CVD Conditions for MWCNTs Production and Their Effects on the Optical and Electrical Properties of PPy/MWCNTs, PANI/MWCNTs Nanocomposites by In Situ Electropolymerization
Abstract
1. Introduction
2. Experimental Procedure
2.1. Synthesis and Purification of Carbon Nanotubes (CNT)
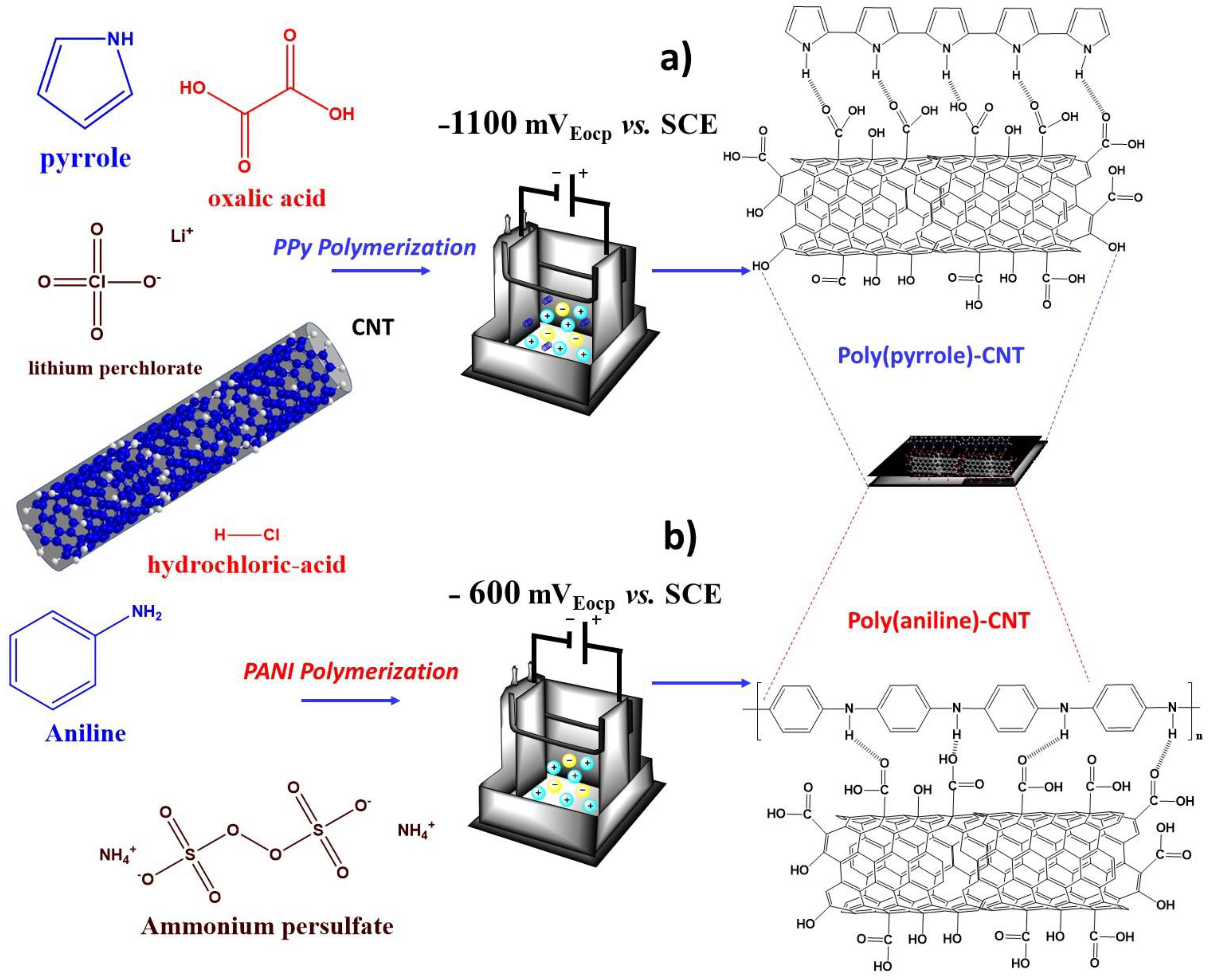
2.2. Fabrication of Polymer/MWCNTs Nanocomposites
2.3. MWCNTs and Nanocomposites Characterization
3. Results and Discussion
3.1. Characterization of MWCNTs before and after Purification Process
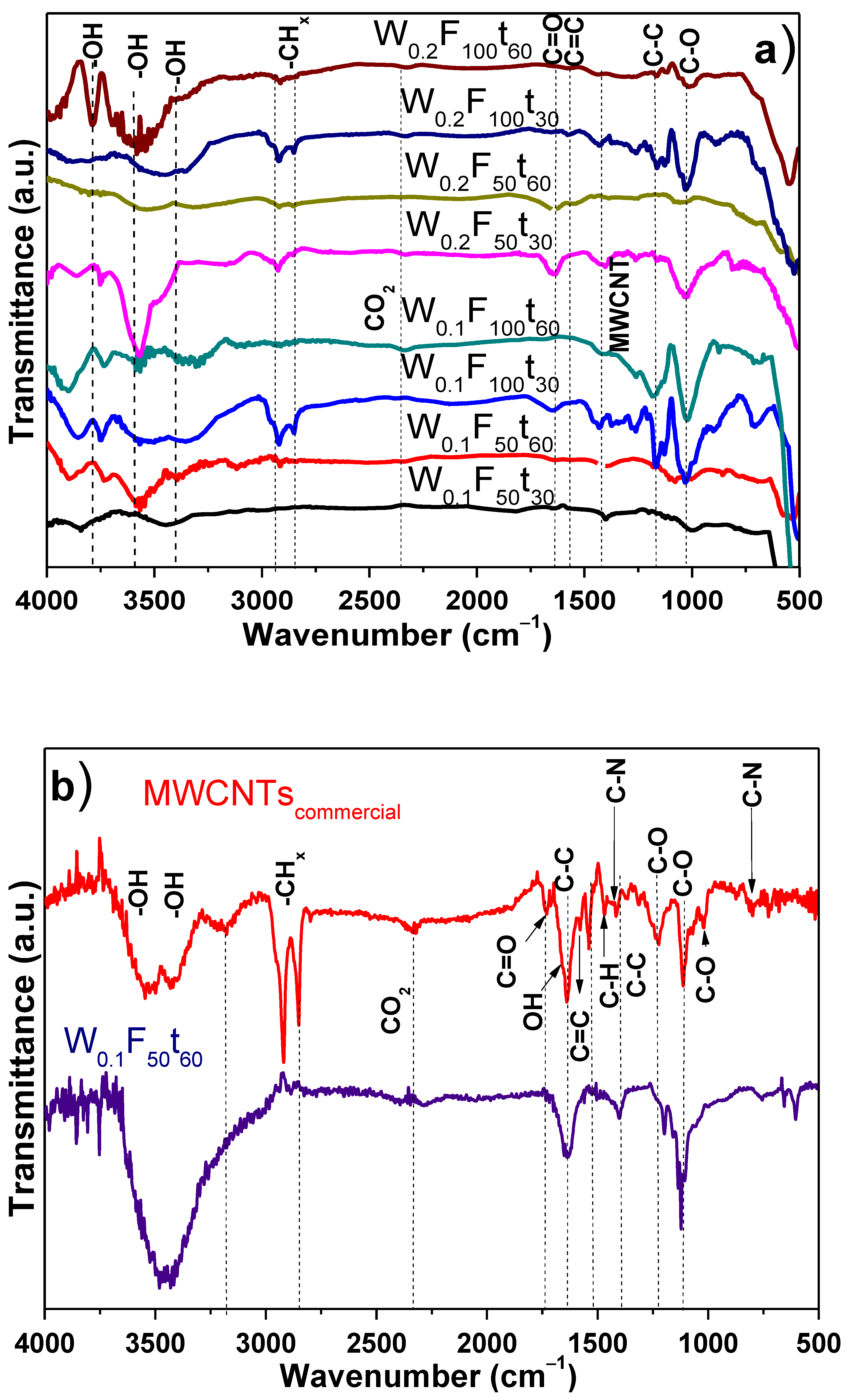
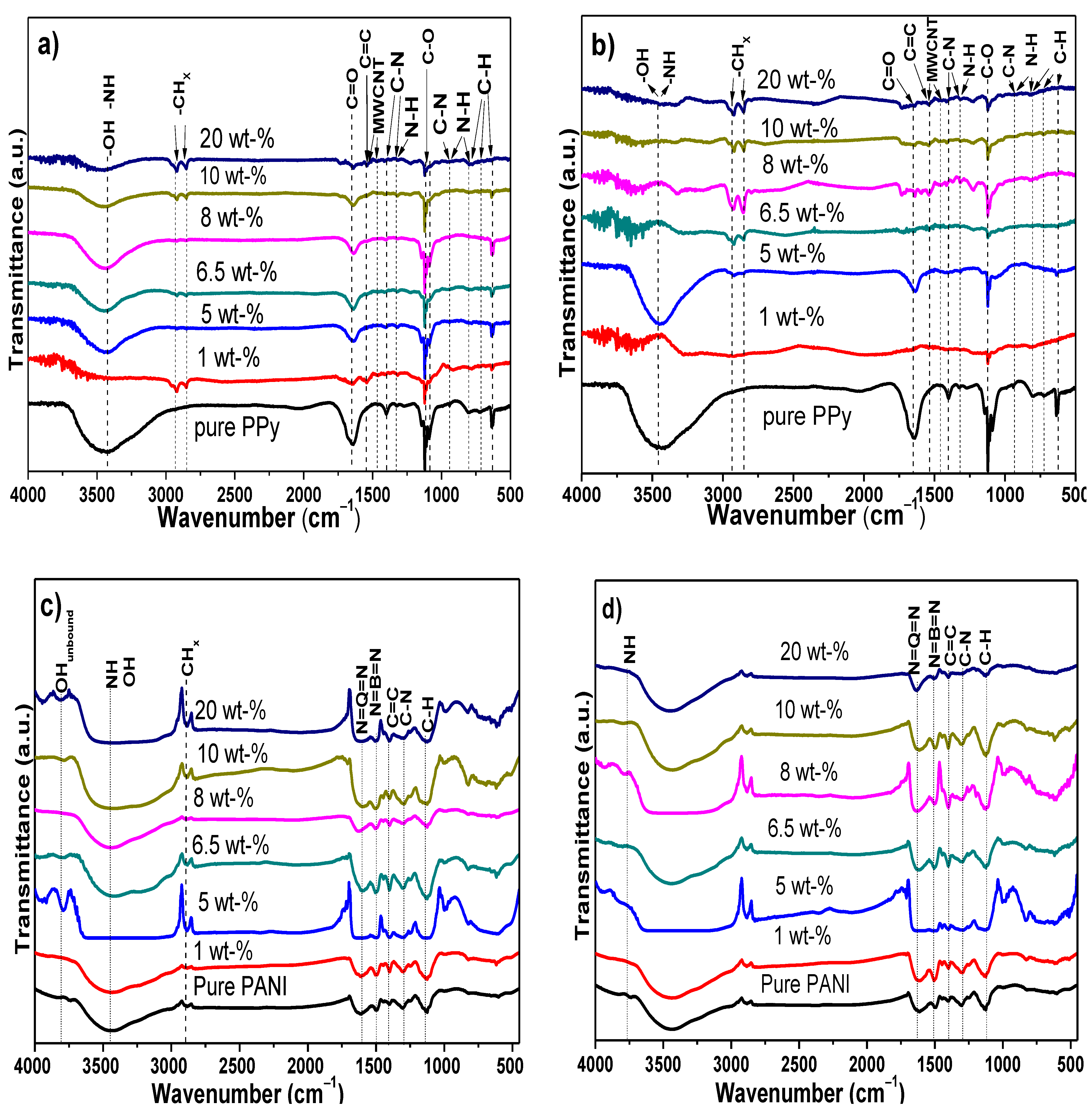
3.2. Chemical Analysis of Electrochemical PPy/MWCNTs, PANI/MWCNTs Composites
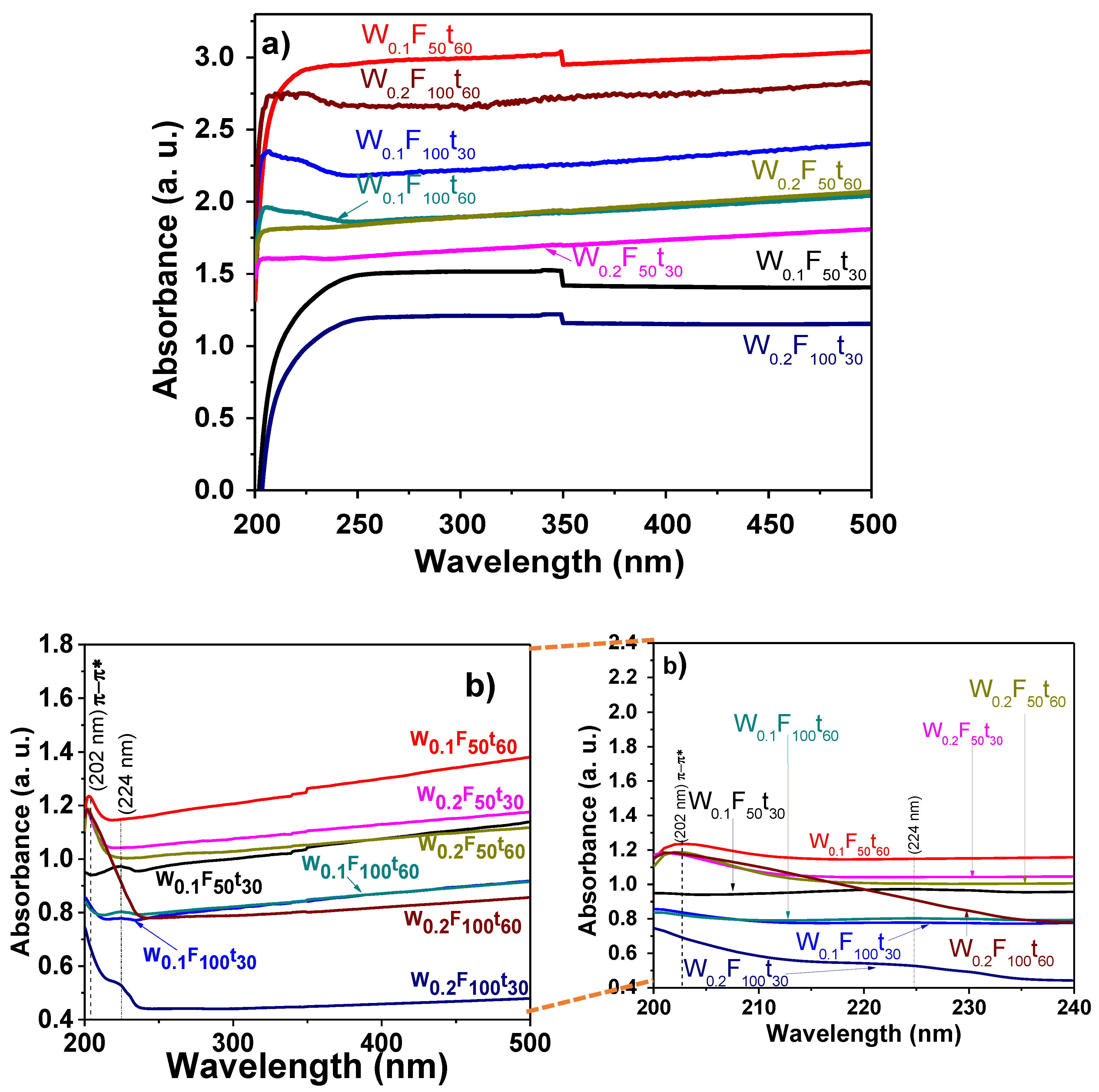
3.3. UV-Visible Optical Absorption Studies of PPy/MWCNTs, PANI/MWCNTs Nanocomposites
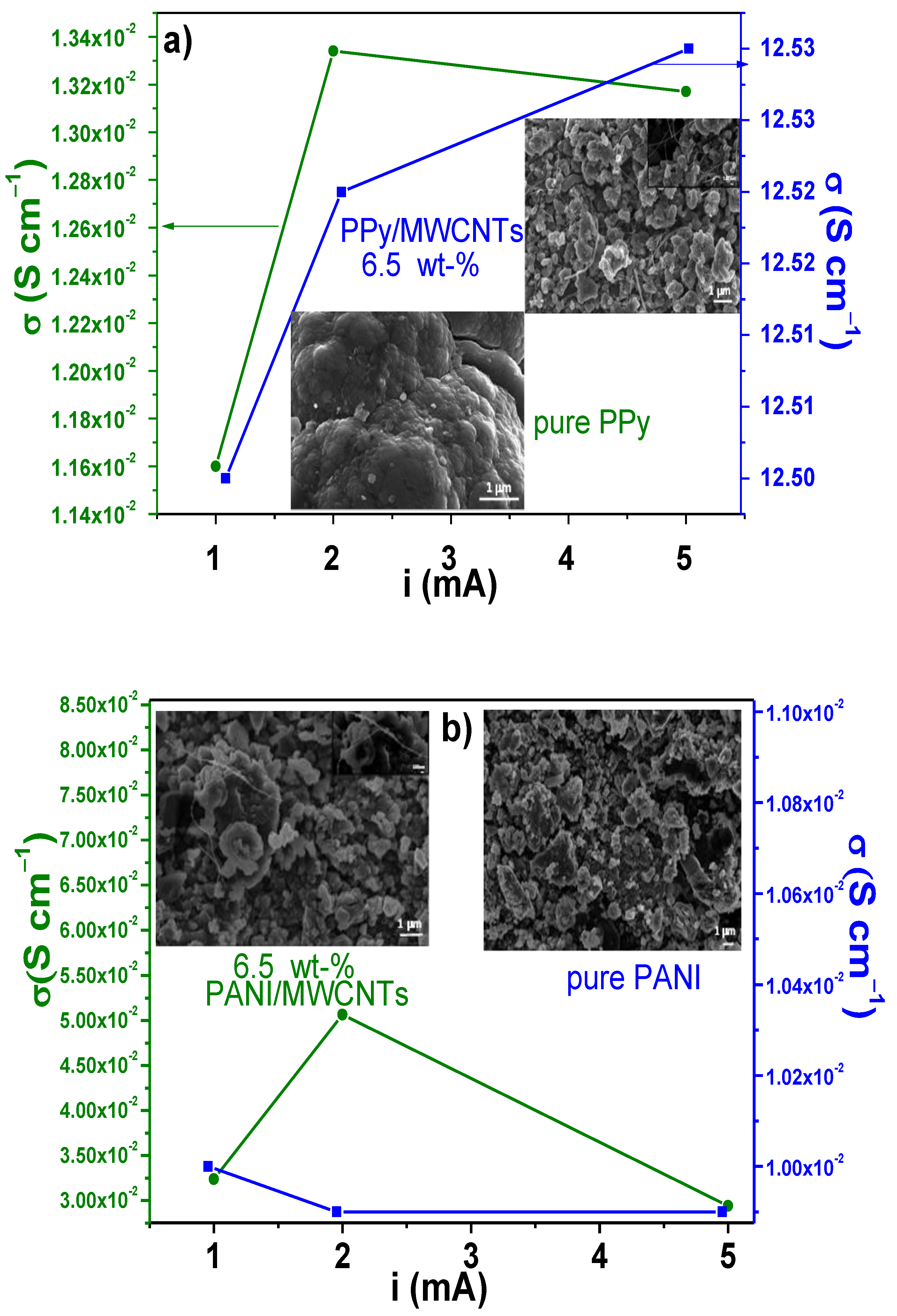
3.4. Electrical Conductivity Measurements
3.5. Hall Measurements
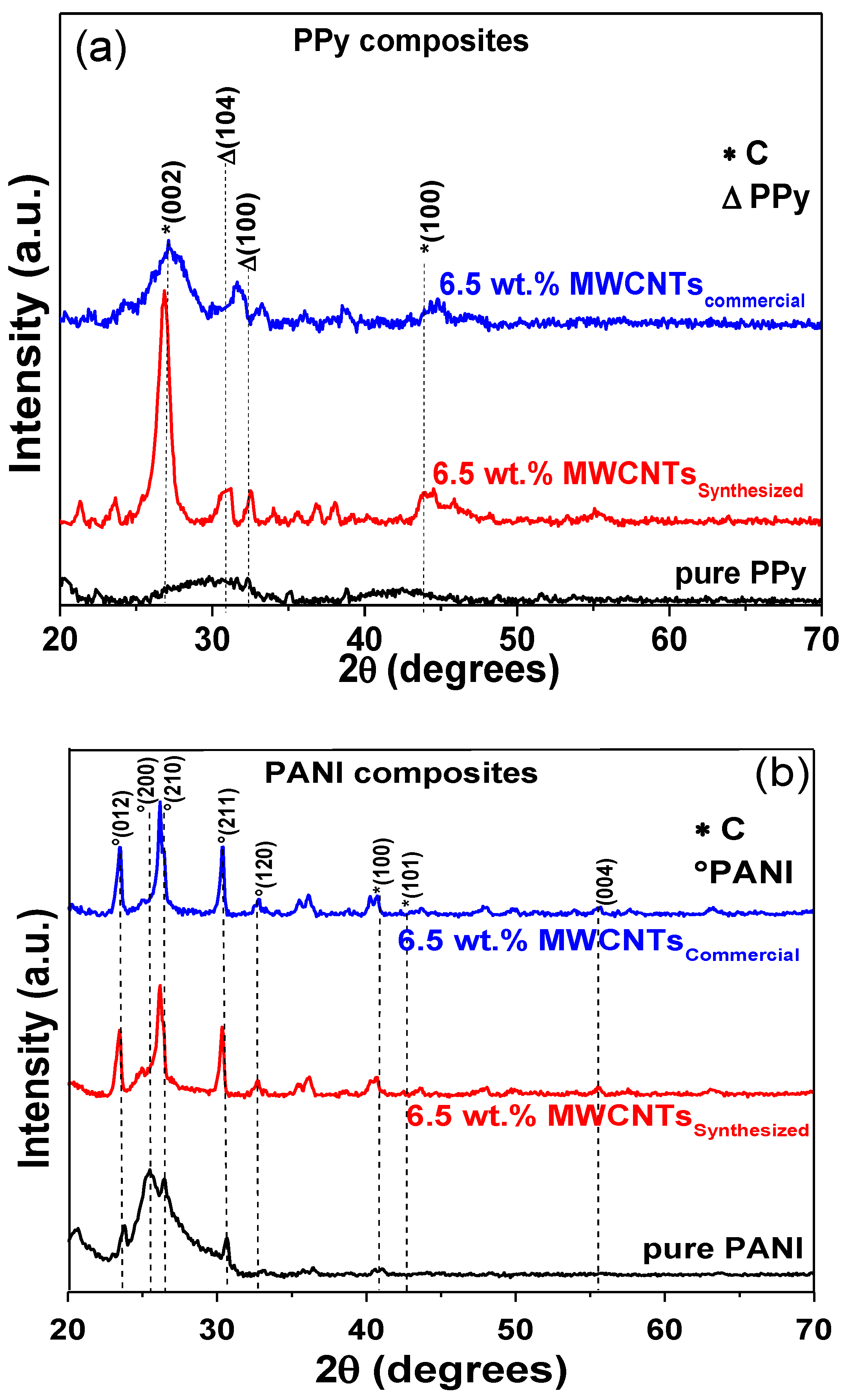
3.6. Structural Changes and Stability of Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.-X.; Yu, G.-F.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Conductive Polymer Ultrafine Fibers via Electrospinning: Preparation, Physical Properties and Applications. Prog. Mater. Sci. 2020, 100704. [Google Scholar] [CrossRef]
- Dias, O.A.T.; Konar, S.; Leão, A.L.; Sain, M. Flexible electrically conductive films based on nanofibrillated cellulose and polythiophene prepared via oxidative polymerization. Carbohydr. Polym. 2019, 220, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, W.; Tański, T.; Smok, W.; Gołombek, K.; Schab-Balcerzak, E. Effect of conductive polymers on the optical properties of electrospun polyacrylonitryle nanofibers filled by polypyrrole, polythiophene and polyaniline. Appl. Surf. Sci. 2020, 509, 145068. [Google Scholar] [CrossRef]
- Taghizadeh, A.; Taghizadeh, M.; Jouyandeh, M.; Yazdi, M.K.; Zarrintaj, P.; Saeb, M.R.; Lima, E.C.; Gupta, V.K. Conductive polymers in water treatment: A review. J. Mol. Liq. 2020, 312, 113447. [Google Scholar] [CrossRef]
- Zhao, P.; Tang, Q.; Zhao, X.; Tong, Y.; Liu, Y. Highly stable and flexible transparent conductive polymer electrode patterns for large-scale organic transistors. J. Colloid Interface Sci. 2018, 520, 58–63. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, J.; Bai, B.; Qiu, A.; Losic, D.; Shi, D.; Chen, M. Free-standing PEDOT/polyaniline conductive polymer hydrogel for flexible solid-state supercapacitors. Electrochim. Acta 2019, 322, 134769. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y. In-situ synthesis of transparent conductive PEDOT coating on PET foil by liquid phase depositional polymerization of EDOT. Synth. Met. 2016, 217, 185–188. [Google Scholar] [CrossRef]
- Norouzian, R.-S.; Lakouraj, M.M. Polyaniline-thiacalix[4]arene metallopolymer, self-doped, and externally doped conductive polymers. Prog. Org. Coat. 2020, 146, 105731. [Google Scholar] [CrossRef]
- Biswas, M.R.U.D.; Cho, K.Y.; Na, J.D.; Oh, W.-C. Highly electro-conductive graphene-decorated PANI-BiVO4 polymer-semiconductor nanocomposite with outstanding photocatalytic performance. Mater. Sci. Eng. B 2019, 251, 114469. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Hubbe, M.A.; Pal, L. Highly conductive carbon nanotubes and flexible cellulose nanofibers composite membranes with semi-interpenetrating networks structure. Carbohydr. Polym. 2019, 222, 115013. [Google Scholar] [CrossRef]
- Zamani, P.; Higgins, D.; Hassan, F.; Jiang, G.; Wu, J.; Abureden, S.; Chen, Z. Electrospun Iron–Polyaniline–Polyacrylonitrile Derived Nanofibers as Non–Precious Oxygen Reduction Reaction Catalysts for PEM Fuel Cells. Electrochim. Acta 2014, 139, 111–116. [Google Scholar] [CrossRef]
- Anwane, R.S.; Kondawar, S.B.; Late, D.J. Bessel’s polynomial fitting for electrospun polyacrylonitrile/polyaniline blend nanofibers based ammonia sensor. Mater. Lett. 2018, 221, 70–73. [Google Scholar] [CrossRef]
- Bai, H.; Zhao, L.; Lu, C.; Li, C.; Shi, G. Composite nanofibers of conducting polymers and hydrophobic insulating polymers: Preparation and sensing applications. Polymer 2009, 50, 3292–3301. [Google Scholar] [CrossRef]
- Kamble, D.B.; Sharma, A.K.; Yadav, J.B.; Patil, V.B.; Devan, R.S.; Jatratkar, A.A.; Yewale, M.A.; Ganbavle, V.V.; Pawar, S.D. Facile chemical bath deposition method for interconnected nanofibrous polythiophene thin films and their use for highly efficient room temperature NO2 sensor application. Sens. Actuators B Chem. 2017, 244, 522–530. [Google Scholar] [CrossRef]
