Recycling of Chrome-Tanned Leather and Its Utilization as Polymeric Materials and in Polymer-Based Composites: A Review
Abstract
1. Aim and Structure of the Review
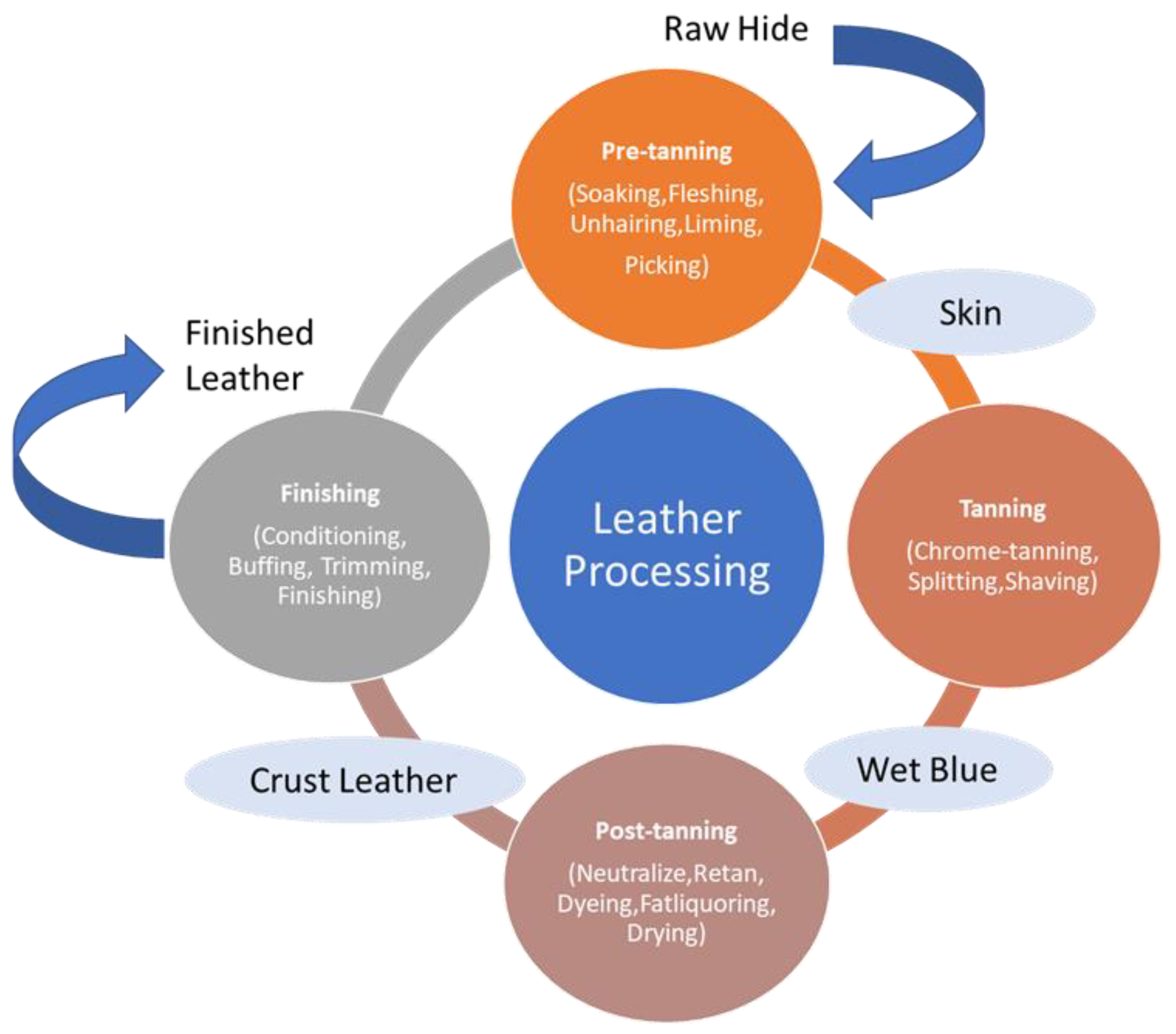
2. Raw Leather Materials and Solid Waste Disposal Issues
2.1. Raw Materials, Reagents, and Emission Factors
2.2. Solid Waste and Disposal Issues
- The combined effect of unsaturated fatty acids present in fat-liquoring agents and photo-ageing with UV light or thermal ageing (e.g., dry heating over 353 K) [29].
- The combined effect of relative humidity and temperature in the storage of fatty-liquored leather.
- The presence of an alkaline medium used, for example, in footwear production [29].
- 2Cr2O3 + 8OH− + 3O2
4CrO4− + 4H2O in alcaline medium (1)
- 2Cr2O3 + 2H2O + 3O2
2Cr2O7− + 4H+ in acid medium (2)
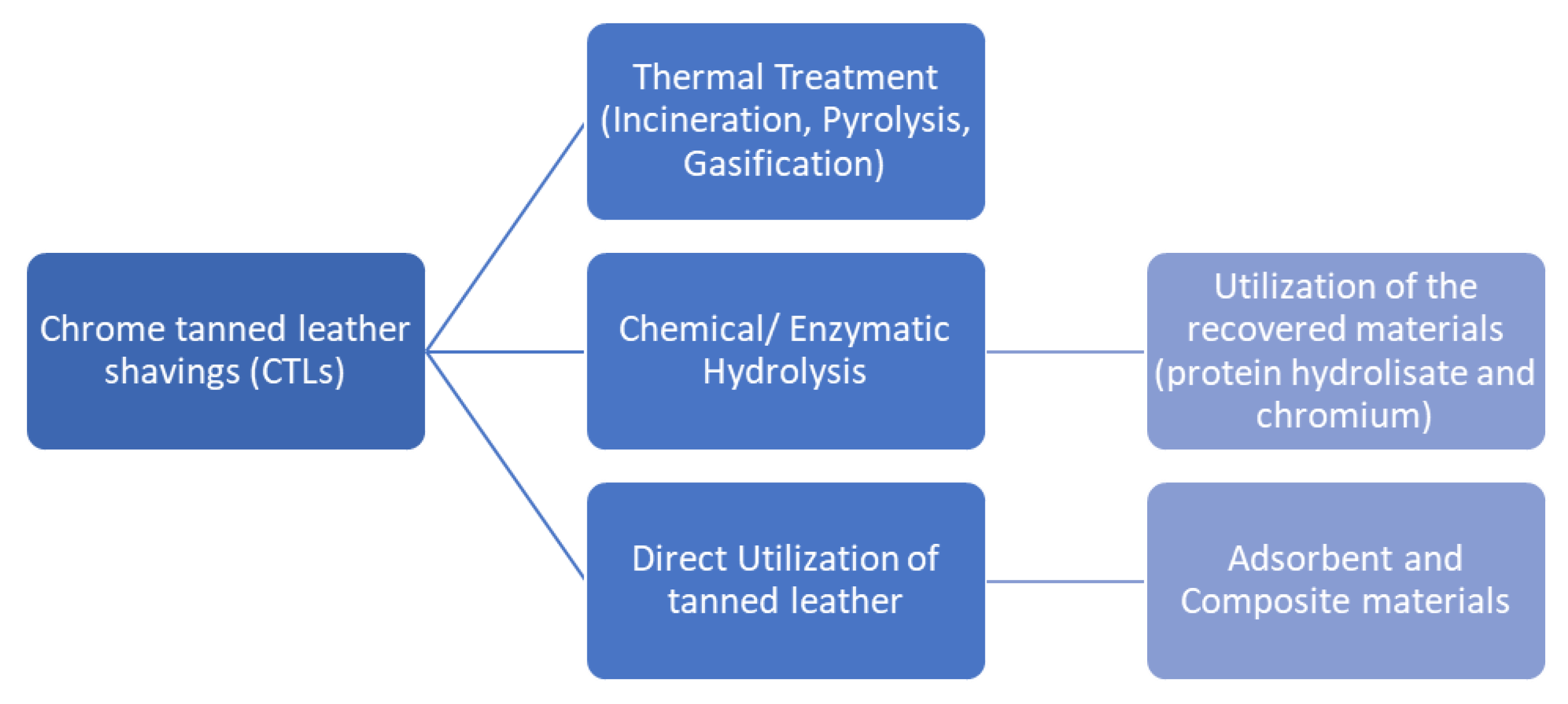
3. Waste Management Methodologies
- Direct use to make composites.
