Experimental Study on Thermosensitive Hydrogel Used to Extinguish Class A Fire
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
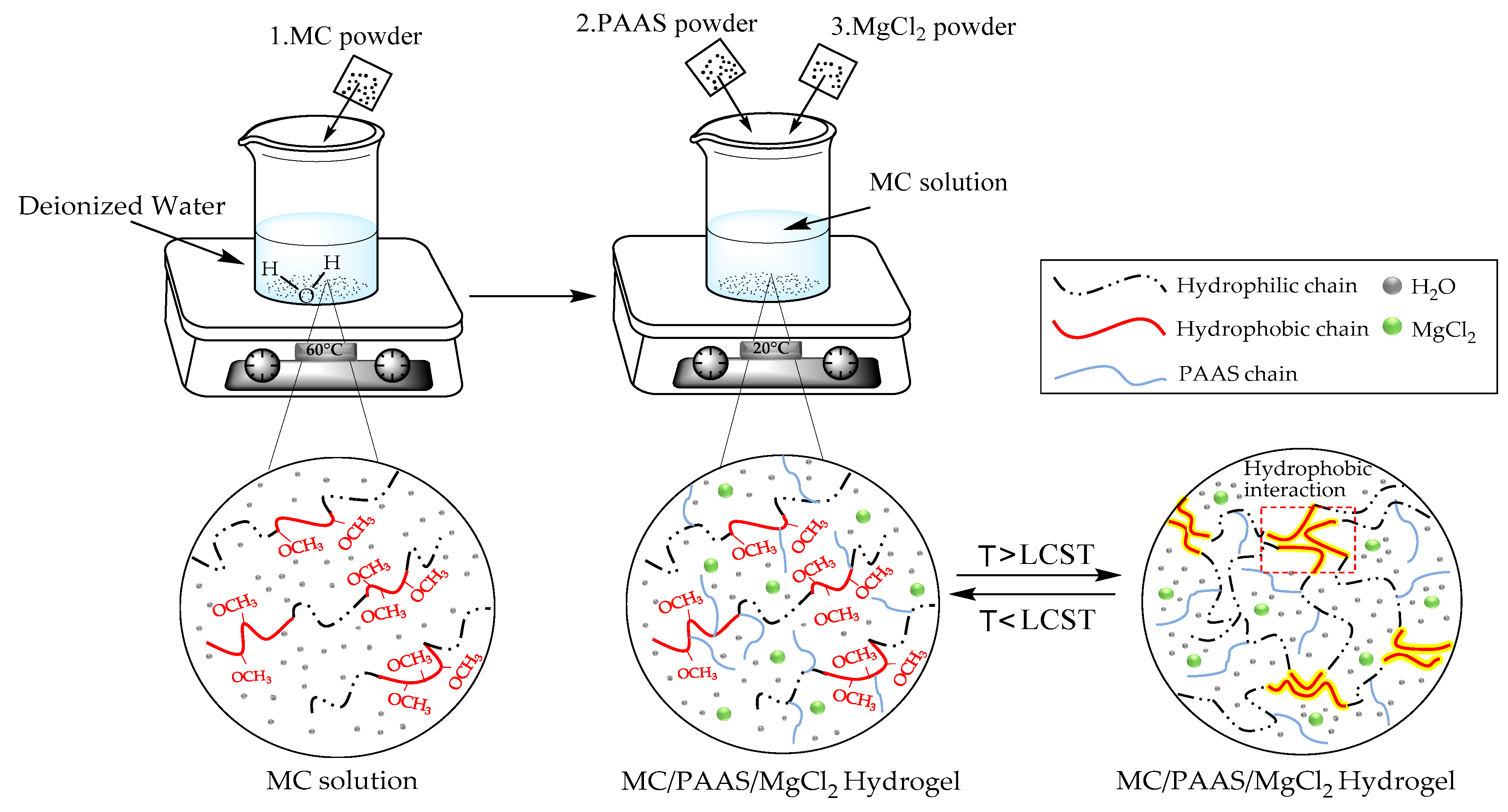
2.2. Gel Preparation
2.3. Performance Testing
2.3.1. Study on the Structure of the Gel
2.3.2. Surface Tension Test
2.3.3. Viscosity Test
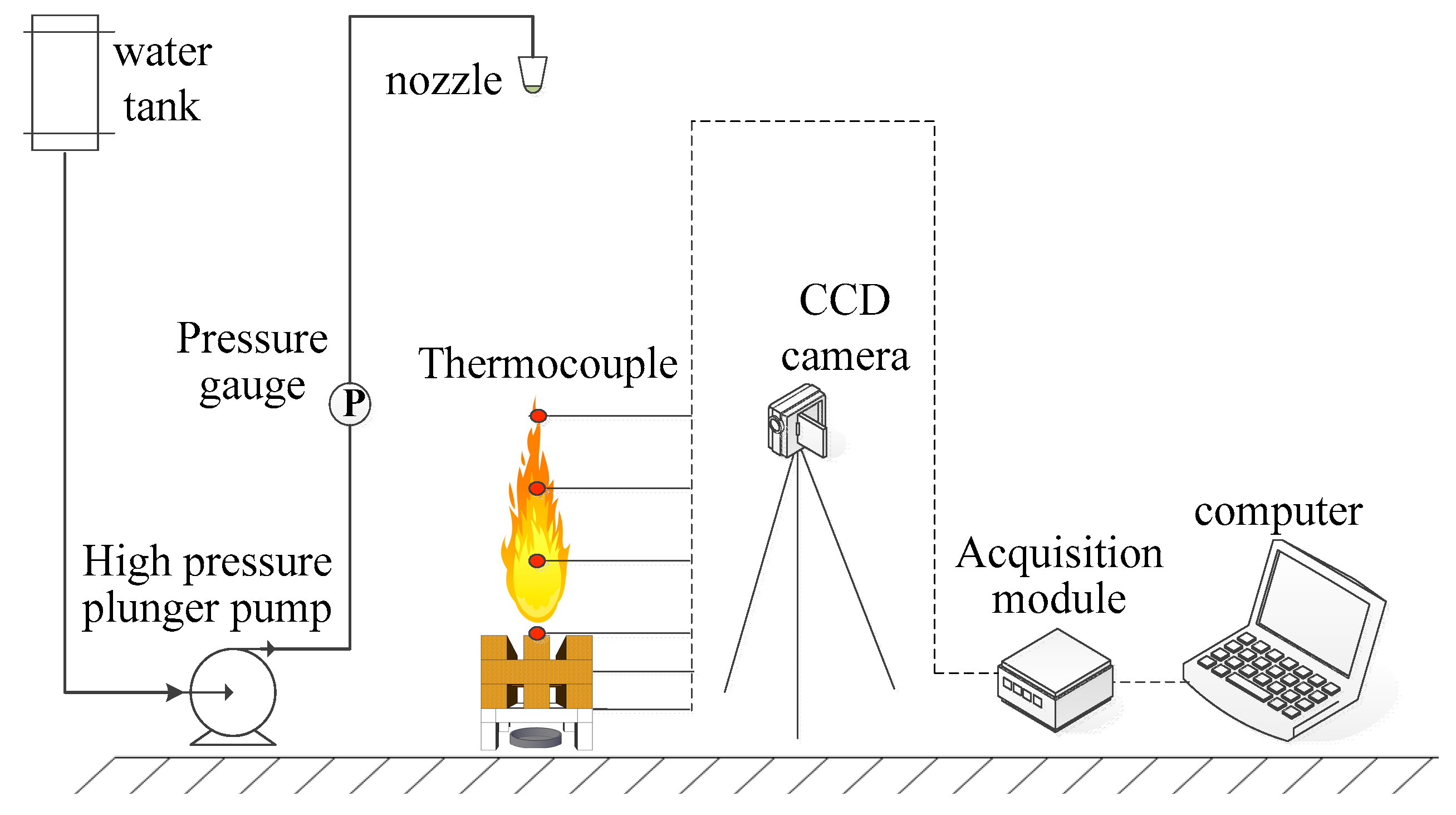
2.4. Fire Extinguishing Experiment
3. Results and Discussion
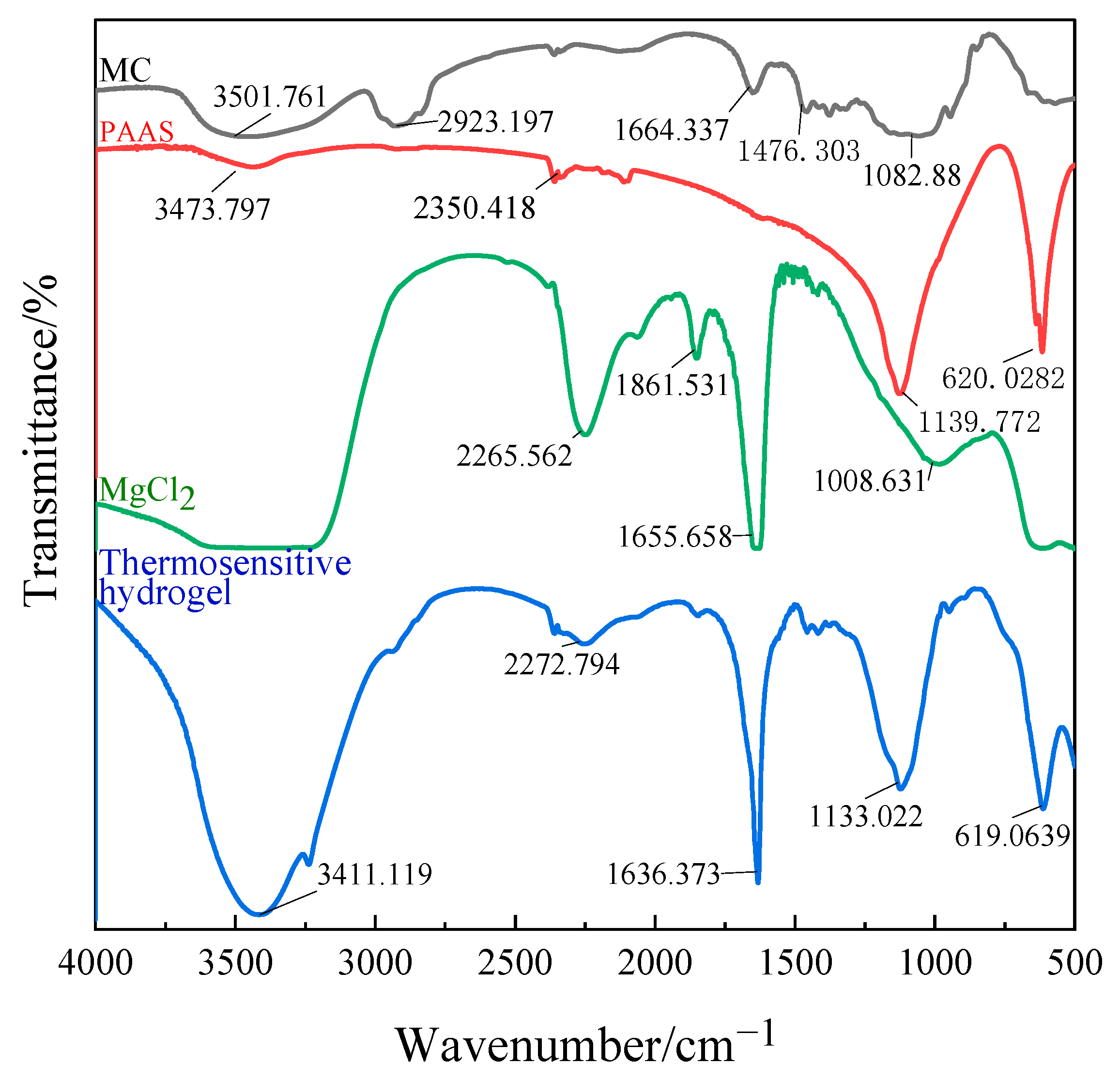
3.1. Infrared Spectrum Analysis
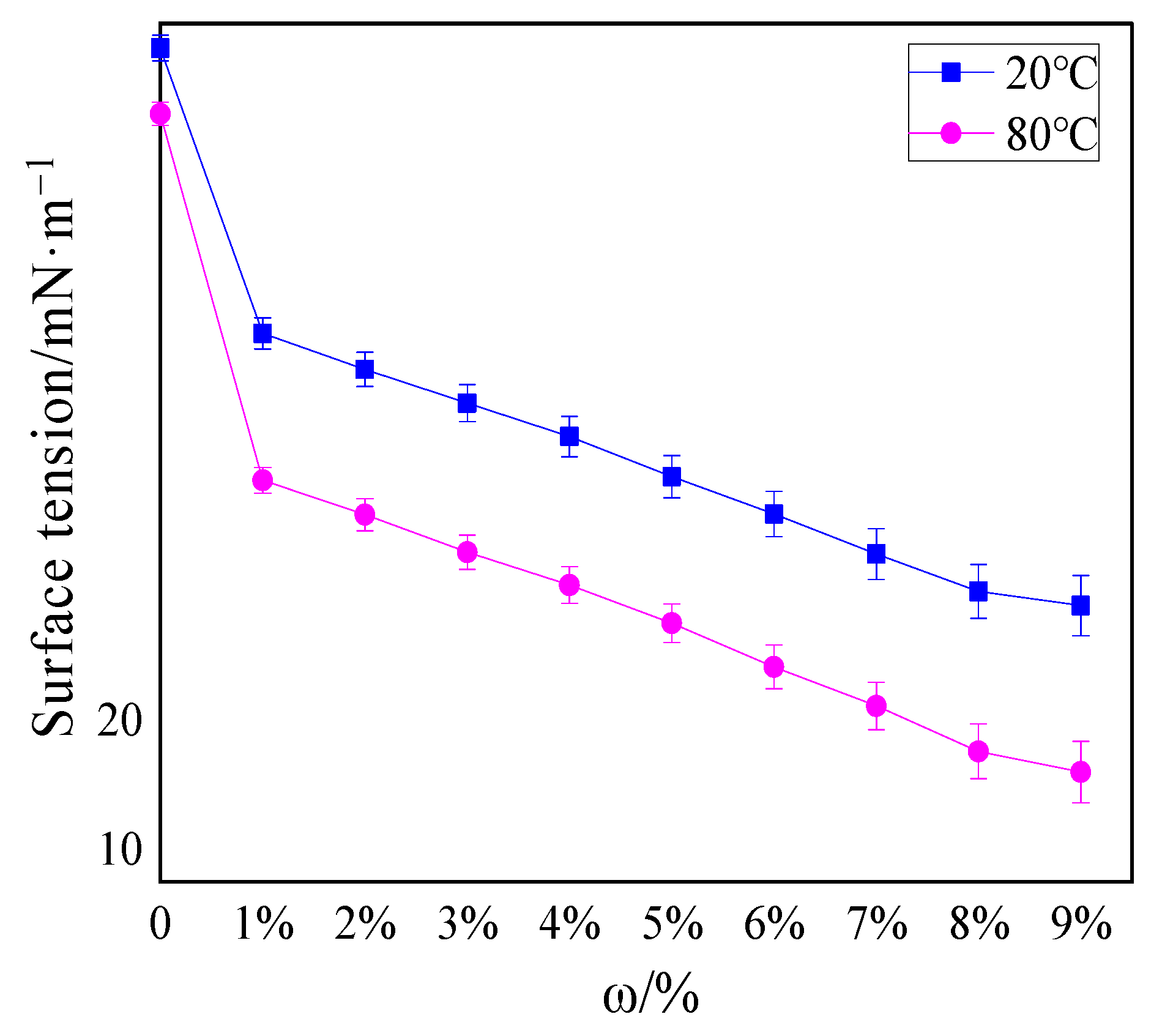
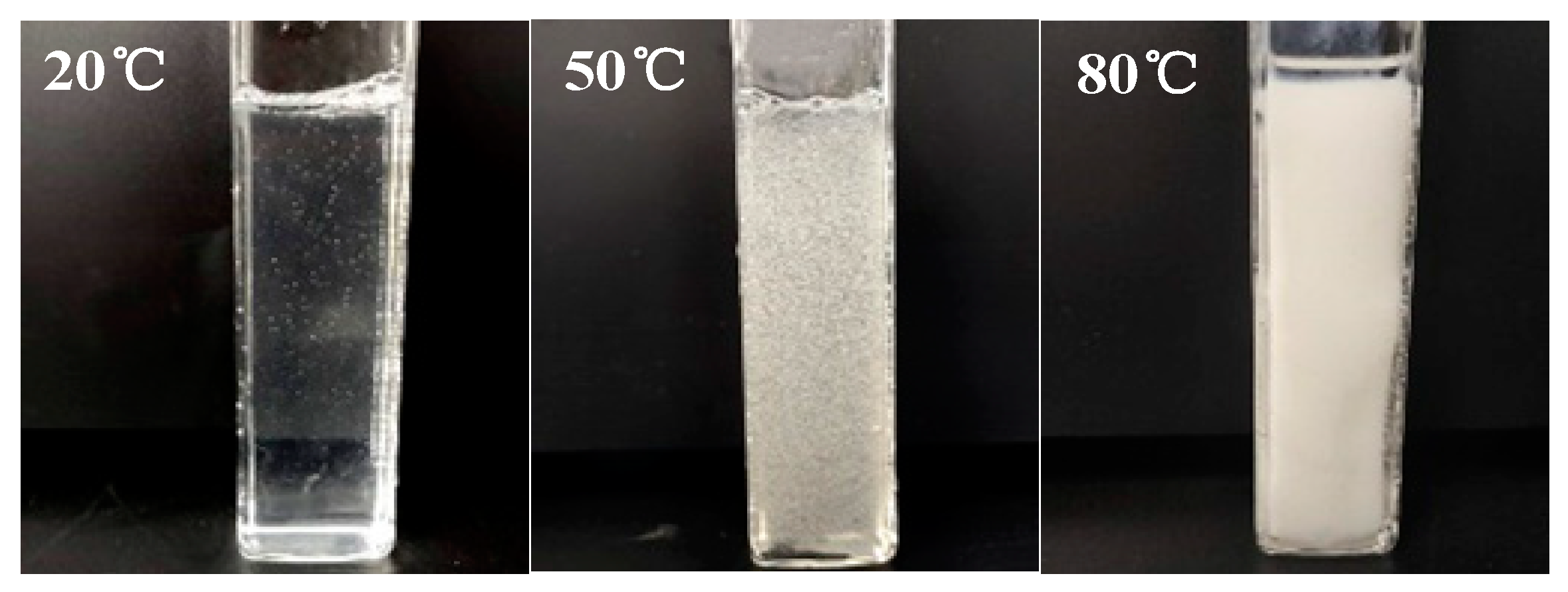
3.2. Surface Tension Analysis
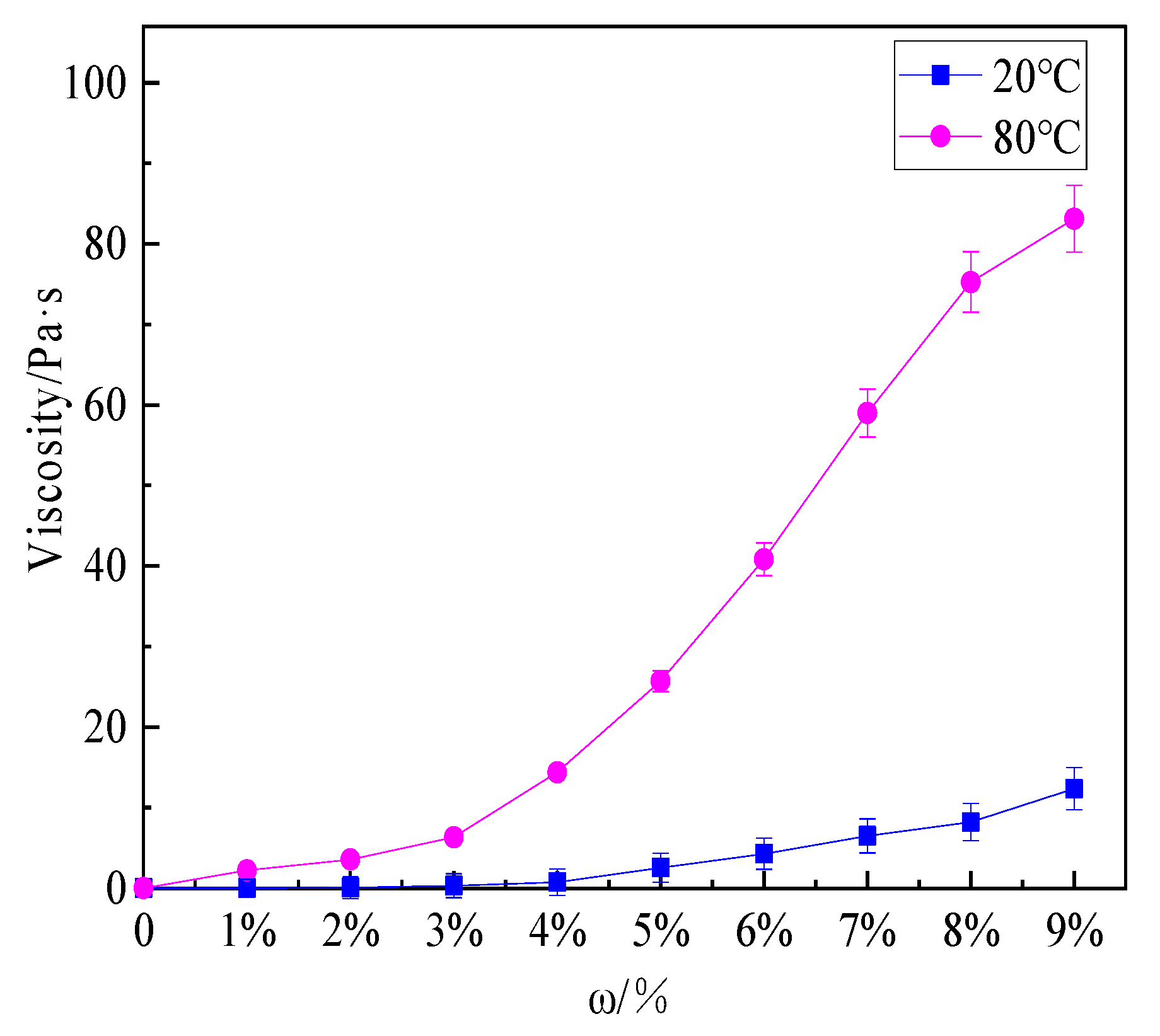
3.3. Viscosity Analysis
3.4. Small-Scale Fire Extinguishing Performance
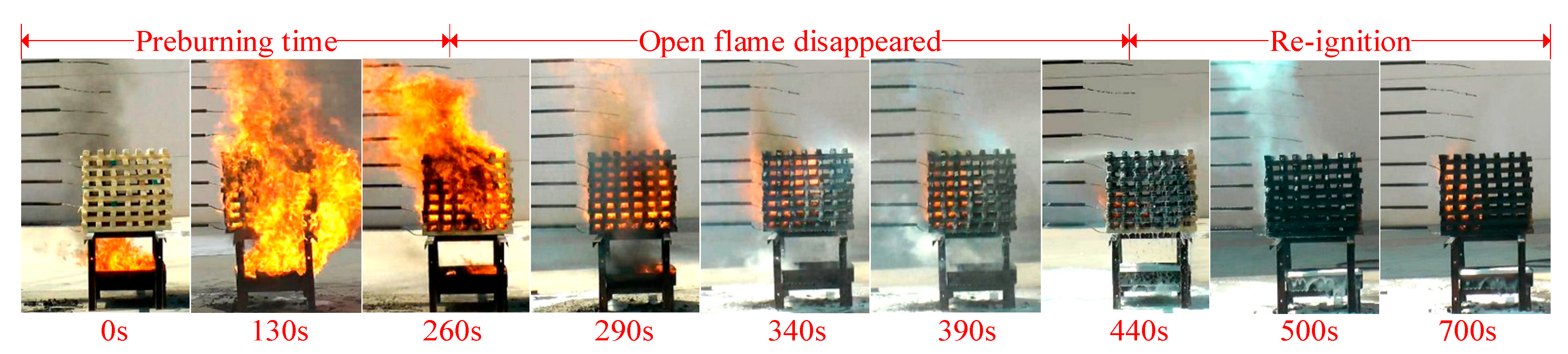
3.5. Large-Scale Fire Extinguishing Experiment Results
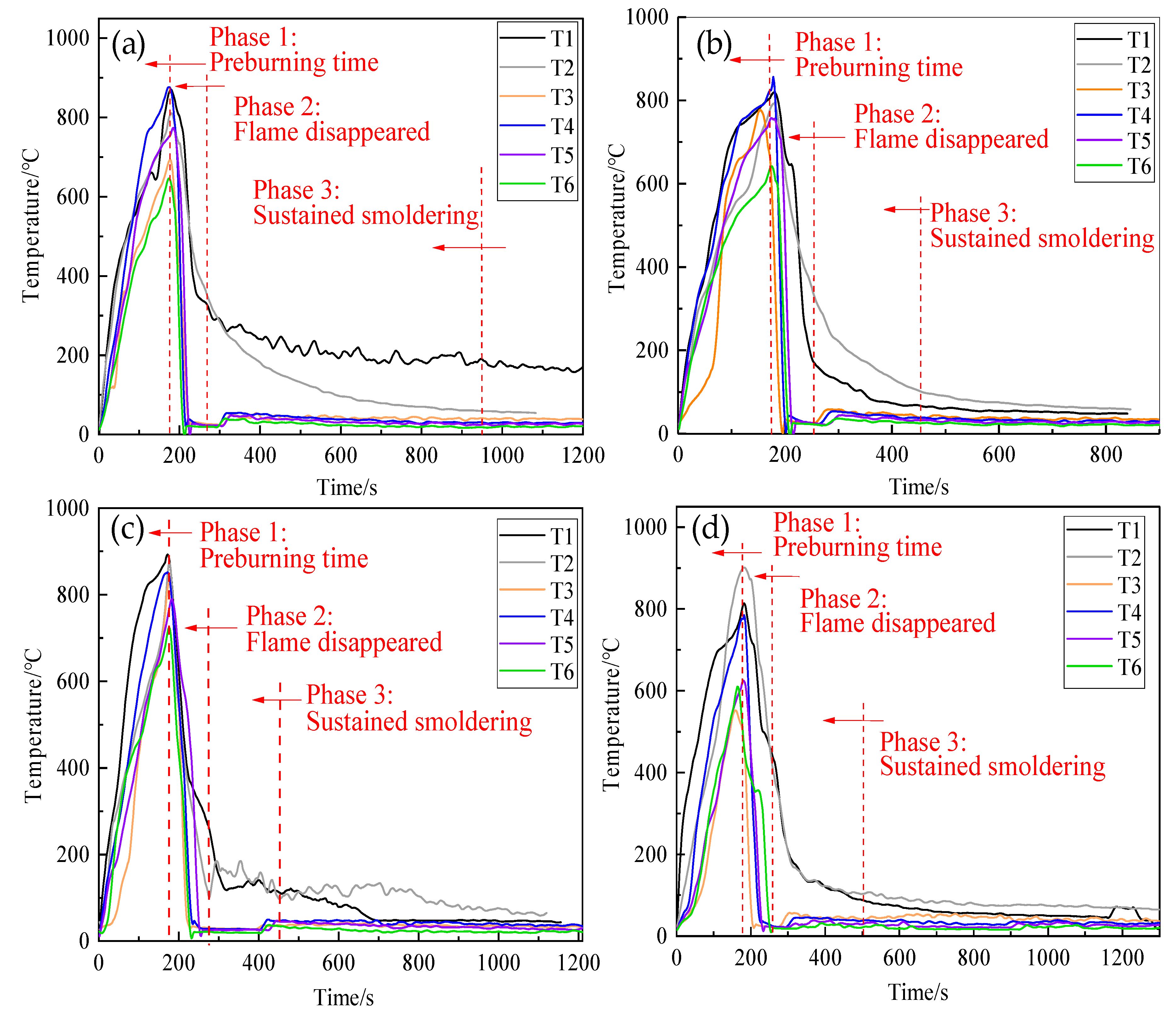
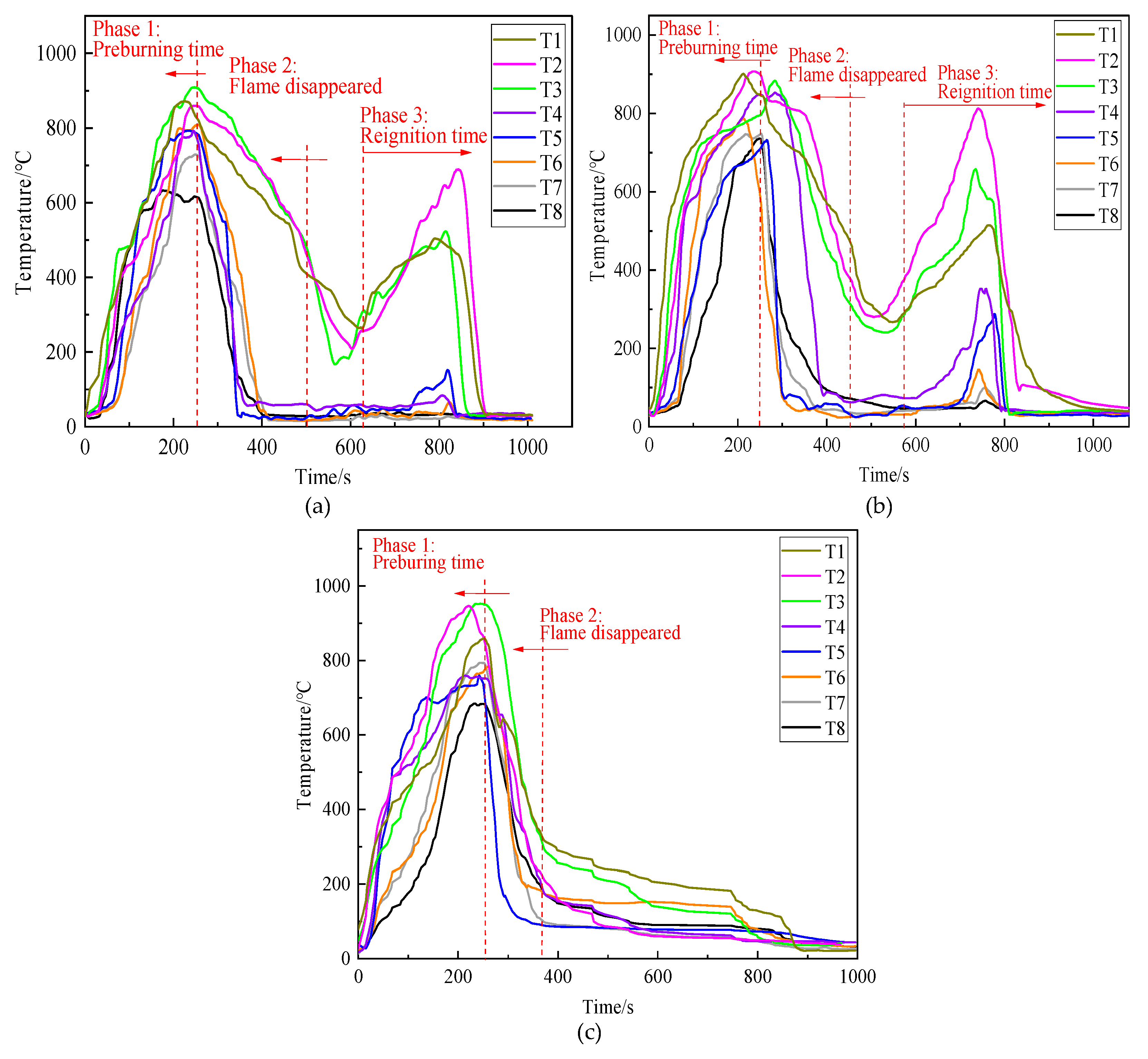
3.5.1. Fire Extinguishing Performance
3.5.2. Cooling Performance
3.5.3. Fire Extinguishing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cascio:, W.E. Wildland fire smoke and human health. Sci. Total Environ. 2018, 624, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yao, J.; Sila-Nowicka, K.; Jin, Y. Urban fire dynamics and its association with urban growth: Evidence from Nanjing, China. ISPRS Int. J. Geo-Inf. 2020, 9, 218. [Google Scholar] [CrossRef]
