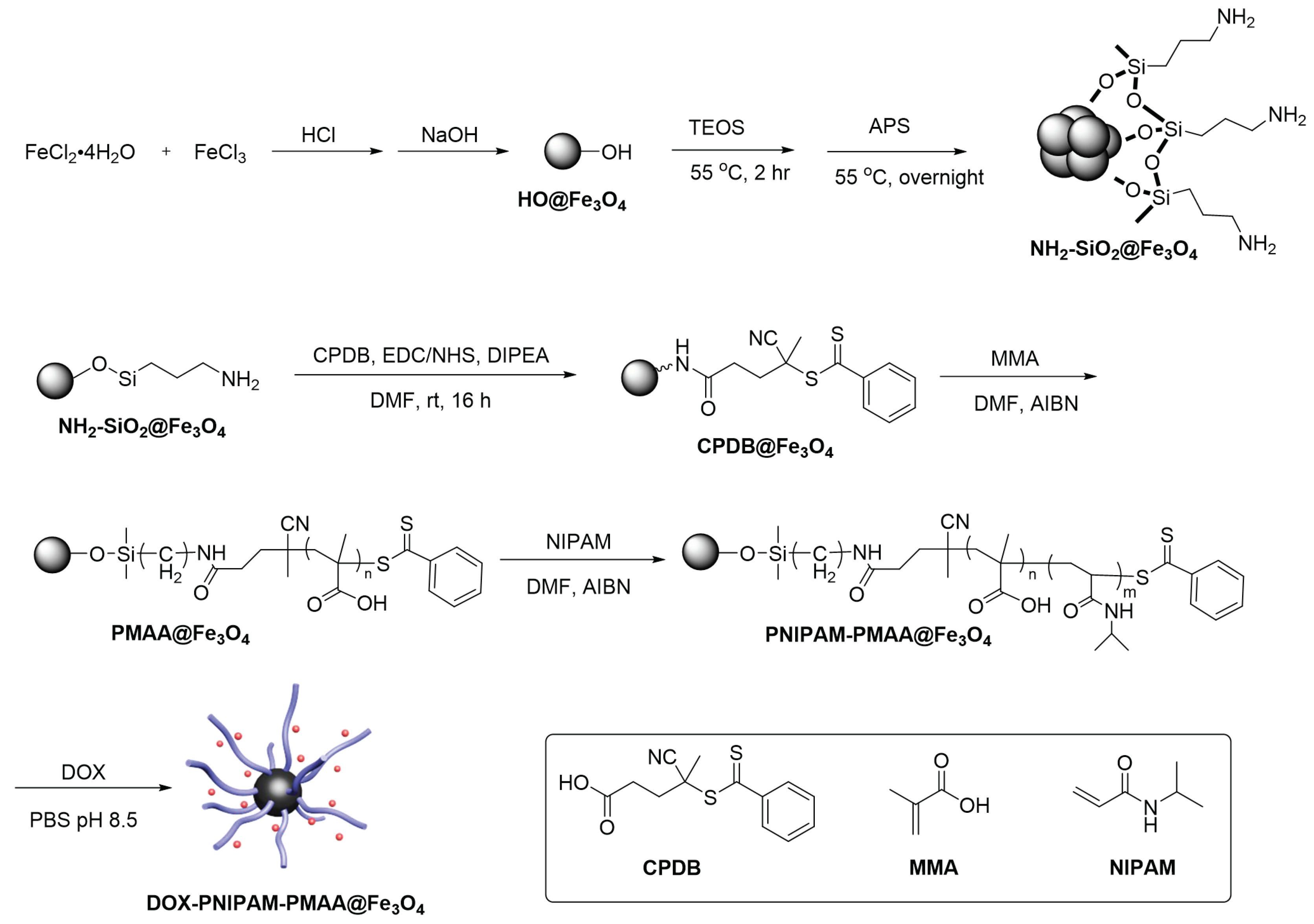
1. Introduction
To attain specific drug delivery while maintaining drug stability has been a significant issue in the research field of tumor therapy [
1,
2]. Modified magnetic nanoparticles (MNPs) have been reported to act as a platform for drug delivery that could overcome drug degradation and cytotoxicity problems [
3,
4,
5]. Tumor cells have greater glycolytic activity, and the corresponding metabolites lower the pH value of tumor sites; on the other hand, the replication and proliferation processes may produce heat and increase the temperature in the tissue [
6,
7]. Based on the above-mentioned aspects, recent research has pointed out several innovations to utilize these abnormal features of the tumor microenvironment (TME) [
8,
9,
10,
11,
12]. For instance, modified MNPs have been used as a vector for drug delivery due to their low cytotoxicity and high bio-compatibility [
5]. Through modification of the particle surface, particles can respond to subtle environment changes and, thus, could be able to perform target-specific drug delivery [
13,
14].
Superparamagnetism is a unique characteristic of magnetic nano-materials. Owing to this feature, magnetic nanoparticles are an easy-to-operate material while being highly bio-compatible, highly stable, and less cytotoxic. Therefore, there is no doubt that the material can be applied in the biomedical field, such as for MRI image improvement, tumor magneto-therapy, and bio-separation [
15,
16,
17]. As for the synthesis method, in addition to cobalt and nickel, most were formed with Fe
3O
4 iron-cored via co-precipitation. Co-precipitation is a synthesis method that features great yield and gentle reaction condition [
18].