- Utilization of recovered collagen from hydrolysis.
- Thermal treatment process.
3.1. Thermal Treatment Process of Chromium-Containing Solid Wastes
3.2. Chemical-Enzymatic Treatments for Collagen Recovery and Chromium Removal
4. Recycling of Collagen Hydrolysate and Its Industrial Applications
5. Direct Utilization of Chrome-Tanned Leather in Polymer-Based Composites
5.1. Composite Materials with Chrome-Tanned Leather (Granules, Dusts and Fibers)
5.1.1. Thermoplastic Composite Materials
5.1.2. Thermosetting Composite Materials
5.1.3. Rubber-Leather Composites
6. Applications of Leather Wastes in Composite with Other Materials
7. Conclusions
- Thermal treatments and energy recovery.
- Hydrolysis of leather to obtain collagen and chromium-containing mixtures.
- Direct utilization of leather.
- Preparation of leather/polymer composites.
7.1. Thermal Treatments
7.2. Hydrolysis of Leather
7.3. Direct Use of Leather
7.4. Preparation of Leather/Polymer Composites
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doble, M.; Kruthiventi, A.K. Industrial Examples. In Green Chemistry and Engineering; Elsevier: Amsterdam, The Netherlands, 2007; pp. 245–296. [Google Scholar]
- Sciences, M.; Yamamoto, G.T.; Campus, K. Marketing Activities in the Leather Industry: Comparative Country Analysis. Int. J. Econ. Manag. Sci. 2011, 1, 37–48. [Google Scholar]
- Beghetto, V.; Zancanaro, A.; Scrivanti, A.; Matteoli, U.; Pozza, G. The Leather Industry: A Chemistry Insight Part I: An Overview of the Industrial Process. Sci. Ca’Foscari 2013, 1, 12–22. [Google Scholar] [CrossRef]
- Covington, A.D. The chemistry of tanning materials. In Conservation of Leather and Related Materials; Taylor and Francis: Abingdon, UK, 2006; pp. 22–35. ISBN 9781136415234. [Google Scholar]
- Dixit, S.; Yadav, A.; Dwivedi, P.D.; Das, M. Toxic Hazards of Leather Industry and Technologies to Combat Threat: A Review. J. Clean. Prod. 2015, 87, 39–49. [Google Scholar] [CrossRef]
- Germann, H.P. The Ecology of Leather Production–Present State and Development Trends. In Proceedings of the XXV IULTCS Congress, Chennai, India, 27–30 January 1999. [Google Scholar]
- Pati, A.; Chaudhary, R.; Subramani, S. A Review on Management of Chrome-Tanned Leather Shavings: A Holistic Paradigm to Combat the Environmental Issues. Environ. Sci. Pollut. Res. 2014, 21, 11266–11282. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.J.; Almeida, M.F. Recycling of Leather Waste Containing Chromium—A Review. Mater. Sci. Res. J. 2011, 5, 327. [Google Scholar]
- Roberts, M.T.; Etherington, D. Bookbinding and the Conservation of Books: A Dictionary of Descriptive Terminology; Library of Congress: Washington, DC, USA, 1982; ISBN 0-8444-0366-0.
