Morphological, Structural, Thermal, Permeability, and Antimicrobial Activity of PBS and PBS/TPS Films Incorporated with Biomaster-Silver for Food Packaging Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of PBS and PBS/TPS Films
2.3. Characterization of PBS and PBS/TPS Films
2.3.1. Morphological Examination
2.3.2. Functional Chemistry Analysis
2.3.3. Thermal Analysis
2.3.4. BET Analysis
2.3.5. Permeability Analysis
2.4. Antimicrobial Activity Determination
2.5. Statistical Analysis
3. Results and Discussions
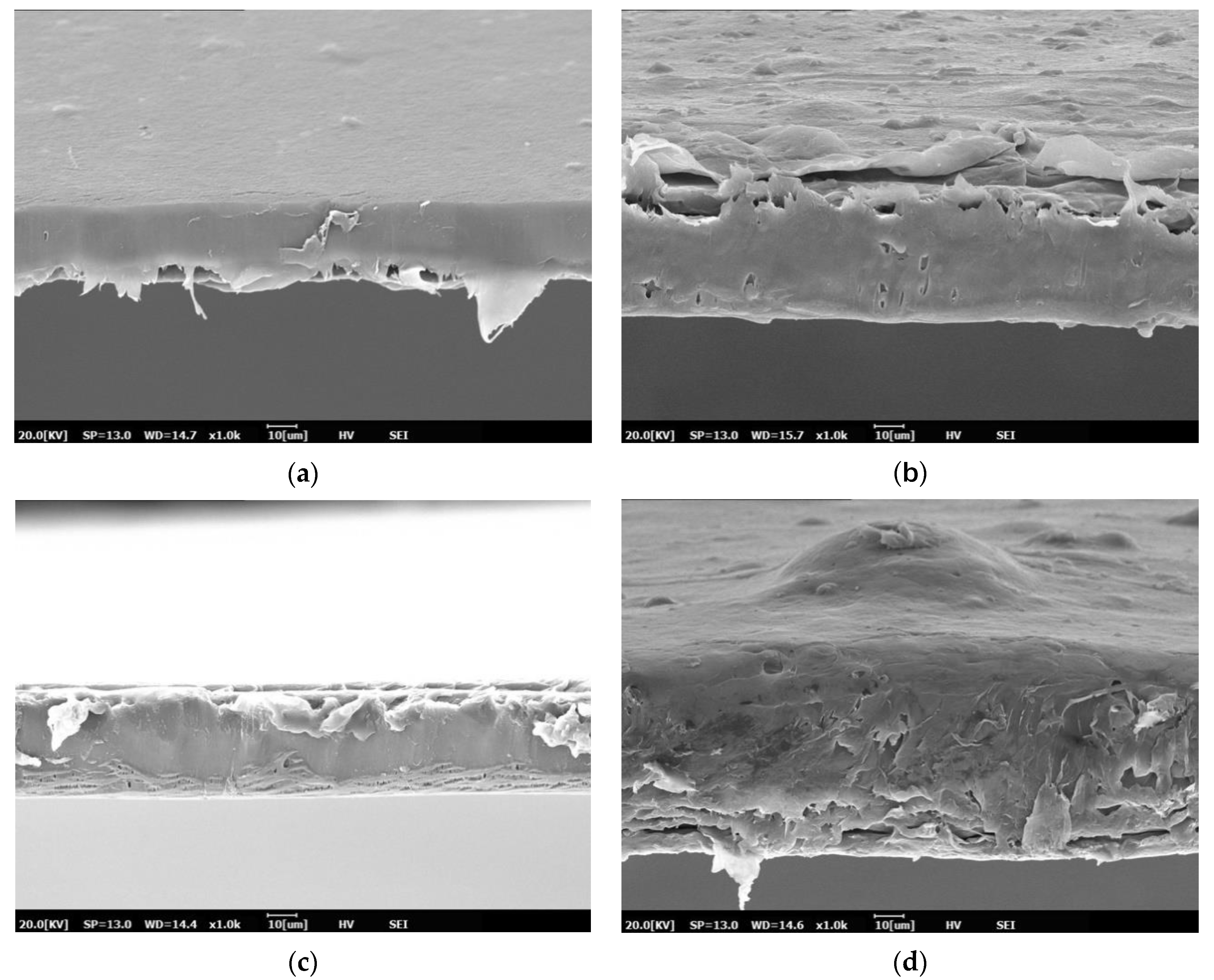
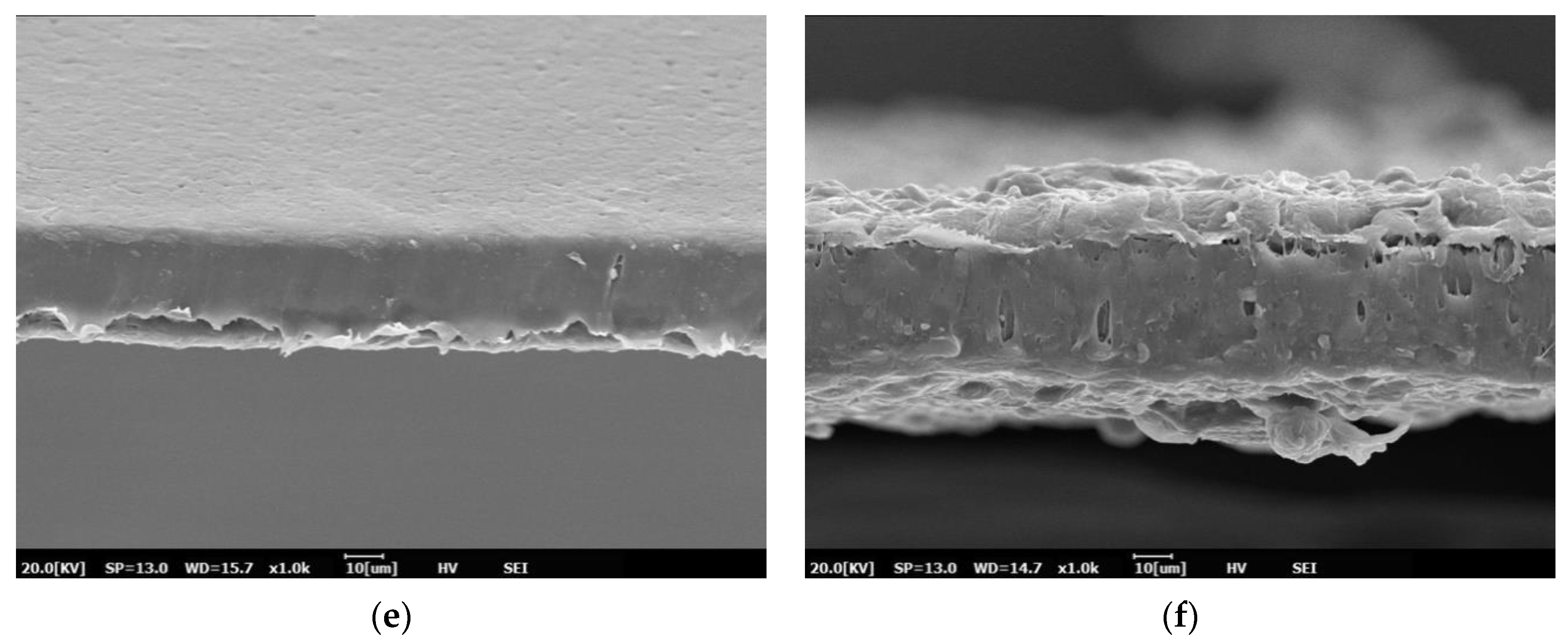
3.1. Morphology of PBS and PBS/TPS Films
3.2. Structural Properties of PBS and PBS/TPS Films
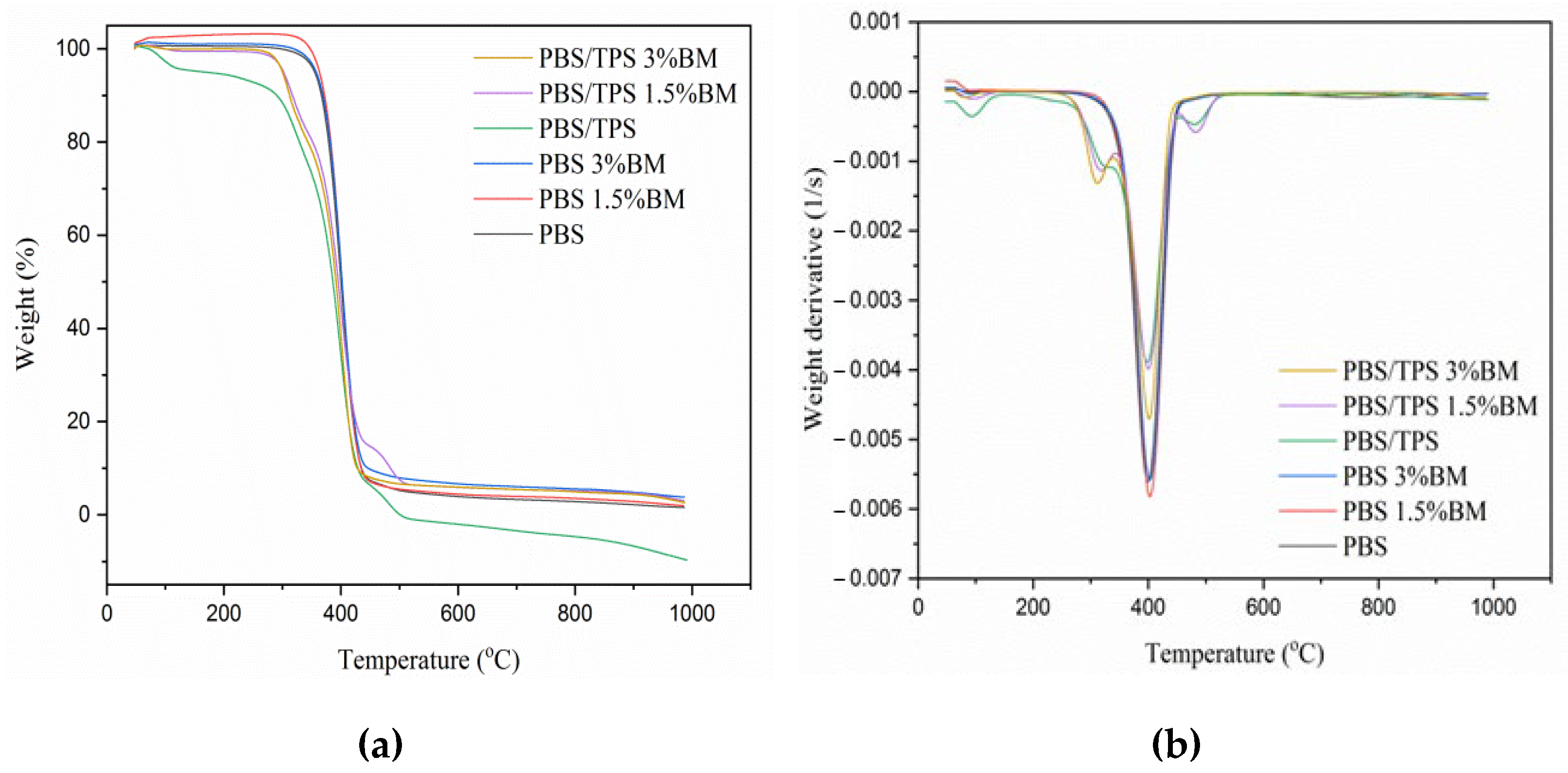
3.3. Thermal Properties of PBS and PBS/TPS Films
3.4. Porosity, Water Vapor, and Oxygen Gas Permeability of PBS and PBS/TPS Films
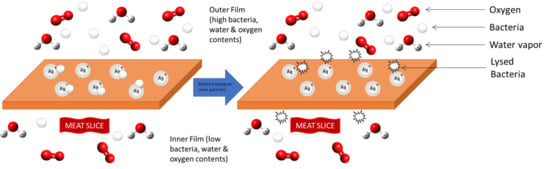
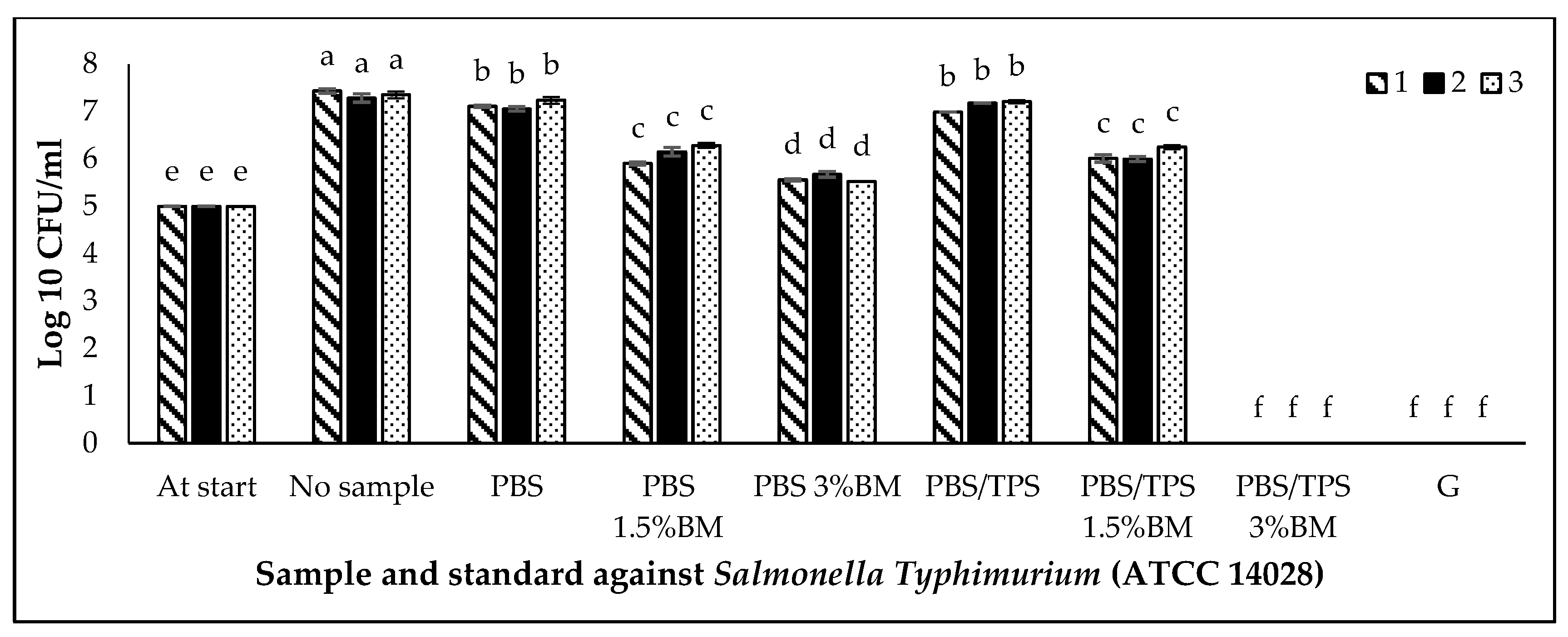
3.5. Antimicrobial Activity of PBS and PBS/TPS Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doi, Y.; Kasuya, K.; Abe, H.; Koyama, N.; Ishiwatari, S.; Takagi, K. Evaluation of biodegradabilities of biosynthetic and chemosynthetic polyesters in river water. Polym. Degrad. Stab. 1996, 51, 281–286. [Google Scholar] [CrossRef]
- Bahari, K.; Mitomo, H.; Enjoji, T.; Yoshii, F.; Makuuchi, K. Radiation crosslinked poly (butylene succinate) foam and its biodegradation. Polym. Degrad. Stab. 1998, 62, 551–557. [Google Scholar] [CrossRef]
- Phua, Y.J.; Chow, W.J.; Ishak, Z.A.M. Mechanical properties and structure development in poly (butylene succinate)/organo-montmorillonite nanocomposites under uniaxial cold rolling. EXPRESS Polym. Lett. 2011, 5, 93–103. [Google Scholar] [CrossRef]