In order to achieve specific targeting to TME, we designed a nanovector for target-specific drug delivery. TMEs in blood vessels, immune cells, fibroblasts, various signaling molecules, and extracellular matrix are different from healthy tissue. Tumor cells obtain large amounts of oxygen and nutrition for rapid growth and cells may secrete vascular endothelial growth factor (VEGF) and facilitate angiogenesis [
19,
20]. Tumor cells have complicated interactions and signaling transduction to enhance carcinoma metastasis, growth, and inflammation [
6,
21]. Tumor-associated macrophages (TAMs) are immune cells in the TME and can be divided into M1 and M2 types. M1-type TAMs can express and produce pro-inflammatory cytokines to induce anti-tumor responses. M2-type TAMs, on the contrary, secrete anti-inflammatory agents such as transforming growth factor-β (TGF-β), interleukin-10 (IL-10), and epidermal growth factor, which inhibit immune responses and induce angiogenesis and carcinoma metastasis [
22]. Nanoparticles and macromolecular drugs may remain in the TME through an enhanced permeability and retention effect (EPR effect). Cancer tissues usually consist of abnormal blood vessels and poorly aligned defective endothelial cells, where inter-endothelial junctions of tumors lie in the range of 40–80 nm while the inter-endothelial junction of normal endothelial cells is 8 nm [
23]. In addition, cancer cells lack effective lymphatic drainage. Therefore, nanoparticles and macromolecular drugs tend to accumulate at tumor tissues rather than normal tissues due to the EPR effect. When tumor size reaches 200 μm, the TME forms and induces the EPR effect [
19,
20]. We have used this feature and developed nanovectors to target cancer cells [
23,
24].
In this study, the reverse addition-fragmentation chain transfer (RAFT) polymerization method is used for MNP modifications. RAFT is a common radical polymerization mediated by a RAFT agent to achieve radical transfer and chain elongation. In this kind of polymerization, certain dithioester derivatives serve as the RAFT agent, and azobisisobutyronitrile (AIBN) acts as a radical initiator to activate radical formation, propagation, RAFT pre-equilibrium, re-initiation, main RAFT equilibrium, and termination [
25]. RAFT polymerization features a broad application of monomers, gentle condition, and narrow polydispersity index (PDI), traits that are key to modifying functionalized nanoparticles [
12,
26].
To date, several cancer-specific responsive nanoparticles have been developed [
27]. For example, a silica nanoparticle was designed to be both pH- and thermo-responsive via RAFT polymerization [
27]. When the nanoplatform was subjected to low pH values and high temperature, carboxylate groups on the surface protonated to form the COOH group. In addition, when
N-isopropylacrylamide (NIPAM) aggregates above the lower critical solution temperature (LCST), it results in a synergistic effect of doxorubicin (DOX) releasing. The formation of the COOH group would weaken the electrostatic attraction between drugs and particle surface. Furthermore, when the reaction temperature is higher than the LCST of NIPAM, NIPAM on the particle surface would be insoluble, resulting in aggregation of MNPs that spread DOX. This finding definitely improved the low effect on drug releasing of temperature that was reported in the previous literature.
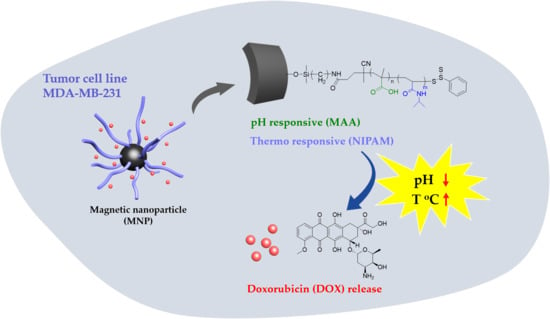
This series of experiments was designed based on the above-mentioned literature review. We aimed to synthesize Fe
3O
4-cored nanoparticles via co-precipitation and utilize the sol-gel method to complete amine functionalization. RAFT polymerization is taken to modify the particle surface with responsive moiety. Doxorubicin, an anti-tumor drug, is then loaded onto the particle and subjected to in vitro drug release (
Scheme 1).
2. Materials and Methods
2.1. Material
FeCl3 (Alfar Aesar), FeCl2·4H2O (Alfar Aesar), tetraethyl orthosilicate (TEOS, Sigma-Aldrich, St. Louis, MO, USA), 3-aminopropyltrimethoxysilane (APS, Alfar Aesar, Haverhill, MA, USA), 2-propanol (MACRON, Radnor, PA, USA), ammonium hydroxide solution (25%, MACRON), 4-nitrobenzaldehyde (NPC, Alfar Aesar), triethyl amine (TEA, Alfar Aesar), ethanolamine (Sigma-Aldrich), 4-Cyano-4-(phenylcarbonothioylthio)pentanoic acid (CPDB, STREM, Newburyport, MA, USA), 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC, Sigma-Aldrich), N-hydroxysuccinimide (NHS, TCI, Tokyo, Japan), methacrylic acid (MAA, ACROS), azobisisobutyronitrile (AIBN, UniRegion Bio-Tech), ethanolamine (Sigma-Aldrich), N,N-Diisopropylethylamine (DIPEA, TCI), Dimethyl formaldehyde (DMF, DUKSAN), tetrahydrofuran (THF, DUKSAN, Gyeonggi-do, Korea), dimethyl sulfoxide (DMSO, Fisher), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) were used as received. Analytical thin-layer chromatography (TLC) was performed using pre-coated plates (Silica Gel 60 F254, Merck, Kenilworth, NJ, USA).
