X-ray Photoelectron Spectroscopy Analysis of Chitosan–Graphene Oxide-Based Composite Thin Films for Potential Optical Sensing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Prepartion of Chemicals
2.2. Prepartion of Thin Films
2.3. X-ray Photoelectron Spectroscopy
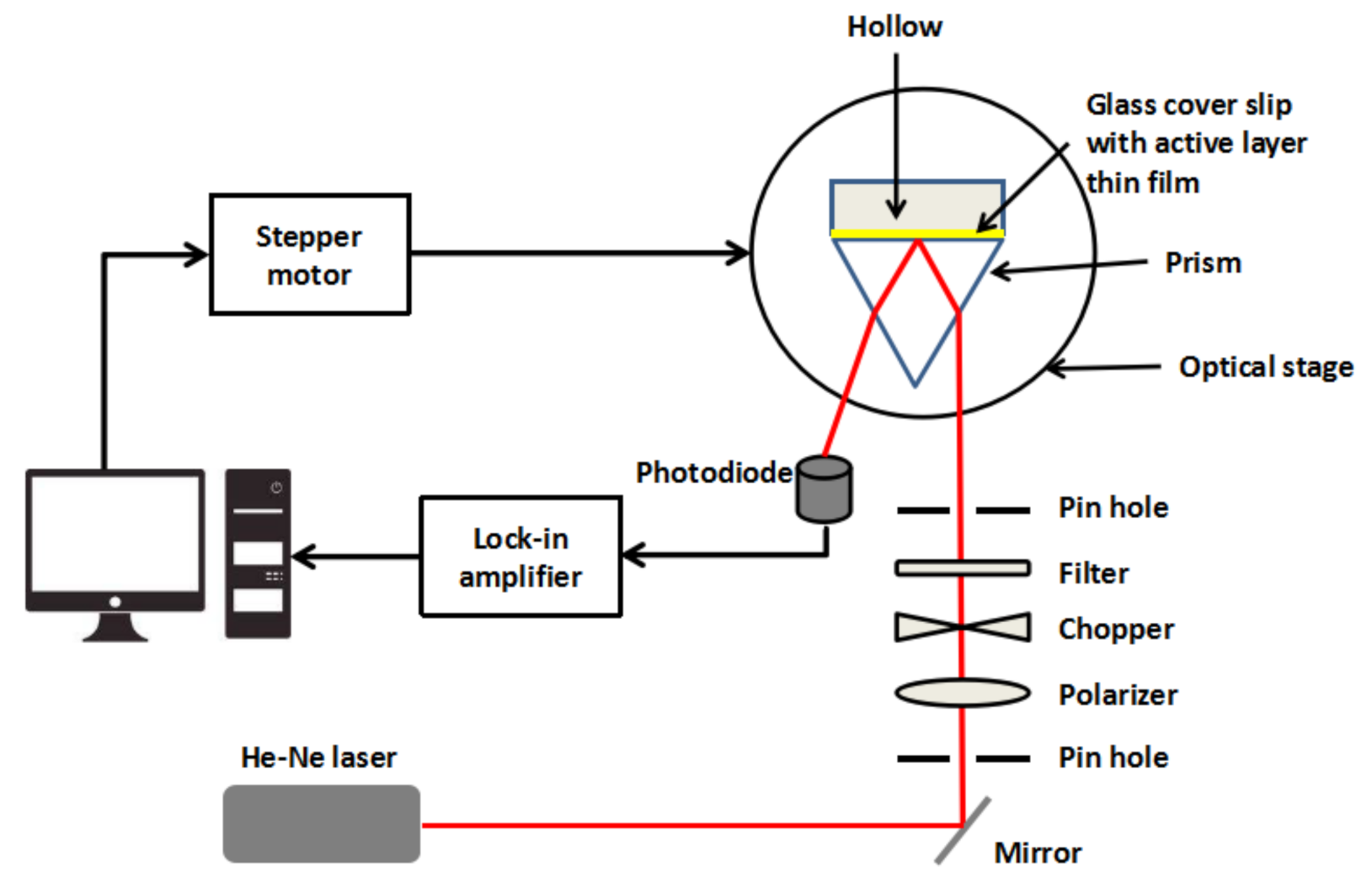
2.4. Surface Plasmon Resonance Spectroscopy
3. Results and Discussion
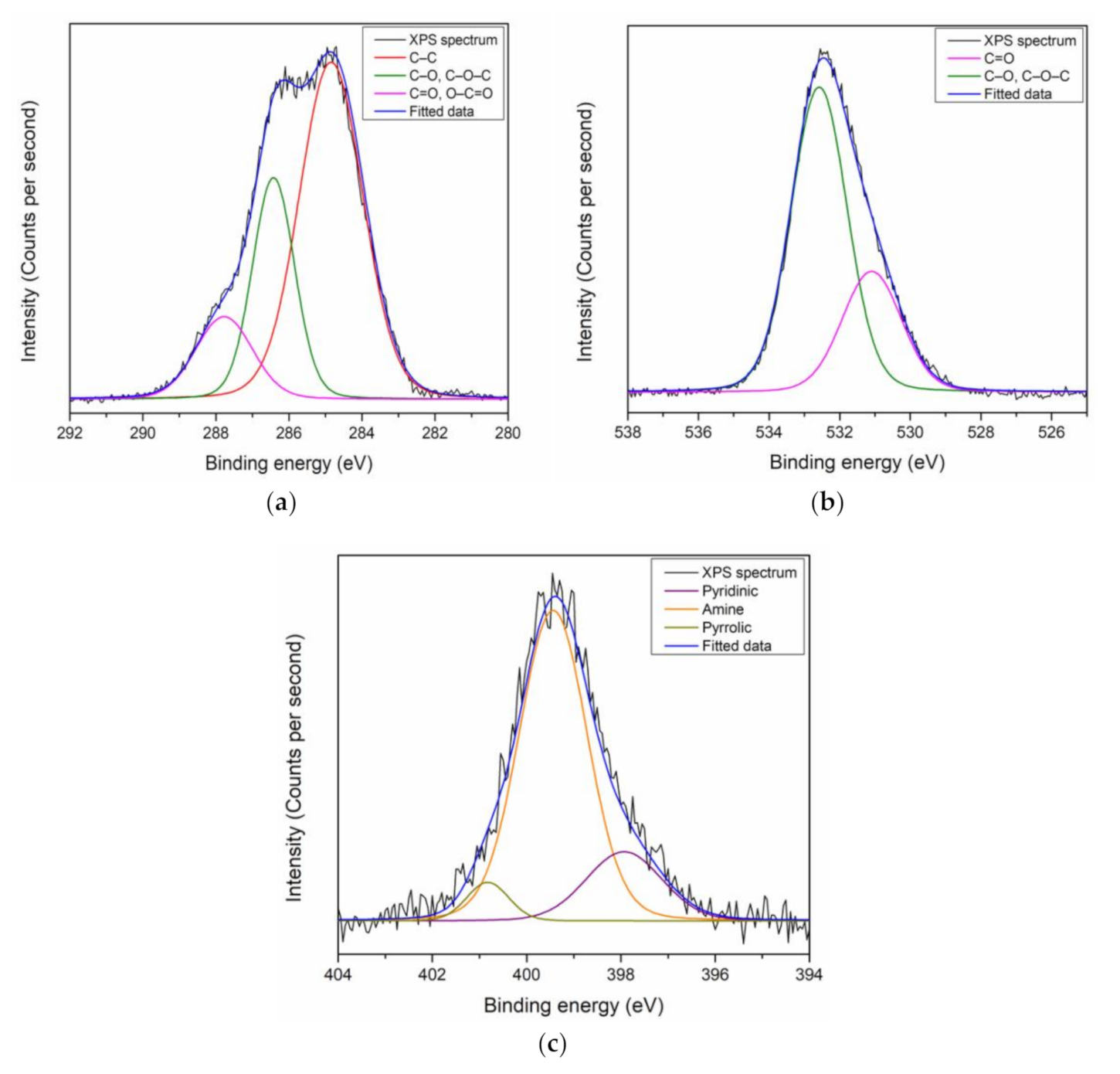
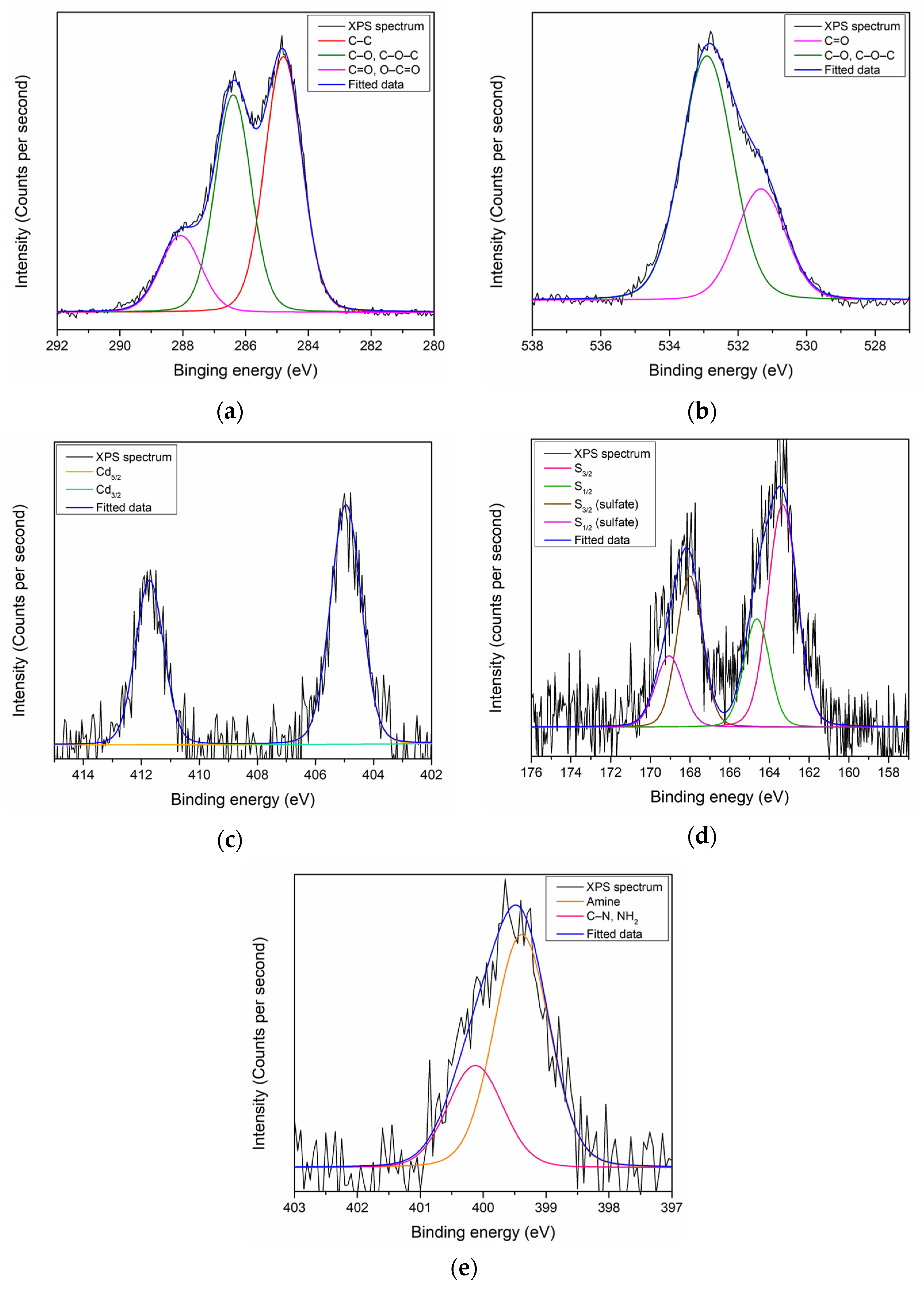
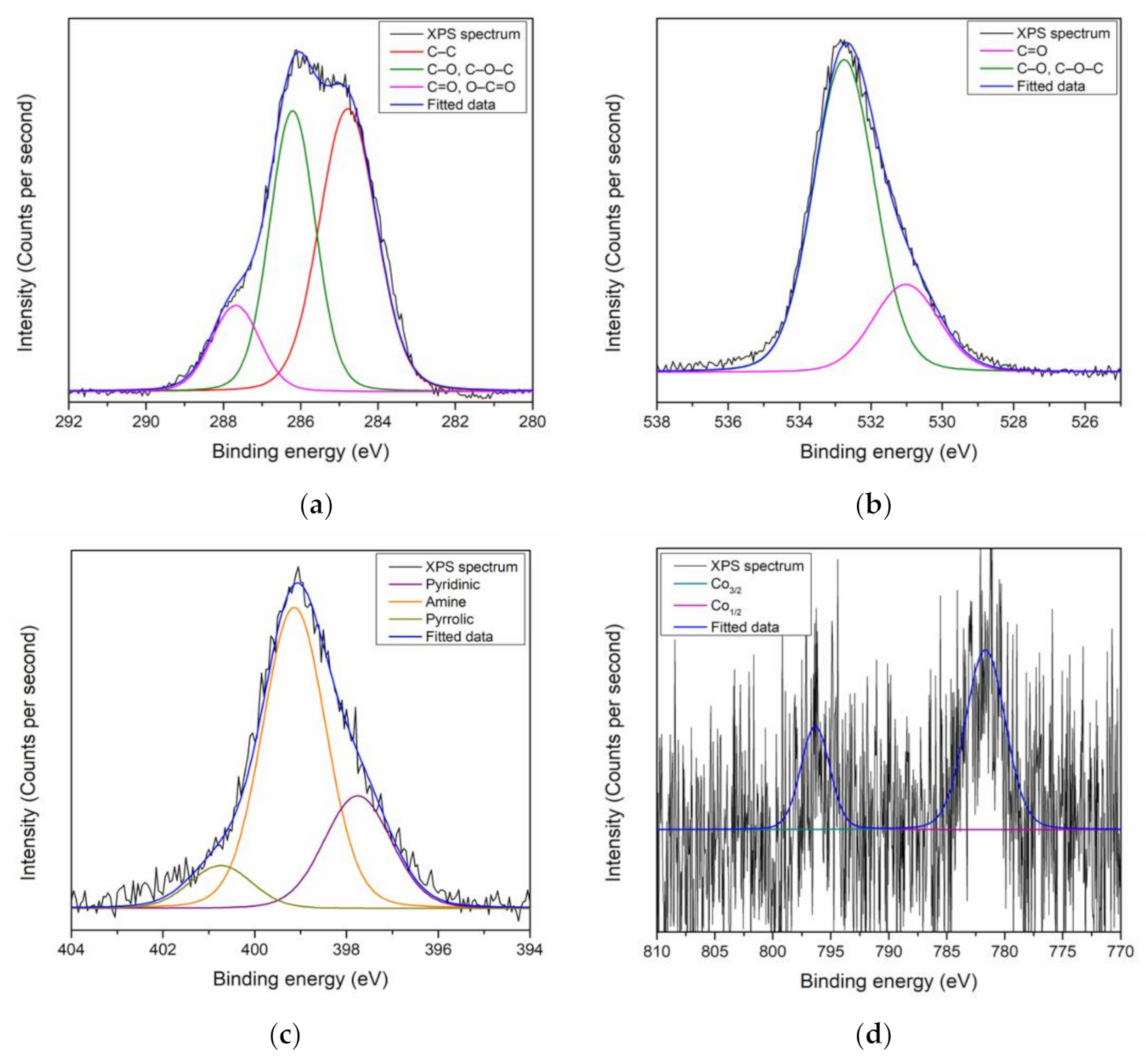
3.1. XPS Characterization of Thin Films
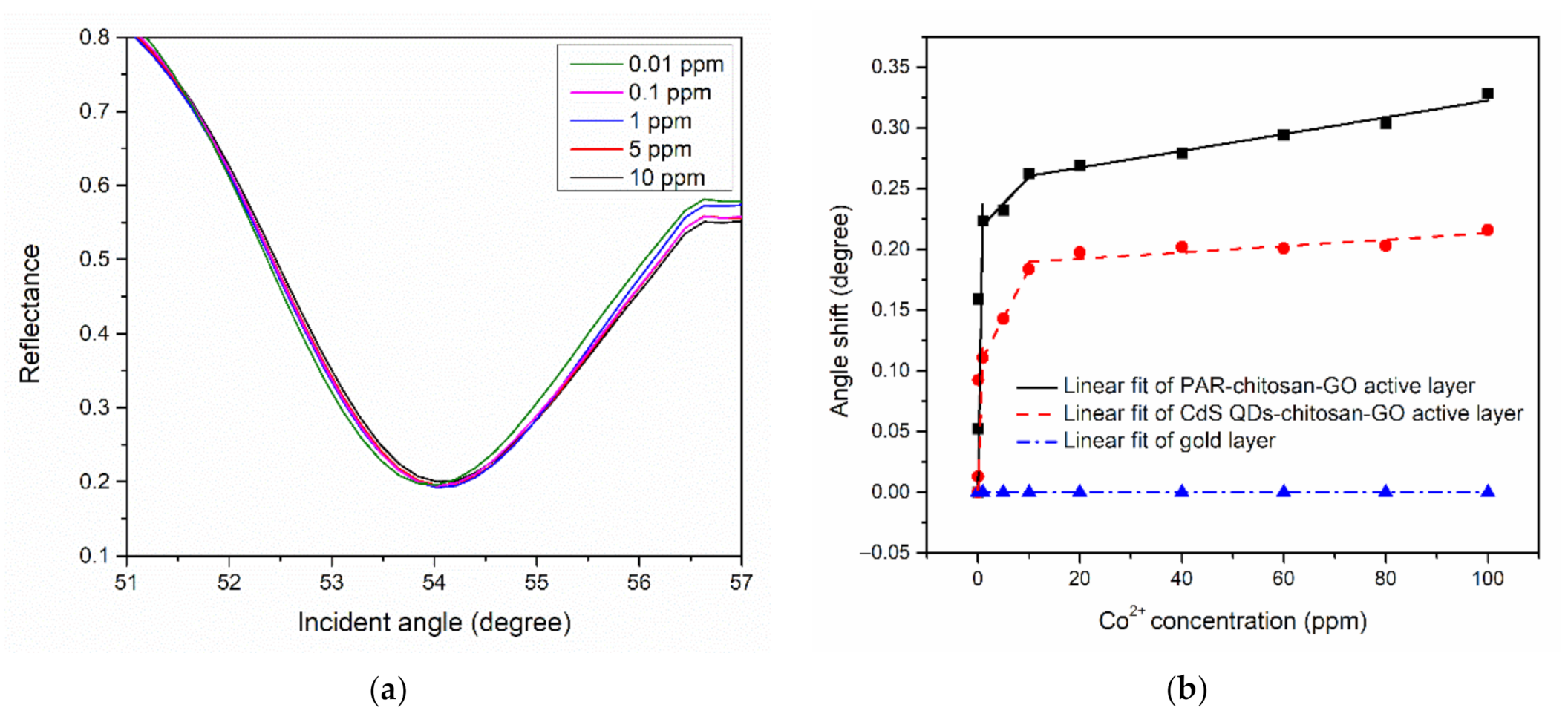
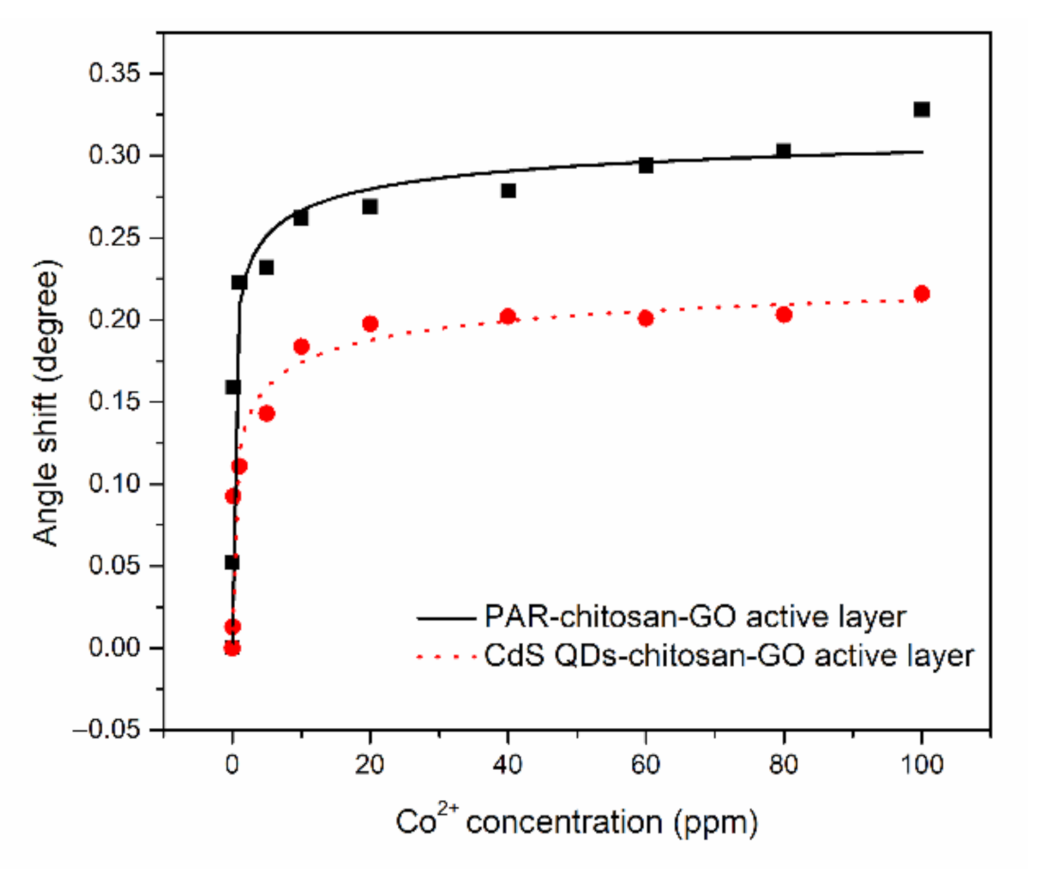
3.2. SPR Response of Thin Films With Co2+
3.3. Evidence of Co2+ Interaction with the Thin Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banerjee, A.; Ganguly, S. Alginate–chitosan composite hydrogel film with macrovoids in the inner layer for biomedical applications. J. Appl. Polym. Sci. 2019, 136, 30–33. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite nanotubes coated by chitosan for the controlled release of khellin. Polymers 2020, 12, 1766. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Lazzara, G. Polysaccharides/Halloysite nanotubes for smart bionanocomposite materials. Carbohydr. Polym. 2020, 245, 116502–116513. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels—A review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Avouris, P. Graphene: Electronic and photonic properties and devices. Nano Lett. 2010, 10, 4285–4294. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1535. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S. Structural, optical and sensing properties of ionophore doped graphene based bionanocomposite thin film. Optik 2017, 144, 308–315. [Google Scholar]
- Hunt, A.; Kurmaev, E.Z.; Moewes, A. Band gap engineering of graphene oxide by chemical modification. Carbon 2014, 75, 366–371. [Google Scholar] [CrossRef]
- Chen, P.; Cao, Z.-F.; Wen, X.; Wang, J.; Yang, F.; Wang, S.; Zhong, H. In situ nano-silicate functionalized graphene oxide composites to improve MB removal. J. Taiwan Inst. Chem. Eng. 2017, 81, 87–94. [Google Scholar] [CrossRef]
- Wang, W.L.; Jang, J.; Nguyen, V.H.; Auxilia, F.M.; Song, H.; Jang, K.; Jin, E.M.; Lee, G.Y.; Gu, H.B.; Ham, M.H. Cerium vanadate and reduced graphene oxide composites for lithium-ion batteries. J. Alloy. Compd. 2017, 724, 1075–1082. [Google Scholar] [CrossRef]
- Yang, X.; Tu, Y.; Li, L.; Shang, S.; Tao, X. Well-dispersed chitosan/graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 1707–1713. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Saleviter, S.; Omar, N.A.S. Preparation and characterization of hexadecyltrimethylammonium bromide modified nanocrystalline cellulose/graphene oxide composite thin film and its potential in sensing copper ion using surface plasmon resonance technique. Optik 2018, 173, 71–77. [Google Scholar] [CrossRef]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Chemically derived graphene oxide: Towards large-area thin-film electronics and optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef]
- Jiang, J.; Feng, C.; Qian, W.; Zhu, L.; Han, S.; Lin, H. Effect of graphene oxide nanosheets and ultrasonic electrodeposition technique on Ni–Mo/graphene oxide composite coatings. Mater. Chem. Phys. 2017, 199, 239–248. [Google Scholar] [CrossRef]
- Cheng, Z.-L.; Li, Y.; Liu, Z. Fabrication of graphene oxide/silicalite-1 composites with hierarchical porous structure and investigation on their adsorption performance for rhodamine B. J. Ind. Eng. Chem. 2017, 55, 234–243. [Google Scholar] [CrossRef]
- Yu, B.; Xu, J.; Liu, J.H.; Yang, S.T.; Luo, J.; Zhou, Q.; Wan, J.; Liao, R.; Wang, H.; Liu, Y. Adsorption behavior of copper ions on graphene oxide-chitosan aerogel. J. Environ. Chem. Eng. 2013, 1, 1044–1050. [Google Scholar] [CrossRef]