- Cheema, U.; Ananta, M.; Mudera, V. Collagen: Applications of a Natural Polymer in Regenerative Medicine. Regener. Med. Tissue Eng. Cells Biomater. 2011, 287–300. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Veit, G.; Kobbe, B.; Keene, D.R.; Paulsson, M.; Koch, M.; Wagener, R. Collagen XXVIII, a Novel von Willebrand Factor A Domain-Containing Protein with Many Imperfections in the Collagenous Domain. J. Biol. Chem. 2006, 281, 3494–3504. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Suo, Z.; Avci, R.; Asara, J.M.; Allen, M.A.; Arce, F.T.; Horner, J.R. Analyses of Soft Tissue from Tyrannosaurus Rex Suggest the Presence of Protein. Science 2007, 316, 277–280. [Google Scholar] [CrossRef]
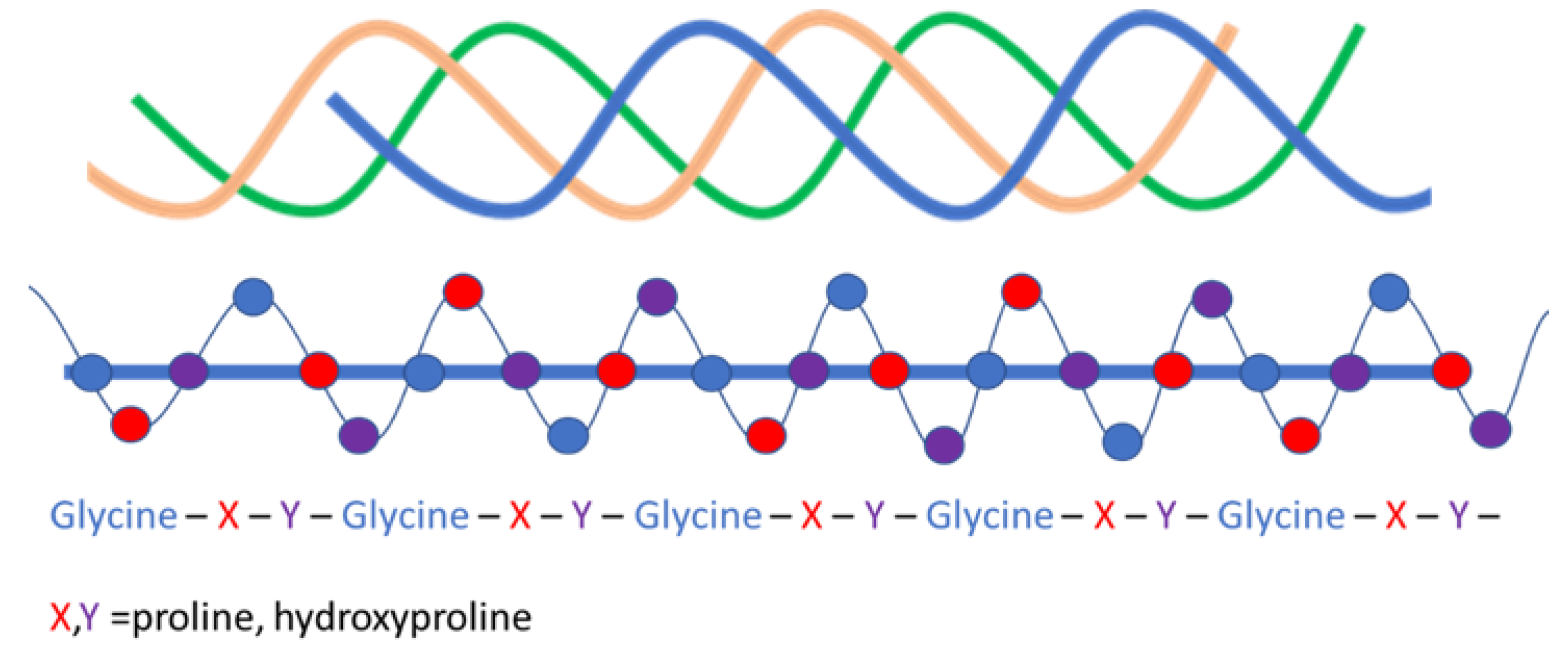
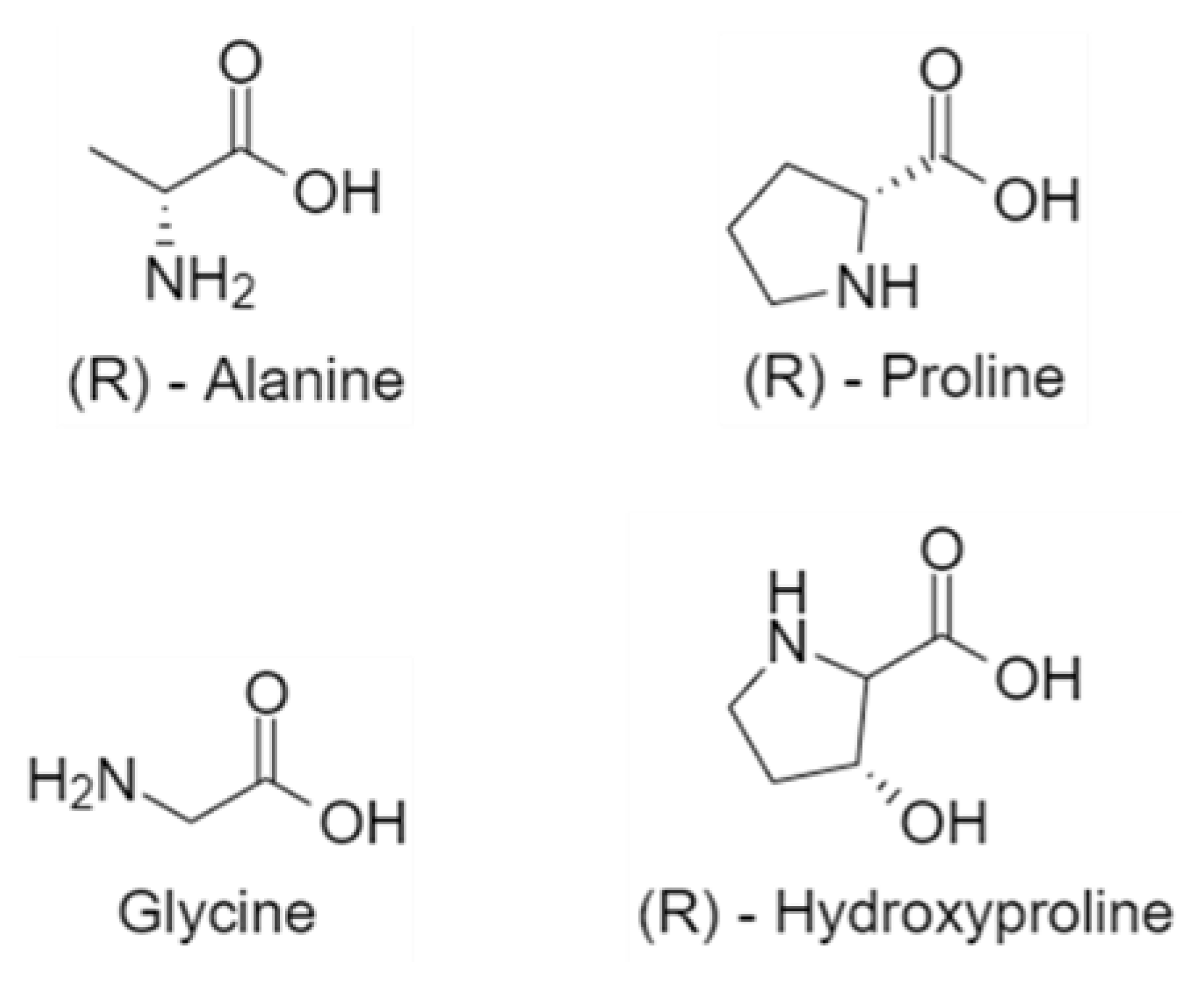
- Ramshaw, J.A.M.; Shah, N.K.; Brodsky, B. Gly-XY Tripeptide Frequencies in Collagen: A Context for Host–Guest Triple-Helical Peptides. J. Struct. Biol. 1998, 122, 86–91. [Google Scholar] [CrossRef]
- Toshiharu, M. Relationship between Amino-Acid Composition and Differentiation of Collagen. Int. J. Biochem. 1972, 3, 265–274. [Google Scholar] [CrossRef]
- Reich, G. From Collagen to Leather—The Theoretical Background; BASF: Ludwigshafen, Germany, 2007. [Google Scholar]
- Bella, J.; Eaton, M.; Brodsky, B.; Berman, H.M. Crystal and Molecular Structure, of a Collagen-like Peptide at 1.9 Å Resolution. Science 1994, 266, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Komanowsky, M. Thermal Stability of Hide and Leather at Different Moisture Contents. J. Am. Leather Chem. Assoc. 1993, 86, 269–280. [Google Scholar]
- Santos, R.J.; Agostini, D.L.S.; Cabrera, F.C.; Budemberg, E.R.; Job, A.E. Recycling Leather Waste: Preparing and Studying on the Microstructure, Mechanical, and Rheological Properties of Leather Waste/Rubber Composite. Polym. Compos. 2015, 36, 2275–2281. [Google Scholar] [CrossRef]
- Gustavson, K.H. Evidence for the Rupture of Intermolecularly Coordinated Peptide Bonds in the Heat Denaturation (Shrinkage) of Collagen. J. Am. Leather Chem. Assoc. 1946, 41, 47–58. [Google Scholar]
- Kanagaraj, J.; Velappan, K.C.; Chandra Babu, N.K.; Sadulla, S. Solid Wastes Generation in the Leather Industry and Its Utilization for Cleaner Environment—A Review. J. Sci. Ind. Res. 2006, 65, 541–548. [Google Scholar] [CrossRef]
- Ramasami, T. Beam House and Tanning Operations: Process Chemistry Revisited. J. Soc. Leather Technol. Chem. 1999, 83, 39–45. [Google Scholar]
- Jiang, H.; Liu, J.; Han, W. The Status and Developments of Leather Solid Waste Treatment: A Mini-Review. Waste Manag. Res. 2016, 34, 399–408. [Google Scholar] [CrossRef]
- Sundar, V.J.; Gnanamani, A.; Muralidharan, C.; Chandrababu, N.K.; Mandal, A.B. Recovery and Utilization of Proteinous Wastes of Leather Making: A Review. Rev. Environ. Sci. Biotechnol. 2011, 10, 151–163. [Google Scholar] [CrossRef]
- Kirk, D.W.; Chan, C.C.Y.; Marsh, H. Chromium Behavior during Thermal Treatment of MSW Fly Ash. J. Hazard. Mater. 2002, 90, 39–49. [Google Scholar] [CrossRef]
- Saravanabhavan, S.; Sreeram, K.J.; Raghava Rao, J.; Unni Nair, B. The Three Pot Solution for Chromium, Tannins and Solid Wastes: Recovery and Reuse Technique for Spent Semi-Chrome Liquor and Chrome Shavings. J. Soc. Leather Technol. Chem. 2004, 88, 202–207. [Google Scholar]
- Pati, A.; Chaudhary, R.; Subramani, S. Biochemical Method for Extraction and Reuse of Protein and Chromium from Chrome Leather Shavings: A Waste to Wealth Approach. J. Am. Leather Chem. Assoc. 2013, 108, 365–372. [Google Scholar]
- Costa, M.; Klein, C.B. Toxicity and Carcinogenicity of Chromium Compounds in Humans. Crit. Rev. Toxicol. 2006, 36, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Kolomaznik, K.; Adamek, M.; Andel, I.; Uhlirova, M. Leather Waste—Potential Threat to Human Health, and a New Technology of Its Treatment. J. Hazard. Mater. 2008, 160, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Christian, S.; Elpida Constantinou, E. Chemical Kinetic Mechanism for Atmospheric Chromium. Environ. Sci. Technol. 1995, 29, 222–231. [Google Scholar]
- Houghton, J.T. Climate Change 2001: The Scientific Basis; The Press Syndacate of the University of Cambridge: Cambridge, UK, 2001. [Google Scholar]
- Yılmaz, O.; Kantarli, I.C.; Yuksel, M.; Saglam, M.; Yanik, J. Conversion of Leather Wastes to Useful Products. Resour. Conserv. Recycl. 2007, 49, 436–448. [Google Scholar] [CrossRef]
- Lollar, R.M. Potential Uses of Tanned Collagen. J. Am. Leather Chem. Assoc. 1981, 76, 192–193. [Google Scholar]
- Langmaier, F.; Kolomaznik, K.; Sukop, S.; Mladek, M. Products of Enzymic Decomposition of Chrome-Tanned Leather Waste. J. Soc. Leather Technol. Chem. 1999, 83, 187–195. [Google Scholar]