- Thurber, H.; Curtzwiler, G.W. Suitability of poly (butylene succinate) as a coating for paperboard convenience food packaging. Int. J. Biobased Plast. 2020. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Syafiq, R.; Sherwani, S.F.K. Nanocellulose reinforced thermoplastic starch (TPS), polylactic acid (PLA), and polybutylene succinate (PBS) for food packaging applications. Front. Chem. 2020, 8, 213. [Google Scholar] [CrossRef]
- Doles, P.E.; Babu, S.M.; Ittera, N.; John, I.; John, L.; John, N. Tapioca starch based antimicrobial food packaging material. Int. J. Eng. Res. Technol. 2014, 3, 1051–1056. [Google Scholar]
- Wang, T.; Drzal, L.T. Cellulose-nanofiber-reinforced poly (lactic acid) composites prepared by a water-based approach. ACS Appl. Mater. Interfaces 2012, 4, 5079–5085. [Google Scholar] [CrossRef]
- Yin, Q.; Chen, F.; Zhang, H.; Liu, C. Fabrication and characterisation of thermoplastic starch/poly(butylene succinate) blends with maleated poly(butylene succinate) as compatibiliser. Plast. Rubber Compos. 2015, 44, 362–367. [Google Scholar] [CrossRef]
- Warsiki, E.; Bawardi, J.T. Assessing Mechanical Properties And Antimicrobial Activity Of Zinc Oxide-Starch Biofilm. In Proceedings of the 3rd International Conference on Biomass: Accelerating the Technical Develop-ment and Commercialization for Sustainable Bio-based Products and Energy, Bogor, Indonesia, 1–2 August 2018; IOP Conference Series Earth Environmental Science: Bristol, UK, 2018. [Google Scholar]
- Ayu, R.S.; Khalina, A.; Harmaen, A.S.; Zaman, K.; Jawaid, M.; Lee, C.H. Effect of modified tapioca starch on mechanical, thermal, and morphological properties of PBS blends for food packaging. Polymers 2018, 10, 1187. [Google Scholar] [CrossRef]
- Wyrwa, J.; Barska, A. Innovations in the food packaging market: Active packaging. Eur. Food Res. Technol. 2017, 243, 1681–1692. [Google Scholar] [CrossRef]
- Zhao, S.; Yao, J.; Fei, X.; Shao, Z.; Chen, X. An antimicrobial film by embedding in situ synthesized silver nanoparticles in soy protein isolate. Mater. Lett. 2013, 95, 142–144. [Google Scholar] [CrossRef]