- Anandhavelu, S.; Thambidurai, S. Single step synthesis of chitin/chitosan-based graphene oxide-ZnO hybrid composites for better electrical conductivity and optical properties. Electrochim. Acta 2013, 90, 194–202. [Google Scholar] [CrossRef]
- Kamaruddin, N.H.; Bakar, A.A.A.; Yaacob, M.H.; Mahdi, M.A.; Zan, M.S.D.; Shaari, S. Enhancement of chitosan-graphene oxide SPR sensor with a multi-metallic layers of Au-Ag-Au nanostructure for lead (II) ion detection. Appl. Surf. Sci. 2016, 361, 177–184. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, T.; Bao, H.; Li, L. Green fabrication of chitosan films reinforced with parallel aligned graphene oxide. Carbohydr. Polym. 2011, 83, 1908–1915. [Google Scholar] [CrossRef]
- Kamaruddin, N.; Bakar, A.A.; Mobarak, N.; Zan, M.S.; Arsad, N. Binding affinity of a highly sensitive Au/Ag/Au/chitosan-graphene oxide sensor based on direct detection of Pb2+ and Hg2+ ions. Sensors 2017, 17, 2277. [Google Scholar] [CrossRef]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Al-Rekabi, S.H.; Mahdi, M.A.; Omar, N.A.S. Incorporation of surface plasmon resonance with novel valinomycin doped chitosan-graphene oxide thin film for sensing potassium ion. Spectrochim. Acta Mol. Biomol. Spectrosc. 2018, 191, 111–115. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Fauzi, N.I.M.; Hashim, H.S.; Ramdzan, N.S.M.; Omar, N.A.S. Recent advances in surface plasmon resonance optical sensors for potential application in environmental monitoring. Sens. Mater. 2020, 32, 4191–4200. [Google Scholar]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Hashim, H.S.; Fauzi, N.I.M.; Chanlek, N.; Mahdi, M.A. X-ray photoelectron study on gold/nanocrystalline cellulose-graphene oxide thin film as surface plasmon resonance active layer for metal ion detection. Thin Solid Film. 2020, 713, 138340–138350. [Google Scholar] [CrossRef]
- Florence, T.M.; Farrar, Y. Spectrophotometric determination of uranium with 4-(2-pyridylazo) resorcinol. Anal. Chem. 1963, 35, 1613–1616. [Google Scholar] [CrossRef]
- Starosta, R.; Nitek, W.; Komarnicka, U.K.; Stochel, G. Synthesis and characterization of copper (I) coordination compounds with (1-(2-pyridylazo)-2-naphthol) and (4-(2-pyridylazo) resorcinol). Polyhedron 2014, 68, 357–364. [Google Scholar]
- Karmakar, A.; Singh, B. Charge-transfer interaction of 4-(2-pyridylazo) resorcinol with nitroaromatics: Insights from experimental and theoretical results. J. Mol. Liq. 2017, 236, 135–143. [Google Scholar] [CrossRef]
- Pollard, F.H.; Hanson, P.; Geary, W.J. 4-(2-pyridylazo) resorcinol as a possible analytical reagent for the colorimetric estimation of cobalt, lead, and uranium. Anal. Chim. Acta 1959, 20, 26–31. [Google Scholar] [CrossRef]
- Zi, L.; Huang, Y.; Yan, Z.; Liao, S. Thioglycolic acid-capped CuInS2/ZnS quantum dots as fluorescent probe for cobalt ion detection. J. Lumin. 2014, 148, 359–363. [Google Scholar] [CrossRef]
- Amjadi, M.; Shokri, R.; Hallaj, T. A new turn-off fluorescence probe based on graphene quantum dots for detection of Au (III) ion. Spectrochim. Acta Mol. Biomol. Spectrosc. 2016, 153, 619–624. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Ramdzan, N.S.M. Highly sensitive surface plasmon resonance optical detection of ferric ion using CTAB/hydroxylated graphene quantum dots thin film. J. Appl. Phys. 2020, 128, 1–10. [Google Scholar] [CrossRef]
- Manan, F.A.A.; Hong, W.W.; Abdullah, J.; Yusof, N.A.; Ahmad, I. Nanocrystalline cellulose decorated quantum dots based tyrosinase biosensor for phenol determination. Mater. Sci. Eng. 2019, 99, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, C.; Sharma, A.; Kurchania, R. Synthesis of cds quantum dots using wet chemical co-precipitation method. J. NonOxide Glas. 2014, 6, 23–26. [Google Scholar]