A UV–Vis spectrum reader (BioTek, SYNERGY Mx, Winooski, VT, USA), a fluorescence spectrometer (Leica DMI4000 B, Wetzlar, Germany), dynamic light scattering (DLS) (HORIBA, SZ-100, Kyoto, Japan), FTIR (Perkin Elmer Intruments, Spectrum One, Waltham, MA, USA), TGA/DSC (Mettler-Toledo, 2-HT, Columbus, OH, USA), a centrifuge (HERMLE, Gosheim, Germany), and a fluorescence microscope (HORIBA, Fluorolog, Kyoto, Japan) were used for analytical purposes.
2.2. Preparation of HO@Fe3O4
The particle HO@Fe3O4 was synthesized by co-precipitation of FeCl3 and FeCl2·4H2O under 1.5 N NaOH(aq). FeCl3 (2.0 g, 12.3 mmol) and FeCl2·4H2O (5.2 g, 26.2 mmol) were mixed in 25 mL of 12.1 N HCl. The mixture was then added dropwise into 250 mL of 1.5 N NaOH. The black precipitate was collected after 4000 rpm centrifugation for 15 min. Then, 500 mL of 0.01 N HCl solution was added into the collected residues under vigorous vortex. HO@Fe3O4 was then obtained and washed with deionized water.
2.3. Preparation of NH2-SiO2@Fe3O4
An amine moiety was attached to HO@Fe3O4 by a sol-gel method that used tetraethyl orthosilicate (TEOS) and 3-aminopropyltrimethoxysilane (APS). HO@Fe3O4 (500 mg) and TEOS (0.42 mL) were mixed in 11 mL of 2-propanol. Ammonium hydroxide (1.58 mL) was then added. The flask was placed in an oil bath at 55 °C for 2 h. Then, 0.42 mL of APS was added into the above mixture and reacted for 15 h. The final product was washed twice by 2-propanol and deionized water consecutively and then dried under vacuum.
2.4. Quantification of Amine Functional Group
NH2-SiO2@Fe3O4 (5.0 mg) was suspended in 1.0 mL of MeOH and then subjected to sonication for 30 min. The particles were washed using THF and DCM three times each. Next, the particles were treated with 10 mg of 4-nitrobenzaldehyde (NPC) and 10 μL trimethylamine (TEA) for 1 h. NPC@Fe3O4 was then obtained and washed thrice using DCM. MeOH (1 mL), 10 μL ethanolamine, and 10 μL TEA were added into the flask and left for 30 min. A 4-nitrobenzaldehyde solution was obtained via hydrolysis followed by ethanolamine and was subsequently estimated by comparison of the calibration curve. The results showed that the amine concentration attained was around 120 nmol per milligram of particle.
2.5. Attachment of RAFT Agent to Particle Surface
NH2-SiO2@Fe3O4 (5 mg) was dissolved in DMF. EDC (11.50 mg, 0.06 mmol), NHS (6.91 mg, 0.06 mmol), DIPEA (3.2 μL, 0.06 mmol), and CPDB (16.76 mg, 0.06 mmol) were added to the above solution and placed at room temperature for 16 h. The product CPDB@Fe3O4 was then obtained after washing twice with DMF and dried under vacuum.
2.6. Synthesis of PMAA@Fe3O4
CPDB@Fe3O4 (10.4 mg, 1.25 × 10−3 mmol) and methacrylic acid (MAA, 275 mg, 3.2 mmol) were dissolved in 1 mL THF in a 10-mL flask. AIBN (32.5 μg, 198 μmol) was then added. The mixture was then degassed by the typical three freeze–pump–thaw cycle. After being backfilled with argon, the flask was placed in an oil bath at 60 °C for 3 h. The RAFT polymerization was quenched by 1 mL ether in an ice bath.
2.7. Synthesis of PNIPAM-PMAA@Fe3O4
PMAA @Fe3O4 (23.4 mg) and N-Isopropylacrylamide (NIPAM, 158.4 mg, 1.4 mmol) were dissolved in 1 mL DMF. AIBN (23 μg, 140 μmol) was then added. The mixture was then degassed by the typical three freeze–pump–thaw cycle. After being backfilled with argon, the flask was placed in an oil bath at 80 °C overnight. The RAFT polymerization was quenched by 1 mL ether in an ice bath. The PNIPAM-PMAA@ Fe3O4 was dried under vacuum for the next experiment.
2.8. Preparation of DOX-PNIPAM-PMAA@Fe3O4
PNIPAM-PMAA@Fe3O4 (1.5 mg) and DOX (500 μg) were mixed in 1 mL PBS, pH 8.5. The mixture was placed at room temperature overnight. After ultracentrifugation at 10,000 rpm for 30 min, the supernatant was collected and subjected to UV–Vis analysis. The mass of DOX attached to magnetic nanoparticles was estimated by comparing with the standard curve.