- Mauriello, G.; De Luca, E.; La Storia, A.; Villani, F.; Ercolini, D. Antimicrobial activity of a nisin-activated plastic film for food packaging. Lett. Appl. Microbiol. 2005, 41, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, M.; Lopez-de-Dicastillo, C.; Lopez-Carballo, G.; Velez, D.; Munoz, P.H.; Gavara, R. Active films based on cocoa extract with antioxidant, antimicrobial and biological applications. Food Chem. 2013, 139, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hongsriphan, N.; Sanga, S. Antibacterial food packaging sheets prepared by coating chitosan on corona-treated extruded poly (lactic acid)/poly (butylene succinate) blends. J. Plast. Film Sheeting 2018, 34. [Google Scholar] [CrossRef]
- Petchwattana, N.; Naknaen, P. Utilization of thymol as an antimicrobial agent for biodegradable poly (butylene succinate). Mater. Chem. Phys. 2015, 163, 369–375. [Google Scholar] [CrossRef]
- Wiburanawong, S.; Petchwattana, N.; Covavisaruch, S. Carvacrol as an antimicrobial agent for poly (butylene succinate): Tensile properties and antimicrobial activity observations. Adv. Mat. Res. 2014, 931, 111–115. [Google Scholar] [CrossRef]
- Naknaen, P. Utilization possibilities of antimicrobial biodegradable packaging produced by poly (butylene succinate) modified with zinc oxide nanoparticles in fresh-cut apple slices. Int. Food Res. J. 2014, 21, 2413–2420. Available online: http://www.ifrj.upm.edu.my (accessed on 20 April 2020).
- Threepopnatkul, P.; Wongnarat, C.; Intolo, W.; Suato, S.; Kulsetthanchalee, C. Effect of TiO2 and ZnO on thin film properties of PET/PBS blend for food packaging applications. Energy Procedia 2014, 56, 102–111. [Google Scholar] [CrossRef]
- Xu, Y.; Rehmani, N.; Alsubaie, L.; Kim, C.; Sismour, E.; Scales, A. Tapioca starch active nanocomposite films and their antimicrobial effectiveness on ready-to-eat chicken meat. Food Packag. Shelf Life 2018, 16, 86–91. [Google Scholar] [CrossRef]