- Liu, S.; Na, W.; Pang, S.; Su, X. Fluorescence detection of Pb2+ based on the DNA sequence functionalized CdS quantum dots. Biosens. Bioelectron. 2014, 58, 17–21. [Google Scholar] [CrossRef]
- Uppa, Y.; Kulchat, S.; Ngamdee, K.; Pradublai, K. Silver ion modulated CdS quantum dots for highly selective detection of trace Hg2+. J. Lumin. 2016, 178, 437–445. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Tang, H. Ultrasensitive electrochemical detection of tumor cells based on multiple layer CdS Quantum dots-functionalized polystyrene microspheres and graphene oxide–polyaniline composite. Biosens. Bioelectron. 2018, 100, 1–7. [Google Scholar] [CrossRef]
- Li, Z.; Du, Y.; Zhang, Z.; Pang, D. Preparation and characterization of CdS quantum dots chitosan biocomposite. React. Funct. Polym. 2003, 55, 35–43. [Google Scholar] [CrossRef]
- Tshemese, Z.; Mlowe, S.; Revaprasadu, N.; Deenadayalu, N. Synthesis of CdS quantum dots in an imidazolium based ionic liquid. Mater. Sci. Semicond. Process. 2017, 71, 258–262. [Google Scholar] [CrossRef]
- Koneswaran, M.; Narayanaswamy, R. Mercaptoacetic acid capped CdS quantum dots as fluorescence single shot probe for mercury (II). Sens. Actuators Chem. 2009, 139, 91–96. [Google Scholar] [CrossRef]
- Veerathangam, K.; Pandian, M.S.; Ramasamy, P. Photovoltaic performance of Ag-doped CdS quantum dots for solar cell application. Mater. Res. Bull. 2017, 94, 371–377. [Google Scholar]
- Choi, H.-I.; Hong, J.A.; Kim, M.-S.; Lee, S.E.; Jung, S.-H.; Yoon, P.W.; Song, J.S.; Kim, J.-J. Severe cardiomyopathy due to arthroprosthetic cobaltism: Report of two cases with different outcomes. Cardiovasc. Toxicol. 2018, 19, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.S.; Fen, Y.W.; Saleviter, S.; Daniyal, W.M.E.M.M.; Anas, N.A.A.; Ramdzan, N.S.M.; Roshidi, M.D.A. Development of a graphene-based surface plasmon resonance optical sensor chip for potential biomedical application. Materials 2019, 12, 1928. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Zaid, M.H.M.; Daniyal, W.M.E.M.M.; Mahdi, M.A. Sensitive surface plasmon resonance performance of cadmium sulfide quantum dots-amine functionalized graphene oxide based thin film towards dengue virus E-protein. Opt. Laser Technol. 2019, 114, 204–208. [Google Scholar] [CrossRef]
- Ortega, R.; Bresson, C.; Fraysse, A.; Sandre, C.; Devès, G.; Gombert, C.; Tabarant, M.; Bleuet, P.; Seznec, H.; Simionovici, A.; et al. Cobalt distribution in keratinocyte cells indicates nuclear and perinuclear accumulation and interaction with magnesium and zinc homeostasis. Toxicol. Lett. 2009, 188, 26–32. [Google Scholar] [CrossRef]
- Battaglia, V.; Compagnone, A.; Bandino, A.; Bragadin, M.; Rossi, C.A.; Zanetti, F.; Colombatto, S.; Grillo, M.A.; Toninello, A. Cobalt induces oxidative stress in isolated liver mitochondria responsible for permeability transition and intrinsic apoptosis in hepatocyte primary cultures. Int. J. Biochem. Cell Biol. 2009, 41, 586–594. [Google Scholar] [CrossRef]
- Karovic, O.; Tonazzini, I.; Rebola, N.; Edström, E.; Lövdahl, C.; Fredholm, B.B.; Daré, E. Toxic effects of cobalt in primary cultures of mouse astrocytes. Similarities with hypoxia and role of HIF-1α. Biochem. Pharmacol. 2007, 73, 694–708. [Google Scholar] [CrossRef]
- Lombaert, N.; Lison, D.; Van Hummelen, P.; Kirsch-Volders, M. In vitro expression of hard metal dust (WC-Co)-responsive genes in human peripheral blood mononucleated cells. Toxicol. Appl. Pharmacol. 2008, 227, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Daniyal, W.M.E.M.M.; Saleviter, S.; Fen, Y.W. Development of surface plasmon resonance spectroscopy for metal ion detection. Sens. Mater. 2018, 30, 2023–2038. [Google Scholar] [CrossRef]