- Ingason, H.; Li, Y.Z. Large scale tunnel fire tests with different types of large droplet fixed fire fighting systems. Fire Saf. J. 2019, 107, 29–43. [Google Scholar] [CrossRef]
- Tang, Z.; Fang, Z.; Yuan, J.P.; Merci, B. Experimental study of the downward displacement of fire-induced smoke by water sprays. Fire Saf. J. 2013, 55, 35–49. [Google Scholar] [CrossRef]
- Kim, N.K.; Rie, D.H. A study on the fire extinguishing characteristics of deep-seated fires using the scale model experiment. Fire Saf. J. 2016, 80, 38–45. [Google Scholar] [CrossRef]
- Myers, T.M.; Marshall, A.W. A description of the initial fire sprinkler spray. Fire Saf. J. 2016, 84, 1–7. [Google Scholar] [CrossRef]
- Dong, K.; Wang, J.; Zhang, Y.; Liang, Z.; Shi, Q. Performance of Fire Extinguishing Gel with Strong Stability for Coal Mine. Combust. Sci. Technol. 2020, 00, 1–17. [Google Scholar] [CrossRef]
- Li, S.; Zhou, G.; Wang, Y.; Jing, B.; Qu, Y. Synthesis and characteristics of fire extinguishing gel with high water absorption for coal mines. Process Saf. Environ. Prot. 2019, 125, 207–218. [Google Scholar] [CrossRef]
- Cui, X.F.; Zheng, W.J.; Zou, W.; Liu, X.Y.; Yang, H.; Yan, J.; Gao, Y. Water-retaining, tough and self-healing hydrogels and their uses as fire-resistant materials. Polym. Chem. 2019, 10, 5151–5158. [Google Scholar] [CrossRef]