- Jia, X.; Ge, Y.; Shao, L.; Wang, C.; Wallace, G.G. Tunable Conducting Polymers: Toward Sustainable and Versatile Batteries. ACS Sustain. Chem. Eng. 2019, 7, 14321–14340. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Rincón, M.E.; Gutierrez-Granados, S.; Chahma, M.H.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical–Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation and Electrochemical Evaluation of Polyaniline and Polypyrrole Nanocomposites Based on Glucose Oxidase and Gold Nanostructures. Polymers 2020, 12, 3026. [Google Scholar] [CrossRef]
- Barsan, M.M.; Enache, T.A.; Preda, N.; Stan, G.; Apostol, N.G.; Matei, E.; Kuncser, A.; Diculescu, V.C. Direct Immobilization of Biomolecules through Magnetic Forces on Ni Electrodes via Ni Nanoparticles: Applications in Electrochemical Biosensors. ACS Appl. Mater. Interfaces 2019, 11, 19867–19877. [Google Scholar] [CrossRef]
- Farka, Z.; Juřík, T.; Kovář, D.; Trnková, L.; Skládal, P. Nanoparticle-Based Immunochemical Biosensors and Assays: Recent Advances and Challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Li, P.; Yuan, D.; Zhou, X.; Sun, J.; Lu, X.; He, C. Interfacial control and carrier tuning of carbon nanotube/polyaniline composites for high thermoelectric performance. Carbon 2018, 136, 292–298. [Google Scholar] [CrossRef]
- Cho, C.; Wallace, K.L.; Tzeng, P.; Hsu, J.-H.; Yu, C.; Grunlan, J.C. Outstanding Low Temperature Thermoelectric Power Factor from Completely Organic Thin Films Enabled by Multidimensional Conjugated Nanomaterials. Adv. Energy Mater. 2016, 6, 1502168. [Google Scholar] [CrossRef]
- Toshima, N. Recent progress of organic and hybrid thermoelectric materials. Synth. Met. 2017, 225, 3–21. [Google Scholar] [CrossRef]
- Victorious, A.; Clifford, A.; Saha, S.; Zhitomirsky, I.; Soleymani, L. Integrating TiO2 Nanoparticles within a Catecholic Polymeric Network Enhances the Photoelectrochemical Response of Biosensors. J. Phys. Chem. C 2019, 123, 16186–16193. [Google Scholar] [CrossRef]
- Barkauskas, J.; Mikoliunaite, L.; Paklonskaite, I.; Genys, P.; Petroniene, J.J.; Morkvenaite-Vilkonciene, I.; Ramanaviciene, A.; Samukaite-Bubniene, U.; Ramanavicius, A. Single-walled carbon nanotube based coating modified with reduced graphene oxide for the design of amperometric biosensors. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, J.; Gao, D.; Zhou, Y.; Li, C.; Zha, J.; Zhang, J. Preparation of amino-functionalized graphene oxide by Hoffman rearrangement and its performances on polyacrylate coating latex. Prog. Org. Coat. 2016, 94, 9–17. [Google Scholar] [CrossRef]
- Xu, R.; Wei, J.; Guo, F.; Cui, X.; Zhang, T.; Zhu, H.; Wang, K.; Wu, D. Highly conductive, twistable and bendable polypyrrole–carbon nanotube fiber for efficient supercapacitor electrodes. RSC Adv. 2015, 5, 22015–22021. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A review and analysis of electrical percolation in carbon nanotube polymer composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Matos, M.A.S.; Tagarielli, V.L.; Baiz-Villafranca, P.M.; Pinho, S.T. Predictions of the electro-mechanical response of conductive CNT-polymer composites. J. Mech. Phys. Solids 2018, 114, 84–96. [Google Scholar] [CrossRef]
- Kazazi, M. High-performance electrode based on electrochemical polymerization of polypyrrole film on electrophoretically deposited CNTs conductive framework for supercapacitors. Solid State Ion. 2019, 336, 80–86. [Google Scholar] [CrossRef]
- Kazazi, M.; Vaezi, M.R.; Kazemzadeh, A. Enhanced rate performance of polypyrrole-coated sulfur/MWCNT cathode material: A kinetic study by electrochemical impedance spectroscopy. Ionics 2014, 20, 635–643. [Google Scholar] [CrossRef]
- Zheng, B.; Li, Y.; Liu, J. CVD synthesis and purification of single-walled carbon nanotubes on aerogel-supported catalyst. Appl. Phys. A Mater. Sci. Process. 2002, 74, 345–348. [Google Scholar] [CrossRef]
- Ando, Y.; Zhao, X.; Sugai, T.; Kumar, M. Growing carbon nanotubes. Mater. Today 2004, 7, 22–29. [Google Scholar] [CrossRef]
- Novoselova, I.; Oliinyk, N.F.; Volkov, S.; Konchits, A.; Yanchuk, I.; Yefanov, V.S.; Kolesnik, S.P.; Karpets, M. Electrolytic synthesis of carbon nanotubes from carbon dioxide in molten salts and their characterization. Phys. E Low-Dimens. Syst. Nanostruct. 2008, 40, 2231–2237. [Google Scholar] [CrossRef]
- Flamant, G.; Laplaze, D. Solar synthesis of single-walled carbon nanotubes at medium scale. Carbon 2005, 43, 2302–2310. [Google Scholar] [CrossRef]
