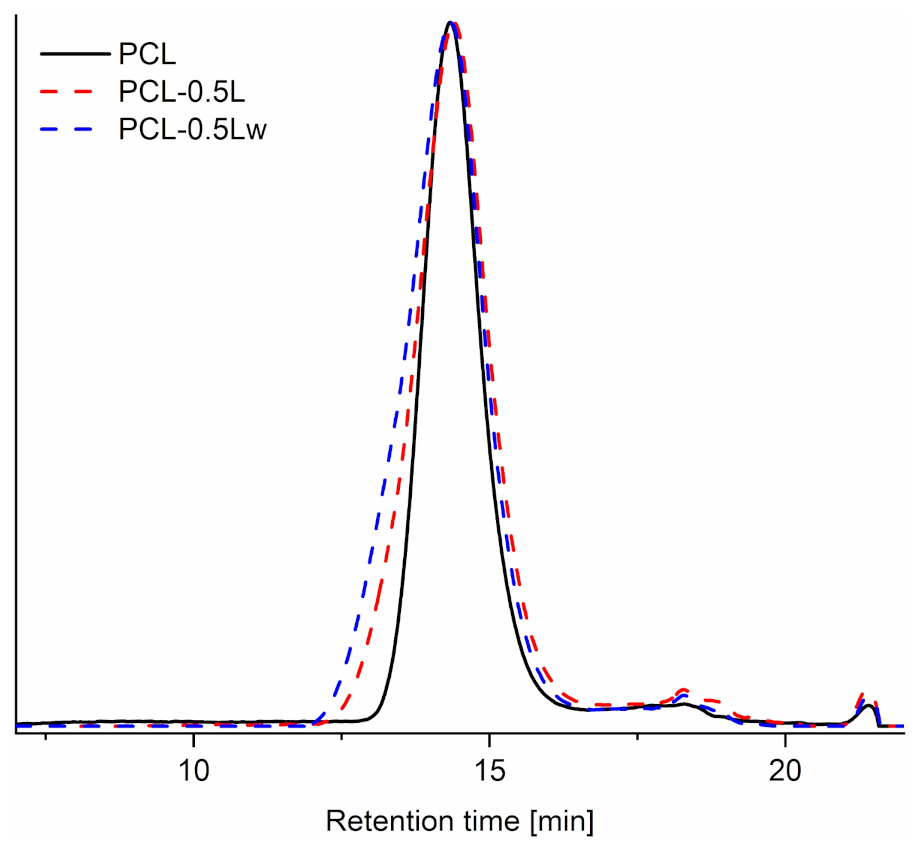

| Material | Ð | ||
|---|---|---|---|
| PCL | 77,000 | 150,000 | 2 |
| PCL-0.5L | 74,000 | 220,000 | 2.92 |
| PCL-0.5Lw | 86,000 | 290,000 | 3.37 |
| Material | T5% (°C) | Tc (°C) | χXRD (%) | D110 (Å) |
|---|---|---|---|---|
| PCL | 363 | 35.1 | 38.2 | 223 |
| PCL-0.5L | 331 | 36.7 | - | - |
| PCL-0.5Lw | 325 | 36.4 | - | - |
| PCL-1L | 345 | 35.5–40.2 | 41.4 | 260 |
| PCL-1Lw | 347 | 41.2 | 42.8 | 291 |
| Material | Tg (°C) | Tα (°C) | DF | E’ −70 °C (MPa) | E’20 °C (MPa) |
|---|---|---|---|---|---|
| PCL | −56.2 | −49.4 | 0.095 | 3920 | 613 |
| PCL-0.5L | −54.8 | −46.6 | 0.089 | 3770 | 621 |
| PCL-0.5Lw | −54.4 | −45.7 | 0.090 | 3405 | 528 |
| PCL-1L | −54.0 | −45.7 | 0.075 | 3544 | 669 |
| PCL-1Lw | −52.1 | −42.9 | 0.088 | 4051 | 591 |
| Material | Young’s Modulus (MPa) | Yield Stress (MPa) | Ultimate Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| PCL | 284 ± 5 | 19.2 ± 0.5 | > 38.7 * | > 1200 * |
| PCL-0.5L | 248 ± 6 | 18.4 ± 0.1 | > 38.8 * | > 1175 * |
| PCL-0.5Lw | 218 ± 8 | 16.0 ± 0.4 | > 39.8 * | > 1200 * |
| PCL-1L | 242 ± 13 | 17.3 ± 1 | 33.6 ± 1.3 | 941 ± 57 |
| PCL-1Lw | 225 ± 14 | 17.1 ± 0.5 | 44 ± 1.2 | 1035 ± 48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avella, A.; Mincheva, R.; Raquez, J.-M.; Lo Re, G. Substantial Effect of Water on Radical Melt Crosslinking and Rheological Properties of Poly(ε-Caprolactone). Polymers 2021, 13, 491. https://doi.org/10.3390/polym13040491
Avella A, Mincheva R, Raquez J-M, Lo Re G. Substantial Effect of Water on Radical Melt Crosslinking and Rheological Properties of Poly(ε-Caprolactone). Polymers. 2021; 13(4):491. https://doi.org/10.3390/polym13040491
Chicago/Turabian StyleAvella, Angelica, Rosica Mincheva, Jean-Marie Raquez, and Giada Lo Re. 2021. "Substantial Effect of Water on Radical Melt Crosslinking and Rheological Properties of Poly(ε-Caprolactone)" Polymers 13, no. 4: 491. https://doi.org/10.3390/polym13040491
APA StyleAvella, A., Mincheva, R., Raquez, J.-M., & Lo Re, G. (2021). Substantial Effect of Water on Radical Melt Crosslinking and Rheological Properties of Poly(ε-Caprolactone). Polymers, 13(4), 491. https://doi.org/10.3390/polym13040491