- Basch, C.Y.; Jagus, R.J.; Flores, S.K. Physical and antimicrobial properties of tapioca starch-HPMC edible films incorporated with nisin and/or potassium sorbate. Food Bioproc. Technol. 2013, 6, 2419–2428. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Bragg, P.D.; Rainnie, D.J. The effect of silver ions on the respiratory chain of Escherichia coli. Can. J. Microbiol. 1974, 20, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Belly, R.T.; Kydd, G.C. Silver Resistance in Microorganisms. In Proceedings of the 38th General Meeting of the Society for Industrial Microbiology—Volume 23, Richmond, VA, USA, 9–14 August 1981; Developments Industrial Microbiology: Richmond, VA, USA, 1981; pp. 567–577. [Google Scholar]
- Furr, J.R.; Russell, A.D.; Turner, T.D.; Andrews, A. Antibacterial activity of Actisorb Plus, Actisorb and silver nitrate. J. Hosp. Infect. 1994, 27, 201–208. [Google Scholar] [CrossRef]
- Thurman, R.B.; Gerba, C.P. The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. Crit. Rev. Environ. Sci. Technol. 1989, 18, 295–315. [Google Scholar] [CrossRef]
- ISO 22196. Plastics—Measurement of Antibacterial Activity on Plastics Surfaces; International Standard Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Zhang, M.; Li, Y.; Wang, L.; Li, S. Compatibility and mechanical properties of gelatin-filled polybutylene succinate composites. J. Appl. Polym. Sci. 2019, 137, 48881. [Google Scholar] [CrossRef]
- Cao, C.; Wang, Y.; Zheng, S.; Zhang, J.; Li, W.; Li, B.; Yu, J. Poly (butylene adipate-co-terephthalate)/titanium dioxide/silver composite biofilms for food packaging application. LWT. Food Sci. Technol. 2020, 109874. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, C.; Zheng, S.; Li, W.; Li, B.; Xie, X. Poly (butylene adipate-co-terephthalate)/magnesium oxide/silver ternary composite bio films for food packaging application. Food Packag. Shelf Life 2020, 24, 100487. [Google Scholar] [CrossRef]