- Ren, X.; Xue, D.; Li, Y.; Hu, X.; Shao, Z.; Cheng, W.; Dong, H.; Zhao, Y.; Xin, L.; Lu, W. Novel sodium silicate/polymer composite gels for the prevention of spontaneous combustion of coal. J. Hazard. Mater. 2019, 371, 643–654. [Google Scholar] [CrossRef]
- Huang, Z.; Yan, L.; Zhang, Y.; Gao, Y.; Liu, X.; Liu, Y.; Li, Z. Research on a new composite hydrogel inhibitor of tea polyphenols modified with polypropylene and mixed with halloysite nanotubes. Fuel 2019, 253, 527–539. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, H. Development of a novel bentonite–acrylamide superabsorbent hydrogel for extinguishing gangue fire hazard. Powder Technol. 2018, 323, 486–494. [Google Scholar] [CrossRef]
- Qin, B.; Dou, G.; Wang, Y.; Xin, H.; Ma, L.; Wang, D. A superabsorbent hydrogel–ascorbic acid composite inhibitor for the suppression of coal oxidation. Fuel 2017, 190, 129–135. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Leroux, J.C. In situ-forming hydrogels—Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.C.; da Silva, A.A.; Costa, D.B.; Cesarino, I.; Costa, C.M.; Lanceros-Méndez, S.; Pawlicka, A.; Silva, M.M. Thermo-sensitive chitosan–cellulose derivative hydrogels: Swelling behaviour and morphologic studies. Cellulose 2014, 21, 4531–4544. [Google Scholar] [CrossRef]
- Klouda, L. Thermoresponsive hydrogels in biomedical applications A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef]