- Manzo, G.; Fedele, G.; Colurcio, A. Utilizzazione Della Rasatura Nella Concia Al Cromo. Cuoio Pelli Mat. Conci. 1993, 69, 63–69. [Google Scholar]
- Wojciech, L.; Miesczyslzw, G.; Ursula, M. Leather Treatment to Remove Chromium. WO 1998003685A1, 29 January 1998. [Google Scholar]
- Ferreira, M.J.; Almeida, M.F.; Pinho, S.C.; Santos, I.C. Finished Leather Waste Chromium Acid Extraction and Anaerobic Biodegradation of the Products. Waste Manag. 2010, 30, 1091–1100. [Google Scholar] [CrossRef]
- Kašpárková, V.; Kolomaznik, K.; Burketova, L.; Šašek, V.; Šimek, L. Characterization of Low-Molecular Weight Collagen Hydrolysates Prepared by Combination of Enzymatic and Acid Hydrolysis. J. Am. Leather Chem. Assoc. 2009, 104, 46–51. [Google Scholar]
- Bataille, P.; Gagnon, F.; Smith, W.E. Upgrading Leather and Felt Scrap into Proteins. J. Am. Leather Chem. Assoc. 1983, 78, 328–337. [Google Scholar]
- Ferreira, M.J.; Pinto, V.V.; Rodrigues, J.; Pinho, S.; Azevedo, M.; Almeida, M.F. Advanced and Clean Technologies for Chromium Tanned Leather Waste Recycling and Green Energy Production. In Proceedings of the 15th Romanian Textiles and Leather Conference, Poiana Brașov, Romania, 3–6 September 2014. [Google Scholar]
- Reis, M.A.; Beleza, V. Utilisation of Leather Waste—Animal Feedstuff from Chrome Shavings: Part 1 Pilot Plant Study. J. Soc. Leather Technol. Chem. 1991, 75, 45–47. [Google Scholar]
- Zhang, X.; Yang, D.; GaO, Q.; Liu, H. Process for Extracting Protein from Waste Leather. CN 1313352A, 19 September 2001. [Google Scholar]
- Simoncini, A.; Ummarino, G. Possibilita Reali Di Recupero Dei Sottoprodoti Dell’industria Conciaria. Cuoio Pelli Mat. Conci. 1988, 64, 528–539. [Google Scholar]
- Mu, C.; Lin, W.; Zhang, M.; Zhu, Q. Towards Zero Discharge of Chromium-Containing Leather Waste through Improved Alkali Hydrolysis. Waste Manag. 2003, 23, 835–843. [Google Scholar] [CrossRef]
- Malek, A.; Hachemi, M. Effect of the Detoxification on the Shrinkage Temperature and PH of Chromium Leather Waste, Another Promising Way for the Tannery Pollution. Am. J. Appl. Sci. 2008, 5, 1329–1335. [Google Scholar] [CrossRef][Green Version]
- Malek, A.; Hachemi, M.; Didier, V. New Approach of Depollution of Solid Chromium Leather Waste by the Use of Organic Chelates: Economical and Environmental Impacts. J. Hazard. Mater. 2009, 170, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Sarkar, S.K. Enzyme Hydrolysis of Solid Tannery Wastes: Solid State Enzyme Production. J. Soc. Leather Technol. Chem. 1998, 82, 56–58. [Google Scholar]
- Chakraborty, R. Optimization of Hydrolysis of Chrome Shavings by Enzyme from p. Lilacinus. J. Am. Leather Chem. Assoc. 2004, 99, 103–109. [Google Scholar]
- Taylor, M.M.; Diefendorf, E.J.; Na, G.C. Enzymic Treatment of Chrome Shavings. J. Am. Leather Chem. Assoc. 1991, 85, 264–275. [Google Scholar]
- Taylor, M.M.; Diefendorf, E.J.; Na, G.C.; Marmer, W.N. Enzymatic Processing of Materials Containing Chromium and Protein. US 5094946A, 10 March 1992. [Google Scholar]
- Taylor, M.M.; Diffendorf, E.J.; Marmer, W.N. Efficiency of Enzymic Solubilization of Chrome Shavings as Influenced by Choice of Alkalinity-Inducing Agents. J. Am. Leather Chem. Assoc. 1991, 86, 199–208. [Google Scholar]
- Taylor, M.M.; Diefendorf, E.J.; Marmer, W.N. Compendium of Advanced Topics on Leather Technology. In Proceedings of the XXI Congress of the International Union of Leather Technologists and Chemists Societies, Barcelona, Spain, 23 September 1991; Volume 29, pp. 689–703. [Google Scholar]
- Taylor, M.M.; Diefendorf, E.J.; Brown, E.M.; Marmer, W.N. Characterization of Products Isolated by Enzyme Treatment of Chromium-Containing Leather Waste. J. Am. Leather Chem. Assoc. 1994, 87, 380–389. [Google Scholar]
- Cabeza, L.F.; Taylor, M.M.; Dimaio, G.L.; Brown, E.M.; Marmer, W.N.; Carrio, R.; Celma, P.J.; Cot, J. Original Contributions—Processing of Leather Waste: Pilot Scale Studies on Chrome Shavings. Isolation of Potentially Valuable Protein Products and Chromium. Waste Manag. 1998, 18, 211. [Google Scholar] [CrossRef]
- Cabeza, L.F.; McAloon, A.J.; Yee, W.C.; Taylor, M.M.; Brown, E.M.; Marmer, W.N. Process Simulation and Cost Estimation of Treatment of Chromium-Containing Leather Waste. J. Am. Leather Chem. Assoc. 1998, 10, 299–315. [Google Scholar]
- Taylor, M.M.; Diefendorf, E.J.; Thompson, C.J.; Brown, E.M.; Marmer, W.N. Extraction and Characterization of “Chrome-Free” Protein from Chromium-Containing Collagenous Waste Generated in the Leather Industry; ACS Publications: Washington, DC, USA, 1994; ISBN 1947-5918. [Google Scholar]