- Viculis, L.M.; Mack, J.J.; Kaner, R.B. A chemical route to carbon nanoscrolls. Science 2003, 299, 1361. [Google Scholar] [CrossRef]
- Chen, Y.; Conway, M.; Fitz gerald, J.; Williams, J.S.; Chadderton, L.T. The nucleation and growth of carbon nanotubes in a mechano-thermal process. Carbon 2004, 42, 1543–1548. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, T.; Liu, Q.; Chen, Y.; Zhang, G.; Xiong, H.; Zhang, H. The effect of nickel content of composite catalysts synthesized by hydrothermal method on the preparation of carbon nanotubes. Mater. Sci. Eng. B 2006, 127, 198–202. [Google Scholar] [CrossRef]
- Varshney, D.; Weiner, B.R.; Morell, G. Growth and field emission study of a monolithic carbon nanotube/diamond composite. Carbon 2010, 48, 3353–3358. [Google Scholar] [CrossRef]
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for carbon nanotubes synthesis—Review. J. Mater. Chem. 2011, 21, 15872–15884. [Google Scholar] [CrossRef]
- Domínguez-Crespo, M.A.; Torres-Huerta, A.; Onofre-Bustamante, E.; Alanis-Valdelamar, A.; Escudero, M.; Brachetti, B. Corrosion studies of PPy/Ni organic–inorganic hybrid bilayer coatings on commercial carbon steel. J. Solid State Electrochem. 2014, 19, 1073–1089. [Google Scholar] [CrossRef]
- Cabezas, A.L.; Zhang, Z.-B.; Zheng, L.-R.; Zhang, S.-L. Morphological development of nanofibrillar composites of polyaniline and carbon nanotubes. Synth. Met. 2010, 160, 664–668. [Google Scholar] [CrossRef]
- Duc Vu Quyen, N.; Quang Khieu, D.; Tuyen, T.N.; Xuan Tin, D.; Thi Hoang Diem, B. Carbon Nanotubes: Synthesis via Chemical Vapour Deposition without Hydrogen, Surface Modification, and Application. J. Chem. 2019, 2019, 4260153. [Google Scholar] [CrossRef]
- Bhatia, R.; Prasad, V. Synthesis of multiwall carbon nanotubes by chemical vapor deposition of ferrocene alone. Solid State Commun. 2010, 150, 311–315. [Google Scholar] [CrossRef]
- Öncel, Ç.; Yürüm, Y. Carbon Nanotube Synthesis via the Catalytic CVD Method: A Review on the Effect of Reaction Parameters. Fuller. Nanotub. Carbon Nanostruct. 2006, 14, 17–37. [Google Scholar] [CrossRef]
- Yu, J.; Lucas, J.; Strezov, V.; Wall, T. Coal and carbon nanotube production. Fuel 2003, 82, 2025–2032. [Google Scholar] [CrossRef]
- Maruyama, S.; Kojima, R.; Miyauchi, Y.; Chiashi, S.; Kohno, M. Low-temperature synthesis of high-purity single-walled carbon nanotubes from alcohol. Chem. Phys. Lett. 2002, 360, 229–234. [Google Scholar] [CrossRef]
- Zhao, N.; He, C.; Li, J.; Jiang, Z.; Li, Y. Study on purification and tip-opening of CNTs fabricated by CVD. Mater. Res. Bull. 2006, 41, 2204–2209. [Google Scholar] [CrossRef]
- Salaeh, S.; Thitithammawong, A.; Salae, A. Highly enhanced electrical and mechanical properties of methyl methacrylate modified natural rubber filled with multiwalled carbon nanotubes. Polym. Test. 2020, 85, 106417. [Google Scholar] [CrossRef]
- Yu, L.; Shearer, C.; Shapter, J. Recent Development of Carbon Nanotube Transparent Conductive Films. Chem. Rev. 2016, 116, 13413–13453. [Google Scholar] [CrossRef]
- Min, C.; Shen, X.; Shi, Z.; Chen, L.; Xu, Z. The Electrical Properties and Conducting Mechanisms of Carbon Nanotube/Polymer Nanocomposites: A Review. Polym. Plast. Technol. Eng. 2010, 49, 1172–1181. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.-Z.; Mai, Y.-W. Electrically conductive and super-tough polyamide-based nanocomposites. Polymer 2009, 50, 4112–4121. [Google Scholar] [CrossRef]
- Yang, K.; Han, H.; Pan, X.; Chen, N.; Gu, M. The effect of chemical treatment on the crystallinity of multi-walled carbon nanotubes. J. Phys. Chem. Solids 2008, 69, 222–229. [Google Scholar] [CrossRef]
- Yuan, J.-M.; Chen, X.-H.; Chen, X.-H.; Fan, Z.-F.; Yang, X.-G.; Chen, Z.-H. An easy method for purifying multi-walled carbon nanotubes by chlorine oxidation. Carbon 2008, 46, 1266–1269. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Azodpour, J.; Baniadam, M. Microwave assisted purification of multi-walled carbon nanotubes by potassium permanganate; effect of acid to oxidant molar ratio and treatment time. Diam. Relat. Mater. 2019, 98, 107485. [Google Scholar] [CrossRef]
- Tan, J.M.; Karthivashan, G.; Arulselvan, P.; Fakurazi, S.; Hussein, M.Z. Characterization and In Vitro Sustained Release of Silibinin from pH Responsive Carbon Nanotube-Based Drug Delivery System. J. Nanomater. 2014, 2014, 439873. [Google Scholar] [CrossRef]