- Dai, M.; Shang, Y.; Li, M.; Ju, B.; Liu, Y.; Tian, Y. Synthesis and characterization of starch ether/alginate hydrogels with reversible and tunable thermoresponsive properties. Mater. Res. Express 2020, 7. [Google Scholar] [CrossRef]
- Cheng, W.; Hu, X.; Xie, J.; Zhao, Y. An intelligent gel designed to control the spontaneous combustion of coal: Fire prevention and extinguishing properties. Fuel 2017, 210, 826–835. [Google Scholar] [CrossRef]
- Lim, J.W.; Kim, H.J.; Kim, Y.; Shin, S.G.; Cho, S.; Jung, W.G.; Jeong, J.H. An active and soft hydrogel actuator to stimulate live cell clusters by self-folding. Polymers 2020, 12, 583. [Google Scholar] [CrossRef]
- Jiang, P.; Cheng, Y.; Yu, S.; Lu, J.; Wang, H. Study on the effect of 1-butanol soluble lignin on temperature-sensitive gel. Polymers 2018, 10, 1109. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.L.; Kan, C.-w. Thermoresponsive hydrogels and their biomedical applications: Special insight into their applications in textile based transdermal therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yang, Y.; Tang, K. Research on preparation and fire extinguishing performance of temperature-sensitive hygrogel. J. China Univ. Min. Technol. 2014, 43, 1–7. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, W.; Nie, W.; Shao, Z. Synthesis and characterization of a temperature-sensitive hydrogel based on sodium alginate and N-isopropylacrylamide. Polym. Adv. Technol. 2015, 26, 1340–1345. [Google Scholar] [CrossRef]