2.9. UV-Vis Absorption Measurement for Drug Loading
Doxorubicin has a significant peak at the 480 nm endpoint. Therefore, the supernatant of the DOX-PNIPAM-PMAA@Fe3O4 sample was collected to estimate the drug loading content (DL contents, %) and drug encapsulation efficiency (drug EE, %).
2.10. DLS and Zeta-Potential Measurement
Samples of HO@Fe3O4 (1 mg), NH2-SiO2@Fe3O4 (1 mg), CPDB@Fe3O4 (1 mg), PMAA@Fe3O4 (1 mg), and PNIPAM-PMAA@Fe3O4 (1 mg) were collected and dried under high vacuum. Particles of HO@Fe3O4, NH2-SiO2@Fe3O4, and PNIPAM-PMAA@Fe3O4 were dispersed in 2 mL dd-H2O and sonicated for 30 min. Then, the solution was subjected to DLS analysis (HORIBA, SZ-100). The same method was employed for CPDB@Fe3O4 and PMAA@Fe3O4 but the particles were dispersed in DMF for better polydiseperse Index (PDI). For zeta-potential analysis, each sample (1 mg) was collected and dispersed in dd-H2O. After sonication for 30 min, the samples were subjected to zeta-potential analysis.
2.11. Drug Loading Analysis Through Fluorescence Spectrometer
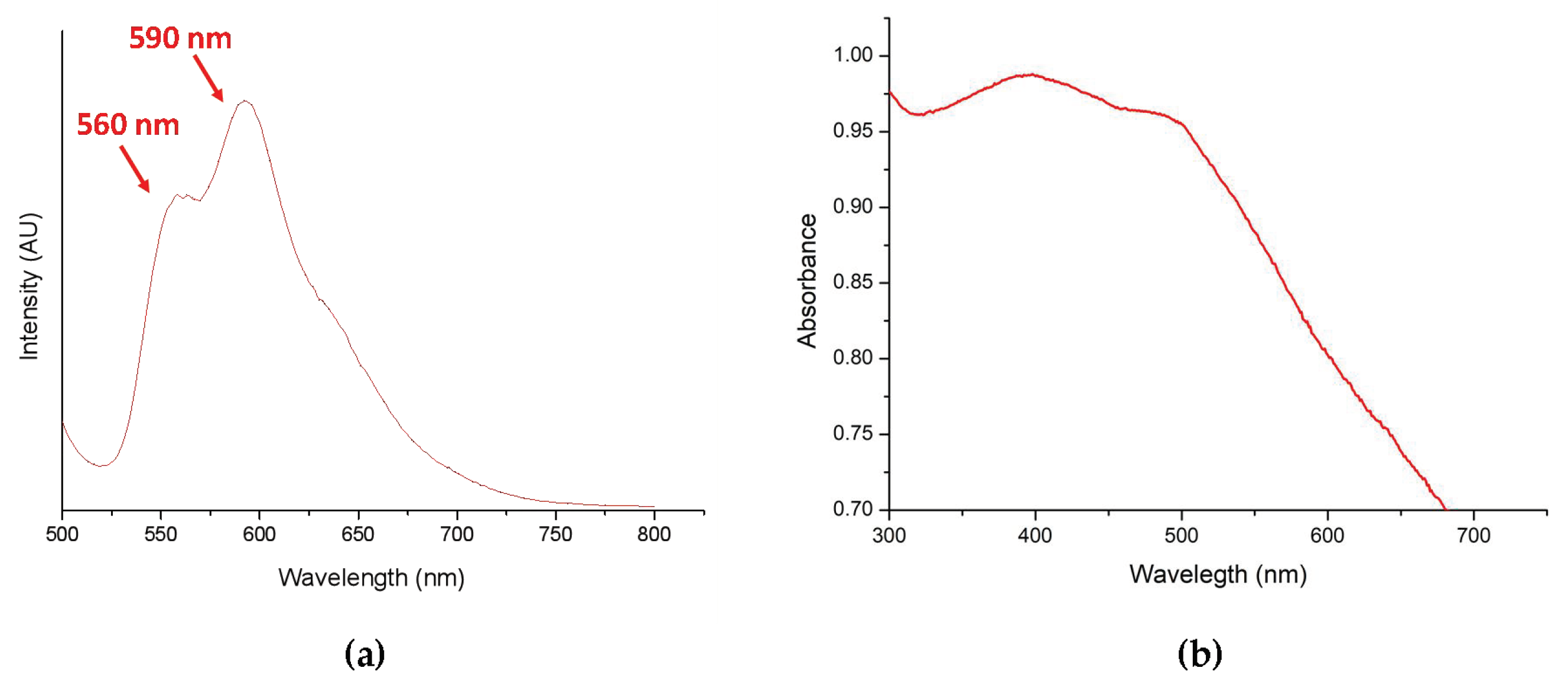
According to the literature, doxorubicin (DOX) has a significant fluorescence emission signal at 560 and 590 nm under 480-nm excitation. As a result, DOX-PNIPAM-PMAA@Fe3O4 was dissolved in H2O and an analysis was conducted using a fluorescence spectrometer to further ensure whether doxorubicin was loaded onto the particle surface.