- Lu, D.; Xiao, C.; Sun, F. Controlled grafting of poly (vinyl acetate) onto starch via RAFT polymerization. J. Appl. Polym. Sci. 2012, 124, 3450–3455. [Google Scholar] [CrossRef]
- Zhao, Y.; Qu, J.; Feng, Y.; Wu, Z. Mechanical and thermal properties of epoxidized soybean oil plasticized polybutylene succinate blends. Polym. Adv. Technol. 2012, 23. [Google Scholar] [CrossRef]
- Wootthikanokkhan, J.; Wongta, N.; Sombatsompop, N.; Kositchaiyong, A.; Ayutthaya, S.I.; Kaabbuathong, N.; Noi, W. Effect of blending conditions on mechanical, thermal, and rheological properties of plasticized poly (lactic acid)/maleated thermoplastic starch blends. J. Appl. Polym. Sci. 2011, 124, 1–8. [Google Scholar] [CrossRef]
- Mathew, S.; Snigdha, S.; Mathew, J.; Radhakrishnan, E.K. Poly (vinyl alcohol): Montmorillonite: Boiled rice water (starch) blend film reinforced with silver nanoparticles; characterization and antibacterial properties. Appl. Clay Sci. 2018, 161, 464–473. [Google Scholar] [CrossRef]
- Khalil, F.; Galland, S.; Cottaz, A.; Joly, C.; Degraeve, P. Polybutylene succinate adipate/starch blends: A morphological study for the design of controlled release films. Carbohydr. Polym. 2014, 108, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Phiriyawirut, M.; Mekaroonluck, J.; Hauyam, T.; Kittilaksanon, A. Biomass-based foam from crosslinked tapioca starch/polybutylene succinate blend. J. Renew. Mater. 2016, 4, 185–189. [Google Scholar] [CrossRef]
- Mizuno, S.; Maeda, T.; Kanemura, C.; Hotta, A. Biodegradability, reprocessability, and mechanical properties of polybutylene succinate (PBS) photografted by hydrophilic or hydrophobic membranes. Polym. Degrad. Stab. 2015, 117, 58–65. [Google Scholar] [CrossRef]
- Lule, Z.; Ju, H.; Kim, J. Effect of surface-modified Al2O3 on the thermomechanical properties of polybutylene succinate/Al2O3 composites. Ceram. Int. 2018, 44, 13530–13537. [Google Scholar] [CrossRef]