- Yu, Z.; Suryawanshi, A.; He, H.; Liu, J.; Li, Y.; Lin, X.; Sun, Z. Preparation and characterisation of fire-resistant PNIPAAm/SA/AgNP thermosensitive network hydrogels and laminated cotton fabric used in firefighter protective clothing. Cellulose 2020, 27, 5391–5406. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Yang, Y.; Wang, C.; Shu, C.M.; Deng, J. Comparison of the inhibition mechanisms of five types of inhibitors on spontaneous coal combustion. Int. J. Energy Res. 2018, 42, 1158–1171. [Google Scholar] [CrossRef]
- Jiang, Z.; Dou, G. Preparation and Characterization of Chitosan Grafting Hydrogel for Mine-Fire Fighting. ACS Omega 2020, 5, 2303–2309. [Google Scholar] [CrossRef]
- Tan, J.; Xie, S.; Wang, G.; Yu, C.W.; Zeng, T.; Cai, P.; Huang, H. Fabrication and Optimization of the Thermo-Sensitive Hydrogel Carboxymethyl Cellulose/Poly(N-isopropylacrylamide-co-acrylic acid) for U(VI) Removal from Aqueous Solution. Polymers 2020, 12, 151. [Google Scholar]
- Kim, M.H.; Park, H.; Shin, J.Y.; Park, W.H. Effect of vitamin derivatives on gelation rate and gel strength of methylcellulose. Carbohydr. Polym. 2018, 196, 414–421. [Google Scholar] [CrossRef]
- Tian, Y.-y.; Wei, S. Research Progress of the Polyacrylic Acid Sodium with Low Molecular Weight. Ind. Guangzhou Chem. 2012, 40, 15–17. [Google Scholar]