- Bankole, M.T.; Abdulkareem, A.S.; Mohammed, I.A.; Ochigbo, S.S.; Tijani, J.O.; Abubakre, O.K.; Roos, W.D. Selected Heavy Metals Removal From Electroplating Wastewater by Purified and Polyhydroxylbutyrate Functionalized Carbon Nanotubes Adsorbents. Sci. Rep. 2019, 9, 4475. [Google Scholar] [CrossRef]
- Granados-Martínez, F.G.; Domratcheva-Lvova, L.; Flores-Ramírez, N.; García-González, L.; Zamora-Peredo, L.; Mondragón-Sánchez, M.d.L. Composite Films from Polystyrene with Hydroxyl end Groups and Carbon Nanotubes. Mater. Res. 2016, 19, 133–138. [Google Scholar] [CrossRef]
- Ahmed, D.S.; Haider, A.J.; Mohammad, M.R. Comparesion of Functionalization of Multi-Walled Carbon Nanotubes Treated by Oil Olive and Nitric Acid and their Characterization. Energy Procedia 2013, 36, 1111–1118. [Google Scholar] [CrossRef]
- Hajighorbani, M.; Hekmati, M. Pd nanoparticles deposited on Isoniazid grafted multi walled carbon nanotubes: Synthesis, characterization and application for Suzuki reaction in aqueous media. RSC Adv. 2016, 6, 88916–88924. [Google Scholar] [CrossRef]
- Mohammadi, M.; Garmarudi, A.B.; Khanmohammadi, M.; Rouchi, M.B. Infrared spectrometric evaluation of carbon nanotube sulfonation. Fuller. Nanotub. Carbon Nanostruct. 2016, 24, 219–224. [Google Scholar] [CrossRef]
- Matranga, C.; Chen, L.; Smith, M.; Bittner, E.; Johnson, J.K.; Bockrath, B. Trapped CO2 in Carbon Nanotube Bundles. J. Phys. Chem. B 2003, 107, 12930–12941. [Google Scholar] [CrossRef]
- Matranga, C.; Bockrath, B. Permanent Trapping of CO2 in Single-Walled Carbon Nanotubes Synthesized by the HiPco Process. J. Phys. Chem. B 2004, 108, 6170–6174. [Google Scholar] [CrossRef] [PubMed]
- Osswald, S.; Havel, M.; Gogotsi, Y. Monitoring oxidation of multiwalled carbon nanotubes by Raman spectroscopy. J. Raman Spectrosc. 2007, 38, 728–736. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Garg, P.; Singh, B.P.; Kumar, G.; Gupta, T.; Pandey, I.; Seth, R.K.; Tandon, R.P.; Mathur, R.B. Effect of dispersion conditions on the mechanical properties of multi-walled carbon nanotubes based epoxy resin composites. J. Polym. Res. 2011, 18, 1397–1407. [Google Scholar] [CrossRef]
- Misra, A.; Tyagi, P.K.; Rai, P.; Misra, D.S. FTIR spectroscopy of multiwalled carbon nanotubes: A simple approach to study the nitrogen doping. J. Nanosci. Nanotechnol. 2007, 7, 1820–1823. [Google Scholar] [CrossRef]
- Kastner, J.; Pichler, T.; Kuzmany, H.; Curran, S.; Blau, W.; Weldon, D.N.; Delamesiere, M.; Draper, S.; Zandbergen, H. Resonance Raman and infrared spectroscopy of carbon nanotubes. Chem. Phys. Lett. 1994, 221, 53–58. [Google Scholar] [CrossRef]
- Njuguna, J.; Vanli, O.A.; Liang, R. A Review of Spectral Methods for Dispersion Characterization of Carbon Nanotubes in Aqueous Suspensions. J. Spectrosc. 2015, 2015, 463156. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, L.; Sun, J. Production of aqueous colloidal dispersions of carbon nanotubes. J. Colloid Interface Sci. 2003, 260, 89–94. [Google Scholar] [CrossRef]
- Yu, J.; Grossiord, N.; Koning, C.E.; Loos, J. Controlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solution. Carbon 2007, 45, 618–623. [Google Scholar] [CrossRef]
- Ryabenko, A.G.; Dorofeeva, T.V.; Zvereva, G.I. UV–VIS–NIR spectroscopy study of sensitivity of single-wall carbon nanotubes to chemical processing and Van-der-Waals SWNT/SWNT interaction. Verification of the SWNT content measurements by absorption spectroscopy. Carbon 2004, 42, 1523–1535. [Google Scholar] [CrossRef]
- Mendoza-Cachú, D.; López-Miranda, J.L.; Mercado-Zúñiga, C.; Rosas, G. Functionalization of MWCNTs with Ag-AuNPs by a green method and their catalytic properties. Diam. Relat. Mater. 2018, 84, 26–31. [Google Scholar] [CrossRef]
- Meng, J.; Yang, M.; Song, L.; Kong, H.; Wang, C.Y.; Wang, R.; Wang, C.; Xie, S.S.; Xu, H.Y. Concentration control of carbon nanotubes in aqueous solution and its influence on the growth behavior of fibroblasts. Colloids Surf. B Biointerfaces 2009, 71, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, I.A.; Euler, W.B.; Karachevtsev, V.A. Photophysics of Carbon Nanotubes Interfaced with Organic and Inorganic Materials; Springer: London, UK, 2012. [Google Scholar]
- He, Z.; Lan, X.; Chen, F.; Wang, K.; Deng, H.; Zhang, Q.; Fu, Q. Effect of surface wettability on transparency in different water conditions. J. Coat. Technol. Res. 2013, 10, 641–647. [Google Scholar] [CrossRef]