- Roshidi, M.D.A.; Fen, Y.W.; Omar, N.A.S.; Saleviter, S.; Daniyal, W.M.E.M.M. Optical studies of graphene oxide/poly amidoamine) dendrimer composite thin film and its potential for sensing Hg2+ using surface plasmon resonance spectroscopy. Sens. Mater. 2019, 31, 1147–1156. [Google Scholar] [CrossRef]
- Ock, K.; Jang, G.; Roh, Y.; Kim, S.; Kim, J.; Koh, K. Optical detection of Cu2+ ion using a SQ-dye containing polymeric thin-film on Au surface. Microchem. J. 2001, 70, 301–305. [Google Scholar] [CrossRef]
- Lee, S.M.; Kang, S.W.; Kim, D.U.; Cui, J.Z.; Kim, S.H. Effect of metal ions on the absorption spectra and surface plasmon resonance of an azacrown indoaniline dye. Dye. Pigment. 2001, 49, 109–115. [Google Scholar] [CrossRef]
- Ramdzan, N.S.M.; Fen, Y.W.; Omar, N.A.S.; Anas, N.A.A.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical and surface plasmon resonance sensing properties for chitosan/carboxyl-functionalized graphene quantum dots thin film. Optik 2019, 178, 802–812. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Wang, Y.; Toledo, F.; Zhou, F. Studies of metal ion binding by apo-metallothioneins attached onto preformed self-assembled monolayers using a highly sensitive surface plasmon resonance spectrometer. Sens. Actuators Chem. 2007, 123, 784–792. [Google Scholar] [CrossRef]
- Forzani, E.S.; Foley, K.; Westerhoff, P.; Tao, N. Detection of arsenic in groundwater using a surface plasmon resonance sensor. Sens. Actuators Chem. 2007, 123, 82–88. [Google Scholar] [CrossRef]
- Hong, S.; Kang, T.; Moon, J.; Oh, S.; Yi, J. Surface plasmon resonance analysis of aqueous copper ions with amino-terminated self-assembled monolayers. Colloids Surf. Physicochem. Eng. Asp. 2007, 292, 264–270. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M. Surface plasmon resonance spectroscopy as an alternative for sensing heavy metal ions: A review. Sens. Rev. 2013, 33, 305–314. [Google Scholar]
- Chen, H.; Gal, Y.-S.; Kim, S.-H.; Choi, H.-J.; Oh, M.-C.; Lee, J.; Koh, K. Potassium ion sensing using a self-assembled calix[4]crown monolayer by surface plasmon resonance. Sens. Actuators Chem. 2008, 133, 577–581. [Google Scholar] [CrossRef]
- Fahnestock, K.J.; Manesse, M.; McIlwee, H.A.; Schauer, C.L.; Boukherroub, R.; Szunerits, S. Selective detection of hexachromium ions by localized surface plasmon resonance measurements using gold nanoparticles/chitosan composite interfaces. Analyst 2009, 134, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, T.; Du, Y.; Chen, C.; Li, B.; Zhou, M.; Dong, S. Au NPs-enhanced surface plasmon resonance for sensitive detection of mercury (II) ions. Biosens. Bioelectron. 2010, 25, 2622–2626. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, S.; Wei, S.C.; Chen, C.Y.; Lin, C.W. An amplified surface plasmon resonance “turn-on” sensor for mercury ion using gold nanoparticles. Biosens. Bioelectron. 2011, 30, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Detection of mercury and copper ions using surface plasmon resonance optical sensor. Sens. Mater. 2011, 23, 325–334. [Google Scholar]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Surface plasmon resonance optical sensor for detection of essential heavy metal ions with potential for toxicity: Copper, zinc and manganese ions. Sens. Lett. 2011, 9, 1704–1711. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A.; Ishak, N.S.; Omar, N.A.S.; Zainudin, A.A. Preparation, characterization and optical properties of ionophore doped chitosan biopolymer thin film and its potential application for sensing metal ion. Optik 2015, 126, 4688–4692. [Google Scholar] [CrossRef]