- Cabeza, L.F.; Taylor, M.M.; DiMaio, G.L.; Brown, E.L.; Marmer, W.N.; Carrio, R.; Celma, P.J.; Cot, J. Cost Effective Processing of Leather Waste: Pilot Scale Studies on Chrome Shavings from Cattle Hides and Sheep Skins Isolation of Potentially Valuable Protein Products and Utilization of Recovered Chromium. In Proceedings of the XXV IULTCS Congress India (Chennai) Waste Manage Technol, Chennai, India, 27–30 January 1999; pp. 465–472. [Google Scholar]
- Taylor, M.M.; Diefendorf, E.J.; Marmer, W.N.; Brown, E.M. Enzymatic Processing of Materials Containing Chromium and Protein. U.S. Patent 5271912A, 21 December 1993. [Google Scholar]
- Cioca, G.; Fertell, P.A. Manufactured Article Based on Leather and a Polyisocyanate. FR 2495054A1, 4 June 1982. [Google Scholar]
- Munoz, J.; Maldonado, M.; Rangel, A. Development of a Tanning Process Based on Using Hydrolyzated Material Collected from Leather Scrap. J. Am. Leather Chem. Assoc. 2002, 97, 83–88. [Google Scholar]
- Langmaier, F.; Mokrejs, P.; Kolomaznik, K.; Mladek, M. Plasticizing Collagen Hydrolysate with Glycerol and Low-Molecular Weight Poly (Ethylene Glycols). Thermochim. Acta 2008, 469, 52–58. [Google Scholar] [CrossRef]
- Langmaier, F.; Kolomazník, K.; Mládek, M. Modifyining Products of Enzymatic Breakdown of Chrome-Tanned Leather Wastes with Glutaraldehyde. J. Soc. Leather Technol. Chem. 2003, 87, 55–61. [Google Scholar]
- Taylor, M.M.; Cabeza, L.F.; Marmer, W.N.; Brown, E.M. Computer-Assisted Method to Measure the Adhesive Properties of Hydrolysis Products from Collagen. J. Am. Leather Chem. Assoc. 1998, 92, 28–37. [Google Scholar]
- Langmaier, F.; Kolomazník, K.; Mládek, M.; Šivarová, J. Curing Urea–Formaldehyde Adhesives with Hydrolysates of Chrome-Tanned Leather Waste from Leather Production. Int. J. Adhes. Adhes. 2005, 25, 101–108. [Google Scholar] [CrossRef]
- Taylor, M.M.; Cabeza, L.F.; Brown, E.M.; Marmer, W.N. Chemical modification of protein products isolated from chromium-containing solid tannery waste and resultant influence on physical and functional pro perties. J. Am. Leather Chem. Assoc. 1999, 94, 171–181. [Google Scholar]
- Taylor, M.M.; Diefendorf, E.J.; Thompson, C.J.; Brown, E.M.; Marner, W.N. Extraction and Characterization of “Chrome-Free” Protein from Chromium-Containing Collagenous Waste Generated in the Leather Industry. Polymer from Agricultural Coproducts; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1994; Volume 575, Chapter 12; pp. 171–187. [Google Scholar]
- Langmaier, F.; Mládek, M.; Kolomazník, K.; Malý, A. Hydrolysates of Chromed Waste as Raw Materials for the Production of Surfactants. Tenside Surfactants Deterg. 2002, 39, 47–51. [Google Scholar]
- Langmaier, F.; Mládek, M.; Kolomazník, K.; Malý, A. Degradation of Chromed Leather Waste Hydrolysates for the Production of Surfactants. Tenside Surfactants Deterg. 2002, 39, 31–34. [Google Scholar]
- Brasack, I.; Böttcher, H.; Hempel, U. Biocompatibility of Modified Silica-Protein Composite Layers. J. Sol Gel Sci. Technol. 2000, 19, 479–482. [Google Scholar] [CrossRef]
- Qiang, X.; Sheng, Z.; Zhang, H. Study on Scale Inhibition Performances and Interaction Mechanism of Modified Collagen. Desalination 2013, 309, 237–242. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Thanikaivelan, P.; Murali, R.; Chandrasekaran, B. Preparation and Characterization of Composite Sheets from Collagenous and Chromium–Collagen Complex Wastes Using Polyvinylpyrrolidone: Two Problems, One Solution. Waste Biom. Valorization 2010, 1, 347–355. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Taylor, M.M.; Brown, E.M.; Marmer, W.N. Potential Applications for Gelatin Isolated from Chromium-Containing Solid Tannery Waste: Microencapsulation. J. Am. Leather Chem. Assoc. 1999, 94, 182–189. [Google Scholar]
- Ocak, B.; Aslan, A.; Gulumser, G. Utilization of Chromium-Tanned Leather Solid Wastes in Microencapsulation. J. Am. Leather Chem. Assoc. 2011, 106, 232–238. [Google Scholar]
- Langmaier, F.; Mokrejs, P.; Kolomaznik, K.; Mladek, M.; Karnas, R. Cross-Linking Epoxide Resins with Hydrolysates of Chrome-Tanned Leather Waste. J. Therm. Anal. Calorim. 2007, 88, 857–862. [Google Scholar] [CrossRef]
- Taylor, M.M.; Liu, C.K.; Latona, N.; Marmer, W.N.; Brown, E.M. Enzymatic Modification of Hydrolysis Products from Collagen Using a Microbial Transglutaminase. II. Preparation of Films. J. Am. Leather Chem. Assoc. 2002, 97, 225–234. [Google Scholar]
- Taylor, M.M.; Liu, C.K.; Marmer, W.N.; Brown, E.M. Enzymatic Modification of Hydrolysis Products from Collagen Using a Microbial Transglutaminase. III. Preparation of Films with Improved Mechanical Properties. J. Am. Leather Chem. Assoc. 2003, 98, 435–444. [Google Scholar]