- Liu, H.; Zhai, J.; Jiang, L. Wetting and anti-wetting on aligned carbon nanotube films. Soft Matter 2006, 2, 811–821. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, K.K.; Jeong, S.J.; An, K.H.; Lee, S.H.; Lee, Y.H. Optical absorption spectroscopy for determining carbon nanotube concentration in solution. Synth. Met. 2007, 157, 570–574. [Google Scholar] [CrossRef]
- Lin, M.F.; Shung, K.W.K. Plasmons and optical properties of carbon nanotubes. Phys. Rev. B 1994, 50, 17744–17747. [Google Scholar] [CrossRef]
- Rance, G.A.; Marsh, D.H.; Nicholas, R.J.; Khlobystov, A.N. UV–vis absorption spectroscopy of carbon nanotubes: Relationship between the π-electron plasmon and nanotube diameter. Chem. Phys. Lett. 2010, 493, 19–23. [Google Scholar] [CrossRef]
- Escobar, B.; Barbosa, R.; Miki Yoshida, M.; Verde Gomez, Y. Carbon nanotubes as support of well dispersed platinum nanoparticles via colloidal synthesis. J. Power Sources 2013, 243, 88–94. [Google Scholar] [CrossRef]
- Zeferino González, I.; Valenzuela-Muñiz, A.M.; Gauvin, R.; Miki-Yoshida, M.; Verde-Gómez, Y. Influence of the synthesis temperature and silicon concentration on the properties of Si doped MWCNT. Diam. Relat. Mater. 2020, 104, 107743. [Google Scholar] [CrossRef]
- Wang, R.; Wu, H.; Chen, R.; Chi, Y. Strong Electrochemiluminescence Emission from Oxidized Multiwalled Carbon Nanotubes. Small 2019, 15, 1901550. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on Carbon Nanotubes and Graphene Raman Spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Audiffred, M.; Elías, A.L.; Gutiérrez, H.R.; López-Urías, F.; Terrones, H.; Merino, G.; Terrones, M. Nitrogen–Silicon Heterodoping of Carbon Nanotubes. J. Phys. Chem. C 2013, 117, 8481–8490. [Google Scholar] [CrossRef]
- Darne, C.; Desforges, A.; Berrada, N.; Fontana, C.; Guichard, Y.; Gaté, L.; Bégin, D.; Le Normand, F.; Valsaque, F.; Ghanbaja, J.; et al. A non-damaging purification method: Decoupling the toxicity of multi-walled carbon nanotubes and their associated metal impurities. Environ. Sci. Nano 2019, 6, 1852–1865. [Google Scholar] [CrossRef]
- Zdrojek, M.; Gebicki, W.; Jastrzebski, C.; Melin, T.; Huczko, A. Studies of Multiwall Carbon Nanotubes Using Raman Spectroscopy and Atomic Force Microscopy. Solid State Phenom. 2004, 99, 265–268. [Google Scholar] [CrossRef]
- Mammadova, S.A.; Huseynov, A.B.; Israfilov, A.O. Synthesis and Optical Properties of Iodinated Multi-walled Carbon Nanotubes. Opt. Spectrosc. 2018, 125, 921–927. [Google Scholar] [CrossRef]
- Ezz, A.A.; Kamel, M.M.; Saad, G.R. Synthesis and characterization of nanocarbon having different morphological structures by chemical vapor deposition over Fe-Ni-Co-Mo/MgO catalyst. J. Saudi Chem. Soc. 2019, 23, 666–677. [Google Scholar] [CrossRef]
- Qi, X.; Zhong, W.; Deng, Y.; Au, C.; Du, Y. Synthesis of helical carbon nanotubes, worm-like carbon nanotubes and nanocoils at 450 °C and their magnetic properties. Carbon 2010, 48, 365–376. [Google Scholar] [CrossRef]
- Cox, B.J.; Hill, J.M. New Carbon Molecules in the Form of Elbow-Connected Nanotori. J. Phys. Chem. C 2007, 111, 10855–10860. [Google Scholar] [CrossRef]
- Zhao, W.; Shan, C.; Elias, A.L.; Rajukumar, L.P.; O’Brien, D.J.; Terrones, M.; Wei, B.; Suhr, J.; Lu, X.L. Hyperelasticity of three-dimensional carbon nanotube sponge controlled by the stiffness of covalent junctions. Carbon 2015, 95, 640–645. [Google Scholar] [CrossRef]
- Vanyorek, L.; Sikora, E.; Balogh, T.; Román, K.; Marossy, K.; Pekker, P.; Szabó, T.J.; Viskolcz, B.; Fiser, B. Nanotubes as polymer composite reinforcing additive materials—A comparative study. Arab. J. Chem. 2020, 13, 3775–3782. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, M.; Nakayama, Y. Growth mechanism of carbon nanocoils. J. Appl. Phys. 2002, 91, 10058–10061. [Google Scholar] [CrossRef]
- Baba, M.; Sano, H.; Zheng, G.-B.; Uchiyama, Y. Effect of Mo in Co-Mo/MgO catalysts on the synthesis yield and structure of carbon nanotubes. J. Ceram. Soc. Jpn. 2009, 117, 654–658. [Google Scholar] [CrossRef]
- Kostić, R.; Raković, D.; Stepanyan, S.A.; Davidova, I.E.; Gribov, L.A. Vibrational spectroscopy of polypyrrole, theoretical study. J. Chem. Phys. 1995, 102, 3104–3109. [Google Scholar] [CrossRef]
- Minisy, I.M.; Gavrilov, N.; Acharya, U.; Morávková, Z.; Unterweger, C.; Mičušík, M.; Filippov, S.K.; Kredatusová, J.; Pašti, I.A.; Breitenbach, S.; et al. Tailoring of carbonized polypyrrole nanotubes core by different polypyrrole shells for oxygen reduction reaction selectivity modification. J. Colloid Interface Sci. 2019, 551, 184–194. [Google Scholar] [CrossRef]