- Wu, Y.; Yao, C.; Hu, Y.; Zhu, X.; Qing, Y.; Wu, Q. Comparative performance of three magnesium compounds on thermal degradation behavior of red gum wood. Materials 2014, 7, 637–652. [Google Scholar] [CrossRef]
- Kawamoto, H.; Yamamoto, D.; Saka, S. Influence of neutral inorganic chlorides on primary and secondary char formation from cellulose. J. Wood Sci. 2008, 54, 242–246. [Google Scholar] [CrossRef]
- Chunlei, J.Y.F.; Fulong, C.; Tao, H. Physicochemical Properties Analysis of a New Thermosensitive Hydrogel Extinguishing Agent. Fire Tech. Prod. Inf. 2018, 31, 73–77. [Google Scholar]
- Rehage, H.; Hoffmann, H.; Hoffmann, H. Molecular Physics: An International Journal at the Interface Between Chemistry and Physics Viscoelastic surfactant solutions: Model systems for rheological research Viscoelastic surfactant solutions: Model systems for rheological research. Mol. Phys. Int. J. Interface Chem. Phys. Mol. Physlcs 1991, 74, 933–973. [Google Scholar]
- Jia, C.L.; Jiang, Z.A.; Yang, Y.; Tang, K. Synthesis of a thermosensitive hydrogel and its fire suppressing performance. J. Funct. Mater. 2013, 1593–1597. [Google Scholar]
- Yang, Y.; Deng, J.; Zhao, D.L.; Guo, J. Mechanism and property of extinguishing temperature-sensitive hydrogels. In Proceedings of the 2014 7th International Conference on Intelligent Computation Technology and Automation, Changsha, China, 25–26 October 2014; pp. 360–364. [Google Scholar] [CrossRef]