- Abidin, A.S.Z.; Yusoh, K.; Jamari, S.S.; Abdullah, A.H.; Ismail, Z. Surface functionalization of graphene oxide with octadecylamine for improved thermal and mechanical properties in polybutylene succinate nanocomposite. Polym. Bull. 2017, 75, 3499–3522. [Google Scholar] [CrossRef]
- Huang, J.; Lu, X.; Zhang, N.; Yang, L.; Yan, M.; Liu, H.; Qu, J. Study on the properties of nano-TiO2/polybutylene succinate composites prepared by vane extruder. Polym. Compos. 2014, 35. [Google Scholar] [CrossRef]
- Xu, A.; Wang, F. Carboxylate ionic liquid solvent systems from 2006 to 2020: Thermal properties and application in cellulose processing. Green Chem. 2020, 22, 7622–7664. [Google Scholar] [CrossRef]
- Mohanty, F.; Swain, S.K. Nano silver embedded starch hybrid graphene oxide sandwiched poly (ethylmethacrylate) for packaging application. Nano. Struct. Nano. Objects 2019, 18, 100300. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, M.; Bai, X.; Dong, X.; Tong, J.; Zhou, J. Effect of postcrosslinking modification with glutaraldehyde on the properties of thermoplastic starch/poly(vinyl alcohol) blend films. J. Appl. Polym. Sci. 2011, 124. [Google Scholar] [CrossRef]
- Wang, Y.; Duo, T.; Xu, X.; Xiao, Z.; Xu, A.; Liu, R.; Jiang, C.; Lu, J. Eco-friendly high-performance poly(methyl methacrylate) film reinforced with methylcellulose. ACS Omega 2020, 5, 24256–24261. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Wang, Y.; Gao, J.; Wang, J. Facile fabrication of a homogeneous cellulose/polylactic acid composite film with improved biocompatibility, biodegradability and mechanical properties. Green Chem. 2019, 21, 4449–4456. [Google Scholar] [CrossRef]
- Messin, T.; Guinault, A.; Delpouve, N.; Sollogoub, C. Biodegradable PLA/PBS multinanolayer membrane with enhanced barrier performances. J. Membr. Sci. 2020, 598, 117777. [Google Scholar] [CrossRef]