2.12. In Vitro DOX Release from Magnetic Nanoparticles
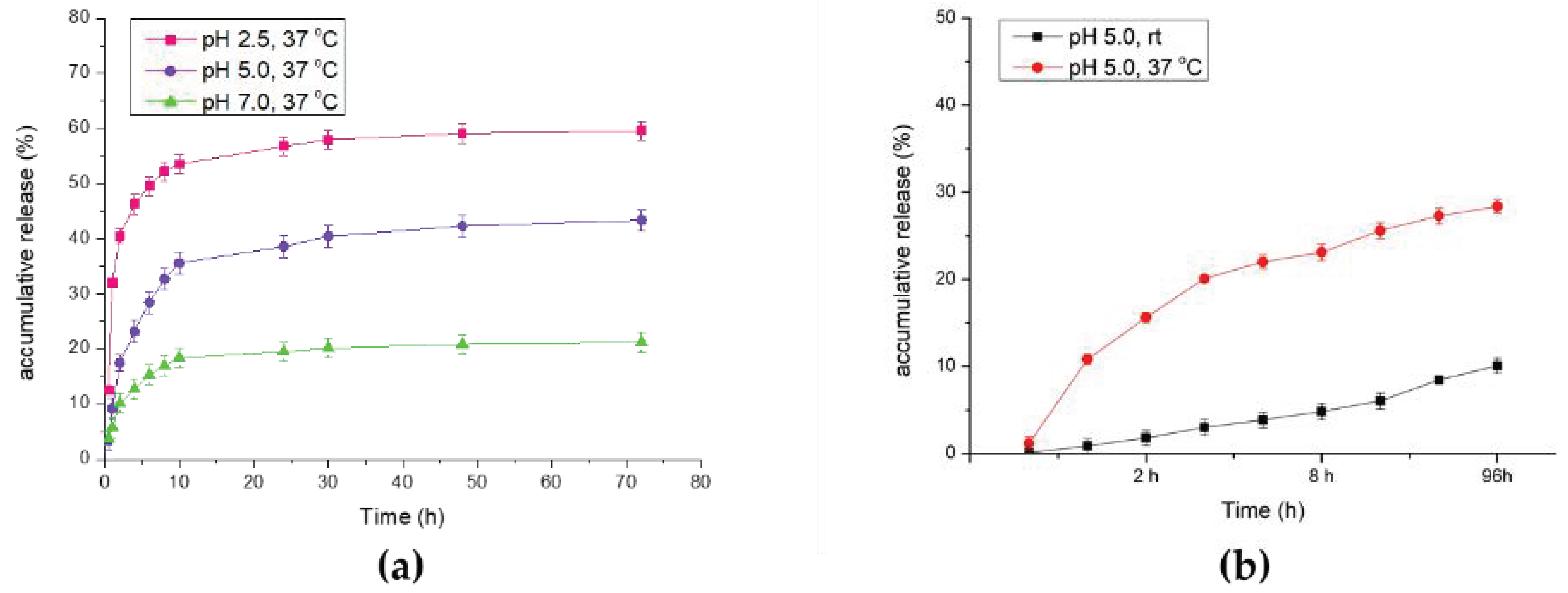
DOX-PNIPAM-PMAA@Fe3O4 (1.5 mg) was washed twice with H2O. DOX-PNIPAM-PMAA@Fe3O4 was well dispersed in different acidic conditions in phosphate-buffered saline (PBS) buffer (pH 7.0, 5.0, or 2.5) and placed at 37 °C for a period of time. Each sample was collected at certain time points and subjected to UV–Vis analysis to estimate the mass of total DOX released from the particles. In the same method, DOX-PNIPAM-PMAA@Fe3O4 (1.5 mg) was well dispersed in phosphate-buffered saline (PBS) buffer (pH 2.5) and placed at room temperature and 40 °C. Each sample was collected and subjected to UV–Vis analysis to estimate DOX release efficiency.