| Concentration of Extinguishing Agent/wt% | Extinguishing Time/s | Smoldering Time/s | Total Fire-Extinguishing Time/s |
|---|---|---|---|
| 2 | 70 | 580 | 650 |
| 4 | 74 | 320 | 404 |
| 6 | 80 | 200 | 280 |
| 8 | 115 | 180 | 296 |
| Types | 20 °C | 80 °C | Extinguishing Open Flame Time/s | Total Fire Extinguishing Time/s | ||
|---|---|---|---|---|---|---|
| Surface Tension/mN·m−1 | Viscosity/Pa·s | Surface Tension/mN·m−1 | Viscosity/Pa·s | |||
| Ordinary gel extinguishing agent | 26.4 | 28.4 | 25.1 | 28.1 | 240 | 340 |
| Foam extinguishing agent | 20.6 | 0.068 | 19.8 | 0.054 | 180 | 240 |
| Thermosensitive hydrogel | 36 | 4.256 | 24.15 | 40.858 | 120 | 120 |
| Curve | Fitting Equation | R2 | Vmax (°C/s) | Vaver (°C/s) | |
|---|---|---|---|---|---|
| Ordinary gel extinguishing agent | T1 | −1.35t + 1149.30 | 0.993 | 7.22 | 3.92 |
| T2 | −1.34t + 1211.27 | 0.971 | |||
| T3 | −1.71t + 1368.59 | 0.970 | |||
| T4 | −5.19t + 2048.99 | 0.961 | |||
| T5 | −7.22t + 2711.39 | 0.904 | |||
| T6 | −5.55t + 2308.62 | 0.962 | |||
| T7 | −4.46t + 1853.51 | 0.987 | |||
| T8 | −4.55t + 1768.50 | 0.968 | |||
| Foam extinguishing agent | T1 | −1.67t + 1250.49 | 0.980 | 6.39 | 3.68 |
| T2 | −2.11t + 1458.21 | 0.809 | |||
| T3 | −2.85t + 1640.42 | 0.837 | |||