- Hur, Y.; Ock, K.; Kim, K.; Jin, S.; Gal, Y.; Kim, J.; Kim, S.; Koh, K. Surface plasmon resonance study on enhanced refractive index change of an Ag+ ion-sensing membrane containing dithiosquarylium dye. Anal. Chim. Acta 2002, 460, 133–139. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Noor, A.S.M.; Moksin, M.M.; Abdi, M.M.; Mohammadi, A. Application of polypyrrole-chitosan layer for detection of Zn (II) and Ni (II) in aqueous solutions using surface plasmon resonance. Int. J. Polym. Mater. 2013, 62, 284–287. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Surface plasmon resonance optical sensor for detection of Pb2+ based on immobilized p-tert-butylcalix[4]arene-tetrakis in chitosan thin film as an active layer. Sens. Actuators Chem. 2012, 171, 287–293. [Google Scholar] [CrossRef]
- Pelossof, G.; Tel-Vered, R.; Willner, I. Amplified surface plasmon resonance and electrochemical detection of Pb2+ ions using the Pb2+-dependent DNAzyme and hemin/G-quadruplex as a label. Anal. Chem. 2012, 84, 3703–3709. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Chung, B.H.; Lee, H.J. Parts per trillion detection of Ni (II) ions by nanoparticle-enhanced surface plasmon resonance. Anal. Chem. 2012, 84, 10091–10096. [Google Scholar] [CrossRef] [PubMed]
- Fen, Y.W.; Yunus, W.M.M.; Moksin, M.M.; Talib, Z.A.; Yusof, N.A. Surface plasmon resonance optical sensor for mercury ion detection by crosslinked chitosan thin film. J. Optoelectron. Adv. Mater. 2011, 13, 279–285. [Google Scholar]
- Verma, R.; Gupta, B.D. Detection of heavy metal ions in contaminated water by surface plasmon resonance based optical fibre sensor using conducting polymer and chitosan. Food Chem. 2015, 166, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Domínguez, D.; Rodriguez, M.; Ramos-Ortiz, G.; Maldonado, J.L.; Luna-Moreno, D.; Ortiz-Gutierrez, M.; Barba, V. A Schiff base derivative used as sensor of copper through colorimetric and surface plasmon resonance techniques. Sens. Actuators Chem. 2016, 225, 221–227. [Google Scholar] [CrossRef]
- May, L.M.; Russell, D.A. Novel determination of cadmium ions using an enzyme self-assembled monolayer with surface plasmon resonance. Anal. Chim. Acta 2003, 500, 119–125. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Naseri, M.; Rashid, S.A. Polypyrrole-chitosan/nickel-ferrite nanoparticle composite layer for detecting heavy metal ions using surface plasmon resonance technique. Opt. Laser Technol. 2017, 93, 216–223. [Google Scholar] [CrossRef]
- Saleviter, S.; Fen, Y.W.; Sheh Omar, N.A.; Daniyal, W.M.E.M.M.; Abdullah, J.; Mahdi, M.A. Label-free binding analysis of 4-(2-Pyridylazo)-resorcinol-based composite layer with cobalt ion using surface plasmon resonance optical sensor. Sens. Mater. 2020, 32, 2877–2889. [Google Scholar] [CrossRef]
- Saleviter, S.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Abdullah, J.; Zaid, M.H.M. Structural and optical studies of cadmium sulfide quantum dot-graphene oxide-chitosan nanocomposite thin film as a novel SPR spectroscopy active layer. J. Nanomater. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Zhao, Y.; Gan, S.; Wu, L.; Zhu, J.; Xiang, Y.; Dai, X. GeSe nanosheets modified surface plasmon resonance sensors for enhancing sensitivity. Nanophotonics 2020, 9, 327–336. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Denizli, A. Highly sensitive detection of Cd (II) ions using ion-imprinted surface plasmon resonance sensors. Microchem. J. 2020, 159, 105572–105583. [Google Scholar] [CrossRef]
- Saleviter, S.; Fen, Y.W.; Alia, N.; Omar, S.; Zainudin, A.A.; Yusof, N.A. Development of optical sensor for determination of Co (II) based on surface plasmon resonance phenomenon. Sens. Lett. 2017, 15, 1–6. [Google Scholar] [CrossRef]