2.13. Cell Viability Analysis via MTT Assay
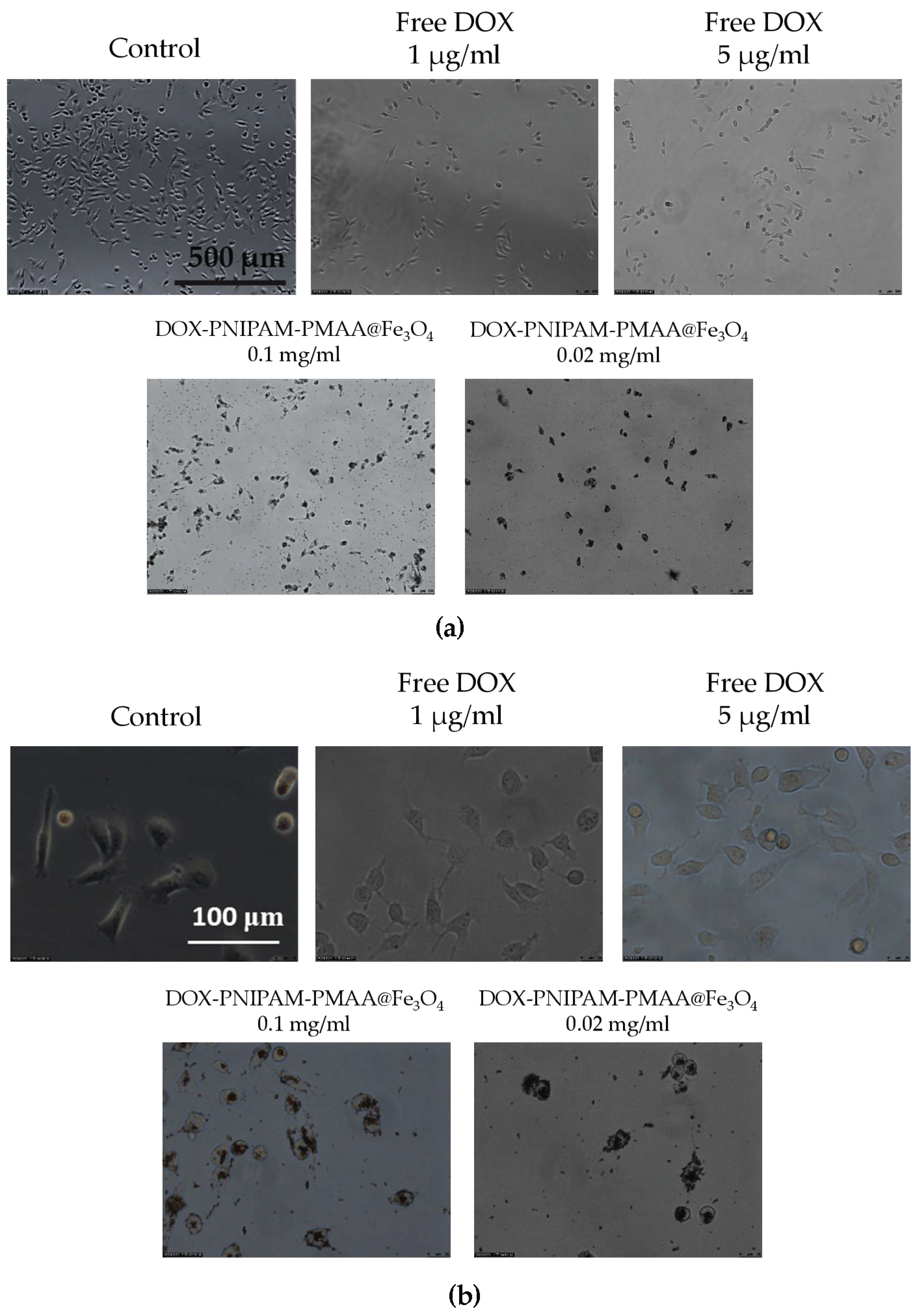
The MDA-MB-231 cell line was used as the cell material for the cell viability analysis. Cells were incubated in a 96-well microplate overnight (104 cells/well). PNIPAM-PMAA@Fe3O samples were acquired through sequencing dilution (0.1 and 0.02 mg/mL for particle concentration). DOX-PNIPAM-PMAA@Fe3O4 (0.1 and 0.02 mg/mL for particle concentration) samples were obtained through the same method (DOX concentration was 5.4 and 1.1 μg/mL for each, respectively). MDA-MB-231 cells were treated with the above-mentioned samples and PBS (control) for three replications. After 24 h, particles were washed away twice with PBS and further placed at 37 °C to incubate for 48 and 72 h. After MTT addition for 4 h, purple crystallization was dissolved with DMSO and subjected to UV-Vis absorption analysis under 570-nm excitation.
2.14. Cellular Uptake Analysis via Microscopy
MBA-MB-231 cells were plated previously in a 12-well plate (2 × 106 cell per well). Cells were treated with free DOX (5 and 1 μg/mL), DOX-PNIPAM-PMAA@Fe3O4 (5 and 1 μg/mL for DOX-loaded concentration; 0.1 and 0.02 mg/mL for particle concentration), and PBS as control. All the treatments lasted for 4 h. After 4 h of incubation, particles were washed away with PBS. Then, each well was observed under a microscope.