- Xu, A.; Wang, F.; Zhang, L.; Xu, X.; Xiao, Z.; Liu, R. Composites from biodegradable and biocompatible methylcellulose, poly(d,l-lactide-co-glycolide) and poly(1,4-butylene succinate) with enhanced properties. J. Appl. Polym. Sci. 2020, 50320. [Google Scholar] [CrossRef]
- Wei, Z.; Gu, J.; Ye, Y.; Fang, M.; Lang, J.; Yang, D. Biodegradable poly (butylene succinate) nanofibrous membrane treated with oxygen plasma for super hydrophilicity. Surf. Coat. Technol. 2019, 381, 125147. [Google Scholar] [CrossRef]
- Wattanawong, N.; Chatchaipaiboon, K.; Sreekirin, N.; Aht-Ong, D. Migration, physical and antibacterial properties of silver zeolite/poly (butylene succinate) composite films for food packaging applications. J. Reinf. Plast. Compos. 2020, 39, 3–4. [Google Scholar] [CrossRef]
- Petchwattana, N.; Covavisaruch, S.; Wibooranawong, S.; Naknaen, P. Antimicrobial food packaging prepared from poly (butylene succinate) and zinc oxide. Measurement 2016, 93, 442–448. [Google Scholar] [CrossRef]


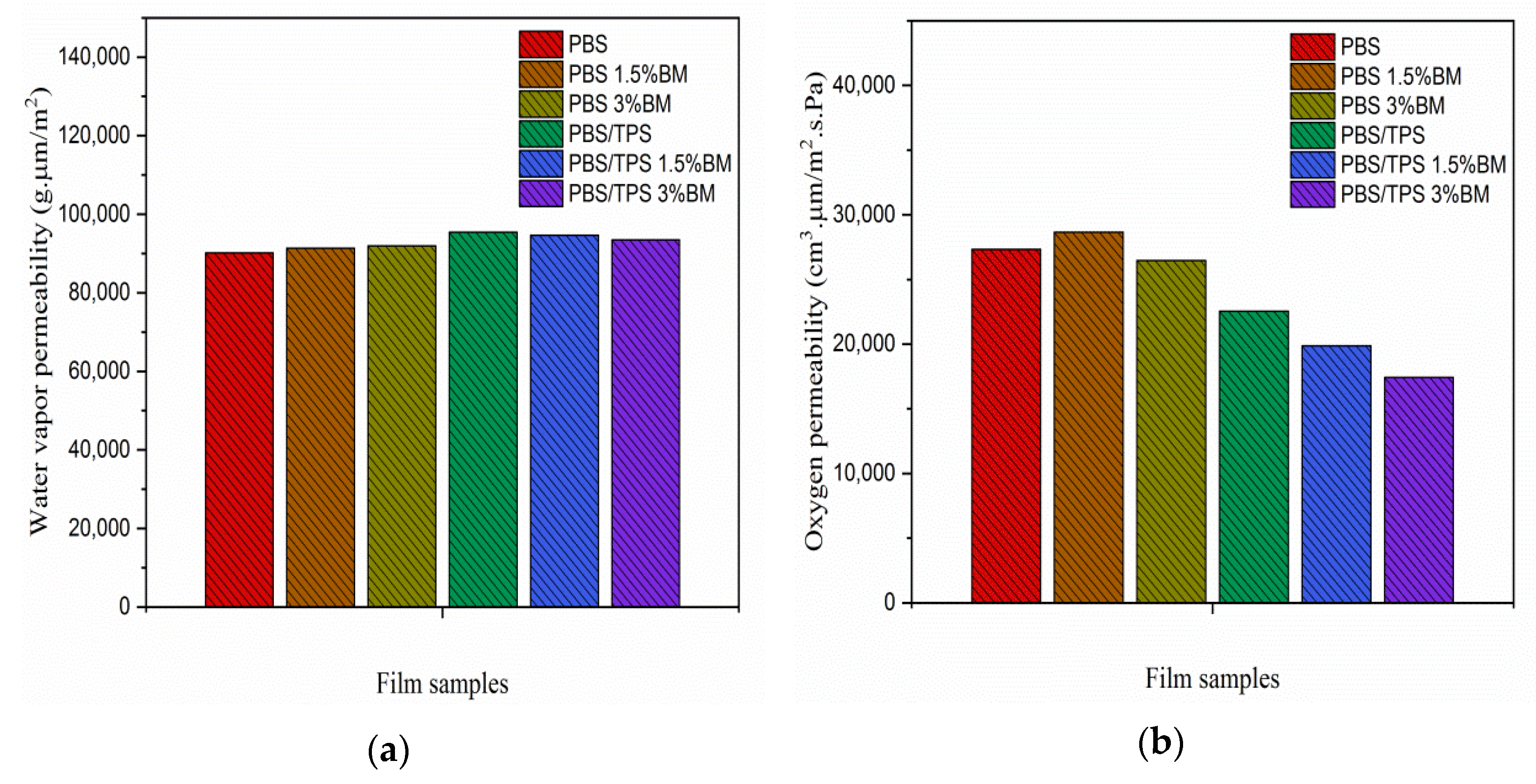

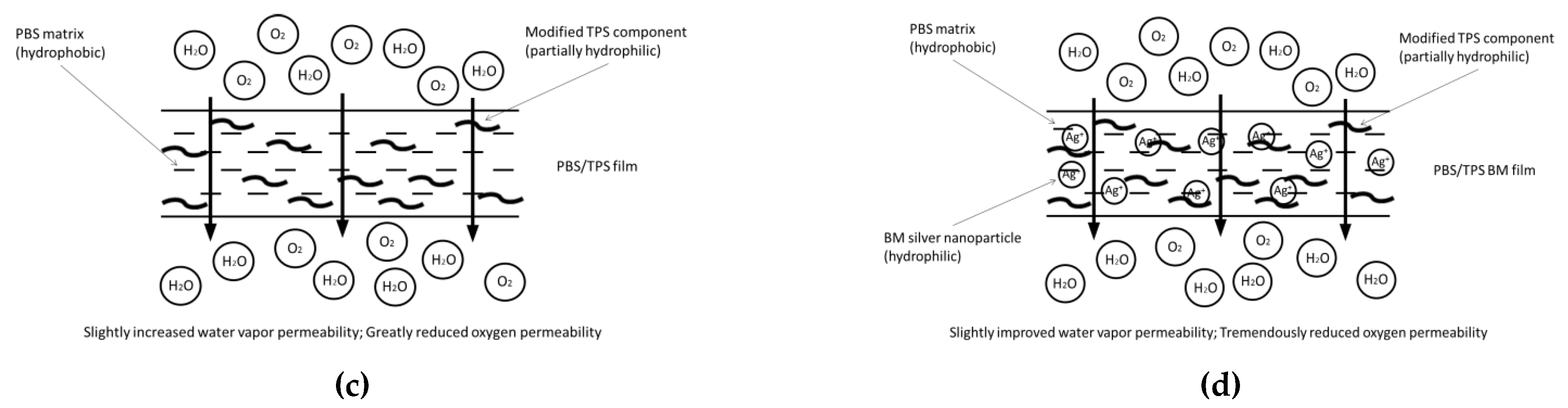
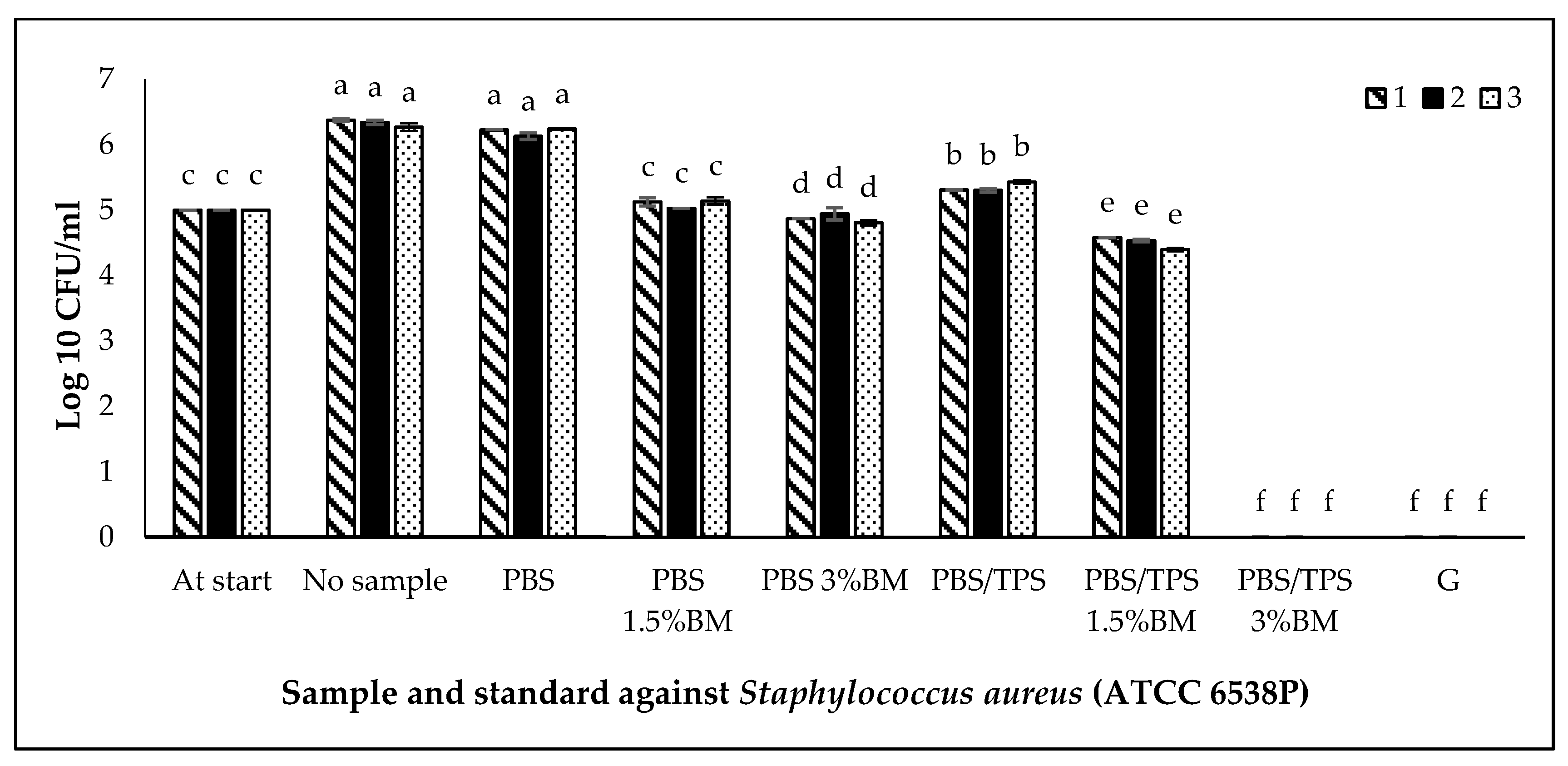
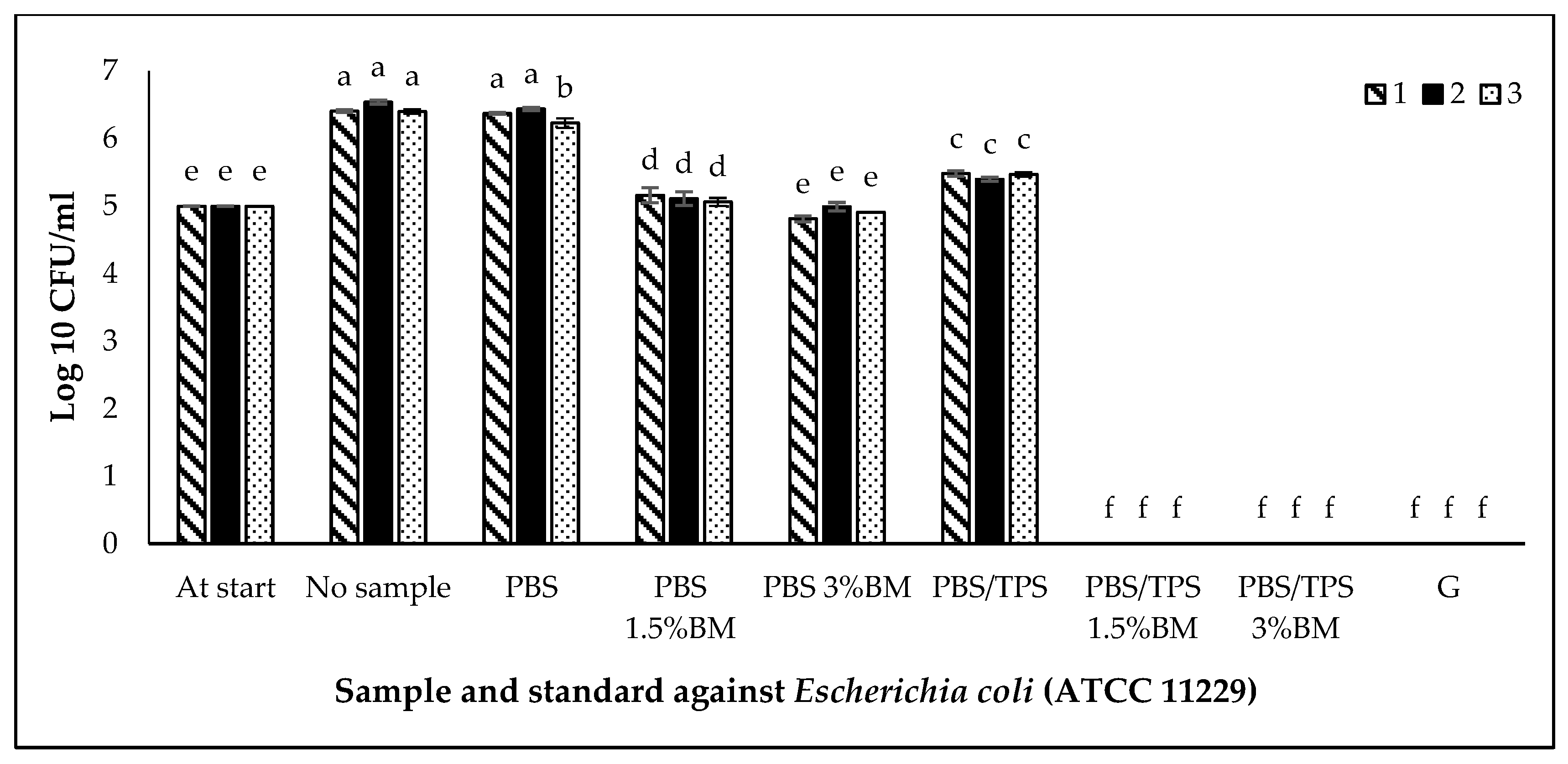
| No. | Film Denotations | Amount of TPS (wt %) | Amount of BM (wt %) |
|---|---|---|---|
| 1 | PBS | N/A | N/A |
| 2 | PBS 1.5% BM | N/A | 1.5 |
| 3 | PBS 3% BM | N/A | 3 |
| 4 | PBS/TPS | 40 | N/A |
| 5 | PBS/TPS 1.5% BM | 40 | 1.5 |
| 6 | PBS/TPS 3% BM | 40 | 3 |
| Samples | TON (°C) a | TPD (°C) b | WML (J/g) c | RF (%) d |
|---|---|---|---|---|
| PBS | 355.1 | 399.3 | 95.62 | 4.75 |
| PBS 1.5% BM | 357.0 | 403.2 | 97.81 | 5.36 |
| PBS 3% BM | 356.8 | 402.5 | 93.04 | 7.80 |
| PBS/TPS | 283.7 | 399.5 | 96.08 | 1.19 |
| PBS/TPS 1.5% BM | 288.7 | 399.8 | 93.11 | 6.30 |
| PBS/TPS 3% BM | 290.2 | 401.9 | 93.38 | 6.50 |
| Samples | Tc (°C) a | Tm (°C) b | ∆Hm (J/g) c | XC (%) d |
|---|---|---|---|---|
| PBS | 94.4 | 113.3 | 69.42 | 34.71 |
| PBS 1.5% BM | 95.3 | 113.7 | 63.53 | 32.25 |
| PBS 3% BM | 92.2 | 113.5 | 71.89 | 37.06 |