- Sakamoto, H.; Kumazawa, Y.; Motoki, M. Strength of Protein Gels Prepared with Microbial Transglutaminase as Related to Reaction Conditions. J. Food Sci. 1994, 59, 866–871. [Google Scholar] [CrossRef]
- Chiellini, E.; Cinelli, P.; Fernandes, E.G.; Kenawy, E.R.S.; Lazzeri, A. Gelatin-Based Blends and Composites. Morphological and Thermal Mechanical Characterization. Biomacromolecules 2001, 2, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Křesálková, M.; Hnaníčková, L.; Kupec, J.; Kolomazník, K.; Alexy, P. Application of Protein Hydrolysate from Chrome Shavings for Polyvinyl Alcohol-Based Biodegradable Material. J. Am. Leather Chem. Assoc. 2002, 97, 143–149. [Google Scholar]
- Mokrejs, P.; Janacova, D.; Langmaier, F.; Mladek, M.; Kolomaznik, K.; Vasek, V. Thermal Properties of Hydrolysates of Chrome-Tanned Leather Scraps. Indian Chem. Eng. 2007, 49, 108. [Google Scholar]
- Langmaier, F.; Stibora, M.; Mládek, M.; Kolomazník, K. Gel-Sol Transitions of Chrome Tanned Leather Waste Hydrolysate. J. Soc. Leather Technol. Chem. 2001, 85, 100–105. [Google Scholar]
- Castiello, D.; Chiellini, E.; Cinelli, P.; Corti, A.; D’Antone, S.; Puccini, M.; Salvadori, M.; Vitolo, S. Polyethylene-Collagen Hydrolizate Thermoplastic Blends: A New Reutilization Route to Transform a Waste of the Leather Industry into Environmentally Degradable Plastics. In Proceedings of the XXIX Congress of the International Union of Leather Technologists and Chemists Societies, Washington, DC, USA, 20–24 June 2007; pp. 24–25. [Google Scholar]
- Langmaier, F.; Mokrejs, P.; Karnas, R.; Mládek, M.; Kolomazník, K. Modification of Chrome Tanned Leather Waste Hydrolysate with Epichlorhydrin. J. Soc. Leather Technol. Chem. 2006, 90, 29. [Google Scholar]
- Chakarska, L.; Goshev, I.; Idakieva, K.; Todinova, S.; Apostolov, G. Cross-Linking Phosphoric Acid Hydrolysates of Collagen with Cyanuric Chloride. J. Soc. Leather Technol. Chem. 2008, 92, 81–84. [Google Scholar]
- Murali, R.; Anumary, A.; Ashokkumar, M.; Thanikaivelan, P.; Chandrasekaran, B. Hybrid Biodegradable Films from Collagenous Wastes and Natural Polymers for Biomedical Applications. Waste Biom. Valorization 2011, 2, 323–335. [Google Scholar] [CrossRef]
- Shin, E.C.; Lee, S.C.; Kim, W.J. Development of Regenerated Protein Fibers from a Collagen-Polyvinyl Alcohol Complex. J. Am. Leather Chem. Assoc. 2007, 102, 315–321. [Google Scholar]
- Wang, Y.; Ke, Y.; Ren, L.; Wu, G.; Chen, X.; Zhao, Q. Surface Engineering of PHBV by Covalent Collagen Immobilization to Improve Cell Compatibility. J. Biomed. Mater. Res. Part A 2009, 88, 616–627. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Vatankhah, E.; Ramakrishna, S. Electrospun Aligned PHBV/Collagen Nanofibers as Substrates for Nerve Tissue Engineering. Biotechnol. Bioeng. 2013, 110, 2775–2784. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Gao, C.; Gong, Y.; Shen, J. Cartilage Tissue Engineering PLLA Scaffold with Surface Immobilized Collagen and Basic Fibroblast Growth Factor. Biomaterials 2005, 26, 1253–1259. [Google Scholar] [CrossRef]
- MacDonald, R.A.; Laurenzi, B.F.; Viswanathan, G.; Ajayan, P.M.; Stegemann, J.P. Collagen-Carbon Nanotube Composite Materials as Scaffolds in Tissue Engineering. J. Biomed. Mater. Res. Part A 2005, 74, 489–496. [Google Scholar] [CrossRef]
- Pati, A.; Chaudhary, R. Studies on the Generation of Biogas from Collagen Hydrolysate Obtained from Chrome Shavings by Alkaline Hydrolysis: A Greener Disposal Method. Res. J. Recent Sci. 2013, 2, 234. [Google Scholar]
- Zuchowski, J. Utilisation of Hide Wastes for the Production of High-Protein Feed Meals on the Basis of a ’collagen Amino-Acid Index’. J. Soc. Leather Technol. Chem. 1987, 71, 15–16. [Google Scholar]
- Kolomaznik, K.; Mladek, M.; Langmaier, F.; Janacova, D.; Taylor, M.M. Experience in Industrial Practice of Enzymatic Dechromation of Chrome Shavings. J. Am. Leather Chem. Assoc. 2000, 95, 55–63. [Google Scholar]
- Montoneri, E.; Rizzi, G.; Rizzi, A.; Mordenti, A.; Bauli, A.; Riolfatti, M.; Pellegrini, L. Hydrolysis of Tannery Wastes to Protein Meal for Animal Feedstuffs: A Process and Product Evaluation. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 1994, 59, 91–99. [Google Scholar] [CrossRef]
- Kolomaznik, K.; Vasek, V.; Zelinka, I.; Mladek, M.; Langmaier, F. Automatic Control of Recycling Technology for Chromium from Liquid and Solid Tannery Waste. J. Am. Leather Chem. Assoc. 2005, 100, 119–123. [Google Scholar]
- Cabeza, L.F.; Taylor, M.M.; DiMaio, G.L.; Brown, E.M.; Marmer, W.N.; Carrió, R.; Celma, P.J.; Cot, J. Processing of Leather Waste: Pilot Scale Studies on Chrome Shavings. Part II. Purification of Chrome Cake and Tanning Trials. J. Am. Leather Chem. Assoc. 1998, 93, 83–98. [Google Scholar]