4. Discussion
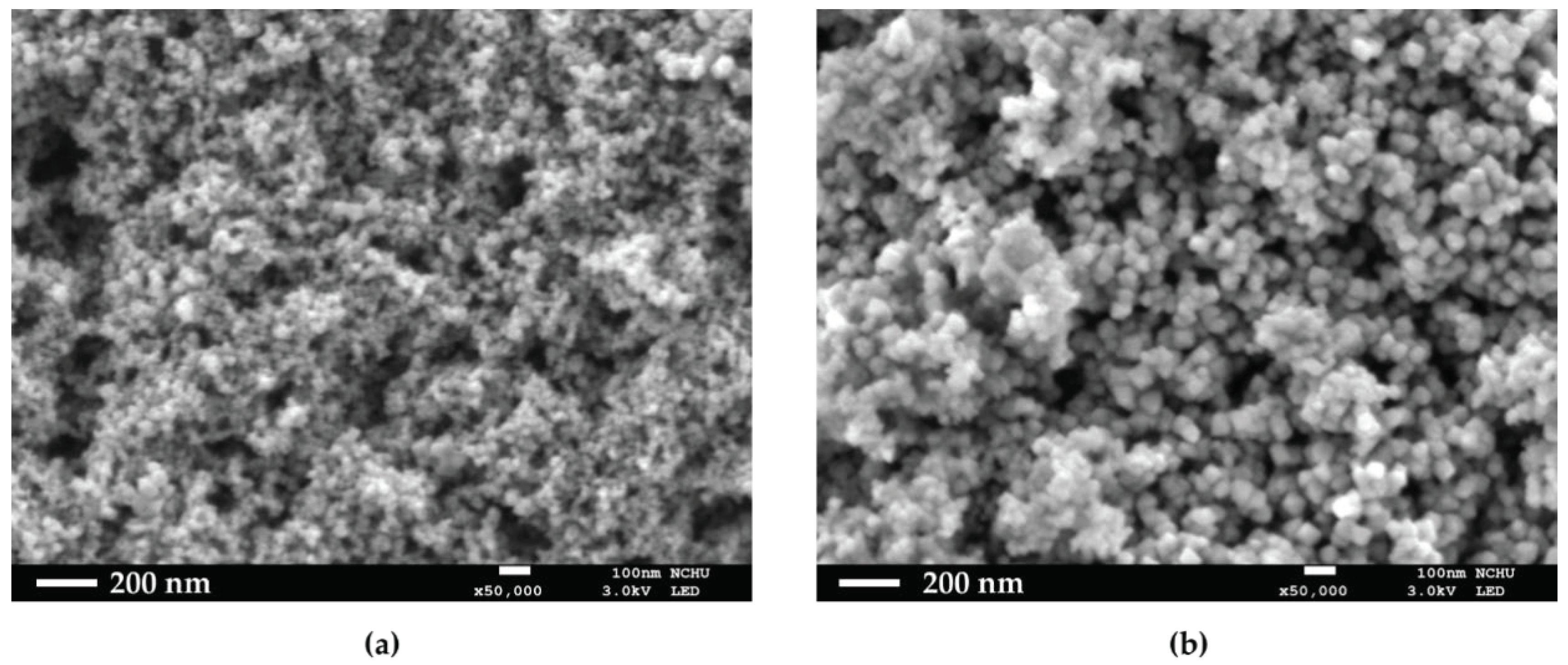
In this series of experiments, numerous methods and analysis means were used to identify whether monomers or RAFT agents were linked on particles. In the first section, we used co-precipitation to form Fe3O4-cored nanoparticles, and the product was well suspended in water after a sequence of modification. As for the two-step sol-gel method to synthesize NH2-SiO2@Fe3O4, 4-nitrobenzaldehyde was used to react with NH2-SiO2@Fe3O4, and then, the moiety was cleaved off by ethanolamine. After the reaction, the supernatant became light yellow and was subsequently examined by UV-Vis absorption to estimate the number of amine groups on each unit of particles. Besides, SEM was used to see the actual structures of HO@Fe3O4 and NH2-SiO2@Fe3O4.
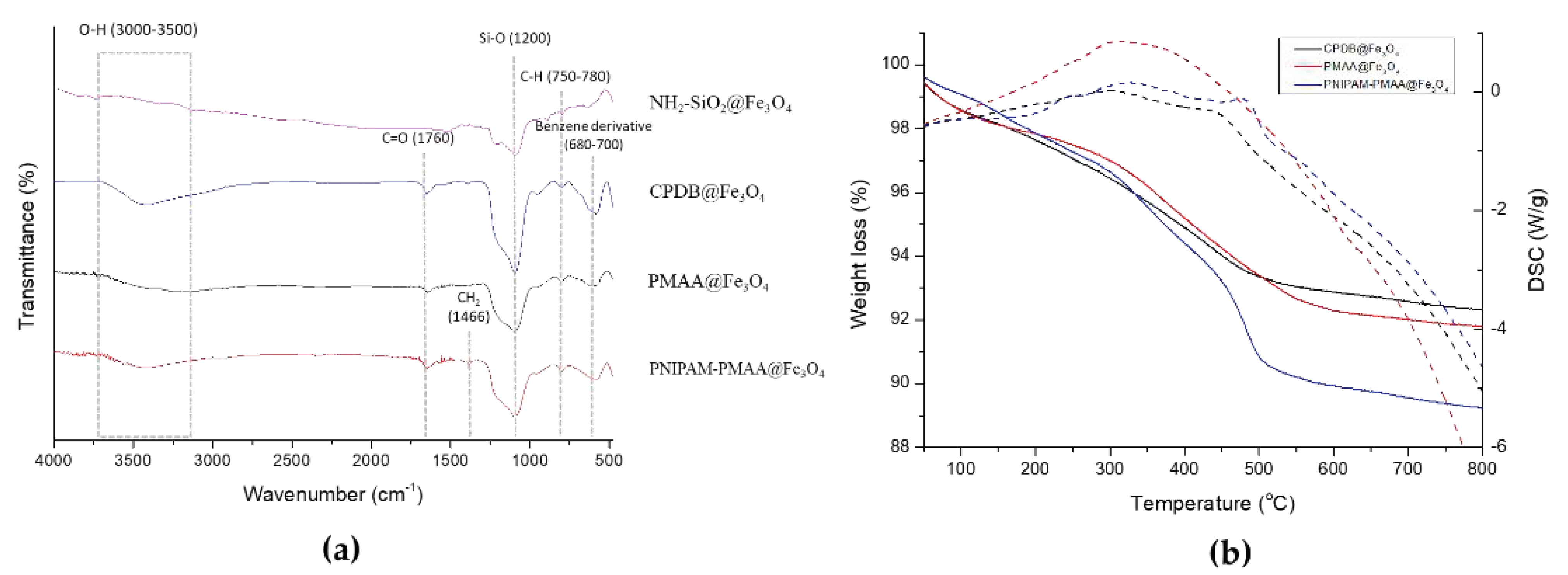
CPDB@Fe3O4, PMAA@Fe3O4, and PNIPAM-PMAA@Fe3O4 were rather hard to analyze since the particles are made up of iron core, a trait that poses a limitation to many analytical instruments. However, we were still able to identify the differences among these particles through some observed phenomena. Through DLS data, we found that particle hydrodynamic size increased as the monomers were polymerized on the particles. As for zeta-potential analysis, it was clear that after modification of the RAFT agent, the potential of the surface switched from a positive to a negative charge (+16.6 mV for NH2-SiO2@Fe3O4 and −20.95 mV for CPDB@Fe3O4). Furthermore. after polymerization of MAA and NIPAM, the charge differed subtly, as mentioned in the result section. FTIR was also used to observe the monomer distribution of the particles; however, no evident peaks were observed in the spectrum. This may be due to the fact that iron interrupted the transmittance of other moieties and made it difficult for analysis. TGA was applied to determine the organic polymer mass of the PNIPAM-PMAA@Fe3O4. The pattern of PNIPAM-PMAA@Fe3O4 it differed from those of CPDB@Fe3O4 and PMAA@Fe3O4 with a significant weight loss when the temperature was heated up to 500 °C. Additionally, the total loss amounted to 89% compared to that of PMAA@Fe3O4. The total loss of PMAA@Fe3O4 came to 91%, which was 1% higher than CPDB@Fe3O4. Though we could not accurately estimate the degree of polymerization, the obtained data still verify the thermo-behavior of particles.
Since DLS, zeta-potential, and FTIR analyses may be time consuming for sample preparation, we introduced a rapid method to determine the characteristics and suspensions in different solution. This method is less persuasive because the results are determined through the naked eye; however, there is no doubt that it is an effective and quick method. In the last section, we loaded particles with the anti-cancer drug doxorubicin. Under basic conditions (pH = 8.5), the electrostatic attraction between carboxyl groups and DOX was enhanced, and the result could be estimated by drug loading content and drug encapsulation efficiency. Furthermore, DOX-PNIPAM-PMAA@Fe3O4 was subjected to fluorescence spectrometry analysis, in which, under 480-nm excitation, we could obviously observe two peaks at 560 and 590 nm. The results indicated that DOX was attached to particles.