- Hien, H.T.; Van Tuan, C.; Anh Thu, D.T.; Ngan, P.Q.; Thai, G.H.; Doanh, S.C.; Giang, H.T.; Van, N.D.; Trung, T. Influence of surface morphology and doping of PPy film simultaneously polymerized by vapour phase oxidation on gas sensing. Synth. Met. 2019, 250, 35–41. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. A facile method for synthesis of polyaniline nanospheres and effect of doping on their electrical conductivity. Polym. Int. 2011, 60, 1291–1295. [Google Scholar] [CrossRef]
- Al-Hussaini, A.S.; Elias, A.M.; Abd El-Ghaffar, M.A. New Poly(aniline-co-o-phenylenediamine)/Kaolinite Microcomposites for Water Decontamination. J. Polym. Environ. 2017, 25, 35–45. [Google Scholar] [CrossRef]
- Karbownik, I.; Rac-Rumijowska, O.; Fiedot-Toboła, M.; Rybicki, T.; Teterycz, H. The Preparation and Characterization of Polyacrylonitrile-Polyaniline (PAN/PANI) Fibers. Materials 2019, 12, 664. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, G.M.; Codognoto, L.; Simões, F.R. Self-assembled electrodes based on polyaniline grafted with reduced graphene oxide and polystyrene sulfonate. J. Solid State Electrochem. 2020, 24, 1857–1866. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Resonance Raman Spectroscopy of Conducting Polypyrrole Nanotubes: Disordered Surface versus Ordered Body. J. Phys. Chem. A 2018, 122, 9298–9306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xiao, F.; Dong, Z.H.; Shi, W. Synthesis of polypyrrole wrapped graphene hydrogels composites as supercapacitor electrodes. Electrochim. Acta 2013, 114, 125–132. [Google Scholar] [CrossRef]
- Kumar, V.; Mahajan, R.; Bhatnagar, D.; Kaur, I. Nanofibers synthesis of ND:PANI composite by liquid/liquid interfacial polymerization and study on the effect of NDs on growth mechanism of nanofibers. Eur. Polym. J. 2016, 83, 1–9. [Google Scholar] [CrossRef]
- Shao, D.; Hu, J.; Chen, C.; Sheng, G.; Ren, X.; Wang, X. Polyaniline Multiwalled Carbon Nanotube Magnetic Composite Prepared by Plasma-Induced Graft Technique and Its Application for Removal of Aniline and Phenol. J. Phys. Chem. C 2010, 114, 21524–21530. [Google Scholar] [CrossRef]
- Thakur, A.K.; Deshmukh, A.B.; Choudhary, R.B.; Karbhal, I.; Majumder, M.; Shelke, M.V. Facile synthesis and electrochemical evaluation of PANI/CNT/MoS2 ternary composite as an electrode material for high performance supercapacitor. Mater. Sci. Eng. B 2017, 223, 24–34. [Google Scholar] [CrossRef]
- Saheeda, P.; Jayaleksmi, S. Liquid/liquid interfacial polymerization as an effective synthesis approach for polypyrrole/MWCNTs nanocomposite with impressive nonlinear optical properties. Opt. Mater. 2020, 104, 109940. [Google Scholar] [CrossRef]
- Shi, K.; Zhitomirsky, I. Polypyrrole nanofiber–carbon nanotube electrodes for supercapacitors with high mass loading obtained using an organic dye as a co-dispersant. J. Mater. Chem. A 2013, 1, 11614–11622. [Google Scholar] [CrossRef]
- Patil, B.H.; Shinde, S.S.; Lokhande, C.D. Synthesis of polypyrrole thin film by SILAR method for supercapacitor application. AIP Conf. Proc. 2013, 1536, 1179–1180. [Google Scholar] [CrossRef]
- Thakur, A.V.; Lokhande, B.J. Effect of the molar concentration of pyrrole monomer on the rate of polymerization, growth and hence the electrochemical behavior of highly pristine PPy flexible electrodes. Heliyon 2019, 5, e02909. [Google Scholar] [CrossRef] [PubMed]
- Huyen, D.N.; Tung, N.T.; Vinh, T.D.; Thien, N.D. Synergistic Effects in the Gas Sensitivity of Polypyrrole/Single Wall Carbon Nanotube Composites. Sensors 2012, 12, 7965–7974. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation of Polyaniline and Polypyrrole Nanocomposites with Embedded Glucose Oxidase and Gold Nanoparticles. Polymers 2019, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Monfared, A.; Jamshidi, M. Synthesis of polyaniline/titanium dioxide nanocomposite (PAni/TiO2) and its application as photocatalyst in acrylic pseudo paint for benzene removal under UV/VIS lights. Prog. Org. Coat. 2019, 136, 105257. [Google Scholar] [CrossRef]
- Salem, A.A.; Grgur, B.N. Corrosion of Mild Steel with Composite Alkyd Polyanilinebenzoate Coating. Int. J. Electrochem. Sci. 2017, 12, 8683–8694. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, R.; Liao, X.; Ma, Z.; Huang, Y.; Li, Q. Polyaniline/Copper Composite Anode Current Collectors Prepared through Electrochemical Polymerization for Lithium-Ion Batteries. ChemElectroChem 2020, 7, 2896–2904. [Google Scholar] [CrossRef]