- Chah, S.; Yi, J.; Zare, R.N. Surface plasmon resonance analysis of aqueous mercuric ions. Sens. Actuators Chem. 2004, 99, 216–222. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S. Exploration of surface plasmon resonance for sensing copper ion based on nanocrystalline cellulose-modified thin film. Opt. Express 2018, 26, 34880–34893. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.C.; Lai, E.P.C.; Sadeghi, S. Surface plasmon resonance sensor for Hg (II) detection by binding interactions with polypyrole and 2-mercaptobenzothiazole. Sens. Actuators Chem. 2004, 101, 236–241. [Google Scholar] [CrossRef]
- Wu, C.M.; Lin, L.Y. Immobilization of metallothionein as a sensitive biosensor chip for the detection of metal ions by surface plasmon resonance. Biosens. Bioelectron. 2004, 20, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Lin, L.Y. Utilization of albumin-based sensor chips for the detection of metal content and characterization of metal-protein interaction by surface plasmon resonance. Sens. Actuators Chem. 2005, 110, 231–238. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical properties of chitosan/hydroxyl-functionalized graphene quantum dots thin film for potential optical detection of ferric (III) ion. Opt. Laser Technol. 2019, 120, 105724–105732. [Google Scholar] [CrossRef]
- Forzani, E.S.; Zhang, H.; Chen, W.; Tao, N. Detection of heavy metal ions in drinking water using a high-resolution differential surface plasmon resonance sensor. Environ. Sci. Technol. 2005, 39, 1257–1262. [Google Scholar] [CrossRef]
- Moon, J.; Kang, T.; Oh, S.; Hong, S.; Yi, J. In situ sensing of metal ion adsorption to a thiolated surface using surface plasmon resonance spectroscopy. J. Colloid Interface Sci. 2006, 298, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Fen, Y.W.; Yunus, W.M.M.; Talib, Z.A. Analysis of Pb (II) ion sensing by crosslinked chitosan thin film using surface plasmon resonance spectroscopy. Optik 2013, 124, 126–133. [Google Scholar] [CrossRef]
- Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Saleviter, S.; Abdullah, J. Structural, optical and potential sensing properties of tyrosinase immobilized graphene oxide thin film on gold surface. Optik 2020, 212, 164786–164797. [Google Scholar] [CrossRef]
- Crist, B.V. XPS in industry-problems with binding energies in journals and binding energy databases. J. Electron Spectros. Relat. Phenom. 2019, 231, 75–87. [Google Scholar] [CrossRef]
- Shinotsuka, H.; Yoshikawa, H.; Murakami, R.; Nakamura, K.; Tanaka, H. Automated information compression of XPS spectrum using information criteria. J. Electron Spectros. Relat. Phenom. 2020, 239, 146903–146910. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Saleviter, S.; Daniyal, W.M.E.M.M.; Hashim, H.S.; Nasrullah, M. Nanostructured chitosan/maghemite composites thin film for potential optical detection of mercury ion by surface plasmon resonance investigation. Polymers 2020, 12, 1497. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Talib, Z.A.; Yusof, N.A. Development of surface plasmon resonance sensor for determining zinc ion using novel active nanolayers as probe. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015, 134, 48–52. [Google Scholar] [CrossRef]
- Bennettand, H.; Ouver, G.J. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database. (Beamson, G.; Briggs, D.). J. Chem. Educ. 1993, 70, A25. [Google Scholar]
- De Moraes, A.C.M.; Lima, B.A.; De Faria, A.F.; Brocchi, M.; Alves, O.L. Graphene oxide-silver nanocomposite as a promising biocidal agent against methicillin- resistant Staphylococcus aureus. Int. J. Nanomed. 2015, 10, 6847–6861. [Google Scholar] [CrossRef]
- Liu, Q.; Li, D.; Zhu, Z.; Yu, S.; Zhang, Y.; Yu, D.; Jiang, Y. N-doped carbon dots from phenol derivatives for excellent colour rendering WLEDs. RSC Adv. 2018, 8, 4850–4856. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Ramdzan, N.S.M.; Saleviter, S. Development of graphene quantum dots-based optical sensor for toxic metal ion detection. Sensors 2019, 19, 3850. [Google Scholar] [CrossRef]