Furthermore, the drug release efficacy result matched what we had estimated, because drug release is primarily induced by the acidic microenvironment. In the analysis of DOX release under different temperatures, we also found that higher temperatures could actually enhance drug release efficiency [
28]. Thus, we evaluated the release rate under different pH conditions, and as we expected, the maximum drug release rate of particles was observed in the most acidic treatment. Last, we evaluated cell viability and cellular uptake through MTT assay and microscopy observation. As mentioned in the results section, we observed toxicity in the particles without a drug attachment [
27], and it was not a positive outcome as it may confine medical applications. We found that after modification of two monomers, the particles could exhibit a cytotoxic property and caused the number of cancer cells to decrease; this effect was dose-dependent. After being loaded with the anti-cancer agent DOX, the anti-cancer effect could be obviously enhanced. Since the modified particles without DOX loading already showed cancer cell inhibition ability, the result might not be very acceptable. However, this could be improved by further modification of the particles surface, such as by polyethylene glycol addition [
29]; that is what we will work on in the future. As for the cellular uptake analysis, we could observe that particle scratches and fragments may remain and aggregate in the cells after washing with PBS several times. The results also indicated that with higher concentration, DOX-PNIPAM-PMAA@Fe
3O
4 may cause damage to cancer cells and, thus, attain a cancer therapy effect. In this research, we employed co-precipitation method to prepare iron-cored nanoparticles. This method allows users to quickly obtain product, and the yield was quite considerable as well. Furthermore, the method was easy and required no sophisticated instruments compared to other methods [
30]. The HO@Fe
3O
4 was then modified with an amine group and further polymerization of MAA and NIPAM through RAFT polymerization. The polymerization method is now recognized as a proper way to modify particles since it is more understandable and it could be applied to a wide range of monomers. MAA and NIPAM, the selected monomers, could make the particles dual-responsive. Besides, the iron-cored nanoparticle itself already exhibited magnetic properties. Therefore, the PNIPAM-PMAA@Fe
3O
4 could be more functional compared to previous research [
31]. This is more prospective and will be developed in the near future. There is more work to be done to improve the particle characterization, as we mentioned in the above contents.