- Long, Y.-Z.; Chen, Z.; Zhang, X.; Zhang, J.; Liu, Z. Electrical properties of multi-walled carbon nanotube/polypyrrole nanocables: Percolation-dominated conductivity. J. Phys. D Appl. Phys. 2004, 37, 1965. [Google Scholar] [CrossRef]
- Imani, A.; Farzi, G.; Ltaief, A. Facile synthesis and characterization of polypyrrole-multiwalled carbon nanotubes by in situ oxidative polymerization. Int. Nano Lett. 2013, 3, 52. [Google Scholar] [CrossRef]
- Smitha, M.G.; Murugendrappa, M.V. Structural, Electrical, Thermal and Transport Properties of Poly Pyrrole/La0.7Ca0.3MnO3 Perovskite Manganite Nano Composite Studies Above Room Temperature. J. Inorg. Organomet. Polym. Mater. 2020, 30, 841–858. [Google Scholar] [CrossRef]
- Wu, T.-M.; Chang, H.-L.; Lin, Y.-W. Synthesis and characterization of conductive polypyrrole/multi-walled carbon nanotubes composites with improved solubility and conductivity. Compos. Sci. Technol. 2009, 69, 639–644. [Google Scholar] [CrossRef]
- Lim, T.; Oh, K.W.; Kim, S. Polypyrrole/MWCNT-gr-PSSA Composite for Flexible and Highly Conductive Transparent Film. J. Appl. Polym. Sci. 2012, 123, 388–397. [Google Scholar] [CrossRef]
- Milakin, K.A.; Capáková, Z.; Acharya, U.; Vajďák, J.; Morávková, Z.; Hodan, J.; Humpolíček, P.; Bober, P. Biocompatible and antibacterial gelatin-based polypyrrole cryogels. Polymer 2020, 197, 122491. [Google Scholar] [CrossRef]
- Li, Y.; Bober, P.; Trchová, M.; Stejskal, J. Polypyrrole prepared in the presence of methyl orange and ethyl orange: Nanotubes versus globules in conductivity enhancement. J. Mater. Chem. C 2017, 5, 4236–4245. [Google Scholar] [CrossRef]
- Stejskal, J.; Prokeš, J. Conductivity and morphology of polyaniline and polypyrrole prepared in the presence of organic dyes. Synth. Met. 2020, 264, 116373. [Google Scholar] [CrossRef]
- Karaer Yağmur, H. Synthesis and characterization of conducting polypyrrole/bentonite nanocomposites and in-situ oxidative polymerization of pyrrole: Adsorption of 4-nitrophenol by polypyrrole/bentonite nanocomposite. Chem. Eng. Commun. 2020, 207, 1171–1183. [Google Scholar] [CrossRef]
- Gomes, E.C.L.; Oliveira, M.A.S. Chemical Polymerization of Aniline in Hydrochloric Acid (HCl) and Formic Acid (HCOOH) Media. Differences Between the Two Synthesized Polyanilines. Am. J. Polym. Sci. 2012, 2, 5–13. [Google Scholar] [CrossRef]
- Bahrami, A.; Talib, Z.A.; Yunus, W.M.M.; Behzad, K.; Abdi, M.; Din, F.U. Low temperature hall effect investigation of conducting polymer-carbon nanotubes composite network. Int. J. Mol. Sci. 2012, 13, 14917–14928. [Google Scholar] [CrossRef]
- Kaneto, K.; Yoshino, K. Hall Effect and Reflectance in Conducting Polypyrrole Films. J. Phys. Soc. Jpn. 1986, 55, 4568–4569. [Google Scholar] [CrossRef]
- Xie, J.; Pan, W.; Guo, Z.; Jiao, S.S.; Ping Yang, L. In situ polymerization of polypyrrole on cotton fabrics as flexible electrothermal materials. J. Eng. Fibers Fabr. 2019, 14. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, G.-S.; Yu, Z.-Z.; Qi, J.-S. The effect of graphite oxide on the thermoelectric properties of polyaniline. Carbon 2012, 50, 3064–3073. [Google Scholar] [CrossRef]
- Zhang, A.-Q.; Xiao, Y.-H.; Lu, L.-Z.; Wang, L.-Z.; Li, F. Polypyrrole/MnO2 composites and their enhanced electrochemical capacitance. J. Appl. Polym. Sci. 2013, 128, 1327–1331. [Google Scholar] [CrossRef]
- Zhang, A.-Q.; Zhang, Y.; Wang, L.-Z.; Li, X.-F. Electrosynthesis and capacitive performance of polyaniline–polypyrrole composite. Polym. Compos. 2011, 32, 1–5. [Google Scholar] [CrossRef]
- Daş, E.; Yurtcan, A.B. Effect of carbon ratio in the polypyrrole/carbon composite catalyst support on PEM fuel cell performance. Int. J. Hydrogen Energy 2016, 41, 13171–13179. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, X.; Zhao, X.; Gai, X.; An, W.; Fang, G.; Zhang, A.; Chen, X. Polypyrrole coated Fe3O4 nanoparticles decorated carbon nanotubes nanocomposites and the microwave absorption properties. J. Mater. Sci. Mater. Electron. 2019, 30, 17333–17341. [Google Scholar] [CrossRef]
- Petrovski, A.; Paunović, P.; Avolio, R.; Errico, M.E.; Cocca, M.; Gentile, G.; Grozdanov, A.; Avella, M.; Barton, J.; Dimitrov, A. Synthesis and characterization of nanocomposites based on PANI and carbon nanostructures prepared by electropolymerization. Mater. Chem. Phys. 2017, 185, 83–90. [Google Scholar] [CrossRef]
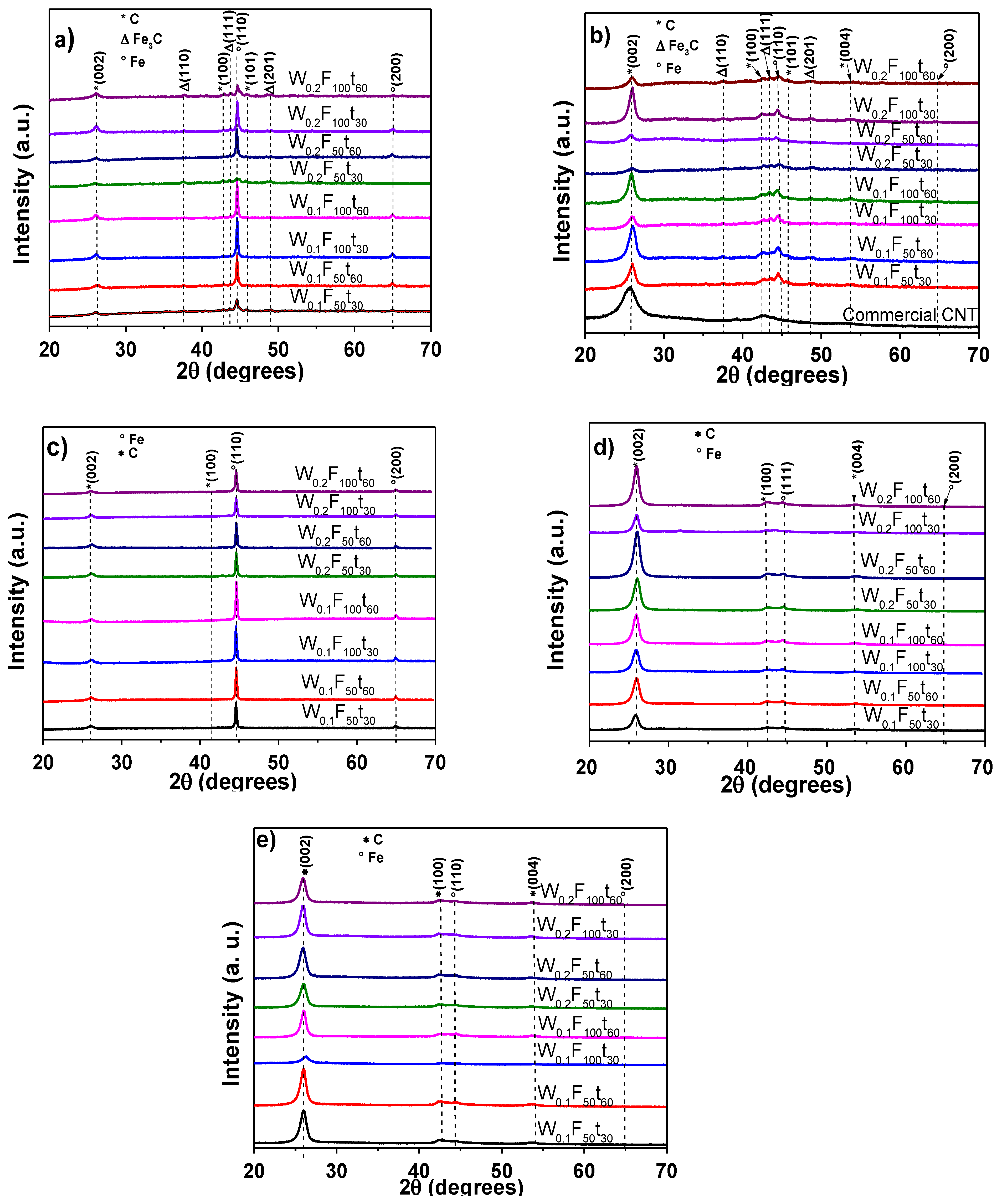