| T4 | −6.39t + 2669.48 | 0.878 | |||
| T5 | −5.52t + 2027.79 | 0.673 | |||
| T6 | −3.11t + 1180.40 | 0.663 | |||
| T7 | −4.14t + 1588.64 | 0.782 | |||
| T8 | −3.67t + 1502.39 | 0.841 | |||
| Thermosensitive hydrogel | T1 | −4.09t + 1830.95 | 0.944 | 8.77 | 5.19 |
| T2 | −5.03t + 2080.56 | 0.951 | |||
| T3 | −5.49 + 2353.12 | 0.943 | |||
| T4 | −4.59t + 1917.28 | 0.937 | |||
| T5 | −8.77t + 2833.85 | 0.805 | |||
| T6 | −5.15t + 2030.13 | 0.800 | |||
| T7 | −4.64t + 1849.30 | 0.859 | |||
| T8 | −3.76t + 1585.72 | 0.917 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Huang, X.; Sheng, Y.; Liu, X.; Wei, G. Experimental Study on Thermosensitive Hydrogel Used to Extinguish Class A Fire. Polymers 2021, 13, 367. https://doi.org/10.3390/polym13030367
Ma L, Huang X, Sheng Y, Liu X, Wei G. Experimental Study on Thermosensitive Hydrogel Used to Extinguish Class A Fire. Polymers. 2021; 13(3):367. https://doi.org/10.3390/polym13030367
Chicago/Turabian StyleMa, Li, Xiao Huang, Youjie Sheng, Xixi Liu, and Gaoming Wei. 2021. "Experimental Study on Thermosensitive Hydrogel Used to Extinguish Class A Fire" Polymers 13, no. 3: 367. https://doi.org/10.3390/polym13030367
APA StyleMa, L., Huang, X., Sheng, Y., Liu, X., & Wei, G. (2021). Experimental Study on Thermosensitive Hydrogel Used to Extinguish Class A Fire. Polymers, 13(3), 367. https://doi.org/10.3390/polym13030367