- Rao, J.R.; Thanikaivelan, P.; Sreeram, K.J.; Nair, B.U. Green Route for the Utilization of Chrome Shavings (Chromium-Containing Solid Waste) in Tanning Industry. Environ. Sci. Technol. 2002, 36, 1372–1376. [Google Scholar] [CrossRef]
- Berry, F.J.; Costantini, N.; Smart, L.E. Recovery and Reuse of Chromium from Leather Shavings: Formation of Chromium-Containing Ceramic Pigments. J. Am. Leather Chem. Assoc. 2001, 96, 46–53. [Google Scholar]
- Azzi, M.; Tahiri, S.; Albizane, A.; Messaoudi, A.; Bouhria, M.; Sibari, A. Processing of Chrome-Tanned Solid Waste Generated in the Leather Industry: Recovery of Proteins and Synthesis of a Pigment for Paint. J. Am. Leather Chem. Assoc. 2001, 99, 1–8. [Google Scholar]
- Cheng, F.; Zhang, H.; Zhang, Y.; Li, W.; Ji, X. Preparation of Chrome Liquor Using Chrome Shavings as Reducing Agent. J. Am. Leather Chem. Assoc. 2005, 100, 217–224. [Google Scholar]
- Rao, J.R.; Thanikaivelan, P.; Sreeram, K.J.; Nair, B.U. Tanning Studies with Basic Chromium Sulfate Prepared Using Chrome Shavings as a Reductant: A Call for’wealth from Waste’approach to the Tanning Industry. J. Am. Leather Chem. Assoc. 2004, 77, 170–177. [Google Scholar]
- Przepiorkowska, A.; Janowska, G.; Slusarski, L.; Wolniak, S. Leather Powder as an Agent for Removing Environmentally Hazardous Organic Compounds. J. Soc. Leather Technol. Chem. 2003, 87, 223–226. [Google Scholar]
- Oliveira, D.Q.L.; Gonçalves, M.; Oliveira, L.C.A.; Guilherme, L.R.G. Removal of As (V) and Cr (VI) from Aqueous Solutions Using Solid Waste from Leather Industry. J. Hazard. Mater. 2008, 151, 280–284. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Aguilar-Vega, M.J.; Márquez-Lucero, A.; Vázquez-Moreno, F. Production of Leather-like Composites Using Chemically Modified Short Leather Fibers. I: Chemical Modification by Emulsion Polymerization. Polym. Compos. 2002, 23, 49–60. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Li, L. Reuse of Leather Shavings as a Reinforcing Filler for Poly (Vinyl Alcohol). J. Thermoplast. Compos. Mater. 2016, 29, 327–343. [Google Scholar] [CrossRef]
- Reis, K.C.; Pereira, L.; Melo, I.C.N.A.; Marconcini, J.M.; Trugilho, P.F.; Tonoli, G.H.D. Particles of Coffee Wastes as Reinforcement in Polyhydroxybutyrate (PHB) Based Composites. Mater. Res. 2015, 18, 546–552. [Google Scholar] [CrossRef]
- Nanni, A.; Ricci, A.; Versari, A.; Messori, M. Wine Derived Additives as Poly (Butylene Succinate)(PBS) Natural Stabilizers for Different Degradative Environments. Polym. Degrad. Stab. 2020, 182, 109381. [Google Scholar] [CrossRef]
- Nanni, A.; Messori, M. Effect of the Wine Wastes on the Thermal Stability, Mechanical Properties, and Biodegradation’s Rate of Poly (3-hydroxybutyrate). J. Appl. Polym. Sci. 2020, 138, 49713. [Google Scholar] [CrossRef]
- Arjmandi, R.; Hassan, A.; Majeed, K.; Zakaria, Z. Rice Husk Filled Polymer Composites. Int. J. Polym. Sci. 2015, 2015, 501471. [Google Scholar] [CrossRef]
- Ambone, T.; Joseph, S.; Deenadayalan, E.; Mishra, S.; Jaisankar, S.; Saravanan, P. Polylactic Acid (PLA) Biocomposites Filled with Waste Leather Buff (WLB). J. Polym. Environ. 2017, 25, 1099–1109. [Google Scholar] [CrossRef]
- Duan, J.K.; Fan, C.X.; Li, Y.; Jiang, L.; Shao, S.X. Preparation and Characterization of the Covalent-Integrated Poly(Lactic Acid) and Scrap Leather Fiber Composites. J. Shanghai Jiaotong Univ. 2012, 17, 586–592. [Google Scholar] [CrossRef]
- Donati, F.; Puccini, V.; Vitolo, S. Procedimento Di Produzione Di Materiale Similpelle e Materiale Così Ottenuto. EP 3536830A1, 25 March 2019. [Google Scholar]
- Moses, A.J.; Babu, A.S.; Kumar, S.A. Fabrication and Characterization of Leather Waste and LDPE/LLDPE Blend Composites Fabrication and Characterization of Leather Waste and LDPE/LLDPE Blend Composites. In Proceedings of the International Conference on Advances in Production and Industrial Engineering, Tiruchirappalli, India, 20–21 February 2015; pp. 342–346. [Google Scholar]
- Adhikary, K.B.; Pang, S.; Staiger, M.P. Dimensional Stability and Mechanical Behaviour of Wood-Plastic Composites Based on Recycled and Virgin High-Density Polyethylene (HDPE). Compos. Part B Eng. 2008, 39, 807–815. [Google Scholar] [CrossRef]
- Babanas, K.; Tarantili, P.A.; Andreopoulos, A.G. Plasticized Poly(Vinyl Chloride) Filled with Waste Leather Particles. J. Elastomers Plast. 2001, 33, 72–85. [Google Scholar] [CrossRef]
- Nahar, S.; Khan, M.A.; Khan, R.A.; Abdullah, E.C.B.; Khan, M.J.H.; Islam, R.; Karim, F.; Rahman, M.; Rahman, A.; Mahmood, A.A.; et al. An Approach to Utilize Crust Leather Scrapes, Dumped into the Land, for the Production of Environmental Friendly Leather Composite. Eng. J. 2013, 17, 17–24. [Google Scholar] [CrossRef][Green Version]