| CVD at 1023 K, Purified with HCl + H2O2 | |||||
| Sample | 2θ(deg) | t(nm), BP | t(nm),AP | d(nm),AP | Crystallinity(%) |
| Commercial CNT | 25.73 | 3.00 | 2.92 | 0.3459 | 85.30 |
| W0.1F50t30 | 25.95 | 9.01 | 8.46 | 0.3431 | 88.67 |
| W0.1F50t60 | 26.00 | 8.90 | 7.69 | 0.3424 | 91.36 |
| W0.1F100t30 | 25.93 | 10.88 | 8.76 | 0.3433 | 97.78 |
| W0.1F100t60 | 25.85 | 12.15 | 8.73 | 0.3443 | 97.03 |
| W0.2F50t30 | 25.93 | 7.90 | 7.02 | 0.3433 | 97.54 |
| W0.2F50t60 | 25.77 | 10.77 | 9.52 | 0.3454 | 96.96 |
| W0.2F100t30 | 25.94 | 11.28 | 9.81 | 0.3432 | 91.54 |
| W0.2F100t60 | 25.92 | 7.87 | 9.18 | 0.3434 | 98.07 |
| CVD at 1123 K, Purified with HCl + H2O2 | |||||
| Commercial CNT | 25.73 | 3.00 | 2.93 | 0.3459 | 85.30 |
| W0.1F50t30 | 25.82 | 12.42 | 8.73 | 0.3448 | 96.37 |
| W0.1F50t60 | 25.93 | 11.59 | 8.64 | 0.3433 | 96.56 |
| W0.1F100t30 | 25.88 | 10.75 | 8.55 | 0.3441 | 96.59 |
| W0.1F100t60 | 25.88 | 12.41 | 9.04 | 0.3440 | 96.44 |
| W0.2F50t30 | 26.02 | 10.06 | 8.30 | 0.3422 | 89.52 |
| W0.2F50t60 | 26.00 | 10.40 | 8.63 | 0.3424 | 94.56 |
| W0.2F100t30 | 25.94 | 13.81 | 8.47 | 0.3433 | 85.07 |
| W0.2F100t60 | 25.92 | 10.69 | 9.41 | 0.3434 | 95.11 |
| CVD at 1123 K, Purified with HNO3 + H2SO4 | |||||
| W0.1F50t30 | 25.96 | 12.42 | 8.75 | 0.3429 | 94.20 |
| W0.1F50t60 | 25.94 | 11.59 | 9.11 | 0.3432 | 84.41 |
| W0.1F100t30 | 26.22 | 10.75 | 8.51 | 0.3396 | 96.21 |
| W0.1F100t60 | 25.98 | 12.41 | 10.41 | 0.3427 | 86.83 |
| W0.2F50t30 | 25.95 | 10.06 | 8.43 | 0.3431 | 95.45 |
| W0.2F50t60 | 25.88 | 10.40 | 8.34 | 0.3439 | 95.38 |
| W0.2F100t30 | 25.89 | 13.81 | 9.74 | 0.3438 | 95.21 |
| W0.2F100t60 | 25.89 | 10.69 | 8.96 | 0.3438 | 87.32 |
| Content of MWCNTs | PPy/Synthesized MWCNTs | PPy/Commercial MWCNTs | PANI/Synthesized MWCNTs | PANI/Commercial MWCNTs |
|---|---|---|---|---|
| 1 wt% | 0.953 | 1.444 | 0.805 | 1.132 |
| 5 wt% | 0.903 | 1.731 | 0.750 | 0.761 |
| 6.5 wt% | 0.745 | 1.183 | 0.869 | 1.096 |
| 8 wt% | 0.733 | 1.492 | 1.038 | 0.785 |
| 10 wt% | 0.790 | 1.043 | 0.913 | 0.938 |
| 20 wt% | 0.692 | 1.671 | 0.977 | 0.923 |
| Sample | Ω*cm | S cm−1 | Sample | Ω*cm | S cm−1 |
|---|---|---|---|---|---|
| PPy | 86.3 ± 0.6 | 1.16 × 10−2 ± 8.49 × 10−4 | PANI | 90.6 ± 7.2 | 1.10 × 10−2 ± 8.80 × 10−4 |
| 1 wt% | 35.4 ± 0.4 | 2.82 × 10−2 ± 3.90 × 10−4 | 1 wt% | 64.4 ± 14.1 | 1.55 × 10−2 ± 3.41 × 10−3 |
| 5 wt% | 15.3 ± 0.0 | 6.5 × 10−2 ± 0.0 | 5 wt% | 55.3 ± 18.8 | 1.80 × 10−2 ± 5.94 × 10−3 |
| 6.5 wt% | 0.08 ± 0.01 | 12.5 ± 1.90 | 6.5 wt% | 30.8 ± 0.6 | 3.24 × 10−2 ± 6.48 × 10−4 |
| 8 wt% | 10.9 ± 0.0 | 9.1 × 10−2 ± 0.0 | 8 wt% | 8.3 ± 0.2 | 1.2 × 10−1 ± 3.6 × 10−3 |
| 10 wt% | 1.8 ± 0.3 | 5.5 × 10−1 ± 1.15 × 10−2 | 10 wt% | 3.8 ± 1.0 | 2.6 × 10−1 ± 7.28 × 10−2 |
| 20 wt% | 2.0 ± 0.0 | 5.0 × 10−1 ± 0.0 | 20 wt% | 0.4 ± 0.0 | 2.5 ± 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brachetti-Sibaja, S.B.; Palma-Ramírez, D.; Torres-Huerta, A.M.; Domínguez-Crespo, M.A.; Dorantes-Rosales, H.J.; Rodríguez-Salazar, A.E.; Ramírez-Meneses, E. CVD Conditions for MWCNTs Production and Their Effects on the Optical and Electrical Properties of PPy/MWCNTs, PANI/MWCNTs Nanocomposites by In Situ Electropolymerization. Polymers 2021, 13, 351. https://doi.org/10.3390/polym13030351
Brachetti-Sibaja SB, Palma-Ramírez D, Torres-Huerta AM, Domínguez-Crespo MA, Dorantes-Rosales HJ, Rodríguez-Salazar AE, Ramírez-Meneses E. CVD Conditions for MWCNTs Production and Their Effects on the Optical and Electrical Properties of PPy/MWCNTs, PANI/MWCNTs Nanocomposites by In Situ Electropolymerization. Polymers. 2021; 13(3):351. https://doi.org/10.3390/polym13030351
Chicago/Turabian StyleBrachetti-Sibaja, Silvia Beatriz, Diana Palma-Ramírez, Aidé Minerva Torres-Huerta, Miguel Antonio Domínguez-Crespo, Héctor Javier Dorantes-Rosales, Adela Eugenia Rodríguez-Salazar, and Esther Ramírez-Meneses. 2021. "CVD Conditions for MWCNTs Production and Their Effects on the Optical and Electrical Properties of PPy/MWCNTs, PANI/MWCNTs Nanocomposites by In Situ Electropolymerization" Polymers 13, no. 3: 351. https://doi.org/10.3390/polym13030351
APA StyleBrachetti-Sibaja, S. B., Palma-Ramírez, D., Torres-Huerta, A. M., Domínguez-Crespo, M. A., Dorantes-Rosales, H. J., Rodríguez-Salazar, A. E., & Ramírez-Meneses, E. (2021). CVD Conditions for MWCNTs Production and Their Effects on the Optical and Electrical Properties of PPy/MWCNTs, PANI/MWCNTs Nanocomposites by In Situ Electropolymerization. Polymers, 13(3), 351. https://doi.org/10.3390/polym13030351