| PBS/TPS | 94.5 | 113.3 | 51.50 | 28.61 |
| PBS/TPS 1.5% BM | 94.0 | 113.6 | 56.53 | 31.94 |
| PBS/TPS 3% BM | 92.0 | 112.6 | 62.20 | 35.75 |
| Samples | BET | Permeability | |||
|---|---|---|---|---|---|
| Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) | Water Vapor (g μm/m2) | Oxygen (cm3 μm/m2 s Pa) | |
| PBS | 3.598 | 0.211 | 117.21 | 90,170 | 27,320 |
| PBS 1.5% BM | 3.572 | 0.414 | 231.62 | 91,370 | 28,650 |
| PBS 3% BM | 3.030 | 0.436 | 287.44 | 91,940 | 26,470 |
| PBS/TPS | 0.336 | 0.102 | 604.90 | 95,400 | 22,540 |
| PBS/TPS 1.5% BM | 1.504 | 0.097 | 128.97 | 94,660 | 19,860 |
| PBS/TPS 3% BM | 4.872 | 0.081 | 33.07 | 93,500 | 17,420 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziman, N.; Kian, L.K.; Jawaid, M.; Sanny, M.; Alamery, S. Morphological, Structural, Thermal, Permeability, and Antimicrobial Activity of PBS and PBS/TPS Films Incorporated with Biomaster-Silver for Food Packaging Application. Polymers 2021, 13, 391. https://doi.org/10.3390/polym13030391
Aziman N, Kian LK, Jawaid M, Sanny M, Alamery S. Morphological, Structural, Thermal, Permeability, and Antimicrobial Activity of PBS and PBS/TPS Films Incorporated with Biomaster-Silver for Food Packaging Application. Polymers. 2021; 13(3):391. https://doi.org/10.3390/polym13030391
Chicago/Turabian StyleAziman, Nurain, Lau Kia Kian, Mohammad Jawaid, Maimunah Sanny, and Salman Alamery. 2021. "Morphological, Structural, Thermal, Permeability, and Antimicrobial Activity of PBS and PBS/TPS Films Incorporated with Biomaster-Silver for Food Packaging Application" Polymers 13, no. 3: 391. https://doi.org/10.3390/polym13030391
APA StyleAziman, N., Kian, L. K., Jawaid, M., Sanny, M., & Alamery, S. (2021). Morphological, Structural, Thermal, Permeability, and Antimicrobial Activity of PBS and PBS/TPS Films Incorporated with Biomaster-Silver for Food Packaging Application. Polymers, 13(3), 391. https://doi.org/10.3390/polym13030391