- Gu, Y.; Tang, L.; Guo, X.; Xiang, J.; Seng Teng, K.; Ping Lau, S. Preparation and photoelectric properties of cadmium sulfide quantum dots. Chin. Phys. 2019, 28, 47803. [Google Scholar] [CrossRef]
- Fantauzzi, M.; Elsener, B.; Atzei, D.; Rigoldi, A.; Rossi, A. Exploiting XPS for the identification of sulfides and polysulfides. RSC Adv. 2015, 5, 75953–75963. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Optical properties of crosslinked chitosan thin film as copper ion detection using surface plasmon resonance technique. Opt. Appl. 2011, 41, 999–1013. [Google Scholar]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Anas, N.A.A.; Omar, N.A.S.; Ramdzan, N.S.M.; Nakajima, H.; Mahdi, M.A. Enhancing the sensitivity of a surface plasmon resonance-based optical sensor for zinc ion detection by the modification of a gold thin film. RSC Adv. 2019, 9, 41729–41736. [Google Scholar] [CrossRef]
- Lokman, N.F.; Bakar, A.A.A.; Suja, F.; Abdullah, H.; Rahman, W.B.W.A.; Huang, N.M.; Yaacob, M.H. Highly sensitive SPR response of Au/chitosan/graphene oxide nanostructured thin films toward Pb (II) ions. Sens. Actuators Chem. 2014, 195, 459–466. [Google Scholar] [CrossRef]
- Ho, Y.S.; Porter, J.F.; McKay, G. Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: Copper, nickel and lead single component systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, A.S. Lable-free optical spectroscopy for characterizing binding properties of highly sensitive nanocrystalline cellulose-graphene oxide based nanocomposite towards nickel ion. Spectrochim. Acta Mol. Biomol. Spectrosc. 2018, 212, 25–31. [Google Scholar] [CrossRef]
- Rosddi, N.N.M.; Fen, Y.W.; Anas, N.A.A.; Omar, N.A.S.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M. Cationically modified nanocrystalline cellulose/carboxyl-functionalized graphene quantum dots nanocomposite thin film: Characterization and potential sensing application. Crystals 2020, 10, 875. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Chik, C.E.N.C.E.; Mahdi, M.A. Development of an optical sensor based on surface plasmon resonance phenomenon for diagnosis of dengue virus E-protein. Sens. Biosensing Res. 2018, 20, 16–21. [Google Scholar] [CrossRef]
- Saleviter, S.; Fen, Y.W.; Daniyal, W.M.E.M.M.; Abdullah, J.; Sadrolhosseini, A.R.; Omar, N.A.S. Design and analysis of surface plasmon resonance optical sensor for determining cobalt ion based on chitosan-graphene oxide decorated quantum dots-modified gold active layer. Opt. Express 2019, 27, 32294–32307. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Kamil, Y.M.; Daniyal, W.M.E.M.M.; Sadrolhosseini, A.R.; Mahdi, M.A. Sensitive detection of dengue virus type 2 E-proteins signals using self-assembled monolayers/reduced graphene oxide-pamam dendrimer thin film-SPR optical sensor. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Shafie, S.; Rashid, S.A.; Mahdi, M.A. Surface plasmon resonance measurement of arsenic in low concentration using polypyrrole-graphene quantum dots layer. Measurement 2020, 10, 108546–108557. [Google Scholar] [CrossRef]

| Sample | Element | Corrected RSF | Area | Corrected Area | Ratio (%) |
|---|---|---|---|---|---|
| PAR–chitosan–GO | C | 1.2070 | 15,701 | 13,008.3 | 66.41 |
| O | 3.1048 | 17,005 | 5477.0 | 27.96 | |
| N | 2.0520 | 2264 | 1103.3 | 5.63 |
| Sample | Element | Corrected RSF | Area | Corrected Area | Ratio (%) |
|---|---|---|---|---|---|
| CdS QD–chitosan–GO | C | 1.2070 | 15,033 | 12,454.8 | 67.53 |
| O | 3.1048 | 16,396 | 5280.9 | 28.63 | |
| Cd | 25.9923 | 1147 | 44.1 | 0.24 | |
| S | 2.4319 | 742 | 305.1 | 1.65 | |
| N | 2.0520 | 738 | 359.6 | 1.95 |
| Sample | K (ppm−1) | R2 | n | (°) |
|---|---|---|---|---|
| PAR–chitosan–GO | 1.649 | 0.96 | 0.400 | 0.334 |