- Senthil, R.; Vedakumari, S.W.; Hemalatha, T.; Das, B.N.; Sastry, T.P. Leather Fibres as Reinforcement for Epoxy Composites: A Novel Perspective. Fibers Polym. 2015, 16, 181–187. [Google Scholar] [CrossRef]
- Senthil, R.; Sastry, T.P.; Saraswathy, G.; Das, B.N.; Gobi, N. Leather Insole with Acupressure Effect: New Perspectives. J. Polym. Environ. 2018, 26, 175–182. [Google Scholar] [CrossRef]
- Natchimuthu, N.; Ravichandran, K. Natural Rubber-Leather Composites. Polím. Ciênc. Tecnol. 2013, 15, 102–108. [Google Scholar]
- Sumathi, V.; Senthil, R. Physico-Chemical Properties of Reconstituted Fibers Composite Prepared from Leather Waste. Int. J. Pharma Bio Sci. 2016, 7, P105–P110. [Google Scholar] [CrossRef]
- El-Sabbagh, S.H.; Mohamed, O.A. Recycling of Chrome-tanned Leather Waste in Acrylonitrile Butadiene Rubber. J. Appl. Polym. Sci. 2011, 121, 979–988. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Freitas, F.; Almeida, M.F. The Effect of Leather Fibers on the Properties of Rubber-Leather Composites. J. Compos. Mater. 2010, 44, 2801–2817. [Google Scholar] [CrossRef]
- Meşe, P.; Karaağaç, B.; Uyanık, N. Investigating Effect of Chrome Tanned Leather Scraps in Ethylene Propylene Diene Monomer Rubber. Prog. Rubber Plast. Recycl. Technol. 2018, 34, 89–103. [Google Scholar]
- Nashy, E.-S.H.A.; Al-Ashkar, E.; Moez, A.A. Optical and Spectroscopic Studies on Tannery Wastes as a Possible Source of Organic Semiconductors. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 86, 33–38. [Google Scholar]
- Krummenauer, K.; de Oliveira Andrade, J.J. Incorporation of Chromium-Tanned Leather Residue to Asphalt Micro-Surface Layer. Constr. Build. Mater. 2009, 23, 574–581. [Google Scholar]
- Zăinescu, G.; Deselnicu, V.; Constantinescu, R. Composite Structures Containing Leather Fibers with Applications in Constructions Industry. In Proceedings of the ICAMS 2018–7th International Conference on Advanced Materials and Systemshttps, Bucharest, Romania, 18–20 October 2018; pp. 593–598. [Google Scholar] [CrossRef]




| Topic | Subtopic | Number of Papers |
|---|---|---|
| Leather market | 6 | |
| Leather chemical structure | 12 | |
| Emission factors in leather industry | 8 | |
| Formation of chromium (VI) | 2 | |
| Thermal treatment of leather | 4 | |
| Leather hydrolysis | 27 | |
| Acidic hydrolysis | 6 | |
| Alkaline hydrolysis | 9 | |
| Enzymatic hydrolysis | 12 | |
| Applications of recycled collagen | 39 | |
| Use of collagen in leather processes | 4 | |
| Preparation of hydrogels | 3 | |
| Preparation of adhesives | 3 | |
| Coagulant and surfactants | 3 | |
| Collagen biocomposites | 3 | |
| Encapsulating agents | 3 | |
| Collagen-based films | 12 | |
| Collagen-based fibres | 1 | |
| Biomedical applications | 4 | |
| Biomethanization | 1 | |
| Animal feed | 3 | |
| Chromium recovery | 5 | |
| Direct use in tanning process | 4 | |
| Direct use in composites | 24 | |
| Thermoplastic composites | 12 | |
| Thermosetting composites | 2 | |
| Rubber-leather composites | 7 | |
| Other applications of leather wastes | 3 |
| Solid Waste | Quantity (kg/ton Processed) |
|---|---|
| raw hide trimmings | 120–150 |
| fleshings | 70–230 |
| tanned splits | 115–140 |
| CTLSs and tanned leather trimmings | 100–120 |
| buffing dust | 2–5 |
| finished leather trimmings | 30–40 |
| Parameters | Values |
|---|---|
| Moisture content (wt%) | 120–150 |
| Cr2O3 (wt%) | 70–230 |
| Inorganic ash content (wt%) | 115–140 |
| Nitrogen content trimmings (wt%) | 100–120 |
| Oils and fat content (wt%) | 2–5 |
| pH in 10% aqueous leather | 30–40 |
| Apparent density (g/mL) | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, M.; Nanni, A.; Colonna, M. Recycling of Chrome-Tanned Leather and Its Utilization as Polymeric Materials and in Polymer-Based Composites: A Review. Polymers 2021, 13, 429. https://doi.org/10.3390/polym13030429
Parisi M, Nanni A, Colonna M. Recycling of Chrome-Tanned Leather and Its Utilization as Polymeric Materials and in Polymer-Based Composites: A Review. Polymers. 2021; 13(3):429. https://doi.org/10.3390/polym13030429
Chicago/Turabian StyleParisi, Mariafederica, Alessandro Nanni, and Martino Colonna. 2021. "Recycling of Chrome-Tanned Leather and Its Utilization as Polymeric Materials and in Polymer-Based Composites: A Review" Polymers 13, no. 3: 429. https://doi.org/10.3390/polym13030429
APA StyleParisi, M., Nanni, A., & Colonna, M. (2021). Recycling of Chrome-Tanned Leather and Its Utilization as Polymeric Materials and in Polymer-Based Composites: A Review. Polymers, 13(3), 429. https://doi.org/10.3390/polym13030429