| CdS QD–chitosan–GO | 0.939 | 0.96 | 0.400 | 0.213 |
| Sample | Element | Corrected RSF | Area | Corrected Area | Ratio (%) |
|---|---|---|---|---|---|
| PAR–chitosan–GO | C | 1.2070 | 16,139 | 13,371.2 | 63.51 |
| O | 3.1048 | 19,169 | 6174 | 29.32 | |
| N | 2.0520 | 2998 | 1461 | 6.94 | |
| Co | 18.5764 | 858 | 46.2 | 0.22 |
| Sample | Element | Corrected RSF | Sub-Peak | Area | Corrected Area | Ratio (%) |
|---|---|---|---|---|---|---|
| PAR–chitosan–GO before contact with Co2+ | C | 1.2070 | C–C | 9491.2 | 7863.5 | 60.28 |
| C–O, C–O–C | 4159.1 | 3445.8 | 26.41 | |||
| C=O, O–C=O | 2095.1 | 1735.8 | 13.31 | |||
| O | 3.1048 | O–C, C–O–C | 11,196.8 | 3606.3 | 70.50 | |
| O=C | 4685.2 | 1509.0 | 29.50 | |||
| N | 2.0520 | Pyridinic | 442.4 | 214.6 | 19.41 | |
| Amine | 1705.1 | 830.9 | 74.79 | |||
| Pyrrolic | 132.3 | 64.5 | 5.80 | |||
| PAR–chitosan–GO after contact with Co2+ | C | 1.2070 | C–C | 7526.9 | 6236.0 | 48.68 |
| C–O, C–O–C | 5985.3 | 4958.8 | 38.71 | |||
| C=O, O–C=O | 1949.8 | 1615.4 | 12.61 | |||
| O | 3.1048 | O–C, C–O–C | 14,541.3 | 4683.5 | 76.80 | |
| O=C | 4392.7 | 1414.8 | 23.15 | |||
| N | 2.0520 | Pyridinic | 772 | 376.21 | 25.87 | |
| Amine | 1939.9 | 946.29 | 65.01 | |||
| Pyrrolic | 272.1 | 132.60 | 9.12 |
| Sample | Element | Corrected RSF | Area | Corrected Area | Ratio (%) |
|---|---|---|---|---|---|
| CdS QD–chitosan–GO | C | 1.2070 | 18,606 | 15,415.1 | 66.60 |
| O | 3.1048 | 20,161 | 6493.5 | 28.05 | |
| Cd | 25.9923 | 1608 | 61.9 | 0.27 | |
| S | 2.4319 | 1267 | 521 | 2.25 | |
| N | 2.0520 | 1230 | 599.4 | 2.59 | |
| Co | 18.5764 | 1070 | 57.6 | 0.29 |
| Sample | Element | Corrected RSF | Sub-Peak | Area | Corrected Area | Ratio (%) |
|---|---|---|---|---|---|---|
| CdS QD–chitosan–GO before contact with Co2+ | C | 1.2070 | C–C | 6594.5 | 5463.5 | 46.45 |
| C–O, C–O–C | 5438.9 | 4506.1 | 38.31 | |||
| C=O, O–C=O | 2163.6 | 1792.5 | 15.24 | |||
| O | 3.1048 | O–C, C–O–C | 10,782.7 | 3472.9 | 69.57 | |
| O=C | 4716.3 | 1519.0 | 30.43 | |||
| N | 2.0520 | Amine | 515.9 | 251.4 | 70.01 | |
| C–N | 221.1 | 107.7 | 29.91 | |||
| CdS QD–chitosan–GO after contact with Co2+ | C | 1.2070 | C–C | 7542.7 | 6249.1 | 44.56 |
| C–O, C–O–C | 7332.8 | 6075.2 | 43.32 | |||
| C=O, O–C=O | 2051.6 | 1699.8 | 12.12 | |||
| O | 3.1048 | O–C, C–O–C | 14,528.3 | 4679.3 | 73.29 | |
| O=C | 5294.7 | 1705.3 | 26.71 | |||
| N | 2.0520 | Amine | 935.1 | 455.7 | 67.32 | |
| C–N | 453.9 | 221.2 | 32.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniyal, W.M.E.M.M.; Fen, Y.W.; Saleviter, S.; Chanlek, N.; Nakajima, H.; Abdullah, J.; Yusof, N.A. X-ray Photoelectron Spectroscopy Analysis of Chitosan–Graphene Oxide-Based Composite Thin Films for Potential Optical Sensing Applications. Polymers 2021, 13, 478. https://doi.org/10.3390/polym13030478
Daniyal WMEMM, Fen YW, Saleviter S, Chanlek N, Nakajima H, Abdullah J, Yusof NA. X-ray Photoelectron Spectroscopy Analysis of Chitosan–Graphene Oxide-Based Composite Thin Films for Potential Optical Sensing Applications. Polymers. 2021; 13(3):478. https://doi.org/10.3390/polym13030478
Chicago/Turabian StyleDaniyal, Wan Mohd Ebtisyam Mustaqim Mohd, Yap Wing Fen, Silvan Saleviter, Narong Chanlek, Hideki Nakajima, Jaafar Abdullah, and Nor Azah Yusof. 2021. "X-ray Photoelectron Spectroscopy Analysis of Chitosan–Graphene Oxide-Based Composite Thin Films for Potential Optical Sensing Applications" Polymers 13, no. 3: 478. https://doi.org/10.3390/polym13030478
APA StyleDaniyal, W. M. E. M. M., Fen, Y. W., Saleviter, S., Chanlek, N., Nakajima, H., Abdullah, J., & Yusof, N. A. (2021). X-ray Photoelectron Spectroscopy Analysis of Chitosan–Graphene Oxide-Based Composite Thin Films for Potential Optical Sensing Applications. Polymers, 13(3), 478. https://doi.org/10.3390/polym13030478








